Quorum sensing, the monitoring of population density by bacteria, is used to coordinately control gene expression and therefore particular behaviors under conditions of high cell density. Such group behaviors provide advantages to organisms under certain conditions, such as during pathogenic colonization when virulence traits are induced by a group of bacteria. In the accompanying paper, Lupp and Ruby describe the requirement for a quorum-sensing system at relatively low cell density during colonization by Vibrio fischeri of its symbiotic host, Euprymna scolopes (18).
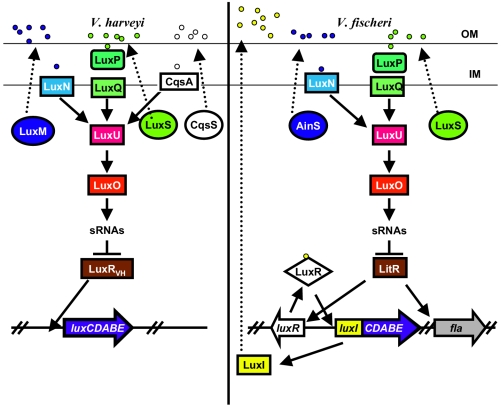
Quorum-sensing systems in Vibrio fischeri and Vibrio harveyi.
Quorum sensing is a field built from laboratory-based investigations of luminous marine bacteria, namely, Vibrio fischeri and Vibrio harveyi (25). It was in V. fischeri that the quorum-sensing regulators LuxR (a transcriptional activator) and LuxI (a signal synthase) first were discovered (reviewed in reference 6). The LuxI-produced signaling molecule, an acyl homoserine lactone (acyl-HSL), diffuses out of and then (under conditions of high cell density) back into the cell, where it activates LuxR and thus the lux (luminescence) genes, which are under LuxR control (11, 14). Homologs of these proteins have been found in most gram-negative quorum-sensing bacteria studied to date.
Studies of quorum sensing in V. harveyi subsequently revealed a much more complex system for lux control (Fig. 1) (reviewed in reference 28). This organism uses three sensor kinase proteins to detect its three quorum-sensing molecules (one of which is an acyl-HSL) (1, 2, 13). At low cell density, the sensor proteins autophosphorylate. The phosphate is sequentially transferred to LuxU, a phosphotransferase protein, and LuxO, a transcriptional activator. Phosphorylated LuxO activates the transcription of five small RNA (sRNA) genes (16). The resulting sRNAs work in conjunction with the Hfq protein to destabilize the transcript encoding LuxRVH, a transcriptional regulator that is not homologous to V. fischeri LuxR (16). The consequence is that LuxRVH is not synthesized, and thus the lux genes are not transcribed. Increasing population densities sequentially signal the three sensor proteins to switch from kinases to phosphatases. The consequence is dephosphorylation of LuxO, loss of sRNA synthesis, increased translation of LuxR, and thus transcription of lux and production of light.
FIG. 1.
A comparison of the V. harveyi and V. fischeri quorum-sensing systems. Parts of the pathway in V. fischeri are modeled after those described for V. harveyi and are described further in the text. The colors reflect proteins that are homologous between the two species. The acyl-HSL synthases are depicted as ovals. Although the V. fischeri AinS and LuxS proteins are homologous to LuxM and LuxS of V. harveyi, the acyl-HSL signaling molecules (represented by small circles) produced by the different bacteria are distinct. It is not known whether sRNAs are involved in bioluminescence control in V. fischeri. OM, outer membrane; IM, inner membrane.
For some time, it has been known that V. fischeri encodes a second acyl-HSL synthase, termed AinS (8). AinS is homologous to LuxM of V. harveyi yet produces a distinct acyl-HSL (8, 15). More recently, homologs of the other V. harveyi quorum-sensing components have been identified (Fig. 1) (7, 17, 19, 24). Although only a few of these components have been examined at a molecular level, the results to date suggest that this second V. fischeri system functions like that of V. harveyi. The ain system is integrated with the lux system at LitR, a LuxRVH homolog that is controlled by LuxO and itself controls transcription of luxR (Fig. 1) (7, 24), thereby controlling lux expression.
Roles for AinS and LuxO in symbiotic initiation.
Lupp and Ruby report the novel finding that the acyl-HSL synthase AinS is required for initiation of symbiotic colonization of the squid E. scolopes by V. fischeri (18). Loss of ainS delayed both colonization and the luminescence emission that results from the symbiotic association (Fig. 2A). No such delay of colonization was observed for lux mutants, suggesting that AinS may control factors other than lux that are required for symbiotic initiation. Surprisingly, a mutation in luxO, which might be expected to counteract the consequences of an ainS mutation, also prevented normal symbiotic initiation. Together, these data suggested that an optimal level of AinS/LuxO-controlled (non-lux) target gene transcription is necessary for symbiotic initiation.
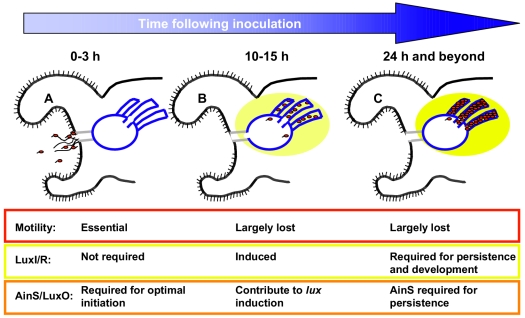
FIG. 2.
Time course of bacterial colonization in the V. fischeri-E. scolopes symbiosis. Panels A to C depict a one side of the bilaterally symmetric light organ from juvenile E. scolopes, containing one of three crypts (outlined in blue). Each panel represents a different stage of symbiotic colonization. V. fischeri bacteria are shown as red ovals, with or without flagella. The yellow ovals surrounding the crypt spaces represent bioluminescence emission from the bacteria. The states of motility and of LuxI/R and AinS/LuxO activity are indicated below each stage. The cartoon reflects data reviewed in reference 26.
To identify potential AinS-controlled genes, Lupp and Ruby used microarray analysis (the first such published for the recently sequenced V. fischeri [27]). Their screen yielded 30 positively and negatively controlled genes, including, notably, negatively controlled motility genes (18). The effect of the AinS signal on motility was supported experimentally by the finding that the ainS mutant migrated through soft agar faster than the wild-type strain. Is the increase in motility sufficient to account for the colonization defect? Perhaps. Previous work has demonstrated that motility is essential for symbiotic colonization: nonmotile bacteria fail to colonize, while hypermotile mutants exhibit a severe delay (9, 22). Interestingly, a luxO mutant also displayed a defect in motility, in this case a decreased rate of migration through soft agar. These data support the idea that mutations in ainS and luxO can differentially unbalance regulation of a downstream target. Besides motility genes, however, there were a number of other, equally interesting, targets identified that could be the cause of the initiation defect observed and which will presumably be the focus of future investigations.
Sequential activation of two quorum-sensing systems.
In laboratory culture, induction of lux depends primarily upon ainS. Whereas luxI mutants exhibit near-wild-type levels of light emission, no luminescence can be detected from ainS mutants (19, 29). During symbiotic colonization, the opposite is true: luxI mutants fail to produce any detectable light, while ainS mutants exhibit only a small decrease in light. These data led Lupp and Ruby to suggest a model in which the AinS signal promotes luminescence at low cell densities, which occur in culture and the early stages of symbiotic colonization; their results that ainS, but not luxI, is required for initiation support this low cell density role for AinS. At higher cell densities, which occur in the symbiotic light organ, LuxI produces the signal necessary for high-level light production.
Colonization by V. fischeri occurs in a series of stages that occur immediately after hatching of the juvenile squid (Fig. 2) (reviewed in reference 26). First, V. fischeri cells in the seawater aggregate in mucus on the surface of the symbiotic (light) organ (Fig. 2A). Next, after a short period of time, during which signaling likely occurs between the bacteria themselves (as suggested by the results of Lupp and Ruby) as well as between the bacteria and their host, motile bacteria migrate into the light organ. After entry into nutrient-rich crypts, the bacteria begin to multiply to high cell density, induce the lux genes, and lose their flagella (Fig. 2B). The last stage, termed persistence, occurs once the animals are fully colonized (Fig. 2C). This stage requires the adaptation of the microbe to a changing environment: certain host developmental events are triggered by the bacteria, including an increase in size of the epithelial cells lining the bacterium-containing crypts. Furthermore, 90% of V. fischeri cells are expelled each morning, while the remaining 10% regrow to high cell density.
At the persistence stage of colonization, mutants defective for one of the quorum regulators, luxR and luxI, or the LuxR/I target gene, luxA (encoding one subunit of the luciferase enzyme), fail to achieve the same levels of colonization (regrowth) as the wild-type strain (29) (Fig. 2C). Furthermore, normal host development requires luxA, as luxA mutants fail to induce the typical epithelial cell swelling (29). It is not yet understood how the function (or lack thereof) of this quorum-sensing-controlled gene is communicated to the host.
Lupp et al. (19) previously reported that the acyl-HSL synthase AinS is similarly required for symbiotic persistence (Fig. 2C). Whereas mutations in luxI abolish symbiotic bioluminescence, mutations in ainS only slightly decrease light levels. Thus, although the colonization defect of the luxI mutant can be attributed to control of lux transcription, it is not clear that the same is true for the defect of the ainS mutant. Indeed, in culture, this mutant is unable to achieve the same growth yield as the wild-type strain, suggesting that an AinS-controlled factor other than lux may be involved in symbiotic persistence. The array experiments performed by Lupp and Ruby thus may also yield a (non-lux) factor required for symbiotic persistence as well. Taken together, these studies support sequential roles in symbiotic colonization for the AinS-produced signal (early control of a lux-independent target and later control of lux and/or a lux-independent target) and the LuxI-produced signal (later control of lux) (Fig. 2). Thus, it has been shown, for the first time, that sequential signaling by two acyl-HSLs occurs, and is important, during colonization of host tissue.
Quorum sensing in Vibrio spp.
Although LuxI/LuxR homologs have been found in most gram-negative quorum-sensing organisms studied to date, homologs of the V. harveyi quorum-sensing components have been found only in other Vibrio species, including V. cholerae (10, 21, 30), V. vulnificus (3), V. parahaemolyticus (12, 20), V. anguillarum (4, 5, 23), and V. fischeri (17, 19). Of the four sequenced Vibrio species (V. cholerae, V. parahaemolyticus, V. vulnificus, and V. fischeri), only V. fischeri appears to encode both LuxR/LuxI- and LuxO-based systems of quorum sensing. Why are the vibrios distinct from other gram-negative bacteria in the mechanism by which quorum sensing occurs, and why does V. fischeri contain both systems? These questions await further study, including genomic sequencing of additional Vibrio strains (including other luminescent isolates), but insights into these questions may be gained from research into the roles of the two systems in V. fischeri.
In summary, this work represents an important leap forward in terms of elucidating factors necessary for the V. fischeri-squid symbiosis. Significantly, the dissection by Lupp and Ruby of the relative roles of two distinct quorum-sensing systems at specific stages of colonization, and in particular during an early, low-cell-density stage, provides important insights for the larger field of quorum sensing as well.
Acknowledgments
I am grateful to Emily Yip for her contribution to Fig. 2 and to Bonnie Bassler for her helpful comments.
Research on the Vibrio fischeri-E. scolopes symbiosis in the Visick lab is supported by NIH grant GM59690.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Bassler, B. L., M. Wright, R. E. Showalter, and M. R. Silverman. 1993. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 9:773-786. [DOI] [PubMed] [Google Scholar]
- 2.Bassler, B. L., M. Wright, and M. R. Silverman. 1994. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 13:273-286. [DOI] [PubMed] [Google Scholar]
- 3.Chen, C. Y., K. M. Wu, Y. C. Chang, C. H. Chang, H. C. Tsai, T. L. Liao, Y. M. Liu, H. J. Chen, A. B. Shen, J. C. Li, T. L. Su, C. P. Shao, C. T. Lee, L. I. Hor, and S. F. Tsai. 2003. Comparative genome analysis of Vibrio vulnificus, a marine pathogen. Genome Res. 13:2577-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croxatto, A., V. J. Chalker, J. Lauritz, J. Jass, A. Hardman, P. Williams, M. Camara, and D. L. Milton. 2002. VanT, a homologue of Vibrio harveyi LuxR, regulates serine, metalloprotease, pigment, and biofilm production in Vibrio anguillarum. J. Bacteriol. 184:1617-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croxatto, A., J. Pride, A. Hardman, P. Williams, M. Camara, and D. L. Milton. 2004. A distinctive dual-channel quorum-sensing system operates in Vibrio anguillarum. Mol. Microbiol. 52:1677-1689. [DOI] [PubMed] [Google Scholar]
- 6.Dunlap, P. V. 1999. Quorum regulation of luminescence in Vibrio fischeri. J. Mol. Microbiol. Biotechnol. 1:5-12. [PubMed] [Google Scholar]
- 7.Fidopiastis, P. M., C. M. Miyamoto, M. G. Jobling, E. A. Meighen, and E. G. Ruby. 2002. LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Mol. Microbiol. 45:131-143. [DOI] [PubMed] [Google Scholar]
- 8.Gilson, L., A. Kuo, and P. V. Dunlap. 1995. AinS and a new family of autoinducer synthesis proteins. J. Bacteriol. 177:6946-6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graf, J., P. V. Dunlap, and E. G. Ruby. 1994. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J. Bacteriol. 176:6986-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammer, B. K., and B. L. Bassler. 2003. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 50:101-104. [DOI] [PubMed] [Google Scholar]
- 11.Hanzelka, B. L., and E. P. Greenberg. 1995. Evidence that the N-terminal region of the Vibrio fischeri LuxR protein constitutes an autoinducer-binding domain. J. Bacteriol. 177:815-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henke, J. M., and B. L. Bassler. 2004. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol. 186:3794-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henke, J. M., and B. L. Bassler. 2004. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J. Bacteriol. 186:6902-6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan, H. B., and E. P. Greenberg. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 163:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo, A., N. V. Blough, and P. V. Dunlap. 1994. Multiple N-acyl-l-homoserine lactone autoinducers of luminescence in the marine symbiotic bacterium Vibrio fischeri. J. Bacteriol. 176:7558-7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenz, D. H., K. C. Mok, B. N. Lilley, R. V. Kulkarni, N. S. Wingreen, and B. L. Bassler. 2004. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118:69-82. [DOI] [PubMed] [Google Scholar]
- 17.Lupp, C., and E. G. Ruby. 2004. Vibrio fischeri LuxS and AinS: comparative study of two signal synthases. J. Bacteriol. 186:3873-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lupp, C., and E. G. Ruby. 2005. Vibrio fischeri uses two quorum-sensing systems for the regulation of early and late colonization factors. J. Bacteriol. 187:3620-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lupp, C., M. Urbanowski, E. P. Greenberg, and E. G. Ruby. 2003. The Vibrio fischeri quorum-sensing systems ain and lux sequentially induce luminescence gene expression and are important for persistence in the squid host. Mol. Microbiol. 50:319-331. [DOI] [PubMed] [Google Scholar]
- 20.Makino, K., K. Oshima, K. Kurokawa, K. Yokoyama, T. Uda, K. Tagomori, Y. Iijima, M. Najima, M. Nakano, A. Yamashita, Y. Kubota, S. Kimura, T. Yasunaga, T. Honda, H. Shinagawa, M. Hattori, and T. Iida. 2003. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae. Lancet 361:743-749. [DOI] [PubMed] [Google Scholar]
- 21.Miller, M. B., K. Skorupski, D. H. Lenz, R. K. Taylor, and B. L. Bassler. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303-314. [DOI] [PubMed] [Google Scholar]
- 22.Millikan, D. S., and E. G. Ruby. 2002. Alterations in Vibrio fischeri motility correlate with a delay in symbiosis initiation and are associated with additional symbiotic colonization defects. Appl. Environ. Microbiol. 68:2519-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milton, D. L., A. Hardman, M. Camara, S. R. Chhabra, B. W. Bycroft, G. S. A. B. Stewart, and P. Williams. 1997. Quorum sensing in Vibrio anguillarum: characterization of the vanI/vanR locus and identification of the autoinducer N-(3-oxodecanoyl)-l-homoserine lactone. J. Bacteriol. 179:3004-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyamoto, C. M., P. V. Dunlap, E. G. Ruby, and E. A. Meighen. 2003. LuxO controls luxR expression in Vibrio harveyi: evidence for a common regulatory mechanism in Vibrio. Mol. Microbiol. 48:537-548. [DOI] [PubMed] [Google Scholar]
- 25.Nealson, K. H., and J. W. Hastings. 1979. Bacterial bioluminescence: its control and ecological significance. Microbiol. Rev. 43:496-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyholm, S. V., and M. J. McFall-Ngai. 2004. The winnowing: establishing the squid-Vibrio symbiosis. Nat. Rev. Microbiol. 2:632-642. [DOI] [PubMed] [Google Scholar]
- 27.Ruby, E. G., M. Urbanowski, J. Campbell, A. Dunn, M. Faini, R. P. Gunsalus, P. Lostroh, C. Lupp, J. McCann, D. Millikan, A. Schaefer, E. Stabb, A. Stevens, K. Visick, C. Whistler, and E. P. Greenberg. 2005. Complete genome sequence of Vibrio fischeri: a symbiotic bacterium with pathogenic congeners. Proc. Natl. Acad. Sci. USA 102:3004-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schauder, S., and B. L. Bassler. 2001. The languages of bacteria. Genes Dev. 15:1468-1480. [DOI] [PubMed] [Google Scholar]
- 29.Visick, K. L., J. Foster, J. Doino, M. McFall-Ngai, and E. G. Ruby. 2000. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J. Bacteriol. 182:4578-4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu, J., M. B. Miller, R. E. Vance, M. Dziejman, B. L. Bassler, and J. J. Mekalanos. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 99:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]