Abstract
The crgA gene of Neisseria meningitidis, which codes for a LysR-type regulator, is divergently oriented with respect to the mdaB gene, which codes for a hypothetical NADPH-quinone oxidoreductase. Transcriptional studies of the intergenic region between crgA and mdaB showed that two overlapping and divergent promoters, PcrgA and PmdaB, control transcription of these genes. Deletion of the crgA gene led to a strong increase in transcription from the PcrgA promoter and a concomitant strong decrease in transcription from the PmdaB promoter, indicating that CrgA acts both as an autorepressor of transcription at its own promoter and as an activator of transcription at the mdaB promoter. Addition of α-methylene-γ-butyrolactone (MBL), an inducer of NADPH-quinone oxidoreductase, to wild-type N. meningitidis cells specifically resulted in further activation of transcription of the PmdaB promoter and more repression of transcription of the PcrgA promoter. No such regulation was observed when MBL was added to crgA-deficient cells, indicating that the transcriptional response to MBL is CrgA mediated. Under the same experimental conditions, no regulation of transcription by either CrgA or MBL was detected at the pilus and capsule genes. The role of CrgA in the regulation of gene expression during the infectious cycle of N. meningitidis is discussed.
The human pathogen Neisseria meningitidis is a common colonizer of the nasopharynx, and in a small percentage of carriers, this bacterium can cross the epithelial barrier to enter the bloodstream, causing septicemia, and then further cross the blood-brain barrier, causing meningitis. The ability to interact with host cells plays a major role in the ability of N. meningitidis to establish a productive infection. Numerous bacterial attributes have been identified as factors that play a role in these interactions. Among these, the type IV pili play an essential role by allowing the initial adhesion of bacteria to host cells via the adhesin PilC1 (20, 22). The expression of PilC1 is upregulated during the initial interaction of the bacteria with the cells; this upregulation is required for complete adhesion of the bacterium (28). It has been proposed that regulation of PilC1 expression is controlled by a 150-bp sequence located upstream of pilC1. This 150-bp element was designated CREN, for contact regulatory element of Neisseria (28). Further analysis demonstrated that a 150-bp sequence very similar to that found upstream of pilC1 corresponds to a sequence repeat, designated Rep2, containing a ribosome binding site upstream of an ATG codon, which is the predicted start codon of a downstream open reading frame (ORF) (23). This full-length element is present 16 times in the N. meningitidis genome. Fourteen of 16 ORFs located downstream of Rep2 are upregulated during the initial contact of the bacteria with the cells in a manner similar to that of pilC1 (21), suggesting that these 14 Rep2-associated genes are coordinately upregulated in the initial interaction of N. meningitidis with host cells. Interestingly, one of the CREN/Rep2 elements lies upstream of a gene designated crgA (contact-regulated gene A) (7), which encodes a 299-amino-acid protein belonging to the LysR family of transcriptional regulators (14, 26, 32).
It has been reported that in the absence of epithelial cells, crgA is expressed at low levels from two transcription start sites, P1 and P2, which map upstream of and within the CREN/Rep2 element, respectively. RNA analyses led to the hypothesis that transcription starting at P2 is responsible for inducing crgA expression when N. meningitidis comes into contact with target cells (7). As a consequence, it has been proposed that the product of this gene, CrgA, represses the expression of several genes, including the pilC1, pilE, and sia genes involved in adhesin, pilin, and capsule biosynthesis, respectively. However, there are reasons to doubt aspects of the previous model. For example, the transcript starting at the P2 site poorly resembles the −24/−12 GG-N10-GC consensus sequence characteristic of a sigma 54-dependent promoter (1). Furthermore, in N. meningitidis the sigma 54 factor encoded by the rpoN gene is inactive (17, 29), and in vitro the P2 promoter appears to be transcribed by the Escherichia coli RNA polymerase containing sigma 70, the housekeeping sigma factor (4). Although it has been suggested that the P1 and P2 transcriptional start sites of the crgA gene arise from two distinct promoters, no functional evidence has been provided yet, and the nature of the mapped 5′ ends of RNA remains unclear.
CrgA has been reported to function by binding to the promoters of the crgA and pilC1 genes (both harboring a CREN/Rep2 element), as well as the pilE and sia genes (both devoid of a CREN/Rep2 element), repressing transcription upon adhesion of bacteria to target epithelial cells (5). This suggested that, independent of the CREN/Rep2 element, CrgA regulates all four promoters by a similar mechanism (3, 5-7), possibly by binding to a T-N11-A motif characteristic of other LysR regulators (9).
In this paper, we report functional identification of the crgA gene promoter and provide evidence that the CREN/Rep2 repeat element is not involved in initiation of gene transcription. We show that CrgA is a regulatory protein controlling transcription both as a repressor and as an activator of overlapping and divergent promoters. Furthermore, activation and repression of transcription controlled by CrgA are enhanced by the addition of an inducer of NADPH-quinone oxidoreductase to N. meningitidis cells. It is likely that this inducer, or a similar inducer, activates the CrgA protein to control the expression of sets of genes; however, transcription of pilus and capsule genes appeared to be unaltered irrespective of CrgA and α-methylene-γ-butyrolactone (MBL).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are listed in Table 1. The N. meningitidis strains are MC58 derivatives (29) and were routinely cultured in GC-based (Difco) agar supplemented with Kellogg's supplement I (15) at 37°C in a 5% CO2-95% air atmosphere at 95% humidity. Strains were stocked in 10% skim milk and stored at −80°C. For liquid cultures, N. meningitidis strains were grown overnight on solid medium, resuspended in phosphate-buffered saline (PBS) to an optical density at 600 nm of 1, and inoculated at a 1:20 dilution into GC broth supplemented with Kellogg's supplement I, 12.5 μM Fe(NO3)3, and, when required, erythromycin, kanamycin, and/or chloramphenicol added at final concentrations of 5, 100, and 5 μg/ml, respectively. For transformation by naturally competent N. meningitidis, four or five single colonies of a freshly grown overnight culture were resuspended in 20 μl of PBS, spotted onto GC agar plates to which 5 to 10 μg of linearized plasmid DNA was added, allowed to dry, and incubated for 6 to 8 h at 37°C. Transformants were then selected on plates containing erythromycin (5 μg/ml), kanamycin (150 μg/ml), and/or chloramphenicol (5 μg/ml), and single colonies were restreaked on selective media for further analysis. Single colonies were resuspended in 50 μl of distilled water, placed in a boiling water bath for 5 min, and centrifuged in a bench top centrifuge for 5 min at 8,000 × g. One microliter of the sample was used as a template for PCR analysis. E. coli DH5α cultures were grown in Luria-Bertani medium, and when required, ampicillin, kanamycin, and chloramphenicol were added at final concentrations of 100, 25, and 30 μg/ml, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| E. coli strain DH5α | supE44 hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 12 |
| N. meningitidis strains | ||
| MC58 | Clinical isolate; sequenced strain | 29 |
| wtA (MC-PcrgA1) | crgA promoter region from position −63 to position 179, fused to lacZ, is inserted between ORFs NMB1074 and NMB1075; Eryr | This study |
| Δ35 (MC-PcrgA2) | crgA promoter region from position −15 to position 179, fused to lacZ, is inserted between ORFs NMB1074 and NMB1075; Eryr | This study |
| Δ10 (MC-PcrgA3) | crgA promoter region from position −6 to position 179, fused to lacZ, is inserted between ORFs NMB1074 and NMB1075; Eryr | This study |
| ΔP (MC-PcrgA4) | crgA promoter region from position 44 to position 179, fused to lacZ, is inserted between ORFs NMB1074 and NMB1075; Eryr | This study |
| ΔR (MC-PcrgA5) | crgA promoter region from position −63 to position 43, fused to lacZ, is inserted between ORFs NMB1074 and NMB1075; Eryr | This study |
| ΔRCrgA (MC-C−PcrgA5) | CrgA null mutant, derivative of ΔR (MC-PcrgA5); Eryr Kmr | This study |
| ΔRCrgA-C (MC-C−CindPcrgA5) | CrgA complemented mutant, derivative of ΔRCrgA (MC-C−PcrgA5) containing the crgA gene under the control of the Ptac promoter and lacI repressor located between ORFs NMB1428 and NMB1429; Eryr Kmr Cmr | This study |
| Plasmids | ||
| pGem3Z | Cloning vector, Ampr | Promega |
| pSL1190 | Cloning vector, Ampr | Pharmacia |
| pCMVβ | Plasmid containing the lacZ gene of E. coli | Clontech |
| pAT110 | Plasmid containing the ermAM erythromycin resistance gene | 30 |
| pDT2548 | Plasmid containing the chloramphenicol resistance cassette from Campylobacter coli | 31 |
| pILL600 | Plasmid containing the kanamycin cassette from Campylobacter coli | 16 |
| pMMB206 | Plasmid containing the Ptac promoter and the lacI gene | 19 |
| pSL-Fla-Ery | Plasmid consisting of a promoterless lacZ gene and the ermAM erythromycin resistance gene flanked by upstream and downstream regions for allelic replacement at a chromosomal location between ORFs NMB1074 and NMB1075 | This study |
| pSL-Pcrg1 | Derivative of pSL-Fla-Ery containing the full crgA promoter region from position −63 to position 179 fused to the lacZ gene | This study |
| pSL-Pcrg2 | Derivative of pSL-Fla-Ery containing the crgA promoter region from position −15 to position 179 fused to the lacZ gene | This study |
| pSL-Pcrg3 | Derivative of pSL-Fla-Ery containing the crgA promoter region from position −6 to position 179 fused to the lacZ gene | This study |
| pSL-Pcrg4 | Derivative of pSL-Fla-Ery containing the crgA promoter region from position 44 to position 179 fused to the lacZ gene | This study |
| pSL-Pcrg5 | Derivative of pSL-Fla-Ery containing the crgA promoter region from position −63 to position 43 fused to the lacZ gene | This study |
| pG3cr:Km | Plasmid for knockout of crgA gene containing the upstream and downstream regions of the crgA locus flanking a kanamycin resistance cassette | This study |
| pSLComCmr | Plasmid consisting of the chloramphenicol resistance gene flanked by upstream and downstream regions for allelic replacement at a chromosomal location between ORFs NMB1428 and NMB1429 | This study |
| pPindcrgA | Plasmid for complementation of the CrgA null mutant, derivative of pSLComCmr containing a copy of the crgA gene under the control of the Ptac promoter and the lacI repressor | This study |
DNA techniques.
DNA manipulations were carried out routinely as described by Sambrook et al. (25). Small-scale plasmid DNA preparation and large-scale plasmid DNA preparation were carried out with a QIAprep Spin mini kit and a plasmid midi kit (QIAGEN, Inc.) used according to the manufacturer's instructions. DNA fragments or PCR-amplified products were purified from agarose gels with a QiaEX DNA purification kit (QIAGEN, Inc.). Each PCR was performed with a Perkin-Elmer 2400 thermal cycler with Platinum Taq polymerase (Invitrogen). One microliter of each reaction mixture contained 10 to 50 ng of chromosomal DNA or 1 μl of bacterial sample (see above), 100 pmol of the required primers, and 200 μM of each deoxynucleotide in 100 μl of 1× PCR buffer containing MgCl2 (New England Biolabs, Inc.). After the initial denaturing step at 95°C for 5 min, 30 cycles of denaturation at 95°C, annealing at the appropriate temperatures for the specific primers, and elongation at 72°C were carried out. DNA fragments were sequenced by the dideoxy chain termination method by using [α-32P]dATP (Amersham) and a T7 sequencing kit (Pharmacia).
Construction of chromosomally located transcriptional lacZ fusions.
To generate transcriptional lacZ fusions of the promoters studied at a chromosomal location between two converging ORFs, NMB1074 and NMB1075, a series of plasmids for allelic exchange in N. meningitidis strains were constructed (Table 1). A plasmid consisting of a promoterless lacZ gene and the ermAM erythromycin resistance genes flanked by upstream and downstream regions for allelic replacement was generated by cloning fragments into the multiple-cloning site of the pSL1190 vector in the following order: a 510-bp SpeI-XhoI fragment consisting of the upstream flanking region amplified from MC58 chromosomal DNA with primers Fla-UP-L and Fla-UP-R (Table 2), a 1.1-kb XhoI-PstI fragment carrying the ermAM genes amplified from plasmid pAT110 using primers Eryt-DO and Eryt-UP, a 3.4-kb SmaI-BamHI fragment carrying the lacZ gene from pCMVβ, and a 909-bp BamHI-XmaI fragment consisting of a downstream flanking region amplified from the MC58 chromosome with primers Fla-DO-L and Fla-DO-R. This plasmid was named pSL-Fla-Ery. The promoter region of crgA or portions of this region were then amplified with primer pairs Pcrg1R-N-Pcrg1L, Pcrg2R-N-Pcrg1L, Pcrg3R-N-Pcrg1L, Pcrg4R-N-Pcrg1L, and Pcrg1R-N-Pcrg2L and cloned as an NsiI-SphI fragment in pSL-Fla-Ery. The resulting plasmids were designated pSL-Pcrg1, pSL-Pcrg2, pSL-Pcrg3, pSL-Pcrg4, and pSL-Pcrg5, respectively (Table 1). These plasmids were used for transformation of the MC58 strain. Transformants were first selected for erythromycin resistance and then tested for the double-crossover at the flanking regions by PCR using primer pairs Fla-UP-C-Ery-DO-C and Fla-DO-C2-LAC-DO-C. The resulting strains were wtA (MC-PcrgA1), Δ35 (MC-PcrgA2), Δ10 (MC-PcrgA3), ΔP (MC-PcrgA4), and ΔR (MC-PcrgA5), respectively (Table 1).
TABLE 2.
Primers used in this study
| Olignucleotide | Sequencea | Siteb |
|---|---|---|
| adk-PE | CGCGCCTAAAAGTAATGC | |
| Com1 | attcagcccgggGCGTGGCTGATCAAACGCACCG | XmaI |
| Com2 | gttatgcatAGGTCTCGAATTGTGGATCCCACGC | NsiI-BamHI |
| Com3 | ggatccACAATTCGAGACCTATGCATTAACATAG | NsiI-BamHI |
| Com4 | attcagactagtAATGCCGCCTCCGTCGGTTTGC | SpeI |
| crdw-L | ccgggaattcTTAGGCTACGGCATCTGCGCC | EcoRI |
| crdw-R | attcgcggatccGTCGGCGGCAATGGCAAT | BamHI |
| CrgA-N | attcgcatATGAAAACCAATTCAGAAGAACTGACC | NdeI |
| Crg-R | attcgatgcatAGAATTATCCACAGAGATTGTTTTC | NsiI |
| crup-L2 | attcgcggatcCTTGAACAAATACGGTCAG | BamHI |
| crup-R2 | aactgcagCATAGCCGGCATCAATCACGG | PstI |
| Ery-DO-C | CAGGTTACTAAAGGGAATGGAG | |
| Eryt-DO | CCGTAGGCGCTcGaGACCTCTTTAGCTTCTTG | XhoI |
| Eryt-UP | GCAAACTgcAGAGTGTGTTGATAG | PstI |
| Fla-DO-C2 | CTCGAAACCGGTTCTGACGG | |
| Fla-DO-L | ATAAATGTAAAGGaTCCGTTTCATAGCTAAGG | BamHI |
| Fla-DO-R | CGCCGTCAACCCgGgTGCCGAGCTGGAAAAAGAGC | XmaI |
| Fla-UP-C | CTGAAGCAAAGTCGGAAAACGCCGGC | |
| Fla-UP-L | GGTTCCGTACTAgTTGTACTGTCTGC | SpeI |
| Fla-UP-R | aatttaactcgagCCACCAATCCCACACCACCCTTACC | XhoI |
| LAC-DO-C | CGCTACCATTACCAGTTGGTCTGG | |
| LAC | CTTGTTGGTCAAAGTAAACGACAT | |
| mdaB-PE | CCGTGAGAATGTCCGAACGC | |
| MDER-PE1 | GATACTGCACTATCAACACA | |
| NMB1856L | ATTGCGCGCCTTCTTCCGTC | |
| NMB1856R | CATAGCCGGCATCAATCACG | |
| Pcrg1L | atatatgcatgcTCTGAATTGGTTTTTATCGTGTTTCC | SphI |
| Pcrg1R-N | atatatatgcatTTGTTACCTCGTTTGTGAATTGATG | NsiI |
| Pcrg2L | atatatgcatgcCTGATTATTTCATTTGACGCAAAAG | SphI |
| Pcrg2R-N | atatatatgcatCTTTATAATTTAAAAGTGCAAAAATAAG | NsiI |
| Pcrg3R-N | atatatatgcatTTAAAAGTGCAAAAATAAGAAAACA | NsiI |
| Pcrg4R-N | atatatatgcatATGTTCCAACACACGGGATGGCAC | NsiI |
| pilC-PE2 | TTTTAAAGTTTTATTCATCG | |
| pilE-PE1 | GAAGGGTGTTCATAAAATTAC | |
| Pind-F | attcgggatccGCGTTGCGCTCACTGCCCGC | BamHI |
| Pind-R | aatgcatgcatggtcatatgTGTTTCCTGTGTGAAATTG | NsiI-NdeI |
| sia-PE1 | ACCTGTAATGCAAAGAATTC |
Uppercase letters in Roman type indicate N. meningitidis-derived sequences, italicized uppercase letters indicate E. coli-derived sequences, lowercase letters indicate sequences added for cloning purposes, and underlined letters indicate recognition sites.
Restriction enzyme sites added for cloning purposes.
Construction of the crgA mutant of N. meningitidis and complementation.
To construct a crgA deletion mutant, the crgA gene was replaced with a kanamycin cassette by double crossing over. To do this, plasmid pG3cr:Km was generated as follows. Upstream and downstream flanking regions of crgA were amplified from the MC58 chromosome with primer pairs crup-L2-crup-R2 and crdw-L-crdw-R and cloned as 428-bp PstI/BamHI and 489-bp BamHI/EcoRI fragments into pGem3Z, respectively; a kanamycin cassette from plasmid pILL600 was cloned as a 1.4-kb BamHI fragment into the BamHI site between the two flanking regions. This plasmid was used to transform N. meningitidis strain ΔR (MC-PCrgA5). Transformants were selected for kanamycin resistance and analyzed by PCR for correct insertion by a double homologous recombination event. The resulting mutant was named ΔRCrgA (MC-C−PcrgA5). Complementation of CrgA was achieved by insertion of a copy of the crgA gene under the control of the inducible promoter Ptac and the LacI repressor in the noncoding region of the ΔRCrgA (MC-C−PcrgA5) chromosome between the converging ORFs NMB1428 and NMB1429. To do this, a 500-bp XmaI/NsiI fragment downstream of NMB1428 and a 430-bp BamHI/SpeI fragment upstream of NMB1429 were amplified with oligonucleotides Com1 and Com2 and oligonucleotides Com3 and Com4, respectively. These fragments are consecutive fragments that have overlapping ends bearing the BamHI and NsiI restriction sites for cloning purposes. These two fragments were used as templates to amplify an 885-bp XbaI/BamHI fragment with the Com1 and Com4 primers, which was cloned as an XmaI/SpeI fragment in pSL1190. Subsequently, an 800-bp XbaI/BamHI fragment containing the chloramphenicol resistance cassette was added to this construct, generating plasmid pSLComCmr. The 1,550-bp BamHI/NsiI lacI-Ptac region was amplified from plasmid pMMB206 with oligonucleotides Pind-F and Pind-R, and a 910-bp NdeI/NsiI fragment of the crgA gene was amplified from N. meningitidis strain MC58 with oligonucleotides CrgA-N and Crg-R. The PCR products were cloned in the BamHI/NdeI sites of the pSLComCmr plasmid. The resulting plasmid, pPindcrgA, was used to transform ΔRCrgA (MC-C−PcrgA5). Transformants were selected for chloramphenicol resistance, and correct insertion was verified by PCR. The selected strain was named ΔRCrgA-C (MC-C−CindPcrgA5). Induction of the protein was achieved by growing the strain in GC broth with isopropyl-β-d-thiogalactopyranoside (IPTG) to the logarithmic phase.
Primer extension analysis.
An oligonucleotide (3 pmol) was 5′ end labeled in the presence of [γ-32P]ATP (5,000 Ci/mmol; Perkin-Elmer) and T4 polynucleotide kinase (New England Biolabs). One hundred femtomoles of the labeled oligonucleotide was coprecipitated with 30 μg of total RNA and resuspended in 5 μl of water, 2 μl of 2 mM deoxynucleoside triphosphates, and 2 μl of 5× reverse transcription buffer (Roche). The mixture was incubated for 2 min at 95°C and for 1 min at room temperature, and then reverse transcription was started by adding 1 μl of reverse transcriptase (Roche) and incubating the reaction mixture at 45°C for 45 min. The sample was then incubated for 10 min at room temperature with 1 μg of RNase A for RNA digestion, extracted once with an equal volume of phenol-chloroform (1:1), ethanol precipitated, and resuspended in 5 μl of sequencing loading buffer. After denaturation at 95°C for 2 min, samples were subjected to 6% urea-polyacrylamide gel electrophoresis and autoradiographed.
S1 nuclease mapping.
A 441-bp probe corresponding to the promoter region of crgA and the 5′ region of the crgA gene (Probe1) (Fig. 1A), labeled at the 5′ end of the noncoding strand, was obtained by PCR amplification with the primer pair NMB1856L-NMB1856R, 5′ end labeled in the presence of [γ-32P]ATP and T4 polynucleotide kinase (New England Biolabs), and subsequently digested with SspI. Approximately 20 fmol of the probe was coprecipitated with 40 μg of total N. meningitidis RNA and resuspended in 20 μl of hybridization buffer (80% formamide, 60 mM Tris-HCl, pH 7.5, 400 mM NaCl, 0.4 mM EDTA). The mixture was overlaid with 5 μl of mineral oil, denatured at 100°C for 2 min, and incubated overnight at 55°C. Subsequently, 180 μl of ice-cold S1 buffer (33 mM sodium acetate, pH 5.2, 5 mM ZnSO4, 250 mM NaCl) and 1 μl of S1 nuclease (150 U/μl; Invitrogen) were added, and digestion was carried out for 30 min at 37°C. Samples were extracted with phenol-chloroform (1:1), ethanol precipitated, and resuspended in 5 μl of sequencing buffer. After denaturation at 95°C for 2 min, samples were subjected to 6% urea-polyacrylamide gel electrophoresis and autoradiographed.
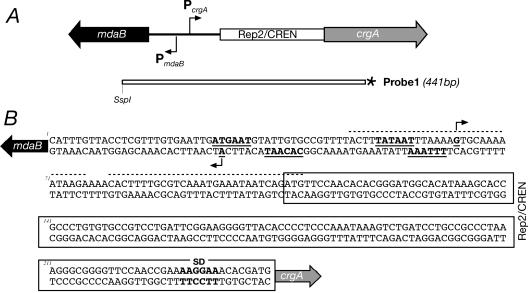
FIG. 1.
(A) Structural organization of N. meningitidis crgA and mdaB genes (not to scale). Genes are indicated by their designations. The open box represents the 138-bp Rep2/CREN repetitive DNA element. crgA codes for a 299-amino-acid protein belonging to the LysR family (NMB1856), and mdaB codes for an NADPH-quinone oxidoreductase (192 amino acids). The arrows indicate the direction of transcription. Probe1 represents the DNA segment used as a probe in S1 nuclease protection experiments; the asterisk indicates the radioactively labeled end. (B) Nucleotide sequence of the mdaB-crgA intergenic sequence. The shaded arrows indicate directions of translation. The bent arrows indicate transcriptional start sites. Underlined boldface letters indicate −10 and −35 promoter consensus sequences. The Rep2/CREN DNA sequence is enclosed in a box. The dotted lines indicate the CrgA binding site according to Deghmane et al. (3). SD, Shine-Dalgarno sequence.
Western blot analysis. To prepare sera against CrgA, 20 μg of recombinant CrgA protein obtained under denaturing conditions was used to immunize 6-week-old CD1 female mice (Charles River Laboratories) according the procedure described by Pizza et al. (24). One milliliter of a single culture in the exponential growth phase was harvested by centrifugation at 8,000 × g and resuspended in 100 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer, and 5 μl of each total protein sample was fractionated on a sodium dodecyl sulfate-12.5% polyacrylamide gel and transferred onto a nitrocellulose filter by standard methods (25). Filters were blocked overnight at 4°C by agitation in blocking solution (5% skim milk and 0.05% Tween 20 in PBS) and incubated for 1 h with a 1:1,500 dilution of the anti-CrgA protein sera in blocking solution. After washing, the filters were incubated with a 1:2,000 dilution of peroxidase-conjugated anti-mouse immunoglobulin (Dako) in blocking solution for 1 h, and the resulting signal was detected with the Supersignal West Pico chemiluminescent substrate (Pierce).
RESULTS
Intergenic region between the crgA and mdaB genes of N. meningitidis contains two overlapping and divergent promoters.
The structure of the locus and the nucleotide sequence of the 238-bp intergenic region between the two divergent genes crgA and mdaB are shown in Fig. 1. This region contains a 138-bp DNA element, termed Rep2 or CREN (21, 28), directly upstream of the putative ATG start codon of the crgA gene. To define the start point of transcription of crgA and mdaB, we carried out an S1 nuclease protection assay and primer extension analysis for total RNA extracted from N. meningitidis.
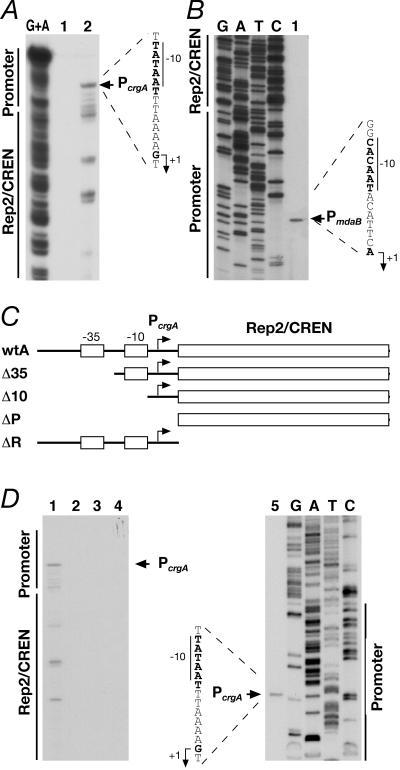
Figure 2A shows the results of urea-acrylamide gel electrophoresis in an S1 nuclease protection experiment carried out by hybridizing Probe1 (Fig. 1A) to RNA of wild-type N. meningitidis. The results show a major S1-resistant band migrating at a position corresponding to 375 nucleotides, which defined the PcrgA start site of transcription (Fig. 2A, lane 2). Other faster-migrating bands may have been derived from the presence of weaker start points or from in vivo degradation of the RNA, and most of these 5′ ends of RNA mapped within the CREN/Rep2 region. Primer extension analysis (Fig. 2D, lane 5) confirmed the position of the PcrgA start site of transcription at a position 179 nucleotides from the ATG start codon of the crgA gene and minor 5′ ends of RNA mapping within the CREN/Rep2 element.
FIG. 2.
Mapping of the promoters in the crgA-mdaB intergenic region. (A) Identification of the crgA transcripts. The 441-bp DNA of Probe1 (Fig. 1A) was end labeled at one extremity, hybridized to N. meningitidis total RNA, and used for S1 nuclease digestion to map 5′ ends of transcription products synthesized by the PcrgA promoter. The G+A lane contained a G+A sequence reaction mixture for the DNA probe used as a size marker (18). A control sample was processed identically but contained no RNA (lane 1). Lane 2 contained 40 μg RNA. The nucleotide sequence of the coding strand upstream of the transcriptional start site is shown on the right, and the −10 promoter element is indicated by a vertical bar. The PcrgA promoter in strain MC58 appears to be localized 10 bp upstream of the P1 promoter mapped by Deghmane and coworkers (7) in strain C8013. The nature of this discrepancy was not investigated. (B) Identification of mdaB transcript. Total RNA (30 μg) from N. meningitidis was hybridized to the end-labeled mdaB-PE oligonucleotide (Table 2) and elongated with reverse transcriptase to map 5′ ends of transcription products synthesized by the PmdaB promoter (lane 1). Precise mapping was performed by sequencing the cloned region in plasmid pG3cr:Km (Table 1) with the same primer (lanes G, A, T, and C). DNA regions corresponding to promoter and Rep2/CREN elements are indicated on the left. (C) Schematic representation (not to scale) of the chromosomal promoter-Rep2 mutations fused to the lacZ gene and inserted between the NMB1074 and NMB1075 ORFs of the N. meningitidis genome. The strains are referred to by their short names; the full names are given in Table 1. (D) Detection of the transcript generated by the promoter-Rep2 mutations fused to lacZ. Lanes 1 to 5 contained 30 μg of total RNA extracted from strains wtA, Δ35, Δ10, ΔP, and ΔR, respectively, hybridized to the end-labeled LAC oligonucleotide (Table 2), and elongated with reverse transcriptase. Lanes 1 and 5 contained RNA synthesized by the PcrgA promoter; no bands were de-tected in lanes 2 to 4. Similar band patterns have been obtained with RNA extracted from E. coli transformed with plasmids carrying the promoter mutations shown in panel C. Precise mapping was performed by sequencing the cloned region in plasmid pSL-Pcrg5 (Table 1) with primer LAC (lanes G, A, T, and C).
To define the start point of the mRNA encoded by the mdaB gene, we carried out primer extension of total RNA extracted from N. meningitidis. Figure 2B shows a unique extended product mapping at a position 24 nucleotides upstream of the ATG start codon of the mdaB gene, indicating the position of the PmdaB start site of RNA transcription.
These results indicate that the intergenic region between the crgA and mdaB genes contains at least two promoters, which we called PcrgA and PmdaB. Analysis of the DNA sequence revealed the presence of −10-TATAAT and −35-ATGAAT regions upstream of PcrgA and −10-CACAAT and −35-TTTTAA regions upstream of PmdaB. These sequences show conservation with the E. coli sigma 70 −10-TATAAT and −35-TTGACA recognized promoters and are likely to define the N. meningitidis PcrgA and PmdaB promoters (Fig. 1B). Analysis of the sequences upstream of the 5′ ends mapping within the CREN/Rep2 element revealed no sequence conservation with known promoter consensus sequences.
In order to obtain information on the nature of the 5′ ends of transcripts mapping downstream of the PcrgA promoter, we generated progressive deletions of the PcrgA promoter region fused to lacZ, introduced into the N. meningitidis MC58 genome by double recombination, and assayed for activity. As the beta-galactosidase activity values were very close to the background levels for all constructs, we decided to investigate transcription by primer extension analyses. Figure 2C shows a diagrammatic representation of the strains harboring the PcrgA deletion mutations. Total RNA was extracted from each mutant strain and used in primer extension analyses. Figure 2D shows that the PcrgA promoter was active in the strain carrying the full-length promoter construct (strain wtA) (lane 1), as well as in the strain carrying the deletion of the CREN/Rep2 region (strain ΔR) (lane 5). No extension products were detected with RNA extracted from strains Δ35, Δ10, and ΔP (lanes 2, 3, and 4), which harbored deletions from position −63 to position −15, from position −63 to position −6, and from position −63 to position 43 of PcrgA, respectively. Therefore, transcription of the region studied was abolished when deletions affected the −35 or −10 regions of the PcrgA promoter. Consequently, we concluded that the 5′ ends mapping within the CREN/Rep2 region are PcrgA dependent and that no promoters map within this region. These results were confirmed by primer extension of RNA extracted from E. coli transformed with plasmids carrying the crgA promoter mutations (data not shown). Furthermore, five independent beta-galactosidase experiments with the E. coli system gave Miller unit values of 61.5 ± 6.3, 6.8 ± 1.4, 9.8 ± 1.6, 5.4 ± 1.2, and 98.4 ± 3.3 for the wtA, Δ35, Δ10, ΔP, and ΔR constructs, respectively.
We concluded that the crgA gene is transcribed from the PcrgA promoter upstream of the Rep2/CREN element.
CrgA represses transcription from PcrgA and activates transcription from PmdaB.
To establish the role of CrgA in transcription of the PcrgA and PmdaB promoters, we decided to assay transcription from these promoters in a crgA deletion mutant. To do this, we substituted the crgA gene with a kanamycin cassette in strain ΔR (Fig. 2C), which carried the PcrgA promoter fused to lacZ in a heterologous locus, generating strain ΔRCrgA (Table 1). Expression of CrgA in these strains was assessed by Western blot analysis of total protein extracts, whereas transcription from the PcrgA and the PmdaB promoters was assayed by primer extension analysis.
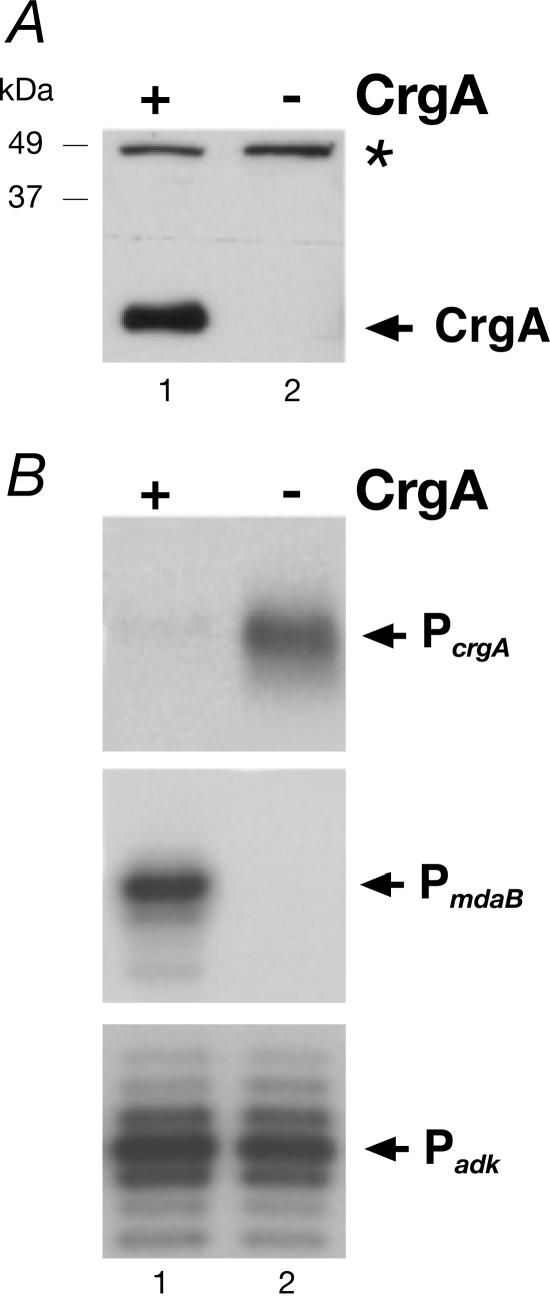
Figure 3A shows a Western blot of protein extracts from strains ΔR (CrgA+) and ΔRcrgA (CrgA−). As expected, in the wild-type background a protein band corresponding to CrgA was detected (lane 1), while this band was not detected in the crgA deletion mutant (lane 2).
FIG. 3.
(A) Western blot analysis with anti-CrgA antisera. Total cell lysates from strains ΔR (lane 1) and ΔRcrgA (lane 2) were used to detect CrgA. The asterisk marks a cross-reactive band. (B) Regulation of transcription of the PcrgA and PmdaB promoters. Total RNA from strains ΔR (lanes 1) and ΔRcrgA (lanes 2) was hybridized to primers MDER-PE1 (upper panel) and LAC (middle panel) and elongated with reverse transcriptase. As a control (lower panel), RNA from strains ΔR and ΔRcrgA was elongated with primer adk-PE for adk mRNA.
Total RNA was extracted from these strains, and primer extension was carried out with primers hybridizing to RNA synthesized from the PcrgA and PmdaB promoters in the heterologous genomic location. Figure 3B shows that transcription from the PcrgA promoter was increased in the CrgA mutant (lane 2) compared to RNA extracted from the wild-type background (lane 1). Surprisingly, the amount of transcripts at the PmdaB promoter was strongly reduced and transcripts were undetectable in the CrgA mutant (lane 2) compared to the wild-type background (lane 1). As a control, primer extension was carried out with a primer complementary to the adenylate kinase gene (adk), and this experiment showed no variation in the amount of mRNA (lanes 1 and 2). These results indicate that CrgA represses transcription from the PcrgA promoter and activates transcription from the PmdaB promoter.
Complementation of the crgA mutation.
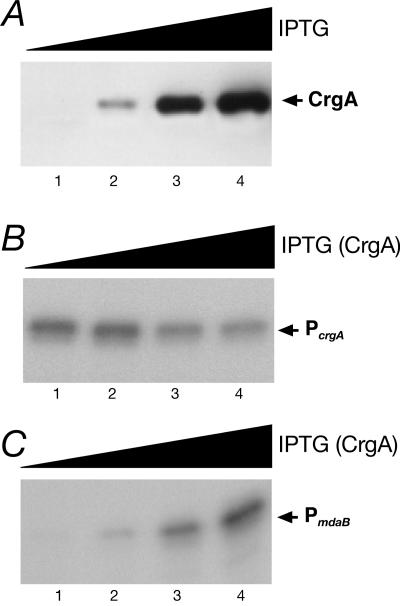
To complement the crgA mutation and to obtain a better understanding of the regulation of transcription by CrgA, we constructed a strain expressing the crgA gene under the control of an inducible promoter to monitor accumulation of the CrgA protein and correlate it to regulation of transcription. In this strain, ΔRCrgA-C (Table 1), the expression of the crgA gene was inducible by addition of IPTG, as its transcription was under the control of the Ptac promoter and the LacI repressor. Cells were grown in liquid cultures in the presence of increasing amounts of IPTG to the mid-log phase, and aliquots of each sample were collected and used to prepare total protein extracts and total RNA.
The Western blot in Fig. 4A shows that the CrgA protein was detected when cells were grown in the presence of 10 μM IPTG (lane 2) and that the amount increased with increasing amounts of IPTG in the culture medium (lanes 3 to 4). Primer extension analysis showed that transcription from the PcrgA promoter was repressed in cells grown in the presence of 30 μM IPTG or in the presence of higher concentrations of IPTG (Fig. 4B, compare lanes 3 and 4 with lanes 1 and 2). By contrast, at the same concentrations of IPTG the PmdaB promoter was increasingly activated (Fig. 4C, lanes 1 to 4). In a control experiment, transcription from the adk promoter showed no variation in the amount of RNA in response to IPTG (data not shown). We concluded that CrgA controls transcription from the PcrgA and PmdaB promoters in a dose-dependent manner.
FIG. 4.
(A) Western blot analysis of CrgA expression in the complementing strain (ΔRCrgA-C) grown with increasing amounts of IPTG. Lanes 1 to 4 contained total lysates from strains grown in the presence of 0, 10, 30, and 100 μM IPTG, respectively. Total RNA was extracted from samples of the same cultures and used in primer extensions to monitor accumulation of RNA synthesized from the PcrgA (B) and PmdaB (C) promoters.
Activity of the CrgA regulator is induced by α-methylene-γ-butyrolactone.
In E. coli, the mdaB gene encodes a protein with NADPH-specific quinone oxidoreductase activity that has been shown to be induced more than 25-fold by MBL (13). To investigate whether addition of this small molecule to N. meningitidis cells influences transcription of the mdaB gene in a CrgA-dependent manner and in turn transcription of the crgA gene, we extracted RNA from the wild-type background (ΔR), the crgA mutant (ΔRCrgA), and the complementing (ΔRCrgA-C) strains before treatment and after 10 min of treatment of the cells with 2 mM MBL. These RNA preparations were used in primer extension experiments to monitor accumulation of RNA at the PmdaB and PcrgA promoters, and the results are shown in Fig. 5.
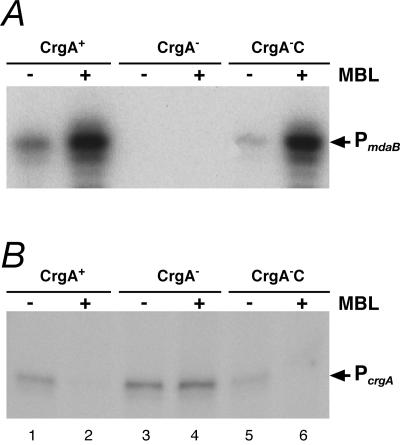
FIG. 5.
Transcriptional response of the PmdaB (A) and PcrgA (B) promoters to MBL treatment. RNA was extracted from the strains indicated at the top, which were treated (+) or not treated (−) with MBL. Samples CrgA+, CrgA−, and CrgA−C were samples from strains ΔR, ΔRCrgA, and ΔRCrgA-C, respectively. Strain ΔRCrgA-C was grown in the presence of 1 mM IPTG.
Treatment of the ΔR strain (CrgA+) with MBL clearly resulted in an increase in the amount of transcript from the PmdaB promoter (Fig. 5A, lanes 1 and 2). Independent of MBL addition, no extended products were detected in the CrgA mutant (lanes 3 and 4); however, the pattern of RNA accumulation in response to MBL was restored in the CrgA complemented strain (CrgA−C) (lanes 5 and 6). The same RNA preparations were then used in primer extension experiments to monitor transcription from the PcrgA promoter. Figure 5B shows that in the wild-type background, the amount of transcript from the PcrgA promoter was decreased upon MBL treatment (lanes 1 and 2) and was not influenced in the mutant (lanes 3 and 4) and that these changes could be complemented by CrgA (lanes 5 and 6).
From these results, we concluded that addition of MBL to growing cells activates transcription of the PmdaB promoter and represses transcription from the PcrgA promoter. Furthermore, these modulations of transcription are CrgA dependent.
CrgA and MBL have no effect on transcription of pilus and capsule genes.
The results described above prompted us to investigate whether other CrgA-regulated promoters are similarly regulated. As it has been reported that CrgA regulates transcription of the pilC1, pilE, and sia genes (5), we selected these genes to study the CrgA-mediated MBL response. To do this, we carried out primer extensions of the pilE, sia, and pilC1 mRNA using the same RNA sample that was used for the experiment whose results are shown in Fig. 5, and the results are shown in Fig. 6.
FIG. 6.
Mapping and regulation of the pilE, sia, and pilC1 promoters. Primer extension analyses were performed with the same RNA used for the experiment shown in Fig. 5 and with primers pilE-PE1 (A), sia-PE1 (B), and pilC-PE2 (C) to assess regulation of the PpilC1, PpilE, and Psia promoters, respectively. Sequencing reactions carried out with each cloned promoter fragment served as size markers (lanes G, A, T, and C). Independent of the strain and of MBL treatment, major bands show no appreciable variation in the amount of elongated products. Analyses of the DNA sequence upstream of the identified major bands revealed the presence of −10 and −35 regions similar to the E. coli sigma 70 consensus sequences, −10-TATAAT and −35-TTGACA. While no promoter consensus sequences were identified upstream of the other 5′ ends of RNA mapping downstream of the PpilE and PpilC1 promoters, a putative promoter sequence, AATAAA-N17-TATAAT, was detected upstream of the 5′ end of RNA mapping 48 nucleotides upstream of the sia genes (faster-migrating band in panel B).
Figure 6A shows the extended products of the pilE mRNA, with a major band mapping 90 nucleotides upstream of the ATG start codon, which identified the PpilE start site of RNA transcription. This is in agreement with previous studies (2). Surprisingly, regardless of MBL addition to the cells the intensity of this band remained unchanged in the three strains used (lanes 1 to 6). Figure 6B shows two extended products of the sia mRNA, with a major band identifying the Psia start site of RNA transcription 106 nucleotides upstream of the ATG start codon. This corresponds to a previously identified promoter (27), which showed no variation in the amount of RNA among strains or upon MBL treatment (lanes 1 to 6). Figure 6C shows the extended products of the pilC1 mRNA. The slowly migrating band mapped 255 nucleotides upstream of the ATG start codon of the gene and corresponded to the PpilC1 start site of RNA transcription (28). This band and the faster-migrating bands had similar intensities in RNA extracted from the three strains and from cells treated or not treated with MBL (lanes 1 to 6). These results indicate that transcription from the pilC1, pilE, and sia promoters is regulated neither by CrgA nor by MBL addition.
It is worth noting that the 5′ ends mapping downstream of the PpilC1 promoter are located in the CREN/Rep2 region of the pilC1 gene; thus, analogous to results obtained for the PcrgA promoter (Fig. 2), these 5′ ends of RNA and those mapping downstream of the PpilE promoter may arise from in vivo processing of longer mRNAs.
DISCUSSION
The CrgA protein of N. meningitidis is a LysR-type transcriptional regulator (29), which is upregulated during the initial phase of adhesion of the bacterium to the target cells, and this protein was proposed to be a repressor controlling expression of a set of genes during bacterial adhesion to epithelial cells (7). The gene encoding the CrgA regulator, crgA, is preceded by a CREN/Rep2 repetitive DNA element and maps divergently with respect to another gene, the mdaB gene coding for an NADPH-quinone reductase.
In this study, we identified a single promoter, PcrgA, which is responsible for transcription of the crgA gene. We carried out deletion and 5′ end mapping analyses, which showed that the 5′ ends of transcripts mapping within the CREN/Rep2 region depend on transcription from the upstream PcrgA promoter (Fig. 2). Furthermore, the PmdaB promoter of the upstream gene is divergently oriented and overlaps the PcrgA promoter (Fig. 1 and 2). This promoter architecture is compatible with coordinated regulation of transcription of the crgA and mdaB genes. Accordingly, the amounts of transcripts synthesized from the PcrgA and PmdaB promoters are increased and decreased in a crgA knockout background, respectively (Fig. 3). Furthermore, repression of the PcrgA promoter and activation of the PmdaB promoter are restored in a complementing strain (Fig. 4). In addition, the degree of complementation of the PcrgA and PmdaB transcriptional regulation correlates well with the intracellular amount of CrgA (Fig. 4). Thus, the autoregulatory mechanism of gene transcription primarily controls the intracellular concentration of CrgA, a condition used by many regulatory proteins to modify their activities in response to environmental changes. In addition, CrgA activates the divergently oriented upstream gene, another typical feature of LysR-type regulators. Interestingly, two CrgA binding sites have been mapped within this region (4, 5). One CrgA binding site spans positions −16 to 13 of the PcrgA promoter, and this region corresponds to positions −20 to −49 of the PmdaB promoter. Another CrgA binding site spans positions 17 to 46 of the PcrgA promoter that correspond to positions −53 to −82 of the PmdaB promoter (Fig. 1B). Therefore, the two CrgA binding sites are located close to the transcriptional start site of the PcrgA promoter and upstream of the PmdaB promoter, respectively. These DNA binding positions could be compatible with a mechanism of repression and activation of transcription of the PcrgA and PmdaB promoters, respectively.
It has been proposed that a second promoter, termed P2, controls expression of the crgA gene mapping within the Rep2/CREN element (7). We have no evidence of promoters mapping within this region. The possibility that this element is responsible for posttranscriptional regulation of crgA upon cell contact should be considered. Indeed, mRNA processing and degradation by nucleases depend on the secondary structure, including the presence of stem-loops at the 5′ ends (11). Accordingly, a transcript carrying a CREN/Rep2 region at its 5′ end might fold into a secondary structure that could be targeted by specific nucleases during cell contact, resulting in changes in the stability of mRNA. This hypothesis could account for the observed upregulation of transcripts mapping at the P2 site described by Deghmane and coworkers (7).
It has been reported that the activity of the NADPH-quinone reductase encoded by the mdaB gene of E. coli is induced more than 25-fold by the addition of MBL to growing cells (13). As this could result from increased expression of the enzyme, we decided to investigate the effect of MBL on transcription of the CrgA-regulated gene promoters in N. meningitidis. Indeed, cells treated with MBL showed strong induction of transcription from the PmdaB promoter and, in parallel, strong repression of the PcrgA promoter, and both induction and repression were CrgA dependent (Fig. 5). This suggests that MBL could act as a cofactor or effector molecule that activates the CrgA regulatory protein. Interestingly, transcriptome analyses have highlighted the finding that transcription of the mdaB gene is increased upon interaction of N. meningitidis with epithelial cells (8, 10). Therefore, it is tempting to speculate that MBL or a similar molecule could be available to N. meningitidis during adhesion to activate CrgA, which in turn would control transcription of a specific set of genes.
It has been reported that CrgA functions by binding to the promoters of the pilC1, pilE, and sia genes in the regions spanning positions −94 to −58, −163 to −133, and −39 to 13 with respect to the corresponding transcriptional start sites, respectively (3, 5). Consequently, we investigated the effect of MBL-specific and CrgA-dependent regulation of transcription of these genes (Fig. 6). Surprisingly, independent of the CrgA protein, transcription of these genes was not altered in cells treated with MBL. These results do not exclude the possibility that pilC1, pilE, and sia could be regulated by CrgA in response to cell contact. However, it is interesting that while transcription of mdaB and crgA is regulated by CrgA and in response to MBL in a CrgA-dependent manner, transcription of pilC1, pilE, and sia is not affected by CrgA or by addition of MBL to cultured cells. These results are compatible with two hypotheses: (i) regulation by CrgA occurs only upon cell contact by perception of a signal that activates CrgA in a fashion different from that observed for MBL, and (ii) the CrgA binding observed in vitro at these promoters does not occur in vivo or has no biological significance. In support of the latter hypothesis are the findings of Morelle and coworkers (21), who demonstrated that a crgA mutant was capable of adhering to the same extent as the wild-type strain, thus providing functional evidence that pilus expression is not controlled by crgA during bacterial adhesion to eukaryotic cells. Furthermore, these workers reported that the crgA mutant loses its pili during late adhesion stages at a rate similar to that observed with the wild-type strain, thus demonstrating that downregulation of pili is not via crgA (21).
In conclusion, we established that CrgA acts as a repressor of transcription of its own gene and as an activator of transcription of the mdaB gene and that its action is enhanced by the presence of MBL. As crgA is upregulated during the initial phase of adhesion (5), it would be interesting to understand the role of MBL or a similar inducer in the coordination of CrgA-regulated genes during infection.
Acknowledgments
We are grateful to Silvana Savino and the MenB group of Chiron for the donation of antiserum raised against the CrgA protein. We thank Catherine Mallia for manuscript editing and Giorgio Corsi for artwork.
This work was supported by Chiron and partially by a grant from MIUR and the University of Bologna to V.S.
REFERENCES
- 1.Barrios, H., B. Valderrama, and E. Morett. 1999. Compilation and analysis of σ54-dependent promoter sequences. Nucleic Acids Res. 27:4305-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrick, C. S., J. A. Fyfe, and J. K. Davies. 1997. The normally silent σ54 promoters upstream of the pilE genes of both Neisseria gonorrhoeae and Neisseria meningitidis are functional when transferred to Pseudomonas aeruginosa. Gene 198:89-97. [DOI] [PubMed] [Google Scholar]
- 3.Deghmane, A. E., and M. K. Taha. 2003. The Neisseria meningitidis adhesion regulatory protein CrgA acts through oligomerization and interaction with RNA polymerase. Mol. Microbiol. 47:135-143. [DOI] [PubMed] [Google Scholar]
- 4.Deghmane, A. E., D. Giorgini, L. Maigre, and M. K. Taha. 2004. Analysis in vitro and in vivo of the transcriptional regulator CrgA of Neisseria meningitidis upon contact with target cells. Mol. Microbiol. 53:917-927. [DOI] [PubMed] [Google Scholar]
- 5.Deghmane, A. E., D. Giorgini, M. Larribe, J. M. Alonso, and M. K. Taha. 2002. Down-regulation of pili and capsule of Neisseria meningitidis upon contact with epithelial cells is mediated by CrgA regulatory protein. Mol. Microbiol. 43:1555-1564. [DOI] [PubMed] [Google Scholar]
- 6.Deghmane, A. E., M. Larribe, D. Giorgini, D. Sabino, and M. K. Taha. 2003. Differential expression of genes that harbor a common regulatory element in Neisseria meningitidis upon contact with target cells. Infect. Immun. 71:2897-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deghmane, A. E., S. Petit, A. Topilko, Y. Pereira, D. Giorgini, M. Larribe, and T. K. Taha. 2000. Intimate adhesion of Neisseria meningitidis to human epithelial cells is under the control of the crgA gene, a novel LysR-type transcriptional regulator. EMBO J. 19:1068-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich, G., S. Kurz, C. Hubner, C. Aepinus, S. Theiss, M. Guckenberger, U. Panzner, J. Weber, and M. Frosch. 2003. Transcriptome analysis of Neisseria meningitidis during infection. J. Bacteriol. 185:155-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goethals, K., P. Mergaert, M. Gao, D. Geelen, M. Van Montagu, and M. Holsters. 1992. Identification of a new inducible nodulation gene in Azorhizobium caulinodans. Mol. Plant-Microbe Interact. 5:405-411. [DOI] [PubMed] [Google Scholar]
- 10.Grifantini, R., E. Bartolini, A. Muzzi, M. Draghi, E. Frigimelica, J. Berger, G. Ratti, R. Petracca, G. Galli, M. Agnusdei, M. M. Giuliani, L. Santini, B. Brunelli, H. Tettelin, R. Rappuoli, F. Randazzo, and G. Grandi. 2002. Previously unrecognized vaccine candidates against group B meningococcus identified by DNA microarrays. Nat. Biotechnol. 20:914-921. [DOI] [PubMed] [Google Scholar]
- 11.Grunberg-Manago, M. 1999. Messenger RNA stability and its role in control of gene expression in bacteria and phages. Annu. Rev. Genet. 33:193-227. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi, M., H. Ohzeki, H. Shimada, and T. Unemoto. 1996. NADPH-specific quinone reductase is induced by 2-methylene-4-butyrolactone in Escherichia coli. Biochim. Biophys. Acta 1273:165-170. [DOI] [PubMed] [Google Scholar]
- 14.Henikoff, S., G. W. Haughn, J. M. Calvo, and J. C. Wallace. 1988. A large family of bacterial activator proteins. Proc. Natl. Acad. Sci. USA 85:6602-6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and C. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labigne-Roussel, A., P. Courcoux, and L. Tompkins. 1988. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J. Bacteriol. 170:1704-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laskos, L., J. P. Dillard, H. S. Seifert, J. A. Fyfe, and J. K. Davies. 1998. The pathogenic neisseriae contain an inactive rpoN gene and do not utilize the pilE σ54 promoter. Gene 208:95-102. [DOI] [PubMed] [Google Scholar]
- 18.Maxam, A. M., and W. Gilbert. 1977. A new method for sequencing DNA. Proc. Natl. Acad. Sci. USA 74:560-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales, V. M., A. Backman, and M. Bagdasarian. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39-47. [DOI] [PubMed] [Google Scholar]
- 20.Morand, P. C., E. Bille, S. Morelle, E. Eugene, J. L. Beretti, M. Wolfgang, T. F. Meyer, M. Koomey, and X. Nassif. 2004. Type IV pilus retraction in pathogenic Neisseria is regulated by the PilC proteins. EMBO J. 23:2009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morelle, S., E. Carbonelle, and X. Nassif. 2003. The REP2 repeats of the genome of Neisseria meningitidis are associated with genes coordinately regulated during bacterial cell interaction. J. Bacteriol. 185:2618-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nassif, X., M. Marceau, C. Pujol, B. Pron, J. L. Beretti, and M. K. Taha. 1997. Type-4 pili and meningococcal adhesiveness. Gene 192:149-153. [DOI] [PubMed] [Google Scholar]
- 23.Parkhill, J., M. Achtman, K. D. James, S. D. Bentley, C. Churcher, S. R. Klee, G. Morelli, D. Basham, D. Brown, T. Chillingworth, R. M. Davies, P. Davis, K. Devlin, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, S. Leather, S. Moule, K. Mungall, M. A. Quail, M. A. Rajandream, K. M. Rutherford, M. Simmonds, J. Skelton, S. Whitehead, B. G. Spratt, and B. G. Barrell. 2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502-506. [DOI] [PubMed] [Google Scholar]
- 24.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Arico, M. Comanducci, J. G. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 27.Swartley, J. S., J. H. Ahn, L. J. Liu, C. M. Kahler, and D. S. Stephens. 1996. Expression of sialic acid and polysialic acid in serogroup B Neisseria meningitidis: divergent transcription of biosynthesis and transport operons through a common promoter region. J. Bacteriol. 178:4052-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taha, M. K., P. C. Morand, Y. Pereira, E. Eugene, D. Giorgini, M. Larribe, and X. Nassif. 1998. Pilus-mediated adhesion of Neisseria meningitidis: the essential role of cell contact-dependent transcriptional upregulation of the PilC1 protein. Mol. Microbiol. 28:1153-1163. [DOI] [PubMed] [Google Scholar]
- 29.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 30.Trieu-Cuot, P., C. Poyart-Salmeron, C. Carlier, and P. Courvalin. 1990. Nucleotide sequence of the erythromycin resistance gene of the conjugative transposon Tn1545. Nucleic Acids Res. 18:3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, Y., and D. E. Taylor. 1990. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene 94:23-28. [DOI] [PubMed] [Google Scholar]
- 32.Zaim, J., and A. M. Kierzek. 2003. The structure of full-length LysR-type transcriptional regulators. Modeling of the full-length OxyR transcription factor dimer. Nucleic Acids Res. 31:1444-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]