Abstract
Background
Cannabis is used by patients with Crohn’s disease (CD) and ulcerative colitis (UC) as an alternative to, or in combination with, conventional therapies to treat symptoms such as abdominal pain, poor sleep, and reduced appetite. The clinical efficacy of cannabis for these disorders is controversial, with some studies showing harmful outcomes associated with its use. Previous studies suggest that cannabis is used by ~12% of patients with UC and ~16% of patients with CD in the USA despite legal prohibition.
Methods
We conducted a prospective cohort study of adult patients with inflammatory bowel diseases (IBD) followed in a Canadian tertiary care center. Patients completed an online 40-question survey that included demographics, IBD disease history, cannabis use, and the Short Inflammatory Bowel Disease Questionnaire (SIBDQ).
Results
Completed surveys were obtained from 254 participants (148 with CD, 90 with UC, and 16 with indeterminate colitis). Recent cannabis use was reported by 41% of CD and 31% of UC participants. Interestingly, only 46% of participants who used cannabis discussed their use with their physician. Participants who recently used cannabis reported more abdominal pain, poor appetite, and flatulence, and importantly this was associated with lower SIBDQ scores (recent use 37 vs non-recent use 40).
Conclusions
Cannabis use among patients with IBD has more than doubled since its legalization. Cannabis use is associated with worse abdominal symptoms and quality of life. Physicians should inquire about cannabis use and optimize symptom control with evidence-based therapies.
Keywords: Cannabis, marijuana, colitis, Crohn’s disease, inflammatory bowel disease
Investigating cannabis use post-legalization in Canadian IBD patients revealed widespread lifetime usage. Recent use was linked to a decline in reported quality of life, indicating potential considerations for IBD management.
Key Messages.
What is already known? In IBD, cannabis use for symptom relief is infrequent and its medicinal efficacy is debated amid contradictory findings on its impact on inflammation.
What is new here? The legalization of cannabis in Canada has led to an increased prevalence of its use among patients with IBD, and this usage is associated with worsened symptom severity.
How can this help patient care? The study informs healthcare professionals about the association between cannabis use and symptom severity, encouraging discussions with patients regarding cannabis use in the management of IBD.
Introduction
The inflammatory bowel diseases (IBD), Crohn’s disease (CD), and ulcerative colitis (UC) are characterized by chronic and relapsing inflammation of the gastrointestinal tract. A diagnosis of IBD impacts quality of life. A cohort of Norwegian patients with IBD reported a significantly lower quality of life if they had moderate or severe symptoms compared to individuals without active symptoms.1 Indeed, the persistence of symptoms despite the advent of novel therapies is often problematic for patients. Conversely, in the absence of physical symptoms, patients can also continue to describe feeling stress, anxiety, fear about pain, and worry about the consequences of their disease. This often compels patients to seek out alternative therapeutic options.2
Marijuana, cultivated from the flowering plant cannabis sativa, is a frequently used herb for symptom control in patients with IBD. Cannabis is reported to improve stress levels, sleep, and symptoms such as abdominal pain, diarrhea, and reduced appetite.3–5 The clinical effects of cannabis are thought to be due to endocannabinoid receptors present within the gastrointestinal tract, central and peripheral nervous system, and lymphoid tissues. Interestingly, the medicinal effects of cannabis in IBD are controversial; several studies suggest a potential benefit of cannabis, while others report no effect or association with worse outcomes.6–9 A meta-analysis of 15 non-randomized and 5 randomized trials examining cannabis in IBD concluded there may be some benefit with respect to patient-reported outcomes, but not the reduction of inflammation.6 Glickman similarly conducted the largest population-based study examining cannabis use and mortality risk in IBD and reported a positive association with negative outcomes such as hospitalization and corticosteroid use but no increased risk of surgery or death.9
Cannabis was legalized in Canada in 2018 and this has led to a sudden rise in its use as producers increase their marketing efforts.10 Thus, Canada is in a unique position to study the effects of widespread legalization, and evaluation of the medicinal effects of cannabis on disease. In a 2006 survey study conducted prior to legalization, approximately 50% of Canadian patients with IBD reported lifetime cannabis use.4 In that study, patients with CD reported current cannabis use rates of 12% and 16%, respectively.4 Similar estimates were reported in a study from Massachusetts where medical cannabis was legalized but recreational possession was not.3 Cannabis use, however, was likely underestimated by these studies as study participants may not have answered truthfully at the time due to fear of stigmatization. Indeed, 52% of patients with IBD who never used cannabis in one study stated they would be interested in trying it once it became legally available.3 In the current study, we aimed to determine the prevalence of cannabis use in patients with IBD following its legalization in Canada. We additionally assessed whether patients with IBD who currently use cannabis, report a change in disease severity when compared to non-users.
Methods
Study Design
We performed a prospective cohort study examining the prevalence and effects of cannabis use in patients with IBD. We recruited patients during their scheduled gastroenterology clinic appointments at 2 tertiary care centers in London, Ontario. Clinic appointments occurred either in-person or virtually during the COVID-19 pandemic. Patient recruitment occurred between October 2020 and December 2021. To be eligible for the study, individuals had to be (1) 18 years of age or older, and (2) have a diagnosis of IBD as determined by their gastroenterologist. Patients were eligible if they were diagnosed with either CD, UC, or indeterminant colitis (IC). The study aimed to recruit 292 participants based on similar studies, allowing for interim analysis to assess significant data.3 After being introduced to the study, interested participants were sent an electronic link to the study’s Letter of Information. Consent to participate in the study was collected electronically and stored securely. Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at the Lawson Health Research Institute.11,12 The study was approved by the Western University Ethics Committee.
Survey
The online 40-question survey was distributed and collected by REDCap and data on demographics, IBD disease history, and cannabis use were compiled. Quality of life was assessed using the Short Inflammatory Bowel Disease Questionnaire (SIBDQ), a 10-question survey validated for use in both UC and CD patients.13 The SIBDQ consists of questions addressing bowel, systemic, social, and emotional dimensions that are scored on a 7-point scale. Lower scores indicate lower quality of life. Use of the SIBDQ was approved and licensed by McMaster University. Incomplete surveys were excluded from the final analysis. Recent use was defined as those with any use of cannabis within the past 6 months.
Data Analysis
Descriptive statistics were used to identify the mean and standard deviation for variables of interest. Continuous variables were compared with 2-tailed Student’s t-tests for parametric data and Mann–Whitney test for non-parametric data. Categorical variables were analyzed using the chi-square test. A P-value of less that .05 was considered significant. All data analysis was performed using GraphPad Prism version 9.3.1 for macOS (GraphPad Software, San Diego, California USA).
Results
A total of 437 individuals were recruited for this study over a 13-month period. Of those recruited, 148 individuals did not complete the online consent process or respond to our reminder email (Figure 1). The online consent was additionally completed by 35 individuals who were subsequently excluded due to incomplete survey completion. In total, 254 individuals consented and completed the survey yielding a completion rate of 58.1%. We collected completed surveys from 148 individuals with CD (58.3%), 90 with UC (35.4%), and 16 with IC(6.3%).
Figure 1.
Patient recruitment into study.
Participants’ ages were equally distributed amongst IBD subtypes (Table 1). No gender difference was observed amongst IBD subtypes. Over half of the participants were between 35 and 64 years of age and female. Most study participants identified as Caucasian, with the CD group being comprised of significantly more Caucasian individuals than the UC group (141/148 CD vs 76/90 UC, P < .05). Active tobacco use was not common among participants, but a third did report having a previous smoking history. Tobacco smoking habits did not differ between IBD groups. Fifty-three percent of participants (135/254) responded they had ever (previously and/or currently) used cannabis and this rate did not differ between IBD subtypes (CD 55%, UC 51%, and IC 44%). Thirty-six percent of participants had “recently used cannabis,” which was defined as use within the past 6 months. Participants with CD had higher active use rates when compared to those with UC (CD 41% vs UC 31%, P < .05).
Table 1.
Survey participant demographics.
| CD (n = 148) |
UC (n = 90) |
IC (n = 16) |
||
|---|---|---|---|---|
| Female % | 61 | 47 | 56 | |
| Age, year % | 18–24 | 7 | 8 | 6 |
| 25–34 | 16 | 19 | 0 | |
| 35–44 | 19 | 22 | 38 | |
| 45–54 | 26 | 19 | 13 | |
| 55–64 | 20 | 18 | 19 | |
| 65–74 | 9 | 12 | 19 | |
| 75+ | 3 | 2 | 6 | |
| Race/Ethnicity % | African American | 0 | 1 | 0 |
| Asian | 1 | 3 | 6 | |
| Caucasian | 95* | 84 | 88 | |
| First nations | 0 | 2 | 0 | |
| Hispanic | 1 | 2 | 0 | |
| Other | 3 | 7 | 6 | |
| Tobacco use % | Never | 52 | 57 | 56 |
| Quit | 41 | 33 | 31 | |
| Active | 7 | 10 | 13 | |
| Ever used cannabis? % | 55 | 51 | 44 | |
| Cannabis use in past 6 months? % | 41* | 31 | 25 | |
* P < .05 CD vs UC.
Half of individuals with IC were diagnosed in the preceding 3 years and this was significantly higher when compared to either CD or UC (IC 50% vs 7% CD and 12% UC, P < .05; Table 2). On the other hand, over half of CD and UC participants were diagnosed more than 10 years ago (19% IC vs 66% CD and 54% UC, P < .05).
Table 2.
Inflammatory bowel disease medical history and symptoms.
| CD (n = 148) |
UC (n = 90) |
IC (n = 16) |
||
|---|---|---|---|---|
| Length of IBD diagnosis % | <3 years | 7 | 12 | 50* |
| 3–5 years | 10 | 9 | 19 | |
| 6–10 years | 16 | 24 | 13 | |
| >10 years | 66 | 54 | 19* | |
| IBD medications % | 5-ASA@ | 8 | 52 | 13 |
| Budesonide/prednisone | 7 | 8 | 6 | |
| Methotrexate/azathioprine | 17 | 7 | 0 | |
| Biologics$ | 70 | 42 | 13 | |
| Analgesics | 7 | 6 | 25 | |
| None | 14 | 14 | 50 | |
| Surgery for IBD % | 47# | 16 | 6 | |
| Active GI symptoms % | Nausea or vomiting | 26 | 17 | 38 |
| Poor appetite | 22 | 20 | 25 | |
| Abdominal pain | 64 | 50 | 81 | |
| Dyspepsia | 39 | 34 | 50 | |
| Diarrhea | 55 | 47 | 63 | |
| Constipation | 18 | 19 | 31 | |
| Flatulence | 39 | 38 | 38 | |
| None | 14 | 19 | 13 | |
* P < .05 IC vs CD and UC.
# P < .05 CD vs IC and UC.
@For example Asacol, Salofalk, and Pentasa.
$Including Infliximab (Remicade)/ Adulimumab (Humira)/ Ustekinumab (Stelara)/ Vedolizumab (Entyvio).
Seventy percent of the CD group and 42% of the UC group were treated with biologics. UC participants were commonly treated with 5-ASA products (52% UC vs 8% CD vs 13% IC). Fifty percent of participants with IC currently received no treatment. Surgical rates for IBD were more common amongst patients with CD versus the other IBD subtypes (CD 47% vs UC 16% and IC 6%, P < .05). The most common symptoms experienced by participants included abdominal pain, diarrhea, dyspepsia, and flatulence.
Given that more than half (53%) of survey responders used cannabis in their lifetime, we explored the characteristics of these patients further. We found that approximately half of the survey responders were female (Table 3), and participants stated that they used cannabis by inhalation, ingestion, or both equally. There was no preference for THC-predominant versus CBD-predominant cannabis observed. Only nineteen percent of survey responders had a prescription for cannabis and 30% had been using it for more than 10 years. Interestingly, 51% of participants were new to using cannabis and had started only in the preceding 3 years. Cannabis was used multiple times a week by 57% of those surveyed, while 19% only used it every few years. Survey participants commonly took up to half a gram of cannabis per use. cannabis was commonly used to treat gastrointestinal symptoms (30%), but also used for recreation (27%), and to help with sleep (26%). Gut symptoms relieved by cannabis included abdominal pain (53%), poor appetite (33%) and nausea or vomiting (26%). Cannabis users commonly reported side effects such as dry mouth (44%), anxiety or paranoia (25%), cognitive or concentration issues (24%), and red eyes (23%), whereas, 32% percent of participants, reported no side effects. Despite the common rate of side effects, 79% of users felt that the benefits of cannabis outweighed its unpleasantness. Many participants were aware of harmful consequences of long-term cannabis use including mood disorders (eg, depression, bipolar, and anxiety), psychosis (eg, schizophrenia), decreased sperm production, and even potentially increased risk of cancer. Interestingly, only 46% of cannabis users stated that they had discussed their cannabis use with their family physician or gastroenterologist.
Table 3.
Cannabis use patterns of amongst lifetime users.
| Lifetime use (n = 135) | ||
|---|---|---|
| Female % | 53 | |
| Route of cannabis use % | Inhalation | 27 |
| Ingestion | 36 | |
| Both | 37 | |
| Type of cannabis product preferred % | THC predominant | 23 |
| CBD predominant | 24 | |
| Both | 27 | |
| No preference | 26 | |
| Has cannabis prescription % | 19 | |
| Length of cannabis use % | <6 months | 14 |
| 6–12 months | 13 | |
| 1–3 years | 24 | |
| 4–5 years | 5 | |
| 6–10 years | 11 | |
| >10 years | 30 | |
| Frequency of cannabis use % | Daily | 31 |
| 4–6 times/week | 8 | |
| 1–3 times/week | 12 | |
| Every 1–2 weeks | 6 | |
| Every 3–4 weeks | 5 | |
| Every 1–5 months | 7 | |
| Every 6–11 months | 10 | |
| Every few years | 19 | |
| Amount of cannabis used in one sitting % | 0.1–0.5 grams | 78 |
| 0.6–1.0 grams | 12 | |
| 1–2 grams | 4 | |
| >2 grams | 1 | |
| Main reason for cannabis use % | Recreational | 27 |
| GI symptoms | 30 | |
| Anxiety or depression | 12 | |
| Sleep | 26 | |
| Other | 4 | |
| GI symptoms relieved by cannabis % | Nausea or vomiting | 26 |
| Poor appetite | 33 | |
| Abdominal pain/discomfort | 53 | |
| Diarrhea | 10 | |
| Constipation | 3 | |
| Flatulence | 5 | |
| None | 39 | |
| Biggest side effects of cannabis % | Anxiety or paranoia | 25 |
| Visual hallucinations | 4 | |
| Nausea or vomiting | 1 | |
| Palpitations | 9 | |
| Red eyes | 23 | |
| Dry mouth | 44 | |
| Cognitive or concentration issues | 24 | |
| Other | 9 | |
| None | 32 | |
| Benefits of cannabis outweigh side effects % | 79 | |
| Perceived consequences of chronic cannabis use % | Psychotic disorders& | 39 |
| Mood disorders$ | 41 | |
| Cancer | 24 | |
| Hyperemesis syndrome | 12 | |
| Fatty liver disease | 4 | |
| Worsening HCV@ | 3 | |
| Dental disease | 18 | |
| Oligospermia | 21 | |
| Other | 1 | |
| None | 44 | |
| Family physician or gastroenterologist aware of cannabis use % | 46 | |
&Such as schizophrenia,
$Such as depression, bipolar or anxiety disorder,
@Hepatitis C viral disease.
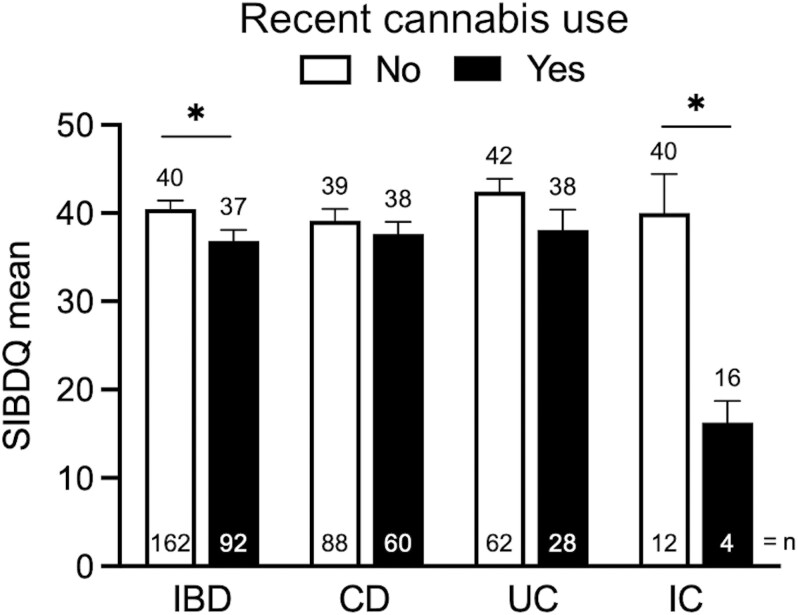
Next, we examined the impact of recent cannabis use on IBD severity. Recent users, defined as those patients who had used cannabis in the preceding 6 months, had similar patterns of IBD medication use as non-active users (Table 4). Furthermore, the two groups did not differ in rates of need for IBD-related surgery (recent use 35% vs. non-recent use 32%). Participants were more likely to report experiencing poor appetite, abdominal pain, and flatulence if they had used cannabis recently. Furthermore, recent cannabis use was associated with lower SIBDQ scores compared to non-recent users (recent use 37 vs non-recent use 40, P < .05) (Figure 2). When SIBDQ responses were analyzed according to the IBD subtype, recent and non-recent users displayed no statistical difference. In the UC subgroup, however, the P-value equaled .083 suggesting a trend towards lower quality of life in those participants who had used cannabis recently (Figure 2). Amongst individuals with IC, patients had significantly lower SIBDQ scores if they used cannabis recently (recent use 16 vs non-recent use 40; Figure 2).
Table 4.
Impact of Inflammatory Bowel Disease severity on cannabis use.
| Recent cannabis use | |||
|---|---|---|---|
| No (n = 162) |
Yes (n = 92) |
||
| IBD medications % | 5-ASA@ | 25 | 22 |
| Budesonide/prednisone | 9 | 5 | |
| Methotrexate/azathioprine | 13 | 11 | |
| Biologics$ | 54 | 61 | |
| Analgesics | 9 | 7 | |
| None | 15 | 17 | |
| Surgery for IBD % | 32 | 35 | |
| Active GI symptoms % | Nausea or vomiting | 20 | 29 |
| Poor appetite | 14* | 36 | |
| Abdominal pain | 54* | 71 | |
| Dyspepsia | 34 | 46 | |
| Diarrhea | 51 | 55 | |
| Constipation | 19 | 18 | |
| Flatulence | 30* | 54 | |
| None | 17 | 13 | |
* P < .05.
@For example Asacol, Salofalk, and Pentasa.
$Including Infliximab (Remicade)/ Adulimumab (Humira)/ Ustekinumab (Stelara)/ Vedolizumab (Entyvio).
Figure 2.
Impact of inflammatory bowel disease (IBD) subtype on the short inflammatory bowel disease questionnaire (SIBDQ) by recent cannabis use. Lower score indicates a lower quality of life. CD, Crohn’s Disease; UC, ulcerative colitis; IC, indeterminate colitis. *P < .05.
Discussion
In this study, we report the results of the first prospective cohort study to survey cannabis use amongst patients with IBD following the legalization of cannabis in Canada. The Cannabis Act was new legislation that was passed in 2018 with the aims of providing access to a quality-controlled supply of cannabis, displacing the illegal cannabis market and preventing youth from accessing cannabis. The Act allows any person over the age of 18 to possess up to 30 grams of legal cannabis and grow up to 4 plants for personal use. At the time the Act was passed, it made Canada one of four countries to legalize recreational use of cannabis. Indeed, the United States Drug Enforcement Administration in contrast continues to list cannabis as a Schedule 1 drug alongside drugs such as heroin. Reasons often cited for this include that approximately 1 in 10 people who experiment with cannabis will become addicted.14 Additionally, chronic daily use of cannabis is associated with impaired respiratory function, psychotic symptoms, impaired educational attainment, and subtle cognitive impairment.15
Consistent with previous reports, we found that 53% of patients with IBD had used cannabis in their lifetime.4,16 In this study, however, we found that rates of recent cannabis use were more than twice that previously reported in studies from 2006 (CD 41% vs 15.9%; UC 31% vs 11.6%).4 The Cannabis Act has likely led to increased reporting of cannabis use by boosting access and lowering the stigma associated with cannabis. Indeed, cannabis use in Canadians increased from 14.0% to 17.5% in the first year after legalization.17 Our findings that 51% of responders started using cannabis only in the preceding 3 years is consistent with legalization increasing its adoption. Interestingly, the rates of recent cannabis use in our study were similar to a 2017 survey study of British Columbia patients with IBD done prior to cannabis legalization (50.6% CD and 35.7% UC).16 While the rates reported in that previous study were high, British Columbia was well known to have a higher rate of cannabis use when compared to the national Canadian average even prior to legalization. In fact, according to the 2017 Canadian Tobacco, Alcohol, and Drugs Survey, 23.4% of British Columbians had reported using cannabis in the previous year versus only 14% of Ontario residents.17 We acknowledge that our study was conducted during the COVID-19 pandemic when Canadians faced unprecedented financial and social upheaval. Despite these stressors, the percentage of individuals using cannabis in Ontario increased marginally from 23% in 2020 to 24% in 2021.18 Thus, the pandemic alone is not likely to have significantly contributed to the large increase in cannabis consumption observed during our study.
In the present study, 30% of survey responders stated they used cannabis to relieve their gastrointestinal symptoms, while others consumed cannabis for reasons applicable to the general public such as recreation. Active users of cannabis also reported more abdominal pain, poor appetite, and flatulence, coinciding with lower SIBDQ scores indicating a lower quality of life when compared to non-users of cannabis. Subgroup analyses revealed that individuals with IC, but not CD or UC, reported lower SIBDQ scores with recent cannabis use. The UC group, however, trended towards lower SIBDQ scores. Pi et al. similarly found lower SIBDQ scores in patients with UC, but not in patients with CD who used cannabis.16 Our findings point to a possible difference in the mechanism of disease between CD and UC. The exact mechanism by which cannabis exerts its action on disease activity, however, is currently unknown. A recent double-blind, randomized, placebo-controlled trial of 32 patients with UC found that daily inhalation of THC-predominant cannabis did not alter endoscopic Mayo scores.19 Other markers of disease activity including serum C-reactive protein levels, hemoglobin, or fecal calprotectin also did not differ between treatment arms. Similarly, other studies have also failed to demonstrate any impact of cannabis on empiric markers of IBD severity.20 In contrast, a trial by Naftali et al., reported an improvement in quality of life scores with cannabis use.19 The discrepancy between our results and that reported by Naftali et al., likely stems from their use of the Short Form Health Survey, a 36-question survey designed to compare generic illness and measure quality of life. In the present study, we used the SIBDQ, a validated IBD-specific questionnaire.21 Thus, the improved quality of life scores observed by Naftali et al. may be attributable to the psychoactive and euphoric effects associated with daily cannabis use.
Cannabis may also be associated with lower SIBDQ scores as a result of higher use amongst those patients with more severe diseases. Indeed, we found a difference in SIBDQ scores in patients with IC. Patients in this group were more likely to be recently diagnosed and have received no IBD-specific therapy. This IBD group likely relied on cannabis to alleviate their symptoms in the absence of adequate medical therapy. In fact, 30 percent of our survey responders reported using cannabis primarily to alleviate their GI symptoms despite numerous studies showing no benefit.6 Complementary and alternative medicines such as herbs, acupuncture, homeopathic medicines, and meditation are commonly used by 30-50% of patients with IBD.22 Consistent with this, predictors of CAM use in IBD have previously been reported to include experiencing side effects or dissatisfaction with conventional medication, extra-intestinal manifestations or long-term progression of disease, and prolonged steroid use.23–25 Similarly, predictors of cannabis use include previous abdominal surgery, use of pain medication, and use of other complementary and alternative medicines.4 Thus, it is reasonable to speculate that individuals with increased disease burden use cannabis when other therapies fail them. While many of our survey responders believed cannabis is safe and innocuous, its use has been reported to be associated with a higher risk for surgery in patients with CD.8
This study clearly highlights the prevalence of cannabis use amongst patients with IBD and its impact on these patients, particularly in the post-legalization era. We acknowledge several limitations of the current study, however, including the reliance on self-reported data, which could introduce recall bias and affect the accuracy of reported cannabis use and symptom severity. The cross-sectional study design also limits our ability to infer causal relationships between cannabis use and IBD outcomes. Moreover, our study sample was drawn from a specific geographical area, healthcare setting, and largely Caucasian population, potentially limiting the generalizability of the findings to other populations. These limitations highlight the need for further research, including longitudinal and randomized controlled trials, to better understand the impacts of cannabis use on IBD. Nevertheless, our study underscores the need for physicians caring for patients with IBD to be aware that cannabis use has significantly increased following its legalization in Canada, and that its use is associated with worse disease outcomes. This association does not necessarily indicate causality but does suggest that the underlying severity of IBD could impact cannabis use or vice-versa.
Thus, physicians should always inquire about cannabis use and look for opportunities to optimize their patients’ symptoms with proven therapies. Additional high-quality randomized controlled studies may be warranted to evaluate the potential benefits or harm of cannabis in IBD management.
Contributor Information
Vadim Iablokov, Division of Gastroenterology, Department of Medicine, University of Western Ontario, Lawson Health Research Institute, London, ON, Canada.
Jamie Gregor, Division of Gastroenterology, Department of Medicine, University of Western Ontario, Lawson Health Research Institute, London, ON, Canada.
Nilesh Chande, Division of Gastroenterology, Department of Medicine, University of Western Ontario, Lawson Health Research Institute, London, ON, Canada.
Terry Ponich, Division of Gastroenterology, Department of Medicine, University of Western Ontario, Lawson Health Research Institute, London, ON, Canada.
Vipul Jairath, Division of Gastroenterology, Department of Medicine, University of Western Ontario, Lawson Health Research Institute, London, ON, Canada.
Reena Khanna, Division of Gastroenterology, Department of Medicine, University of Western Ontario, Lawson Health Research Institute, London, ON, Canada.
Samuel Asfaha, Division of Gastroenterology, Department of Medicine, University of Western Ontario, Lawson Health Research Institute, London, ON, Canada.
Funding
Institute of Cancer Research—Canadian Institutes for Health Research.
Conflict of Interest
The authors declare no conflicts.
References
- 1. Bernklev T, Jahnsen J, Aadland E, et al. ; the IBSEN Study Group. Health‐related quality of life in patients with inflammatory bowel disease five years after the initial diagnosis. Scand J Gastroenterol. 2004;39(4):365-373. doi: 10.1080/00365520310008386 [DOI] [PubMed] [Google Scholar]
- 2. Merker AM, Riaz M, Friedman S, Allegretti JR, Korzenik J.. Legalization of medicinal marijuana has minimal impact on use patterns in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2018;24(11):2309-2314. doi: 10.1093/ibd/izy141 [DOI] [PubMed] [Google Scholar]
- 3. Allegretti JR, Courtwright A, Lucci M, et al. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(13):2809-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lal S, Prasad N, Ryan M, et al. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23(10):891-896. doi: 10.1097/MEG.0b013e328349bb4c [DOI] [PubMed] [Google Scholar]
- 5. Kerlin AM, Long M, Kappelman M, Martin C, Sandler RS.. Profiles of patients who use marijuana for inflammatory bowel disease. Dig Dis Sci. 2018;63(6):1600-1604. doi: 10.1007/s10620-018-5040-5 [DOI] [PubMed] [Google Scholar]
- 6. Doeve BH, Meeberg MM van de, Schaik FDM van, et al. A systematic review with meta-analysis of the efficacy of cannabis and cannabinoids for inflammatory bowel disease. J Clin Gastroenterol. 2021;55(9):798-809. [DOI] [PubMed] [Google Scholar]
- 7. Hansen TM, Sabourin BC, Oketola B, Bernstein CN, Singh H, Targownik LE.. Cannabis use in persons with inflammatory bowel disease and vulnerability to substance misuse. Inflamm Bowel Dis. 2019;26(9):1401-1406. doi: 10.1093/ibd/izz272 [DOI] [PubMed] [Google Scholar]
- 8. Storr M, Devlin S, Kaplan GG, Panaccione R, Andrews CN.. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn’s Disease. Inflamm Bowel Dis. 2014;20(3):472-480. doi: 10.1097/01.MIB.0000440982.79036.d6 [DOI] [PubMed] [Google Scholar]
- 9. Glickman D, Dalessio S, Raup-Konsavage WM, Vrana KE, Coates MD.. The impact of cannabis use on clinical outcomes in inflammatory bowel disease: A population-based Longitudinal Cohort Study. Inflamm Bowel Dis. 2023;29:e42-e43. doi: 10.1093/ibd/izad222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelsall D. Watching Canada’s experiment with legal cannabis. CMAJ. 2018;190(41):E1218-E1218. doi: 10.1503/cmaj.181287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95(1):103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Irvine EJ, Zhou Q, Thompson AK.. The short inflammatory bowel disease questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91(8):1571-1578. [PubMed] [Google Scholar]
- 14. Volkow ND, Baler RD, Compton WM, Weiss SRB.. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219-2227. doi: 10.1056/NEJMra1402309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hall W, Degenhardt L.. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383-1391. doi: 10.1016/S0140-6736(09)61037-0 [DOI] [PubMed] [Google Scholar]
- 16. Pi S, Rosenfeld G, Enns R, et al. Patterns and motivations of Cannabis use amongst patients with inflammatory bowel disease. Gastrohep. 2019;1(3):100-107. doi: 10.1002/ygh2.338 [DOI] [Google Scholar]
- 17. Rotermann M. Analysis of trends in the prevalence of cannabis use and related metrics in Canada. Health Rep. 2019;30(6):3-13. doi: 10.25318/82-003-x201900600001-eng [DOI] [PubMed] [Google Scholar]
- 18. Statistics Canada. Research to Insights: Cannabis in Canada. 2023. https://www150.statcan.gc.ca/n1/pub/11-631-x/11-631-x2023006-eng.htm [Google Scholar]
- 19. Naftali T, Schleider LB-L, Benjaminov FS, et al. Cannabis is associated with clinical but not endoscopic remission in ulcerative colitis: a randomized controlled trial. PLoS One. 2021;16(2):e0246871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kafil TS, Nguyen TM, MacDonald JK, Chande N.. Cannabis for the treatment of Crohn’s disease. Cochrane Database Syst Rev. 2018;11(11):CD012853. doi: 10.1002/14651858.CD012853.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160-164. doi: 10.1136/bmj.305.6846.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheifetz AS, Gianotti R, Luber R, Gibson PR.. Complementary and alternative medicines used by patients with inflammatory bowel diseases. Gastroenterology. 2017;152(2):415-429.e15. doi: 10.1053/j.gastro.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 23. Mountifield R, Andrews JM, Mikocka-Walus A, Bampton P.. Doctor communication quality and Friends’ attitudes influence complementary medicine use in inflammatory bowel disease. World J Gastroenterol. 2015;21(12):3663-3670. doi: 10.3748/wjg.v21.i12.3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Langhorst J, Anthonisen IB, Steder‐Neukamm U, et al. Amount of systemic steroid medication is a strong predictor for the use of complementary and alternative medicine in patients with inflammatory bowel disease. Results From a German National Survey. Inflamm Bowel Dis. 2005;11(3):287-295. [DOI] [PubMed] [Google Scholar]
- 25. Fernández A, Acosta MB, Vallejo N, et al. Complementary and alternative medicine in inflammatory bowel disease patients: Frequency and risk factors. Digest Liver Dis 2012;44(11):904-908. [DOI] [PubMed] [Google Scholar]