Abstract
The sig1 gene, predicted to encode an extracytoplasmic function-type heat shock sigma factor of Deinococcus radiodurans, has been shown to play a central role in the positive regulation of the heat shock operons groESL and dnaKJ. To determine if Sig1 is required for the regulation of additional heat shock genes, we monitored the global transcriptional and proteomic profiles of a D. radiodurans R1 sig1 mutant and wild-type cells in response to elevated temperature stress. Thirty-one gene products were identified that showed heat shock induction in the wild type but not in the sig1 mutant. Quantitative real-time PCR experiments verified the transcriptional requirement of Sig1 for the heat shock induction of the mRNA of five of these genes—dnaK, groES, DR1314, pspA, and hsp20. hsp20 appears to encode a new member of the small heat shock protein superfamily, DR1314 is predicted to encode a hypothetical protein with no recognizable orthologs, and pspA is predicted to encode a protein involved in maintenance of membrane integrity. Deletion mutation analysis demonstrated the importance in heat shock protection of hsp20 and DR1314. The promoters of dnaKJE, groESL, DR1314, pspA, and hsp20 were mapped and, combined with computer-based pattern searches of the upstream regions of the 26 other Sig1 regulon members, these results suggested that Sig1 might recognize both σ70-type and σW-type promoter consensus sequences. These results expand the D. radiodurans Sig1 heat shock regulon to include 31 potential new members, including not only factors with cytoplasmic functions, such as groES and dnaK, but also those with extracytoplasmic functions, like pspA.
Deinococcus radiodurans is a radioresistant, nonpathogenic bacterium whose 16S rRNA sequence is extremely divergent from that of other known bacteria; Thermus and Meiothermus are the only closely related genera (4). The organism is able to survive an acute dose of up to 5 Mrad of γ-irradiation and up to 1,000 J/m2 of UV irradiation without mutation (4, 38). The sequenced and annotated genome of D. radiodurans, containing four high-G+C genetic elements (45), has allowed intensive study of the radioresistance mechanisms of D. radiodurans in recent years.
Despite this interest, little is known about how the organism responds to other environmental stressors. Previous studies of stress response systems in D. radiodurans demonstrated that the organism mounts a regulated protective response against DNA damage induced by UV light (8), γ-irradiation (4, 38, 39), and oxidative stress (15, 41). In addition, recent work showed that D. radiodurans also protects itself in a regulated manner against heat and cold shock (2, 30).
The process of mounting a regulated, protective response to elevated temperature is conserved among all tested bacteria; however, the mechanisms of regulation vary widely. In some organisms, the positive regulation of heat shock genes is governed by an alternative sigma factor. For example, the transient heat shock induction of over 30 genes encoding proteins with cytoplasmic heat shock protective functions is controlled by σ32 in Escherichia coli (47), and more than 20 extracytoplasmic function genes are controlled by σ24 (5). Similarly, in Bacillus subtilis, σB governs the heat shock induction of approximately 100 general stress-protective genes (24, 26). However, the system of negative regulation is more widespread among bacteria, especially the CIRCE (controlling inverted repeat of chaperone expression) DNA element, which binds its cognate repressor, HrcA, to control the heat induction of groESL and/or dnaKJ-grpE operons (20). In the high-G+C gram-positive organisms, such as Streptomyces and Mycobacterium tuberculosis, however, HspR negatively regulates the dnaK operon by binding to HAIR (HspR associated inverted repeat) sequences associated with the promoter (34, 37). Recently, it has been suggested that HspR also controls a much larger regulon in these organisms (34, 36, 37). An additional level of control can be added by other more specialized repressor systems, such as CtsR repression of the clp genes in B. subtilis and other low-G+C gram-positive bacteria (6) and RheA repression of hsp18 in Streptomyces albus G (33).
Our previous work in D. radiodurans indicates that sig1, one of only three sigma factor genes predicted to be present in the genome sequence of D. radiodurans, appears to encode the major heat shock regulator that positively regulates the heat shock induction of groESL and dnaKJ operons (30). In addition, although it appears to control these cytoplasmic function genes, the Sig1 protein sequence clustered most closely with extracytoplasmic function (ECF) sigma factors. Furthermore, orthologs encoding the heat shock negative regulators hrcA, ctsR, and hspR are conspicuously missing from the annotated genome sequence. In order to further investigate the regulation of the heat shock response in D. radiodurans, we sought to determine if Sig1 controls the heat shock induction of genes in addition to dnaKJ and groESL, thereby expanding the understanding of the Sig1 regulon. Therefore, we conducted a combined global transcriptional and proteomic comparison of the wild type and sig1 mutants following temperature upshift, with subsequent determination of a putative promoter consensus sequence for Sig1-dependent genes.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. E. coli cultures were grown in Luria-Bertani (LB) broth or on Luria-Bertani agar at 37°C; D. radiodurans cultures were grown in tryptone-glucose-yeast extract (TGY) broth or on TGY agar at 30°C. For all heat shock experiments, wild-type and mutant cultures were grown to mid-exponential phase (optical density at 600 nm, ∼0.3 to 0.5) and split into two aliquots, one of which was shifted to 48°C, the other held at 30°C as a control. The length of the heat induction varied from 5 to 60 min depending on the experiment as indicated below in Results. Ampicillin (100 μg/ml), kanamycin (E. coli at 50 μg/ml; D. radiodurans at 15 μg/ml for the selection of transformants, 20 μg/ml for screening of mutants and routine growth on plates, and 8 μg/ml for growth in liquid), or chloramphenicol (3 μg/ml for D. radiodurans) was added to culture media as appropriate. Transformations of E. coli were performed using commercially available cells (TOP10 [Invitrogen, Carlsbad, CA] and JM109 [Promega, Madison, WI]). Transformations of D. radiodurans cells were performed by electroporation as previously described (30).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype and description | Source or reference |
|---|---|---|
| E. coli strains | ||
| JM109 | e14−(McrA−) recA1 endA1 gyrA96 thi-1 hsdR17(rK− mK+) supE44 relA1 Δ(lac-proAB) [F′ traΔ36 proAB] lacIqZΔM15] | Promega |
| TOP10 | F−mcrAD (mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 deoR araD139 Δ(ara-leu)7679 galU galK rpsL(Strr) endA1 nupG | Invitrogen |
| D. radiodurans strains | ||
| R1 | Wild-type strain | J. R. Battista |
| AKS10 | R1 sig1::aph (Kmr) [Sig1−]; result of recombination of pAKS10 into chromosome of R1 | 30 |
| ΔDR1314 | R1 ΔDR1314 (Kmr); result of recombination of pDR1314M into chromosome of R1 | This study |
| Δhsp20 | R1 Δhsp20 (Kmr); result of recombination of pHSP20M into chromosome of R1 | This study |
| Plasmids | ||
| pCR2.1-TOPO | Cloning vector for PCR-generated products; Apr Kmr; 3.9 kb; uses covalently linked topoisomerase instead of DNA ligase | Invitrogen |
| pHMR186 | Improved D. radiodurans-E. coli shuttle vector for the deletion of genes in D. radiodurans. Carries loxP sites for the purpose of unmarking mutants by recombinational loss of Kmr marker; Kmr Cmr Ampr | This study |
| pDR1314M | pHMR186 carrying ∼1 kb of sequence flanking the 5′ and 3′ ends of DR1314; for the purpose of deleting the chromosomal copy of DR1314 | This study |
| pHSP20M | Similar to pDR1314M, for the purpose of deleting the chromosomal copy of hsp20 | This study |
| pGRO5′ | pCR2.1-TOPO carrying the ∼500-bp promoter fragment upstream of groESL of D. radiodurans; used as template for sequencing ladder in transcription start site mapping experiments | 16 |
| pPdnaK | Like pGRO5′, but carrying the dnaKJ promoter fragment | 30 |
| pPDR1314 | Like pGRO5′, but carrying the DR1314 promoter fragment | This study |
| pPSPA | Like pGRO5′, but carrying the pspA promoter fragment | This study |
| pPhsp20 | Like pGRO5′, but carrying the hsp20 promoter fragment | This study |
DNA manipulations.
DNA was isolated from E. coli cells by using the alkaline lysis method of Sambrook and Russell (29). D. radiodurans chromosomal DNA for PCR amplification was prepared using a detergent lysis method described previously (30). Restriction enzymes were obtained from New England Biolabs (Beverly, MA), Roche Corp. (Indianapolis, IN), or Promega and were used as the supplier described. T4 DNA ligase was purchased from New England Biolabs. Standard PCRs were performed using Taq polymerase (Promega) and custom primers obtained from Invitrogen.
Protein preparation for two-dimensional gel electrophoresis.
Heat-shocked and control D. radiodurans R1 wild-type and sig1 mutant cultures were harvested at 4,500 × g for 8 min at 4°C and were then resuspended in 1 ml osmotic lysis buffer (0.3% sodium dodecyl sulfate [SDS], 10 mM Tris [pH 7.4] supplemented with 10 μl protease inhibitor cocktail [20 mM aminoethyl benzenesulfonyl fluoride, 1 mg/ml leupeptin, 0.36 mg/ml E-64 EDTA, and 5.6 mg/ml benzamidine]). Lysis was achieved by passing bacterial suspensions twice through a French press at 37 kPa, after which lysates were incubated on ice with 100 μl of nuclease solution for 10 min to reduce lysate viscosity (nuclease stock contains 50 mM MgCl2, 100 mM Tris [pH 7.0], 500 μg/ml RNase A, and 1,000 μg/ml DNase I). Total protein concentration was determined using the bicinchoninic acid method (Pierce, Rockford, IL).
2D-PAGE.
Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) was performed according to the method of O'Farrell (21) by Kendrick Labs, Inc. (Madison, WI). Isoelectric focusing (IEF) was carried out in 2.0-mm inner diameter glass tubes using 2% (wt/vol) pH 4 to 8 ampholines (Gallard-Schlesinger Industries, Inc., Garden City, NY) for 9,600 V-h. One microgram of tropomyosin (MW 33,000 and pI 5.2), an IEF internal standard, was added to each sample prior to loading. After reaching equilibrium in SDS sample buffer (10% [wt/vol] glycerol, 50 mM dithiothreitol, 2.3% [wt/vol] SDS, and 0.0625 M Tris; pH 6.8), each tube gel was sealed to the top of a stacking gel that overlay a 10% (wt/vol) acrylamide slab gel (0.75 mm thick). SDS slab gel electrophoresis was carried out for 4 h at 12.5 mA/gel. The following proteins (Sigma Chemical Co., St. Louis, MO) were added as molecular mass standards to the agarose that sealed the tube gel to the slab gel: myosin (220 kDa), phosphorylase a (94 kDa), catalase (60 kDa), actin (43 kDa), carbonic anhydrase (29 kDa), and lysozyme (14 kDa). These standards appeared as horizontal lines on the Coomassie blue-stained 10% acrylamide slab gels. Gels were dried between sheets of cellophane paper with the acid edge to the left.
Computerized comparison of induced spots from 2D-PAGE analysis.
Gel analysis was carried out by Kendrick Labs, Inc. Duplicate gels were obtained as described above, and one gel from each pair was scanned with a laser densitometer (model PDSI; Molecular Dynamics Inc., Sunnyvale, CA). The scanner was checked for linearity prior to scanning with a calibrated neutral-density filter set (Melles Griot, Irvine, CA). The images were analyzed using Nonlinear Technology Progenesis software (version 1.0) such that all major spots and all changing spots were outlined, quantified, and matched on all the gels. In cases where protein spots were missing from some gels and present in others, a small area of background was outlined appropriately to facilitate matching. The general method of computerized analysis for these pairs included automatic spot finding and quantification, automatic background subtraction (mode of non-spot), and automatic spot matching, in conjunction with detailed manual checking of the spot finding and matching functions.
Identification of protein spots by mass spectrometry.
Protein spots that showed significant induction (induction ratio ≥ 2) at 48°C compared to 30°C in D. radiodurans R1 wild-type but not in sig1 mutant cells were excised from the D. radiodurans R1 wild-type 48°C Coomassie-stained 2D gel using a scalpel and were placed in a siliconized Eppendorf tube (Island Sci, Bainbridge Island, WA). Trypsin digestions were performed either using the ProteoProfile trypsin in-gel digest kit (Sigma) according to the manufacturer's instructions or by the Fred Hutchinson Cancer Research Center Core Proteomics Facility (Seattle, WA) as described in reference 35. Tryptic peptides were then analyzed by either the Core Proteomics Facility or the Pacific Northwest National Laboratory (Richland, WA) as follows. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) was performed using a ThermoFinnigan LCQ as described previously (10), acquiring data in the data-dependent mode in which an MS scan was followed by MS/MS scans of the three most abundant ions from the preceding scan. The MS/MS data were then analyzed using SEQUEST software (ThermoFinnigan, San Jose, CA): the MS/MS data were searched against either the D. radiodurans protein database from The Institute for Genomic Research (TIGR; www.tigr.org) (45) or the NCBI nonredundant protein database (www.ncbi.nlm.nih.gov). The resultant data set was filtered according to mudPIT rules (42).
RNA preparation.
Total RNA was harvested as follows. A 0.1 culture volume of STOP solution (5% [wt/vol] saturated phenol, 95% [vol/vol] ethanol) was added to ∼1 × 108 heat-shocked and control D. radiodurans R1 wild-type and sig1 mutant cells. Cultures were subsequently harvested by centrifugation at 4,500 × g for 6 min at 4°C. Total RNA was extracted from D. radiodurans pellets using the RNeasy Mini kit (QIAGEN, Valencia, CA) according to the manufacturer's Yeast III protocol. Lysis was achieved by 3 min of bead-beating in the Mini-Beadbeater 8 (BioSpec Products, Inc., Bartlesville, OK) with 500 μl of 0.1-mm zirconium-silica beads (BioSpec Products, Inc.). Contaminating DNA was subsequently removed from RNA samples by using 6 to 10 U of DNase I (Ambion, Austin, TX, or Epicentre, Madison, WI), followed by cleanup over an additional RNeasy column (QIAGEN).
QRT-PCR.
cDNA was synthesized in random hexamer-primed reactions from 500 ng to 1 μg of each DNase I-treated RNA sample using the ThermoScript reverse transcription-PCR (RT-PCR) system (Invitrogen) according to the manufacturer's directions. Reactions lacking reverse transcriptase were included for each sample as a control for DNA contamination. One microliter of cDNA was used as template for each quantitative real-time RT-PCR (QRT-PCR) mixture in which 100- to 200-bp sequences within the open reading frames of DR1314, hsp20 (DR1114), dnaK (DR0129), hpi (DR2508), and groES (DR0606) were amplified. QRT-PCR mixtures totaling 20 μl contained 0.2 μM standard Q_fwd and Q_rev primers (see Table 5, below) and SYBR green PCR core reagents (Applied Biosystems, Foster City, CA) in final concentrations of 2 mM MgCl2 and 1 mM deoxynucleoside triphosphate and 0.025 U of Amplitaq Gold polymerase. Quantitative analysis was performed in a RotorGene 3000 (Corbett Research, Sydney, Australia) for 45 cycles with the following parameters: 95°C for 15 min to activate Taq polymerase, melting for 20 s at 95°C, annealing for 20 s at 55°C, and extension and data acquisition for 20 s at 72°C. A melting curve analysis was performed after each run to ensure the specificity of products.
TABLE 5.
Primers used in this study and their sequences
| Purpose and name | Sequence 5′ to 3′ |
|---|---|
| Quantitative real-time PCR | |
| hpiQ_fwd | AGACGCGGTAGGTCACGTTGTCCTG |
| hpiQ_rev | TGTCCTCGAACCCGGCACTGTGAAG |
| DR1314Q_fwd | CAGGTCAAGGACATGAGCGAGTACC |
| DR1314Q_rev | CTTCGAGCAGCTGGAGGCGATCAGG |
| dnaKQ_fwd | TCGGCGGTCAGTTCCTCGAACTTGG |
| dnaKQ_rev | AGCATCTCCCTGCCCTTCATCACCT |
| hsp20Q_fwd | GGCCTCGAACTGACCTTGGACATTC |
| hsp20Q_rev | AAAGGTGCCGTAGGCGCGCTCGACA |
| Cloning and mutagenesisa | |
| DR1314M_fwd5′ | AGATCT-GAGAGGTCAGGCGGTCAGCA |
| DR1314M_rev5′ | GGTACC-TATGGAGGTCACGGTTCTTG |
| DR1314M_fwd3′ | TACGTA-ACCACCAAGCGCAACATCTG |
| DR1314M_rev3′ | GAGCTC-GCTGCTCCATCGTCGCAATC |
| P1314_up | CCAGCTTCACGGCGGCGTTGAAGAC |
| hsp20M_fwd5′ | AGATCT-CCGGACCGGCTTCTCCCTTT |
| hsp20M_rev5′ | GGTACC-ACCTGCGCTCAAGTAGTCCT |
| hsp20M_fwd3′ | TACGTA-ACCGCCGCCACGGAATAACC |
| hsp20M_rev3′ | GAGCTC-TGCCGTTGGCGTGAAGGAGT |
| Phsp20_up | AGATCTGGAACGCGGTGAAGTGGCTG |
| dnaK_fwd2 | GGAAGATCT-CGCACCAATCCGCACCT |
| dnaK_rev2 | CGGCGTTGACGATCACT |
| pspA_up | TAGAAACGGCCCGCGAAGGT |
| Diagnostic PCR | |
| DR1314M_check5′fwd | AGGAGGCGGCCAGTTGGAAG |
| DR1314M_check5′rev | ATCGCGGCCTCGAGCAAGAC |
| DR1314M_check5′fwd | CGGGACGGCGGCTTTGTTGA |
| DR1314M_check3′rev | TGCCGCCTTCGCGCTGCTTT |
| hsp20M_check5′fwd | CCACTGCGGGCCGCAAATTC |
| hsp20M_check5′rev | GCCTCGAGCAAGACGTTTCC |
| hsp20M_check3′fwd | CAACTGGTCCACCTACAACA |
| hsp20M_check3′rev | CGCGGGCCAAGTCGAGATAC |
| CmSCOfwd | AGGACAAATCCGCCGAGCTT |
| CmSCOrev | TGCCGCCTCGACGAATTTCT |
| kan_fwd | AAGCCACGTTGTGTCTCA |
| kan_rev | CCGTCAAGTCAGCGTAAT |
| 0180_304bp_ups | TGCTGGTCCTCGCCTAT |
| 0180_171bp_dnsC | AACGCGCTGATGTCAGG |
| Primer extension | |
| PdnaK_PE1 | AGTTGGTGGTACCCAAATCG |
| PdnaK_PE2 | CCATCACAGCGATCACGGAGTTGGT |
| P1314_PE3 | CATGGTATTTCCTCCGGGAAATGGG |
| P1314_PE4 | GATCTTGTCGCCGTTCACGCCGTAA |
| Phsp20_PE2 | GGTCCATACGCTGGGTCAGTTCTTC |
| Phsp20_PE3 | GCTTGACGCCGGGAATGTCCAAGGT |
| PpspA_PE1 | ATATCCCGCAGGGCCTGATCGATAA |
| PpspA_PE2 | CTTGGCGAGGTCCTTGTGGTTCTGG |
| PgroES_PE30 | CGGCTTCTTCGATAATTTCAACCAGAACGC |
| PgroES_PE | GGAATCGGGGACGTACAG |
Bold lettering indicates restriction site extension on primer.
To establish hpi as an appropriate housekeeping reference gene for D. radiodurans, QRT-PCR analyses were conducted using a standard curve ranging in concentration from 8 to 1,000 ng of RNA prepared from wild-type untreated RNA. The absolute concentration of hpi expressed from 200 ng of wild-type or sig1 mutant RNA at 30°C compared to that at 48°C RNA was then calculated. No change greater than 1.6-fold at 30°C relative to 48°C was observed for the sig1 mutant and wild-type strains in duplicate experiments. Using hpi as a reference gene, data for dnaK, groES, DR1314, and hsp20 were analyzed using the relative quantitation method from the website www.gene-quantification.info. Relative expression levels greater than 1 represent gene induction at 48°C relative to 30°C. Figures represent the averages plus or minus standard deviations of triplicate runs using RNA prepped on three different days, with triplicates of every sample included in each run.
Mutagenesis and diagnostic PCR.
The vector pHMR186 (28) was modified as follows to generate pHSP20M and pDR1314M for the purpose of deleting hsp20 and DR1314, respectively (Table 1). Approximately 1 kbp of sequence flanking the 5′ and 3′ ends of the gene of interest was PCR amplified from D. radiodurans wild-type chromosomal DNA using Pfu Ultra polymerase (Stratagene, La Jolla, CA) with the following primers: hsp20_5′fwd and hsp20_5′rev for the sequences upstream of hsp20, hsp20_3′fwd and hsp20_3′rev for the sequences downstream of hsp20, and primers with similar names for mutating DR1314 (see Table 5). PCR products were then cloned into pCR2.1-TOPO (Invitrogen), sequenced, and compared to the D. radiodurans genome database (www.tigr.org) to confirm the absence of mutations. Sequences upstream (5′) of the gene of interest were cloned into pHMR186 as BglII-KpnI fragments, followed by cloning of the downstream (3′) sequences as SacI-SnaBI fragments to generate pHSP20M and pDR1314M. Deletion plasmids pHSP20M and pDR1314M were then transformed into D. radiodurans R1 to disrupt the chromosomal copies of hsp20 and DR1314, respectively.
Transformants were screened for double-crossover mutants exhibiting kanamycin resistance and chloramphenicol sensitivity, and positive clones were verified by diagnostic PCR from chromosomal DNA to (i) be devoid of the wild-type gene copy, (ii) contain the kanamycin resistance gene, (iii) lack the chloramphenicol resistance gene, and (iv) contain the upstream and downstream flanking sequences in the correct chromosomal position (see Table 5, below, for primers). Heat shock growth and survival assays were performed with D. radiodurans R1 wild type, Δhsp20, and ΔDR1314 as described previously (30). Mutant strains were found to be unstable. Therefore, for all phenotypic experiments, mutants were obtained by freshly transforming the wild type with the pHSP20M and pDR1314M deletion vectors prior to every survival experiment. Resultant mutant clones were screened by diagnostic PCR to ensure the correct genotype.
Microarray design and construction.
Microarrays were generated by The Institute for Genome Research as reported previously (39). PCR primers were designed to amplify each open reading frame from the fully sequenced D. radiodurans R1 genome (45). PCR products represent internal portions of annotated sequences with a size range of 100 to 800 bp. Primer pairs were designed for 3,180 open reading frames. PCR products were generated by combining 20 ng of genomic DNA from strain R1 with oligonucleotide primers (0.2 μmol each; average Tm = 55°C) and 0.1 U of Taq DNA polymerase (Perkin-Elmer, Wellesley, MA) in a total volume of 100 μl. The other reaction components were as specified by the manufacturer, except that 0.3 M betaine was included in the reaction mixture to aid in denaturing D. radiodurans genomic DNA. PCR amplification successes were scored (single band, correct size, >50 ng/μl). Failed PCRs were repeated with an additional 2% success, for an overall efficiency of 93%. PCR products were spotted in triplicate onto UltraGAPS-coated slides (Corning Life Sciences, Corning, NY) using a Lucidea array spotter (Amersham Pharmacia, Piscataway, NJ) at a redundancy of 3.0. PCR products were immobilized to the slide surface using a Stratalinker UV cross-linker (Stratagene, La Jolla, CA). All slides were stored in a desiccator at room temperature before use.
Microarray probe preparation.
cDNA probes for microarray hybridization were prepared from five biological replicate total RNA samples each of wild-type and sig1 mutant cultures at 30°C and 48°C as follows. Two micrograms of D. radiodurans RNA was annealed to 300 pmol of random hexamer primers (Invitrogen) in a total volume of 18.5 μl by incubating for 10 min at 70°C and subsequent snap-freezing in a dry ice-ethanol bath. cDNA was synthesized at 42°C overnight in 30.7-μl reaction mixtures containing 6 μl of 5× first-strand buffer, 1 μM dithiothreitol, 0.5 mM deoxynucleoside triphosphate mix containing amino allyl-dUTP (Amersham Biosciences, Piscataway, NJ), and 400 U of SuperScript II reverse transcriptase (Invitrogen). RNA was hydrolyzed by adding EDTA to 100 mM and NaOH to 200 mM, incubating at 65°C for 15 min, and then neutralizing with 25 μl of 1 M Tris (pH 7.0). Unincorporated free amino allyl-dUTPs were removed by washing over Microcon 30 columns (Millipore, Bedford, MA), and resultant cDNA samples were coupled to 1 pmol of Cy3 and Cy5 dyes (Amersham) in 0.1 M sodium carbonate buffer for 2 h at room temperature in the dark. Unincorporated dyes were removed by passage over QIAquick MinElute PCR purification columns (QIAGEN). Hybridization of probes to prehybridized microarray slides (1 h incubation at 42°C in 5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.1% SDS, 1% bovine serum albumin) was conducted as previously described (25).
Microarray data acquisition and analysis.
Hybridized slides were scanned on a GenePix 4000B imager (Axon, Union City, CA) at both 532-nm and 635-nm visible light. Data were processed using the TIGR TM4 software analysis package (www.tigr.org/software/tm4). Using the TIGR_Spotfinder program, hybridization signals were quantified by the following formula: spot median > background median + background standard deviation (roughly two times background), and any signals below this threshold were eliminated from subsequent analysis. Hybridization signals were then normalized to total slide intensity with the TIGR_MIDAS program. Using the TIGR_MeV program, resultant raw intensity data were analyzed as Cy5/Cy3 ratios averaged from 15 total hybridization signals for each gene: five biological replicate RNA samples, each of which hybridized to triplicate spots on the array.
A perl script was used to analyze the expression ratio data. Genes exhibiting average expression ratios of ≥2 in four of five wild-type replicate data sets were considered induced. The Sig1 data sets were then mined for Sig1-dependent genes by Pavlidis template matching clustering using the template genes dnaK, hsp20, groES, and DR1314, which were determined to be members of the Sig1 regulon by previous proteomics and QRT-PCR experiments. Only genes that were represented in the results from both the perl script and Pavlidis template matching analyses were considered Sig1 dependent.
Transcription start site mapping by primer extension.
Primer extension reactions were performed on 5 μg of total RNA with 20 pmol of 18- to 30-mer end-labeled oligonucleotide primers (see Table 5) and ThermoScript reverse transcriptase (Invitrogen) as described by the manufacturer. Primers were labeled using T4 polynucleotide kinase (Roche) with 50 μCi of [γ-32P]ATP (Perkin-Elmer Life Sciences, Inc., Wellesley, MA). Sequencing ladders were generated using Sequenase 7.0 (U.S. Biochemicals Corp., Cleveland, OH) according to the manufacturer's protocol from cloned promoter fragments with the same primer that was used to generate the primer extension products. Promoter fragments were PCR amplified from D. radiodurans wild-type chromosomal DNA using Expand polymerase (Roche), sequenced to confirm the absence of mutations, and cloned into pCR2.1-TOPO (Table 1). Transcription start sites were mapped by running primer extension reactions alongside the corresponding sequencing ladder on gradient polyacrylamide sequencing gels (National Diagnostics, Atlanta, GA). Each promoter map was confirmed with duplicate RNA samples prepared on different days and two primers annealing in different locations to the transcript of interest. Primer extension images were quantified by average intensity measurement of the whole band using Kodak 1D 3.6 image quantitation software. Intensities for each band were normalized to the untreated wild-type band for each gene image. Representative experiments are shown below in Fig. 4.
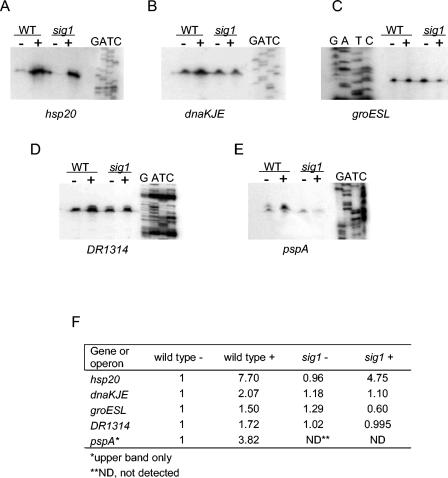
FIG. 4.
Mapping the transcription start site of potential Sig1-dependent genes. (A) hsp20; (B) dnaKJE; (C) groESL; (D) DR1314; (E) pspA. Primer extension reactions were performed on RNA prepared from heat-shocked (+) and untreated (-) D. radiodurans R1 wild-type and sig1 mutant cultures. Adjacent sequencing reactions (GATC) were conducted using the same primer that was used for the primer extension reactions. (F) Quantification of primer extension images. Induction ratios of relative intensities measured for each band relative to the untreated wild type for each gene are reported.
RESULTS
Proteomic analysis of the heat shock response in wild-type and sig1 mutant D. radiodurans strains.
Our previous work indicated that Sig1 not only protects against the stress of elevated temperature, but also appears to be required for the heat shock induction of groESL and dnaKJE operons (30). To further investigate the Sig1 heat shock regulon, 2D gel electrophoresis was performed on whole-cell protein extracts prepared from D. radiodurans wild-type and sig1 mutant cultures that had been shifted to heat shock at 48°C for 1 hour, conditions which had previously been shown to be sufficient to induce the expression of heat shock genes in D. radiodurans (16, 30). In addition, we have shown that cultivation at 48°C for 1 hour inhibits growth but is not lethal to D. radiodurans cells (30). Protein extracted from half of each culture incubated at 30°C served as a control.
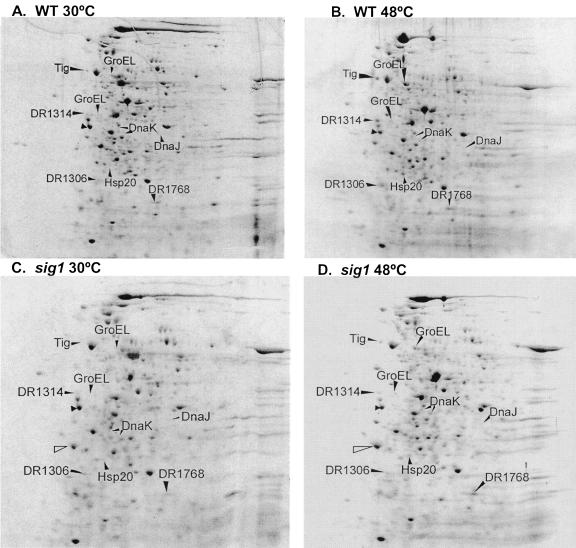
Over 300 protein spots were resolved on all gels (Fig. 1; see also Fig. S1 in the supplemental material). In wild-type cells, the expression of 28 of these protein spots was increased under heat shock at 48°C compared to nonstressed cells incubated at 30°C, and an additional 37 protein spots were only visible at 48°C (Fig. 1). In contrast, the sig1 mutant was either partially or completely impaired for induction of 15 of the 65 total spots induced in wild-type cells. These 15 spots were extracted from the gel and analyzed by LC-MS as described in Materials and Methods. Of the 15 spots tested, 10 were successfully identified with strong hits to the D. radiodurans genome database (Table 2). Of the five unsuccessful identifications, one spot was detected only on the sig1 mutant gels (Fig. 1). The complex mixture of protein fragments detected for this spot suggests that it is composed of either aggregated or degraded protein.
FIG. 1.
Comparison of two-dimensional electrophoresis of total protein from heat-shocked and nonstressed D. radiodurans R1 wild-type and sig1 mutant cells. (A) Wild type at 30°C; (B) wild type at 48°C; (C) sig1 mutant at 30°C; (D) sig1 mutant at 48°C. Two-dimensional electrophoresis was performed in the pI range of 4 to 8 on whole-cell protein extracts isolated from wild-type and sig1 mutant cultures shifted to heat shock for 1 h at 48°C; control cultures were maintained at 30°C. Computerized and manual comparisons between panels A and B indicated spots that were induced under heat shock in wild-type cultures. Comparison between panels C and D indicated heat shock-induced spots in the sig1 mutant, and comparison between panels B and D indicated spots that were induced in the wild type but not the sig1 mutant. Labeled arrows point to spots that are induced in wild-type cultures but not in sig1 mutants, and labels correspond to the LC-MS identifications shown in Table 2. The filled unlabeled arrow indicates the IEF internal standard. The open unlabeled arrow indicates a spot observed only in the sig1 mutant that had no hits to the D. radiodurans genome database. GroEL and DnaK fragments are indicated in parentheses.
TABLE 2.
LC-MS identification of spots isolated from 2D gels
| Gene name | ORF no.a | Mass (kDa)
|
Putative function | Induction ratio at 48 vs 30°C
|
||
|---|---|---|---|---|---|---|
| Predicted | Observed | R1b | sig1 | |||
| tig | DR1948 | 51.8 | 65.1 | Trigger factor | 3.55 | 1.27 |
| groEL | DR0607 | 57.8 | 62.8 | Chaperone hsp60 | 9.75 | + |
| DR1314 | 36.5 | 38.8 | Hypothetical protein | 7.84 | 1.94 | |
| groEL | DR0607 | 57.8 | 38.7 | Chaperone hsp60 | 3.17 | + |
| dnaK | DR0129 | 67.7 | 35.3 | Chaperone hsp70 | +++ | −19.42 |
| dnaK | DR0129 | 67.7 | 34.2 | Chaperone hsp70 | +++ | 4.34 |
| dnaJ1 | DR0126 | 33.0 | 33.1 | Protein folding | +++ | −3.1 |
| DR1306 | 24.9 | 23.8 | Hypothetical protein | 3.50 | 1.04 | |
| hsp20 | DR1114 | 20.2 | 26.4 | Small heat shock protein | 8.10 | 4.18 |
| DR1768 | 15.2 | 20.0 | Hypothetical protein | 3.05 | 1.99 | |
Annotated open reading frame (ORF) numbers come from the D. radiodurans complete genome sequence (45).
+++, the spot was detected only at 48°C; +, detection only at 48°C, but the 48°C spot density was very low in the mutant compared to wild type.
Of the 10 successful identifications, two polypeptides were identified for each of DnaK and GroEL. The molecular mass of each of the DnaK polypeptides was lower than the predicted 70 kDa (Table 2). The combined molecular mass of both spots was 69.7 kDa, an observation consistent with the hypothesis that these spots represent nonspecific degradation products of DnaK. This notion is also supported by the fact that full-length GroEL (observed mass, 62.8 kDa) was isolated from the gels in addition to a smaller spot with an observed mass of 38.7 kDa. Therefore, a total of eight gene products were identified by this analysis (Table 2).
After comparison of the eight identified polypeptides with the annotated genome, two predicted functional categories were obtained. (i) Heat shock-protective functions were obtained (five polypeptides), which included DnaK and GroEL, as expected from our previous studies (30), but also included DnaJ1, trigger factor (Tig) (a protein that works together with DnaK to fold nascent polypeptides emerging from the ribosome [7]), and Hsp20, a putative small heat shock protein (Table 2). GroES was not identified in this analysis, since it has a predicted pI of 9 and our gels were run in the pI range of 4 to 8. Both Tig and Hsp20 show low identities with known homologs, 31% to Tig from B. subtilis and 31% to Hsp18 from S. albus (32). (ii) Hypothetical proteins of unknown function (three polypeptides) were found, including DR1314, DR1306, and DR1768, none of which are orthologous to known bacterial genes in the sequence database.
QRT-PCR verification of proteomics data.
Sig1-dependent heat shock induction of polypeptides implies a transcriptionally based regulatory mechanism. This hypothesis was tested for a subset of the genes, DR1314, groESL, dnaK, and hsp20, using real-time QRT-PCR. Since our previous results have indicated that groESL and dnaKJ operons appear to be regulated by Sig1 (30), these genes were included as internal controls for the validity of the QPCR assay. RNA was harvested from heat-shocked and nonstressed control cultures of sig1 and wild-type D. radiodurans cells, cDNA was synthesized, and real-time PCR was conducted using primers corresponding to 100- to 200-bp sequences internal to the gene of interest. Data were quantified employing a relative technique that uses hpi as a housekeeping reference gene. hpi is thought to encode the major surface layer protein in D. radiodurans (22). In our hands, hpi exhibited no change in expression greater than 1.6-fold at 48°C relative to that at 30°C in replicate experiments (data not shown) (see Materials and Methods) and was therefore chosen as an appropriate reference gene for relative quantitation. In this scheme, values greater than 1 indicate gene induction at 48°C relative to 30°C expression levels, values equal to 1 represent no change, and values less than 1 indicate repression.
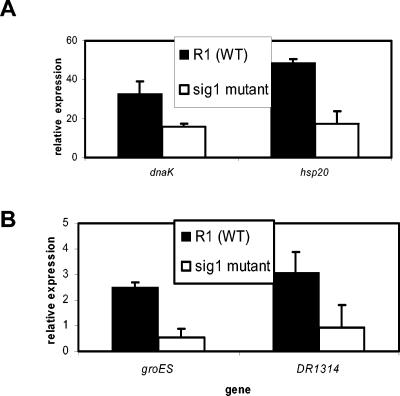
In the D. radiodurans wild-type background, dnaK and hsp20 were induced up to 40 units and 55 units of relative gene expression, respectively, at 1 h after induction to heat shock. In contrast, sig1 mutant cells failed to induce either of these genes to the same extent, exhibiting an impairment of approximately one-third to one-half the wild-type induction level (Fig. 2A). The heat shock induction of groES and DR1314 was also tested. Since preliminary QRT-PCR experiments indicated that these genes were not induced at 60 min after a shift to 48°C (not shown), we harvested RNA over a time course and found that the expression of both groES and DR1314 peaked at 5 min after induction to heat shock (data not shown). Data from this 5-min time point are shown in Fig. 2B. In the wild-type background, groES and DR1314 were induced to a moderate level (2 to 4 units of gene expression) upon heat shock. In contrast, sig1 mutant cells failed to induce DR1314 to the same level, showing only up to 2 units of relative expression (Fig. 2B). In addition, the sig1 mutant cells failed to induce groES transcription above the levels observed at 30°C.
FIG. 2.
Quantitative RT-PCR assessment of transcriptional heat shock induction in D. radiodurans R1 wild-type (WT) compared to sig1 mutant cells. (A) hsp20 and dnaK 1 h after induction to heat shock at 48°C. (B) DR1314 and groES expression 5 min after heat shock. RNA was harvested from wild-type and sig1 cells following shift to heat shock at 48°C for 5 min (B) or 1 h (A); 30°C cultures served as a control. Following cDNA synthesis, QPCR was performed using primers that anneal to 100- to 200-bp sequences internal to the gene of interest. Data were quantified using a relative scheme in which relative expression values greater than 1 indicate heat shock induction. Data shown represent the averages ± standard deviations of at least three biological replicate experiments.
Role of Hsp20 and DR1314 in protection of D. radiodurans against heat shock.
Since the proteomics and RT-PCR data suggested that hsp20 and DR1314 were both induced under heat shock conditions, mutants were generated in these genes to determine if their gene products were involved in protection against the stress of increased temperature. To disrupt the chromosomal copies of DR1314 and hsp20, deletion plasmids pDR1314M and pHSP20M (Table 1) were transformed into D. radiodurans cells and screened for kanamycin resistance and chloramphenicol sensitivity. Resultant mutant clones, designated ΔDR1314 and Δhsp20, were verified by diagnostic PCR from chromosomal DNA as described in Materials and Methods.
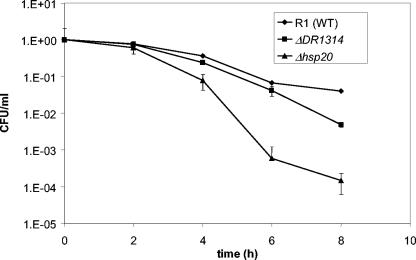
Following diagnostic PCR, D. radiodurans R1 wild-type, ΔDR1314, and Δhsp20 strains were tested for growth and survival at 30°C, 40°C, 42°C, and 48°C. The sig1 mutant was also tested as a reference for severe heat shock phenotypes. No significant growth or survival differences were observed among wild-type, ΔDR1314, and Δhsp20 mutant strains at 30°C, 40°C, or 42°C (data not shown). However, at 48°C Δhsp20 exhibited an impairment of up to 3 orders of magnitude in survival compared to the wild type (Fig. 3). In addition, the ΔDR1314 mutant was up to 10-fold more susceptible to heat shock than the wild type (Fig. 3). The sig1 mutant cultures also displayed the expected survival impairment of 2 orders of magnitude compared to the wild type (data not shown) (30), indicating that the stress conditions were accurately reproduced in this experiment.
FIG. 3.
Survival of wild-type (WT) D. radiodurans R1 compared to ΔDR1314 and Δhsp20 mutant strains under heat shock. Wild-type, ΔDR1314, and Δhsp20 cells were grown in TGY broth to mid-exponential phase (optical density at 600 nm = 0.3 to 0.4) and shifted to heat shock at 48°C (time zero). Half of each culture was continued at 30°C as a control (not shown). Samples were removed from each culture every 2 h, and duplicate serial dilutions were spotted in 5-μl drops onto TGY in triplicate to assess survival. Results are normalized to initial CFU/ml. Error bars represent standard deviations from the averages of four biological replicate experiments.
Global transcriptional analysis of the Sig1 regulon.
To perform a more exhaustive and global search for Sig1-dependent genes, we conducted whole-genome microarray analysis using arrays spotted in triplicate with PCR products corresponding to each of 3,180 unique D. radiodurans open reading frames (40). Five biological replicate array hybridizations were conducted comparing cDNA from samples heat shocked for 5 minutes to untreated controls for each of the D. radiodurans R1 wild-type and sig1 mutant strains. As a control for hybridization quality and reproducibility, competitive hybridizations were performed comparing replicate cDNA samples from the same strain at 30°C. We observed good reproducibility in Cy5/Cy3 ratios, ranging from 0.4 to 2 in three replicate samples (not shown).
Under heat shock, a total of 136 genes were induced greater than twofold in the wild type, the 25 most highly induced of which are listed in Table 3. Employing a clustering algorithm (Pavlidis template matching [www.tigr.org/software/tm4]) in which hsp20, DR1314, dnaK, and groES were used as template genes, the sig1 mutant was found to be partially or completely impaired for induction of 31 of the 136 genes during heat shock (Table 4). These genes were grouped into four categories according to their predicted functions from the annotated genome sequence (Table 4): (i) housekeeping genes, which included a phosphoenolpyruvate carboxykinase homolog (DR0977); (ii) hypothetical factors and putative transposases of unknown function, including DR1314 and DR1768, which were identified as Sig1-dependent polypeptides in the proteomic analysis (Table 2). All of the genes in this category except DR0194 have no orthologs in the bacterial sequence database. Interestingly, DR0194 bears 45% amino acid identity to B. subtilis YugP, a putative zinc-dependent metalloprotease. The other two categories were (iii) ABC transporters and membrane proteins and (iv) heat shock and stress response-related functions, including hsp20, dnaK, and groES, corroborating the proteomic (Fig. 1) and QRT-PCR (Fig. 2) data. All other members of the dnaKJE and groESL operons were expressed at similar levels to the first gene of the transcriptional unit. In addition, four new Sig1-dependent genes with predicted stress response functions were discovered, including two putative response regulators bearing amino acid identity to those of T. thermophilus (DR0743, 71%) and Agrobacterium tumefaciens (DR0408, 55%); dps, which bears low amino acid homology to E. coli dps, whose gene product is known to bind and protect DNA during general stress (3); and pspA, whose product exhibits 28% identity at the amino acid level to E. coli PspA, 27% to the B. subtilis PspA homolog, YdjF, and 38% homology to cyanobacterial VIPP1. E. coli PspA functions in maintenance of membrane integrity under a variety of stress conditions (13, 43) but has also been implicated as a negative regulator of the psp phage shock operon (9). Cyanobacterial VIPP1 is essential for photosynthesis and biogenesis of thylakoid membranes (14, 44). Surprisingly, of all genes identified by microarray analysis as Sig1 dependent, pspA was the most severely affected by the sig1 mutation, exhibiting a sevenfold impairment in heat shock expression compared to the wild type (Table 4), and was therefore chosen for inclusion in subsequent analyses.
TABLE 3.
The 25 most highly induced genes in the wild-type strain as determined by microarray analysis, sorted by induction ratio
| Locus | Gene name | Induction ratio R1a | Predicted functionb |
|---|---|---|---|
| DR1114 | hsp20 | 11.99 | Small heat shock protein |
| DR0127 | 11.06 | Hypothetical | |
| DR0128 | grpE | 10.77 | Chaperone |
| DR1046 | clpB | 8.02 | ATP-dependent Clp protease |
| DRA0199 | 7.95 | Nodulation protein N-related protein | |
| DR0126 | dnaJ | 7.90 | Chaperone |
| DR0129 | dnaK | 7.49 | Chaperone |
| DR2381 | 7.35 | Aldehyde dehydrogenase | |
| DR0561 | 6.07 | Maltose ABC transporter, periplasmic maltose-binding protein | |
| DR0178 | 5.91 | Transposase, putative | |
| DR0349 | lon | 5.80 | ATP-dependent protease |
| DR0997 | 5.41 | Transcriptional regulator, FNR/CRP family | |
| DR1082 | lrpA | 5.30 | Light-repressed protein A, putative |
| DR1019 | 4.99 | Glycerol-3-phosphate dehydrogenase | |
| DR0220 | 4.83 | Amidophosphoribosyltransferase | |
| DR2307 | 4.76 | Multidrug efflux transporter, putative | |
| DR2386 | paaA | 4.70 | Phenylacetic acid degradation protein |
| DRA0200 | 4.62 | Oxidoreductase, short-chain dehydrogenase/reductase family | |
| DR1473 | pspA | 4.50 | Phage shock related protein |
| DRC0007 | 4.38 | Hypothetical protein | |
| DRB0012 | 4.35 | Cobyric acid synthase | |
| DR1314 | 4.35 | Conserved hypothetical protein | |
| DR0392 | 4.34 | Hypothetical protein | |
| DRB0083 | 4.32 | Potassium-transporting ATPase, B subunit | |
| DR0194 | 4.27 | Conserved hypothetical protein |
Numbers represent average ratios of 48°C and 30°C intensity values from five replicate data sets.
Functional annotations are taken from the D. radiodurans completed genome sequence (45; www.tigr.org).
TABLE 4.
The Sig1 regulon as determined by microarray analysis
| Locus | Gene name | R1 | sig1 | Annotated function |
|---|---|---|---|---|
| Heat shock, stress response, or regulatory function genes | ||||
| DR0126 | dnaJ | 7.90 | 4.39 | Chaperone |
| DR0127 | 11.06 | 7.07 | Hypothetical | |
| DR0128 | grpE | 10.77 | 7.67 | Chaperone |
| DR0129a | dnaK | 7.49 | 3.95 | Chaperone |
| DR0606 | groES | 2.36 | 1.24 | Chaperone |
| DR0607 | groEL | 3.03 | 1.60 | Chaperone |
| DR1114 | hsp20 | 11.99 | 8.77 | Small heat shock protein |
| DR2263 | dps | 2.76 | 1.01 | DNA binding stress response protein |
| DR1473 | pspA | 4.50 | 0.607 | Phage shock protein A |
| DR0743 | 2.78 | 1.74 | Response regulator | |
| DR0408 | 3.18 | 1.53 | Response regulator | |
| Housekeeping gene | ||||
| DR0977*b | 3.25 | 1.48 | Phosphoenolpyruvate carboxykinase | |
| Hypothetical function and transposase genes | ||||
| DR0194 | 4.27 | 2.34 | Conserved hypothetical; 45% identity to B. subtilis yugP, putative Zn-dependent metallopeptidase | |
| DR0227 | 3.42 | 1.35 | Hypothetical | |
| DR0370 | 2.77 | 1.58 | Hypothetical | |
| DR0762 | 2.70 | 1.48 | Hypothetical | |
| DR0972 | 2.90 | 1.11 | Hypothetical | |
| DR1314 | 4.35 | 1.55 | Hypothetical | |
| DR1315 | 4.06 | 1.85 | Hypothetical | |
| DR1768* | 2.51 | 1.3 | Hypothetical | |
| DR1816 | 2.75 | 1.19 | Hypothetical | |
| DR1815 | 3.12 | 1.31 | Hypothetical | |
| DR2165* | 2.40 | 1.59 | Hypothetical | |
| DR2527 | 3.91 | 1.47 | Hypothetical | |
| DR2528 | 2.98 | 1.57 | Hypothetical | |
| DR2572 | 2.73 | 1.11 | Hypothetical | |
| DRB0059 | 2.81 | 1.7 | Transposase, putative | |
| DRC0015* | 3.01 | 1.52 | Hypothetical | |
| ABC transporter membrane protein genes | ||||
| DR1185 | 3.41 | 1.3 | S-layer-like array-related | |
| DR1655 | 2.59 | 1.85 | ABC transporter, periplasmic substrate binding | |
| DR1438 | 3.66 | 1.65 | ABC transporter, periplasmic substrate binding |
Items in bold lettering represent genes that have been identified by QRT-PCR and/or 2D-PAGE as Sig1 dependent.
Loci labeled with an asterisk designate genes with no recognizable predicted Sig1-dependent promoter.
Transcription start site mapping and determination of a putative Sig1-dependent promoter consensus sequence.
To confirm the dependence of hsp20, DR1314, groES, dnaK, and pspA on Sig1 for their heat shock regulation and to identify a potential Sig1 consensus promoter sequence, we performed transcription start site mapping by primer extension. RNAs prepared from heat-shocked and untreated sig1 mutant and wild-type cultures were used as templates to conduct primer extension reactions using primers listed in Table 5. The resultant transcription start site maps are shown in Fig. 4. We observed that transcription of each of hsp20 and the dnaKJ-grpE and DR1314/1315 operons initiated from a single promoter that was induced under heat shock in wild-type cells (7.7-fold, 2-fold, and 1.7-fold, respectively) (Fig. 4A, B, D, and F). In contrast, the sig1 mutant was completely impaired for dnaKJ-grpE and DR1314/1315 transcriptional induction at 48°C (Fig. 4B, D, and F). Furthermore, transcript signal intensities of hsp20 were weaker at 48°C in the sig1 mutant background (4.75-fold induction) (Fig. 4F) compared to those in the wild type, though the signals in both backgrounds were of extremely low intensity at 30°C (Fig. 4A). The shadowy band observed just upstream of the primer extension product of DR1314/1315 (Fig. 4D) is a primer artifact, since it was not observed in replicate experiments with the P1314_PE3 primer (data not shown) (Table 5).
In the wild-type strain, a strong, mildly heat shock-inducible (1.5-fold) primer extension signal was detected for the groESL operon promoter, and transcription was observed to initiate from a single promoter located 76 bp upstream of the predicted ATG (Fig. 4C and F). The minor start site found in previous mapping experiments of the groES promoter was not detected (16). In sharp contrast to the wild-type situation, in the sig1 mutant, the level of groES promoter transcription was much reduced under heat shock (0.6-fold repressed) (Fig. 4C and F).
Transcription of pspA was observed to start from two promoters (Fig. 4E): transcription from pspA P1 was present at low constitutive levels in both wild-type and sig1 mutant strains, whereas P2 was strongly inducible (3.8-fold) in the wild type (Fig. 4E and F). However, a P2 signal was completely absent under both heat shock and nonstress conditions in sig1-defective cells, indicating complete dependence of this promoter on Sig1-associated RNA polymerase for its transcription.
Interestingly, the −35 and −10 regions of pspA P2 bore strong resemblance to the known σW consensus from B. subtilis (TGAAAC-N16-CGTA) (12), diverging at only one nucleotide position (Fig. 5B). In contrast, the −35 and −10 positions of pspAP1 and DR1314/1315 appeared to resemble the divergent σ70-type promoters previously identified in D. radiodurans (Fig. 5A) (16). In addition, each of the single promoters of hsp20, dnaKJE, and groESL was well conserved with the E. coli σ70 consensus, diverging only at the −10 region (Fig. 5A). To determine if either of these putative σW- or σ70-type consensus sequences was present upstream of the other Sig1-dependent genes identified by microarray analysis, the promoters mapped by primer extension were used in a sequence pattern findingprogram (rsat.ulb.ac.be/rsat/dna_pattern_form.cgi) toquery regions located 500 bp upstream and 150 bp downstream of the predicted ATG for each of the Sig1 regulon genes. Only hits to potential −35 and −10 regions that were located within 200 bp and that contained a predicted Shine-Dalgarno sequence within 12 bp of the predicted ATG were included in the final consensus list, the results of which are aligned in Fig. 5. As a control, an additional search was performed in which the upstream sequences were queried with the E. coli σE (5) and B. subtilis σB consensus sequences (23), but no hits were yielded with this search.
FIG. 5.
Alignment of putative Sig1-dependent promoters and determination of a potential Sig1 consensus sequence. The −10/−35 regions of mapped Sig1-dependent promoters (bracketed on left) were used to search regions 500 bp upstream and 150 bp downstream of the predicted ATG of the 31 Sig1 regulon members identified by microarray analysis. Resultant −35/−10 regions for the genes listed on the left (see Table 4 for annotation) are aligned and in boldface type. Italicized characters represent predicted ribosome binding sites. Predicted cotranscribed genes are listed together, separated by a slash. Nucleotide positions at which transcription starts are capitalized and marked with an asterisk. The Sig1 consensus sequences listed below each alignment were determined by calculating the percentage occurrence of each nucleotide at each position. Nucleotides with the highest occurrence at a given position were chosen for the consensus.
As shown in Fig. 5B, 8 of the 31 Sig1 regulon genes contained a predicted σW-type promoter, all of which belonged to the hypothetical or heat shock and stress response functional categories (Table 4). As shown in Fig. 5A, 18 of the 31 genes, organized into 12 operons, possessed a predicted σ70-type promoter. Both consensus sequences were found upstream of the hypothetical operon DR1816/DR1815. No predicted −35 or −10 promoter regions were found upstream of the remaining four genes, which included phosphoenolpyruvate carboxykinase (DR0977) and three hypothetical genes (DR1768, DR2165, and DRC0015). All four of these genes were predicted to be monocistronic based on the transcriptional direction of the surrounding genes. A putative promoter was found upstream of all genes with predicted heat shock and stress response functions.
DISCUSSION
Results from previous studies (30) suggested that Sig1 is the major heat shock sigma factor of D. radiodurans that both protects against elevated temperature stress and is involved in the positive regulation of cytoplasmic heat shock operons dnaKJE and groESL. We therefore undertook the study reported here to further define the role of Sig1 in the heat shock response of D. radiodurans. The results of the 2D-PAGE proteomics, quantitative PCR, mutant analysis, and microarray experiments suggest that Sig1 is involved in controlling a larger regulon consisting not only of cytoplasmic heat shock and stress response factors but also of extracytoplasmic factors and previously unidentified genes that do not have orthologs in the known bacterial domain.
Thirty-one genes were identified as being fully or partially impaired for induction in sig1 mutant cells compared to D. radiodurans R1 wild-type cells in a combined global survey using 2D-PAGE proteomics and microarray techniques. Eight of these genes, organized into five predicted transcriptional units, are known heat shock factors in other bacteria: dnaKJ-grpE, groESL, dps, pspA, and hsp20, of which dnaKJ-grpE and groESL had previously been shown to be involved in the heat shock response in D. radiodurans (30). hsp20 encodes a 20-kDa protein predicted to be a member of the small heat shock protein (sHSP) superfamily, since its amino acid sequence contains an alpha-crystalline domain (18; www.tigr.org), and is within the predicted small heat shock protein size range of 15 to 30 kDa. In addition, like the S. albus sHSP hsp18 mutant, Δhsp20 mutant cells exhibited an extreme impairment in survival at a growth-inhibitory temperature (Fig. 3) (32, 33). However, in S. albus, hsp18 is controlled by a specific negative regulator (RheA) (33), while in D. radiodurans it appears this sHSP is part of the Sig1 regulon. No ortholog of RheA is present in the D. radiodurans genome.
D. radiodurans pspA, another member of the Sig1 regulon identified in the microarrays with predicted heat shock functions, bears 28% homology to E. coli pspA, which encodes a peripheral membrane protein exhibiting high induction in response to diverse environmental stresses (reviewed in reference 17). Intriguingly, D. radiodurans pspA also bears 27% identity to B. subtilis ydjF, which has been shown to be highly induced by alkali stress and phage infection in a σW-dependent manner (46). Similarly, our microarray data (Table 4) and primer extension results (Fig. 4) indicate a clear transcriptional dependence of D. radiodurans pspA on Sig1, an ECF-type sigma factor (30). In addition, the −35/−10 sequences of the pspA P2 promoter were nearly identical to the B. subtilis σW ECF-type consensus (12), suggesting a possible functional similarity between Sig1 and σW.
E. coli PspA has also been implicated in maintenance of outer membrane integrity (13). Interestingly, several other Sig1-regulated heat shock genes identified here are also predicted to encode proteins with membrane functions, including two ABC transporters, DR1185 (a putative surface layer gene) and DR1314, which appears to be a novel Sig1-regulated heat shock gene containing predicted membrane-spanning domains and bearing predicted structural homology to membrane proteins (data not shown) (www.sbg.bio.ic.ac.uk/∼3Dpssm). These results are consistent with the hypothesis that Sig1 may also function as a regulator of factors with periplasmic or extracytoplasmic functions. Therefore, Sig1 appears to be a truly unique ECF factor, involved in the heat shock regulation of genes whose products perform both cytoplasmic and extracytoplasmic functions, which are usually regulated by two different sigma factors (e.g., σH and σE in E. coli [47]).
In addition to the σW-type promoter upstream of pspA and other genes, we also detected an E. coli σ70-type consensus upstream of the remaining genes identified by microarray analysis (Fig. 5). Surprisingly, transcription initiating from the single σ70-type promoters of each of dnaKJE, groESL, DR1314/1315, and hsp20 appears to be responsible for expression of all these genes in both wild-type and sig1-deficient cells in the absence of stress, as evidenced by the persistence of the primer extension signals in the sig1 mutant and wild type at 30°C. However, at least part of the increase in expression from these σ70-type promoters upon temperature upshift appears to require the presence of Sig1, suggesting that Sig1 may recognize this promoter only at high temperature and that another sigma factor may be responsible for the transcription of the same promoter in the absence of stress. Mounting evidence suggests that multiple sigma factors do recognize the same promoter sequences, as is the case with B. subtilis σX and σW overlapping regulons (11) and σH and σE dual recognition of the σB promoter in M. tuberculosis (27). In addition, ECF sigma factors appear to recognize broader consensus sequences, as is the case with E. coli σE (5). Alternatively, it remains possible that Sig1 involvement in control of these σ70-type promoters is indirect through another regulator that depends on Sig1 for its positive regulation, for example, the putative response regulators DR0743 and DR0408, which were partially abrogated in their heat shock response in cells defective for sig1 (Table 4).
It was also noteworthy that hypothetical factors DR0326 and DR0972, proteins of unknown function that have been observed to be heat shock inducible by 2D gel analysis in other studies (1), were not detected in the current 2D gel study. Also of note is the fact that, although Airo and colleagues detected the heat shock inducibility of a putative cold shock protein, Csp (DR0907), we observed repression of csp gene transcription under heat shock in our microarray analysis (average ratio, 0.318) (data not shown). It should also be noted that the heat shock protocols used by Airo and colleagues were significantly different from those used in this study. However, given the differences in protocols, the results between the two studies were surprisingly similar.
The other surprising finding, that transcription of dnaK, hsp20, and DR1314 was still partially induced in sig1-defective cells in our microarray and QRT-PCR experiments, suggests that an additional mode of regulation may exist that controls these genes during elevated temperature stress. In fact, we have evidence that these genes are also subject to negative regulation by an HspR-like system in D. radiodurans (31). Such a phenomenon is not uncommon. For example, A. tumefaciens rpoH mutants are still able to induce groESL transcription to 40% of the wild-type level, since release from repression by hrcA is also required to fully induce the heat shock expression of this operon (19).
In summary, our results represent the first example of a global study of the heat shock response in D. radiodurans and indicate that Sig1 may play a novel role among the ECF sigma factors in the regulation of this response, in at least partial control of both cytoplasmic and extracytoplasmic factors. At least one of the extracytoplasmic factors, pspA, is completely and directly dependent on Sig1 for its transcription. Sig1 appears to be in direct or indirect control of 30 other heat shock-inducible and -protective genes, one of which has been confirmed as a novel heat shock-protective gene (DR1314).
Supplementary Material
Acknowledgments
This work was funded by a grant to Mary E. Lidstrom from the DOE EMSP (ER20294), by a grant to John R. Battista from the DOE MGP (DEFG0201ER63151), and in part by a University of Washington Molecular and Cellular Biology Training Grant (GM0720). Part of this research was performed in the Environmental Molecular Sciences Laboratory (a national scientific user facility sponsored by the U.S. DOE Office of Biological and Environmental Research) located at Pacific Northwest National Laboratory and operated by Battelle for the DOE.
We are indebted to Dhileep Sivam for writing the perl script to analyze the microarray data. We also thank Ashlee Earl for her invaluable advice concerning the quantitative real-time RT-PCR assays and microarrays and Heather Rothfuss for her support, helpful comments, and the use of her pHMR186 vector system.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Airo, A., S. L. Chan, Z. Martinez, M. O. Platt, and J. D. Trent. 2004. Heat shock and cold shock in Deinococcus radiodurans. Cell Biochem. Biophys. 40:277-288. [DOI] [PubMed] [Google Scholar]
- 2.Allen, S. P., J. O. Polanzzi, J. K. Gierse, and A. M. Easton. 1992. Two novel heat shock genes encoding proteins produced in response to heterologous protein expression in Escherichia coli. J. Bacteriol. 174:6938-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almiron, M., A. J. Link, D. Furlong, and R. Kolter. 1992. A novel DNA binding protein with regulatory and protective roles in starved E. coli. Genes Dev. 6:1646-1654. [DOI] [PubMed] [Google Scholar]
- 4.Battista, J. R. 1997. Against all odds: the survival strategies of Deinococcus radiodurans. Annu. Rev. Microbiol. 51:203-224. [DOI] [PubMed] [Google Scholar]
- 5.Dartigalongue, C., D. Missiakas, and S. Raina. 2001. Characterization of the Escherichia coli σE regulons. J. Biol. Chem. 276:20866-20875. [DOI] [PubMed] [Google Scholar]
- 6.Derre, I., G. Rapoport, and T. Msadek. 1999. CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in gram-positive bacteria. Mol. Microbiol. 31:117-131. [DOI] [PubMed] [Google Scholar]
- 7.Deuerling, E., A. Schulze-Specking, T. Tomoyasu, A. Mogk, and B. Bukau. 1999. Trigger factor and DnaK cooperate in folding of newly synthesized proteins. Nature 400:693-696. [DOI] [PubMed] [Google Scholar]
- 8.Earl, A. M., S. K. Rankin, K.-P. Kim, O. N. Lamendola, and J. R. Battista. 2002. Genetic evidence that the uvsE gene product of Deinococcus radiodurans R1 is a UV damage endonuclease. J. Bacteriol. 194:1003-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elderkin, S., S. Jones, J. Schumacher, D. Studholme, and M. Buck. 2002. Mechanism of action of the Escherichia coli phage shock protein PspA in repression of the AAA family transcription factor PspF. J. Mol. Biol. 320:23-37. [DOI] [PubMed] [Google Scholar]
- 10.Gatlin, C. L., G. R. Kleemann, L. G. Hays, A. J. Link, and J. R. Yate III. 1998. Protein identification at the low femtomole level from silver-stained gels using a new fritless electrospray interface for liquid chromatography-microspray and nanospray mass spectrometry. Anal. Biochem. 263:93-101. [DOI] [PubMed] [Google Scholar]
- 11.Huang, X., K. L. Frederick, and J. D. Helmann. 1998. Promoter recognition by Bacillus subtilis σW: autoregulation and partial overlap with the σX regulon. J. Bacteriol. 180:3765-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, X., A. Gaballa, M. Cao, and J. D. Helmann. 1999. Identification of target promoters for the Bacillus subtilis extracytoplasmic function sigma factor, σW. Mol. Microbiol. 31:361-371. [DOI] [PubMed] [Google Scholar]
- 13.Kleerebezem, M., W. Crielaard, and J. Tommassen. 1996. Involvement of stress protein PspA (phage shock protein A) of Escherichia coli in maintenance of the proton-motive force under stress conditions. EMBO J. 15:162-171. [PMC free article] [PubMed] [Google Scholar]
- 14.Kroll, D., K. Meierhoff, N. Bechtold, M. Kinoshita, S. Westphal, U. C. Vothknecht, J. Soll, and P. Westhoff. 2001. VIPP1, a nuclear gene of Arabidopsis thaliana essential for thylakoid membrane formation. Proc. Natl. Acad. Sci. USA 98:4238-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markillie, L. M., S. M. Varnum, P. Dradecky, and K.-K. Wong. 1999. Targeted mutagenesis by duplication insertion in the radioresistant bacterium Deinococcus radiodurans: radiation sensitivities of catalase (katA) and superoxide dismutase (sodA) mutants. J. Bacteriol. 181:666-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meima, R., H. M. Rothfuss, L. Gewin, and M. E. Lidstrom. 2001. Promoter cloning in the radioresistant bacterium Deinococcus radiodurans. J. Bacteriol. 183:3169-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Model, P., G. Jovanovic, and J. Dworkin. 1997. The Escherichia coli phage-shock-protein (psp) operon. Mol. Microbiol. 42:255-261. [DOI] [PubMed] [Google Scholar]
- 18.Münchbach, M., A. Nocker, and F. Narberhaus. 1999. Multiple small heat shock proteins in rhizobia. J. Bacteriol. 181:83-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakahigashi, K., E. Z. Ron, H. Yanagi, and T. Yura. 1999. Differential and independent roles of a σ32 homolog (RpoH) and an HrcA repressor in the heat shock response of Agrobacterium tumefaciens. J. Bacteriol. 181:7509-7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narberhaus, F. 1999. Negative regulation of bacterial heat shock genes. Mol. Microbiol. 31:1-8. [DOI] [PubMed] [Google Scholar]
- 21.O'Farrell, P. H. 1975. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250:4007-4021. [PMC free article] [PubMed] [Google Scholar]
- 22.Peters, J., M. Peters, F. Lottspeich, W. Schafer, and W. Baumeister. 1987. Nucleotide sequence analysis of the gene encoding the Deinococcus radiodurans surface protein, derived amino acid sequence, and complementary protein chemical studies. J. Bacteriol. 169:5216-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersohn, A., J. Bernhardt, U. Gerth, D. Höper, T. Koburger, U. Völker, and M. Hecker. 1999. Identification of σB-dependent genes in Bacillus subtilis using a promoter consensus-directed search and oligonucleotide hybridization. J. Bacteriol. 181:5718-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersohn, A., M. Brigulla, S. Haas, J. D. Hoheisel, U. Völker, and M. Hecker. 2001. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 183:5617-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson, S., R. T. Cline, H. Tettelin, V. Sharov, and D. A. Morrison. 2000. Gene expression analysis of the Streptococcus pneumoniae competence regulons by use of DNA microarrays. J. Bacteriol. 182:6192-6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price, C. W., P. Fawcett, H. Cérémonie, N. Su, C. K. Murphy, and P. Youngman. 2001. Genome-wide analysis of the general stress response in Bacillus subtilis. Mol. Microbiol. 41:757-774. [DOI] [PubMed] [Google Scholar]
- 27.Raman, S., T. Song, X. Puyang, S. Bardarov, W. R. Jacobs, Jr., and R. N. Husson. 2001. The alternative sigma factor SigH regulates major components of oxidative and heat stress responses in Mycobacterium tuberculosis. J. Bacteriol. 183:6119-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothfuss, H. M. 2004. Ph.D. thesis. University of Washington, Seattle.
- 29.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Schmid, A. K., and M. E. Lidstrom. 2002. Involvement of two putative alternative sigma factors in stress response of the radioresistant bacterium Deinococcus radiodurans. J. Bacteriol. 184:6182-6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmid, A. K., H. A. Howell, J. R. Battista, S. N. Peterson, and M. E. Lidstrom. 2005. HspR is a global negative regulator of heat shock gene expression in Deinococcus radiodurans. Mol. Microbiol. 55:1579-1590. [DOI] [PubMed] [Google Scholar]
- 32.Servant, P., and P. Mazodier. 1995. Characterization of Streptomyces albus 17-kilodalton heat shock-responsive protein. J. Bacteriol. 177:2998-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Servant, P., G. Rapoport, and P. Mazodier. 1999. RheA, the repressor of hsp18 in Streptomyces albus G. Microbiology 145:2385-2391. [DOI] [PubMed] [Google Scholar]
- 34.Servant, P., and P. Mazodier. 2001. Negative regulation of the heat shock response in Streptomyces. Arch. Microbiol. 176:237-242. [DOI] [PubMed] [Google Scholar]
- 35.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 36.Sobczyk, A., A. Bellier, J. Viala, and P. Mazodier. 2002. The lon gene, encoding an ATP-dependent protease, is a novel member of the HAIR/HspR stress-response regulon in Actinomycetes. Microbiology 148:1931-1937. [DOI] [PubMed] [Google Scholar]
- 37.Stewart, G. R., L. Wernisch, R. Stabler, J. A. Mangan, J. Hinds, K. G. Laing, D. B. Young, and P. D. Butcher. 2002. Dissection of the heat-shock response in Mycobacterium tuberculosis using mutants and microarrays. Microbiology 148:3129-3138. [DOI] [PubMed] [Google Scholar]
- 38.Sweet, D. M., and B. E. B. Moseley. 1976. The resistance of Micrococcus radiodurans to killing and mutation by agents that damage DNA. Mutat. Res. 34:175-186. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka, A., H. Hirano, M. Kikiuchi, S. Kitayama, and H. Watanabe. 1996. Changes in cellular proteins of Deinococcus radiodurans following γ-irradiation. Radiat. Environ. Biophys. 35:95-99. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka, M., A. E. Earl, H. A. Howell, M.-J. Park, J. A. Eisen, S. A. Peterson, and J. R. Battista. 2004. Analysis of Deinococcus radiodurans' response to ionizing radiation and desiccation reveals novel proteins that contribute to extreme radioresistance. Genetics 168:21-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, P., and H. E. Schellhorn. 1995. Induction of resistance to hydrogen peroxide and radiation in Deinococcus radiodurans. Can. J. Microbiol. 41:170-176. [DOI] [PubMed] [Google Scholar]
- 42.Washburn, M. P., D. Wolters, and J. R. Yates III. 2001. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat. Biotechnol. 19:242-247. [DOI] [PubMed] [Google Scholar]
- 43.Weiner, L., J. L. Brissette, and P. Model. 1991. Stress-induced expression of the Escherichia coli phage shock operon is dependent on σ54 and modulated by positive and negative feedback mechanisms. Genes Dev. 5:1912-1923. [DOI] [PubMed] [Google Scholar]
- 44.Westphal, S., L. Heins, J. Soll, and U. C. Vothknecht. 2001. Vipp1 deletion mutant of Synechocystis: a connection between bacterial phage shock and thylakoid biogenesis? Proc. Natl. Acad. Sci. USA 98:4243-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White, O., J. A. Eisen, J. F. Heidelberg, E. K. Hickey, J. D. Peterson, R. J. Dodson, D. H. Haft, M. L. Gwinn, W. C. Nelson, D. L. Richardson, K. S. Moffat, H. Qin, L. Jiang, W. Pamphile, M. Crosby, M. Shen, J. J. Vamathevan, P. Lam, L. McDonald, T. Utterback, C. Zalewski, K. S. Makarova, L. Aravind, M. J. Daly, K. W. Minton, R. D. Fleischmann, K. A. Ketchum, K. E. Nelson, S. Salzberg, H. O. Smith, J. C. Venter, and C. M. Fraser. 1999. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science 286:1571-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiegert, T., G. Homuth, S. Versteeg, and W. Schumann. 2001. Alkaline shock induces the Bacillus subtilis σW regulon. Mol. Microbiol. 41:59-71. [DOI] [PubMed] [Google Scholar]
- 47.Yura, T., M. Kanemori, and M. T. Morita. 2000. The heat shock response: regulation and function, p. 3-18. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.