Abstract
Shewanella oneidensis MR-1 is a free-living gram-negative γ-proteobacterium that is able to use a large number of oxidizing molecules, including fumarate, nitrate, dimethyl sulfoxide, trimethylamine N-oxide, nitrite, and insoluble iron and manganese oxides, to drive anaerobic respiration. Here we show that S. oneidensis MR-1 is able to grow on vanadate as the sole electron acceptor. Oxidant pulse experiments demonstrated that proton translocation across the cytoplasmic membrane occurs during vanadate reduction. Proton translocation is abolished in the presence of protonophores and the inhibitors 2-heptyl-4-hydroxyquinoline N-oxide and antimycin A. Redox difference spectra indicated the involvement of membrane-bound menaquinone and cytochromes c, which was confirmed by transposon mutagenesis and screening for a vanadate reduction-deficient phenotype. Two mutants which are deficient in menaquinone synthesis were isolated. Another mutant with disruption in the cytochrome c maturation gene ccmA was unable to produce any cytochrome c and to grow on vanadate. This phenotype could be restored by complementation with the pEC86 plasmid expressing ccm genes from Escherichia coli. To our knowledge, this is the first report of E. coli ccm genes being functional in another organism. Analysis of an mtrB-deficient mutant confirmed the results of a previous paper indicating that OmcB may function as a vanadate reductase or may be part of a vanadate reductase complex.
One of the primary tasks of a microorganism is to catalyze chemical reactions in order to obtain energy for metabolic growth from its environment. The most well-known electron acceptor is O2. However, in the absence of oxygen, some microorganisms can grow by coupling the oxidation of simple organic acids, alcohols, H2, or aromatic compounds to the reduction of Fe(III) or Mn(IV) (21). Fe(III) and Mn(IV) reduction has an important impact on the organic and inorganic geochemistry of anaerobic aquatic sediments and groundwater (20). Many other metals, including Fe(III), Mn(IV), Mn(III), Cr(VI), Hg(II), Au(III), Ag(I), Mo(VI), Co(III), Pd(II), As(V), Se(VI), Se(IV), U(VI), Tc(VII), Te(IV), V(V), can be enzymatically reduced, but microorganisms are not necessarily able to conserve energy from the reduction process (20, 43). A distinction is made between respiratory metal reduction, in which the electron flow is coupled to a proton-translocating complex to allow ATP generation, and dissimilatory reduction, in which electrons are transferred without the generation of a proton motive force (24). Vanadate is a known electron acceptor for anaerobic respiration (9, 23, 35, 52), but only limited data are available on the biological vanadium reduction process, concomitant precipitation effects, and the geochemical implications. Although vanadium is not abundant, it is omnipresent in nature (51). Of importance is the comparatively large amount of vanadium in seawater, which contains vanadate at an average concentration of 30 nM (7). This makes vanadium the second most common transition metal in seawater, surpassed only by molybdenum (100 nM), and it is clearly more abundant than iron (0.02 to 1 nM). However, this concentration is rather low for use of vanadium as an electron acceptor for anaerobic respiration. The vanadium content of soils is related to that of the parent rocks from which they are formed and ranges from 3 to 310 mg/kg (51). Although vanadate species are soluble at neutral pH, it has been found that very little soil vanadium is released into rivers (0.001 mg/liter), in which it is present predominantly in suspended form (87%) and to a lesser extent in solution (13%) (15, 17). Bacterial vanadium reduction may play a significant part in the element's low mobility in soil and may contribute to the suspended, vanadium-containing colloid fraction present in rivers. In addition to colloid deposition by sedimentation (37, 45), bacterial reduction may also contribute to the high vanadium concentrations associated with organic material, like those often found in ocean floor silts. Shewanella oneidensis MR-1 is a gram-negative facultative anaerobic organism which can grow by reduction of a variety of compounds, including manganese(IV) oxides, iron(III) oxides, fumarate, nitrate, trimethylamine N-oxide (TMAO), and many other compounds (26, 27, 31). In a previous report, we showed that S. oneidensis can reduce V(V) using a number of electron donors, which results in extracellular precipitation of reduced vanadate products (9). In a recent study, Myers et al. (33) demonstrated that menaquinone-, cymA-, and omcB-deficient mutants are severely limited in the capacity to reduce vanadate. Here, we provide evidence that the reduction of V(V) is an energy-conserving process, resulting in proton translocation and growth. By using a different approach, we also confirmed the findings of Myers et al. (33) that menaquinone and membrane-localized c-type cytochromes play a crucial role in electron transfer to V(V).
MATERIALS AND METHODS
Bacterial strains and plasmids.
S. oneidensis MR-1 (= LMG 19005) was obtained from the LMG culture collection. S. oneidensis MR-1R is a spontaneous rifampin-resistant mutant of strain MR-1 that was isolated in-house. The construct pEC86 is a pACYC184 derivative containing the ccmABCDEFGH genes from Escherichia coli (4).
Growth conditions and analytical techniques.
S. oneidensis cultures were routinely grown aerobically in Luria-Bertani (LB) broth on a rotary shaker (200 rpm) at 28°C. Anaerobic growth was performed in SM defined medium as described by Myers and Nealson (30). When growth on V(V) was analyzed, the SM medium was replaced by VM medium that had a pH of 7.0 and consisted of 50 mM lactate, 18 mM NH4Cl, 6 mM HEPES, 1.3 mM KH2PO4, 2.3 mM K2HPO4, 2.0 mM NaHCO3, 1.0 mM MgSO4, 0.49 mM CaCl2, 0.2 g/liter vitamin-free Casamino Acids, and trace elements as described previously (30); vanadate was added at various concentrations. The vanadate reduction capacities of cultures grown under different conditions were tested as described previously (9). For testing growth on vanadate, VM medium was inoculated with washed cells grown anaerobically in SM mineral medium containing fumarate (9). Anaerobic conditions were obtained using a Coy anaerobic chamber (Coy Laboratories, Grass Lake, MI) containing an atmosphere of 90% N2, 8% CO2, 2% H2. When the presence of H2 was undesirable, the medium was made anaerobic by flushing it with oxygen-free N2 gas. For measuring V(V) reduction, a vanadate detection assay (DPC assay) was used, as described previously (9). Fe(III) reduction was monitored by measuring Fe(II) production using the phenanthroline method (46). Mn(IV) reduction was monitored by measuring Mn(II) formation by the formaldoxime method (3).
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) analysis was performed as described by Laemmli (19). Proteins were visualized by Coomassie brilliant blue R-250 staining. Heme staining was performed for detection of cytochromes c, and the specific cytochrome content was determined as described previously (28, 47). Culture turbidity was measured at 500 nm. To determine the protein concentration, washed cells were lysed in 1 N NaOH and analyzed using the Bio-Rad protein assay (Bio-Rad, Munich, Germany). A vanadate stock solution was prepared from V2O5 (Sigma-Aldrich, St. Louis, MO) as described by Carpentier et al. (9).
Oxidant pulse experiments.
Experiments were performed by using the oxidant pulse technique (41, 50) at room temperature as previously described by Myers and Nealson (31). A Jenway 924001 combination pH electrode (Jenway, Felsted, England) and gas inlet and outlet needles were inserted through the closure. An LF351 operational amplifier (STMicroelectronics, Geneva, Switzerland) was used in a unity gain configuration as an interface for the pH electrode, a digital voltmeter, and an REC 112 x/t analogue recorder (Amersham Pharmacia, Uppsala, Sweden) set to a full-scale sensitivity of 1 mV. As described by Myers and Nealson (31), late-exponential-phase fumarate-grown MR-1 cells were harvested and washed twice in oxygen-free KKG buffer (100 mM KSCN, 50 mM KCl, 1.5 mM glycylglycine, pH 7.1) before they were resuspended in 5 ml of anaerobic KKG buffer containing 2 mM lactate at a density of 2 mg of total cellular protein per ml. Cells grown anaerobically on vanadate were not tested because this type of growth resulted in association of the cells with vanadium reduction products that could not be washed away completely. Solutions were tested for residual oxygen content with a Beckman oxygen analyzer (Beckman Coulter, Fullerton, CA). Anaerobic solutions of sulfate, fumarate, iron (ferric citrate), and V(V) were prepared in KKG buffer at a concentration of 2 mM at pH 7.1. Various amounts of the electron acceptors were injected into cell suspensions with an N2-flushed gas-tight microsyringe (Hamilton, Bonaduz, Sweden). Pulse amplitudes were quantified by the graphic method of Scholes and Mitchell (41). Changes in pH were calibrated by micromolar increments using a 1 mM HCl solution in N2-saturated KKG buffer. H+/2e− ratios were evaluated from independent triplicate experiments. Samples were taken from the proton translocation assays for spectrophotometric confirmation of the presence of the vanadyl ion using 4,5-dihydroxy-1,3-benzenedisulphonate, as described by Lyalikova et al. (23). Pulsing with sulfate was used as a negative control for every batch of solutions, because sulfate cannot be used as electron acceptor (31). Iron and fumarate H+/2e− ratios served as positive controls, and the values were compared to those obtained in a previous study (31).
Mutagenesis and screening.
Transposon mutants were generated as described by Beliaev and Saffarini using the Tn5 transposon (5). Mutagenized clones were inoculated into 96-well microplates and grown in 100 μl LB broth to the late stationary phase. Optical density at 600 nm was measured using a microplate reader. Cultures that deviated by more than 10% from the average optical density were disregarded during the rest of the experiment. Microplates were placed in a Coy anaerobic chamber (Coy Laboratories, Grass Lake, MI) for 15 min prior to the addition of 100 μl anaerobic assay buffer (9). Vanadate reduction resulted in a blue-green discoloration within 60 min. The lack of discoloration or reduced discoloration enabled identification of mutant cultures. The DNA that flanks the transposon insertions was identified using arbitrary PCR (8), as described by O'Toole and Kolter (36). The PCR products obtained were purified from the agarose gel using the QIAquick Spin purification procedure (QIAGEN, Venlo, The Netherlands) as described by the manufacturer. The products were sequenced and were analyzed on the basis of the genome sequence of S. oneidensis (14).
Preparation and analysis of loosely attached proteins.
The procedure for preparation and analysis of loosely attached proteins was performed essentially as described by DiChristina et al., with minor adaptations (12). Briefly, cell cultures were grown to the late exponential phase and harvested by centrifugation (at 4°C and 8,000 × g). Cells were washed once in 10 mM Tris-HCl, pH 7.2, and subsequently washed with 0.5 M KCl. Spent KCl wash buffer was filtered through 0.22-μm-pore-size filters, and the filtrate was concentrated on Millipore filters (5-kDa cutoff) before SDS-PAGE analysis.
Redox difference spectra.
Crude membrane fractions were prepared as described by Schumacher and Holliger (43). Difference spectra for the membrane fractions were recorded using a Uvikon 943 double-beam spectrophotometer (Kontron Instruments, Watford, United Kingdom) and quartz cuvettes containing ∼1 mg of protein per ml (determined using the Bio-Rad protein assay [Bio-Rad, Munich, Germany]) in O2-free 100 mM Tris-HCl buffer, pH 7. The membrane fractions in the reference cuvette and in the sample cuvette were suspended in H2-saturated buffer and reduced for at least 15 min. The reduced membranes were reoxidized by introducing 0.1 to 0.5 mM V(V) into the sample cuvette. The V(V)-oxidized-minus-reduced difference spectrum of the membrane fraction was recorded after 10 min to allow for complete reoxidation.
Quinone preparation.
Quinones were prepared as described previously (18), resolved by thin-layer chromatography (16) on Merck Kieselgel 60 F-254 plates, and developed with petroleum ether-diethyl ether (9/1 [vol/vol]). The plates were examined under reflective UV light. Ubiquinone-6 and menaquinone-4 were used as reference compounds. The quinones were identified by relative migration, as described previously (27).
RESULTS
Inducibility of vanadate reduction.
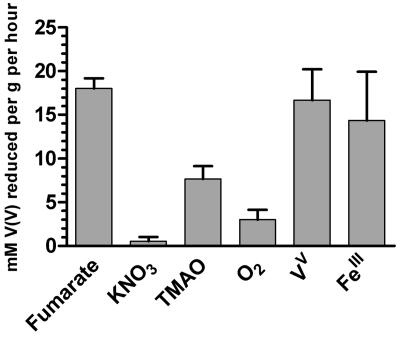
To enable characterization of the V(V) reduction process, we determined the growth conditions in which the highest V(V) reduction capacity was expressed. The results (Fig. 1) demonstrate that cells grown anaerobically on fumarate, Fe(III) citrate, and vanadate express the highest reduction capacity. Interestingly, cells grown anaerobically on nitrate reduced almost no V(V).
FIG. 1.
Vanadate reduction capacity of S. oneidensis MR-1 cells grown in SM medium either aerobically or anaerobically on fumarate, KNO3, Fe(III) citrate, TMAO, or V(V) (in VM medium). Values were obtained from the initial reduction rates, as described previously (9) and are expressed as mM V(V) reduced per gram (wet weight) of cells per hour.
Proton translocation.
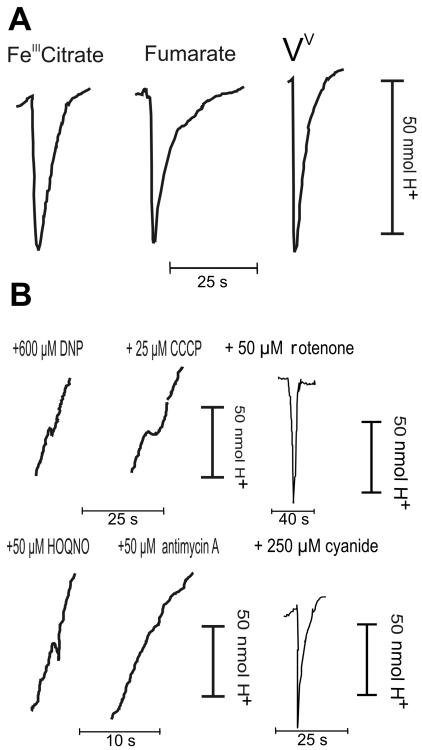
If vanadium reduction is a respiratory process, then the electron flow should be coupled to a proton-translocating complex to allow ATP generation. If electrons are transferred without the generation of a proton motive force, the process is strictly dissimilatory (24). To distinguish between the possibility that vanadium reduction is a dissimilatory process and the possibility that vanadium reduction is a respiratory process, proton liberation measurements were obtained using the classical oxidant pulse method (41). Limiting amounts (10 to 150 nmol) of an electron acceptor were added to fumarate-grown resting cells that were saturated with lactic acid (2 mM). The reaction was started by addition of nanomolar amounts of V(V), after which changes in the pH of the suspension were recorded. Actual reduction of V(V) was confirmed by the DPC assay. Reduction of vanadate was clearly associated with rapid (half-time, 2 ± 1 s) proton liberation into the medium (Fig. 2A). The H+/2e− ratio for proton liberation per V(V) molecule reduced was 0.62 ± 0.18. This value is low compared to the value for fumarate, since the fumarate/succinate couple has an Eh of 0.033 V (31). The Fe(III)/FeII couple, however, has an Eh of 0.77 and an H+/2e− ratio of 0.57. Compared to this value, the H+/2e− ratio for vanadate (Eh = 0.13 V) is rather high. The efficiency of proton translocation is of course related to the specific nature of the electron transfer pathway and the specific proteins involved. No significant irreversibility of the initial acidification pulse was detected. Such an irreversible acidification, or possibly alkalinization, may be the result of chemical proton consumption or liberation during the formation of the vanadyl ion. In control experiments, the ratios for ferric citrate and fumarate were determined to be 0.50 ± 0.11 and 0.39 ± 0.12, respectively. Acidification was followed by slow (half-time, 15 to 30 s) alkalinization due to proton backflow into the cells. Addition of the electron transport inhibitor 2-heptyl-4-hydroxyquinoline N-oxide (HOQNO) (50 μM) dramatically reduced proton translocation to vanadate, while addition of antimycin A (50 μM) completely abolished proton liberation (Fig. 2B). Inhibition of proton translocation by HOQNO and antimycin A in response to ferric citrate was observed in a control reaction and was consistent with the results described previously (31). We therefore believe that HOQNO and antimycin A are effective inhibitors of V(V) reduction by MR-1. Their action confirms that actual electron transport is required to generate the proton pulse. Addition of 50 μM rotenone or 250 μM cyanide did not inhibit proton translocation (Fig. 2B) to V(V), fumarate, or ferric citrate. Cyanide inhibits the oxidase and would therefore have abolished the pulse if it were the result of oxygen contaminants in the electron acceptor solutions. The inability of rotenone to inhibit electron transport has been demonstrated previously for ferric iron reduction (31). Because actual V(V) reduction was measured during the proton pulse experiments and because of the effect of the inhibitors HOQNO and antimycin A, the observed proton pulses could not have been generated by antiporter activity or ATPases. Such activities would not be influenced by the inhibition of electron transfer and would not result in V(V) reduction. Inhibition of proton pulses by protonophores can be expected if proton pulses are really the result of a proton gradient across the membrane. We found that addition of the protonophores carbonyl cyanide m-chlorophenylhydrazone (CCCP) (25 μM) and dinitrophenol (600 μM) resulted in markedly lower proton pulses to V(V) (Fig. 2B), fumarate, and Fe(III) citrate. In the presence of either of the protonophores, rapid alkalinization occurred, indicating that the extracellular environment was acidic compared to the cell cytoplasmic space.
FIG. 2.
(A) Proton pulse traces obtained with fumarate-grown S. oneidensis MR-1 cells in response to 100 nmol ferric citrate, fumarate, and vanadate. (B) Pulse traces from 100 nmol vanadate in the presence of dinitrophenol (DNP), CCCP, HOQNO, antimycin A, rotenone, and cyanide at the concentrations indicated.
Growth of S. oneidensis MR-1 on vanadate.
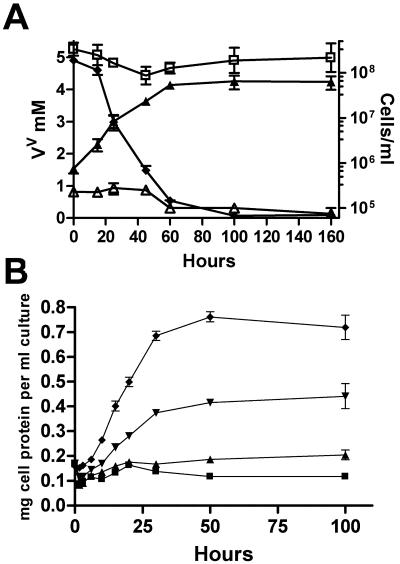
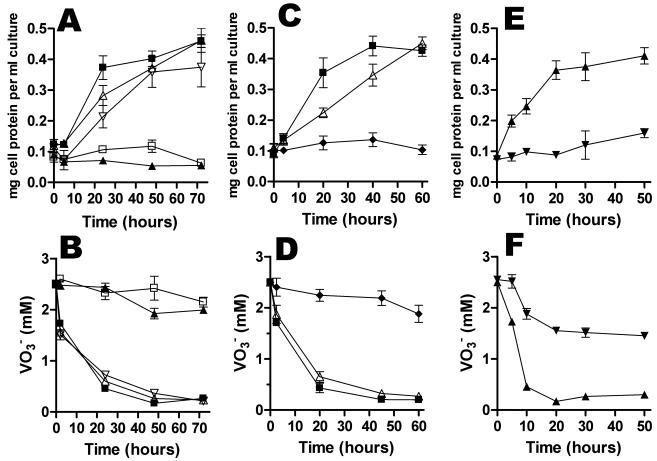
Since proton pulse experiments indicated that S. oneidensis MR-1 performs proton translocation in response to V(V), the organism is likely to be able to conserve energy for growth by V(V) respiration. As described by Myers et al. (33), we confirmed that anaerobic growth in vanadate-supplemented SM medium (pH 7.4) is not possible. However, growth occurred in an adapted SM medium, designated VM medium, which was used for further experiments. For typical growth experiments, cells were pregrown anaerobically in defined medium (SM medium) containing 20 mM fumarate, before they were inoculated into VM medium using V(V) as an electron acceptor. To eliminate a lag phase and to obtain the maximal growth rate, cells previously grown on V(V) were then washed anaerobically and reinoculated into VM medium. Growth was monitored by determining the amount of total cellular protein per ml of washed cells, by measuring culture turbidity at 500 nm, and by determining colony counts for serial dilutions. The results are presented in Fig. 3, which clearly shows that growth and concomitant vanadate reduction occurred (Fig. 3A). An average biomass doubling time of 10 h ± 1.3 h was routinely observed. The biomass yield was directly proportional to the amount of vanadate used (Fig. 3B). A potential for negative interference by V(V) and V(IV) existed for the determination of protein concentration, and a potential for positive interference existed for turbidity measurements based on the V(IV) formed. This could conceivably have resulted in underestimation and overestimation of the growth, respectively. Therefore, turbidity and the protein concentration were measured simultaneously in each experiment. Control samples with added V(V) and V(IV) were subjected to the same procedures. The good correlation between the protein measurements and the turbidity, as well as control experiments, confirmed that the residual sample concentrations of vanadium were too low to interfere with these measurements.
FIG. 3.
(A) Anaerobic growth in VM medium under an N2 atmosphere (▴) and concomitant reduction of 5 mM V(V) (⧫); V(V) reduction with cells omitted (□); and growth with vanadate omitted (▵). (B) Cellular protein formed using 0.5 (▪), 1 (▴), 2.5 (▾), and 5 (♦) mM of vanadate as electron acceptor in VM medium under an N2 atmosphere. All measurements at each time were obtained by calculating the average values from at least three independent incubations.
Menaquinones and cytochromes are involved in V(V) reduction.
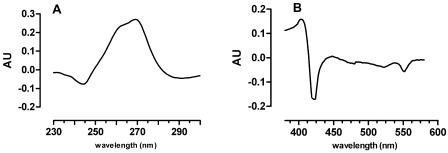
It has been shown that S. oneidensis produces quinones that are involved in a number of reduction processes (2, 32), including reduction of vanadate (33). The redox response to V(V) of the quinone constituents of the membrane fraction was therefore studied by difference spectroscopy. Incubation of the membrane fraction of anaerobic cells grown on fumarate in H2-saturated Tris buffer in the sample and reference cuvettes was followed by addition of vanadate to the sample cuvette. This resulted in a V(V)-oxidized-minus-reduced difference spectrum with an absorbance minimum at 245 and an absorbance maximum at 260 to 270 nm (Fig. 4A). This corresponds to the spectrum of a menaquinone and not the spectrum of a ubiquinone compound which has a typical maximum at 275 nm (2, 32, 42), although the possibility of reduction of a limited amount of ubiquinone cannot be excluded as it may have been obscured by the partially overlapping menaquinone spectrum. To determine whether membrane-localized cytochromes take part in the reduction of vanadate, the redox difference spectra of anaerobic H2-reduced crude membrane fractions in the reference cell and anaerobic vanadate reoxidized membranes were obtained spectrophotometrically. The difference spectrum clearly displayed a Soret γ-absorption trough at 420 nm. Additionally, the decreases in absorbance of the β peak and the α peak at 523 nm and 552 nm, respectively, are characteristic of cytochrome c oxidation (Fig. 4B).
FIG. 4.
Redox difference spectra [V(V) oxidized minus reduced] of the membrane fraction of S. oneidensis MR-1. The difference spectra were recorded in the quinone (A) and cytochrome (B) absorption ranges. AU, absorption units.
Isolation of mutants defective in V(V) reduction.
To identify the genes encoding the proteins that enable S. oneidensis to reduce vanadate ions, a transposon mutagenesis strategy was employed. Transposon insertion mutants were made as described by Beliaev and Saffarini (5). For screening, colonies were inoculated into 96-well microplates, grown, and assayed for their vanadate reduction capacities. Upon reduction, blue-green discoloration enabled visual scoring of the individual wells. From a total of 18,000 mutants that were screened for their vanadate reduction capacities, 4 mutants with lower capacities were isolated and analyzed further in the present study. The mutants were designated VRD1, VRD2, VRD3, and VRD4. To identify the point of insertion of the Tn5 transposon, an arbitrarily primed PCR strategy was used.
Menaquinone-deficient mutants.
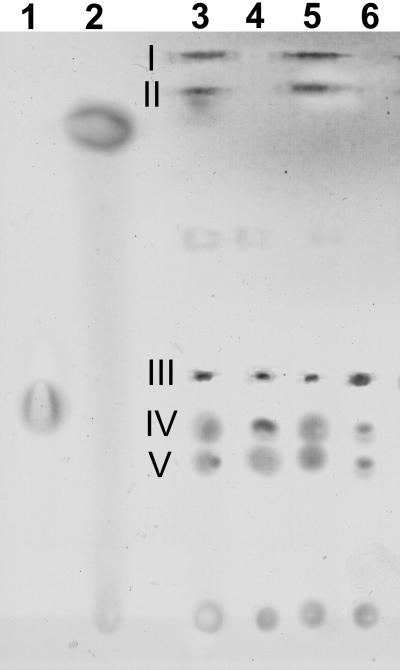
VRD1 is a menB disruption mutant. The menB gene product catalyzes a crucial step in menaquinone synthesis. VRD2 has a disruption in menD, which is part of a four-gene cluster that is necessary for menaquinone synthesis. Compared to the control cultures, both the VRD1 and VRD2 strains lost the capacity to reduce vanadate and were unable to grow (Fig. 5A and B). Additionally, both strains were severely limited in the capacity for anaerobic growth on fumarate and dimethyl sulfoxide (DMSO) but not TMAO (Table 1). The mutants were also deficient in reduction of Fe(III) citrate, solid Fe(III) oxide, and Mn(IV) oxide, while aerobic growth was not affected (Table 1). To confirm the defects in menaquinone synthesis, quinones were extracted and resolved by thin-layer chromatography (Fig. 6). Although growth on fumarate was severely limited for both VRD1 and VRD2, prolonged growth for several weeks in SM medium allowed us to harvest sufficient biomass for extraction of quinones. This showed that both menaquinone and methylmenaquinone were absent, while ubiquinones were present at normal levels.
FIG. 5.
(A and B) Anaerobic growth in VM medium (A) and concomitant reduction of vanadate (B) for VRD1 (□), VRD2 (▴), MR-1R (▪), VRD1 (▿) amended with 50 μM vitamin K2, and VRD2 (▵) amended with 50 μM vitamin K2. (C and D) Growth of (C) and concomitant reduction of vanadate by (D) mutant VRD3 (♦) and VRD3/pEC86 (▵), compared to MR-1R (▪). (E and F) Growth of (E) and concomitant reduction of vanadate by (F) mutant VRD4 (▾), compared to MR-1R (▴). All measurements at each time were obtained by calculating the average values for at least three independent incubations.
TABLE 1.
Growth and metal reduction capacities of wild-type strain MR-1 and isolated mutants
| Strain | Electron acceptora
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Fumarateb | DMSOb | TMAOb | V(V)b | Fe(III) citratec | Fe2O3c | MnO2d | O2b | |
| MR-1 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| MR-1R | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| VRD1 | − | − | ++ | − | − | − | − | ++ |
| VRD2 | − | − | ++ | − | − | − | − | ++ |
| VRD3 | − | − | − | − | − | − | − | ++ |
| VRD4 | ++ | ++ | ++ | + | + | − | − | ++ |
++, normal growth [for fumarate, DMSO, TMAO, V(V), and O2] or metal reduction [for Fe(III) citrate, Fe2O3, and MnO2] compared to wild-type strain MR-1; +, significantly lower but nonetheless detectable growth [for fumarate, DMSO, TMAO, V(V), and O2] or metal reduction [for Fe(III) citrate, Fe2O3, and MnO2] compared to MR-1; −, no detectable growth [for fumarate, DMSO, TMAO, V(V), and O2] or metal reduction [for Fe(III) citrate, Fe2O3, and MnO2]. Data were obtained from at least three independent experiments.
Growth was measured spectrophotometrically after 24 h.
Fe(II) formation was measured after 24 h.
Mn(II) formation was measured after 24 h.
FIG. 6.
Thin-layer chromatogram of quinone standards and quinones isolated from S. oneidensis cells grown anaerobically with fumarate as the electron acceptor. Lanes 1 and 2 were loaded with ubiquinone-6 and menaquinone-4, respectively. The other lanes were loaded with quinone extracts isolated from cells of the following strains: lane 3, MR-1R; lane 4, VRD1; lane 5, MR-1R; lane 6, VRD2. Slight differences in migration of quinone standards compared to the quinones from MR-1R were due to differences in the length and composition of the isoprenyl side chain. Spots I (methylmenaquinone), II (menaquinone), and III to V (ubiquinones) were identified by relative migration (27).
We demonstrated that the wild-type phenotype could be restored almost completely upon addition of 0.05 mM vitamin K2 to the medium for both VRD1 and VRD2, resulting in vanadate reduction and growth that proceeded at levels similar to the levels observed for the MR-1R strain (Fig. 5A and B).
Analysis of a c-type cytochrome-deficient mutant.
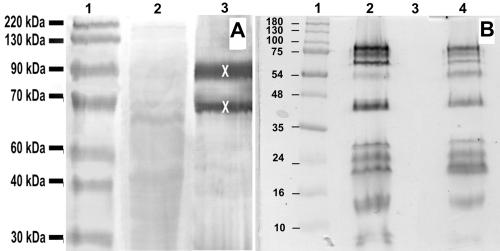
Mutant VRD3 has a Tn5 transposon insertion in the ccmA gene, the first gene of the ccmABCDE operon encoding proteins essential for cytochrome c maturation and heme delivery (1, 49). Compared to the control cultures, the VRD3 strain had lost the capacity to reduce vanadate and grow (Fig. 5C and D). While the rates of aerobic growth in LB medium, as well as in SM medium, were normal, the strain was unable to grow anaerobically on fumarate, TMAO, DMSO, Fe(III) citrate, Fe2O3, or MnO2 (Table 1). SDS-PAGE analysis and heme staining of protein extracts from MR-1R and VRD3 showed a complete absence of cytochromes in VRD3 (Fig. 7B). Interestingly, the phenotype was shown to be fully complemented in trans with the pEC86 plasmid (4) that expresses the E. coli ccm genes. SDS-PAGE analysis and heme staining of extracts of the complemented VRD3 mutant revealed wild-type cytochrome levels (Fig. 7B). Also, anaerobic growth with V(V) and V(V) reduction were restored to almost wild-type levels (Fig. 5C and D). For mutant VRD3, no menaquinone reoxidation by vanadate was detected in the redox difference spectra.
FIG. 7.
(A) Heme-stained SDS-PAGE analysis of loosely attached proteins from cells grown anaerobically on fumarate. Equal amounts (∼30 μg protein) of sample were loaded. Lane 1, molecular weight reference sample; lane 2, S. oneidensis MR1-R protein extract; lane 3, mutant VRD4 protein extract. X indicates differences at ∼65 and ∼90 kDa. (B) Heme-stained SDS-PAGE analysis of total cellular protein of cells grown aerobically in LB broth. Lane 1, molecular weight reference sample; lane 2, S. oneidensis MR1-R; lane 3, VRD3; lane 4, VRD3/pEC86.
Analysis of a mutant deficient in MtrB.
VRD4 has a disrupted mtrB gene, which is part of a seven-gene cluster consisting of mtrDEF, omcAB, and mtrAB. While MtrA and MtrD are periplasmic cytochromes, MtrF, OmcA, and OmcB are outer membrane decaheme cytochromes. MtrB is known to be essential for proper incorporation of the outer membrane cytochromes OmcA and OmcB in the outer membrane (28). VRD4 was deficient in growth with V(V) as the terminal electron acceptor, and accordingly, a limited amount of V(V) was reduced by the cells (Fig. 5E and F). Of the different electron acceptors tested, only small amounts of the organic ion adduct Fe(III) citrate were reduced. However, there was no detectable growth with any of the metal substrates (Table 1). VRD4 was able to grow normally on TMAO, fumaric acid, and DMSO. The mutation is clearly specific for the metals tested and not for organic electron acceptors. This suggests that MtrB affects, or is part of, a metal reductase pathway which is shared by at least Fe(III), Mn(VI), and V(V).
To examine VRD4 for mislocalization of outer membrane proteins, peripherally or loosely attached proteins were extracted. The protein extracts obtained were subsequently analyzed by SDS-PAGE, which showed differences in protein profiles after cytochrome c staining. The stain revealed proteins with apparent molecular masses of approximately 65 and 90 kDa which were extracted from the mutant VRD4 strain but not from MR-1R (Fig. 7A). These cytochromes were identified by N-terminal sequencing to be OmcB and OmcA, respectively. Mislocalization of these cytochromes and the resulting inability to perform their redox function are consistent with the results obtained by Myers et al. (28, 33). As for VRD3, no reoxidation with vanadate was detected in the redox difference experiments with this mutant either.
DISCUSSION
Respiration of S. oneidensis MR-1 on vanadate.
We previously reported on the vanadate reduction capacity of S. oneidensis (9). We show here that the vanadate reductase activity is not specifically induced by growth on V(V) but that anaerobic growth using fumarate or Fe(III) citrate also induces the V(V) reductase activity. This suggests either that there is common regulation of individual pathways specific for the different metals or that it is conceivable that the terminal metal reductase is rather unspecific in terms of the substrate. As the standard redox potential for the reduction of V(V) to V(IV) (VO2+ → VO2+) at pH 7.4 is 0.127 V (33), the reduction should enable energy conservation by the microorganism using several organic and inorganic electron donors. To distinguish between the possibility that vanadate reduction is a dissimilatory process and the possibility that vanadate reduction is a respiratory process, we examined whether the electron flow results in proton translocation across the cytoplasmic membrane. Respiration-dependent proton translocation in response to the acceptor provides evidence that energy is conserved from the reduction of the electron acceptor. Proton extrusion and energy conservation have been demonstrated for iron and manganese in several species of respiratory bacteria, including S. oneidensis (30, 31, 43). We therefore set out to examine this type of translocation linked to vanadate in S. oneidensis. Proton liberation measurements were obtained by the classical oxidant pulse method (41). The ratio determined, one proton per two electrons for the electron transport from lactate to vanadate, was similar to the value obtained for ferric citrate. It seems that the proton liberation is coupled to transmembrane electron transport, as indicated by the H+ conductance mediated by the protonophores CCCP and dinitrophenol, which abolish fast proton liberation. The effect of the inhibitors HOQNO and antimycin A suggests involvement of b- and c-type cytochromes in the electron transport chain (11, 44). Both the effect of the protonophores CCCP and dinitrophenol on the one hand and the effect of HOQNO and antimycin A on the other hand are similar to the results obtained in iron- and manganese-linked proton translocation experiments (31). In addition, the H+/2e− ratios obtained for iron and fumarate confirm the values obtained in a previous study (31). Regardless of stoichiometries, MR-1 is clearly capable of translocating protons in response to V(V) and is therefore capable of generating a proton motive force via anaerobic respiration.
S. oneidensis MR-1 can grow on vanadate.
Growth data presented here suggest that MR-1 is indeed capable of using vanadate as a terminal external electron acceptor, at least under the growth conditions used. Adaptation of the medium composition proved to be necessary for successful growth on vanadate. In a recent report that characterized the vanadate reduction by S. oneidensis MR-1, no growth could be demonstrated under anaerobic conditions that are known to sustain growth on iron and manganese (33). We confirmed that under these conditions growth does not occur. However, the complex interdependence of pH, concentration, redox potential, and complexation for vanadium speciation in solution and its plethora of possible interactions with medium components, such as bicarbonate, phosphate, and lactate, must be taken into consideration (10, 13, 38, 39). In doing this, the SM medium traditionally used for respiratory growth of the organism was adapted until growth was obtained. Compared to the SM medium, the lactate concentration, which has a chelating effect on vanadate, was increased, the pH was lowered to 7.0, and the phosphate concentration was slightly lowered. Under these conditions, cells grew with an average doubling time of 10 h, and the biomass yield was proportional to the amount of V(V) available (Fig. 3A and C). The growth data, combined with the proton translocation data, provide unequivocal evidence for energy conservation linked to the reduction of vanadate. The environmental relevance of V(V) reduction in a marine environment is questionable due to the typically low concentrations (30 nM) of vanadium present. The vanadium present in soils and aquifers may be associated to a large extent with solids and may be chelated by humics and organic acids. Although the respiration on soluble, unbound vanadate may not be representative of typical environmental conditions, S. oneidensis is clearly metabolically and biochemically capable of V(V) respiration.
Menaquinones and cytochromes are electron carriers in anaerobic respiration on V(V).
The inhibition of proton translocation by antimycin A and HOQNO points toward a cytochrome-mediated exocytoplasmic site of reduction. We examined this further, reasoning that, if membrane cytochromes effectively take part in the process, the exposure of reduced cytochrome to vanadate should result in cytochrome oxidation. To determine this, V(V)-oxidized-minus-reduced difference spectra of the cell membranes of anaerobically grown MR-1 were recorded spectrophotometrically. The difference spectrum clearly shows the typical Soret trough, in addition to disappearance of the α and β peaks, which is consistent with cytochrome c reoxidation, indicating involvement of c-type cytochromes in the electron transfer pathway (Fig. 4B). Although the wavelength maximum of the α peak is a specific feature of each cytochrome c, the abundance of cytochromes present in the membrane (25) and the fact that many cytochromes in S. oneidensis contain multiple hemes (14) did not allow us to determine the identity of the cytochrome(s) involved. Myers and coworkers have found that a chemically mutagenized S. oneidensis strain which is unable to produce menaquinone cannot reduce vanadate (33). We confirmed the involvement of menaquinone by redox difference spectroscopy. The observed difference spectrum is consistent with a menaquinone and not a ubiquinone compound (32, 34, 48). Although we could not strictly exclude the possibility of reoxidation of ubiquinone in this experiment, menaquinone is clearly the major component involved.
Mutants confirm the requirement for menaquinones and cytochromes c.
In an attempt to identify the molecular components that constitute the respiratory chain for vanadate reduction, 18,000 transposon mutants were screened. Four mutants isolated from the screening were examined further.
The menB gene and the menD gene were found to be disrupted in mutants VRD1 and VRD2, respectively. Both of these genes code for proteins which catalyze crucial steps in the naphthoquinone (menaquinone and dimethylmenaquinone) synthesis pathway of S. oneidensis. Transposon mutants deficient in menaquinone biosynthesis have been described previously (40) in a study on Fe(III) respiration. We confirmed that the mutants deficient in menB and menD are unable to reduce ferric iron, Mn(IV), and a number of other electron acceptors, including V(V). Our findings thus show that menaquinone synthesis is crucial not only for iron and manganese reduction but also for growth on vanadate, which is in accordance with the results described by Myers and coworkers (33). As Fig. 5A and B show, the defects can be overcome by addition of vitamin K2 to the growth medium. These results indicate that the key role of menaquinone for electron transport across the cell membrane is shared for iron, manganese, and vanadate reduction.
The VRD3 strain lost vanadate reduction and growth capacity (Fig. 5C and D). The disrupted ccmA gene is part of a five-gene operon known to take part in cytochrome c maturation and heme delivery (1, 49). Interestingly, the phenotype could be restored by complemention in trans with the pEC86 plasmid constitutively expressing the complete ccm operon from E. coli. Complementation resulted in complete restoration of the cytochrome content and enabled vanadate reduction and growth at almost wild-type levels (Fig. 5C and D).
As ccmA is the first gene of the ccmABCDE operon, the Tn5 insertion in mutant VRD3 most likely prevents transcription of the downstream ccm genes as a result of polar effects. This means that in addition to CcmA, the other E. coli Ccm proteins might be functional in S. oneidensis as well. To our knowledge, for CcmA, this is the first report that E. coli ccm gene products are functional in another organism. Our results, combined with the effect of oxidant pulse inhibitors and the cytochrome redox response, unambiguously show the importance of membrane cytochromes in the electron transfer pathway to vanadate.
Mutant VRD4 had the Tn5 insertion in the mtrB gene and was unable to grow on vanadate (Fig. 5E and F). Although VRD4 was still capable of aerobic growth and anaerobic growth using fumarate and DMSO, the strain was deficient in iron and manganese reduction (Table 1). The mtrB gene, together with the mtrA gene preceding it, is essential in Mn(IV) and Fe(III) reduction (5, 6). It is known that MtrB is necessary for proper incorporation of cytochromes OmcA and OmcB into the outer membrane of MR-1 (28) and that both of these cytochromes are exposed on the outer face of the membrane (29). We found that treatment of VRD4 with 0.5 M KCl readily released both OmcA and OmcB, which was not the case for strain MR-1, in which these cytochromes are embedded in the outer membrane (Fig. 7A). The localization of cytochromes in the outer membrane suggests a plausible mechanism by which electrons can be transferred to metals at the cell surface, an idea which has been underscored by atomic force microscopy studies that indicated the presence of Fe(III) reductase in the outer membrane (22). In the recent study of Myers et al. (33), an omcB-deficient mutant was shown to be severely limited in its vanadate reduction capacity, but its growth on vanadate was not examined. The finding that a V(V) reduction-deficient mutant has a disruption in the mtrB gene, in addition to the known involvement of OmcB and CymA (33), indicates that vanadate reduction may share, at least in part, the electron transfer pathway with iron and manganese reduction. OmcB is therefore likely to be functional as a terminal vanadate reductase. The total evidence now available suggests that there is a multicomponent pathway consisting of at least CymA and menaquinone in the cytoplasmic membrane and the outer membrane component OmcB, in which MtrB plays a crucial role in properly localizing this outer membrane cytochrome.
Conclusion.
A chemiosmotic mechanism of energy conservation from metal reduction has now been demonstrated for V(V). Based on what is known for vanadate reduction from this and previous studies (9, 33) and the fact that proton translocation is associated with the process, we concluded that the reduction of vanadate is linked to a membrane-localized respiratory electron transport chain. This chain consists of the key components menaquinone and CymA in the cytoplasmic membrane and the outer membrane-localized cytochrome OmcB, properly incorporated by MtrB. The available evidence suggests that OmcB is the terminal vanadate reductase. However, the mechanism of electron transfer from CymA across the periplasmic space to the outer membrane cytochromes remains unknown.
Acknowledgments
We thank L. Thöny-Meyer for the generous gift of pEC86. We also thank D. A. Saffarini for the pSUP1011 plasmid and I. Vandenberghe for N-terminal sequencing.
This work was supported by the Institute for the Promotion and Innovation of Science and Technology in Flanders (IWT) through STWW research grant 174IWT20. W. Carpentier was supported by Bijzonder Onderzoeksfonds (BOF), University of Ghent, through VEO research grant B/04165 and by the Fund for Scientific Research Flanders through grant G.0282.01.
REFERENCES
- 1.Ahuja, U., and L. Töny-Meyer. 2003. Dynamic features of a heme delivery system for cytochrome c maturation. J. Biol. Chem. 278:52061-52070. [DOI] [PubMed] [Google Scholar]
- 2.Akagawamatsushita, M., T. Itoh, Y. Katayama, H. Kuraishi, and K. Yamasato. 1992. Isoprenoid quinone composition of some marine Alteromonas, Marinomonas, Deleya, Pseudomonas and Shewanella species. J. Gen. Microbiol. 138:2275-2281. [Google Scholar]
- 3.Armstrong, P. B., W. B. Lyons, and H. E. Gaudette. 1979. Application of formaldoxime colorimetric method for the determination of manganese in the pore water of anoxic estuarine sediments. Estuaries 2:198-201. [Google Scholar]
- 4.Arslan, E., H. Schulz, R. Zufferey, P. Kunzle, and L. Thöny-Meyer. 1998. Overproduction of the Bradyrhizobium japonicum c-type cytochrome subunits of the cbb3 oxidase in Escherichia coli. Biochem. Biophys. Res. Commun. 251:744-747. [DOI] [PubMed] [Google Scholar]
- 5.Beliaev, A. S., and D. A. Saffarini. 1998. Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction. J. Bacteriol. 180:6292-6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beliaev, A. S., D. A. Saffarini, J. L. McLaughlin, and D. Hunnicutt. 2001. MtrC, an outer membrane decahaem c cytochrome required for metal reduction in Shewanella putrefaciens MR-1. Mol. Microbiol. 39:722-730. [DOI] [PubMed] [Google Scholar]
- 7.Butler, A. 1998. Acquisition and utilization of transition metal ions by marine organisms. Science 281:207-210. [DOI] [PubMed] [Google Scholar]
- 8.Caetano-Annoles, G. 1993. Amplifying DNA with arbitrary oligonucleotide primers. PCR Methods Appl. 3:85-92. [DOI] [PubMed] [Google Scholar]
- 9.Carpentier, W., K. Sandra, I. De Smet, A. Brigé, L. De Smet, and J. Van Beeumen. 2003. Microbial reduction and precipitation of vanadium by Shewanella oneidensis. Appl. Environ. Microbiol. 69:3636-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crans, D., S. Amin, and A. Keramidas. 1998. Chemistry of relevance to vanadium in the environment, p. 73-96. In J. Nriagu (ed.), Vanadium in the environment, part 1. Chemistry and biochemistry. John Wiley & Sons, New York, N.Y.
- 11.De Vries, W., H. G. D. Niekus, H. van Berchum, and A. H. Stouthammer. 1982. Electron transport-linked proton translocation at nitrite reduction in Campylobacter sputorum subspecies bubulus. Arch. Microbiol. 131:132-139. [DOI] [PubMed] [Google Scholar]
- 12.DiChristina, T. J., C. M. Moore, and C. A. Haller. 2002. Dissimilatory Fe(III) and Mn(IV) reduction by Shewanella putrefaciens requires ferE, a homolog of the pulE (gspE) type II protein secretion gene. J. Bacteriol. 184:142-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorzsas, A., I. Andersson, and L. Pettersson. 2003. Speciation in the aqueous H+/H2VO4-/H2O2/L-(+)-lactate system. Dalton Transact. 12:2503-2511. [Google Scholar]
- 14.Heidelberg, J. F., I. T. Paulsen, K. E. Nelson, E. J. Gaidos, W. C. Nelson, T. D. Read, J. A. Eisen, R. Seshadri, N. Ward, B. Methe, R. A. Clayton, T. Meyer, A. Tsapin, J. Scott, M. Beanan, L. Brinkac, S. Daugherty, R. T. DeBoy, R. J. Dodson, A. S. Durkin, D. H. Haft, J. F. Kolonay, R. Madupu, J. D. Peterson, L. A. Umayam, O. White, A. M. Wolf, J. Vamathevan, J. Weidman, M. Impraim, K. Lee, K. Berry, C. Lee, J. Mueller, H. Khouri, J. Gill, T. R. Utterback, L. A. McDonald, T. V. Feldblyum, H. O. Smith, J. C. Venter, K. H. Nealson, and C. M. Fraser. 2002. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat. Biotechnol. 20:1118-1123. [DOI] [PubMed] [Google Scholar]
- 15.Holodov, V. N. 1968. Vanadium. Nauka Publishing House, Moscow, USSR.
- 16.Itoh, T., H. Funabashi, Y. Katayama-Fujimura, S. Iwasaki, and H. Kuraishi. 1985. Structure of methylmenaquinone-7 isolated from Alteromonas putrefaciens IAM 12079. Biochim. Biophys. Acta 840:51-55. [Google Scholar]
- 17.Konovalov, G. S., A. A. Ivanova, and T. C. H. Kolesnikov. 1968. Scattered rare elements dissolved in water contained in colloidal substances of the major USSR rivers, p. 72-87. In Documentation of the Seventh All-Union Conference on Lithology of the Academy of Sciences of the USSR. Nauka Publishing House, Moscow, USSR.
- 18.Kröger, A., and V. Dadák. 1969. On the role of quinones in bacterial electron transport: the respiratory system of Bacillus megaterium. Eur. J. Biochem. 11:328-340. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd, J. R. 2003. Microbial reduction of metals and radionuclides. FEMS Microbiol. Rev. 27:411-425. [DOI] [PubMed] [Google Scholar]
- 21.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lower, S. K., M. F. Hochella, and T. J. Beveridge. 2001. Bacterial recognition of mineral surfaces: nanoscale interactions between Shewanella and alpha-FeOOH. Science 292:1360-1363. [DOI] [PubMed] [Google Scholar]
- 23.Lyalikova, N. N., and N. A. Yurkova. 1992. Role of microorganisms in vanadium concentration and dispersion. Geomicrobiol. J. 10:15-26. [Google Scholar]
- 24.Moreno-Vivián, C., and S. J. Ferguson. 1998. Definition and distinction between assimilatory, dissimilatory and respiratory pathways. Mol. Microbiol. 29:664. [DOI] [PubMed] [Google Scholar]
- 25.Myers, C. R., and J. M. Myers. 1992. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J. Bacteriol. 174:3429-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers, C. R., and J. M. Myers. 1994. Ferric iron reduction-linked growth yields of Shewanella putrefaciens MR-1. J. Appl. Bacteriol. 76:253-258. [DOI] [PubMed] [Google Scholar]
- 27.Myers, C. R., and J. M. Myers. 1997. Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate, and nitrate by Shewanella putrefaciens MR-1. J. Bacteriol. 179:1143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myers, C. R., and J. M. Myers. 2002. MtrB is required for proper incorporation of the cytochromes OmcA and OmcB in the outer membrane of Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 68:5585-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers, C. R., and J. M. Myers. 2003. Cell surface exposure of the outer membrane cytochromes of Shewanella oneidensis MR-1. Lett. Appl. Microbiol. 37:254-258. [DOI] [PubMed] [Google Scholar]
- 30.Myers, C. R., and K. H. Nealson. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319-1321. [DOI] [PubMed] [Google Scholar]
- 31.Myers, C. R., and K. H. Nealson. 1990. Respiration-linked proton translocation coupled to anaerobic reduction of manganese(IV) and iron(III) in Shewanella putrefaciens MR-1. J. Bacteriol. 172:6232-6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers, J. M., and C. R. Myers. 2000. Role of the tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone. J. Bacteriol. 182:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers, J. M., W. E. Antholine, and C. R. Myers. 2004. Vanadium(V) reduction by Shewanella oneidensis MR-1 requires menaquinone and cytochromes from the cytoplasmic and outer membranes. Appl. Environ. Microbiol. 70:1405-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishijima, M., M. Araki-Sakai, and H. Sano. 1997. Identification of isoprenoid quinones by frit-FAB liquid chromatography-mass spectrometry for the chemotaxonomy of microorganisms. J. Microbiol. Methods 28:113-122. [Google Scholar]
- 35.Ortiz-Bernad, I., R. T. Anderson, H. A. Vrionis, and D. R. Lovley. 2004. Vanadium respiration by Geobacter metallireducens: novel strategy for in situ removal of vanadium from groundwater. Appl. Environ. Microbiol. 70:3091-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 37.Petkevich, A. N., G. E. Viller, and B. A. Vorotnikov. 1967. Some trace elements in natural waters of Kama's district, p. 223-227. In Trace elements in the biosphere and their application in agriculture and medicine in Siberia and the Far East. Ulan-Ude Book Publishers, Ulan-Ude, USSR.
- 38.Rehder, D. 1992. Structure and function of vanadium compounds in living organisms. Biometals 5:3-12. [DOI] [PubMed] [Google Scholar]
- 39.Rehder, D. 2003. Biological and medicinal aspects of vanadium. Inorg. Chem. Commun. 6:604-617. [Google Scholar]
- 40.Saffarini, D. A., S. L. Blumerman, and K. J. Mansoorabadi. 2002. Role of menaquinones in Fe(III) reduction by membrane fractions of Shewanella putrefaciens. J. Bacteriol. 184:846-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scholes, P., and P. Mitchell. 1970. Respiration-driven proton translocation in Micrococcus denitrificans. J. Bioenerg. 1:309-323. [DOI] [PubMed] [Google Scholar]
- 42.Schumacher, W., and C. Holliger. 1996. The proton/electron ratio of the menaquinone-dependent electron transport from dihydrogen to tetrachloroethene in Dehalobacter restrictus. J. Bacteriol. 178:2328-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Short, K. A., and R. P. Blakemore. 1986. Iron respiration-driven proton translocation in aerobic bacteria. J. Bacteriol. 167:729-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singer, T. P. 1979. Mitochondrial electron-transport inhibitors. Methods Enzymol. 55:454-462. [DOI] [PubMed] [Google Scholar]
- 45.Strahov, N. M. 1968. On the theory of geochemical process in humid zones, p. 102-134. In A collection of papers on the geochemistry of sedimentary rocks and ores. Nauka Publishing House, Moscow, USSR.
- 46.Stucki, J. W. 1981. The quantitative assay of minerals for Fe2+ and Fe3+ using 1,10-phenanthroline. II. A photochemical method. Soil Sci. Soc. Am. J. 45:638-641. [Google Scholar]
- 47.Thomas, P. E., D. Ryan, and W. Levin. 1976. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal. Biochem. 75:168-176. [DOI] [PubMed] [Google Scholar]
- 48.Thomson, R. H. 1971. Naturally occurring quinones, p. 41-81. Academic Press, New York, N.Y.
- 49.Thöny-Meyer, L. 2000. Haem-polypeptide interactions during cytochrome c maturation. Biochim. Biophys. Acta 1459:316-324. [DOI] [PubMed] [Google Scholar]
- 50.van Verseveld, H. W., K. Krab, and A. H. Stouthamer. 1981. Proton pump coupled to cytochrome c oxidase in Paracoccus denitrificans. Biochim. Biophys. Acta 635:525-534. [DOI] [PubMed] [Google Scholar]
- 51.Vinogradov, A. P. 1957. Geochemistry of rare and dispersed chemical elements in soils, 2nd ed. (revised), p. 122-129 and 216-217. USSR Academy of Sciences, Moscow, USSR.
- 52.Yurkova, N. A., and N. N. Lyalikova. 1990. New vanadate-reducing facultative chemolithotrophic bacteria. Microbiology 59:672-677. [Google Scholar]