Abstract
In response to the in vivo environment, the Salmonella enterica serovar Typhimurium lipopolysaccharide (LPS) is modified. These modifications are controlled in part by the two-component regulatory system PmrA-PmrB, with the addition of 4-aminoarabinose (Ara4N) to the lipid A and phosphoethanolamine (pEtN) to the lipid A and core. Here we demonstrate that the PmrA-regulated STM4118 (cptA) gene is necessary for the addition of pEtN to the LPS core. pmrC, a PmrA-regulated gene necessary for the addition of pEtN to lipid A, did not affect core pEtN addition. Although imparting a similar surface charge modification as Ara4N, which greatly affects polymyxin B resistance and murine virulence, neither pmrC nor cptA plays a dramatic role in antimicrobial peptide resistance in vitro or virulence in the mouse model. Therefore, factors other than surface charge/electrostatic interaction contribute to resistance to antimicrobial peptides such as polymyxin B.
In order to survive in the mammalian host, salmonellae have evolved means of evading killing by antimicrobial peptides (AP), central components of innate immunity. Such mechanisms have been demonstrated to be important for survival within hosts and host cells (9, 16, 27, 28). A common mode of increasing AP resistance involves alteration of the bacterial surface. In Salmonella enterica serovar Typhimurium, the two-component regulatory systems (TCRS) PhoP-PhoQ and PmrA-PmrB have been characterized as contributing to resistance to AP by mediating alterations to the outer membrane and lipopolysaccharide (LPS) (reviewed in reference 8). Both TCRS respond to specific cues from the host environment and have been shown to be activated within host cell vacuoles (1, 17). PhoP-PhoQ can be activated under conditions of low magnesium or low pH in vitro (3) and can activate PmrA-PmrB via induction of PmrD, whose product affects the phosphorylation state of PmrA (15, 19-21). PmrA can also be activated independent of PhoP in response to mild acid pH or high iron concentrations (11, 30). Such activation results in the induced transcription of pmrE and pmrHFIJKLM (also called pbg and arn elsewhere [12, 35]), which encode enzymes involved in biosynthesis of aminoarabinose (Ara4N) and its addition primarily to the 4′-phosphate of the lipid A portion of LPS (14).
PmrA is also predicted to control modification of LPS with phosphoethanolamine (pEtN), because PmrA mutants with increased polymyxin B (PM) resistance show enhanced pEtN substitutions to the lipid A and core oligosaccharide (OS) fractions of LPS (18). Recently, the PmrA-regulated pmrC gene product was shown to mediate the addition of pEtN to the 1-position of lipid A and affect resistance to PM (22). In Neisseria meningitidis, loci necessary for transferring pEtN to the heptose II (HepII) of the LPS core or to lipid A were also recently identified (5, 23, 38). Inactivation of N. meningitidis lpt-3 resulted in the absence of pEtN from HepII of the core and bestowed increased resistance to bactericidal killing and decreased opsonophagocytosis by LPS-specific monoclonal antibody B5 (23). Similarly, inactivation of lptA led to loss of pEtN replacement of lipid A phosphates (5), and lpt6 was required for the addition of pEtN-6 on HepII of the core. However, it is not known if these pEtN phosphotransferases play a role in neisserial AP resistance or animal virulence.
We recently identified a number of new Salmonella PmrA-regulated genes, including one locus with homology to both lpt-3 and lptA of N. meningitidis (34). In this work, we describe the further characterization of this gene, its role in pEtN modification of LPS, and the role of pEtN modification of LPS in AP resistance and virulence of Salmonella.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains used in this study are described in Table 1. Salmonellae were grown in Luria-Bertani broth at 37°C with aeration. Antibiotics were used, when appropriate, at the following concentrations: chloramphenicol, 25 μg/ml; kanamycin, 45 μg/ml; tetracycline, 25 μg/ml.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Description | Reference(s) |
|---|---|---|
| Strains | ||
| CS019 | phoN2 zxx::6251dTn10-cam | 24 |
| JSG210 | Wild type, ATCC 14028s | 24 |
| JSG421 | pmrA::Tn10d-tet | 15 |
| JSG435 | pmrA505 zjd::Tn10d-cam | 15 |
| JSG485 | pmrA505 zjd::Tn10d-cam pmrF::Tn10d-Tet | 14 |
| JSG1311 | rpsL phoN2 zxx::6251dTn10-cam | This study |
| JSG1800 | pmrA505 zjd::Tn10d-cam STM4118::Kan | This study |
| JSG1863 | pmrA505 zjd::Tn10d-cam ΔSTM4118 | This study |
| JSG1900 | pmrA505 zjd::Tn10d-cam STM4118::lacZ | This study |
| JSG1901 | pmrA::Tn10d-tet STM4118::lacZ | This study |
| JSG1960 | 14028s STM4118::Kan | This study |
| JSG2264 | pmrA505 zjd::Tn10d-cam STM3635::Kan | This study |
| JSG2266 | pmrA505 zjd::Tn10d-cam pmrC::Kan | This study |
| JSG2268 | pmrA505 zjd::Tn10d-cam STM0834::Kan | This study |
| JSG2271 | pmrA505 zjd::Tn10d-cam ΔSTM3635 | This study |
| JSG2273 | pmrA505 zjd::Tn10d-cam ΔpmrC | This study |
| JSG2275 | pmrA505 zjd::Tn10d-cam ΔSTM0834 | This study |
| JSG2278 | pmrA505 zjd::Tn10d-cam STM3635::lacZ | This study |
| JSG2279 | pmrA::Tn10d-tet STM3635::lacZ | This study |
| JSG2280 | pmrA505 zjd::Tn10d-cam pmrC::lacZ | This study |
| JSG2281 | pmrA::Tn10d-tet pmrC::lacZ | This study |
| JSG2282 | pmrA505 zjd::Tn10d-cam STM0834::lacZ | This study |
| JSG2283 | pmrA::Tn10d-tet STM0834::lacZ | This study |
| JSG2284 | 14028s STM3635::Kan | This study |
| JSG2285 | 14028s pmrC::Kan | This study |
| JSG2286 | 14028s STM0834::Kan | This study |
| JSG2339 | pmrA505 zjd::Tn10d-cam ΔSTM4118 pmrF::Tn10d-Tet | This study |
| JSG2341 | pmrA505 zjd::Tn10d-cam ΔpmrC pmrF::Tn10d-Tet | This study |
| JSG2435 | pmrA505 zjd::Tn10d-cam ΔpmrC STM4118::Kan | This study |
| JSG2449 | STM4118::Kan ΔpmrC::Cam | This study |
| JSG2512 | pmrA505 zjd::Tn10d-cam ΔpmrC ΔSTM4118 pmrF::Tn10d-Tet | This study |
| Plasmids | ||
| pKD46 | bla PBADgam bet exo pSC101 oriTS | 6 |
| pKD4 | bla FRT aph FRT PS1 PS2 oriR6K | 6 |
| pCP20 | bla cat cl857 λPRflppSC101 oriTS | 4, 6 |
| pCE36 | aph FRT lacZY+ this oriR6K | 7 |
Generation of mutations in Salmonella homologues to lpt-3 of N. meningitidis.
Initially, mutations were generated in PmrAc (JSG435, pmrA505 zjd::Tn10d-cam) and PmrA-null (pmrA::Tn10d-tet) backgrounds using the lambda Red recombinase and FLP-mediated recombination techniques developed by Datsenko and Wanner (6). Briefly, the kanamycin resistance fragment was amplified from pKD4 using primers flanked with homology to pmrC, STM4118, STM3635, or STM0834 (Table 2). PCR fragments were transformed into PmrAc and PmrA-null target strains in which lambda Red recombinase had been induced from pKD46. Correct integration of the kanamycin resistance gene insertion was verified by PCR. All strains and plasmids described herein are listed in Table 1.
TABLE 2.
Primers used in this study
| Primer | Sequencea |
|---|---|
| STM3635 | 5′ ACGCAACAGAAACTTAGTTTCTTGCTTGCGTGTGTAGGCTGGACGTGCTTC 3′ |
| 5′ TATCGTATCGTATAGCTCCACATGACGACGCATATGAATATCCTCCTTA 3′ | |
| STM4293 | 5′ CAGGTACTACAAGACCTACCGTTAAACTCGTGTGTA |
| (pmrC) | GGCTGGAGCTGCTTC 3′ |
| 5′ CCGTCATTATCATTCCACAGTACGTTAATCCCATATGAATATCCTCCTTA 3′ | |
| STM4118 | 5′ CGATTAAAGACAGGCTCTCATTATGCAATCCTGTGTAGGCTGGAGCTGCTTC 3′ |
| 5′ GCGTTATCGGGCAACATACAGGCTATTGATTCATATGAATATCCTCCTTA 3′ | |
| STM0834 | 5′ GCGTTTACCTGCCTGTTGCTGTTGCTCTGGTGTGTAGGCTGGAGCTGCTTC 3′ |
| 5′ CTGCCCAGATGGTTATAGTCCACTTTCTGTCATATGAATATCCTCCTTA 3′ |
Italicized sequences are complementary to sequence flanking the aph gene in pKD4 (6).
The insertion was eliminated using pCP20 as described previously, leaving a nonpolar deletion of the target gene and reintroducing a ribosome binding site (6). Transcriptional fusions (lacZ) were generated to each gene in both the PmrAc and PmrA-null backgrounds by using site-specific recombination between the FLP-recognition target “scar site” generated in the deletion strains and that in pCE36 (7).
Mutations in STM4118, STM3635, STM0834, and pmrC were also introduced into a wild-type 14028s (JSG224) background using P22 phage-mediated transduction, generating JSG1960, JSG2284, JSG2285, and JSG2286.
pmrF double mutants in selected backgrounds were generated by transducing phage propagated on JSG485 (pmrA505 zjd::Tn10d-cam pmrF::Tn10d-tet) into JSG1863, JSG2271, JSG2273, and JSG2275. Tetracycline-, chloramphenicol-resistant colonies were selected and designated JSG2339 to -2342.
AP resistance assays.
MIC assays were performed as described elsewhere (32). AP survival assays were performed by diluting logarithmic-phase bacteria to ∼105 CFU/ml followed by the addition of an AP at a set concentration. At various times after the addition of the AP (as well as prior to the addition of AP), aliquots of the bacteria were washed, diluted, and plated onto solid medium to enumerate the surviving bacteria.
Virulence assays.
Survival assays were performed as described previously (16). Wild-type 14028s, JSG1960 (14028s STM4118::kan), JSG2285 (14028s pmrC::kan), and JSG2449 (14028s ΔSTM4118::kan ΔpmrC::cat) were included in the assay. Briefly, each strain was grown to stationary phase (16 h) at 37°C. Approximately 5 × 106 stationary-phase bacteria (1 log unit above the 50% lethal dose) were washed and resuspended in 20 to 200 μl of phosphate-buffered saline, pH 7.4. Female BALB/c mice (weighing 16 to 18 g) were inoculated orally using the swallowing reflex or with a feeding needle. Five mice were used per strain. Dilutions of the stationary-phase cultures were plated to enumerate bacteria present in the inocula. Infected mice were observed for 21 days postinoculation. For competition assays, wild-type (JSG1311) and mutant (JSG2449) bacteria were inoculated into five BALB/c mice at a 1:1 ratio (1 × 106 of each in 200 μl total) and, at 4 days postinoculation, organs were harvested, macerated, and plated onto appropriate solid media to select for each of the competing strains.
LPS and lipid A isolation and gel analysis.
LPS was purified from stationary-phase cells using the hot phenol-water method as previously described (2). Deoxycholine-polyacrylamide gel electrophoresis (DOC-PAGE) was performed with an 18% polyacrylamide gel. The LPS samples were dissolved at a concentration of 4 μg/μl, and 1 μl was loaded in each well. The samples were electrophoresed at 30 mA constant current mode and were developed by silver staining (Bio-Rad).
Size exclusion chromatography.
For the LPS samples, chromatography was performed with a Sephacryl S-200 (HR) column (120 cm by 1 cm). The samples were eluted with Tris-EDTA buffer (pH 9.2), and the eluted fractions were monitored by an on-line refractive index detector and assayed by DOC-PAGE. Samples were pooled according to the molecular size, dialyzed extensively to remove the DOC, and lyophilized. This fractionated LPS was used for further analysis.
Isolation and purification of the oligosaccharide.
The LPS samples were delipidated with 1% acetic acid at 100°C for 2 h. The lipid precipitate was removed by centrifugation at 10,000 rpm for 10 min. The supernatant was fractionated with a Bio-Gel P10 column, using water as eluent. There was a single major fraction of each oligosaccharide that was lyophilized and used for further analysis.
NMR analysis.
Proton-nuclear magnetic resonance (NMR) of each oligosaccharide (2 mg) was performed after exchanging three times with 99.8% D2O (Aldrich) and dissolving in 0.5 ml D2O (100%) (Cambridge Isotopes). All of the NMR experiments were accomplished with a 600-MHz Varian-Inova instrument at 30°C. Two-dimensional NMR (2D-NMR) was performed using standard parameters as provided by Varian. The gCOSY (gradient sensitive 1H-1H correlation spectroscopy) spectra were measured over a spectral width of 2.5 kHz using a data set of (t1 × t2) of 256 by 2,048 points, and 16 scans were acquired. For g-HSQC (gradient-sensitive 1H-13C heteronuclear single quantum coherence spectroscopy), the spectral widths in proton and carbon dimensions were selected to be 2.5 and 13.9 kHz, respectively, and 96 scans were acquired.
MALDI mass spectrometry.
The oligosaccharide samples were dissolved in Milli-Q water at a concentration of 1 μg/μl and mixed with 2,5-dihydroxy azobenzoic acid matrix at a 1:1 ratio. The samples were spotted on a 100-well stainless steel matrix-assisted laser desorption ionization (MALDI) plate and air dried. A 337-nm N2 laser was used to desorb the molecules, and spectra were collected in delayed, linear, and negative mode using an acceleration voltage of 20,000.
RESULTS
Identification of Salmonella homologues to N. meningitidis phosphoethanolamine phosphotransferases and their regulation by PmrA.
The predicted protein sequences of lpt-3 (NMB2010), lptA (NMB1638), and lpt6 (NMA0408) were compared to the S. enterica serovar Typhimurium LT-2 genome sequence using the NCBI database (BLASTX and BLASTP). Similar searches and results that were restricted to the use of lpt-3 have been previously reported (22). The products of four S. enterica serovar Typhimurium loci showed significant similarity (E value < 4e−7) to Lpt-3: STM3635 (yhjW; 24% identity, 41% similarity), STM4293 (pmrC yjdB; 24% identity, 43% similarity), STM4118 (yijP; 23% identity, 38% similarity), and STM0834 (ybiP; 23% identity, 41% similarity). LptA showed similar levels of identity and similarity to the same S. enterica serovar Typhimurium loci but demonstrated a greater degree of conservation to STM4293 (43% identity, 62% similarity). Lpt6 showed no significant similarity to Salmonella proteins. STM3635 is predicted to encode a membrane-associated, metal-dependent hydrolase. STM4118, pmrC, and STM0834 encode putative integral membrane proteins, consistent with the predicted location of an LPS phosphoethanolamine phosphotransferase, and PmrC has been demonstrated to be necessary for the transfer of pEtN to the 1-phosphate of lipid A (22).
Two of these loci, pmrC and yijP (STM4118, hereafter called cptA), have been previously shown to be regulated by PmrA (15, 31, 34), while the role of PmrA-PmrB in the regulation of STM0834 and STM3635 was unknown. Therefore, to determine whether PmrA activated transcription of these genes, expression of lacZ transcriptional fusions to STM3635 and STM0834 in PmrAc and PmrA-null backgrounds was compared. This analysis demonstrated that neither STM0834 nor STM3635 was regulated by PmrA (data not shown). Because the transferase that catalyzes addition of pEtN to the Salmonella LPS core is predicted to be regulated by PmrA (18, 40), STM0834 and STM3635 were not further characterized.
Structural analysis of LPS demonstrates that cptA, but not pmrC, is required for pEtN addition to the LPS core.
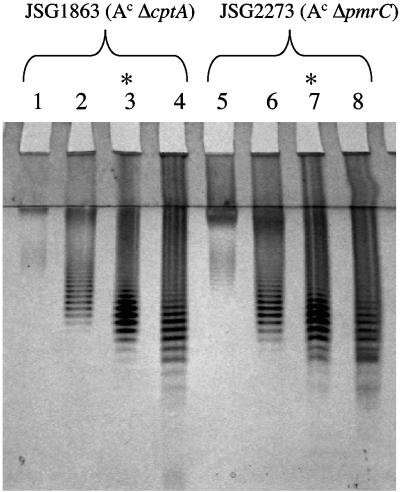
To further examine the role of pmrC and cptA in LPS modification, deletions in each gene were created in a JSG435 (PmrA-constitutive, PmrAc) background. The PmrAc strain possesses high-level PM resistance and high levels of Ara4N- and pEtN-modified LPS (18). Following the isolation of LPS from these mutants, the DOC-PAGE profile showed the characteristic ladder pattern, indicating molecular heterogeneity in the LPS. No obvious differences were detected in the patterns of the LPS between the cptA and pmrC mutants, or in comparison to the controls (PmrA-null or PmrAc). After separation by size exclusion chromatography, LPS fractions were identified that were enriched in LPS molecules bearing a low number of O-antigenic repeats (Fig. 1). Compositional analysis showed that higher-molecular-weight material contained more mannose and rhamnose sugars compared to the low-molecular-weight materials (data not shown). Appropriate low-molecular-weight fractions were chosen, and the OS was prepared from the core-enriched LPS molecule by mild acid hydrolysis.
FIG. 1.
DOC-PAGE analysis of LPS fractions. Sephacryl S-200 fractions of whole LPS from strains JSG1863 and JSG2273 were collected and electrophoresed. Four lanes corresponding to different fractions are shown for each strain (lanes 1 to 4, JSG1863; lanes 5 to 8, JSG2273). Similar profiles of strains JSG421 and JSG435 were performed but are not shown. Asterisks mark the lanes with the polysaccharide chosen for further analysis.
The NMR data indicated a mixture of molecules, as expected from LPS heterogeneity. The anomeric region indicated that most of the sugars are α-linked and lie between 5.8 and 4.8 ppm; however, there was one β-linked sugar at 4.5 ppm, as was evident from 2D-NMR spectroscopy. The other reporting group signals were 6-deoxy methyl doublets from rhamnose at 1.0 to 1.35 ppm and N-acetyl methyl singlets at 2.1 ppm. The H-3 methylene protons of the Kdo residue resonate at 1.9 and 2.2 ppm. The other nonanomeric signals from ring protons overlap between 4.4 and 3.5 ppm. For the controls, the characteristic chemical shift for the distal methylene (-CH2-) protons from the phosphorylated ethanolamine group (pEtN/PEA) was found in both JSG435 (PmrAc) and also in JSG421 (PmrA-null) at 3.3 ppm, which was evident from the NMR spectra (Fig. 2). However, the ratio of the integral area for the methylene to anomeric proton at 5.21 ppm was 2:1 for JSG435 and 1.5:1 for JSG421, indicating that the latter strain contained less pEtN. 2D-NMR further confirmed the presence of the pEtN group on both OS preparations from JSG435 and JSG421 in that there was a distinct COSY correlation between the distal and proximal -CH2- protons at 3.3 and 4.2 ppm, respectively (data not shown). Further, the presence of pEtN was confirmed by C-H one-bond heteronuclear g-HSQC analysis, which showed that the distal -CH2 protons at 3.3 ppm were correlated to the nitrogen-bearing carbon of the pEtN group, which resonated at 40.0 ppm (data not shown).
FIG. 2.
Proton NMR spectra of LPS core-enriched polysaccharide preparations. The proton signals are as indicated in the figure. Strain JSG1863 (PmrAc ΔcptA) is missing the signal at 3.3 ppm corresponding to the chemical shift for the distal methylene (-CH2-) protons of the pEtN (also called -PEA). All other samples, including that of JSG2273 (PmrAc ΔpmrC) and JSG421 (pmrA::Tn10d-tet), still possess a signal at 3.3 ppm (although the signal is less intense in JSG421). The signals at about 2.9 ppm (marked with an asterisk) are due to an unidentified contaminant.
MALDI was also performed on the controls. The MALDI mass spectrum showed the presence of more than one molecular species. The 123 mass unit difference between m/z at 2426 and 2549 in the JSG435 OS indicates the presence of pEtN in this mutant (Fig. 3). There also was a molecular distribution of oligosaccharides with a larger number of repeating units (OS1) that have the pEtN-bearing oligosaccharide. We also observed similar ions in JSG421 samples, with a 123 mass difference indicating the presence of pEtN distribution on that oligosaccharide (Fig. 3). However, again, the intensity of pEtN-bearing signals was less in the JSG421 OS than in JSG435 OS.
FIG. 3.
The MALDI-TOF mass spectrometry spectrum of LPS core-enriched polysaccharide preparations. As indicated in the figure, the clusters of signals are due to polysaccharides comprised of the core oligosaccharide plus one O-chain repeat unit, core plus two repeat units, etc. The core (after mild acid hydrolysis) is defined as that published by Olsthoorn et al. (25). The structures corresponding to m/z 2644.6 and m/z 3245.0, 220 mass units larger than m/z 2424.8 and m/z 3025.0, respectively, are likely due to structures that still contain the second Kdo residue of the core oligosaccharide. Ion peaks at 2446.76 and 3044.34 in JSG2273 (2446.63 and 3045.15 in JSG1863) are due to sodiated (+22 mass units) forms of other apparent ions. These spectra clearly reveal the presence of structures with an added pEtN (PEA) component in JSG345, JSG421, and JSG2273, i.e., ions that are increased 123 mass units, as indicated in the figure. The lack of a 123-mass unit increase in any observed peak confirms proton NMR data concerning the lack of pEtN in the LPS core of strain JSG1863 (PmrAc ΔcptA).
Examination of JSG1863 (PmrAc ΔcptA) and JSG2273 (PmrAc ΔpmrC) by NMR and MALDI was also performed. As with the controls, the distal (with respect to phosphate) methylene protons (-CH2-) of the pEtN group had a characteristic chemical shift near 3.3 ppm. The 1D-1H-NMR of the OS isolated from JSG1863 and JSG2273 showed the presence of the distal -CH2 signal of pEtN at 3.3 ppm in the latter, while it was completely absent on the former (Fig. 2). 2D-NMR further detected the presence of the pEtN group on the OS from JSG2273, where there is a distinct COSY correlation between the distal and proximal -CH2- at 3.3 and 4.2 ppm, respectively. The presence of the pEtN signal was confirmed by the C-H one-bond heteronuclear g-HSQC spectrum, where the distal -CH2 protons at 3.3 ppm correlated with a nitrogen-bearing carbon of the pEtN group that resonated at 40.0 ppm (data not shown). In the MALDI mass spectrum, the presence of the pEtN signal in JSG2273 was observed (the −123 mass unit difference at m/z at 2417.3 and 2540.3 in the JSG2273 OS) (Fig. 3). However, there was no indication of −123 mass differences in the JSG1863 mutant, demonstrating the lack of pEtN in the core of this mutant. Other minor differences were noted between JSG1863 and controls, but they did not correlate with pEtN modification. Therefore, the core of the LPS in JSG2273 (PmrAc ΔpmrC) still possesses pEtN, whereas the LPS core of JSG1863 (PmrAc ΔcptA) does not.
pmrC and cptA have a modest effect on resistance to PM.
Strains with various combinations of deletions in pmrC and cptA (in a PmrAc background; JSG1863 and JSG2273) were tested for increased susceptibility to PM using standard MIC assays. Strains JSG1863 and JSG2273 each showed a twofold decrease in PM resistance (4 μg/ml PM) compared to the parent PmrAc strain (8 μg/ml PM). Because the loss of Ara4N has been shown to have a dramatic effect on PM resistance in S. enterica serotypes, mutants containing defined pmrHFIJKLM operon insertions, or those deficient in this operon plus either pmrC or cptA, were examined in MIC assays. Such double mutants were shown to be no more susceptible to PM than mutation of pmrF alone (0.03 μg/ml PM). While these data suggested that elimination of pEtN phosphotransferase genes does not amplify PM susceptibility beyond that conferred by abolishing Ara4N modification of lipid A, this analysis was furthered with additional PM sensitivity assays. Survival assays using 0.5 μg/ml polymyxin B and strains containing pmrC, cptA, or pmrC cptA double mutations in a PmrAc background were performed. As shown in Fig. 4A, these mutants possessed no statistically significant differences in their levels of survival in comparison to the PmrAc parental strain, further demonstrating that pEtN modification of LPS plays a modest role in regard to PM resistance. When these mutations (pmrC, cptA, or pmrC cptA) were examined in a PmrAc pmrF::Tn10d background, both cptA and pmrC resulted in a statistically significant reduction in survival compared to the PmrAc pmrF::Tn10d strain (Fig. 4B). However, while the cptA mutation resulted in a ∼2-fold drop in survival, the PmrAc pmrF::Tn10d pmrC strain (as well as the PmrAc pmrF::Tn10d pmrC cptA strain) demonstrated a 94-fold drop in survival, which obtained a level similar to the survival of a PmrA-null strain. Thus, cptA appears to play a minor role in PM resistance, which can be primarily observed in the absence of Ara4N modification of lipid A. The pmrC gene appears to play a larger role in PM resistance, but it also primarily plays a role only in the absence of Ara4N lipid A modification.
FIG. 4.
PM resistance assays of strains deficient in LPS modifications. Strains were incubated in the presence of 0.5 μg/ml PM for 1 h at room temperature followed by plating to determine the percent survival. (A) Strains with mutations in cptA, pmrC, or both genes were examined for PM sensitivity in a PmrA-constitutive (PmrAc) background. Neither mutation had a significant effect on PM resistance in this background compared to the PmrAc parent strain (t test or analysis of variance [ANOVA], P > 0.05). (B) Strains with mutations in cptA, pmrC, or both genes were examined for PM sensitivity in a PmrAc pmrF (aminoarabinose-deficient) background. Strains containing cptA, pmrC, or both mutations in the PmrAc pmrF background demonstrated a level of sensitivity to PM that was significantly different from the PmrAc pmrF parent strain (t test or ANOVA, P < 0.05). Error bars indicate the standard errors.
Strains lacking both cptA and pmrC, but not those lacking either gene alone, show a modest virulence defect in the mouse model of typhoid fever.
Because the loss of pEtN plays a modest role in resistance to AP and AP resistance has been shown to play a role in virulence, pEtN mutants were examined for virulence defects in the mouse model. Mice infected by the intraperitoneal or oral routes with strains containing cptA, pmrC, or cptA pmrC double deletions in a wild-type background showed no differences in the 50% lethal dose compared to controls (data not shown).
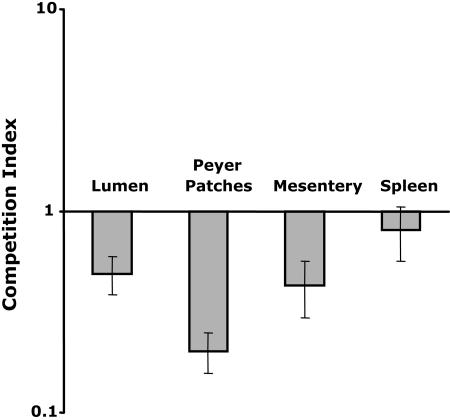
To further examine the in vivo relevance of pEtN LPS modification on virulence, competition infection experiments were performed. While single mutations in cptA or pmrC had no defect in competition experiments when evaluated against the wild-type strain (data not shown), a cptA pmrC double mutant showed a consistent, but less than 10-fold decrease in survival in all tissues examined (Fig. 5). Therefore, the inability to modify LPS in vivo with pEtN on both core and lipid A regions can be shown to play a minor role in virulence.
FIG. 5.
Competition infection experiments of the wild type (JSG1311) and double mutant (cptA pmrC; JSG2449). At 4 days postinfection, selected organs and tissues were harvested and the contents were examined for surviving bacteria by culture on selective, solid medium. Error bars indicate the standard errors.
DISCUSSION
The expression of mechanisms for resistance to AP is an important determinant of pathogenicity, and susceptibility of bacteria to AP is associated with attenuation of virulence (9, 10, 13, 28). The TCRS PmrA-PmrB of S. enterica serovar Typhimurium has been shown to be activated within host cell vacuoles (1, 17) and has been characterized as contributing to AP resistance by mediating alterations to the outer membrane and LPS (reviewed in reference 8). PmrA controls the expression of pmrE and pmrHFIJKLM, which encode enzymes involved in producing Ara4N substitutions on lipid A (16). In addition, PM-sensitive strains have been reported to lack the ability to produce pEtN substitutions in the lipid A and core oligosaccharide fractions of LPS (18, 40).
Four Salmonella open reading frames (STM3635, STM4293 [pmrC], STM4118 [cptA], and STM0834) were shown to possess significant similarity to the N. meningitidis genes (lpt-3 and lptA) that encode pEtN phosphotransferases (5, 23). Two of these genes, STM4293 (pmrC) and STM4118 (cptA), were previously shown to be PmrA regulated (34). STM3635 and STM0834 are not PmrA regulated and their function remains unknown, although by homology they appear to be phosphoethanolamine phosphotransferases. pmrC, the first open reading frame in the pmrCAB operon, has been shown to be necessary for the transfer of pEtN to lipid A (22). However, it was not shown in this work whether PmrC could add pEtN to other positions in the LPS, such as the core. We hypothesized that PmrC may be a phosphoethanolamine phosphotransferase specific for lipid A and cptA may encode a phosphoethanolamine phosphotransferase specific for the LPS core.
NMR and mass spectrometry of PmrAc pmrC and PmrAc cptA mutants demonstrated that cptA but not pmrC is necessary for adding pEtN to the LPS core. Therefore, there exist distinct PmrA-regulated phosphoethanolamine phosphotransferases of the LPS core and lipid A (as it is likely that CptA does not add pEtN to lipid A). It is highly probable that the pEtN group observed in this work is that added to heptose I of the core. A second PmrA-regulated modification of the inner core Kdo with pEtN has been reported (18) but not confirmed. Such a modification could not have been observed in our NMR or mass spectroscopy studies due to the biochemical processing of the oligosaccharide prior to analysis, but it will be the subject of future studies with additional PmrA-regulated gene mutants. Also of interest in these biophysical studies was the identification of pEtN in the LPS core of the PmrA-null strain. This was surprising, because PmrA regulates the expression of cptA, which has a clear pEtN-deficient phenotype. This may suggest that core pEtN modification and/or cptA expression is not completely dependent upon PmrA.
Increased substitution of lipid A with Ara4N results in increased resistance to AP, including PM, neutrophil granule AP azurocidin (CAP-37), and bactericidal permeability-increasing protein (CAP-57) (18, 29, 33, 36). It is surmised that substitution of the anionic phosphates of LPS with moieties such as Ara4N reduces the electrostatic attraction and binding between the LPS and cationic AP. LPS from PM-resistant Salmonella binds PM poorly in vitro (37). Because pEtN addition to lipid A and core phosphate moieties would also result in decreased anionic charge of the LPS, it was expected that pEtN modification of LPS should play a similar role in AP resistance. This reasoning is also in part due to the results of Yethon et al., which demonstrated that the loss of waaP, which catalyzes the phosphorylation of the HepI of the Salmonella LPS core and is required for further pEtN modification of this Hep residue, affected both PM resistance and virulence (39). Here, we show that the lack of PmrC or CptA activity reduced the MIC of PM 2-fold, whereas elimination of Ara4N modification reduced resistance to PM 100-fold (14, 16). Similar major effects of Ara4N and minor effects of pEtN were observed in PM survival assays. Therefore, while both modifications decrease the anionic charge of LPS, only Ara4N substitution dramatically affects the ability of PM to associate with LPS. These results suggest that LPS charge is only partially responsible for interactions between PM and LPS and that the structure and/or location of the Ara4N modification to lipid A has an inhibitory effect on PM binding. With respect to pmrC, these results are somewhat contradictory to the results of Lee et al. (22). In that work, a strain with a mutation in pmrC had a significant effect on PM resistance but, similar to our findings, the role of pmrC-mediated LPS modification was inferior to that of Ara4N LPS modification with regard to PM resistance. As a possible explanation of these contradictory results, variations in experimental conditions may have led to differential pmrC expression and/or PM killing that influenced in vitro bacterial survival.
The PmrA-PmrB TCRS, and also the dependent AP resistance, has been demonstrated to affect virulence of S. enterica serovar Typhimurium in the mammalian host (16). A null mutation in pmrA, pmrE, or pmrHFIJKLM decreases AP resistance and results in 10- to 1,000-fold decreased survival of the bacteria in the murine model of infection (16). Therefore, it was of interest to determine whether the PmrA-mediated pEtN substitution of core and lipid A contributed to AP resistance and/or survival in the host. Mutation of pmrC or cptA did not result in decreased virulence as individual mutations but, when combined, resulted in a consistent but less than 10-fold decrease in the competitive index compared to a wild-type strain. This decrease in competitive index was even observed in the intestinal lumen, an early point after infection. This may suggest that antimicrobial factors in the lumen (possibly including AP) substantially contribute to the observed reduction in the competitive index. However, the decreased AP resistance evinced by loss of pEtN modifications was not sufficient to be dramatically detrimental to survival of Salmonella in the murine model. The previously mentioned waaP mutation had a dramatic affect on virulence, which might have been due to the increased susceptibility to AP in this mutant (39). However, since both this waaP and the cptA mutant are missing pEtN on the core but do not have equal virulence defects, it is likely that the virulence attenuation of the waaP mutant can be attributed to its other known membrane effects, such as an increase in outer membrane phospholipids.
Activation of pmrC and cptA by PmrA suggests that these loci, and the consequent pEtN modifications, are up-regulated during infection of the mouse (17), but the function of pEtN LPS modifications remain unclear. Evidence in N. meningitidis indicates that LOS is a receptor for complement component C4b and that substitution of core with pEtN affects binding by C4b (26). Specifically, pEtN substitutions on HepII form amide bonds with C4b and augment complement-mediated killing in serum bactericidal assays. Bacteria with pEtN additions at position 6 of HepII were more efficiently eliminated than those with substitutions on position 3, and strains with pEtN at position 3 of HepII were more likely to be found in clinical isolates (26). Salmonella has been shown to add pEtN to Kdo and HepI, but not HepII; however, pEtN substitution of the core may likewise affect complement killing in Salmonella. If so, AP resistance attributed to pEtN substitution may be negated by increased recognition and elimination by complement. Further studies of the effect of pEtN substitution of LPS on resistance to AP other than PM and on immune recognition are required in order to understand the physiological role of pEtN modification in vivo.
Acknowledgments
This work was funded by grants from the National Institutes of Health to J.S.G. (AI43521 and AI43521-S1) and a CCRC center grant from the DOE to R.C. (DE-FG-02-93ER20097).
REFERENCES
- 1.Alpuche Aranda, C. M., J. A. Swanson, W. P. Loomis, and S. I. Miller. 1992. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc. Natl. Acad. Sci. USA 89:10079-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apicella, M. A., J. M. Griffiss, and H. Schneider. 1994. Isolation and characterization of lipopolysaccharides, lipooligosaccharides, and lipid A. Methods Enzymol. 235:242-252. [DOI] [PubMed] [Google Scholar]
- 3.Bearson, B. L., L. Wilson, and J. W. Foster. 1998. A low pH-inducible, PhoPQ-dependent acid tolerance response protects Salmonella typhimurium against inorganic acid stress. J. Bacteriol. 180:2409-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9-14. [DOI] [PubMed] [Google Scholar]
- 5.Cox, A. D., J. C. Wright, J. Li, D. W. Hood, E. R. Moxon, and J. C. Richards. 2003. Phosphorylation of the lipid A region of meningococcal lipopolysaccharide: identification of a family of transferases that add phosphoethanolamine to lipopolysaccharide. J. Bacteriol. 185:3270-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellermeier, C. D., A. Janakiraman, and J. M. Slauch. 2002. Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene 290:153-161. [DOI] [PubMed] [Google Scholar]
- 8.Ernst, R. K., T. Guina, and S. I. Miller. 2001. Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microbes Infect. 3:1327-1334. [DOI] [PubMed] [Google Scholar]
- 9.Fields, P. I., E. A. Groisman, and F. Heffron. 1989. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science 243:1059-1062. [DOI] [PubMed] [Google Scholar]
- 10.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia Vescovi, E., F. C. Soncini, and E. A. Groisman. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165-174. [DOI] [PubMed] [Google Scholar]
- 12.Groisman, E. A., J. Kayser, and F. C. Soncini. 1997. Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J. Bacteriol. 179:7040-7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groisman, E. A., C. Parra-Lopez, M. Salcedo, C. J. Lipps, and F. Heffron. 1992. Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc. Natl. Acad. Sci. USA 89:11939-11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunn, J. S., K. B. Lim, J. Krueger, K. Kim, L. Guo, M. Hackett, and S. I. Miller. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 27:1171-1182. [DOI] [PubMed] [Google Scholar]
- 15.Gunn, J. S., and S. I. Miller. 1996. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J. Bacteriol. 178:6857-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunn, J. S., S. S. Ryan, J. C. Van Velkinburgh, R. K. Ernst, and S. I. Miller. 2000. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 68:6139-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heithoff, D. M., C. P. Conner, P. C. Hanna, S. M. Julio, U. Hentschel, and M. J. Mahan. 1997. Bacterial infection as assessed by in vivo gene expression. Proc. Natl. Acad. Sci. USA 94:934-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helander, I. M., I. Kilpelainen, and M. Vaara. 1994. Increased substitution of phosphate groups in lipopolysaccharides and lipid A of the polymyxin-resistant pmrA mutants of Salmonella typhimurium: a 31P-NMR study. Mol. Microbiol. 11:481-487. [DOI] [PubMed] [Google Scholar]
- 19.Kato, A., and E. A. Groisman. 2004. Connecting two-component regulatory systems by a protein that protects a response regulator from dephosphorylation by its cognate sensor. Genes Dev. 18:2302-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato, A., T. Latifi, and E. A. Groisman. 2003. Closing the loop: the PmrA/PmrB two-component system negatively controls expression of its posttranscriptional activator PmrD. Proc. Natl. Acad. Sci. USA 100:4706-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kox, L. F., M. M. Wosten, and E. A. Groisman. 2000. A small protein that mediates the activation of a two-component system by another two-component system. EMBO J. 19:1861-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, H., F. F. Hsu, J. Turk, and E. A. Groisman. 2004. The PmrA-regulated pmrC gene mediates phosphoethanolamine modification of lipid a and polymyxin resistance in Salmonella enterica. J. Bacteriol. 186:4124-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackinnon, F. G., A. D. Cox, J. S. Plested, C. M. Tang, K. Makepeace, P. A. Coull, J. C. Wright, R. Chalmers, D. W. Hood, J. C. Richards, and E. R. Moxon. 2002. Identification of a gene (lpt-3) required for the addition of phosphoethanolamine to the lipopolysaccharide inner core of Neisseria meningitidis and its role in mediating susceptibility to bactericidal killing and opsonophagocytosis. Mol. Microbiol. 43:931-943. [DOI] [PubMed] [Google Scholar]
- 24.Miller, S. I., A. M. Kukral, and J. J. Mekalanos. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. USA 86:5054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsthoorn, M. M., B. O. Petersen, J. Duus, J. Haverkamp, J. E. Thomas-Oates, K. Bock, and O. Holst. 2000. The structure of the linkage between the O-specific polysaccharide and the core region of the lipopolysaccharide from Salmonella enterica serovar Typhimurium revisited. Eur. J. Biochem. 267:2014-2027. [DOI] [PubMed] [Google Scholar]
- 26.Ram, S., A. D. Cox, J. C. Wright, U. Vogel, S. Getzlaff, R. Boden, J. Li, J. S. Plested, S. Meri, S. Gulati, D. C. Stein, J. C. Richards, E. R. Moxon, and P. A. Rice. 2003. Neisserial lipooligosaccharide is a target for complement component C4b. Inner core phosphoethanolamine residues define C4b linkage specificity. J. Biol. Chem. 278:50853-50862. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberger, C. M., R. L. Gallo, and B. B. Finlay. 2004. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc. Natl. Acad. Sci. USA 101:2422-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salzman, N. H., D. Ghosh, K. M. Huttner, Y. Paterson, and C. L. Bevins. 2003. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422:522-526. [DOI] [PubMed] [Google Scholar]
- 29.Shafer, W. M., S. G. Casey, and J. K. Spitznagel. 1984. Lipid A and resistance of Salmonella typhimurium to antimicrobial granule proteins of human neutrophil granulocytes. Infect. Immun. 43:834-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soncini, F. C., E. Garcia Vescovi, F. Solomon, and E. A. Groisman. 1996. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: identification of PhoP-regulated genes. J. Bacteriol. 178:5092-5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soncini, F. C., and E. A. Groisman. 1996. Two-component regulatory systems can interact to process multiple environmental signals. J. Bacteriol. 178:6796-6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinberg, D. A., M. A. Hurst, C. A. Fujii, A. H. Kung, J. F. Ho, F. C. Cheng, D. J. Loury, and J. C. Fiddes. 1997. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 41:1738-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stocker, B. A., and P. H. Makela. 1978. Genetics of the (gram-negative) bacterial surface. Proc. R. Soc. London B 202:5-30. [DOI] [PubMed] [Google Scholar]
- 34.Tamayo, R., A. M. Prouty, and J. S. Gunn. 2005. Identification and functional analysis of Salmonella enterica serovar Typhimurium PmrA-regulated genes. FEMS Immunol. Med. Microbiol. 43:249-258. [DOI] [PubMed] [Google Scholar]
- 35.Trent, M. S., A. A. Ribeiro, S. Lin, R. J. Cotter, and C. R. Raetz. 2001. An inner membrane enzyme in Salmonella and Escherichia coli that transfers 4-amino-4-deoxy-l-arabinose to lipid A: induction on polymyxin-resistant mutants and role of a novel lipid-linked donor. J. Biol. Chem. 276:43122-43131. [DOI] [PubMed] [Google Scholar]
- 36.Vaara, M., T. Vaara, M. Jensen, I. Helander, M. Nurminen, E. T. Rietschel, and P. H. Makela. 1981. Characterization of the lipopolysaccharide from the polymyxin-resistant pmrA mutants of Salmonella typhimurium. FEBS Lett. 129:145-149. [DOI] [PubMed] [Google Scholar]
- 37.Vaara, M., T. Vaara, and M. Sarvas. 1979. Decreased binding of polymyxin by polymyxin-resistant mutants of Salmonella typhimurium. J. Bacteriol. 139:664-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright, J. C., D. W. Hood, G. A. Randle, K. Makepeace, A. D. Cox, J. Li, R. Chalmers, J. C. Richards, and E. R. Moxon. 2004. lpt6, a gene required for addition of phosphoethanolamine to inner-core lipopolysaccharide of Neisseria meningitidis and Haemophilus influenzae. J. Bacteriol. 186:6970-6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yethon, J. A., J. S. Gunn, R. K. Ernst, S. I. Miller, L. Laroche, D. Malo, and C. Whitfield. 2000. Salmonella enterica serovar Typhimurium waaP mutants show increased susceptibility to polymyxin and loss of virulence in vivo. Infect. Immun. 68:4485-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou, Z., A. A. Ribeiro, S. Lin, R. J. Cotter, S. I. Miller, and C. R. Raetz. 2001. Lipid A modifications in polymyxin-resistant Salmonella typhimurium: PmrA-dependent 4-amino-4-deoxy-l-arabinose, and phosphoethanolamine incorporation. J. Biol. Chem. 276:43111-43121. [DOI] [PubMed] [Google Scholar]