Abstract
Protein kinases have a diverse array of functions in bacterial physiology, with a distinct role in the regulation of development, stress responses, and pathogenicity. pknF, one of the 11 kinases of Mycobacterium tuberculosis, encodes an autophosphorylating, transmembrane serine/threonine protein kinase, which is absent in the fast-growing, nonpathogenic Mycobacterium smegmatis. Herein, we investigate the physiological role of PknF using an antisense strategy with M. tuberculosis and expressing PknF and its kinase mutant (K41M) in M. smegmatis. Expression of PknF in M. smegmatis led to reduction in the growth rate and shortening and swelling of cells with constrictions. Interestingly, an antisense strain of M. tuberculosis expressing a low level of PknF displayed fast growth and a deformed cell morphology compared to the wild-type strain. Electron microscopy showed that most of the cells of the antisense strain were of a smaller size with an aberrant septum. Furthermore, nutrient transport analysis of these strains was conducted using 3H-labeled and 14C-labeled substrates. A significant increase in the uptake of d-glucose but not of glycerol, leucine, or oleic acid was observed in the antisense strain compared to the wild-type strain. The results suggest that PknF plays a direct/indirect role in the regulation of glucose transport, cell growth, and septum formation in M. tuberculosis.
Protein phosphorylation, catalyzed by protein kinases, is associated with central functions in growth and proliferation of cells and is particularly important in the signal transduction pathways (25, 29). The eukaryotic protein kinases belong to the Ser, Thr, and Tyr superfamily; however, in prokaryotes, protein kinases belong to the superfamily of His kinases. Genome sequence databases and experimental evidences revealed the presence of eukaryotic-type serine/threonine protein kinases (STPKs) in several prokaryotes (19, 22, 23). Many STPK-encoding genes of Streptomyces, Cyanobacteria, and Myxococcus have been reported to be involved in different processes, such as regulation of growth, development (19, 23, 24, 36, 37), and stress responses (34). Besides their role in cellular activities, STPKs are also known to be involved in host-pathogen interactions. YpkA, a secretory STPK of Yersinia pseudotuberculosis, has been implicated in the survival of this intracellular pathogen in the host by disrupting the eukaryotic cytoskeleton and by reprogramming the signaling network of the host (9, 11). Recently, the genome sequence of Mycobacterium smegmatis was made available (www.tigr.org), and sequence analysis revealed the presence of many putative serine/threonine kinases.
It has been reported that the Mycobacterium tuberculosis genome encodes 11 eukaryotic-type STPKs (3), and the physiological significance of few of these kinases is known (18, 32). PknA of M. tuberculosis was shown to regulate the morphological changes associated with the cell division when Escherichia coli was used as a surrogate host (2). Recently, PknG has been shown to mediate mycobacterial survival in host cells (4), and PknH can phosphorylate EmbR, a protein suspected to be involved in the regulation of genes coding for arabinosyl transferases (21, 31). In a previous study, we showed that PknF is autophosphorylated at serine and threonine residues and was capable of phosphorylating serine/threonine residues of an exogenous substrate (17). PknF was found to be a transmembrane protein, and Southern blot analysis and immunoblotting indicated that pknF was absent in M. smegmatis (17).
It has been shown that the autophosphorylated form of PknF of M. tuberculosis interacts and phosphorylates an ATP-binding cassette (ABC) transporter that is localized next to pknF in the genome sequence (20). But how the phosphorylation affect the activity of the ABC transporter is not known. M. tuberculosis has members of all the main transport protein families, including the ABC transporters, the major facilitator superfamilies, and the resistance nodulation division superfamily (26, 27, 28). ABC transporters help in the uptake and export of a wide variety of biomolecules, including sugars, lipids, peptides, complex organic molecules, and ions, across the plasma membrane, and this activity is coupled with their ATPase activity (15). Phosphorylation has been shown to modify the activity and stability of many ABC transporters. Moreover, in Saccharomyces cerevisiae, STPKs like Ca2+-calmodulin-dependent protein kinase and casein kinase I isoforms (Yck1p and Yck2p) were found to regulate the activity and stability of Pdr12 (ABC transporter) and Pdr5p (drug resistance protein), respectively (6, 16).
As reported herein, we investigated the role of PknF using M. smegmatis as a surrogate host and by an antisense RNA approach. Expression of PknF antisense RNA in M. tuberculosis led to an enhanced uptake of glucose. The results also indicate that PknF is required for maintaining cell morphology and proper septation during cell division in M. tuberculosis.
MATERIALS AND METHODS
Reagents, bacterial strains, and growth conditions.
M. tuberculosis H37Rv and M. smegmatis LR222 were grown in Middlebrook 7H9 liquid medium and on Middlebrook 7H10 agar. These media were supplemented with 10% (vol/vol) albumin dextrose complex (Becton Dickinson, Maryland), containing 0.5% glycerol, 0.02% (vol/vol) Tween 80, and antibiotic as appropriate (20 μg/ml kanamycin). E. coli DH5α was used for all subcloning procedures and was grown in LB medium/agar. DNA-modifying enzymes were obtained from New England Biolabs. [14C]glucose (140 μCi/μmol), [3H]glycerol (2.5 Ci/mmol), [14C]oleic acid (53 mCi/mmol), and l-[3H]leucine (173 Ci/mmol) were obtained from Amersham Biosciences.
Cloning and expression of PknF and its mutant in M. smegmatis.
Genomic DNA isolated from M. tuberculosis H37Rv was used for PCR amplification of pknF. The nucleotide sequence of primers used were 5′-CTACGATGCCCATATGCCGCTCGC-3′ with an NdeI site at the 5′ end (forward primer) and 5′-CAGGCAAGCACGCGTTCACGGC CAG-3′ with an MluI site at the 3′ end (reverse primer). PCR was carried out for 30 cycles (denaturation, 95°C for 1 min per cycle; annealing, 55°C for 1 min per cycle; elongation, 72°C for 1 min 30 s per cycle). Replacement of lysine at position 41 of PknF by methionine was carried out by site-directed mutagenesis using overlapping PCR as described earlier (17). The oligonucleotide used for mutagenesis of pknF was 5′-GGCCCGCAGTACCATGAGCGCGTCCTG-3′ (underlined bases indicate the change from lysine to methionine). The amplicons were digested with NdeI-MluI and cloned in pSD5 (Mycobacterium-E. coli shuttle vector) (5) under the transcriptional control of hsp60, a strong constitutive promoter. The plasmids containing pknF and mutated pknF were designated as pSD5-pknF and pSD5-K41 M pknF, respectively. The nucleotide sequence of the cloned fragments was confirmed by Automated Sequence Analyzer (Applied Biosystem Genetic Analyzer, model 3100). The recombinant plasmids were electroporated in M. smegmatis using a cell porator (Biometra; Whatman) as described earlier (5), and the recombinants were selected on 7H10 agar containing kanamycin. The following recombinant strains were derived from LR222: MS5, having pSD5; MSF, having pSD5-pknF; and MSKM, having pSD5-K41 M pknF. Expression of PknF and its mutant (K41 M) was analyzed in log-phase cultures (optical density at 600 nm [OD600], ∼1.5) by immunoblotting using polyclonal antisera raised against PknF in rabbits as described earlier (17).
Construction of pknF antisense expression vector.
The M. tuberculosis pknF coding region was amplified by PCR (denaturation, 95°C for 1 min per cycle; annealing, 55°C for 1 min per cycle; elongation, 72°C for 1 min 30 s per cycle, for 30 cycles) using the oligonucleotide primer 5′-CTACGATGCCACGCGTCCGCTCGC-3′ with an MluI site at the 5′ end (forward primer) and 5′-CAGGCAAGCACGCGTTCACGGCCAG-3′ with an MluI site at the 3′ end (reverse primer) and cloned in antisense orientation into pSD5. The recombinant plasmid was electroporated in M. tuberculosis as described earlier (5) to obtain a strain expressing the antisense mRNA of pknF (ASF). The expression level of PknF was determined in log-phase cultures (OD600, ∼1.5) of ASF and wild-type M. tuberculosis strains by immunoblotting.
Growth curve analysis.
Starter cultures were prepared by inoculating M. smegmatis wild type (WT), MS5, MSF, and MSKM and M. tuberculosis (wild type and antisense strain) from plates or from glycerol stocks into 3 ml of 7H9 medium. These cultures were grown at 37°C in a shaking incubator (200 rpm) until they reached stationary phase. To measure growth kinetics, 50 ml of medium was inoculated to give an OD600 of about 0.05. Samples from individual cultures were collected at different time intervals, passed five times through a 26-gauge needle, and vortexed. The serial dilutions (in phosphate-buffered saline with 0.02% Tween 80) were plated on 7H10 agar plates to determine numbers of CFU after incubation at 37°C.
Electron microscopy.
The log-phase cultures of different M. smegmatis strains (WT, MS5, MSF, and MSKM) and M. tuberculosis strains (wild type and ASF) were harvested, washed three times with phosphate buffer (pH 7.2), and then fixed for 8 h in 2.5% glutaraldehyde and 2% paraformaldehyde in 100 mM phosphate buffer (pH 7.2). The cells were dehydrated using 30%, 50%, 70%, and then 100% acetone (critical drying), and dried cells were mounted on microscope stubs and sputter coated on the sputter coater. The samples were then examined on a LEO 435 VP scanning electron microscope.
Transmission electron microscopy was done after fixing cells for 24 h in 100 mM sodium cacodylate buffer (pH 7.4) containing 4% paraformaldehyde and 2.5% glutaraldehyde. The cells were fixed in Karnovsky's solution containing 0.5% OsO4-0.8% K3Fe(CN)6 followed by 1% tannic acid and stained overnight in 1% uranyl acetate. Samples were dehydrated in a graded ethanol series and embedded in Spurr's resin. Thin sections were cut with an ultramicrotome (Leica; Ultracut E), stained with 1% uranyl acetate and Reynold's lead citrate, and observed at 80 kV in a Philips CM-10 transmission electron microscope.
Nutrient uptake experiments.
The M. tuberculosis wild-type strain and ASF were grown to mid-log phase, harvested, washed three times with basal salts medium (0.1% KH2PO4, 0.25% NaH2PO4, 0.5% NH4Cl, 0.2% K2SO4, 0.5% glycerol) plus Tween 80 at 0.05%, and concentrated in basal salts medium to a final OD600 of 3.0. The uptake was performed in 2 ml of medium containing either [14C]glucose, [3H]glycerol, [14C]oleic acid, or l-[3H]leucine (40 to 50 μCi/μmol) and 100 μM of unlabeled substrate as described earlier (30). Incorporation of radiolabeled substrate was terminated by removal of 0.5-ml samples at various times to filters (Whatman GF/F; 0.45 μm) prewetted with basal salts medium. The cells were washed quickly three times with 7 ml of ice-cold basal salts medium with air vacuum. The filters were dried and transferred to scintillation vials, and counts were determined in a liquid scintillation counter (Beckman Instruments).
RESULTS AND DISCUSSION
Many signal transduction pathways involve phosphorylation/dephosphorylation in response to environmental alterations. Among 11 kinases of M. tuberculosis, PknF is a 51-kDa transmembrane kinase, absent in M. smegmatis. In the present work, efforts were made to decipher the role of pknF either by expressing it in M. smegmatis or by inhibiting the mRNA of PknF in M. tuberculosis by antisense RNA.
Altered growth and morphology of M. smegmatis expressing pknF.
Genome sequence analysis of M. smegmatis revealed the presence of many eukaryotic-type STPKs. Sequence comparison of PknF of M. tuberculosis with kinases of M. smegmatis showed that the closest homologue, MSMEG3682, has 61% amino acid sequence similarity with PknF. But a closer comparison between PknF and MSMEG3682 using CLUSTAL W (1.82) revealed that kinase subdomain I at the NH2 terminus of MSMEG3682 lacks the GXGXXGV consensus motif required for covering and anchoring the nontransferable phosphates of ATP (12). Moreover, they share only 38% homology in 140 amino acids at the COOH-terminal end. Our earlier results also confirmed that PknF is absent in M. smegmatis (17).
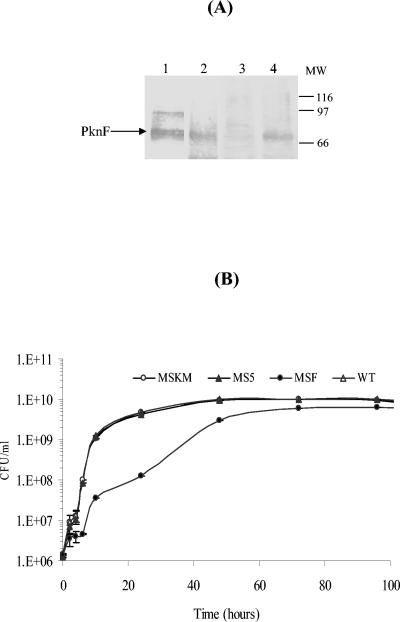
In our study, we used M. smegmatis as a surrogate host to express pknF and its kinase mutant under the control of hsp60 promoter. The expression in cell lysates was determined by immunoblotting. The immunoblot analysis revealed that PknF and its mutant migrated as a ∼70-kDa protein (Fig. 1A), which is higher than the predicted molecular mass of the protein (51 kDa). The slow migration of PknF and its kinase mutant could be due to posttranslational modifications of proteins (17, 13, 22). Moreover, no corresponding band to PknF was detected in MS5.
FIG. 1.
Expression of PknF in M. smegmatis. (A). Analysis of PknF in M. smegmatis by Western blotting. Different strains were grown to log phase and harvested, cellular lysates were prepared, and approximately 40 μg protein from each sample was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blotting was performed using anti-PknF antibodies. Lane 1, lysate of M. tuberculosis H37Rv; lane, 2 to 4, lysates from MSKM, MS5, and MSF, respectively. (B) Cell growth of M. smegmatis carrying various plasmids. Bacterial cultures were grown for 4 days in 50 ml of 7H9 liquid medium. At various time points, CFU were counted for growth analysis. Each point is the mean ± standard error of two values obtained from two different experiments.
The results of growth kinetics indicated that WT, MS5, and MSKM showed normal lag phase and the exponential phase initiated after 6 h of lag phase, followed by stationary phase starting after 48 h (Fig. 1B), whereas MSF displayed exponential phase after 10 h of lag period and the stationary phase started after 72 h, indicating that the expression of PknF in M. smegmatis resulted in slow growth (Fig. 1B). The reduction in growth displayed by MSF suggests its role in cell division and growth. In a similar study, it has been shown earlier that elevated expression of M. tuberculosis FtsZ in M. smegmatis interfered with the cell division process and inhibited growth (7).
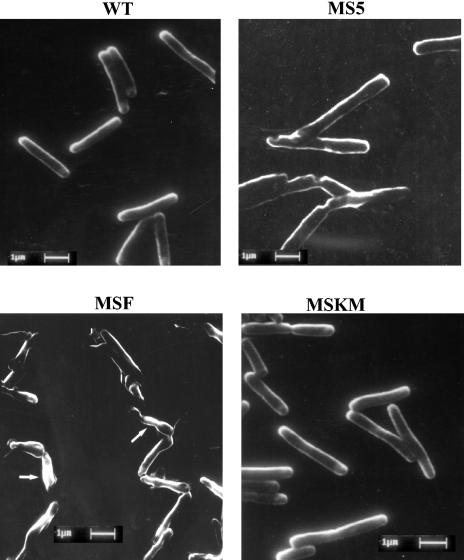
To investigate the morphological changes associated with pknF expression in M. smegmatis, the strains were visualized by scanning electron microscopy. Cells expressing PknF were short rods with constrictions, and some cells contained bulbous structures at one end (Fig. 2). Cell length measurements revealed that cells of the MSF strain were 1.5 to 2 times shorter than the control cells (WT, MS5, and MSKM). The small size and beaded morphology of MSF indicated the deviant cell division. Interestingly, MSKM strain, expressing a kinase mutant of PknF (K41M), showed a growth pattern (Fig. 1B) and phenotype (Fig. 2) similar to those of the wild-type strain. It can be conjectured here that phosphorelay events triggered by PknF lead to growth inhibition and changes in the cell morphology of MSF.
FIG. 2.
Scanning electron microscopy of M. smegmatis strains. Scanning electron microscopy was performed as detailed in Materials and Methods. Arrows indicates septum and bulbous structures. The bar in each panel indicates magnification (bar = 1 μm).
Expression of pknF antisense RNA in M. tuberculosis.
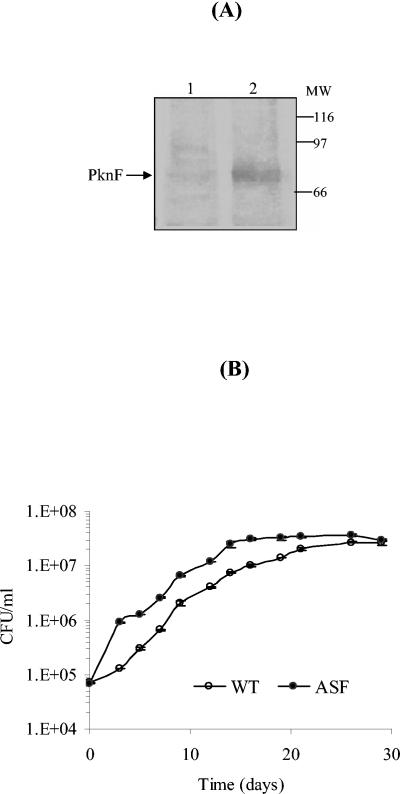
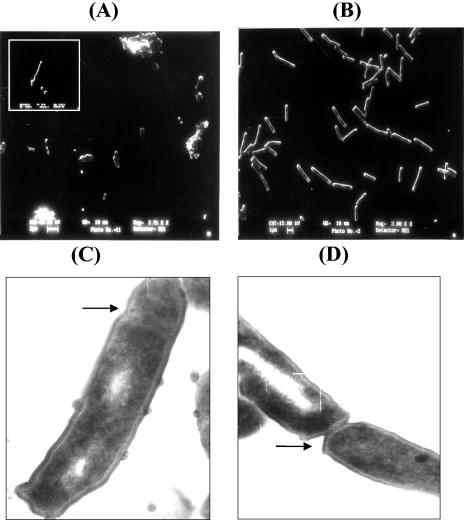
The antisense strategy, employed to elucidate the role of PknF, has provided conclusive evidence for the above-stated hypothesis. Earlier, the antisense approach had also been used for elucidating the roles of SodA (8), AhpC (33), and DnaA (10) in mycobacteria. It has also been shown that incubation of M. tuberculosis with antisense oligodeoxyribonucleotides directed against glutamine synthetase I inhibited the expression and activity of glutamine synthetase and inhibited bacterial growth (14). In the present study, a reverse coding sequence of pknF, under the control of the hsp60 promoter, led to the down-regulation of PknF protein synthesis in the ASF strain (Fig. 3A). M. tuberculosis expressing antisense RNA against PknF grew 1.5-fold faster than the wild-type strain (Fig. 3B). To investigate the morphological changes associated with reduced PknF levels, the ASF cells were visualized by scanning electron microscopy. Most of the ASF cells were shorter (approximately 1.4 times) than control cells and had bulbous structures at one or both ends (Fig. 4A and B). ASF cells showed various cell lengths, ranging from 1.5 to 2.2 μm, compared to 2.1 to 3.4 μm for wild-type cells. Moreover, transmission electron microscopy revealed ASF cells with abnormal septa or incomplete septa compared to control cells (Fig. 4C). Conjointly, these results implied that PknF affects septum formation and cell morphology. Similar phenotypic changes, such as aggregation of cells and reduced cell size, were observed in the pknA mutant of Anabaena PCC 7120 (35). Likewise, in E. coli the pbpA mutant exhibited fast growth and the pbpA ftsZ double mutant showed swollen cells with no sign of septum formation (1). The results implicate that PknF has a role in regulating different stages of cell division, such as elongation and septum formation.
FIG. 3.
Expression of antisense RNA in M. tuberculosis. (A) Western blot analysis. Lysates were resolved on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and proteins were electroblotted on nitrocellulose. The blot was probed with anti-PknF antibodies and developed with ECL reagent. Lane 1, lysate of ASF strain; lane 2, lysate of M. tuberculosis H37Rv. (B) Growth curve analysis. Mycobacterial cultures were grown for 30 days in 50 ml of 7H9 liquid medium. At each time point CFU were counted for growth analysis. Each point is the mean ± SE of CFU obtained from two cultures of two different experiments.
FIG. 4.
Morphological changes in M. tuberculosis expressing antisense RNA against PknF. Scanning and transmission electron microscopy were performed as described in Materials and Methods. Scanning electron microscopy of (A) ASF with inset showing bulbous cell and (B) wild-type M. tuberculosis. Scale bars indicate the magnification. Transmission electron microscopy of (C) ASF and (D) wild-type M. tuberculosis at magnifications of ×31,000 and ×21,500, respectively. Arrow indicates septum.
Nutrient uptake in M. tuberculosis.
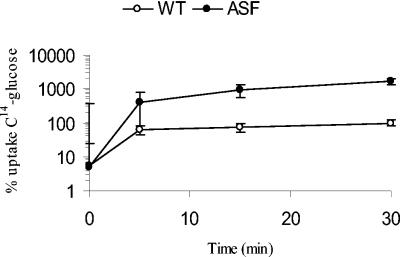
The genome sequence analysis of M. tuberculosis divulged the presence of a putative ABC transporter (Rv1747) downstream of pknF (Rv1746). Recently, Molle et al. showed that PknF phosphorylates the ABC transporter and suggested that this reaction involved interaction between the phosphorylated form of PknF and two FHA domains of ABC transporter (20). In view of these facts, uptake of various radiolabeled metabolites was studied. The results showed that uptake of [14C]glucose in the antisense strain was 16-fold higher than that in the WT strain (Fig. 5). There was no difference between WT and ASF strains in the uptake of [3H]glycerol, [14C]oleic acid, and l-[3H]leucine (data not shown). These observations suggest that expression of pknF antisense has no effect on cell wall permeability, and the difference in uptake of glucose might be mediated through PknF. In view of the earlier report that PknF phosphorylates an ABC transporter (20) and our results that PknF regulates glucose transport, it is tempting to speculate that PknF negatively regulates transport of glucose through an ABC transporter. Earlier, kinases have been shown to phosphorylate and regulate, either directly or indirectly, the activities of ABC transporters (6, 16).
FIG. 5.
Uptake of radiolabeled substrates. ASF and wild-type strains were incubated in basal salts medium containing labeled and unlabeled glucose, as described in Materials and Methods. Each point represents the mean ± standard error of percentage uptake of [14C]glucose obtained from triplicate experiments. The results are expressed as percentage uptake of [14C]glucose, with the maximal uptake by wild-type M. tuberculosis after 30 min taken as 100%.
In conclusion, our findings suggest that expression of PknF in M. smegmatis and M. tuberculosis affects cell division, growth rate, morphology, and glucose transport. Currently, experiments are in progress to determine whether PknF directly affects these processes via an ABC transporter or additional intermediates are involved in PknF mediated signaling.
Acknowledgments
Financial support by the Council of Scientific and Industrial Research (SMM 0003) is acknowledged.
REFERENCES
- 1.Begg, K. J., and W. D. Donachie. 1985. Cell shape and division in Escherichia coli: experiments with shape and division mutants. J. Bacteriol. 163:615-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaba, R., M. Raje, and P. K. Chakraborti. 2002. Evidence that a eukaryotic-type serine/threonine protein kinase from Mycobacterium tuberculosis regulates morphological changes associated with cell division. Eur. J. Biochem. 269:1078-1085. [DOI] [PubMed] [Google Scholar]
- 3.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 4.Cowley, S., M. Ko, N. Pick, R. Chow, K. J. Downing, B. G. Gordhan, J. C. Betts, V. Mizrahi, D. A. Smith, R. W. Stokes, and Y. Av-Gay. 2004. The Mycobacterium tuberculosis protein serine/threonine kinase PknG is linked to cellular glutamate/glutamine levels and is important for growth in vivo. Mol. Microbiol. 52:1691-1702. [DOI] [PubMed] [Google Scholar]
- 5.Das Gupta, S. K., M. D. Bashyam, and A. K. Tyagi. 1993. Cloning and assessment of mycobacterial promoters by using a plasmid shuttle vector. J. Bacteriol. 175:5186-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decottignies, A., G. Owsianik, and M. Ghislain. 1999. Casein kinase I-dependent phosphorylation and stability of the yeast multidrug transporter Pdr5p. J. Biol. Chem. 274:37139-37146. [DOI] [PubMed] [Google Scholar]
- 7.Dziadek, J., M. V. Madiraju, S. A. Rutherford, M. A. Atkinson, and M. Rajagopalan. 2002. Physiological consequences associated with overproduction of Mycobacterium tuberculosis FtsZ in mycobacterial hosts. Microbiology 148:961-971. [DOI] [PubMed] [Google Scholar]
- 8.Edwards, K. M., M. H. Cynamon, R. K. Voladri, C. C. Hager, M. S. DeStefano, K. T. Tham, D. L. Lakey, M. R. Bochan, and D. S. Kernodle. 2001. Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 164:2213-2219. [DOI] [PubMed] [Google Scholar]
- 9.Galyov, E. E., S. Hakansson, A. Forsberg, and H. Wolf-Watz. 1993. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature 361:730-732. [DOI] [PubMed] [Google Scholar]
- 10.Greendyke, R., M. Rajagopalan, T. Parish, and M. V. Madiraju. 2002. Conditional expression of Mycobacterium smegmatis dnaA, an essential DNA replication gene. Microbiology 148:3887-3900. [DOI] [PubMed] [Google Scholar]
- 11.Hakansson, S., E. E. Galyov, R. Rosqvist, and H. Wolf-Watz. 1996. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol. Microbiol. 20:593-603. [DOI] [PubMed] [Google Scholar]
- 12.Hanks, S. K., and T. Hunter. 1995. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576-596. [PubMed] [Google Scholar]
- 13.Hanlon, W. A., M. Inouye, and S. Inouye. 1997. Pkn9, a Ser/Thr protein kinase involved in the development of Myxococcus xanthus. Mol. Microbiol. 23:459-471. [DOI] [PubMed] [Google Scholar]
- 14.Harth, G., P. C. Zamecnik, J. Y. Tang, D. Tabatadze, and M. A. Horwitz. 2000. Treatment of Mycobacterium tuberculosis with antisense oligonucleotides to glutamine mRNA inhibits glutamine synthetase activity, formation of the poly-glutamate/glutamine cell wall structure, and bacterial replication. Proc. Natl. Acad. Sci. USA 97:418-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins, C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 16.Holyoak, C. D., S. Thompson, C. C. Ortiz, K. Hatzixanthis, B. Bauer, K. Kuchler, P. W. Piper, and P. J. Coote. 2000. Loss of Cmk1 Ca(2+)-calmodulin-dependent protein kinase in yeast results in constitutive weak organic acid resistance, associated with a post-transcriptional activation of the Pdr12 ATP-binding cassette transporter. Mol. Microbiol. 37:595-605. [DOI] [PubMed] [Google Scholar]
- 17.Koul, A., A. Choidas, A. K. Tyagi, K. Drlica, Y. Singh, and A. Ullrich. 2001. Serine/threonine protein kinases PknF and PknG of Mycobacterium tuberculosis: characterization and localization. Microbiology 147:2307-2314. [DOI] [PubMed] [Google Scholar]
- 18.Koul, A., T. Herget, B. Klebl, and A. Ullrich. 2004. Interplay between mycobacteria and host signalling pathways. Nat. Rev. Microbiol. 2:189-202. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto, A., S. K. Hong, H. Ishizuka, S. Horinouchi, and T. Beppu. 1994. Phosphorylation of the AfsR protein involved in secondary metabolism in Streptomyces species by a eukaryotic-type protein kinase. Gene 146:47-56. [DOI] [PubMed] [Google Scholar]
- 20.Molle, V., D. Soulat, J. M. Jault, C. Grangeasse, A. J. Cozzone, and J. F. Prost. 2004. Two FHA domains on an ABC transporter, Rv1747, mediate its phosphorylation by PknF, a Ser/Thr protein kinase from Mycobacterium tuberculosis. FEMS Microbiol. Lett. 234:215-223. [DOI] [PubMed] [Google Scholar]
- 21.Molle, V., L. Kremer, C. Girard-Blanc, G. S. Besra, A. J. Cozzone, and J. F. Prost. 2003. An FHA phosphoprotein recognition domain mediates protein EmbR phosphorylation by PknH, a Ser/Thr protein kinase from Mycobacterium tuberculosis. Biochemistry 42:15300-15309. [DOI] [PubMed] [Google Scholar]
- 22.Motley, S. T., and S. Lory. 1999. Functional characterization of a serine/threonine protein kinase of Pseudomonas aeruginosa. Infect. Immun. 67:5386-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz-Dorado, J., S. Inouye, and M. Inouye. 1991. A gene encoding a protein serine/threonine kinase is required for normal development of M. xanthus, a gram negative bacterium. Cell 67:995-1006. [DOI] [PubMed] [Google Scholar]
- 24.Nadvornik, R., T. Vomastek, J. Janecek, Z. Technikova, and P. Branny. 1999. Pkg2, a novel transmembrane protein Ser/Thr kinase of Streptomyces granaticolor. J. Bacteriol. 181:5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nurse, P. 1990. Universal control mechanism regulating onset of M-phase. Nature 344:503-508. [DOI] [PubMed] [Google Scholar]
- 26.Pao, S. S., I. T. Paulsen, and M. H. Saier, Jr. 1998. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62:1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulsen, I. T., M. H. Brown, and R. A. Skurray. 1996. Proton-dependent multidrug efflux systems. Microbiol. Rev. 60:575-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulsen, I. T., M. K. Sliwinski, and M. H. Saier, Jr. 1998. Microbial genome analyses: global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J. Mol. Biol. 277:573-592. [DOI] [PubMed] [Google Scholar]
- 29.Pelech, S. L., and J. S. Sanghera. 1992. Mitogen-activated protein kinases: versatile transducers for cell signaling. Trends Biochem. Sci. 17:233-238. [DOI] [PubMed] [Google Scholar]
- 30.Seth, A., and N. D. Connell. 2000. Amino acid transport and metabolism in mycobacteria: cloning, interruption, and characterization of an L-arginine/γ-aminobutyric acid permease in Mycobacterium bovis BCG. J. Bacteriol. 182:919-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma, K., H. Chandra, P. K. Gupta, M. Pathak, A. Narayan, L. S. Meena, R. C. D'Souza, P. Chopra, S. Ramachandran, and Y. Singh. 2004. PknH, a transmembrane Hank's type serine/threonine kinase from Mycobacterium tuberculosis is differentially expressed under stress conditions. FEMS Microbiol. Lett. 233:107-113. [DOI] [PubMed] [Google Scholar]
- 32.Sharma, K., P. Chopra, and Y. Singh. 2004. Recent advances towards identification of new drug targets for Mycobacterium tuberculosis. Expert Opin. Ther. Targets 8:79-93. [DOI] [PubMed] [Google Scholar]
- 33.Wilson, T., G. W. de Lisle, J. A. Marcinkeviciene, J. S. Blanchard, and D. M. Collins. 1998. Antisense RNA to ahpC, an oxidative stress defence gene involved in isoniazid resistance, indicates that AhpC of Mycobacterium bovis has virulence. Microbiology 144:2687-2695. [DOI] [PubMed] [Google Scholar]
- 34.Yang, X., C. M. Kang, M. S. Brody, and C. W. Price. 1996. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 10:2265-2275. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, C. C. 1993. A gene encoding a protein related to eukaryotic protein kinases from the filamentous heterocystous cyanobacterium Anabaena PCC 7120. Proc. Natl. Acad. Sci. USA 90:11840-11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, C. C., and L. Libs. 1998. Cloning and characterisation of the pknD gene encoding an eukaryotic-type protein kinase in the cyanobacterium Anabaena sp. PCC7120. Mol. Gen. Genet. 258:26-33. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, C. C., A. Friry, and L. Peng. 1998. Molecular and genetic analysis of two closely linked genes that encode, respectively, a protein phosphatase 1/2A/2B homolog and a protein kinase homolog in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 180:2616-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]