Abstract
We have previously reported that a receptor-type adenylyl cyclase (CyaA) of Myxococcus xanthus undergoes an osmosensor mainly during spore germination (Y. Kimura et al., J. Bacteriol. 184:3578-3585, 2002). In the present study, we cloned another receptor-type adenylyl cyclase gene (cyaB) and characterized the function of the cyaB-encoded protein. Disruption of cyaB generates a mutant that showed growth retardation at high ionic (NaCl) or high nonionic (sucrose) osmolarity. When vegetative cells were stimulated with 0.15 M NaCl, the increases in intracellular cyclic AMP levels of cyaB mutant cells were lower than those of wild-type cells. Under nonionic osmostress, the cyaB mutant exhibited reduced spore germination; however, the germination rate of the cyaB mutant was significantly higher than that of the cyaA mutant.
The ability to adapt to changes in environmental osmolarity is fundamental to survival of organisms (20, 24). Myxococcus xanthus is a gram-negative soil bacterium that undergoes multicellular development when starved for essential nutrients (23). During development, high densities of cells (105 to 106) cooperate to produce a mound in which cells differentiate into spores. At least five extracellular signals, known as the A, B, C, D, and E signals, are required for this multicellular development process (7, 8, 13). When spores are exposed to a nutrient-rich environment, they germinate and become vegetative rod-shaped cells. In M. xanthus, we demonstrated that MokA and CyaA are required for osmotic tolerance and involved in sensing extracellular osmolarity (10, 11). MokA consists of a sensor domain in its amino-terminal half and a histidine kinase domain and a response regulator domain in its carboxy-terminal half, making it a typical transmembrane hybrid-type histidine kinase. mokA mutant showed growth retardation at high osmolarity and reduced sporulation under starvation conditions. CyaA is a receptor- or sensor-type adenylyl cyclase that is composed of an amino-terminal sensor domain and a carboxy-terminal catalytic domain of adenylyl cyclase. The cyaA mutant exhibited a marked reduction in spore formation and spore germination under conditions of osmostress (10).
In this study, we cloned another receptor-type adenylyl cyclase gene (cyaB) and characterized the function of the cyaB-encoded protein. The predicted cyaB gene product had structural similarity to M. xanthus CyaA, and CyaB acted as an osmotic sensor during the growth phase, suggesting that two receptor-type adenylyl cyclases, CyaA and CyaB, function as osmotic sensors at different phases of the M. xanthus life cycle.
Cloning of M. xanthus cyaB.
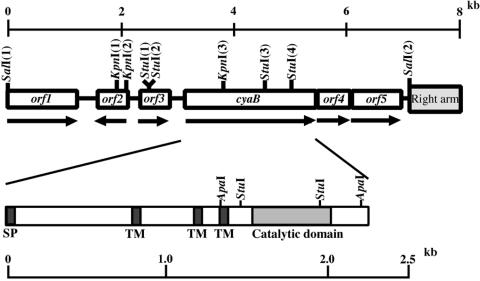
We attempted to clone adenylyl cyclase genes from an M. xanthus FB (5) (IFO [Institute for Fermentation, Osaka] 13542) genomic library (11) by using three oligonucleotide probes. The three oligonucleotides (Cya1:GACAAGTA/TCATCGGC/GGACG/TCC/GVTC/GATG;Cya2: GTC/GAAC/GCTC/GGCC/GTCC/GCGC/GCTC/GGAG; Cya3: TACACC/GGTC/GA/CTC/GGGC/GGACGC/GC/GGT, where V is A, C, or G) were deduced from the conserved catalytic domain of the adenylyl cyclase and were labeled with digoxigenin-11-dUTP using an oligonucleotide tailing kit (Roche Diagnostics GmbH). Three positive clones were obtained by plaque hybridization, and various DNA restriction fragments of the clones were sequenced. The sequence analysis revealed that two clones contained cyaA genes and one clone contained another adenylyl cyclase gene (cyaB). A 7.1-kb SalI fragment of the clone containing cyaB was found to contain six open reading frames (ORFs) (Fig. 1). cyaB encoded 765 amino acid residues deduced from its nucleotide sequence with a molecular mass of 82.6 kDa. A computer search using the BLAST program revealed that the C-terminal region of CyaB shares significant sequence homology to the catalytic domains of adenylyl cyclases. The catalytic domain of CyaB showed 30% identity to the CyaA of M. xanthus (10), 37% identity to the putative adenylyl cyclase of Bdellovibrio bacteriovorus (22), and 41% identity to the putative adenylyl cyclase of Leptospira interrogans (21) (Fig. 2A). The amino-terminal region of CyaB showed similarity to the amino-terminal region of the CyaA of M. xanthus (27% identity) (10), the putative adenylyl/guanylyl cyclase of Bradyrhizobium japonicum USDA 110 (25% identity) (9), and the putative adenylyl/guanylyl cyclase of Rhodopseudomonas palustris (27% identity) (14) (Fig. 2B). Hydropathy analysis of the cyaB gene product suggested that CyaB possesses a signal peptide (Met-1 to Asp-19) and three transmembrane regions (Ala-261 to Gly-283, Ala-390 to Thr-412, and Val-445 to Phe-464) (Fig. 1). These results suggested that M. xanthus CyaB is a receptor-type adenylyl cyclase that belongs to the class III adenylyl cyclases as described by Danchin (4). An adenylyl cyclase, AC1, of the myxobacterium Stigmatella aurantiaca contains a 17-amino-acid motif, which is a signature of G-protein-coupled receptors, in membrane domain (3), but the motif did not exist in CyaB.
FIG. 1.
Restriction map of the cyaB gene of M. xanthus. Lines with arrows indicate orientation. The 0.5-kb StuI fragment was replaced by the Kmr gene. The 7.1-kb SalI fragment contains a partial right arm of λEMBL3 vector. SP, signal peptide; TM, transmembrane domain.
FIG. 2.
Alignment of adenylyl cyclase sequences. (A) Alignment of the deduced catalytic domain of CyaB with the adenylyl cyclase CyaA of M. xanthus (CyaA), the putative adenylyl cyclase of Bdellovibrio bacteriovorus (Bba), and the putative adenylyl-guanylyl cyclase of Leptospira interrogans (Lin). Four conserved motifs (I to IV) in class III adenylyl cyclases are overlined (17). The asterisks and the closed circles mark amino acid residues that are involved in substrate definition and coordination of metal ions (Mg2+ or Mn2+), respectively (2, 17). (B) Alignment of the deduced amino-terminal region of CyaB with the CyaA of M. xanthus (CyaA), the putative adenylyl/guanylyl cyclase of Bradyrhizobium japonicum (Bja), and the putative adenylyl/guanylyl cyclase of Rhodopseudomonas palustris (Rpa). Amino acid residues in agreement for more than two residues are indicated by filled boxes. Gray shading indicates degrees of similarity among amino acid residues. The sequence of the M. xanthus cyaB gene has been deposited in the DNA Data Bank of Japan sequence library under accession number AB188227.
The orf4 gene was located 27 base pairs downstream from the stop codon of the cyaB coding region, and the start codon of the orf5 gene overlapped with the stop codon of orf4. The predicted Orf4 and Orf5 proteins were 192 and 328 amino acids, respectively; however, these predicted gene products showed no extensive homology with other proteins or low homology with unknown proteins in the GenBank database.
Expression of cyaB.
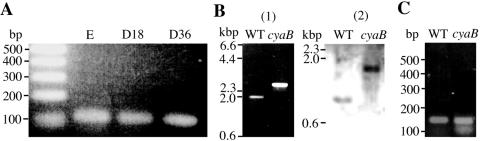
We investigated the expression of the cyaB gene in M. xanthus cells during growth and development by a reverse transcription-PCR (RT-PCR) (Fig. 3A). Total RNA was isolated from M. xanthus at the exponential growth phase and during development as described previously (15), and 0.1 μg of RNA was used for cDNA synthesis with BcaBEST polymerase in accordance with the manufacturer's protocol (Takara shuzo, Kyoto). PCR was performed with Bca-Optimized Taq polymerase, a 5′ gene-specific primer (5′-CTCGGGCCATCGTGTTCG-3′), a 3′ gene-specific primer (5′-AGGTAGAAG GGGATGGACG-3′), and the synthesized cDNA. The expected 105-bp RT-PCR product was amplified from RNA of vegetative cells. The cyaB gene was also expressed at similar levels in developing cells at the mound formation stage (18 h) and the early stage of fruiting body formation (36 h). As a control, the expected product was not amplified without reverse transcription, indicating that there was no DNA contamination in the mRNA (data not shown).
FIG. 3.
RT-PCR analysis of cyaB gene expression in M. xanthus and characterization of the cyaB mutant. (A) RT-PCR analysis of cyaB gene expression in M. xanthus. Total RNA prepared from cultures at the exponential growth phase (E) and during development at 18 h (D18) and 36 h (D36) was used for RT-PCR analysis. Molecular sizes of DNA fragments are given in bases. (B) Confirmation of cyaB deletion-insertion by PCR amplification (1) and hybridization (2) (1). PCR was carried out using genomic DNA as templates and cyaB-specific primers (2). Genomic DNA was restricted with ApaI. Southern blots were probed with a 0.9-kb ApaI fragment of cyaB. (C) Detection of the orf4 transcript in wild type (WT) and cyaB mutant strains. Total RNA prepared from vegetative cells was treated with reverse transcriptase. WT, wild type.
Phenotypes of cyaB mutant.
To investigate the biological function of CyaB, we constructed a cyaB deletion-insertion mutant. A plasmid containing a 3.3-kb KpnI-SalI fragment was digested with StuI, and 1.2 kb of kanamycin-resistant (Kmr) cassette digested with SmaI was ligated into the StuI sites of the plasmid (Fig. 1). cyaB::Kmr was amplified by PCR using a pair of primers (5′-CCATTCCCGTGCTGGTGG-3′ and 5′-TGGGTGCCATTCCCTTGC-3′) and introduced into M. xanthus cells by electroporation (19). We confirmed the replacement by PCR analysis and Southern hybridization (Fig. 3B). PCR of genomic DNA from wild type and cyaB mutant with the above primers had a 2.0-kb band and 2.7-kb band, respectively. Southern analysis of ApaI-digested genomic DNA with a probe (0.9-kb ApaI fragment of cyaB gene) revealed bands of 0.9 kb for the wild type and of 1.6 kb for the mutant strain. These results indicated that the 0.5-kb StuI fragment of cyaB gene was replaced by the 1.2-kb fragment containing a Kmr cassette. Using an RT-PCR, we also confirmed that orf4, which is located downstream of cyaB, was transcribed in the cyaB mutant. A primer set (5′-ATGCGAAGCACAAGCGATGG-3′ and 5′-ACTTCTTCGGATCCACCTGC-3′) was used for PCR analysis. The expected 144-bp product containing part of orf4 was amplified from total RNA of the cyaB mutant, suggesting that the phenotypes of cyaB mutant would not be due to polar effects (Fig. 3C).
The cyaB mutant showed normal growth in Casitone-yeast extract (CYE) medium (1). However, when 0.15 M NaCl or sucrose was added to early exponentially growing cultures, cyaB mutant showed almost twofold longer lag times than the wild type (Fig. 4A and B). No significant differences were found between the final cell densities and maximum growth rates of the wild type and cyaB mutant under osmotic stress. These results indicate that the deletion of cyaB did not affect the final biomass but extended the time needed for cells to adapt to growth under osmotic stress.
FIG. 4.
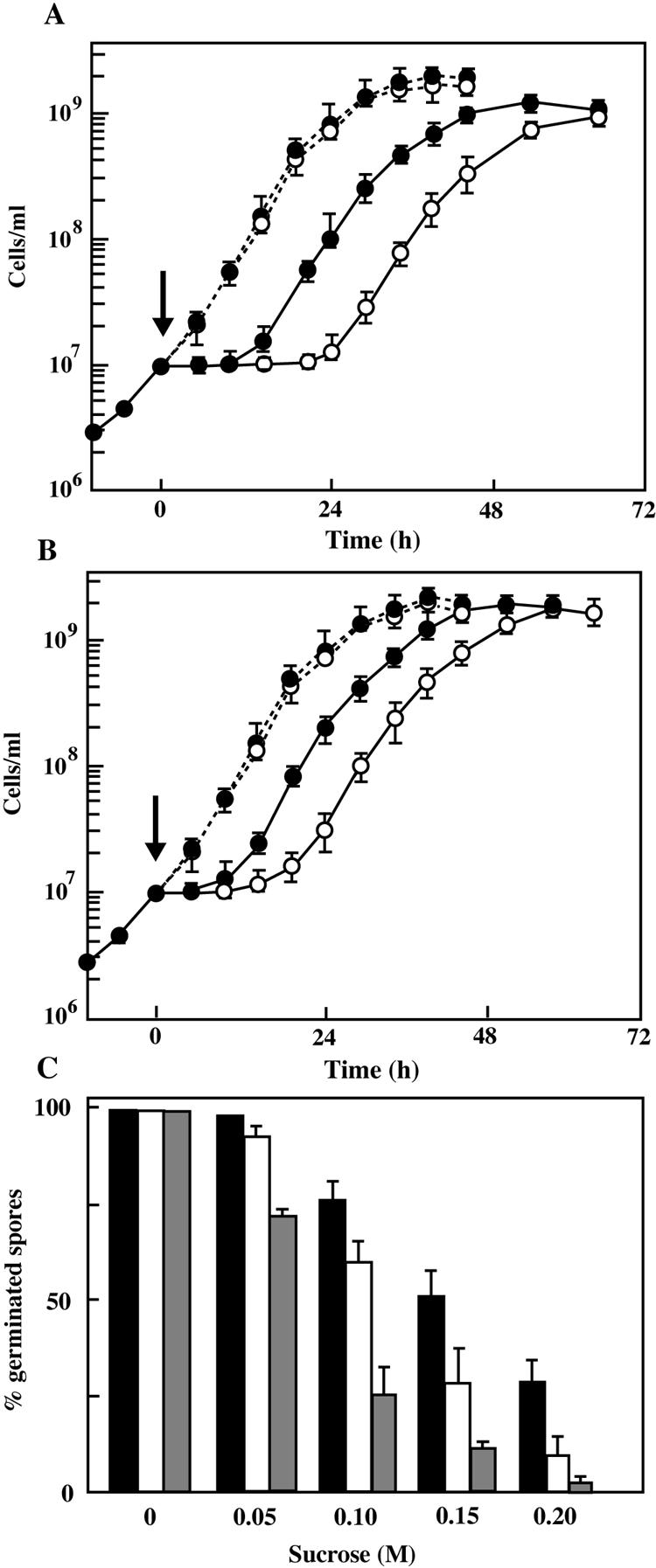
Effect of the cyaB mutation on M. xanthus growth and spore germination under osmotic stress. Growth of M. xanthus wild-type (closed symbols) and cyaB mutant (open symbols) cells in CYE medium with 0.15 M NaCl (A) or 0.15 M sucrose (B) or without either (dashed lines). NaCl or sucrose was added at the point indicated by the arrow. The cells were cultured at 30°C with shaking, and the cell densities were determined with a hemacytometer. The data are expressed as the means for triplicate experiments. (C) Spore germination of M. xanthus wild-type (closed bars), cyaB mutant (open bars), and cyaA mutant (shaded bars) spores in CYE medium containing various concentrations of sucrose. Spores were harvested from 6- to 8-day-old fruiting bodies on CF plates, sonicated for 2 min, and treated with heat (60°C for 15 min). The spores were inoculated to 107 cells/ml in CYE medium containing up to 0.2 M sucrose and incubated at 30°C with continuous shaking until almost all spores in the medium without addition or sucrose were germinated, and then the number of ungerminated spores in each culture was counted with a hemacytometer. The percent germination (germinated spores per inoculated spores) is the mean of duplicate experiments. The standard deviations are shown by error bars.
In contrast to the mokA mutant (6), the cyaB mutant developed normally on medium clone fruiting (CF). The wild-type and cyaB mutant strains formed fruiting bodies after 3 days of incubation on CF plates with 1.5% agar. Within the fruiting bodies of the wild type and mutant, vegetative cells were converted into spherical spores. The numbers of total and viable spores of cyaB mutant counted at 8 days of development were similar to those of the wild-type strain (Table 1). When the developing cells incubated on CF plates for 20 h were harvested and inoculated on CF plates containing up to 0.2 M NaCl or sucrose, the total spores, estimated by direct counts in microscopy, of the cyaB mutant strain on CF agar containing 0.15 M NaCl or sucrose at 8 days of development was approximately 70% or 90% of that of the wild-type strain, respectively. The cyaB mutant under osmotic stress showed higher spore yields compared to a previously reported cyaA mutant (10). On the other hand, the number of the viable spores of cyaB mutant on CF plates containing 0.15 M NaCl or sucrose was 40% or 78% of that of wild-type strain. There were no significant differences in spore formation on CF plates containing less than 0.1 M NaCl or sucrose between the wild-type and cyaB mutant cells.
TABLE 1.
Sporulation of the wild type and cyaB mutant under osmotic stressa
| Addition | Total spores
|
Viable spores
|
||
|---|---|---|---|---|
| Wild type | cyaB mutant | Wild type | cyaB mutant | |
| No addition | 3.7 × 108 | 3.6 × 108 | 2.9 × 107 | 2.8 × 107 |
| 0.10 M NaCl | 1.8 × 108 | 1.7 × 108 | 7.2 × 105 | 7.0 × 105 |
| 0.15 M NaCl | 6.5 × 107 | 4.6 × 107 | 2.0 × 104 | 7.9 × 103 |
| 0.20 M NaCl | 5.9 × 106 | 3.5 × 106 | 9.1 × 102 | 1.4 × 102 |
| 0.10 M Sucrose | 2.0 × 108 | 2.0 × 108 | 1.8 × 106 | 1.7 × 106 |
| 0.15 M Sucrose | 7.4 × 107 | 6.5 × 107 | 3.3 × 105 | 2.6 × 105 |
| 0.20 M Sucrose | 3.0 × 107 | 2.2 × 107 | 7.5 × 104 | 1.8 × 104 |
The developing cells incubated on CF plates for 20 h were harvested and inoculated on CF plates containing 0.1 M to 0.2 M NaCl or sucrose. After 8 days, spots were scraped, resuspended in TM (10 mM Tris-HCl, pH 7.6, and 8 mM MgSO4) buffer, sonicated for 1 min, and treated with heat (2 h at 55°C). The number of spores was counted with a hemacytometer (total spores); the number of viable spores was determined by plating out serial dilutions on CYE plates. Data are the means of duplicate experiments.
When spores were harvested from CF plates and inoculated in CYE medium containing 0 to 0.2 M NaCl, the cyaB mutant spores germinated at the same rate as wild-type spores (data not shown). Meanwhile the cyaB mutant spores in CYE medium containing more than 0.10 M sucrose exhibited a lower germination rate than the wild-type spores (Fig. 4C). However, the germination of the cyaB mutant spores was less inhibited by sucrose than that of the cyaA mutant spores. The cyaB mutant exhibited normal phenotypes with respect to stresses such as temperature shifts and pH changes.
Effects of osmotic stress on levels of cAMP.
Figure 5 shows the changes in intracellular levels of cyclic AMP (cAMP) when vegetative cells were exposed to medium with 0.15 M NaCl for 10 min. The level of cAMP in wild-type cells increased immediately after exposure of the cells to medium with 0.15 M NaCl and reached a maximum level of 7.0 pmol/mg protein at 5 min. In the cyaB mutant, addition of 0.15 M NaCl resulted in a 1.3-fold increase in the cAMP level, with a peak at 1 min. The maximum level was 5.3 pmol/mg protein. Addition of 0.15 M sucrose to wild-type vegetative cells induced an increase in the accumulation of cAMP, but the increased levels (maximum of 1.2-fold) were lower than those after NaCl treatment. cAMP production by mutant vegetative cells was only weakly stimulated by addition of 0.15 M sucrose (data not shown).
FIG. 5.
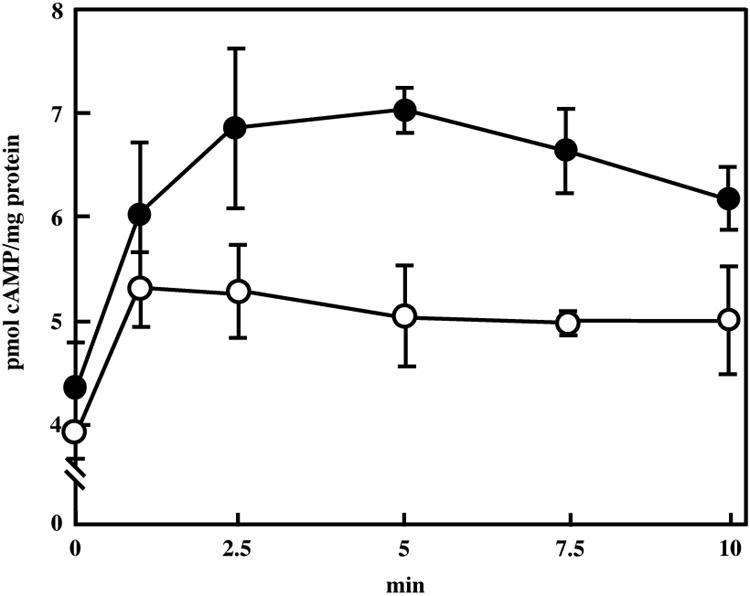
Changes in intracellular levels of cAMP during osmotic stress in M. xanthus. Wild-type (closed circles) and cyaB mutant cells (open circles) were incubated in CYE medium with 0.15 M NaCl. cAMP assays were performed as described previously (10). Experiments were repeated three times. The standard deviations are shown by error bars.
We found no reports of increases in intracellular levels of cAMP in response to osmotic stress during the growth phase of bacteria. In Zygosaccharomyces rouxii, the cAMP levels increased 4.6-fold in response to osmotic stress (1 M NaCl) (18). Compared with the increases in yeast, the increases in the intracellular cAMP levels of M. xanthus in response to osmotic stress were low, but the increase in cAMP may result in the induction of adaptation to osmotic stress in M. xanthus. Recently, we reported that an M. xanthus CbpB containing two cAMP-binding domains was involved in osmotic tolerance (12). CbpB is a hydrophilic protein, and its structural features were partially similar to those of PKA regulatory subunits. The cbpB mutant showed growth retardation under osmotic stress. These data suggest that the cAMP signaling plays an important role in osmotic adaptation in M. xanthus.
During 1 to 2 h of incubation with 0.15 M NaCl or sucrose, the intracellular cAMP levels of wild-type and cyaB mutant cells decreased by 20 to 30% compared with the 0 h value. The cAMP levels then returned to the initial values after 4 h of incubation (data not shown). This temporal decrease is possibly due to the damage of cells by osmotic stress.
We suggest that M. xanthus CyaB and MokA and CyaA function as osmosensors mainly during vegetative growth and germination, respectively. CyaA is also required for osmotic tolerance in fruiting formation and sporulation (10). Recently, we were able to run a BLAST search on the Institute for Genomic Research (TIGR) database. We searched for other receptor-type adenylyl cyclase genes in the TIGR database and found one. The gene encoded a membrane-spanning protein with five to six transmembrane domains and consisting of 375 amino acids, but the encoded adenylyl cyclase has only two short periplasmic regions (13 and 6 amino acids). On the other hand, it is reported that four histidine kinases, Hik16, Hik33, Hik34, and Hik41, act as sensors in the perception of salt stress in Synechocystis sp. strain PCC 6803 (16). We estimate that M. xanthus has about 100 ORFs that encode proteins containing domains homologous to the histidine protein kinase region of MokA. M. xanthus may have other osmosensor histidine kinases and have the ability to adapt to changes in environmental osmolarity using complicated signal transduction pathways.
Nucleotide sequence accession numbers.
The sequence of the M. xanthus cyaB gene has been deposited in the DNA Data Bank of Japan sequence library under accession number AB188227.
REFERENCES
- 1.Campos, M. J., J. Geisselesoder, and D. R. Zusman. 1978. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J. Mol. Biol. 119:167-178. [DOI] [PubMed] [Google Scholar]
- 2.Cann, M. J., A. Hammer, J. Zhou, and T. Kanacher. 2003. A defined subset of adenylyl cyclases is regulated by biacarbonate ion. J. Biol. Chem. 278:35033-35038. [DOI] [PubMed] [Google Scholar]
- 3.Coudart-Cavalli, M. P., O. Sismeiro, and A. Danchin. 1997. Bifunctional structure of two adenylyl cyclases from the myxobacterium Stigmatella aurantiaca. Biochimie 79:757-767. [DOI] [PubMed] [Google Scholar]
- 4.Danchin, A. 1993. Phylogeny of adenylyl cyclases. Adv. Second Messenger Phosphoprotein Res. 27:109-162. [PubMed] [Google Scholar]
- 5.Dworkin, M. 1963. Nutritional regulation of morphogenesis in Myxococcus xanthus. J. Bacteriol. 86:67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagen, C. D., P. A. Bretscher, and D. Kaiser. 1979. Synergism between morphogenic mutants of Myxococcus xanthus. Dev. Biol. 64:284-296. [DOI] [PubMed] [Google Scholar]
- 7.Jelsbak, L., and L. Søgaard-Andersen. 2003. Cell behavior and cell-cell communication during fruiting body morphogenesis in Myxococcus xanthus. J. Microbiol. Methods 55:829-839. [DOI] [PubMed] [Google Scholar]
- 8.Kaiser, D. 2004. Signaling in Myxobacteria. Annu. Rev. Microbiol. 58:75-98. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko, T., Y. Nakamura, S. Sato, K. Minamisawa, T. Uchiumi, S. Sasamoto, A. Watanabe, K. Idesawa, M. Iriguchi, K. Kawashima, M. Kohara, M. Matsumoto, S. Shimpo, H. Tsuruoka, T. Wada, M. Yamada, and S. Tabata. 2003. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 9:189-197. [DOI] [PubMed] [Google Scholar]
- 10.Kimura, Y., Y. Mishima, H. Nakano, and K. Takegawa. 2002. An adenylyl cyclase, CyaA, of Myxococcus xanthus functions in signal transduction during osmotic stress. J. Bacteriol. 184:3578-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura, Y., H. Nakano, H. Terasaka, and K. Takegawa. 2001. Myxococcus xanthus mokA encodes a histidine kinase-response regulator hybrid sensor required for development and osmotic tolerance. J. Bacteriol. 183:1140-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura, Y., H. Nakato, K. Ishibashi, and S. Kobayashi. 2005. A Myxococcus xanthus CbpB containing two cAMP-binding domains is involved in temperature and osmotic tolerances. FEMS Microbiol. Lett. 244:75-83. [DOI] [PubMed] [Google Scholar]
- 13.Kuspa, A., and D. Kaiser. 1989. Genes required for developmental signaling in Myxococcus xanthus: three asg loci. J. Bacteriol. 171:2762-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larimer, F. W., P. Chain, L. Hauser, J. Lamerdin, S. Malfatti, L. Do, D. A. Pelletier, T. J. Beatty, A. S. Lang, F. R. Tabita, J. L. Gibson, T. E. Hanson, Y. Torres, J. Torres, C. Peres, F. H. Harrison, J. Gibson, and C. S. Harwood. 2004. Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nat. Biotechnol. 22:55-61. [DOI] [PubMed] [Google Scholar]
- 15.Lee, M., and L. J. Shimkets. 1994. Cloning and characterization of the socA locus which restores development to Myxococcus xanthus C-signaling mutants. J. Bacteriol. 176:2200-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marin, K., I. Suzuki, K. Yamaguchi, K. Ribbeck, H. Yamamoto, Y. Kanesaki, M. Hagemann, and N. Murata. 2003. Identification of histidine kinases that act as sensors in the perception of salt stress in Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. USA 100:9061-9066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCue, L. A., K. A. McDonough, and C. E. Lawrence. 2000. Functional classification of cNMP-binding proteins and nucleotide cyclases with implications for novel regulatory pathways in Mycobacterium tuberculosis. Genome Res. 10:204-219. [DOI] [PubMed] [Google Scholar]
- 18.Nishi, T., and T. Yagi. 1993. Participation of cAMP in the induction of the synthesis of glycerol for the osmoregulatory response in the salt-tolerant yeast Zygosaccharomyces rouxii. J. Gen. Appl. Microbiol. 39:493-503. [Google Scholar]
- 19.Plamann, L., J. M. Davis, B. Cantwell, and J. Mayor. 1994. Evidence that asgB encodes a DNA-binding protein essential for growth and development of Myxococcus xanthus. J. Bacteriol. 176:2013-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Record, M. T., E. S. Courtenay, Jr., D. S. Cayley, and H. Guttman. 1998. Responses of E. coli to osmotic stress: large changes in amounts of cytoplasmic solutes and water. Trends Biochem. Sci. 23:143-148. [DOI] [PubMed] [Google Scholar]
- 21.Ren, S. X., G. Fu, X. G. Jiang, R. Zeng, Y. G. Miao, H. Xu, Y. X. Zhang, H. Xiong, G. Lu, L. F. Lu, H. Q. Jiang, J. Jia, Y. F. Tu, J. X. Jiang, W. Y. Gu, Y. Q. Zhang, Z. Cai, H. H. Sheng, H. F. Yin, Y. Zhang, G. F. Zhu, M. Wan, H. L. Huang, Z. Qian, S. Y. Wang, W. Ma, Z. J. Yao, Y. Shen, B. Q. Qiang, Q. C. Xia, X. K. Guo, A. Danchin, I. Saint Girons, R. L. Somerville, Y. M. Wen, M. H. Shi, Z. Chen, J. G. Xu, and G. P. Zhao. 2003. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature 422:888-893. [DOI] [PubMed] [Google Scholar]
- 22.Rendulic, S., P. Jagtap, A. Rosinus, M. Eppinger, C. Baar, C. Lanz, H. Keller, C. Lambert, K. J. Evans, A. Goesmann, F. Meyer, R. E. Sockett, and S. C. Schuster. 2004. A predator unmasked: life cycle of Bdellovibrio bacteriovorus from a genomic perspective. Science 303:689-692. [DOI] [PubMed] [Google Scholar]
- 23.Shimkets, L. J. 1990. Social and developmental biology of the Myxobacteria. Microbiol. Rev. 54:473-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood, J. M. 1999. Osmosensing by bacteria: signals and membrane-based sensors. Microbiol. Mol. Biol. Rev. 63:230-262. [DOI] [PMC free article] [PubMed] [Google Scholar]