Abstract
In Bradyrhizobium japonicum, the N2-fixing root nodule endosymbiont of soybean, a group of genes required for microaerobic, anaerobic, or symbiotic growth is controlled by FixK2, a key regulator that is part of the FixLJ-FixK2 cascade. FixK2 belongs to the family of cyclic AMP receptor protein/fumarate and nitrate reductase (CRP/FNR) transcription factors that recognize a palindromic DNA motif (CRP/FNR box) associated with the regulated promoters. Here, we report on a biochemical analysis of FixK2 and its transcription activation activity in vitro. FixK2 was expressed in Escherichia coli and purified as a soluble N-terminally histidine-tagged protein. Gel filtration experiments revealed that increasing the protein concentration shifts the monomer-dimer equilibrium toward the dimer. Purified FixK2 productively interacted with the B. japonicum σ80-RNA polymerase holoenzyme, but not with E. coli σ70-RNA polymerase holoenzyme, to activate transcription from the B. japonicum fixNOQP, fixGHIS, and hemN2 promoters in vitro. Furthermore, FixK2 activated transcription from the E. coli FF(−41.5) model promoter, again only in concert with B. japonicum RNA polymerase. All of these promoters are so-called class II CRP/FNR-type promoters. We showed by specific mutagenesis that the FixK2 box at nucleotide position −40.5 in the hemN2 promoter, but not that at −78.5, is crucial for activation both in vivo and in vitro, which argues against recognition of a potential class III promoter. Given the lack of any evidence for the presence of a cofactor in purified FixK2, we surmise that FixK2 alone is sufficient to activate in vitro transcription to at least a basal level. This contrasts with all well-studied CRP/FNR-type proteins, which do require coregulators.
The Bradyrhizobium japonicum FixK2 protein plays an essential role in the transcriptional regulation of a wide range of genes required for microaerobic, anaerobic, or symbiotic growth (41, 46; for reviews, see references 11 and 12). It is part of the FixLJ-FixK2 cascade that controls expression of an expanding group of genes in response to the “low-oxygen” signal. Conditions of decreased oxygen tension are sensed by the FixL hemoprotein, which, in its deoxygenated form, undergoes autophosphorylation (17, 18). In turn, the phosphoryl group is transferred to the cognate response regulator FixJ that activates transcription of the fixK2 gene. The FixK2 protein distributes the low-oxygen signal to the expression of various genes involved in microaerobic or anaerobic energy metabolism. Apart from being activated by FixJ-phosphate, the fixK2 gene is repressed (directly or indirectly) by its own product (negative autoregulation) (46).
FixK2 belongs to the cyclic AMP (cAMP) receptor protein (CRP) and the fumarate and nitrate reductase (FNR) activator protein superfamily of transcription factors that trigger physiological changes in response to a variety of metabolic and environmental cues (for reviews, see references 20 and 30). All members of this family are predicted to be structurally related to CRP. They consist of four functionally distinct domains: (i) a sensor domain, (ii) a series of β-strands (β-roll structure) that form a loop-like structure making contact with the RNA-polymerase holoenzyme (RNAP), (iii) a long α-helix acting as a dimerization interface, and (iv) a C-terminal helix-turn-helix motif (H-T-H) involved in DNA binding.
The mode of transcriptional activation of the different CRP/FNR family members is largely comparable to that of CRP, which is understood in great structural and mechanistic detail (reviewed in references 8 and 35). The first step involves binding of an allosteric factor, which leads to conformational changes, specific DNA binding, and transcriptional regulation. In response to glucose starvation, CRP binds its allosteric factor, cAMP, which induces a conformational change that switches CRP from the “off” state that does not bind DNA to the “on” state that does (9, 10, 51). In the Rhodospirillum rubrum carbon monoxide-sensing protein CooA, carbon monoxide is the factor that binds to a b-type heme in the sensing domain and induces a conformational change that switches CooA from the “off” to the “on” state (10, 27, 34). Unlike CRP and CooA, FNR bears an N-terminal 30-amino-acid extension containing three essential cysteine residues (C20, C23, and C29) which, together with a fourth central cysteine (C122), are involved in the binding of an oxygen-labile [4Fe-4S]2+ cluster as the sensor of changes toward inhibiting O2 concentrations (36, 37; reviewed in reference 28). Upon a switch to oxic conditions, FNR is inactivated by oxidation of the [4Fe-4S]2+ cluster to a [2Fe-2S]2+ cluster and then converted to apoFNR (clusterless FNR) in a superoxide-dependent manner, which is accompanied by protein monomerization (54).
Transcription activation by CRP/FNR-type proteins requires (i) direct contact between them and different parts of RNAP and (ii) binding to an imperfect palindromic DNA sequence with a consensus of AAATGTGA-N6-TCACATTT (CRP box) or TTGAT-N4-ATCAA (FNR box; critical residues in every half site are underlined) (8, 20). Amino acid residues involved in specific interaction with DNA are located in the DNA recognition helix (αF) of the H-T-H DNA binding motif (αE-αF). Three charged residues, R180, E181, and R185, of CRP-αF make contacts with each CRP box half-site, whereas the FNR residues E209, R213, and S212 interact with each FNR box half-site (20). Thus, S212 of FNR and R180 of CRP provide the discriminatory contacts between the regulators and their respective targets.
CRP/FNR-dependent promoters can be grouped into three classes (I, II, and III) based on the number and the position of CRP/FNR binding sites relative to the start of transcription as well as on the mechanism for transcription activation (8). The upstream DNA binding site in class I promoters is centered either at position −61.5 (i.e., its axis of symmetry is between positions −61 and −62) or one to three helical turns further upstream (i.e., −71.5, −82.5, or −92.5). At class II promoters, the symmetry axis of the binding site is located at position −41.5 relative to the transcription start site, thus overlapping with the −35 region. Class III promoters comprise twin DNA sites for CRP or FNR (9, 21); that is,, they require binding of two (or more) CRP/FNR dimers or a combination with other activators to achieve maximal transcription activation. Although the specific contacts between the CRP/FNR dimer and the RNAP depend upon the architecture of particular promoters, three patches of surface-exposed amino acids (so-called activating region 1 [AR1], AR2, and AR3) have been identified as the key domains (8, 20). Functional counterparts of all three ARs of CRP have been found in the redox-responsive Escherichia coli FNR protein (7) as well as in CooA (38).
Whereas the N-terminal domain of B. japonicum FixK2 differs significantly from its homologs CRP and FNR, the DNA-binding region and the (putative) binding sites for FixK2 resemble those of FNR (46). FixK2 does not have the FNR-specific cysteine residues necessary to bind [4Fe-4S]2+ clusters, and it does not possess the CRP-specific residues involved in cAMP binding.
In vivo studies revealed the existence of at least thirteen FixK2-controlled genes or operons that are associated with a putative FixK2 binding site (41, 46, 55). They have the typical class II architecture, with the location of the binding site at −41.5 and the overlap with the −35 promoter element. The hemN2 promoter might be an exception in that it comprises two identical (putative) FixK2 binding sites at −78.5 and −40.5, which makes it a candidate for being a class III promoter.
In this work, we report on the functional characterization of recombinant B. japonicum FixK2 by in vitro transcription experiments with genuine B. japonicum targets and also with the heterologous FNR-dependent FF(−41.5) model promoter (3). Our findings that these promoters are direct targets of the FixK2-mediated activation and that purified FixK2 protein is active under aerobic conditions answer two important questions that had not been addressed in our previous in vivo experiments.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Bacterial strains used in this study are listed in Table 1. E. coli cells were routinely grown in Luria-Bertani (LB) medium (42) at 37°C. E. coli BL21(DE3) cells for the overproduction of the FixK2 recombinant protein were incubated at 30°C. Where appropriate, antibiotics were used at the following concentrations (in μg per ml): ampicillin, 200; kanamycin, 30; tetracycline, 10. Peptone-salts-yeast extract medium (49) supplemented with 0.1% l-arabinose was used in routine aerobic cultures of B. japonicum. Microaerobic cultures (0.5% O2) were grown as described previously (13). Concentrations of antibiotics for use in B. japonicum cultures were as follows (in μg per ml): chloramphenicol, 20; spectinomycin, 100; kanamycin, 100; streptomycin, 50; tetracycline, 50 (solid media) or 25 (liquid media).
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 gyrA96 thi-1 relA1 | Bethesda Research Laboratories, Inc., Gaithersburg, Md. |
| S17-1 | Smr-SprhsdR (RP4-2 kan::Tn7 tet::Mu, integrated in the chromosome) | 53 |
| BL21 (DE3) | F−opmT hsdSB(rB− mB−) gal dcm (DE3) | Novagen Inc. |
| B. japonicum | ||
| 110spc4 | Spr wild type | 49 |
| 9043 | Spr SmrfixK2::Ω | 46 |
| Plasmids | ||
| pET-28a(+) | Kmr expression vector | Novagen Inc. |
| pBBR1MCS-2 | Kmr cloning vector | 31 |
| pRKPol2 | Tcr (pRK290) part of polylinker from pBluescript II SK(+) | 19 |
| pME3535 | Apr (pNM480) transcriptional lacZ fusion vector | 26 |
| pBBR3535 | Kmr (pBBR1MCS-2) 3.203-kb EcoRI-DraI fragment from pME3535, transcriptional lacZ fusion vector | This work |
| pRKPol2-3535 | Tcr (pRKPol2) 3.251-kb SpeI-XhoI fragment from pBBR3535, transcriptional lacZ fusion vector | This work |
| pRJ9601 | Apr [pBluescript SK(+)] B. japonicum rrn promoter and rrn terminator on a 468-bp SacI-SmaI fragment | 5 |
| pRJ9519 | Apr [(pBluescript SK(+)] 308-bp BstXI-KpnI fragment containing the B. japonicum rrn terminator cloned into the HincII and KpnI sites | 5 |
| pRJ9041 | Apr (pUC19) fixJ′, ORF138 and fixK2 on a 2.288-kb SalI fragment | D. Nellen-Anthamatten, unpublished |
| pRJ9058 | Apr (pRJ9041) NdeI site introduced at fixK2 start codon | This work |
| pRJ9059 | Kmr [pET-28a(+)] His6-fixK2 on a 1.322-kb NdeI/NotI fragment | This work |
| pRJ8808 | Kmr (pBBR1MCS-2) ORF138′ and His6-fixK2 on a 1.912-kb BamHI-XbaI fragment | This work |
| pRJ8816 | Apr (pRJ9519) fixNOQP promoter on a 563-bp BamHI-EcoRI fragment | This work |
| pRJ8817 | Apr (pRJ9519) fixGHIS promoter on a 524-bp XbaI-EcoRI fragment | This work |
| pRJ8823 | Apr (pRJ9519) hemN2 promoter on a 135-bp BamHI-EcoRI fragment | This work |
| pRJ8827 | Apr (pRJ9519) hemN2 promoter on a 135-bp BamHI-EcoRI fragment, mutated FixK2 box at −40.5 | This work |
| pRJ8828 | Apr (pRJ9519) hemN2 promoter on a 135-bp BamHI-EcoRI fragment, mutated FixK2 box at −78.5 | This work |
| pRJ8829 | Apr (pRJ9519) hemN2 promoter on a 135-bp BamHI-EcoRI fragment, mutated FixK2 boxes at −40.5 and −78.5 | This work |
| pRJ8834 | Tcr (pRKPol2) hemN2-lacZ fusion | This work |
| pRJ8835 | Tcr (pRKPol2) hemN2-lacZ fusion, mutated FixK2 box at −40.5 | This work |
| pRJ8836 | Tcr (pRKPol2) hemN2-lacZ fusion, mutated FixK2 box at −78.5 | This work |
| pRJ8837 | Tcr (pRKPol2) hemN2-lacZ fusion, mutated FixK2 boxes at −40.5 and −78.5 | This work |
| pAMB452 | Apr (pSR); FF(−41.5) promoter on a 143-bp EcoRI-HindIII fragment cloned upstream of the oop terminator | 3 |
Plasmid construction.
Plasmids used in this study are listed in Table 1. Primers are available at http://www.micro.biol.ethz.ch/re/re_hennecke/Table_S1.doc. Plasmid pRJ9059 was created by insertion of a 1.32-kb NdeI-NotI fragment from pRJ9058 into pET-28a(+) (Novagen, Madison, WI). The NdeI site of pRJ9058 was created in pRJ9041 using the QuikChange method (Stratagene).
Plasmids used as transcription templates were based on pRJ9519 which contains a B. japonicum rrn transcriptional terminator (5). Plasmid pRJ8816 bears a BamHI/EcoRI-digested 563-bp fixNOQP promoter fragment that was amplified by PCR, using the fixN4-for and fixN4-rev primers. The fixGHIS template pRJ8817 contains an XbaI-EcoRI 524-bp PCR fragment amplified with primers fixG4-for and fixG3-rev.
To test the functionality of the FixK2 boxes associated with hemN2, four hemN2 promoter variants were created by PCR-based site-directed mutagenesis according to a slightly modified version of the overlap-extension method described by Ho et al. (25). Both FixK2 boxes were mutated individually or simultaneously by systematic exchange of T residues with C residues and A residues with G residues (and vice versa) at positions 1 to 5 and 10 to 14 of the 14-bp palindrome that constitutes the FixK2 boxes. To do so, two forward primers (hemN7-for and hemN8-for) and two reverse primers (hemN7-rev and hemN8-rev) that contain a 24-bp overlapping 3′ end (http://www.micro.biol.ethz.ch/re/re_hennecke/Table_S1.doc) were individually combined together with two additional flanking primers (hemN6-for and hemN6-rev) to give the four 135-bp BamHI-EcoRI hemN2 promoter fragments. The following combinations led to the different promoter derivatives: hemN7-for with hemN7-rev (in plasmid pRJ8823), hemN7-for with hemN8-rev (pRJ8827), hemN8-for with hemN7-rev (pRJ8828), and hemN8-for with hemN8-rev (pRJ8829). The correct nucleotide sequences of all PCR-amplified fragments cloned into the corresponding vectors were confirmed by sequencing.
For construction of the transcriptional hemN2-lacZ fusions, 141-bp SpeI-EcoRI fragments from pRJ8823, pRJ8827, pRJ8828, and pRJ8829 were fused with a promoterless lacZ gene and eventually cloned into the broad host-range vector pRKPol2 (19), which resulted in plasmids pRJ8834, pRJ8835, pRJ8836, and pRJ8837. These four plasmids and the control plasmid pRKPol2-3535 were transferred via conjugation into B. japonicum 110spc4 using E. coli S17-1 as donor as previously described (23). Exconjugants were selected for tetracycline resistance, and the presence of the plasmid was verified by plasmid isolation and E. coli transformation (50).
β-Galactosidase tests.
β-Galactosidase activity assays were carried out as described previously (13).
Overproduction and purification of FixK2 as a protein carrying an N-terminal six-histidine tag.
LB medium (150 ml) containing kanamycin was inoculated with freshly transformed E. coli BL21(DE3) cells carrying plasmid pRJ9059 and incubated at 37°C until cells reached an optical density at 600 nm of 0.4. Then, cultures were incubated at 30°C until they reached an optical density at 600 nm of 0.8, where production of the recombinant protein was induced by the addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After 2 h, cells were harvested (10 min at 4°C; 3,000 × g), resuspended in 5 ml of binding buffer (20 mM Tris-HCl, pH 7.9, 500 mM NaCl, 5 mM imidazole), and disrupted by three passages through a cold French pressure cell at 12,000 lb in−2. The lysate was centrifuged at 17,200 × g for 30 min at 4°C. Purification of the FixK2 protein was carried out at 4°C by affinity chromatography under nondenaturing conditions with Ni2+-nitrilotriacetic acid (Ni-NTA) agarose (QIAGEN). The 0.6-ml Ni-NTA column was preequilibrated with binding buffer. After application of the crude extract, the column was washed with buffers of increasing imidazole concentrations (5 to 50 mM). FixK2 protein was eluted by raising the imidazole concentration to 200 mM. Eluted protein was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), carried out as described by Laemmli (32); the gel was stained with Coomassie brilliant blue as described by Sambrook and Russell (50). Protein-containing fractions were desalted and buffer exchanged by passing them through a prepacked Sephadex G-25 M column (PD-10; Amersham Pharmacia Biotech) equilibrated with in vitro transcription buffer (40 mM Tris-HCl, pH 8.0, 10 mM MgCl2, 0.1 mM EDTA, 0.1 mM dithiothreitol [DTT], 150 mM KCl, 0.4 mM K3PO4, 0.1 mg bovine serum albumin ml−1). Protein concentrations were determined by using the Bio-Rad assay (Bio-Rad Laboratories, Richmond, Calif.) with bovine serum albumin as the standard. FixK2 protein concentrations reported in this study refer to the dimeric state.
Gel filtration.
Analytical size-exclusion chromatography of the FixK2 protein was performed at room temperature on a Superdex 75 H/R 30/10 column (Amersham Pharmacia Biotech) using a BioCAD perfusion chromatography system (PerSeptive Biosystems). After equilibrating the column with elution buffer (40 mM Tris-HCl, pH 8.0, 10 mM MgCl2, 0.1 mM EDTA, 0.1 mM DTT, 150 mM KCl, 0.4 mM K3PO4), 200-μl protein samples were injected and separated at a flow rate of 0.5 ml min−1. Absorbance was recorded at 280 nm. Fractions (500 μl) were collected and precipitated with chloroform-methanol (56). Sediments were resuspended in 40 μl of sample buffer (2% SDS, 10% glycerol, 62.5 mM Tris-HCl, pH 6.8, 50 mM DTT, 0.01% bromophenol blue) and analyzed by SDS-PAGE. The following standards were used for calibration: bovine serum albumin (67 kDa), ovalbumin (43 kDa), and bovine pancreas RNase A (13.7 kDa), all from Amersham Pharmacia Biotech. Gel filtration experiments were repeated at least three times with protein obtained from independent preparations.
In vitro transcription.
Multiple-round in vitro transcription assays were carried out in a volume of 20 μl under standard conditions as described previously (5). Different amounts of FixK2 protein (0.1 to 3.75 μM) were added to the reaction mixture. The reaction was started by adding 1.4 μg of B. japonicum RNA polymerase (100 nM; purified as described previously [5]) or 1 U of commercial E. coli RNA polymerase (Roche Diagnostics) and incubated for 30 min at 37°C. Suitable RNA size markers were prepared in vitro with T3 RNA polymerase according to Liggit et al. (40). The templates, pRJ9601 and pRJ8817, were cut with BstXI and BglII to yield runoff transcripts of 286 and 180 nucleotides, respectively. Transcripts were visualized with a phosphorimager. For quantification, signal intensities were determined with the Bio-Rad Quantity One software (version 4.5.2), and the ratio between FixK2-dependent transcripts and the vector-encoded FixK2-independent RNA I reference transcript was calculated.
Primer extension experiments.
The transcription start site of the fixNOQP transcript synthesized in vitro was determined as described previously (5), using primer 9519-1 that hybridizes with the pRJ9519 plasmid sequence located immediately downstream of the HindIII site.
RESULTS
Overproduction and purification of FixK2.
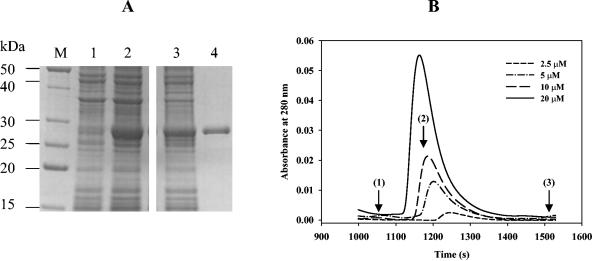
The starting point was the overexpression and purification of FixK2 as an N-terminally His-tagged protein. Due to the fact that FixK2 lacked the cysteine motif present in E. coli FNR and was not, therefore, supposed to be oxygen sensitive, all procedures were carried out under aerobic conditions as described in Material and Methods. Protein fractions were analyzed by electrophoresis on a 14% SDS-polyacrylamide gel (Fig. 1A). A crude extract of E. coli BL21(DE3) cells harboring the pRJ9059 expression plasmid presented a major band of approximately 28 kDa only after induction with 0.1 mM IPTG (Fig. 1A, lane 2). This band corresponds to the theoretically expected size of His6-FixK2 (27,785 Da). For simplification, the prefix His6-, referring to His6-tagged derivatives, will hereafter be omitted from all protein designations. FixK2 was found to be soluble after cell disruption and centrifugation for 30 min at 17,200 × g (Fig. 1A, lane 3). With one affinity chromatography purification step on an Ni-NTA column, we obtained FixK2 protein of ≥95% purity (Fig. 1A, lane 4). Mass spectrometry confirmed the predicted molecular mass of purified, tagged FixK2 protein (data not shown) and, thus, did not hint at the presence of a covalently bound cofactor. The yield of FixK2 was about 20 mg of protein per liter of bacterial culture. The purified protein was recognized by anti-tetra-His serum (QIAGEN) (data not shown). The N-terminal histidine tag did not affect in vivo FixK2 activity, because in trans complementation of the B. japonicum fixK2 mutant 9043 with pRJ8808, expressing the histidine-tagged FixK2 protein from its natural fixK2 promoter, completely restored FixK2-dependent transcription (data not shown).
FIG. 1.
Overproduction, purification, and determination of the oligomeric state of FixK2. (A) E. coli BL21(DE3) cells carrying plasmid pRJ9059 were grown in LB medium. Shown is a Coomassie blue-stained 14% SDS-polyacrylamide gel which was loaded with the following samples: extract of boiled uninduced (lane 1) and IPTG-induced cells (2 h in 0.1 mM IPTG; lane 2), French press extract of induced cells after centrifugation (30 min at 17,200 × g; lane 3), and NTA affinity chromatography-purified FixK2 protein (lane 4; for details, see Material and Methods). Lane M contains a mixture of marker proteins with the indicated molecular masses. Note that all the samples were run in parallel on the same gel. (B) Determination of the FixK2 oligomeric state by Superdex 75 HR 30/10 size exclusion chromatography. Shown is the concentration-dependent elution profile of the FixK2 protein monitored by the absorbance at 280 nm. Arrows indicate the elution time of standards used for calibration: bovine serum albumin (1; 67 kDa), ovoalbumin (2; 43 kDa), and bovine pancreas RNase A (3; 13.7 kDa).
Oligomeric state of purified FixK2 is concentration dependent.
FixK2 belongs to the family of H-T-H proteins that typically function as dimers. To assess the oligomeric state of the FixK2 protein, its molecular mass was determined by size exclusion chromatography at different protein concentrations (Fig. 1B). A concentration-dependent elution profile was observed with an apparent molecular weight ranging from 50,000 (corresponding approximately to a dimer) to 28,000 (corresponding approximately to a monomer). At a concentration of 20 μM (Fig. 1B, solid line) the majority eluted as a dimer. However, as the protein concentration in the sample loaded onto the column was decreased to 10 and 2.5 μM, the elution times were attenuated, reflecting a possible shift to monomeric species. The concentration dependence of the elution profiles and the nonsymmetrical and broad nature of the peaks suggest that some fraction of the dimeric protein population dissociated while moving through the column. An increase of the ionic strength of the elution buffer (up to 500 mM KCl)—to rule out adsorption to the matrix as the possible reason for the tailing profile—did not result in more symmetric peaks (data not shown). SDS-PAGE analysis of the eluted fractions always showed a single 28-kDa band, which excluded the possibility of protein degradation (data not shown). Incubation of FixK2 (10 μM) with either cAMP (0.5 mM) or cyclic GMP (0.5 mM) for 30 min at room temperature prior to loading the samples onto the column did not alter the protein concentration-dependent elution behavior and, hence, did not stabilize the FixK2 dimer (data not shown).
FixK2 is sufficient to activate transcription of genuine B. japonicum promoters and the FF(−41.5) FNR-dependent promoter in vitro.
A total of 13 FixK2-dependent promoters associated with putative FixK2-binding sites have been identified previously in B. japonicum through gene expression studies in vivo (41, 46, 55). To confirm that FixK2 mediates transcription activation at such promoters directly, we monitored RNA synthesis from the fixNOQP and fixGHIS promoters by multiple-round in vitro transcription (Fig. 2).
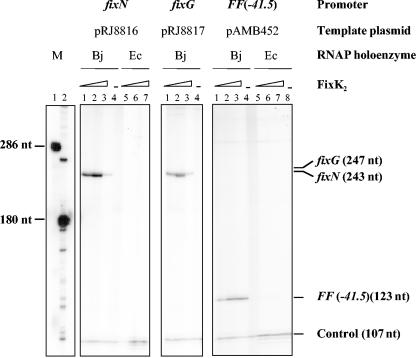
FIG. 2.
Activation of in vitro transcription by FixK2. Supercoiled template plasmids containing either of the indicated promoters cloned upstream of a strong transcriptional terminator were used for multiple-round in vitro transcription assays with increasing amounts of purified FixK2 protein and RNAP from B. japonicum (Bj) or E. coli (Ec). FixK2 concentrations were as follows: no protein (lanes 4 and 8), 0.4 μM (lanes 1 and 5), 1.25 μM (lanes 2 and 6), and 3.75 μM (lanes 3 and 7). Transcripts synthesized in vitro in the presence of [α-32P]UTP were separated on a 6% denaturing polyacrylamide gel and visualized by phosphorimager analysis of the dried gel. The sizes of individual transcripts in nucleotides (nt) are indicated at the right border. Marker transcripts (M) loaded in lanes M1 and M2 were generated by in vitro transcription with T3 RNA polymerase of the plasmids pRJ9061 and pRJ8817 linearized with BstXI and BglII, respectively. The 107-nucleotide transcript present in all lanes originates from a promoter located on the plasmid vector and serves as an internal control. Shown are the results from a typical transcription experiment that was repeated at least once for each individual promoter.
Without FixK2, B. japonicum RNAP did not transcribe from either of these two promoters efficiently (Fig. 2, lane 4), whereas it produced a vector-encoded transcript of 107 nucleotides that served as a useful control. Most likely, this transcript corresponds to the 107-nucleotide RNA I transcript, which, by mistake, was previously assigned 80 nucleotides by Beck et al. (5). In the presence of different concentrations of FixK2 (0.4, 1.25, and 3.75 μM), B. japonicum RNAP transcribed these promoters efficiently, producing theoretically expectable transcripts of 243 and 247 nucleotides, with almost no change in the intensity of the 107-nucleotide control (Fig. 2, lanes 1 to 3 of the fixN and fixG panels). Unexpectedly, at high FixK2 concentrations (3.75 μM), we detected a strong inhibitory effect on FixK2-dependent transcription from the fixN and fixG promoter but no effect or only a weak effect on transcription from the FF(−41.5) promoter and on the FixK2-independent transcription of RNA I (Fig. 2, lanes 3). No FixK2-dependent transcript (either with the fixNQQP or with the fixGHIS template) was detected when the E. coli RNAP was used (Fig. 2, lanes 5 to 7 of the fixN panel, and data not shown).
To analyze more quantitatively the dependency of the fixNOQP promoter on FixK2, we performed a titration experiment using 0, 0.1, 0.25, 0.4, 1.25, 2.5, or 3.75 μM FixK2 in individual transcription reactions (primary data not shown). Transcription activity was maximal (100%) with 1.25 μM FixK2 (see Materials and Methods), while it dropped to 95% and 13% with 2.5 and 3.75 μM FixK2, respectively. With a FixK2 concentration of 0.4 μM or less, transcription activity was ≤10%.
To address whether FixK2 can activate an FNR-dependent promoter, we analyzed transcription from the FF(−41.5) promoter, a derivative of the E. coli melR promoter carrying a consensus FNR binding site centered at position −41.5 and whose transcription absolutely depended on FNR (Fig. 2, right panel). The 107-nucleotide RNA I transcript that is encoded by the pSR vector plasmid (29) served as an internal control to quantify FixK2-dependent transcript formation. When E. coli RNAP was used, only the 107-nucleotide transcript was detected, regardless of the presence (Fig. 2, lanes 5 to 7, right panel) or the absence (Fig. 2, lane 8, right panel) of FixK2. In cooperation with the B. japonicum RNAP, however, FixK2 led to specific transcription from the FF(−41.5) promoter, giving rise to a transcript of the expected size (123 nucleotides; Anne Barnard, personal communication) (Fig. 2, lanes 1 to 3, right panel). Apart from the RNA I transcript, there was no transcription with B. japonicum RNAP alone (Fig. 2, lane 4, right panel).
The start site of the fixNOQP transcript synthesized in vitro is identical to that formed in vivo.
To test the fidelity of the in vitro transcription system, we determined the 5′ end of the transcript generated by FixK2-dependent in vitro transcription from the fixNOQP promoter and compared it with that of the corresponding in vivo transcript that was described previously (48). RNA synthesized in vitro by B. japonicum RNAP with plasmid pRJ8816 as template and purified FixK2 protein (1.25 μM) was isolated and used for primer extension with oligonucleotide 9519-1 (see Material and Methods). Extension products were run on a sequencing gel next to a sequence ladder generated with the same oligonucleotide and plasmid pRJ8816. The 5′ end of the in vitro synthesized fixNOQP transcript was found to be identical to the start site of the in vivo transcript (data not shown) located 41.5 bp downstream of the FixK2 binding site.
Functional analysis of the hemN2 promoter.
The hemN2 promoter is peculiar in that it comprises two identical (putative) FixK2 binding sites located at positions −78.5 and −40.5. This architecture is characteristic for CRP/FNR-dependent class III promoters in which a pair of two dimers may bind simultaneously to both binding sites (8). To test the functionality of the FixK2 boxes associated with hemN2, we constructed a set of hemN2 promoter derivatives (see Materials and Methods). In the resulting plasmids, the original sequence (TTGCG-N4-CGCAA) of the FixK2 box around −78.5 (pRJ8828 and pRJ8836) or −40.5 (pRJ8827 and pRJ8835) or at both locations (pRJ8829 and pRJ8837) was thus altered to CCATA-N4-TATGG (Fig. 3A).
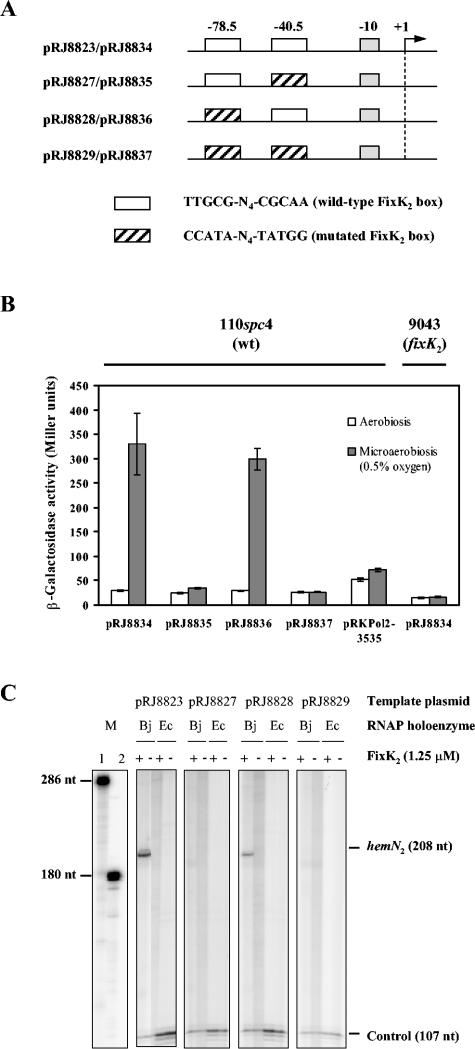
FIG. 3.
In vivo and in vitro activity from hemN2 promoter derivatives. (A) Schematic representation of the hemN2 promoter region present on the template plasmids used for this study (not drawn to scale). The −10 promoter region and the two (putative) FixK2 binding sites located at −78.5 and −40.5 are symbolized by boxes. The sequences of the wild-type and mutated FixK2 binding sites are shown at the bottom. The transcriptional start site of hemN2 is marked by +1. (B) β-Galactosidase activity from hemN2 promoter derivatives. The control plasmid pRKPol2-3535 contains a promoterless lacZ gene. Cultures of B. japonicum wild-type (wt; 110spc4) and fixK2 mutant (9043) cells containing plasmid-encoded hemN2-lacZ fusions were grown aerobically or microaerobically (0.5% O2, 99.5% N2) for 72 h in peptone-salts-yeast extract medium before β-galactosidase activities were determined. Values are the means ± standard errors from at least two independent experiments with two cultures assayed in duplicate. (C) Transcripts generated by multiple-round in vitro transcrip-tion using 1.25 μM FixK2 protein. Shown is the phosphorimager analysis of individual lanes of a 6% denaturing polyacrylamide gel. The composition of individual reactions is specified at the top of the gel (Bj, B. japonicum; Ec, E. coli). The position and size of the hemN2 transcript (208 nucleotides), the vector-encoded control transcript (107 nucleotides), and the RNA size markers (286 and 180 nucleotides) are emphasized. Note that all the samples were run in parallel on the same gel.
For in vivo experiments, plasmids pRJ8834, pRJ8835, pRJ8836, pRJ8837, and the control plasmid pRKPol2-3535 (promoterless lacZ gene) were transferred to B. japonicum 110spc4 (wild type) and 9043 (fixK2 null mutant), and β-galactosidase activity was measured in aerobically or microaerobically grown cells (Fig. 3B). When cells were grown aerobically, hemN2-lacZ expression was basal. Under microaerobic conditions, expression from the wild-type hemN2 promoter (pRJ8834) was induced 12-fold compared with aerobic conditions, and a very similar expression pattern was observed with the promoter lacking the upstream FixK2 box (pRJ8836). No microaerobic induction was observed in the wild-type background containing the plasmids that were mutated in the downstream FixK2 box (pRJ8835) or in both boxes (pRJ8837). Also, no induction occurred in the fixK2 mutant strain regardless of which plasmid was present (data shown only for pRJ8834). These results suggested that the FixK2 box around −40.5 but not that around −78.5 is crucial for in vivo activation of the hemN2 promoter.
The function of the individual FixK2 boxes in the hemN2 promoter was also tested in vitro. Plasmids pRJ8823, pRJ8827, pRJ8828, and pRJ8829 were used as a template for in vitro transcription experiments with either B. japonicum or E. coli RNAP in the presence (1.25 μM) or absence of FixK2 protein (Fig. 3C). A transcript of the expected size (208 nucleotides) was synthesized when the control plasmid pRJ8823 (both FixK2 boxes unaltered) or pRJ8828 (mutated FixK2 box around −78.5) was used as a template and both FixK2 and B. japonicum RNAP were present. By contrast, no transcript was obtained with template plasmids pRJ8827 or pRJ8829. Similar to our findings with the fixNOQP, fixGHIS, and the FF(−41.5) promoter, E. coli RNAP was unable to activate the hemN2 promoter.
Thus, results obtained in the in vitro experiments are in agreement with those obtained in vivo, indicating that only the FixK2 binding site at −40.5 is critical for activation by FixK2. This defines the hemN2 promoter as a class II rather than a class III promoter.
DISCUSSION
All in vitro activity tests described here were carried out with a histidine-tagged B. japonicum FixK2 protein. Concerns about its possible malfunction were eliminated by the successful in vivo complementation of a fixK2 deletion mutant. Hence, the N-terminal extension of 20 amino acids (including six histidine residues) did not interfere with FixK2 activity even though very subtle effects might have escaped detection in the in vivo system.
Increasing FixK2 protein concentrations influence the oligomeric state by changing the monomer-dimer equilibrium toward the dimer (Fig. 1B). A similar shift from the dimeric to the monomeric form between 20 and 5 μM protein was also observed by Moore and Kiley (43) when they studied the oligomeric state of the FNR mutant derivatives FNR-M147A, FNR-I151A, and FNR-I158A. These critical amino acids lie on the same face of the putative dimerization helix in FNR (residues 140 to 159), thus creating a hydrophobic surface that is characteristic of the coiled coils required for dimerization. A comparison of the putative dimerization helix in FNR with other CRP/FNR family members revealed many of the hydrophobic residues to be conserved (43). Likewise, the predicted dimerization helix in FixK2 (V128QVARKLWAMTAGELRHAEDHMLLL152) (http://www.sbg.bio.ic.ac.uk/∼3dpssm) contains many hydrophobic residues. Such a hydrophobic interface seems to be a common prerequisite in all CRP/FNR-type proteins to form a coiled coil.
The concentration-dependent dynamic monomer-dimer equilibrium of FixK2 might suggest the following, not mutually exclusive hypotheses: (i) FixK2 at low concentrations in vivo requires an unknown cofactor (which is not copurified when isolated from E. coli) to enhance dimerization, (ii) changes in the oligomeric state are a means to control FixK2 activity, and (iii) FixK2 inherently lacks the ability to dimerize efficiently. In this context, the presence of the artificial N-terminal histidine tag needs to be considered as a possible cause. However, as described above, the coiled coil of native FNR is also not energetically stable, which might in fact be a key property of FNR, allowing regulation of dimerization in response to oxygen (43).
The B. japonicum FixK2 protein activated in vitro transcription from the genuine B. japonicum fixNOQP and fixGHIS promoters and also from the artificial FF(−41.5) model promoter, all belonging to the so-called class II of CRP/FNR-dependent promoters (8). So far, we have not come across a class I promoter in B. japonicum, and the only candidate for a class III promoter, i.e., the hemN2 promoter, was not recognized as such but, instead, represents another class II promoter.
Transcription activation by FixK2 worked only in concert with the RNA polymerase holoenzyme from B. japonicum but not with that from E. coli. This result is in perfect agreement with our previous observation that FixK2 is unable to initiate transcription from FixK2- or FNR-dependent promoters in E. coli (S. Mesa, unpublished data). Although the molecular reason for this is not known, it likely reflects the lack of productive interactions between B. japonicum FixK2 and E. coli RNA polymerase. In this context, it should be recalled that B. japonicum FixK1, an oxygen-responsive FNR-like homolog of FixK2, was previously shown to be an active transcription factor in vivo in concert with the E. coli RNA polymerase (2). Comparison of E. coli FNR with FixK1 and FixK2 (ClustalW; http://www.ebi.ac.uk/clustalw/) revealed that the amino acid sequence of the putative AR1, AR2, and AR3 in both rhizobial proteins is comparably dissimilar from that of FNR (data not shown). Yet it is striking to note that, on the basis of this alignment, a patch of four consecutive amino acids of AR1 corresponding to F186SPR in FNR has a counterpart in FixK1 (G178ASD) but not in FixK2. Since this domain in FNR was predicted to be part of an exposed loop that contacts the C-terminal domain of the RNA polymerase α subunit (α-CTD) (20), it is tempting to speculate that its absence in FixK2 caused the lack of productive interaction with E. coli polymerase. Conversely, it would mean that interaction with B. japonicum RNA polymerase is not strictly dependent on this putative loop. Clearly, additional experiments are required to test this hypothesis.
We are aware that higher FixK2 concentrations (>1 μM) were used in our in vitro experiments compared with analogous studies performed with CRP or CooA (both in the nanomolar range) (16, 24). Yet when Lamberg and Kiley (33) studied the in vitro activity of FNR, they also used micromolar concentrations. It should be noted, however, that in those experiments the FNR-D154A mutant protein was used, which differs from wild-type FNR by its increased dimerization properties and constitutive activity. The requirement of relatively high FixK2 concentrations could mean that only a fraction of the molecules was active in the FixK2 protein preparations used for the in vitro transcription experiments, possibly due to subsaturation with a hypothetical (noncovalently bound) cofactor, or that the histidine tag attached to FixK2 selectively interferes with its in vitro activity, as opposed to the in vivo situation. Alternatively, FixK2 might have a low affinity either for RNA polymerase or for its DNA targets (or both). Unfortunately, the physiological concentration of FixK2 in cells is not known, making it difficult to relate the in vitro data with the in vivo situation.
The gel filtration data suggest that FixK2 was predominantly present as a monomer in our in vitro transcription assays. Yet it seems very unlikely that FixK2 is functional as a monomer, given that the model proteins CRP and FNR act as dimers and the DNA binding motif of all three regulators is symmetric. Possibly, dimerization of FixK2 is facilitated by the presence of target DNA sequences that were absent in the gel filtration experiments. Also, it could be that the half-life of a minor population of individual FixK2 dimers might be in a range that allows transcriptional activation but not physical separation from a major population of monomers during a gel filtration run. An unexpected observation was that high FixK2 concentrations interfered to different extents with in vitro transcription from different promoters. Transcription from the fixN and the fixG promoter was severely impaired in reactions containing 3.75 μM FixK2 protein, whereas no inhibition or only marginal inhibition was observed with the FF(−41.5) and the RNA I promoter. One may speculate that FixK2 acts as a repressor at FixK2-dependent promoters when it is present at high concentrations. To our knowledge, however, such a regulatory switch lacks any precedent among the CRP/FNR-like proteins. In fact, repression by CRP or FNR was described previously (see reference 20 and references therein), yet it involved additional regulatory proteins or the simultaneous presence of tandem binding sites, both absent in our in vitro system. Therefore, we believe, rather, that high FixK2 concentrations had a nonspecific inhibitory effect that differed for disparate promoters.
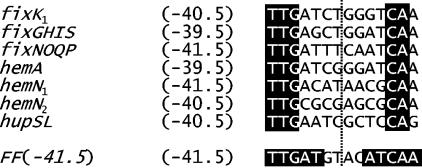
While simultaneous substitution of all five nucleotides in each half site of the −40.5 FixK2-binding site of the hemN2 promoter completely abolishes transcription activation both in vivo and in vitro (Fig. 3B and C), the nucleotides critical for FixK2-mediated activation cannot be identified from this study. Figure 4 shows a comparison of the E. coli consensus FNR box [FF(−41.5)] with the FixK2 box of those B. japonicum FixK2-dependent promoters whose associated transcriptional start site had been mapped. Only three nucleotides of the promoter-distal half site (TTG) and two nucleotides of the proximal half site (CA) are absolutely invariant and identical with those present at these positions in the FNR box. Given that all listed boxes are located in promoters that are activated by FixK2, it is concluded that the nucleotides at nonconserved positions are not absolutely critical for FixK2 binding.
FIG. 4.
FixK2 boxes of FixK2-dependent B. japonicum promoters whose transcriptional start site is known. The E. coli FNR consensus sequence present in the synthetic FF(−41.5) promoter is shown at the bottom. The vertical dotted line marks the axis of dyad symmetry. Numbers indicated at the left refer to the distance of the symmetry axis from the corresponding transcription start site. Invariant nucleotides in the B. japonicum FixK2 boxes and those of the FNR box are highlighted by white letters on a black background.
A systematic single base pair substitution analysis comprising positions 4 to 8 (TGTGA) of the CRP consensus sequence showed that positions 5 and 7 (corresponding to positions 1 and 3 in the FixK2 binding site) are the most critical for CRP binding (22). Green and coworkers defined TGA-N6-TCA as the common core sequence among binding sites of CRP and FNR (20). More recently, Scott et al. (52) showed that nucleotides located between the conserved half sites also contribute to FNR-dependent promoter activation, although they are not in direct contact with the protein. The functional FixK2 binding site at −40.5 in the hemN2 promoter includes a C at position 3 of this motif and a G at position 10. The latter deviation is also found in the FixK2 box of the hemN1 promoter, suggesting that the DNA binding specificity of FixK2 is somewhat relaxed with regard to this position. Interestingly, Bearson et al. (4) made a similar observation when they studied the effects of corresponding nucleotide exchanges on the activity of the FNR-dependent E. coli dmsABC promoter.
Even though FixK2 has only a rather low level of amino acid sequence identity to FNR (28%), the E209XXSR motif of helix F in FNR, which is directly involved in DNA interaction, is also present in the predicted DNA binding motif of FixK2 (L180PMCRRDIGDYLGLTLETVSRALSQLHTQGIL211) (MotifScan, http://www.expasy.org/prosite/). Thus, by analogy with what has been proposed for FNR (20), it is likely that E196, S199, and R200 of FixK2 make contacts with nucleotide positions 3, 1, and 4, respectively, in the FixK2 binding site. Yet additional mutational and structural studies would be required to further support this model.
The fact that a cognate B. japonicum RNA polymerase-FixK2 complex does activate transcription from class II promoters in vitro is remarkable because this might imply that FixK2 alone is necessary and sufficient to activate transcription and that it does not need an additional, low-molecular-weight coregulator for activation. Such a property would be unique among all of the hitherto studied CRP/FNR-type proteins, which do require coregulators (such as cAMP, [4Fe-4S]2+ cluster, and heme-CO complex). Hence, the strength of FixK2-dependent target gene expression in vivo would be adjusted solely by the amount of FixK2 protein synthesized in cells, and no additional physiological signal other than low oxygen would be integrated in the FixLJ-FixK2 cascade. FixK2 synthesis thus equilibrates between the low-oxygen-controlled, FixJ-dependent expression (positive control) and the antagonistic FixK2-dependent repression (negative autoregulation [46]) of the fixK2 gene. A possible means to show this would be to express FixK2 ectopically in a FixJ- and FixK2-independent manner. Yet, for unknown reasons, we were unable to construct this type of a mutant strain (data not shown). Notably, in Sinorhizobium meliloti the fixK gene is also subject to negative autoregulation via an additional regulatory protein, FixT, which inhibits the superimposed FixL sensory kinase (14, 15). Evidence for a fixT-like gene in B. japonicum is currently lacking.
The waiver of a coregulator requirement at the level of a subordinate transcription factor within a signal transduction cascade is not without precedent. A conceptually similar situation as in the B. japonicum FixLJ-FixK2 cascade appears to exist in the NtrBC-nitrogen assimilation control (NAC) cascade of Klebsiella aerogenes and E. coli (6, 44). The nac gene is positively controlled by a two-component regulatory system (NtrBC) and negatively controlled by its own product, and no coregulator requirement was found when the NAC protein was tested in transcription activation assays in vitro (45). Input of the regulatory signal (nitrogen starvation) occurs at the level of NtrBC, and the amount of NAC protein synthesized in cells rules over the quantity of expression from a multitude of target genes. Unlike FixK2, however, NAC is an LysR-type transcription factor.
Another example of a transcriptional factor that does not require additional signals for promoting transcription is the AraC-type protein SoxS, one of the two products of the regulatory soxRS locus of E. coli (1). In response to superoxide-generating agents or nitric oxide, the redox-sensing protein SoxR is first activated; then it enhances the production of SoxS, which in turn triggers transcription of other target genes. Hence, SoxS activity is solely controlled by its concentration as a result of a balance between SoxR activation and its negative autoregulation (39, 47).
Although the lack of a coregulator requirement seems compelling, opposing interpretations of our data cannot formally be ruled out. For example, consider the following possibilities: (i) a low-molecular-weight ingredient present in the in vitro transcription assay (salt ion, nucleotide) might be the activating principle; (ii) what we see as the result of transcription in vitro is just a basal level, which might have the potential to become strongly enhanced upon addition of an as yet unidentified factor; (iii) expression in E. coli and the purification procedure might have converted FixK2 into an enigmatic, activation-competent form or conformation. We will have to keep an eye on such possibilities in future work.
Acknowledgments
We are grateful to Sergiy Chesnov (University of Zürich) for helpful advice in the mass spectrometry analysis of FixK2. We thank Anne Barnard (School of Biosciences, Birmingham, United Kingdom) for providing the pABM452 plasmid. We also thank Stephen J. W. Busby (School of Biosciences, Birmingham, United Kingdom) for valuable suggestions.
This work was supported by a grant from the Swiss Federal Institute of Technology, Zürich, Switzerland.
REFERENCES
- 1.Amábile-Cuevas, C. F., and B. Demple. 1991. Molecular characterization of the soxRS genes of Escherichia coli: two genes control a superoxide stress regulon. Nucleic Acids Res. 19:4479-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthamatten, D., B. Scherb, and H. Hennecke. 1992. Characterization of a fixLJ-regulated Bradyrhizobium japonicum gene sharing similarity with the Escherichia coli fnr and Rhizobium meliloti fixK genes. J. Bacteriol. 174:2111-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnard, A. M., J. Green, and S. J. Busby. 2003. Transcription regulation by tandem-bound FNR at Escherichia coli promoters. J. Bacteriol. 185:5993-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bearson, S. M., J. A. Albrecht, and R. P. Gunsalus. 2002. Oxygen and nitrate-dependent regulation of dmsABC operon expression in Escherichia coli: sites for FNR and NarL protein interactions. BMC Microbiol. 2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck, C., R. Marty, S. Kläusli, H. Hennecke, and M. Göttfert. 1997. Dissection of the transcription machinery for housekeeping genes of Bradyrhizobium japonicum. J. Bacteriol. 179:364-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bender, R. A. 1991. The role of the NAC protein in the nitrogen regulation of Klebsiella aerogenes. Mol. Microbiol. 5:2575-2580. [DOI] [PubMed] [Google Scholar]
- 7.Browning, D. F., D. Lee, J. Green, and S. Busby. 2002. Secrets of bacterial transcription initiation taught by the Escherichia coli FNR protein, p. 127-142. In D. A. Hodgson and C. M. Thomas (ed.), SGM symposium 61: signals, switches, regulons and cascades: control of bacterial gene expression. Cambridge University Press, Cambridge, United Kingdom.
- 8.Busby, S., and R. H. Ebright. 1999. Transcription activation by catabolite activator protein (CAP). J. Mol. Biol. 293:199-213. [DOI] [PubMed] [Google Scholar]
- 9.Busby, S., and A. Kolb. 1996. The CAP modulon, p. 255-280. In E. C. C. Lin and A. S. Lynch (ed.), Regulation and gene expression in Escherichia coli. R. G. Landes and Co., Austin, Tex.
- 10.Chan, M. K. 2000. CooA, CAP and allostery. Nat. Struct. Biol. 7:822-824. [DOI] [PubMed] [Google Scholar]
- 11.Fischer, H. M. 1996. Environmental regulation of rhizobial symbiotic nitrogen fixation genes. Trends Microbiol. 4:317-320. [DOI] [PubMed] [Google Scholar]
- 12.Fischer, H. M. 1994. Genetic regulation of nitrogen fixation in rhizobia. Microbiol. Rev. 58:352-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer, H. M., L. Velasco, M. J. Delgado, E. J. Bedmar, S. Schären, D. Zingg, M. Göttfert, and H. Hennecke. 2001. One of two hemN genes in Bradyrhizobium japonicum is functional during anaerobic growth and in symbiosis. J. Bacteriol. 183:1300-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foussard, M., A. M. Garnerone, F. Ni, E. Soupene, P. Boistard, and J. Batut. 1997. Negative autoregulation of the Rhizobium meliloti fixK gene is indirect and requires a newly identified regulator, FixT. Mol. Microbiol. 25:27-37. [DOI] [PubMed] [Google Scholar]
- 15.Garnerone, A. M., D. Cabanes, M. Foussard, P. Boistard, and J. Batut. 1999. Inhibition of the FixL sensor kinase by the FixT protein in Sinorhizobium meliloti. J. Biol. Chem. 274:32500-32506. [DOI] [PubMed] [Google Scholar]
- 16.Gaston, K., A. Bell, A. Kolb, H. Buc, and S. Busby. 1990. Stringent spacing requirements for transcription activation by CRP. Cell 62:733-743. [DOI] [PubMed] [Google Scholar]
- 17.Gilles-Gonzalez, M. A., G. S. Ditta, and D. R. Helinski. 1991. A haemoprotein with kinase activity encoded by the oxygen sensor of Rhizobium meliloti. Nature 350:170-172. [DOI] [PubMed] [Google Scholar]
- 18.Gong, W., B. Hao, S. S. Mansy, G. Gonzalez, M. A. Gilles-Gonzalez, and M. K. Chan. 1998. Structure of a biological oxygen sensor: a new mechanism for heme-driven signal transduction. Proc. Natl. Acad. Sci. USA 95:15177-15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Göttfert, M., D. Holzhäuser, D. Bäni, and H. Hennecke. 1992. Structural and functional analysis of two different nodD genes in Bradyrhizobium japonicum USDA110. Mol. Plant Microbe Interact. 5:257-265. [DOI] [PubMed] [Google Scholar]
- 20.Green, J., C. Scott, and J. R. Guest. 2001. Functional versatility in the CRP-FNR superfamily of transcription factors: FNR and FLP. Adv. Microb. Physiol. 44:1-34. [DOI] [PubMed] [Google Scholar]
- 21.Guest, J. R., J. Green, A. S. Irvine, and S. Spiro. 1996. The FNR modulon and FNR-regulated gene expression, p. 317-342. In E. C. C. Lin and A. S. Lynch (ed.), Regulation and gene expression in Escherichia coli. Landes and Co., Austin, Tex.
- 22.Gunasekera, A., Y. W. Ebright, and R. H. Ebright. 1992. DNA sequence determinants for binding of the Escherichia coli catabolite gene activator protein. J. Biol. Chem. 267:14713-14720. [PubMed] [Google Scholar]
- 23.Hahn, M., L. Meyer, D. Studer, B. Regensburger, and H. Hennecke. 1984. Insertion and deletion mutation within the nif region of Rhizobium japonicum. Plant Mol. Biol. 3:159-168. [DOI] [PubMed] [Google Scholar]
- 24.He, Y., T. Gaal, R. Karls, T. J. Donohue, R. L. Gourse, and G. P. Roberts. 1999. Transcription activation by CooA, the CO-sensing factor from Rhodospirillum rubrum. The interaction between CooA and the C-terminal domain of the α subunit of RNA polymerase. J. Biol. Chem. 274:10840-10845. [DOI] [PubMed] [Google Scholar]
- 25.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 26.Hojberg, O., U. Schnider, H. V. Winteler, J. Sorensen, and D. Haas. 1999. Oxygen-sensing reporter strain of Pseudomonas fluorescens for monitoring the distribution of low-oxygen habitats in soil. Appl. Environ. Microbiol. 65:4085-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerby, R. L., H. Youn, M. V. Thorsteinsson, and G. P. Roberts. 2003. Repositioning about the dimer interface of the transcription regulator CooA: a major signal transduction pathway between the effector and DNA-binding domains. J. Mol. Biol. 325:809-823. [DOI] [PubMed] [Google Scholar]
- 28.Kiley, P. J., and H. Beinert. 2003. The role of Fe-S proteins in sensing and regulation in bacteria. Curr. Opin. Microbiol. 6:181-185. [DOI] [PubMed] [Google Scholar]
- 29.Kolb, A., D. Kotlarz, S. Kusano, and A. Ishihama. 1995. Selectivity of the Escherichia coli RNA polymerase E-σ38 for overlapping promoters and ability to support CRP activation. Nucleic Acids Res. 23:819-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Körner, H., H. J. Sofia, and W. G. Zumft. 2003. Phylogeny of the bacterial superfamily of Crp-Fnr transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol. Rev. 27:559-592. [DOI] [PubMed] [Google Scholar]
- 31.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 33.Lamberg, K. E., and P. J. Kiley. 2000. FNR-dependent activation of the class II dmsA and narG promoters of Escherichia coli requires FNR-activating regions 1 and 3. Mol. Microbiol. 38:817-827. [DOI] [PubMed] [Google Scholar]
- 34.Lanzilotta, W. N., D. J. Schuller, M. V. Thorsteinsson, R. L. Kerby, G. P. Roberts, and T. L. Poulos. 2000. Structure of the CO sensing transcription activator CooA. Nat. Struct. Biol. 7:876-880. [DOI] [PubMed] [Google Scholar]
- 35.Lawson, C. L., D. Swigon, K. S. Murakami, S. A. Darst, H. M. Berman, and R. H. Ebright. 2004. Catabolite activator protein: DNA binding and transcription activation. Curr. Opin. Struct. Biol. 14:10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazazzera, B. A., D. M. Bates, and P. J. Kiley. 1993. The activity of the Escherichia coli transcription factor FNR is regulated by a change in oligomeric state. Genes Dev. 7:1993-2005. [DOI] [PubMed] [Google Scholar]
- 37.Lazazzera, B. A., H. Beinert, N. Khoroshilova, M. C. Kennedy, and P. J. Kiley. 1996. DNA binding and dimerization of the Fe-S-containing FNR protein from Escherichia coli are regulated by oxygen. J. Biol. Chem. 271:2762-2768. [DOI] [PubMed] [Google Scholar]
- 38.Leduc, J., M. V. Thorsteinsson, T. Gaal, and G. P. Roberts. 2001. Mapping CooA-RNA polymerase interactions. Identification of activating regions 2 and 3 in CooA, the CO-sensing transcriptional activator. J. Biol. Chem. 276:39968-39973. [DOI] [PubMed] [Google Scholar]
- 39.Li, Z., and B. Demple. 1994. SoxS, an activator of superoxide stress genes in Escherichia coli. Purification and interaction with DNA. J. Biol. Chem. 269:18371-18377. [PubMed] [Google Scholar]
- 40.Liggit, P., S. H. Cheng, and E. J. Baker. 1994. Generating customized, long-lived 32P-labeled RNA size markers. BioTechniques 17:465-467. [PubMed] [Google Scholar]
- 41.Mesa, S., E. J. Bedmar, A. Chanfon, H. Hennecke, and H. M. Fischer. 2003. Bradyrhizobium japonicum NnrR, a denitrification regulator, expands the FixLJ-FixK2 regulatory cascade. J. Bacteriol. 185:3978-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Moore, L. J., and P. J. Kiley. 2001. Characterization of the dimerization domain in the FNR transcription factor. J. Biol. Chem. 276:45744-45750. [DOI] [PubMed] [Google Scholar]
- 44.Muse, W. B., and R. A. Bender. 1998. The nac (nitrogen assimilation control) gene from Escherichia coli. J. Bacteriol. 180:1166-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muse, W. B., C. J. Rosario, and R. A. Bender. 2003. Nitrogen regulation of the codBA (cytosine deaminase) operon from Escherichia coli by the nitrogen assimilation control protein, NAC. J. Bacteriol. 185:2920-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nellen-Anthamatten, D., P. Rossi, O. Preisig, I. Kullik, M. Babst, H. M. Fischer, and H. Hennecke. 1998. Bradyrhizobium japonicum FixK2, a crucial distributor in the FixLJ-dependent regulatory cascade for control of genes inducible by low oxygen levels. J. Bacteriol. 180:5251-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nunoshiba, T., E. Hidalgo, Z. Li, and B. Demple. 1993. Negative autoregulation by the Escherichia coli SoxS protein: a dampening mechanism for the soxRS redox stress response. J. Bacteriol. 175:7492-7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Preisig, O. 1995. Genetische und biochemische Charakterisierung der für Bakteroidrespiration essentiellen Häm-Kupfer-Oxidase (cbb3-Typ) von Bradyrhizobium japoncum. Ph.D. thesis. Swiss Federal Institute of Technology, Zürich, Switzerland.
- 49.Regensburger, B., and H. Hennecke. 1983. RNA polymerase from Rhizobium japonicum. Arch. Microbiol. 135:103-109. [DOI] [PubMed] [Google Scholar]
- 50.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 51.Schultz, S. C., G. C. Shields, and T. A. Steitz. 1991. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science 253:1001-1007. [DOI] [PubMed] [Google Scholar]
- 52.Scott, S., S. Busby, and I. Beacham. 1995. Transcriptional co-activation at the ansB promoters: involvement of the activating regions of CRP and FNR when bound in tandem. Mol. Microbiol. 18:521-531. [DOI] [PubMed] [Google Scholar]
- 53.Simon, R., U. Priefer, and A. Pühler. 1983. Vector plasmids for in vivo and in vitro manipulation of gram-negative bacteria, p. 96-108. In A. Pühler (ed.), Molecular genetics of the bacteria-plant interaction. Springer-Verlag, Heidelberg, Germany.
- 54.Sutton, V. R., A. Stubna, T. Patschkowski, E. Munck, H. Beinert, and P. J. Kiley. 2004. Superoxide destroys the [2Fe-2S](2+) cluster of FNR from Escherichia coli. Biochemistry 43:791-798. [DOI] [PubMed] [Google Scholar]
- 55.Velasco, L., S. Mesa, C. Xu, M. J. Delgado, and E. J. Bedmar. 2004. Molecular characterization of nosRZDFYLX genes coding for denitrifying nitrous oxide reductase of Bradyrhizobium japonicum. Antonie Leeuwenhoek 85:229-235. [DOI] [PubMed] [Google Scholar]
- 56.Wessel, D., and U. I. Flügge. 1984. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138:141-143. [DOI] [PubMed] [Google Scholar]