Abstract
SpoIIE is a dual-function protein in Bacillus subtilis that contributes to the switch from medial to polar cell division during sporulation and is responsible for activating the cell-specific transcription factor σF. SpoIIE consists of an N-terminal domain with 10 membrane-spanning segments (region I), a C-terminal phosphatase domain (region III), and a central domain (region II) of uncertain function. To investigate the role of SpoIIE in polar division, we took advantage of a system for efficiently producing polar septa during growth in a SpoIIE-dependent manner using cells engineered to produce the sporulation protein in response to an inducer. The results show that regions II and III play a critical role in polar septum formation and that specific amino acid substitutions in those regions affect the abilities of SpoIIE both to promote polar division and to localize to the division machinery. Additionally, we show that neither the phosphatase function of SpoIIE nor the N-terminal, membrane-spanning region is needed for the switch to asymmetric division.
A hallmark of the process of sporulation by the gram-positive bacterium Bacillus subtilis is the formation of an asymmetrically positioned septum that divides the developing cell into dissimilar-sized progeny called the forespore (the smaller cell) and the mother cell (33). One of the important challenges in the sporulation field is to understand the molecular mechanisms that bring about this switch from medial to polar division. Cytokinesis in bacteria is mediated by the tubulin-like protein FtsZ, which forms a ring-like structure, the cytokinetic or Z-ring, at the future site of cell division (11, 29, 30). Previous work has shown that asymmetric division is accompanied by the formation of bipolar Z-rings and that the formation of these rings involves a helical intermediate of the cytokinetic protein that may be responsible for redeploying molecules of FtsZ from the midcell position to the poles (6, 25). One of the polar Z-rings is converted into a division septum, whereas the other Z-ring is blocked from undergoing cytokinesis by the action of the sporulation genes spoIID, spoIIM, and spoIIP, which are synthesized in the mother cell as a consequence of polar division (10, 34). Redeploying FtsZ from the midcell to the poles is effected by a sporulation-specific increase in FtsZ levels (via a promoter that is recognized by the sporulation regulatory protein σH) and by the activation of the gene for the sporulation protein SpoIIE (via the sporulation regulatory protein Spo0A) (6, 15, 16, 38). Evidence that enhanced FtsZ levels and synthesis of SpoIIE are sufficient to cause polar division comes from the observation that artificial induction of spoIIE during growth in cells harboring an extra copy of ftsZ is sufficient to bring about a switch from medial to asymmetric division (6). Furthermore, a spoIIE mutation prevents polar septum formation in cells engineered to produce a constitutively active form of Spo0A during growth (22).
SpoIIE is an 827-amino-acid-long protein that consists of an N-terminal domain (region I) with 10 membrane-spanning segments (3), a C-terminal domain that is homologous to the PP2C family of phosphatases domain (region III) (1, 27), and a central domain (region II) that shows little similarity to other, nonorthologous proteins in the databases (Fig. 1A). Cytological evidence demonstrates that SpoIIE colocalizes with the Z-ring and that it depends on FtsZ for this colocalization (4, 26). Biochemical evidence additionally indicates that the interaction between SpoIIE and FtsZ is direct (28). Whereas FtsZ and other division proteins exit the developing septum during cytokinesis, SpoIIE remains associated with the polar septum after cytokinesis is complete, when it plays a critical role in the activation of the forespore-specific transcription factor σF. SpoIIE triggers the activation of σF by catalyzing (via its PP2C-like phosphatase domain) the conversion of the inactive phosphoprotein SpoIIAA-P to its active, dephosphorylated form SpoIIAA. SpoIIAA, in turn, reacts with a complex of σF and the anti-σF factor SpoIIAB to effect the release of the transcription factor from its inhibitor (19). Thus, SpoIIE is a dual-function protein. It interacts with the cytokinetic machinery to promote polar division, and it becomes incorporated into the resulting polar septum, where it participates directly in the pathway leading to the activation in the forespore of the transcription factor σF.
FIG. 1.
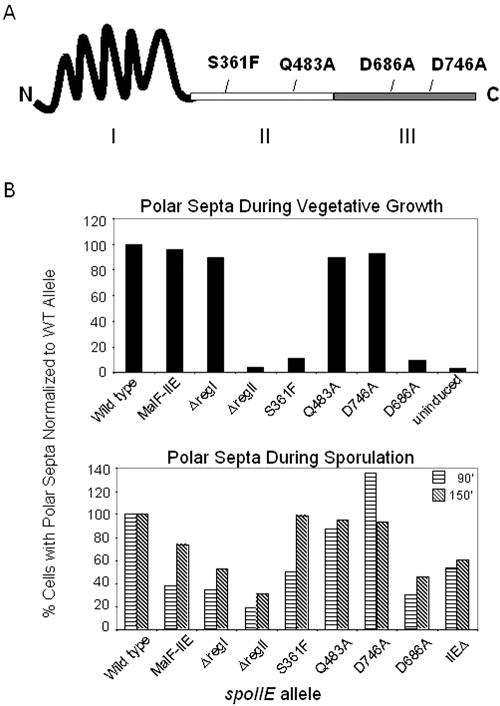
Frequency of polar septation during vegetative growth and sporulation. (A) Schematic diagram representing SpoIIE. The roman numerals represent the membrane-spanning region (I), the central region (II), and the conserved PP2C-like phosphatase region (III). Approximate positions of amino acid substitutions investigated in this study are indicated. (B) Upper graph: the percentage of cells with a polar septum in vegetative cultures induced to express the indicated allele of spoIIE were determined as described in Materials and Methods and are documented in Table 2. The percentage of cells with polar septa in the strain expressing wild-type spoIIE was normalized to 100%, and the values for the other cultures were normalized accordingly. Wild type = SB210, MalF-IIE = KC501, ΔregI = KC541, ΔregII = SB211, S361F = SB250, Q483A = KC500, D746A = SB214, D686A = KC552. Lower graph: the percentage of cells with at least one polar septum after 90 or 150 min of sporulation as visualized by membrane staining as described in Materials and Methods. The percentage of cells with polar septa in the strain expressing wild-type spoIIE was normalized to 100%, and the values for the other cultures were normalized accordingly. Wild type = KC544, MalF-IIE = KC538, ΔregI = KC549, ΔregII = KC548, S361F = KC545, Q483A = KC546, D746A = KC547, D686A = KC554. WT, wild type.
Here we are concerned with the role of SpoIIE in polar division. This is a challenging problem to address because asymmetric division is a composite consequence of the appearance of SpoIIE and a sporulation-specific increase in FtsZ levels. Thus, a mutant lacking SpoIIE but otherwise unimpaired in sporulation-specific FtsZ synthesis is only delayed in septum formation; it eventually produces polar septa at an efficiency of about 50% that of the wild type (5, 6, 20). Further complicating efforts to study the role of SpoIIE in polar division are its indirect effects on septation via its role in the activation of σF. The absence of σF activity in a SpoIIE mutant leads to the formation of aberrant (disporic) sporangia with septa at both poles (20), and premature activation of σF from excess SpoIIE activity prevents polar septation at either pole (8, 18).
To investigate the role of SpoIIE in asymmetric division specifically, we took advantage of a previously described system for producing polar septa during growth (6). This system involves the use of cells that harbor an extra copy of the gene for FtsZ and are engineered to produce SpoIIE in response to an inducer. Such cells undergo asymmetric division robustly when expression of spoIIE is induced but at only a low level when it is not. Moreover, in such cells the contribution of SpoIIE to asymmetric division is uncoupled from its role in the activation of σF. Using this system, we find that neither the phosphatase activity of SpoIIE nor the membrane-spanning segments in its N-terminal region (I) contribute measurably to polar septation but that both the central region (II) of SpoIIE and at least one residue in the C-terminal region (III) play a critical role in the switch to polar division.
MATERIALS AND METHODS
Strain construction.
All strains were derivatives of PY79 (39) and were built with the following constructs: spo and spoIIE phleo (3), amyE::ftsAZ cat (6), and spoIIA::cat and spoIIA::spc (32). To integrate spoIIE alleles at the nonessential chromosomal locus zae-86, plasmids used for integration at the amyE locus (described below) were transformed into JDB326; selected for spectinomycin resistance; and screened for kanamycin sensitivity, chloramphenicol resistance, and the ability to catabolize starch (amy+) (9). To construct 3′ gfp derivatives of spoIIE alleles, either pPE1 (23), pSBY25, pKC70, pCM19, or pCM12 (described below) was integrated via single-crossover recombination into the appropriate strain. Transformants that had integrated the plasmid at the desired site were screened for by linkage to another antibiotic resistance cassette and/or detection of green fluorescent protein (GFP) under the appropriate conditions by fluorescence microscopy. Preparation of competent cells and transformation were performed as described in the work of Harwood and Cutting (17). Antibiotic concentrations used for selection on Luria broth agar were spectinomycin at 100 μg/ml, kanamycin at 10 μg/ml, phleomycin at 0.4 μg/ml, chloramphenicol at 5 μg/ml, and lincomycin at 25 μg/ml plus erythromycin at 1 μg/ml. Table 1 shows strain genotypes.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Genotype | Reference |
|---|---|---|
| Strains | ||
| PY79 | Wild-type parent strain | 39 |
| SB150 | amyE::ftsAZ cat | 6 |
| JDB326 | zae-86::Tn917::pTV21D2::pCB15 kan | 9 |
| SB210 | As SB150, zae-86::Pspank-spoIIE spc | This study |
| SB220 | As SB150, zae-86::Pspank-spoIIE-gfp spc kan | This study |
| SB214 | As SB150, zae-86::Pspank-spoIIE-D746A spc | This study |
| SB250 | As SB150, zae-86::Pspank-spoIIE-S361F spc | This study |
| SB414 | As SB150, zae-86::Pspank-spoIIE-S361F-gfp spc kan | This study |
| KC500 | As SB150, zae-86::Pspank-spoIIE-Q483A spc | This study |
| KC507 | As SB150, zae-86::Pspank-spoIIE-Q483A-gfp spc kan | This study |
| SB211 | As SB150, zae-86::Pspank-spoIIE-ΔregII spc | This study |
| SB221 | As SB150, zae-86::Pspank-spoIIE-ΔregII-gfp spc kan | This study |
| KC541 | As SB150, zae-86::Pspank-spoIIE-ΔregI spc | This study |
| KC542 | As SB150, zae-86::Pspank-spoIIE-ΔregI-gfp spc kan | This study |
| KC501 | As SB150, zae-86::Pspank-malF-spoIIE spc | This study |
| KC506 | As SB150, zae-86::Pspank-malF-spoIIE-gfp spc kan | This study |
| KC552 | As SB150, zae-86::Pspank-spoIIE-D686A-gfp spc kan | This study |
| RL2775 | spoIIA::cat | 32 |
| KC543 | spoIIE::phleo spoIIA::cat | This study |
| KC544 | spoIIE-gfp spc spoIIA::cat | This study |
| KC545 | spoIIE-S361F-gfp spc spoIIA::cat | This study |
| KC546 | spoIIE-Q483A-gfp spc spoIIA::cat | This study |
| KC547 | spoIIE-D746A-gfp spc spoIIA::cat | This study |
| KC548 | As KC543, amyE::spoIIE-ΔregII-gfp cat spc | This study |
| KC549 | As KC543, amyE::spoIIE-ΔregI-gfp cat spc | This study |
| KC538 | spoIIE::phleo spoIIA::spec amyE::malF-spoIIE-gfp spc kan | This study |
| KC554 | spoIIE-D686A-gfp kan spoIIA::cat | This study |
| Plasmids | ||
| pDR110 | bla spc amyE::Pspank | D. Rudner |
| pKC25 | bla spc amyE::Pspank-spoIIE | This study |
| pKC26 | bla spc amyE::Pspank-spoIIE-ΔregII | This study |
| pKC27 | bla spc amyE::Pspank-spoIIE-S361F | This study |
| pKC29 | bla spc amyE::Pspank-spoIIE-D746A | This study |
| pKC63 | bla spc amyE::Pspank-spoIIE-Q483A | This study |
| pKC64 | bla spc amyE::Pspank-malF-spoIIE | This study |
| pKC69 | bla spc amyE::Pspank-spoIIE-ΔregI | This study |
| pKC17 | bla cat amyE::spoIIE-ΔregII | This study |
| pSDE95 | bla cat amyE::spoIIE-ΔregI | 3 |
| pPE1 | bla spc spoIIE(1375-2481)-gfpF64L S65T | 23 |
| pSBY25 | bla kan spoIIE(1540-2481)-gfpF64L S65T | This study |
| pKC70 | bla kan spoIIE(1540-2481, D686A)-gfpF64L S65T | This study |
| pCM12 | bla spc spoIIE-Q483A(1375-2481)-gfpF64L S65T | This study |
| pCM20 | bla spc spoIIE-D746A(1375-2481)-gfpF64L S65T | This study |
| pKC68 | bla spc amyE::malF-spoIIE | This study |
Plasmid construction.
pSBY25 is a derivative of pKL168 (24) containing the 3′ 941 bp of spoIIE linked to the coding sequence of gfp(mut2) by two in-frame codons, ctcgag (encoding Leu-Glu). Positions 1540 to 2481 of spoIIE were PCR amplified from PY79 genomic DNA with oligonucleotides that attached an EcoRI site to the 5′ end and a XhoI site to the 3′ end. The PCR fragment was digested by EcoRI and XhoI and ligated into pKL168 digested with the same enzymes. pKC70 was created by site-directed mutagenesis (Stratagene Quik Change) of pSBY25 to introduce the D686A mutation. pCM20 was created by site-directed mutagenesis (Stratagene Quik Change) of pPE1 (23) to introduce the D746A mutation. pCM12 was created by site-directed mutagenesis of pPE1 to introduce the Q483A mutation. PY79 transformants of pKC70, pCM19, and pCM12 were screened for a Spo− phenotype to generate the spoIIE-D686A-gfp kan, spoIIE-D746A-gfp spc, and spoIIE-Q483A-gfp spc constructs, respectively.
The in-frame deletion of region II of spoIIE under its endogenous promoter was achieved by a three-way ligation between two restriction-digested PCR fragments and the amyE integration vector pDG364 (21). The region stretching from 267 bp upstream of the spoIIE start codon (including the promoter) to bp 969 of the spoIIE coding sequence was PCR amplified from PY79 genomic DNA with oligonucleotides that added an EcoRI site to the 5′ end and a XhoI site to the 3′ end. The region from bp 1753 of the spoIIE coding sequence to 8 bp downstream of the spoIIE stop codon was also PCR amplified from PY79 genomic DNA with oligonucleotides that added a XhoI site to the 5′ end and a BamHI site to the 3′ end. These PCR fragments were digested with the appropriate restriction enzymes and cloned by three-way ligation into pDG364 cut with EcoRI and BamHI to create pKC17, which encodes region I of SpoIIE linked to region III by two residues, Leu-Glu.
Various alleles of spoIIE were placed under control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter Pspank by cloning them into the amyE integration vector pDR110 (gift from David Rudner, Harvard Medical School), which contains the Pspank promoter and its regulatory elements. Pspank is a modified version of Pspac (37) in which a second lac operator was placed 70.5 bp upstream (31). Pspank retains promoter strength similar to that of Pspac under inducing conditions but has significantly lower basal expression in the absence of an inducer (D. Rudner and C. van Ooij, unpublished data). The entire coding sequence of each allele of spoIIE was PCR amplified from the appropriate template (see below) with a forward oligonucleotide (KCO61, 5′ GGAGTCGACGGGACATAAGGAGGAACTACTATGGAAAAAGCAGAAAGAAG) that contained a SalI site and an optimal ribosome binding site (TAAGGAGGA) (36) and a reverse oligonucleotide (KCO35, 5′ GGAGCATGCCGGAAGCGTTATGAAATTTC) that contained a SphI site. The PCR fragments were digested with SalI and SphI and ligated into pDR110 digested with SalI and SphI. For pKC25 (spoIIE) PY79 genomic DNA was the PCR template, for pKC26 (spoIIE-ΔregII) pKC17 (see above) was the PCR template, for pKC27 (spoIIE-S361F) RL65 (12, 23) was the PCR template, for pKC29 (spoIIE-D746A) pKC6 (a derivative of pKC2 [7] that underwent site-directed mutagenesis to introduce the D746A mutation) was the PCR template, for pKC63 (spoIIE-Q483A) pPE27 (7) was the PCR template, and for pKC69 (spoIIE-ΔregI) pSDE95 (3) was the PCR template. For pKC64 (malF-spoIIE) pNK57 (23) was the PCR template, but in this case the reverse oligonucleotide (KCO115, 5′-GGAGCATGCTGAAATTTCTTGTTTGTTTTGAAAGATTGCCGG) contained a stop codon and an SphI site and sequence complementary to the last codons of the spoIIE gene. PCR amplification with KCO115 as the reverse primer amplified the malF-spoIIE coding sequence without the 3′ gfp fusion present in pNK57. A plasmid carrying Pspank-spoIIE-D686A was not constructed. Rather the B. subtilis strain (SB210) containing Pspank-spoIIE+ was transformed with pKC70 (described above), and transformants were screened for the crossover events that incorporated the point mutation into the inducible copy of spoIIE (creating KC552).
malF-spoIIE under its endogenous promoter was cloned into the amyE integration vector pLD30 (14) to create pKC68. The region stretching from 225 bp upstream of the spoIIE start codon (including the promoter) to the end of the malF-spoIIE coding sequence was PCR amplified from pNK57 with a forward oligonucleotide (KCO118) that added an EcoRV site to the 5′ end and a reverse oligonucleotide (KCO117) that added a stop codon and a BamHI site to the 3′ end. KCO117 is the same as KCO115 but with a BamHI site instead of a SalI site. The PCR fragment was digested with EcoRV and BamHI and ligated into pLD30 digested with EcoRV and BamHI.
Septation and localization assays.
For induction of spoIIE alleles during vegetative growth, cells were grown at 30°C in hydrolyzed casein growth medium (17) in the presence or absence of 0.5 mM IPTG. Cells were harvested for microscopy during early to mid-log phase. For examination of sporulating cells, cultures were grown at 37°C and induced to sporulate by resuspension (17). Sporulating cells were harvested for microscopy after shaking in a 37°C water bath for 1.5, 2, or 2.5 h. One-milliliter aliquots of either growing or sporulating cells were briefly centrifuged and resuspended in phosphate-buffered saline supplemented with 50 μM of the vital membrane dye TMA-DPH (Molecular Probes). Samples that were assayed only for polar septa were immobilized on glass microscope slides with poly-l-lysine coated glass coverslips. Samples that were assayed for localization of SpoIIE-GFP derivative proteins were applied to a chambered slide (VWR Scientific) filled with a bed of medium (T base [17] for vegetative cells and resuspension medium [17] for sporulating cells) containing 1% agarose. Fluorescence microscopy was carried out as previously described (10). Random fields of cells were scored for the presence or absence of polar septa.
Immunoblotting.
Cell pellets from 1 ml of a sporulating culture or 2 ml of a vegetative culture were frozen at −80°C. Extracts were prepared by resuspending the pellets in lysis buffer (10 mM Tris, pH 8.0, 10 mM MgCl2, 0.3 mg/ml phenylmethylsulfonyl fluoride, 0.5 mg/ml lysozyme, 0.1 mg/ml DNase I) in a volume proportional to the optical density at 600 nm of the culture at the time the samples were harvested (0.1 ml lysis buffer/1 optical density unit) and incubated in a 37°C water bath for 10 min. Lysates were mixed 5:1 (vol/vol) with 5× sodium dodecyl sulfate (SDS) sample buffer (60 mM Tris, pH 6.8, 25% glycerol, 2% SDS, 0.1% bromophenol blue, 14.4 mM 2-mercaptoethanol) and loaded onto a 12% polyacrylamide-0.8% bis gel. The gels were run for ∼2 h at 150 V with SDS running buffer (50 mM Tris, 380 mM glycine, 4 mM SDS). The protein was electroblotted to an Immobilon-P membrane (Millipore) for 1 h at 200 mA (transfer buffer was 25 mM Tris, 193 mM glycine, 10% methanol). Immobilon-P membranes were blocked in 5% nonfat milk in Tris-buffered saline-0.5% Tween 20 and probed with affinity-purified rabbit anti-GFP (1:10,000) (35), affinity-purified rat anti-SpoIIE (1:4,000) (4), or affinity-purified rabbit anti-σA (1:10,000) (provided by M. Fujita). Primary rabbit antibody was detected by horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Bio-Rad) followed by treatment with Supersignal substrate (Pierce) and exposure to Biomax MR film (Kodak). Primary rat antibody was detected by alkaline phosphatase-conjugated goat anti-rat immunoglobulin G (Promega) followed by treatment with BCIP/NBT color development substrate (Promega).
RESULTS AND DISCUSSION
Region I of SpoIIE is dispensable for polar division.
To carry out our investigation, we built a series of strains harboring two copies of ftsZ and an inducible copy of wild-type or mutated forms of spoIIE and monitored polar septum formation during growth in response to the addition of inducer (IPTG). In confirmation of previous results (6), engineered cells harboring wild-type spoIIE produced polar septa at a frequency of about 21% in the presence of inducer and 0.7% in its absence. To investigate the contribution of region I to polar septation, we used a chimeric gene in which the coding sequence for the 10 membrane-spanning segments was replaced with the coding sequence for the first two membrane-spanning segments of the Escherichia coli integral membrane protein MalF (23). The results show that cells producing the MalF-SpoIIE hybrid protein produced polar septa as efficiently as did cells producing the wild-type protein (Fig. 1B and Table 2).
TABLE 2.
Summary of polar septum formation and localization of the SpoIIE mutant proteinsa
| spoIIE allele | % (n) of cells with polar septa
|
Localization of GFP-tagged protein during:
|
||
|---|---|---|---|---|
| Induced | Uninduced | Growth | Sporulation | |
| WT (SB210) | 21.3 (979) | 0.7 (806) | ++ | ++ |
| malF-spoIIE (KC501) | 20.3 (1,044) | 0.6 (503) | ++ | ++ |
| spoIIE-Δreg I (KC541) | 19.0 (830) | 0.4 (607) | +/− | +/− |
| spoIIE-Δreg II (SB211) | 0.9 (959) | 0.8 (635) | − | − |
| spoIIE-S361F (SB250) | 2.4 (930) | 0.7 (600) | +/− | + |
| spoIIE-Q483A (KC500) | 20.0 (607) | 1.1 (536) | ++ | ++ |
| spoIIE-D746A (SB214) | 19.7 (772) | 1.2 (516) | ND | ++ |
| spoIIE-D686A (KC552) | 2.1 (327) | 0.7 (277) | +/− | +/− |
The percentages of cells with a polar septum in growing cultures either induced or uninduced to express the indicated allele of spoIIE were determined as described in Materials and Methods. The localization of the GFP-tagged mutant proteins to division sites is summarized here from the data in Fig. 3 (third column) and Fig. 4 (fourth column) and data not shown. ND, not determined. ++, normal localization; +/−, partial localization; −, no specific localization. WT, wild type.
Next, we asked whether a truncated SpoIIE protein that simply lacked region I (and hence was a cytoplasmic [soluble] protein rather than an integral membrane protein [3]) would promote asymmetric division. Surprisingly, our data show that cells producing the truncated protein, SpoIIEΔregI, underwent asymmetric division about as efficiently as cells producing full-length SpoIIE (Fig. 1B and Table 2). We conclude that region I is dispensable for asymmetric division and therefore that direct interaction between SpoIIE and the membrane is not required for its interaction with the division machinery.
Region II contributes to polar septation.
To investigate the role of region II in polar division, we tested the effect of an in-frame deletion of its entire coding region (eliminating codons 324 to 584) and the effects of point mutants, S361F (corresponding to the classic spoIIE48 mutation) (5) and Q483A (7), that are blocked in sporulation. Production of the protein from which region II had been removed, SpoIIEΔregII, failed to promote a measurable level of polar septation (Fig. 1B and Table 2). Similarly, synthesis of the SpoIIES361F mutant protein caused only a small increase in polar septation above the background observed in cells that were not treated with inducer (Fig. 1B and Table 2). In contrast, cells producing SpoIIEQ483A underwent asymmetric division as efficiently as did cells producing the wild-type protein (Fig. 1B and Table 2). We conclude that region II and, in particular, some feature of region II that is altered by the S361F substitution play a critical role in facilitating polar division.
Region III also contributes to polar division.
We next investigated the role of the region III phosphatase domain of SpoIIE in polar division. A SpoIIE mutant with an alanine substitution at the catalytic aspartate residue D746 is enzymatically inactive in dephosphorylating SpoIIAA (1; K. Carniol and R. Losick, unpublished results). The results show that cells producing SpoIIED746A were as proficient as the wild type in promoting polar division (Fig. 1B and Table 2), indicating that SpoIIE's enzymatic activity is not important for polar division.
Interestingly, however, another substitution in region III, D686A, which also blocks phosphatase activity (N. King and R. Losick, unpublished results), markedly diminished the capacity of the protein to cause polar division (Fig. 1B and Table 2). We conclude that the phosphatase function of SpoIIE does not contribute to its role in polar division. Nonetheless, at least one residue (Asp686) in region III makes contributions both to enzymatic activity and to asymmetric cytokinesis.
Effects on SpoIIE stability.
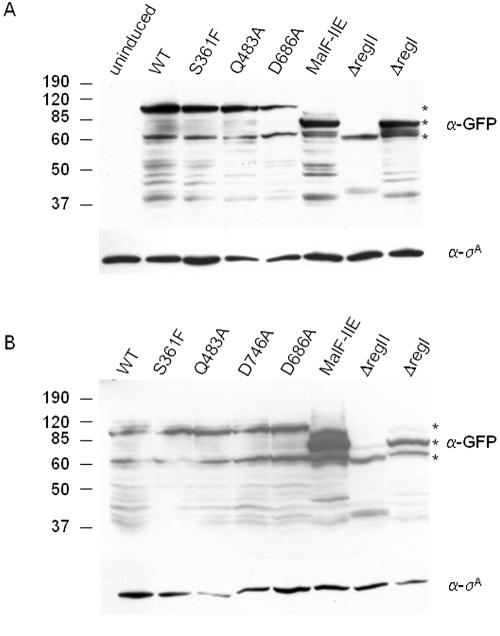
It seemed possible that some or all of the effects observed above were an indirect consequence of the alteration in question to the susceptibility of SpoIIE to proteolysis, resulting in reduced amounts of the protein. To investigate this, we constructed a series of gene fusions in which the coding sequence for GFP was joined in-frame to the 3′ end of spoIIE and its mutant alleles in constructs in which transcription was induced in response to IPTG. Thus, each protein was tagged with an identical epitope (GFP) that could be used to monitor protein levels upon induction. A minor complication was that the GFP tag itself moderately reduced the ability of the proteins to promote polar septation. Thus, the SpoIIE-GFP fusion protein promoted the formation of polar septa at a frequency of 12% compared to 21% for untagged SpoIIE. Nevertheless, the ability of SpoIIE-GFP to promote polar division was robust (well above background), and the GFP tag seemed to affect all of the mutant proteins tested in a commensurate manner (data not shown).
Immunoblot analysis was carried out with lysates from IPTG-treated cells using anti-GFP antibodies. A background of proteolytic fragments was observed for all of the fusion proteins. Nonetheless, the levels of accumulation of SpoIIES361F-GFP, SpoIIEQ483A-GFP, SpoIIED686A-GFP, MalF-SpoIIE-GFP, and SpoIIEΔregI-GFP were approximately the same as that for SpoIIE-GFP (Fig. 2A). These results indicate that the differences observed between the various SpoIIE mutants in their ability to form a polar septum were due to the nature of the alteration and not to differences in the levels of the proteins. Only in the case of SpoIIEΔregII-GFP was the level of accumulation of the fusion protein significantly diminished. Our conclusion that region II is important for promoting polar division is not in jeopardy, however, because the S361F substitution in region II also impaired polar division but had no measurable effect on the stability of the fusion protein (Fig. 2A). Results similar to those of Fig. 2A were also obtained using anti-SpoIIE antibodies instead of anti-GFP antibodies (see Fig. S1 in the supplemental material).
FIG. 2.
Immunoblots of GFP-tagged SpoIIE derivatives during vegetative growth and sporulation with anti-GFP antibodies. (A) SDS-polyacrylamide gel electrophoresis Western blot of lysates prepared from vegetative cells uninduced (SB220) or induced with 0.5 mM IPTG (SB220 [wild-type SpoIIE-GFP], SB414 [SpoIIES361F-GFP], KC507 [SpoIIEQ483A-GFP], KC552 [SpoIIED686A-GFP], KC506 [MalF-SpoIIE-GFP], SB221 [SpoIIEΔregII-GFP], KC542 [SpoIIEΔregI-GFP]) and probed with anti-GFP antibodies (upper panel) and anti-σA antibodies (lower panel). (B) SDS-polyacrylamide gel electrophoresis Western blot of lysates prepared from cells harvested 2 h after the start of sporulation (KC544 [wild-type SpoIIE-GFP], KC545 [SpoIIES361F-GFP], KC546 [SpoIIEQ483A-GFP], KC547 [SpoIIED746A-GFP], KC554 [SpoIIED686A-GFP], KC538 [MalF-SpoIIE-GFP], KC548 [SpoIIEΔregII-GFP], and KC549 [SpoIIEΔregI-GFP]) and probed with anti-GFP antibodies (upper panel) and anti-σA antibodies (lower panel). In each upper panel the top asterisk indicates the position on the gel of full-length SpoIIE-GFP, the middle asterisk marks SpoIIEΔregI-GFP, and the bottom asterisk marks SpoIIEΔregII-GFP. WT, wild type. Numbers at left represent molecular masses in kilodaltons.
Effects on subcellular localization during growth.
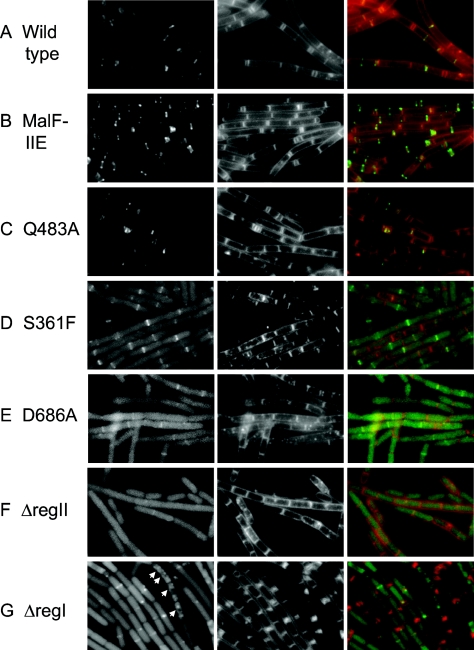
Next, we took advantage of the GFP tags to investigate whether the effect of SpoIIE in promoting polar division during growth was correlated with its interaction with the cytokinetic machinery. We assessed this correlation by observing the localization pattern of fluorescence exhibited by each of the fusion proteins. As expected, GFP fused to the wild-type protein localized to septa and exhibited ring and spiral-like structures at medial and polar positions in the cell in keeping with previous observations (6) (Fig. 3A). MalF-SpoIIE-GFP and SpoIIEQ483A-GFP, both of which promoted polar septum formation as efficiently as wild-type SpoIIE-GFP, showed the same localization pattern as SpoIIE-GFP (Fig. 3B and C). Conversely, SpoIIES361F-GFP, SpoIIEΔregII-GFP, and SpoIIED686A-GFP, which were all impaired in promoting polar septation, did not exhibit discrete patterns of subcellular localization. SpoIIEΔregII-GFP, which was severely impaired in promoting polar septation, appeared completely diffuse and unlocalized (Fig. 3F). SpoIIES361F-GFP and SpoIIED686A-GFP, which were strongly, but not completely, impaired in promoting polar septation, appeared largely diffuse, but occasional enrichment at potential sites of division could be seen (Fig. 3D and E). The enrichment of the GFP signal was observed only at medial division sites in these mutants, consistent with the observation that they formed polar septa inefficiently. We conclude that the capacity of SpoIIE to promote polar division correlates significantly with its capacity to associate with the cytokinetic machinery of the cell.
FIG.3.
Localization of GFP-tagged derivatives of SpoIIE mutants during vegetative growth. SB220 (wild-type SpoIIE) (A), KC506 (MalF-SpoIIE) (B), KC507 (SpoIIEQ483A) (C), SB414 (SpoIIES361F) (D), KC552 (SpoIIED686A) (E), SB221 (SpoIIEΔregII) (F), and KC542 (SpoIIEΔregI) (G) were grown in hydrolyzed casein medium supplemented with 0.5 mM IPTG for 3 h (see Materials and Methods), and the GFP (left panels) and membrane stain (middle panels) were visualized by fluorescence microscopy. Right panels are overlays of GFP (green) and membrane stain (red). Arrowheads in panel G mark punctate spots of fluorescence that may indicate spiral-like structures.
A striking and interesting exception to this correlation was the case of SpoIIEΔregI-GFP, which promoted polar septation as efficiently as did SpoIIE-GFP but did not exhibit a discrete pattern of subcellular localization (Fig. 3G). Rather, it exhibited a diffuse pattern of fluorescence, although in some cells enrichment at a division site, either medial or polar, could be seen. Also, in some cells a punctate pattern could be seen, which could indicate an association with a spiral of FtsZ (arrowheads, Fig. 3G). We interpret these results to indicate that region I or membrane association is necessary for a stable association of SpoIIE with the cytokinetic machinery. Nonetheless, as long as regions II and III are intact, SpoIIE can interact, if only transiently, with FtsZ, and this interaction is sufficient to promote polar division. The localization during growth of each GFP-tagged protein is summarized in Table 2.
Interestingly, mutants of SpoIIE-GFP that failed to localize at division sites demonstrated a diffuse pattern of fluorescence in the cytoplasm rather than uniform membrane localization, as might have been expected for mutants with an intact membrane region, such as SpoIIES361F-GFP and SpoIIED686A-GFP. The immunoblot analysis argues that the cytoplasmic fluorescence was not a result of degradation and release of soluble GFP-containing fragments because similar degradation patterns were observed for the localized wild-type and mutant proteins and the unlocalized mutants (Fig. 2A). A possible explanation for the apparent cytoplasmic localization of the mutant fusion proteins comes from experiments in which cells were depleted of FtsZ. The results show that wild-type SpoIIE-GFP also exhibited diffuse cytoplasmic fluorescence in cells deficient for the cytokinetic protein (see Fig. S2 in the supplemental material). We hypothesize that interaction with FtsZ is required for proper membrane insertion of SpoIIE and that the mislocalization of SpoIIES361F-GFP and SpoIIED686A-GFP during vegetative growth was a consequence of impaired interaction with FtsZ.
Effects during sporulation.
As explained above, the contribution of SpoIIE to polar division during sporulation is partially masked by a sporulation-specific increase in FtsZ levels. We nevertheless attempted to investigate the effects of the mutant proteins described above during sporulation by creating strains containing the mutant alleles under the control of the normal sporulation-specific promoter for spoIIE in place of the wild-type spoIIE gene. To uncouple the contribution of spoIIE to polar division from its role in activation of σF, a deletion of the spoIIA operon was introduced into the strains, thereby removing the gene for σF as well as the gene for the substrate (SpoIIAA-P) of the SpoIIE phosphatase. For each mutant strain we determined the number of cells that had formed at least one polar septum (cells that lack σF activity are disporic and frequently produce two polar septa) (20). Under our conditions, about 60% of the cells producing wild-type SpoIIE formed at least one polar septum by 150 min after the start of sporulation. Cells lacking the spoIIE gene were only modestly impaired in polar division, with about 30% of the mutant cells exhibiting a polar septum by 150 min. Therefore, as expected, sporulation provided only a narrow range within which to assess the effects of the mutant SpoIIE proteins on polar division. The spoIIE-D746A strain and the spoIIE-Q483A strain behaved similarly to the wild-type control with respect to polar septation during sporulation, consistent with their wild-type-like behavior in the vegetative growth assay. The spoIIE-S361F strain appeared to be delayed in polar septation—fewer cells had formed polar septa after 90 min compared to the wild-type control. However, by 150 min the same percentage of SpoIIES361F-producing cells as of the wild-type control had formed polar septa. The strains that appeared the most severely impaired in polar septum formation were the spoIIE-ΔregI, spoIIE-ΔregII, malF-spoIIE, spoIIE-D686A, and spoIIEΔ strains. These results are summarized in Fig. 1B (lower graph).
The defects of SpoIIES361F, SpoIIED686A, and SpoIIEΔregII in promoting polar septation during sporulation are consistent with the defects seen during the vegetative growth assay for polar septation. Interestingly, SpoIIEΔregI and MalF-SpoIIE promoted polar septation with high efficiency during vegetative growth but were somewhat impaired in promoting polar septation during sporulation. This could indicate that there are subtle aspects to the requirements for polar septum formation during sporulation that are not fully mimicked in cells engineered to produce SpoIIE during growth.
Revisiting the role of region I in the localization of SpoIIE to the polar septum during sporulation.
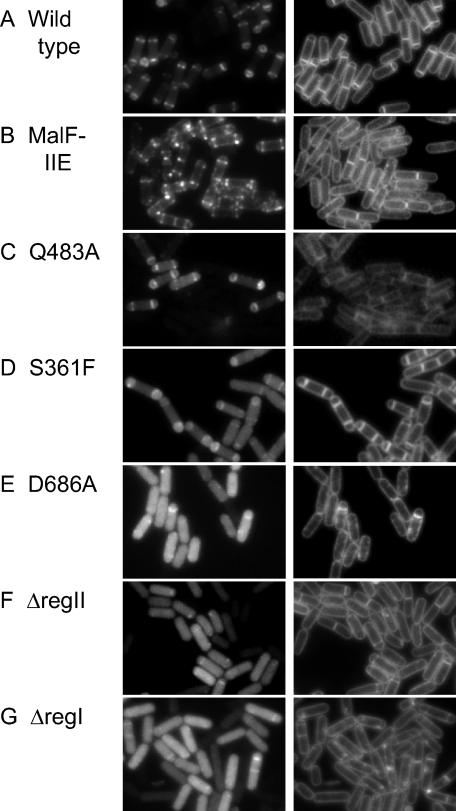
The finding that a mutant form of SpoIIE in which region I was replaced with the first two membrane-spanning segments of MalF (MalF-SpoIIE) localized to septa with high efficiency during growth prompted us to reinvestigate the subcellular localization of the hybrid protein during sporulation. In previous work we had observed a pattern of localization to the cytoplasmic membrane in sporulating cells producing the MalF-SpoIIE-GFP hybrid protein. Such cells activate σF prematurely and at an abnormally high level and are blocked in asymmetric division (23). It therefore seemed possible that the observed localization of the hybrid protein to the cytoplasmic membrane was an indirect consequence of the effect of premature σF activity blocking the formation of polar septa. Accordingly we introduced a spoIIA deletion mutation into a strain in which production of MalF-SpoIIE-GFP was under sporulation control. In such cells MalF-SpoIIE-GFP localized to polar division sites rather than to the cytoplasmic membrane (Fig. 4B). Evidently a heterologous membrane-spanning segment can substitute for region I in allowing SpoIIE to localize to the polar septum. Only when production of polar septa is blocked (e.g., by abnormally high levels of σF activity) does the hybrid membrane protein localize, by default, to the cytoplasmic membrane.
FIG. 4.
Localization of GFP-tagged derivatives of SpoIIE mutants during sporulation in spoIIAΔ backgrounds. KC544 (wild-type SpoIIE) (A), KC538 (MalF-SpoIIE) (B), KC546 (SpoIIEQ483A) (C), KC545 (SpoIIES361F) (D), KC554 (SpoIIED686A) (E), KC548 (SpoIIEΔregII) (F), and KC549 (SpoIIEΔregI) (G) were sporulated by resuspension (see Materials and Methods), and the GFP (left panels) and membrane stain (right panels) were visualized by fluorescence microscopy 2 h after the initiation of sporulation.
When region I was removed but not replaced with a heterologous membrane-spanning segment (i.e., SpoIIEΔregI-GFP), a diffuse pattern of localization throughout the cytoplasm was observed (as had been seen when SpoIIEΔregI-GFP was produced during growth) with occasional enrichment at sites of polar septation (Fig. 4G). We conclude that the membrane-spanning segments of region I help to stabilize the association of SpoIIE with the septum and/or to FtsZ rings but play no measurable role in helping SpoIIE to distinguish septal membranes from the cytoplasmic membrane.
Finally, we address the question of why MalF-SpoIIE-GFP causes σF to become activated prematurely and at abnormally high levels. A possible explanation is provided by the immunoblot analysis of Fig. 2B. As can be seen in Fig. 2B, MalF-SpoIIE-GFP accumulated to a significantly higher level during sporulation than did a fusion of GFP to wild-type SpoIIE (or fusions to any of the other proteins examined). It is known that the timing and level of activation of σF are sensitive to the ratio of SpoIIE to SpoIIAB, which have opposing roles in determining the phosphorylation state of SpoIIAA (19). We therefore suppose that altering the N-terminal region of SpoIIE (by replacing region I with part of the membrane-spanning region of MalF) had stabilized the protein or otherwise led to its accumulation at higher-than-normal levels. This interpretation is consistent with the observation that overexpression of spoIIE causes predivisional σF activity (2), as do mutants of spoIIE that stabilize the protein (13).
Effects of alterations to regions II and III on subcellular localization during sporulation.
We also examined the subcellular localization during sporulation of the other mutant proteins investigated in this study. SpoIIED686A-GFP and SpoIIEΔregII-GFP behaved similarly to SpoIIEΔregI-GFP: they appeared largely in the cytoplasm with occasional instances of enrichment at the polar sites (Fig. 4E and F). In contrast, SpoIIEQ483A-GFP was largely indistinguishable from SpoIIE-GFP in its pattern of localization. In toto, these results are consistent with the subcellular localization results that we had obtained with these fusion proteins during growth and reinforce the view that regions II and III play a critical role in the proper localization of SpoIIE.
We do, however, note one discrepancy. SpoIIES361F-GFP exhibited a normal (wild-type) pattern of localization in sporulating cells (as reported previously), even though (as we have seen) it was defective in localizing to division sites when produced in growing cells. We suppose that the S361F substitution weakens but does not totally abolish the interaction of region II with FtsZ. Perhaps the accumulation of FtsZ to high levels during sporulation helps to compensate for this weakened interaction. The localization during sporulation of each GFP-tagged protein is summarized in Table 2.
Summary.
Taking advantage of a robust system for inducing asymmetric division during growth in a SpoIIE-dependent manner, we have tested a battery of mutants to identify features of the sporulation protein that are important for its ability to promote polar septum formation. Our findings complement and extend those of Lucet et al., who obtained biochemical evidence for a direct interaction between region II and FtsZ (28). In addition, our results implicate region III, although not the phosphatase function of region III, in polar division. Interestingly, and unexpectedly, our data indicate that the multipass, membrane-spanning domain of SpoIIE plays little or no role in polar division. Thus, our results are inconsistent with models of SpoIIE action in which the sporulation protein facilitates polar Z-ring formation by anchoring the spiral intermediate to the inside surface of the cytoplasmic membrane. Finally, we have provided an explanation for the puzzling behavior of a chimeric form of SpoIIE (MalF-SpoIIE-GFP) that was known to cause excessive and premature σF activation. Important challenges for the future will be to elucidate the detailed nature of the interaction of the interaction of regions II and III with FtsZ and how this interaction promotes the switch to polar Z-ring formation.
Supplementary Material
Acknowledgments
We thank M. Fujita for anti-σA antibodies and D. Rudner for anti-GFP antibodies and pDR110.
K.C. and N.K. were supported by National Science Foundation predoctoral fellowships. S.B.-Y. was a postdoctoral fellow of the Human Frontier Science Program (HFSP). This work was supported by NIH grant GM18458 to R.L.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Adler, E., A. Donella-Deana, F. Arigoni, L. A. Pinna, and P. Stragler. 1997. Structural relationship between a bacterial developmental protein and eukaryotic PP2C protein phosphatases. Mol. Microbiol. 23:57-62. [DOI] [PubMed] [Google Scholar]
- 2.Arigoni, F., L. Duncan, S. Alper, R. Losick, and P. Stragier. 1996. SpoIIE governs the phosphorylation state of a protein regulating transcription factor σF during sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 93:3238-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arigoni, F., A. M. Guerout-Fleury, I. Barak, and P. Stragier. 1999. The SpoIIE phosphatase, the sporulation septum and the establishment of forespore-specific transcription in Bacillus subtilis: a reassessment. Mol. Microbiol. 31:1407-1415. [DOI] [PubMed] [Google Scholar]
- 4.Arigoni, F., K. Pogliano, C. D. Webb, P. Stragier, and R. Losick. 1995. Localization of protein implicated in establishment of cell type to sites of asymmetric division. Science 270:637-640. [DOI] [PubMed] [Google Scholar]
- 5.Barak, I., and P. Youngman. 1996. SpoIIE mutants of Bacillus subtilis comprise two distinct phenotypic classes consistent with a dual functional role for the SpoIIE protein. J. Bacteriol. 178:4984-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Yehuda, S., and R. Losick. 2002. Asymmetric cell division in B. subtilis involves a spiral-like intermediate of the cytokinetic protein FtsZ. Cell 109:257-266. [DOI] [PubMed] [Google Scholar]
- 7.Carniol, K., P. Eichenberger, and R. Losick. 2004. A threshold mechanism governing activation of the developmental regulatory protein σF in Bacillus subtilis. J. Biol. Chem. 279:14860-14870. [DOI] [PubMed] [Google Scholar]
- 8.Coppolecchia, R., H. DeGrazia, and C. P. Moran, Jr. 1991. Deletion of spoIIAB blocks endospore formation in Bacillus subtilis at an early stage. J. Bacteriol. 173:6678-6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dworkin, J., and R. Losick. 2001. Differential gene expression governed by chromosomal spatial asymmetry. Cell 107:339-346. [DOI] [PubMed] [Google Scholar]
- 10.Eichenberger, P., P. Fawcett, and R. Losick. 2001. A three-protein inhibitor of polar septation during sporulation in Bacillus subtilis. Mol. Microbiol. 42:1147-1162. [DOI] [PubMed] [Google Scholar]
- 11.Errington, J., R. A. Daniel, and D. J. Scheffers. 2003. Cytokinesis in bacteria. Microbiol. Mol. Biol. Rev. 67:52-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Errington, J., and J. Mandelstam. 1986. Use of a lacZ gene fusion to determine the dependence pattern of sporulation operon spoIIA in spo mutants of Bacillus subtilis. J. Gen. Microbiol. 132:2967-2976. [DOI] [PubMed] [Google Scholar]
- 13.Feucht, A., L. Abbotts, and J. Errington. 2002. The cell differentiation protein SpoIIE contains a regulatory site that controls its phosphatase activity in response to asymmetric septation. Mol. Microbiol. 45:1119-1130. [DOI] [PubMed] [Google Scholar]
- 14.Garsin, D. A., D. M. Paskowitz, L. Duncan, and R. Losick. 1998. Evidence for common sites of contact between the antisigma factor SpoIIAB and its partners SpoIIAA and the developmental transcription factor σF in Bacillus subtilis. J. Mol. Biol. 284:557-568. [DOI] [PubMed] [Google Scholar]
- 15.Gholamhoseinian, A., Z. Shen, J. J. Wu, and P. Piggot. 1992. Regulation of transcription of the cell division gene ftsA during sporulation of Bacillus subtilis. J. Bacteriol. 174:4647-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzy-Treboul, G., C. Karmazyn-Campelli, and P. Stragier. 1992. Developmental regulation of transcription of the Bacillus subtilis ftsAZ operon. J. Mol. Biol. 224:967-979. [DOI] [PubMed] [Google Scholar]
- 17.Harwood, C. R., and S. M. Cutting (ed.). 1990. Molecular biological methods for Bacillus. John Wiley & Sons, New York, N.Y.
- 18.Hilbert, D. W., V. K. Chary, and P. J. Piggot. 2004. Contrasting effects of σE on compartmentalization of σF activity during sporulation of Bacillus subtilis. J. Bacteriol. 186:1983-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilbert, D. W., and P. J. Piggot. 2004. Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol. Mol. Biol. Rev. 68:234-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Illing, N., and J. Errington. 1991. Genetic regulation of morphogenesis in Bacillus subtilis: roles of σE and σF in prespore engulfment. J. Bacteriol. 173:3159-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karmazyn-Campelli, C., L. Fluss, T. Leighton, and P. Stragier. 1992. The spoIIN279(ts) mutation affects the FtsA protein of Bacillus subtilis. Biochimie 74:689-694. [DOI] [PubMed] [Google Scholar]
- 22.Khvorova, A., L. Zhang, M. L. Higgins, and P. J. Piggot. 1998. The spoIIE locus is involved in the Spo0A-dependent switch in the location of FtsZ rings in Bacillus subtilis. J. Bacteriol. 180:1256-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King, N., O. Dreesen, P. Stragier, K. Pogliano, and R. Losick. 1999. Septation, dephosphorylation, and the activation of σF during sporulation in Bacillus subtilis. Genes Dev. 13:1156-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin, P. A., and A. D. Grossman. 1998. Cell cycle and sporulation in Bacillus subtilis. Curr. Opin. Microbiol. 1:630-635. [DOI] [PubMed] [Google Scholar]
- 25.Levin, P. A., and R. Losick. 1996. Transcription factor Spo0A switches the localization of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes Dev. 10:478-488. [DOI] [PubMed] [Google Scholar]
- 26.Levin, P. A., R. Losick, P. Stragier, and F. Arigoni. 1997. Localization of the sporulation protein SpoIIE in Bacillus subtilis is dependent upon the cell division protein FtsZ. Mol. Microbiol. 25:839-846. [DOI] [PubMed] [Google Scholar]
- 27.Lucet, I., R. Borriss, and M. D. Yudkin. 1999. Purification, kinetic properties, and intracellular concentration of SpoIIE, an integral membrane protein that regulates sporulation in Bacillus subtilis. J. Bacteriol. 181:3242-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucet, I., A. Feucht, M. D. Yudkin, and J. Errington. 2000. Direct interaction between the cell division protein FtsZ and the cell differentiation protein SpoIIE. EMBO J. 19:1467-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutkenhaus, J., and S. G. Addinall. 1997. Bacterial cell division and the Z ring. Annu. Rev. Biochem. 66:93-116. [DOI] [PubMed] [Google Scholar]
- 30.Margolin, W. 2000. Themes and variations in prokaryotic cell division. FEMS Microbiol. Rev. 24:531-548. [DOI] [PubMed] [Google Scholar]
- 31.Muller, J., S. Oehler, and B. Muller-Hill. 1996. Repression of lac promoter as a function of distance, phase and quality of an auxiliary lac operator. J. Mol. Biol. 257:21-29. [DOI] [PubMed] [Google Scholar]
- 32.Pan, Q., D. A. Garsin, and R. Losick. 2001. Self-reinforcing activation of a cell-specific transcription factor by proteolysis of an anti-sigma factor in B. subtilis. Mol. Cell 8:873-883. [DOI] [PubMed] [Google Scholar]
- 33.Piggot, P. J., and R. Losick. 2002. Sporulation genes and intercompartmental regulation, p. 483-518. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relative: from genes to cells. ASM Press, Washington, D.C.
- 34.Pogliano, J., N. Osborne, M. D. Sharp, A. Abanes-De Mello, A. Perez, Y. L. Sun, and K. Pogliano. 1999. A vital stain for studying membrane dynamics in bacteria: a novel mechanism controlling septation during Bacillus subtilis sporulation. Mol. Microbiol. 31:1149-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudner, D. Z., and R. Losick. 2002. A sporulation membrane protein tethers the pro-σK processing enzyme to its inhibitor and dictates its subcellular localization. Genes Dev. 16:1007-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vellanoweth, R. L., and J. C. Rabinowitz. 1992. The influence of ribosome-binding-site elements on translational efficiency in Bacillus subtilis and Escherichia coli in vivo. Mol. Microbiol. 6:1105-1114. [DOI] [PubMed] [Google Scholar]
- 37.Yansura, D. G., and D. J. Henner. 1984. Use of the Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 81:439-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.York, K., T. J. Kenney, S. Satola, C. P. Moran, Jr., H. Poth, and P. Youngman. 1992. Spo0A controls the σA-dependent activation of Bacillus subtilis sporulation-specific transcription unit spoIIE. J. Bacteriol. 174:2648-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youngman, P., J. B. Perkins, and R. Losick. 1984. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid 12:1-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.