Abstract
Both gram-negative and gram-positive bacteria possess protein tyrosine phosphatases (PTPs) with a catalytic Cys residue. In addition, many gram-positive bacteria have acquired a new family of PTPs, whose first characterized member was CpsB from Streptococcus pneumoniae. Bacillus subtilis contains one such CpsB-like PTP, YwqE, in addition to two class II Cys-based PTPs, YwlE and YfkJ. The substrates for both YwlE and YfkJ are presently unknown, while YwqE was shown to dephosphorylate two phosphotyrosine-containing proteins implicated in UDP-glucuronate biosynthesis, YwqD and YwqF. In this study, we characterize YwqE, compare the activities of the three B. subtilis PTPs (YwqE, YwlE, and YfkJ), and demonstrate that the two B. subtilis class II PTPs do not dephosphorylate the physiological substrates of YwqE.
Protein tyrosine phosphorylation, mediated by protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs), in Eukarya, in which it participates in regulating important cellular processes, such as signal transduction and oncogenesis, has been extensively studied (12, 21). Protein phosphorylation on tyrosine residues in bacteria was discovered more recently (7) and initially reported to be involved in the regulation of extracellular polysaccharide synthesis and assembly (5, 18, 28, 29). Recently a PTK from Escherichia coli, named Etk, was found to phosphorylate the sigma factor RpoH and the anti-sigma factor RseA, both implicated in the heat shock response (15). Several bacterial UDP-glucose dehydrogenases (the enzymes that convert UDP-glucose to UDP-glucuronate) were also found to be tyrosine phosphorylated by PTKs in both E. coli (10) and Bacillus subtilis (16). Tyrosine phosphorylation of all these proteins regulates their respective physiological activities.
Genes encoding bacterial PTPs are often found within the same operon as PTK-encoding genes. Bacterial PTPs dephosphorylate their cognate autophosphorylated PTKs and PTK substrates (16, 19, 27, 28). In gram-negative bacteria, most PTPs are homologues of Cys-based class II eukaryotic PTPs, often referred to as low-molecular-weight phosphotyrosine (pTyr) protein phosphatases (LMPTPs) (1, 5, 24, 27), while gram-positive bacteria also contain another class of PTPs in addition to LMPTPs, which resemble the phosphoesterase domain of DNA polymerase and histidinol phosphate (PHP) phosphoesterases (3). The first described representative of this new family was the Mn2+-dependent PTP CpsB from Streptococcus pneumoniae (4, 19), which is able to dephosphorylate its cognate PTK, CpsD, and is involved in control of capsular composition and virulence (4, 18, 20).
The B. subtilis operon ywqCDEF encodes the PTK YwqD and its modulator, YwqC, the PTP YwqE, and their substrate, the UDP-glucose dehydrogenase YwqF (16). The PTP YwqE, which is able to dephosphorylate both the autophosphorylated YwqD and its substrate, YwqF, exhibits no homology to LMPTPs but is similar to the Mn2+-dependent PTP CpsB from Streptococcus pneumoniae (19). While bacterial LMPTPs have been well characterized, little is known about the activities, reaction mechanisms, and substrate specificities of CpsB-like PTPs. In addition to YwqE, B. subtilis possesses two LMPTPs, YwlE and YfkJ, which we recently characterized biochemically (L. Musumeci et al., unpublished data). In the present study, we analyze the kinetic properties of B. subtilis YwqE. We also find that the LMPTPs YwlE and YfkJ are very inefficient at dephosphorylating the physiological substrates for YwqE, phospho-YwqD and phospho-YwqF in vitro and in vivo.
MATERIALS AND METHODS
Bioinformatic and statistical analyses.
The comparison between amino acid sequences was performed using the ClustalW program (http://www.ebi.ac.uk/clustalw). Curve fitting and statistical analyses were performed using Prism software (GraphPad Prism version 4.00 for Mac OS X; GraphPad Software, San Diego, CA).
Reagents.
Biomol Green was purchased from Biomol Research Labs (Plymouth Meeting, PA). pTyr peptides were purchased from Celtek Peptides (Nashville, TN); the phosphoserine (pSer) and phosphothreonine (pThr) peptides are commercially available from Upstate (Charlottesville, VA). p-Nitro-phenyl-phosphate disodium (pNPP) and all other reagents were from Sigma (St. Louis, MO) or ICN Biomedicals (Irvine, CA) unless otherwise stated.
Oligonucleotide-directed mutagenesis.
To obtain a set of mutant YwqE proteins, each containing one critical residue replaced by alanine, we performed PCR-based mutagenesis on the ywqE gene from B. subtilis genomic DNA. Mutations in codons 3, 5, and 7 were introduced in a PCR with mutagenic forward primers and the reverse primer YwqE−, which produced mutated full-length ywqE alleles in a single step. For codons 42, 136, 194, and 196, two partially overlapping mutagenic primers were used in separate PCRs, one with the YwqE+ forward primer to amplify the ywqE region upstream of (and including) the mutated codon and the other with the YwqE− reverse primer to amplify the ywqE region downstream of (and including) the mutated codon. The two PCR products were then mixed in the third PCR with the YwqE+ and YwqE− primers to produce full-length mutated versions of ywqE. All final PCR products were integrated in the pQE-30 vector (QIAGEN Inc., Valencia, CA), between the BamHI and the PstI restriction sites, allowing the synthesis of six-His-tagged proteins. All mutations were confirmed by DNA sequencing.
Protein expression and purification.
All wild-type proteins were expressed in an E. coli M15 or BL21 strain transformed with plasmids bearing the corresponding genes cloned on pQE30-derived plasmids for N-terminal six-His-tagged YwqD, YwqF, and YwqE (16), pET15b-derived plasmid for N-terminal six-His-tagged YfkJ and YwlE, and pEGST- (14) and pGEX-2T-derived plasmids, respectively, for glutathione S-transferase (GST)-YfkJ and GST-YwqC-NCter (16). Since mutated versions of six-His-tagged YwqE created in this study had reduced solubility, the proteins in pQE30-derived plasmids were overexpressed in the chaperone overproducing strain M15[pREP4-groESL] (2). Protein synthesis was induced by 1 mM IPTG (isopropyl-α-d-thiogalactopyranoside) during exponential growth. After 3.5 h of induction, cell pellets were sonicated and the tagged proteins were purified by single-step affinity chromatography on Ni-nitrilotriacetic acid agarose (QIAGEN Inc., Valencia, CA) for YwqE (and all its mutants), YfkJ, YwlE, YwqD, and YwqF and glutathione Sepharose 4B (Amersham Biosciences, Piscataway, NJ) for GST-YfkJ and GST-YwqC-NCter. Recombinant proteins were then either desalted on a PD-10 column, as described previously (16), or extensively dialyzed against their final buffer. The purity of recombinant proteins was consistently over 95% as determined by Coomassie staining of gels.
In vitro phosphatase assays.
When phosphopeptides or pNPP was used as a substrate, phosphatase assays were carried out at 30°C, and the release of inorganic phosphate was measured indirectly by reading the absorbance at 620 nm after the addition of Biomol Green to the reaction or measured directly by reading the p-nitro-phenol absorbance at 405 nm. The times of the reactions, amounts of enzyme, and concentrations of substrates were optimized to have linear kinetics. The initial hydrolysis rate was measured in duplicate for phosphopeptides or in triplicate for pNPP. When the kinetic parameters were measured, the initial hydrolysis rate was plotted against the substrate concentration and all datum points were fitted simultaneously to the Michaelis-Menten or Lineweaver-Burk (for vanadate competitive inhibition) equation by using the Prism software. Km, kcat, and Ki values were calculated from a nonlinear fit of the Michaelis-Menten equation by using the same software and an extinction coefficient of 18,000 M−1 cm−1 for p-nitro-phenol or by comparison with a standard curve of inorganic phosphate when Biomol Green was used for detection.
Radiolabeled phospho-YwqD and phospho-YwqF were prepared as described by Mijakovic et al. (16). To phosphorylate its substrate, YwqF, the kinase YwqD needs the presence of a modulator, YwqC, a small transmembrane protein homologous to the central (transmembrane) region of E. coli PTKs Wzc and Etk (16). Briefly, 1 μM YwqD, 1 μM of YwqC-NCter (a fusion between the first 15 amino acids [aa] and the last 50 aa of YwqC [see reference 16]), and 10 μM YwqF were incubated with 50 μM [γ-32P]ATP (20 μCi/mmol), 5 mM MgCl2, and 50 mM Tris-HCl, pH 7.4. To carry out dephosphorylation assays, 32P-labeled proteins were separated from [γ-32P]ATP on a Ni-nitrilotriacetic acid column and transferred to 20 mM NH4HCO3 buffer on a PD-10 column. Radiolabeled proteins were lyophilized and resuspended in 50 mM Tris-HCl, pH 7.4. The dephosphorylation reaction mixture contained approximately 1 μM 32P-YwqD, 10 μM 32P-YwqF (given concentrations refer to total protein, not only the phosphorylated form), 1 μM YwqE or 1 μM YfkJ, and 5 mM MnCl2. The mixtures were incubated at 37°C for the indicated time periods before dephosphorylation was stopped by adding sample buffer for sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and heating at 100°C for 5 min. Proteins were separated by electrophoresis on denaturing SDS-polyacrylamide gels that were subsequently washed in boiling 0.5 M HCl for 10 min to eliminate any phosphohistidine. Radioactive bands were visualized on dried gels with a STORM Phosphoimager (Molecular Dynamics) and quantified with ImageQuant.
Generation of B. subtilis PTP deletion strains.
The ΔywqE B. subtilis strain has already been described (16). Deletions of yfkJ (entailing a polar effect; B. subtilis ΔyfkJIH strain) and of ywlE (B. subtilis ΔywlE strain) were achieved in a B. subtilis JH642 (trpC2 pheA1) background by double-crossover integration of plasmids constructed using vector pJM105A or pJM134A (23) and selecting, respectively, for chloramphenicol or spectinomycin resistance.
Assays of UDP-glucose dehydrogenase activity.
UDP-glucose dehydrogenase activity of B. subtilis was measured as the reduction of NAD+ at 340 nm (two NAD+ reduced for one UDP-glucose oxidized) (16). Dialyzed B. subtilis crude extracts were prepared from wild-type strain 168 and the ΔywqE, ΔyfkJIH, and ΔywlE strains (for extract preparation, see Pagni et al. [22]). In addition to 200 mM Tris-HCl, pH 8.8, the 1-ml reaction mixtures contained 2.5 mM UDP-glucose, 5 mM NAD+, and dialyzed B. subtilis extract (about 0.5 mg total protein). Measurements were carried out every 30 s, in quartz cuvettes thermostated at 37°C, by use of a double-beam spectrophotometer UVIKON 923 (BIO-TEK KONTRON Instruments) and with the reaction mixture without UDP-glucose as a negative control.
RESULTS AND DISCUSSION
Biochemical characterization of B. subtilis YwqE.
Since little is currently known about the activities, reaction mechanisms, and substrate specificities of the CpsB family PTPs, we performed an in vitro biochemical analysis of YwqE, using both pNPP and a standard tyrosine phosphopeptide as substrates.
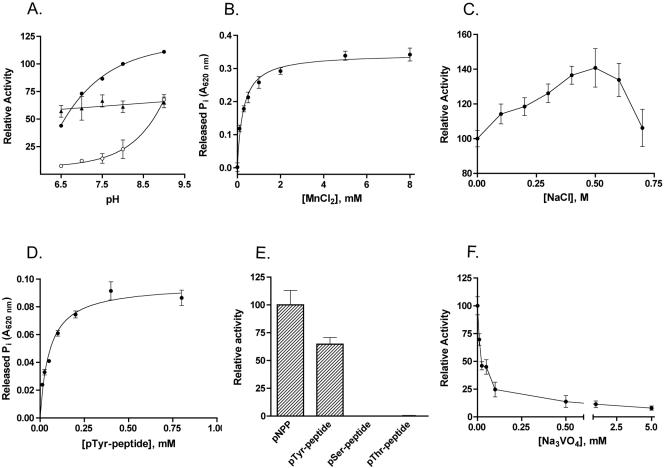
In the absence of cations, the activity of YwqE on pNPP was barely detectable in the reaction mixture in the pH range between 6.5 and 9.0. In the same pH range, 1 mM Mn2+, 1 mM Cu2+, and 1 mM Zn2+ were able to activate the enzyme, while 1 mM Mg2+ or 1 mM Ca2+ did not have any effect (Fig. 1A and data not shown). Figure 1A shows the pH-activity profile of the enzyme in the presence of Mn2+, Cu2+, and Zn2+. In the presence of 1 mM Mn2+ or 1 mM Zn2+, YwqE activity increased with increasing pHs, and there was a significant positive correlation between activity and pH (the Spearman r was 1.00 and the two-tailed P was 0.017 for Mn2+, and the Spearman r was 0.92 and the two-tailed P was 0.027 for Zn2+). On the other hand, activation of the enzyme by Cu2+ was relatively pH independent in the range between pH 6.5 and 9.0, suggesting that Cu2+ affects the catalytic mechanism of the enzyme in a different way than Mn2+ and Zn2+. Since the enzyme exhibited maximal activity at an alkaline pH in the presence of Mn2+, it was further characterized by using a buffer containing MnCl2, pH 8. Figure 1B shows that Mn2+ is an essential activator of YwqE, with a calculated Kmetal ± standard error of 0.274 ± 0.020 mM. This value is very similar to the Kmetal for Mn2+ of murine EyA3(AD), which belongs to a recently described new class of Asp-based eukaryotic PTPs (class IV PTPs [25]). The addition of NaCl to the reaction buffer caused a modest increase of the enzymatic activity for concentrations up to 0.5 M (Fig. 1C). Similar to YwqE, S. pneumoniae CpsB also showed optimal pH in the basic range and Mn2+ dependency (4, 19). The two enzymes might differ in their patterns of sensitivity to other cations, as CpsB has been reported to be activated by Mg2+ and to be insensitive to Cu2+ and Zn2+ (19). Optimal pHs in the basic range and ion dependency clearly differentiate CpsB-like enzymes from Cys-based eukaryotic and prokaryotic PTPs, which have optimal pH in the acidic range and do not require metal ions for their activities. Activation of YwqE by Cu2+ is interesting, as Cu2+ is a known inhibitor of Cys-based PTPs. Given its wide pH range of action, Cu2+ might be a physiological cofactor of the enzyme. Further biochemical and in vivo studies are needed to establish the relevance of different ions for the physiological activity of YwqE.
FIG. 1.
Biochemical characterization of recombinant YwqE. (A) Effect of pH on YwqE enzymatic activity in the presence of 1 mM MnCl2 (closed circles), 1 mM CuCl2 (triangles), or 1 mM ZnCl2 (open circles). The buffer used was 50 mM Tris-HCl. The graph shows datum points and regression. (B) Effect of MnCl2 on the activity of YwqE with pNPP as the substrate. The buffer used was 50 mM HEPES-KOH, pH 8.0, and contained increasing concentrations of MnCl2. The graph shows datum points and a nonlinear fit of the experimental data to the Michaelis-Menten equation. (C) Effect of NaCl on the activity of YwqE with pNPP as the substrate. (D) Analysis of YwqE enzymatic activity using the pTyr peptide KRLIEDNE(pY)TARGQ as a substrate. The graph shows datum points and a nonlinear fit of the experimental data to the Michaelis-Menten equation. (E) Comparison of the activities of YwqE using pNPP, the pTyr peptide KRLIEDNE(pY)TARGQ, the pSer peptide RRA(pS)VA, and the pThr peptide KR(pT)IRR as substrates. The assay mixture contained 0.5 mM pNPP or phosphopeptides as substrates. (F) Effect of Na3VO4 on the activity of YwqE with pNPP as the substrate. The buffer used for panels C and F was 50 mM HEPES-KOH, pH 8.0, and 1 mM MnCl2; the buffer used for panels D and E was 100 mM HEPES-KOH, pH 8.0, and 1 mM MnCl2. For panels A through C and F, the assay mixture contained 3 mM pNPP as a substrate. When error bars are not present in the graphs, they are within the resolution of the datum points.
The kinetic parameters of YwqE activity were then assayed using pNPP as a substrate, in 50 to 100 mM HEPES-KOH buffer, pH 8.0, containing 1 mM MnCl2. YwqE dephosphorylated pNPP following a Michaelis-Menten kinetics (data not shown). The Km ± standard error was 0.345 ± 0.043 mM, which is similar to the values reported for several eukaryotic and prokaryotic Cys-based PTPs and for eukaryotic class IV PTPs (6, 11, 25). The kcat ± standard error for pNPP was 0.022 ± 0.001 s−1. This kcat value is much lower than those reported for other eukaryotic PTPs and for some bacterial PTPs (9, 19, 26, 27; Musumeci et al., unpublished data). The YwqE kcat is only somewhat lower than the one reported for Staphylococcus aureus PtpB (26) and similar to the kcat of B. subtilis YwlE (Musumeci et al., unpublished data).
Figure 1D shows that YwqE was also able to dephosphorylate the tyrosine phosphopeptide KRLIEDNE(pY)TARGQ (derived from the autophosphorylation site of the mammalian tyrosine kinase Lck) following Michaelis-Menten kinetics. The Km ± standard error was 0.054 ± 0.008 mM, and the kcat ± standard error was 0.016 ± 0.001 s−1. As shown in Fig. 1E, YwqE was completely unable to dephosphorylate the serine phosphopeptide RRA(pS)VA and the threonine phosphopeptide KR(pT)IRR, which are good substrates of the serine-threonine phosphatases PP1 and PP2A (8). Among PTP inhibitors, 1 mM iodoacetamide and N-ethylmaleimide (NEM) had minimal effects, while Na3VO4 was an efficient inhibitor of YwqE (Table 1 and Fig. 1F). Vanadate was found to inhibit the enzyme with a competitive mechanism and a Ki ± standard error of 14 ± 3 μM (data not shown). Among classical serine-threonine phosphatase inhibitors, 1 mM sodium fluoride (NaF) did not affect the phosphatase activity (Table 1), while 1 mM sodium pyrophosphate (NaPP) was found to inhibit the phosphatase (Table 1). Inhibition by NaPP was subsequently found to happen only at concentrations higher than 0.5 mM (data not shown), and it might be of physiological relevance, as, depending on the growth conditions, B. subtilis contains between 1.2 and 6 mM pyrophosphate (17). The described pattern of substrate specificity and sensitivity to inhibitors is in line with YwqE being a PTP in vivo. YwqE activity was relatively insensitive to iodoacetamide and NEM, two classical inhibitors of Cys-based PTPs (including LMPTPs) which form covalent adducts with the sulfhydryl group of the catalytic Cys residue. This suggests that the catalytic mechanism of YwqE is different from the one used by LMPTPs and other PTPs and does not involve a Cys residue.
TABLE 1.
Effects of phosphatase inhibitors on YwqE activity
| Phosphatase inhibitor(concn) or condition | % Relative activity ± SEa |
|---|---|
| No inhibitor | 100.0 ± 0.5 |
| Iodoacetamide (1 mM) | 100.9 ± 0.6 |
| NEM (1 mM) | 137.7 ± 0.6 |
| Na3VO4 (1 mM) | 11.4 ± 5.1 |
| NaF (1 mM) | 85.0 ± 1.2 |
| NaPP (1 mM) | 31.2 ± 1.6 |
Inhibitors were added to the reaction buffer at a final concentration of 1 mM. Iodoacetamide and NEM were preincubated with the enzyme for 10 min on ice. The assay mixture contained 300 nM enzyme and 3 mM of pNPP as the substrate. The buffer used was 50 mM HEPES, pH 8.0, and 1 mM MnCl2.
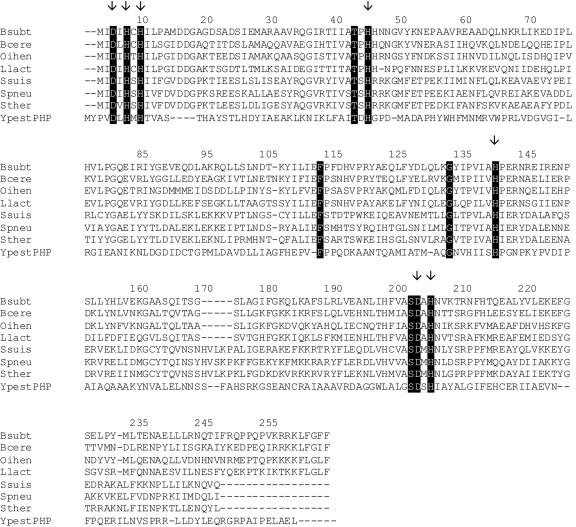
Since the CpsB-like phosphatases share homology with the PHP family of phosphoesterases, a representative of PHP phosphoesterases from Yersinia pestis was aligned with B. subtilis YwqE and its homologues from different gram-positive bacteria (Fig. 2). Our multiple alignment revealed several 100% conserved residues, some of which have been previously suggested to be critical for catalysis (3). Although the active site and catalytic mechanism of CpsB-like phosphatases are currently not defined, the conserved histidines and aspartates were suggested to participate in cation binding (3). We performed site-directed mutagenesis of all conserved charged amino acid residues (two aspartates and five histidines) indicated in Fig. 2 (residues D3, H5, H7, H42, H136, D194, and H196), thus creating a set of YwqE mutant proteins, each bearing an alanine in place of one of these residues. The levels of phosphatase activity of all altered versions of YwqE created in this study were considerably diminished compared to the wild-type protein (Table 2), suggesting that all the conserved charged residues are fundamental for the catalytic integrity of the enzyme. In line with our observations, residues homologous to D194 and H196 have been mutated in S. pneumoniae CpsB and caused a complete loss of its phosphatase activity (19). Further analyses are needed to assess if the reduced activity of each mutant is due to overall structural destabilization of the protein or to the participation of the residue in the enzyme catalytic mechanism.
FIG. 2.
Multiple alignment of Bacillus subtilis (Bsubt) YwqE and its homologues from different gram-positive bacteria, as follows: Bacillus cereus (Bcere), Oceanobacillus iheyensis (Oihen), Lactococcus lactis (Llact), Streptococcus suis (Ssuis), Streptococcus pneumoniae (Spneu), and Streptococcus thermophilus (Sther). The PHP phosphoesterase from Yersinia pestis (YpestPHP) was added as the outgroup. Residues conserved in both PHP phosphoesterases and YwqE homologues are highlighted. Residues mutated in this study are designated by arrows.
TABLE 2.
Mutation of critical residues affects YwqE enzymatic activity
| YwqE protein or mutant | % Relative activity ± SEa |
|---|---|
| Wild type | 100.0 ± 8.4 |
| D3A | 8.1 ± 0.7 |
| H5A | 9.5 ± 0.8 |
| H7A | 6.3 ± 0.5 |
| H42A | 6.8 ± 0.6 |
| H136A | 7.7 ± 0.7 |
| D194A | 5.4 ± 0.5 |
| H196A | 4.1 ± 0.3 |
The assay contained 2 μg YwqE and 3 mM of pNPP as the substrate. The buffer used was 50 mM HEPES, pH 8.0, and 1 mM MnCl2.
B. subtilis LMPTPs are not able to dephosphorylate YwqE substrates in vitro and in vivo.
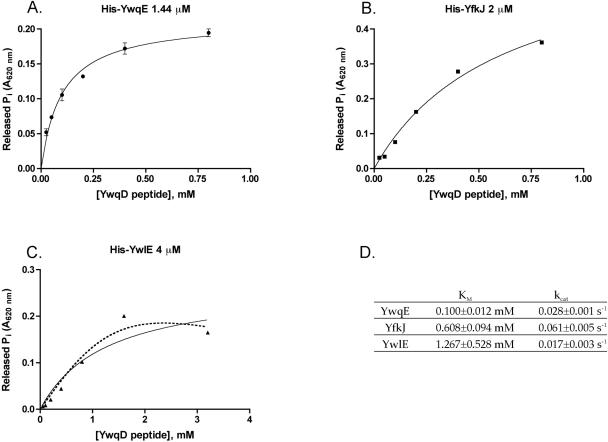
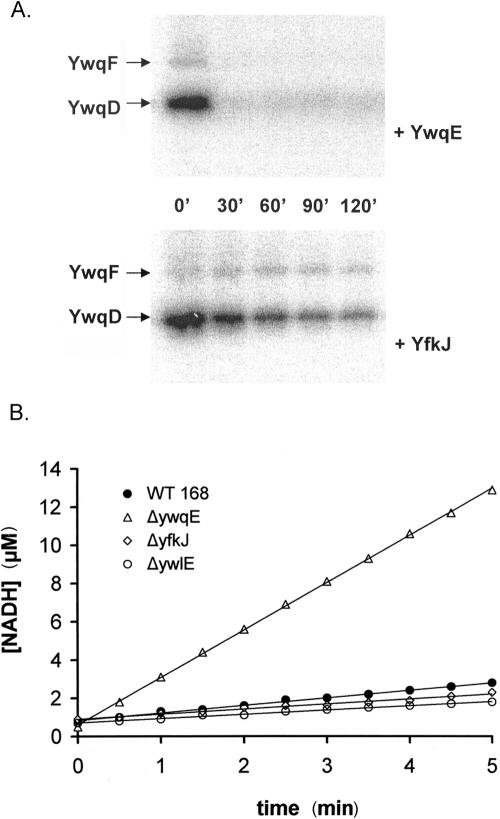
In addition to YwqE, B. subtilis possesses two LMPTPs, YwlE and YfkJ. Gram-negative LMPTPs are known to dephosphorylate substrates encoded in different operons (10, 15) and show much higher activity than YwqE when assayed in vitro with pNPP as a substrate. The E. coli LMPTP Wzb efficiently dephosphorylates its cognate kinase Wzc, which is partly homologous to YwqD (27). We recently characterized the B. subtilis LMPTPs YfkJ and YwlE by using pNPP as a substrate and found that YfkJ has 10-times-higher activity than YwqE, and YwlE has a similar activity compared to YwqE (Musumeci et al., submitted for publication). Despite having similar activity on pNPP, the LMPTP YwlE was very inefficient in dephosphorylating YwqE substrates phospho-YwqD and phospho-YwqF in vitro (16). In order to compare the activities of the three B. subtilis phosphatases on the physiological substrates for YwqE, we assayed them in their optimal buffers with an YwqD-derived phosphopeptide as a substrate. Figure 3A, B, and C show the kinetic parameters of the three B. subtilis PTPs when dephosphorylating the phosphopeptide LSKHSEYGY(pY)GTKDNFMQK, which is derived from the C-terminal tail of YwqD and includes Tyr228, a known target of YwqE-catalyzed dephosphorylation (16). All three phosphatases were able to dephosphorylate the peptide following Michaelis-Menten kinetics to a certain extent, but YwqE was the most efficient and showed the lowest Km, while YwlE was quite inefficient (Fig. 3D). Despite showing a higher Km than YwqE, YfkJ showed activity almost comparable to YwqE in the assays with the phosphopeptide (Fig. 3D). When we repeated the assays using a more physiological buffer at a neutral pH and full-length phospho-YwqD or phospho-YwqF as a substrate, YfkJ was completely unable to dephosphorylate phospho-YwqF and was much less active than YwqE when assayed using phospho-YwqD as a substrate (Fig. 4A). The observed differences in kinetic parameters between YwqE and YfkJ when a synthetic phosphopeptide or full-length substrates were used as in vitro substrates suggest that global steric recognition between the phosphatases and the full-length substrates plays an important role in determining the specificity of YwqE.
FIG. 3.
Comparison of the activities of YwqE (A), YfkJ (B), and YwlE (C) on the phosphopeptide LSKHSEYGY(pY)GTKDNFMQK derived from the last 19 aa of YwqD and including pTyr228, a known dephosphorylation site of YwqE. The buffers used were 100 mM HEPES-KOH, pH 8.0, and 1 mM MnCl2 for YwqE; 100 mM bis-Tris, pH 6.0, and 1 mM dithiothreitol for YfkJ; and 100 mM MES (morpholineethanesulfonic acid), pH 5.5, and 1 mM dithiothreitol for YwlE. Continuous lines in all graphs are nonlinear fits of the experimental data to the Michaelis-Menten equation. When error bars are not present in the graph, they are within the resolution of the datum points. (D) Table with a comparison of the Michaelis-Menten parameters of the three phosphatases for the mentioned peptide. YwlE shows a kinetic of substrate inhibition when using pNPP as a substrate (Musumeci et al., unpublished data), and the dotted line in panel C shows a nonlinear fit of experimental data to the Michaelis-Menten equation for substrate inhibition (see the London South Bank University Faculty of Engineering, Science and the Built Environment website [http://www.lsbu.ac.uk/biology/enztech/inhibition.html]).
FIG. 4.
(A) In vitro dephosphorylation of phospho-YwqD and phospho-YwqF by YwqE and YfkJ. Autoradiograms of SDS-PAGE gels with 1 μM autophosphorylated YwqD and 10 μM phosphorylated UDP-glucose dehydrogenase YwqF. 32P-proteins were incubated with either 1 μM YwqE (upper panel) or 1 μM GST-YfkJ (lower panel), in the presence of 5 mM MnCl2, for the indicated time at 37°C. (B) In vivo analysis of redundancy between YwqE and B. subtilis LMPTPs. The graph shows UDP-glucose dehydrogenase activities of B. subtilis dialyzed protein extracts. The amount of extract used in the assay was normalized with respect to total protein concentration. The different extracts used were prepared from wild-type strain 168 (filled circles), and the ΔywqE (open triangles), ΔyfkJIH (open diamonds), and ΔywlE (open circles) strains. The concentrations of produced NADH were calculated from the absorbance variations at 340 nm.
In order to confirm in vivo the finding that B. subtilis LMPTPs do not or only poorly dephosphorylate YwqE substrates, we compared the effects of ywqE, ywlE, and yfkJ gene inactivation on the enzymatic activity of UDP-glucose dehydrogenases in B. subtilis. Besides containing YwqF, B. subtilis contains another UDP-glucose dehydrogenase, TuaD (22), which is also controlled by tyrosine phosphorylation in the same way as its ortholog YwqF (16). The inactivation of any PTP capable of dephosphorylating UDP-glucose dehydrogenases should provoke the accumulation of the phosphorylated, active form of these enzymes, leading to an increase in the UDP-glucose dehydrogenase activity in B. subtilis. It was previously shown that the UDP-glucose dehydrogenase activity of a dialyzed protein extract prepared from a ΔywqE strain was considerably higher than the activity of the extract from wild-type B. subtilis (16). We measured the UDP-glucose dehydrogenase activity of dialyzed protein extracts prepared from ΔywlE and ΔyfkJIH strains, and the obtained values were almost identical to that of the extract of the wild-type strain (Fig. 4B). This finding strengthened our observation that YwlE and YfkJ are unable to dephosphorylate phospho-YwqD and phospho-YwqF in vitro and suggested that YwqE is the only PTP in B. subtilis with a significant influence on the activity of UDP-glucose dehydrogenases of this organism in vivo described so far. Redundancy between PTPs is not common in Eukarya and should be even less likely in bacteria, as they usually encode a very small number of PTPs in their genomes (13) and should follow the scheme “one phosphatase for many substrates” rather than “one substrate for many phosphatases.”
Acknowledgments
This work was supported by grants of the Institut National de la Recherche Agronomique, the Centre National de la Recherche Scientifique, and the Institut National Agronomique Paris-Grignon (to I.M., D.P., and J.D.), the Danish National Research Council (to I.M.), and the National Institutes of Health (to T.M.).
We thank Marta Perego (The Scripps Research Institute, La Jolla, CA) for helpful discussions and Massimo Bottini (The Burnham Institute, La Jolla, CA) for help with the experimental error evaluations and statistical analyses of the data.
REFERENCES
- 1.Alonso, A., J. Sasin, N. Bottini, I. Friedberg, I. Friedberg, A. Osterman, A. Godzik, T. Hunter, J. Dixon, and T. Mustelin. 2004. The set of genes encoding protein tyrosine phosphatase family members in the human genome. Cell 117:699-711. [DOI] [PubMed] [Google Scholar]
- 2.Amrein, K. E., B. Takacs, M. Stieger, J. Molnos, N. A. Flint, and P. Burn. 1995. Purification and characterization of recombinant human p50csk protein-tyrosine kinase from an Escherichia coli expression system overproducing the bacterial chaperones GroES and GroEL. Proc. Natl. Acad. Sci. USA 92:1048-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aravind, L., and E. V. Koonin. 1998. Phosphoesterase domains associated with DNA polymerases of diverse origins. Nucleic Acids Res. 26:3746-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender, M. H., and J. Yother. 2001. CpsB is a modulator of capsule-associated tyrosine kinase activity in Streptococcus pneumoniae. J. Biol. Chem. 276:47966-47974. [DOI] [PubMed] [Google Scholar]
- 5.Bugert, P., and K. Geider. 1997. Characterization of the amsI gene product as a low molecular weight acid phosphatase controlling exopolysaccharide synthesis of Erwinia amylovora. FEBS Lett. 400:252-256. [DOI] [PubMed] [Google Scholar]
- 6.Cirri, P., T. Fiaschi, P. Chiarugi, G. Camici, G. Manao, G. Raugei, and G. Ramponi. 1996. The molecular basis of the differing kinetic behavior of the two low molecular mass phosphotyrosine protein phosphatase isoforms. J. Biol. Chem. 271:2604-2607. [DOI] [PubMed] [Google Scholar]
- 7.Dadssi, M., and A. J. Cozzone. 1990. Evidence of protein-tyrosine kinase activity in the bacterium Acinetobacter calcoaceticus. J. Biol. Chem. 265:20996-20999. [PubMed] [Google Scholar]
- 8.Donella-Deana, A., H. E. Meyer, and L. A. Pinna. 1991. The use of phosphopeptides to distinguish between protein phosphatase and acid/alkaline phosphatase activities: opposite specificity toward phosphoseryl/phosphothreonyl substrates. Biochim. Biophys. Acta 1094:130-133. [DOI] [PubMed] [Google Scholar]
- 9.Grangeasse, C., P. Doublet, C. Vincent, E. Vaganay, M. Riberty, B. Duclos, and A. J. Cozzone. 1998. Functional characterisation of the low-molecular-mass phosphotyrosine-protein phosphatase of Acinetobacter johnsonii. J. Mol. Biol. 278:339-347. [DOI] [PubMed] [Google Scholar]
- 10.Grangeasse, C., B. Obadia, I. Mijakovic, J. Deutscher, A. J. Cozzone, and P. Doublet. 2003. Autophosphorylation of the Escherichia coli protein kinase Wzc regulates tyrosine phosphorylation of Ugd, a UDP-glucose dehydrogenase. J. Biol. Chem. 278:39323-39329. [DOI] [PubMed] [Google Scholar]
- 11.Howell, L. D., C. Griffiths, L. W. Slade, M. Potts, and P. J. Kennelly. 1996. Substrate specificity of IphP, a cyanobacterial dual-specificity protein phosphatase with MAP kinase phosphatase activity. Biochemistry 35:7566-7572. [DOI] [PubMed] [Google Scholar]
- 12.Hubbard, S. R., and J. H. Till. 2000. Protein tyrosine kinase structure and function. Annu. Rev. Biochem. 69:373-398. [DOI] [PubMed] [Google Scholar]
- 13.Kennelly, P. J. 2002. Protein kinases and protein phosphatases in prokaryotes: a genomic perspective. FEMS Microbiol. Lett. 206:1-8. [DOI] [PubMed] [Google Scholar]
- 14.Kholod, N., and T. Mustelin. 2001. Novel tools for studying protein complexes: vectors for co-expression of two proteins in Escherichia coli. BioTechniques 31:322-328. [DOI] [PubMed] [Google Scholar]
- 15.Klein, G., C. Dartigalongue, and S. Raina. 2003. Phosphorylation-mediated regulation of heat shock response in Escherichia coli. Mol. Microbiol. 48:269-285. [DOI] [PubMed] [Google Scholar]
- 16.Mijakovic, I., S. Poncet, G. Boel, A. Maze, S. Gillet, E. Jamet, P. Decottignies, C. Grangeasse, P. Doublet, P. Le Marechal, and J. Deutscher. 2003. Transmembrane modulator-dependent bacterial tyrosine kinase activates UDP-glucose dehydrogenases. EMBO J. 22:4709-4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mijakovic, I., S. Poncet, A. Galinier, V. Monedero, S. Fieulaine, J. Janin, S. Nessler, J. A. Marquez, K. Scheffzek, S. Hasenbein, W. Hengstenberg, and J. Deutscher. 2002. Pyrophosphate-producing protein dephosphorylation by HPr kinase/phosphorylase: a relic of early life? Proc. Natl. Acad. Sci. USA 99:13442-13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morona, J. K., J. C. Paton, D. C. Miller, and R. Morona. 2000. Tyrosine phosphorylation of CpsD negatively regulates capsular polysaccharide biosynthesis in Streptococcus pneumoniae. Mol. Microbiol. 35:1431-1442. [DOI] [PubMed] [Google Scholar]
- 19.Morona, J. K., R. Morona, D. C. Miller, and J. C. Paton. 2002. Streptococcus pneumoniae capsule biosynthesis protein CpsB is a novel manganese-dependent phosphotyrosine-protein phosphatase. J. Bacteriol. 184:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morona, J. K., D. C. Miller, R. Morona, and J. C. Paton. 2004. The effect that mutations in the conserved capsular polysaccharide biosynthesis genes cpsA, cpsB, and cpsD have on virulence of Streptococcus pneumoniae. J. Infect. Dis. 189:1905-1913. [DOI] [PubMed] [Google Scholar]
- 21.Mustelin, T., G. S. Feng, N. Bottini, A. Alonso, N. Kholod, D. Birle, J. Merlo, and H. Huynh. 2002. Protein tyrosine phosphatases. Front. Biosci. 7:85-142. [DOI] [PubMed] [Google Scholar]
- 22.Pagni, M., V. Lazarevic, B. Soldo, and D. Karamata. 1999. Assay for UDP-glucose 6-dehydrogenase in phosphate-starved cells: gene tuaD of Bacillus subtilis 168 encodes the UDPglucose 6-dehydrogenase involved in teichuronic acid synthesis. Microbiology 145:1049-1053. [DOI] [PubMed] [Google Scholar]
- 23.Perego, M. 1993. Integrational vectors for genetic manipulation in Bacillus subtilis, p. 615-624. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 24.Ramponi, G., and M. Stefani. 1997. Structural, catalytic and functional properties of low Mr phosphotyrosine protein phosphatases. Evidence for a long evolutionary history. Int. J. Biochem. Cell Biol. 29:279-292. [DOI] [PubMed] [Google Scholar]
- 25.Rayapureddi, J. P., C. Kattamuri, B. D. Steinmetz, B. J. Frankfort, E. J. Ostrin, G. Mardon, and R. S. Hegde. 2003. Eyes absent represents a class of protein tyrosine phosphatases. Nature 426:295-298. [DOI] [PubMed] [Google Scholar]
- 26.Soulat, D., E. Vaganay, B. Duclos, A. L. Genestier, J. Etienne, and A. J. Cozzone. 2002. Staphylococcus aureus contains two low-molecular-mass phosphotyrosine protein phosphatases. J. Bacteriol. 184:5194-5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent, C., P. Doublet, C. Grangeasse, E. Vaganay, A. J. Cozzone, and B. Duclos. 1999. Cells of Escherichia coli contain a protein-tyrosine kinase, Wzc, and a phosphotyrosine-protein phosphatase, Wzb. J. Bacteriol. 181:3472-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent, C., B. Duclos, C. Grangeasse, E. Vaganay, M. Riberty, A. J. Cozzone, and P. Doublet. 2000. Relationship between exopolysaccharide production and protein-tyrosine phosphorylation in gram-negative bacteria. J. Mol. Biol. 304:311-321. [DOI] [PubMed] [Google Scholar]
- 29.Wugeditsch, T., A. Paiment, J. Hocking, J. Drummelsmith, C. Forrester, and C. Whitfield. 2001. Phosphorylation of Wzc, a tyrosine autokinase, is essential for assembly of group 1 capsular polysaccharides in Escherichia coli. J. Biol. Chem. 276:2361-2371. [DOI] [PubMed] [Google Scholar]