Abstract
The structural basis for the streptomycin dependence phenotype of ribosomal protein S12 mutants is poorly understood. Here we describe the application of site-directed mutagenesis and gene replacement of Thermus thermophilus rpsL to assess the importance of side chain identity and tertiary interactions as phenotypic determinants of drug-dependent mutants.
Ribosomal protein S12 is a highly conserved protein located at the functional center of the 30S subunit of the ribosome. A direct role for S12 in the tRNA selection process has been extracted from high-resolution X-ray crystal structures of the 30S ribosomal subunit of the extremely thermophilic bacterium Thermus thermophilus (4, 5, 13, 15). Direct contacts between S12 and several important structural elements of 16S rRNA are alternately made and broken during the tRNA selection process (13), and mutations in S12 known to influence the accuracy of decoding are predicted to do so by affecting the stability of these interactions.
Mutations in rpsL, which encodes S12, often affect the cell's response to the aminoglycoside antibiotic streptomycin, an antibiotic which causes misreading of the genetic code. There are several streptomycin phenotypes, including sensitivity (Strs), resistance (Strr), pseudo-dependence (Strp), dependence (Strd), and independence (Stri) (7). The severity of the phenotype can be understood in the context of the open-closed transition observed crystallographically to occur during tRNA selection (13). These mutations may perturb the conformational equilibrium toward the open state to various degrees, with Strd mutations being the most severe, as suggested by Ogle et al. (13). In this case, streptomycin may act by restoring equilibrium. Strp and some Strr strains may also alter this equilibrium but to a lesser extent. Second-site suppressors of streptomycin dependence, as exemplified by the classic S4 and S5 Stri suppressors, may similarly restore equilibrium. However, little is known about the precise structural basis for drug dependence or about the nature of the side chain alterations which might influence this phenotype. We have used site-directed mutagenesis and gene replacement to provide insight into this question.
We have previously reported the isolation of antibiotic-resistant mutants of the extreme thermophile T. thermophilus (3, 9). This organism is amenable to genetic manipulation (3, 9, 10, 11) and offers the possibility that structural effects of such mutations could be ascertained at atomic resolution. Here we describe a genetic analysis of the nature of drug dependence phenotypes resulting from mutations in ribosomal protein S12 and the influence of side chain identity on the severity of phenotype. We also examined the ability of second-site mutations to suppress the phenotypes of such mutations.
Drug-dependent mutants of T. thermophilus.
Selections for paromomycin-dependent mutants of T. thermophilus IB-21 (ATCC 43615) (12) were conducted as previously described (9), except cells were plated on medium containing paromomycin at 20 μg/ml. We identified two rpsL alleles bearing either of the mutations P90R and P90L (Escherichia coli numbering used throughout). The proline at position 90 is extremely well conserved. We previously described the isolation of the P90L mutation during selections for Strd mutants (9). These mutants exhibit a dependence on either paromomycin (Pard) or streptomycin, but the drugs may not be exchanged once a “preference” has been established, as has been described for E. coli (7). Simultaneous binding of both drugs is lethal, while independent binding is tolerated; however, the precise molecular explanation for this observation is unknown. Each drug appears to satisfy a requirement, but by different mechanisms. Growth of the T. thermophilus P90R and P90L Pard mutants on streptomycin could be achieved by transforming a wild-type strain with genomic DNA from the Pard mutants, with selection on plates containing streptomycin (11). A third Pard mutant was isolated and was found to contain two rpsL mutations, P90L and R37C. The appearance of double mutants, consisting of a primary and an ancillary mutation, has also been observed in Strd mutants of E. coli (14).
Influence of side chain identity at S12 position 90 on drug dependence phenotypes.
We sought to determine how different amino acid changes at position 90 would affect the severity of the phenotype. To accomplish this, we used a novel gene replacement strategy, taking advantage of the Strd conditional lethal phenotype of the P90R mutant (Fig. 1). We transformed the P90R mutant with a nonreplicating pUC18 plasmid carrying a mutant rpsL allele, selecting for drug independence. As a result of the highly efficient transformation and homologous recombination characteristic of Thermus spp. (11), the chromosomal rpsL gene is replaced with the plasmid-borne gene. Single-crossover insertions are apparently unstable, such that cointegrates are rapidly resolved. A single-crossover event, which would result in insertion of the plasmid-borne bla gene into the chromosome, was never observed by means of PCR (data not shown). Furthermore, sequencing the resultant chromosomal rpsL gene indicated the presence of only one allele. As an example, we used this gene replacement strategy to construct a novel mutant with a single R37C mutation, which demonstrates that various S12 mutants may be generated, independently of their phenotypes. Similarly, mutants could also be constructed by transformation of wild-type T. thermophilus, with selection for an Strr phenotype. Each mutant described in this study was retransformed to confirm the association between the mutation and the phenotype; that is, the original strain (wild type, in the case of single mutants) was transformed with genomic DNA from the mutant using the same selection. The rpsL gene was then amplified by PCR and sequenced. The mutants and their associated phenotypes are listed in Table 1. This strategy allows us to introduce site-directed mutations into the chromosomal rpsL gene, including novel mutations requiring multiple base substitutions.
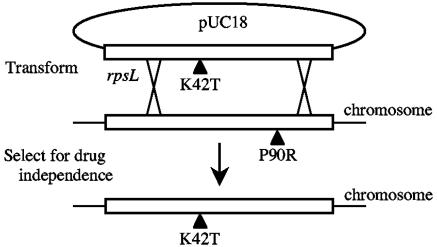
FIG. 1.
Construction of T. thermophilus rpsL mutants by gene replacement. The P90R Strd strain was transformed with plasmid pUC18 containing the rpsL gene with the desired mutation (here, K42T). Plasmid pUC18 does not replicate in T. thermophilus; therefore, replacement of chromosomal rpsL with the plasmid-borne rpsL gene occurs via homologous recombination with selection on antibiotic-free medium.
TABLE 1.
T. thermophilus mutants and their streptomycin phenotypes
| Wild type or mutation(s) | Codon change | Phenotype | Streptomycin MIC (μg/ml) | Paromomycin MIC (μg/ml) |
|---|---|---|---|---|
| IB21 (wild type) | Strs | 25 | 5 | |
| rpsL R37C | CGC→TGC | Strr | 500 | |
| rpsL K42T | AAG→ACG | Strr | 2,000 | |
| rpsL K42C | AAG→TGC | Strr | 2,000 | |
| rpsL P90R | CCG→CGG | Strd Pard | 10a | 1a |
| rpsL P90L | CCG→CTG | Strd Pard | 10a | 1a |
| rpsL P90E | CCG→GAG | Strd | 10a | |
| rpsL P90M | CCG→ATG | Strd | 10a | |
| rpsL P90W | CCG→TGG | Strd | 10a | |
| rpsL P90A | CCG→GCC | Strr | 500 | 5 |
| rpsL P90C | CCG→TGC | Strr | 500 | 5 |
| rpsL P90G | CCG→GGG | Strr | 500 | 5 |
| rpsL K42T, P90R | Strr | >2,000 | ||
| rpsL K42C, P90C | Lethal | |||
| rpsL R37C, P90L | Pard | ND | 1a | |
| rpsL P90R, rpsD E201K | GAG→AAGb | Strr | ND | |
| rpsL P90R, rpsE G103R | GGG→AGGc | Strr | ND |
Minimum concentration that allows growth.
Codon change for rpsD.
Codon change for rpsE.
Compared to the wild-type proline, the bulkier hydrophobic leucine or the long-chain arginine in the two Strd mutants might impose a steric and/or charge-related effect on 16S rRNA, thus favoring the open conformation. To address this possibility, we used gene replacement to generate several mutants with various substitutions at position 90. Small-side chain P90A and P90C mutants were found to exhibit streptomycin resistance, not dependence. A P90G mutant was isolated as a drug-independent pseudorevertant of the P90R mutant and is also Strr, not Strd. However, the P90E, P90M, and P90W mutants, which have bulkier side chains, exhibit dependence on streptomycin. Thus, our results suggest that side chain size, rather than charge, at this position is a determinant of the severity of streptomycin resistance, in which larger side chains strongly favor the open conformation and therefore require drug binding to induce the productive, closed state.
Influence of residue K42 on the drug dependence phenotype of mutations at P90.
Spontaneous suppressors of drug dependence can readily be isolated (9), and here we identified the rpsD E210K (which encodes ribosomal protein S4) and rpsE G103R (which encodes ribosomal protein S5) mutants. In this study, we also identified as an intragenic suppressor mutation the classical Strr mutation K42T. K42R has been found as a suppressor of the P90L mutation in Salmonella enterica serovar Typhimurium (1). These results suggest a functional interaction between these residues, which are within two highly conserved loops of S12 (2) and are in close proximity to one another in the high-resolution crystal structures (Fig. 2) (15). To further address this interaction, we constructed, by site-directed mutagenesis and gene replacement, combinations of mutations at these two positions. Interestingly, K42C and P90C each confer streptomycin resistance. However, attempts to construct a K42C P90C double mutant were unsuccessful. Transformations with DNA from K42T P90R double mutants indicate that these two positions are genetically tightly linked. Nevertheless, transformations using a plasmid encoding the K42C P90C double mutation produced only K42C or P90C single mutants. We conclude from these results that the double mutation produces a dominant lethal phenotype. It is unlikely that the lethality is due to simply the combination of two slow-growth phenotypes, since the doubling times for the K42C and P90C mutants are 59 and 60 min, respectively, compared to 49 min for the wild type. It is noteworthy that the rpsL P90R rpsE G103R double mutant has a doubling time of 95 min, indicating that even more severe growth phenotypes are tolerated. One possible, although speculative, explanation for the lethal phenotype is the formation of a disulfide bridge, which would prevent conformational movements of the two loops necessary for ribosome function.
FIG. 2.
Three-dimensional structure of S12 (blue) showing the location of K42 and P90 (Protein Data Bank accession no. 1FJG). K42 and P90 lie in two highly conserved loops (2), shown in green. K42 contacts streptomycin (strep; red) and 16S RNA (grey) via the phosphate of A913 (13). This figure was illustrated with PyMol (6).
In summary, the phenotype conferred by mutations at P90 of ribosomal protein S12 are highly dependent on side chain identity. The absence of a rigidifying proline side chain appears to be sufficient to prevent inhibition by streptomycin, regardless of the residue side chain. A P90S mutant of Nicotiana tabacum chloroplasts is also Strr (8). Thus, all four Strr P90 alleles introduce smaller side chains (alanine, cysteine, glycine, and serine) than the Strd mutations (leucine, arginine, glutamate, methionine, and tryptophan). Small side chains probably allow for more-localized flexibility than the wild-type proline, which generally promotes a more rigid polypeptide structure. Additionally, the dependence phenotype appears to require bulky side chains, suggesting a steric clash with some structural element of the ribosome or tRNA. Our data are consistent with the hypothesis that mutations in S12 destabilize the closed conformation (13), and we suggest that small-side chain resistance mutations slightly tip the equilibrium to the open state but not as strongly as do the bulky-side chain dependence mutations. Our observation that drug dependence is also influenced by the identity of K42 indicates an important functional interaction between these two highly conserved loops (Fig. 2). Further structural studies, including X-ray crystallographic analysis, will shed more light on the nature of the drug dependence phenotype.
Acknowledgments
This work was supported by a grant (GM19756) from the National Institutes of Health to A.E.D.
REFERENCES
- 1.Bjorkman, J., P. Samuelsson, D. I. Andersson, and D. Hughes. 1999. Novel ribosomal mutations affecting translational accuracy, antibiotic resistance and virulence of Salmonella typhimurium. Mol. Microbiol. 31:53-58. [DOI] [PubMed] [Google Scholar]
- 2.Brodersen, D. E., W. M. Clemons, Jr., A. P. Carter, B. T. Wimberly, and V. Ramakrishnan. 2002. Crystal structure of the 30S ribosomal subunit from Thermus thermophilus: structure of the proteins and their interactions with 16S rRNA. J. Mol. Biol. 316:725-768. [DOI] [PubMed] [Google Scholar]
- 3.Cameron, D., J. Thompson, S. T. Gregory, P. E. March, and A. E. Dahlberg. 2004. Thiostrepton-resistant mutants of Thermus thermophilus. Nucleic Acids Res. 32:3220-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter, A. 2002. Ph.D. thesis. Cambridge University, Cambridge, United Kingdom.
- 5.Carter, A. P., W. M. Clemons, D. E. Brodersen, R. J. Morgan-Warren, B. T. Wimberly, and V. Ramakrishnan. 2000. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 407:340-348. [DOI] [PubMed] [Google Scholar]
- 6.DeLano, W. L. 2002. The PyMol molecular graphics system. DeLano Scientific, San Carlos, Calif.
- 7.Gale, E. F., E. Cundliffe, P. E. Reynolds, M. H. Richmond, and M. J. Waring. 1981. The molecular basis of antibiotic action, p. 402-547. John Wiley & Sons, London, United Kingdom.
- 8.Galili, S., H. Fromm, D. Aviv, M. Edelman, and E. Galun. 1989. Ribosomal protein S12 as a site for streptomycin resistance in Nicotiana chloroplasts. Mol. Gen. Genet. 218:289-292. [DOI] [PubMed] [Google Scholar]
- 9.Gregory, S. T., J. H. D. Cate, and A. E. Dahlberg. 2001. Streptomycin-resistant and streptomycin-dependent mutants of the extreme thermophile Thermus thermophilus. J. Mol. Biol. 309:333-338. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto, Y., T. Yano, S. Kuramitsu, and H. Kagamiyama. 2001. Disruption of Thermus thermophilus genes by homologous recombination using a thermostable kanamycin-resistant marker. FEBS Lett. 506:231-234. [DOI] [PubMed] [Google Scholar]
- 11.Koyama, Y., T. Hoshino, N. Tomizuka, and K. Furukawa. 1986. Genetic transformation of the extreme thermophile Thermus thermophilus and of other Thermus spp. J. Bacteriol. 166:338-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristjansson, J. K., G. O. Hreggvidsson, and G. A. Alfredsson. 1986. Isolation of halotolerant Thermus spp. from submarine hot springs in Iceland. Appl. Environ. Microbiol. 52:1313-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogle, J. M., F. V. Murphy, I. V., M. J. Tarrry, and V. Ramakrishnan. 2002. Selection of tRNA by the ribosome requires a transition from an open to a closed form. Cell 111:721-732. [DOI] [PubMed] [Google Scholar]
- 14.Timms, A. R., and B. A. Bridges. 1993. Double, independent mutational events in the rpsL gene of Escherichia coli: an example of hypermutability? Mol. Microbiol. 9:335-342. [DOI] [PubMed] [Google Scholar]
- 15.Wimberly, B. T., D. E. Brodersen, W. M. Clemons, Jr., R. J. Morgan-Warren, A. P. Carter, C. Vonrhein, T. Hartsch, and V. Ramakrishnan. 2000. Structure of the 30S ribosomal subunit. Nature 407:327-339. [DOI] [PubMed] [Google Scholar]