Many prokaryotes are able to accumulate large amounts of lipophilic compounds as inclusion bodies in the cytoplasm. Members of most genera synthesize polymeric lipids such as poly(3-hydroxybutyrate) (PHB) or other polyhydroxyalkanoates (PHAs) (125), whereas accumulation of triacylglycerols (TAGs) and wax esters (WEs) in intracellular lipid-bodies is a property of only a few prokaryotes (10). Like the formation of PHAs, TAG and WE biosynthesis is also promoted in response to stress imposed on the cells and during imbalanced growth, for example by nitrogen limitation, if an abundant carbon source is present at the same time. All these lipids act as storage compounds for energy and carbon needed for maintenance of metabolism and synthesis of cellular metabolites during starvation and in particular if growth resumes. Although neutral lipid metabolism in bacteria, especially WE biosynthesis, has attracted increasing biotechnological interest, there has so far been little interest in medical research in the formation of prokaryotic lipid bodies. However, recent findings suggest that lipid body formation and accumulation of TAGs play an important role in the metabolism of the pathogenic bacteria like Mycobacterium tuberculosis (37, 52).
In contrast to the restricted occurrence of storage TAGs in prokaryotes, intracellular TAGs are widespread in many eukaryotes (34, 35, 40, 53, 65, 106, 112, 132, 133). In eukaryotes, storage lipids are also deposited as lipid bodies. Their structure and formation have recently been reviewed in detail by several authors, but additional information on prokaryotic lipid-bodies has been only superficial (98, 100, 151). In contrast, intracellular accumulation of WEs as a storage lipid is a great exception in eukaryotes and occurs only in jojoba (Simmondsia chinensis) (147), and PHAs are not at all synthesized as a storage compound in eukaryotes.
The number of studies investigating the enzymatic and structural fundamentals of storage lipid metabolism in bacteria has increased drastically in the last years, providing the knowledge that storage lipids in bacteria have a similar important metabolic function like in eukaryotes. This review will focus on prokaryotic neutral lipid bodies in comparison with the currently discussed models for lipid body structure and their formation in eukaryotes and with PHA inclusions in prokaryotes.
OCCURRENCE AND FUNCTION OF STORAGE LIPIDS IN PROKARYOTES
Accumulation of at least one type of storage lipid can be found in nearly all prokaryotes. Therefore, it is very likely that lipid accumulation is advantageous for survival in natural habitats and that the capability for their synthesis provides a strong advantage during evolution. The few exceptions of prokaryotes lacking the capability to accumulate lipids (for example, lactobacilli, Enterobacteriaceae, and methanogenic bacteria) exist in nutrient-rich habitats, in which accumulation of lipids does not provide an advantage.
Triacylglycerols.
Large amounts of TAGs have been reported mainly in nocardioforms such as Mycobacterium sp., Nocardia sp., Rhodococcus sp., Micromonospora sp., Dietzia sp., and Gordonia sp. and streptomycetes, which accumulate TAG bodies in the cells and the mycelia (2, 5, 10, 17, 62, 105). Furthermore, TAGs are also frequently accumulated in members of the gram-negative genus Acinetobacter, though the amounts are small in comparison to the accumulated WEs (91, 122, 123). In general, TAGs are stored in spherical lipid bodies, with quantities and diameters depending on the respective species, growth stage, and cultivation conditions. For example, cells of Rhodococcus opacus and Streptomyces lividans contain only few TAGs when cultivated in complex media with a high content of carbon and nitrogen; however, the lipid content and the number of TAG bodies increase drastically when the cells were cultivated in mineral salt medium with a low nitrogen-to-carbon ratio, yielding its maximum in the late stationary growth phase (107, 138). At this stage, cells can be almost completely filled with lipid bodies exhibiting diameters ranging from 50 to 400 nm. One interesting example is R. opacus PD630, where lipids can reach more than 70% of the total cellular dry weight (5).
Wax esters.
The first reports on WE biosynthesis in gram-negative bacteria were published more than 30 years ago, mainly involving the genus Acinetobacter (44, 45, 50, 122). Meanwhile, accumulation of WEs was also described for Moraxella, Micrococcus, and Fundibacter (21, 23, 119). WE biosynthesis has also been reported in actinomycetes: for example, in Corynebacterium, M. tuberculosis, and Nocardia (16, 113, 139). In Acinetobacter calcoaceticus, WEs can reach a fraction of about 25% of the cellular dry weight, indicating that WEs act as main storage compound (44, 138). In general, in A. calcoaceticus only one or a few WE bodies can be observed per cell (122, 128, 138). The shape of WE bodies is not restricted to spherical inclusions, and some authors described flat, disk-like, or rectangular inclusions when the cells were cultivated on alkanes or alkanols, respectively (64, 123). WEs are not exclusively produced as intracellular WE bodies, because some strains of Acinetobacter sp. and the marine bacterium Fundibacterium jadensis also produce extracellular WEs from alkanes (21, 41, 91, 123). However, the function of these extracellular WEs and the mechanisms of their export are not known yet.
The main function of TAGs and WEs is to serve as a storage compound for energy and carbon; however, other functions are also considered. Lipid bodies may also act as a deposit for toxic or useless fatty acids during growth on recalcitrant carbon sources, which have to be excluded from the plasma membrane and phospholipid (PL) biosynthesis (9, 11). Furthermore, many TAG-accumulating bacteria are ubiquitous in soil, and in this habitat, water deficiency causing dehydration is a frequent environmental stress. Storage of evaporation-resistant lipids might be a strategy to maintain a basic water supply. Oxidation of the hydrocarbon chains of the lipids under conditions of dehydration would generate considerable amounts of water which could be useful for the cells. In this context, it could be demonstrated that, besides some other effects important during adaption to water stress, a slow degradation of lipid bodies occurred in cells of R. opacus PD630 during prolonged dehydration (12). Currently only little knowledge exists on the mobilization of accumulated neutral lipids under conditions permitting growth or under energy and carbon deficiency, and the enzymes involved in degradation and their regulation are unknown. Alvarez et al. (8) demonstrated mobilization of TAGs in R. opacus and Rhodococcus ruber to occur in the absence of a carbon source and the presence of ammonium. Under these conditions, up to 90% of the accumulated TAGs were used for cell growth and maintenance of metabolism within 120 h.
Poly(hydroxyalkanoates).
Similar to TAGs and WEs, PHAs represent storage compounds for carbon and energy in members of most eukaryotic genera. Accumulation of PHA was first reported nearly 80 years ago in Bacillus megaterium (83). Since then, many reports on the occurrence of PHAs have been published, including reports on members of the halobacteria, whereas PHA could not be detected in lactobacilli, Enterobacteriaceae, and methanogens (58, 66, 67, 115). Almost 150 different hydroxyalkanoic acids have now been described as constituents of PHAs (127). Interestingly, new polythioesters, in which the ester bonds between the building blocks were replaced by thioester bonds, were detected recently (89, 90). The terminology for PHAs is based on the chain length of the incorporated hydroxy fatty acids and refers to PHAscl (short chain length [C3 to C5]) and PHAmcl (medium chain length [C6 to C14]). The best-characterized bacterium with respect to PHA metabolism is Ralstonia eutropha. Accumulation of PHAs in R. eutropha results in the formation of approximately 10 to 20 nearly spherical intracytoplasmic inclusions per cell, with a diameter of up to 500 nm and amounting to up to 90% of the cell dry weight (13). A few actinomycetes, like, for example, R. ruber, are capable of simultaneously synthesizing and accumulating similar amounts of TAGs and the copolyester poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from unrelated carbon sources like glucose (6, 72). Readers more interested in details of PHA metabolism or in the enzymes and genes involved therein should consult one of the recent reviews (114, 129).
STRUCTURE AND PROPERTIES OF PROKARYOTIC LIPID INCLUSIONS
All storage lipids are deposited as intracytoplasmic inclusions (Fig. 1). The general structure of these inclusions seems to be relatively simple. They consist of a hydrophobic core comprising the respective storage lipid and are surrounded by a layer of amphiphilic compounds comprising various proteins and PLs.
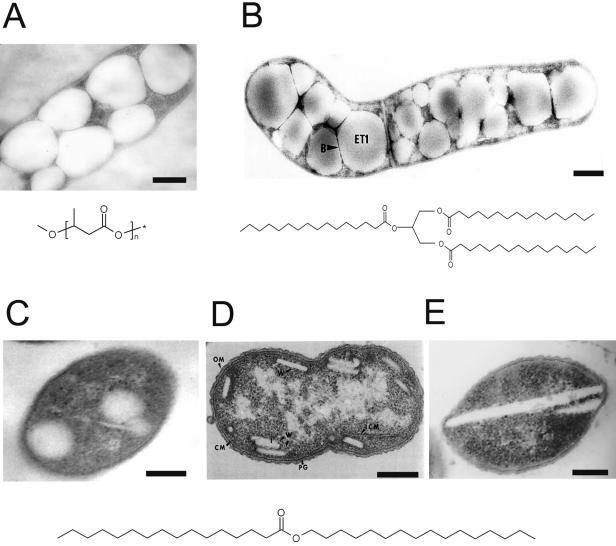
FIG. 1.
Intracellular lipid inclusions in prokaryotes and general structures of the lipids. (A) Cell of R. eutropha H16 accumulating PHB inclusions (109); (B) cell of R. opacus PD630 from late stationary growth phase accumulating large amounts of TAG inclusions (5); (C) cell of A. calcoaceticus ADP1 with three spherical WE inclusions; (D) Acinetobacter sp. strain HO1-N accumulating small rectangular WE inclusions (123); (E) Acinetobacter sp. strain M1 accumulating large, disclike WE inclusions (64). Abbrevations: B, boundary layer; CM, plasma membrane; ET, electron-transparent TAG inclusion; ICM, intracellular plasma membrane; OM, outer membrane; PHA, PHA inclusion; PG, peptidoglycan; W, wax ester inclusion. Bars, 0.2 μm.
Triacylglycerol inclusions.
Already in the 1940s to 1960s, droplets or vacuoles had been described in various species of the genus Mycobacterium (22, 24, 49, 76). However, although their lipophilic nature was recognized, no further investigations were performed. Droplets were also recognized in Streptomyces. However, they were simply referred to as vacuoles or PHA inclusions, without chemical analysis (29, 144). First investigations aimed at revealing the structure of lipid-bodies started only in the 1990s with Streptomyces sp. (107). The authors described electron-transparent lipid bodies in the cytoplasm with no further internal structure, which occurred in the late stationary growth phase and filled a substantial fraction of the cells. These lipid bodies showed some tendency of coalescence, and it was assumed that they possess a thin surrounding membrane; having about half the width of the plasma membrane. TAG bodies were first isolated and chemically investigated from R. opacus strain PD630 by Alvarez et al. (5), who also observed a unique boundary layer (Fig. 1B). These TAG bodies were mainly composed of TAGs (87%), diacylglycerols (∼5%), free fatty acids (∼5%), PLs (1.2%), and proteins (0.8%). TAGs from R. opacus were mainly composed of hexadecanoic acid (36.4% of total fatty acid content) and octadecenoic acid (19.1%) and with considerable amounts of odd-numbered fatty acid residues like heptadecanoic acid (11.4%) and heptadecenoic acid (10.6%) when cells were grown on gluconic acid, acetic acid, or fructose. These carbon sources have to be degraded to acetyl coenzyme A (acetyl-CoA) before fatty acid de novo synthesis occurs, yielding the fatty acids detected in the TAGs. In carbohydrate-grown cells of R. opacus, the stereospecific distribution of the acyl residues on the glycerol backbone is not random. The shorter and saturated fatty acid residues were predominantly esterified to the sn-2 hydroxyl group, whereas unsaturated fatty acids were predominantly bound at position sn-3 (137). The composition of the TAGs strongly varied when the cells were cultivated on propionic acid, which was activated to propionyl-CoA, resulting in a much higher fraction of odd-numbered fatty acids. Furthermore, TAG formation doesn't only depend on fatty acid de novo synthesis, as was revealed by growth experiments using alkanes and phenylalkanes as carbon sources. In this case, alkanes were directly oxidized and activated to the respective acyl-CoA thioesters and incorporated into TAGs (11).
Wax ester inclusions.
As was noted previously, three different forms of WE bodies were reported to occur in Acinetobacter sp. (i) Spherical inclusions in hexadecane-grown cells of A. calcoaceticus HO1-N exhibited average diameters of 200 nm and were first referred to as hydrocarbon inclusions. It was discussed that they could represent an intracellular pool of the unmodified hydrocarbon; however, hexadecane was only a minor component of these inclusions, whereas the WE content was more than 50% of the total lipids (122). Freeze-etching of these inclusions revealed irregular internal fracture faces, and it was suggested that the inclusions were separated from the cytoplasm by a half-unit membrane. Furthermore, intracellular bilayer membranes were described, which were in direct contact with the plasma membrane. In carbohydrate-grown cells of A. calcoaceticus ADP1, WEs also occurred in spherical inclusions with an average diameter of 200 nm, which were also surrounded by PLs (Fig. 1C) (128, 138). Intracellular WEs in strains ADP1 and HO1-N are mainly composed of saturated or monoenic acyl chains with 16 and 18 carbon atoms esterified to fatty alcohols with the same carbon chain length and degree of saturation (71, 123). (ii) Singer et al. (123) reported on electron-transparent, rectangular inclusions with a typical bilayer surface structure in strain HO1-N grown on hexadecanol, measuring 100 to 200 nm in length and 25 to 30 nm in width (Fig. 1D). The authors described the coformation of intracellular cytoplasmic membranes too. These structures could be isolated, and chemical analysis revealed that they consisted of hexadecylpalmitate (85.6%), hexadecanol (4.8%), and PLs (9.6%). In addition, substantial amounts of lipids were also released into the culture broth. These lipids reached a concentration of 320 μmol per liter and were composed mainly of WEs and TAGs as minor compounds. (iii) In hexadecane-grown cells of Acinetobacter sp. strain M-1, WE bodies occurred as electron-transparent, smooth, and disk-like inclusions without a membrane structure (Fig. 1E) (64). These structures grew to about the same diameter as the cell and contained WEs with 32 to 48 total carbon atoms. The authors also performed investigations of the formation of WE bodies in strain M-1 and described the sequential formation of disks, with one disk completion resulting in the formation of a following one, ending in an accumulation of WE bodies resembling a stack of coins. It is not known whether the different carbon sources or costorage of hydrocarbons or long-chain alcohols caused this morphological divergence observed by the different authors or if it was a specificity of the investigated strains.
PHA inclusions.
Light microscopic studies identified PHA inclusions as intensively light-scattering granules inside the cytoplasm, and in electron micrographs they appear as spherical and opaque structures with a diameter of up to about 500 nm (Fig. 1A). These polyesters are synthesized by PHA synthase (PhaC), which utilizes CoA thioesters of the respective hydroxy fatty acids as substrates. Under conditions of carbon and energy deficiency, PHA inclusions are mobilized by intracellular PHA depolymerases (PhaZ), which are also localized on PHA inclusions. In R. eutropha, three active intracellular depolymerases were detected, but this organism possesses at least five depolymerase genes (110, 120, 149). According to the dimensions of the inclusions and to the molecular weight of one PHB molecule, which may consist of up to approximately 35,000 d-(−)-3-hydroxybutyrate moieties, it was assumed that one average PHB inclusion contains more than 40,000 PHB molecules (126). PHA inclusions appear to have a single boundary layer, which was first believed to be a membrane 15 to 20 nm in thickness or a homogenous layer of PhaC (42, 88, 92). Later reports described the inclusions to be surrounded by a PL monolayer with a 4-nm width and with proteins engaged in PHA synthesis, mobilization, and structural function embedded (38, 61, 93). In R. eutropha, four classes of proteins have been demonstrated to occur at the surface of PHA inclusions: PhaC; PhaZ; a transcriptional repressor, PhaR; and phasins (PhaP). PhaP is thought to be a structural protein, because it can be found in large quantities on the inclusions. Recently, it was shown that at least four different PhaPs exist in R. eutropha, which are referred to as PhaP1, PhaP2, PhaP3, and PhaP4 (110). In atomic force microscopy, PHA inclusions from R. eutropha appear as rough structures with a boundary layer 4 nm in thickness, which is in good agreement with the dimensions of a typical PL monolayer. This layer contained globular, pore-like exaltations 35 nm in diameter, which were believed to be composed mainly of PhaP. These exaltations were connected to each other by linear substructures, resembling some kind of cytoskeleton. After elimination of the boundary layer and the globular structures by treatment with detergents, PHA inclusions showed a smooth, network-like surface of parallel 4-nm arrays, assumed to be the naked PHB core (39).
PROTEINS ASSOCIATED WITH PROKARYOTIC LIPID INCLUSIONS
Similar to the isolation of eukaryotic oil bodies and prokaryotic PHA inclusions, neutral lipid bodies can be prepared from lysates of the bacterial cells by centrifugation in density gradients (72, 133, 143). Purification of native lipid inclusions is a first crucial step in identifying the structural or enzymatic proteins associated therewith.
Wax ester bodies.
Scott and Finnerty reported that the protein pattern occurring in purified WE bodies was different from all other membrane fractions isolated from Acinetobacter sp. strain HO1-N (122). About 12 proteins were associated with the inclusions; however, no further characterization of them was done. The first attempts to identify proteins associated with WE bodies were performed with Acinetobacter sp. strain 211 (7). These WE bodies contained one major polypeptide of 39 kDa. The first 9 amino acids of the N terminus of this protein were determined, but further characterization was not achieved at that time. Database search revealed that this N terminus shows a high homology to an outer membrane porin from Acinetobacter baumanii. Copurification of outer membrane porins as the main proteins of WE bodies occurred as well with inclusions from A. calcoaceticus ADP1 (D. Mergelkamp, R. Kalscheuer, and A. Steinbüchel, unpublished results). Recently, it was demonstrated that the wax ester synthase/diacylgycerol acyltransferase (WS/DGAT) is partly located on the surface of WE bodies and also in the cytoplasm and the plasma membrane, as was reported earlier in A. calcoaceticus ADP1 (Table 1) (128, 138).
TABLE 1.
Lipid body proteins and functions
| Cell type | Protein | Function | Reference(s) |
|---|---|---|---|
| Plants | |||
| Seeds and fruits | Oleosin | Structural, maintenance of integrity | 98, 100 |
| Steroleosin | Dehydrogenase/reductase function in signal transduction | 85 | |
| Caleosin | Probably involved in assembly of proteins on the lipid body surface | 32, 46, 101 | |
| Anther pollen | None | 98 | |
| Anther tapetum | Oleosin-like | Structural | 98 |
| Plastids | Plastid lipid-associated protein; fibrillin | Structural | 40, 75, 97, 136 |
| Animals | |||
| Liver hepatocytes, intestine enterocytes | Apolipoprotein | Structural | 98 |
| Adipose enterocytes | Perilipin | Structural | 34, 151 |
| ADRP/TIP47 | Role in lipid body formation and differentiation, control of lipolysis | ||
| Adrenal, testis, ovary (steroidogenic) | Perilipin/ADRP/TIP47 | Control of lipolysis | 151 |
| Mammary epithelium | ADRP/TIP47 | Control of lipolysis | 151 |
| Others (e.g., leukocytes) | ADRP?/TIP47? | Control of lipolysis | 151 |
| Microorganisms | |||
| Yeast | Squalene epoxidase | Lipid metabolism | 82 |
| Sterol-Δ24-methyltransferase | 151 | ||
| Oxidosqualene cyclase | 94 | ||
| Long-chain fatty acid ligases | 151 | ||
| Acylglycerol-3-phosphate dehydrogenase | 151 | ||
| Alcohol acetyltransferase | 135 | ||
| TAG lipase | 15 | ||
| PHA-accumulating | PHA synthase | PHA biosynthesis | 114, 129 |
| prokaryotes | PHA depolymerases | PHA mobilization | 149 |
| Phasins | Structural and involved in PHA inclusion formation | 126, 143 | |
| PhaR | Regulation of phasin expression | 109 | |
| TAG- and WE-accumulating prokaryotes | WS/DGAT? Specific lipases? | Lipid biosynthesis and mobilization | 128, 138 |
Triacyglycerol bodies.
Isolated TAG bodies from R. opacus and R. ruber exhibited a protein pattern strongly resembling the protein pattern of total cell lysates, indicating that a large number of unspecifically bound proteins were copurified (72). This effect is similar to the situation described for oil bodies from plant seeds, which also always contain contaminants of unspecifically bound proteins (95, 133). TAG body-associated proteins were divided into three groups, reflecting their binding properties: (i) probably unspecifically bound proteins by ionic interactions, which could be solubilized by salt treatment or mild detergents; (ii) tightly bound proteins which could be solubilized by treatment with chaotropic reagents; and finally, (iii) extremely tightly bound proteins which even resisted treatment with up to 8% sodium dodecyl sulfate or proteolytic digestion, and which could be only solubilized by sodium dodecyl sulfate plus heat treatment.
Proteins of the latter group were believed to play a role in the structure or formation of TAG bodies and comprised about six proteins in a single bacterium. These proteins were referred to as granule-associated proteins (GAP) and exhibited apparent molecular masses ranging from 15 to 31 kDa. In R. opacus PD630, these were GAP15, GAP17, GAP20, GAP26, and GAP31, with the numeration indicating the apparent molecular weight (72). GAP17 from R. ruber and R. opacus as well as GAP15 from R. opacus showed high homologies to ribosomal L7 proteins from different actinomycetes. L7 proteins are part of the stalk region of 50S ribosomal subunits and exhibit low molecular masses (12 to 13 kDa). These proteins possess hydrophobic domains near their N termini which could interact with the lipid matrix of the lipid bodies. Cloning and characterization of the genes of GAP20 and GAP31 from R. opacus PD630 identified these proteins as outer membrane porins, as revealed by sequence homology alignments with an outer membrane porin (MspA) from M. smegmatis, thus reminding the situation of WE bodies in Acinetobacter sp. (104).
All porin homologous proteins purified from WE and TAG bodies were correctly processed and were devoid of their signal peptides. Obviously, these proteins became artificially bound to the inclusions only during cell disruption. Outer membrane porins of M. smegmatis are oligomeric proteins with a goblet-like tertiary structure, exposing a mostly nonpolar surface at the goblet's stem and base (43), which could also deeply interact with the lipid core of the inclusions, thus accounting for their strong binding properties. It should be noted that these porins are the only proteins which remained associated with the inclusions under the conditions used in the respective experiments. Removal of all proteins did not affect the structural integrity of the inclusions, suggesting that bacteria do not need any structural proteins to maintain the intracellular lipid bodies. For this reason, prokaryotic lipid bodies are most likely comparable to oil bodies in yeast or some plants which also completely lack structural proteins (98). However, in contrast to PHA inclusions, the difficulties in preparing native prokaryotic TAG and WE bodies imply that future and detailed investigations need in situ studies to reveal a deeper knowledge about the surface of prokaryotic lipid-bodies and their protein equipment.
PHA inclusions.
Besides PhaC, PhaZ, and PhaR, the most abundant proteins covering PHA inclusions are low-molecular-mass phasins (14 to 28 kDa) (Table 1) (126). In R. eutropha, PhaP1 can contribute up to 5% of the total cellular protein in cells cultivated under storage conditions (143). In combination with a proposed layer of PLs, their major role in PHA metabolism is believed to separate the PHA core from the cytoplasm and by this prevent PHA inclusions from coalescing with each other because their surface is no longer hydrophobic, similar to the oleosins in plants (55, 70, 93). How PhaP1 binds to PHA inclusions is unclear. In the C-terminal region of the phasin protein of R. ruber, two short stretches of hydrophobic amino acids were detected. Truncated proteins lacking one or both of these stretches were no longer able to bind to PHA inclusions (108). It may be assumed that PhaP1 possesses similar regions at least in the mature protein. In contrast to oleosins of plant seeds, PhaP1 is directly involved in the regulation of the size, quantity, and formation of PHA inclusions and it was shown that the amount of PhaP1 parellels the amount of PHA in the cells (108, 126, 143, 148). The presence or absence of this protein interferes with the shape and number of PHA inclusions, as revealed in mutants overexpressing or lacking PhaP1 (143). In R. eutropha, expression of PhaP1 is regulated by the transcriptional repressor PhaR, which binds upstream of the phaP1 gene in the promoter region. Lowering of the PhaR titer under conditions permissive for PHA synthesis is mediated by binding of PhaR to the PHA inclusions, thus allowing synthesis of PhaP1 (109). The additional phasins, for which genes were detected in the genome of R. eutropha and which occur in much lower concentrations in the cells, also have the capability to bind to PHA inclusions, but their exact function is not known yet (110).
As noted above, in R. ruber significant amounts of TAG and PHA exist simultaneously in inclusions. It was shown that in R. ruber phasin was localized on any inclusions (72, 108). This suggests that in R. ruber the situation is much more complex and that phasins have also a high affinity for TAG bodies, although the processes for formation of both types of inclusions are rather different (see below).
FORMATION OF PROKARYOTIC LIPID BODIES
Although bacterial neutral lipid and PHA inclusions and eukaryotic lipid inclusions share many parallels, the mechanisms of their formation are most likely very different.
Wax ester and triacylglycerol bodies.
In A. calcoaceticus ADP1, both WE biosynthesis and TAG biosynthesis are mediated by WS/DGAT (73). This enzyme comprises a putative membrane-spanning region but shows no sequence homology to the DGAT1 and DGAT2 families from eukaryotes or the WE synthase from jojoba (19, 27, 28, 60, 80, 118, 121, 150). Under in vitro conditions, WS/DGAT shows a broad capability of utilizing a large variety of fatty alcohols and even thiols as acceptors of the acyl moieties of various acyl-CoA thioesters (74). The substrate specifities of this acyltransferase are extraordinary broad, and it can be considered as a promiscuous enzyme. The specific activity of WS/DGAT with diacylglycerol as an acyl acceptor was one magnitude lower than with long-chain fatty alcohols, thus resembling the distribution of both lipid types in this bacterium (73). WS/DGAT from A. calcoaceticus ADP1 represents the first member of a widespread class of bacterial WE and TAG biosynthesis enzymes, because in all bacteria sequenced so far which are known to accumulate neutral lipids, genes for homologous acyltransferases were detected: e.g., in M. tuberculosis CDC1551, almost 15 homologues are present (37, 73).
In cells of strain ADP1, which reached their maximum lipid content, WS/DGAT was found not only at the surface of WE bodies but also in the cytoplasm and at the plasma membrane (128). In contrast, this WS/DGAT is almost exclusively localized at the plasma membrane in cells just beginning to store lipids (138). Corresponding to the cellular localization of WS/DGAT, WE formation starts at special domains at the plasma membrane (Fig. 2) (138).
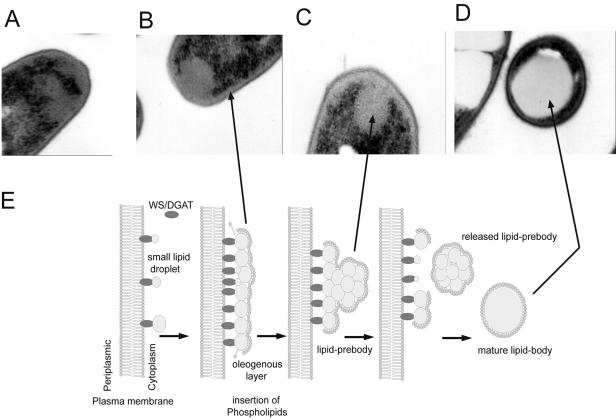
FIG. 2.
Stages of the formation of TAG bodies in R. opacus PD630 and a generalized hypothetical model for the formation of neutral lipid bodies in bacteria. (A) Formation of an oleogenous layer from SLDs at the plasma membrane; (B) lipid prebody formation through conglomeration of SLDs; (C) partly released lipid prebody; (D) mature, cytoplasm-localized lipid body; (E) scheme (138).
Thus, similar to the formation of eukaryotic lipid bodies at the endoplasmic reticulum (ER) membrane, bacterial neutral lipid synthesis is strictly associated with the plasma membrane, with one all-dominant difference: bacterial lipids are not synthesized between the leaflets of a PL bilayer. In bacteria, WE and TAG formation starts with the docking of WS/DGAT to the plasma membrane, and small lipid droplets (SLDs) are formed. These SLDs are only some nanometers in diameter and remain associated with the membrane-docked WS/DGAT. In this phase of lipid accumulation, SLDs form an emulsive, oleogenous layer at the plasma membrane (Fig. 2A). During prolonged lipid synthesis, SLDs leave the membrane-associated acyltransferase and conglomerate to membrane-bound lipid prebodies (Fig. 2B and C). These lipid prebodies reach distinct sizes, e.g., 200 nm in A. calcoaceticus and 300 nm in R. opacus, before they lose contact with the membrane and are released into the cytoplasm (138). Free and membrane-bound lipid prebodies correspond to the lipid domains occurring in the cytoplasm and at the cell wall, as recently observed in M. smegmatis during fluorescence microscopy and also confirmed in R. opacus PD630 and A. calcoaceticus ADP1 (33, 138). Inside the lipid prebodies, SLDs coalesce with each other to form the homogenous lipid core found in mature lipid bodies, which appear opaque in electron microscopy, whereas lipid prebodies exhibit a granulous internal structure (Fig. 2D) (138). Although it was not shown in detail, it is very likely that PLs associate in an early stage with membrane-bound SLDs, because it was demonstrated that mature cytoplasmic lipid bodies are at least partially covered by PLs. This hypothesis (Fig. 2E) is also confirmed by experiments in artificial systems, demonstrating the formation of SLDs and lipid prebodies and their final release from solid-phase-supported membranes by scanning force microscopy and quartz crystal microbalance measurements (138).
However, WS/DGAT activity was demonstrated as well on isolated lipid bodies in vitro and WS/DGAT proteins could be localized on cytoplasmic lipid bodies by cytoimmunological methods (128). This may indicate that lipid biosynthesis also occurs on intracellular lipid bodies in vivo. Despite this, it is questionable if the substrates for TAG and WE biosynthesis can reach the lipid bodies already released into the cytoplasm to a significant extent, because most enzymes for biosynthesis of the precursors are typical membrane proteins (36, 116, 145). Furthermore, there is no explanation of how PLs could reach lipid bodies growing in the cytoplasm, which had already lost contact with the plasma membrane. Thus, although bacterial lipid bodies are quite different in many aspects from eukaryotic lipid bodies, they probably also obey the eukaryotic dogma, which means that lipid biosynthesis does not occur on lipid bodies per definitionem (100).
Poly(hydroxyalkanoate) inclusions.
Although the enzymatic and molecular fundamentals of PHA metabolism have been intensively investigated, our knowledge about formation of PHA inclusions and mobilization of the accumulated PHA is still very scarce. However, the formation of PHAs has to start from soluble substrates which are finally found in insoluble inclusions. Two different models have been proposed for the formation of PHA inclusions (70, 129).
The micelle model.
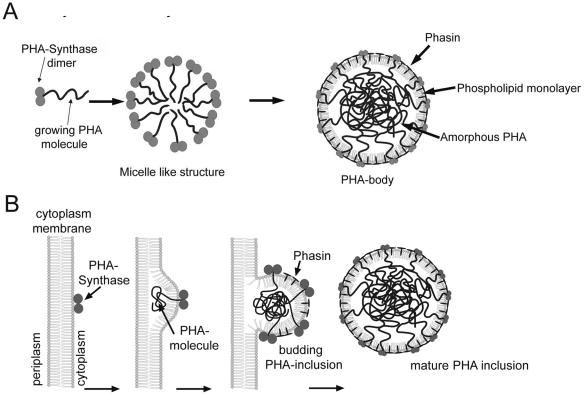
The micelle model (Fig. 3A) involves the formation of micelle-like structures from primed PhaC, which itself is a cytoplasmic and hydrophilic enzyme. There is obviously no region of the enzyme protein which mediates binding to hydrophobic surfaces like the PHA inclusions or the plasma membrane. The conversion to an amphiphilic enzyme-product complex is mediated by prolonged synthesis of the hydrophobic PHA molecule. This amphiphilic enzyme-product complex could form micelle-like structures with the growing PHA molecules representing the hydrophobic core and PhaC itself representing the head group. In this model, PhaC remains at the surface of the growing inclusion and is bound covalently to the growing PHA molecule therein. With increasing volume of the inclusion due to increased chain length of the PHA molecules, the PhaC proteins are separated and dispersed on the surface. Therefore, an increasing fraction of the hydrophobic PHA core would be directly exposed to the cytoplasm, if these regions are not covered by other amphiphilic molecules. This is obviously done by phasins and PLs, which occupy the empty spaces at the surface and prevent the inclusions from coalescing with each other. Although this model is supported by the strict regulation of phasin expression and analysis of the composition of PHA inclusions, there is no explanation of how PLs are inserted and whether they are required at all. In principle, phasins alone may be sufficient to provide the amphiphilic layer at the surface of the inclusions. It is worth mentioning that PLs had so far only been detected in isolated PHA inclusions; there are no reports on in situ studies actually demonstrating the presence of PLs in the inclusions as they occur in the cells.
FIG. 3.
Suggested models for the formation of PHA inclusions in bacteria. (A) The “micelle” model; (B) the “budding membrane” model (70, 129).
The budding membrane model.
The budding membrane model (Fig. 3B) is partially analogous to the model of lipid body formation in plant seeds. It depends on attachment of PhaC to the plasma membrane. Membrane-bound PhaC could then synthesize a growing PHA molecule into the hydrophobic core between the PL bilayer, thus causing a swelling of the plasma membrane. Similar to the formation of TAGs within the ER membrane in seeds, budding of a PHA inclusion surrounded by a PL monolayer derived from the inner membrane leaflet, could occur. This model would provide an easy explanation for the incorporation of PLs. In principal, it may be questioned whether or not phasins are at all required for this model or whether PLs would be sufficient for generation of the amphiphilic layer at the surface of the PHA inclusions. This is in contrast to the observation that PHAscl inclusions without phasins were hitherto not observed. Incorporations of phasins will be faciliated if they accumulate at or close to the plasma membrane, from which they are selectively inserted into the nascent PHA inclusion, similar to the proposed targeting of oleosins into seed oil bodies.
NONPROKARYOTIC LIPID BODIES
To deepen our understanding of bacterial lipid inclusions, it may be helpful to compare their properties with those of eukaryotic lipid bodies, which have been the subject of intensive research in the last decades.
Plants.
In oleogenous plants like sunflower or rape, the major location of lipid storage is the seed. In oilseeds, lipids are stored in oil bodies with 0.6 to 2.5 μm diameter (133). The predominant storage lipids are TAGs, which can constitute up to 75% of the seed weight (98). Interestingly, seeds of jojoba store lipids as long-chain WEs (147). Similar to prokaryotic lipid bodies, plant oil bodies are composed of a core of neutral lipids surrounded by a half-unit membrane of PLs (146) and some proteins embedded therein. The amount of total proteins associated with seed oil bodies varies between 0.2% in peanuts and up to 20% in rape (98, 99). Many proteins have been identified which are associated with the oil bodies in plants. This includes caleosins (31, 32, 46, 101), steroleosins (85), and additional proteins of yet unknown function (69). However, the main surface proteins associated with desiccation-tolerant seed oil bodies are oleosins (Table 1) (98). Oleosins are structural proteins of low molecular masses (15 to 26 kDa), depending on the isoforms and species in which they occur. Due to their secondary structure, oleosins form a meshwork-like organization on the lipid body surface. Oleosins are composed of two amphiphilic regions at the N and C termini, whereas the central part represents an expanded conserved domain composed of hydrophobic amino acids, which directly contacts the lipid matrix (30, 78). The secondary structure of this hydrophobic domain was predicted to be an antiparallel β-strain in solvent (63), but more recent experiments performed on native oleosins suggest that it presents mostly as an α-helix (78). A proline knot motif is located at the central loop of this region and was recognized as a putative targeting signal to the TAG core (1). It is assumed that oleosins play a key role in oil body stability in desiccating seeds, since they prevent them from coalescence and attack of unspecific cytosolic lipases or phospholipases (84, 134). Although oleosins play a major role in the structural organization of seed oil bodies, it is improbable that they have an essential function in their formation, because they are absent from oil bodies of many seeds that do not undergo dehydration and from large oil bodies in the mesocarp tissue of olives and avocados, which are totally devoid of associated proteins (98, 117).
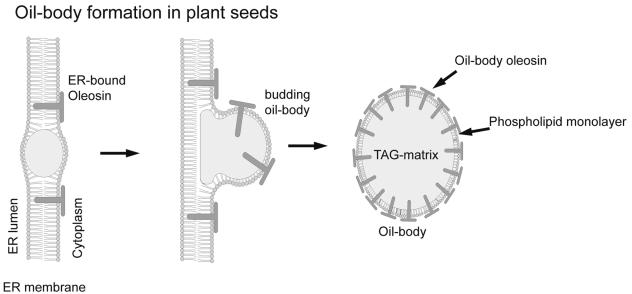
Several different models have been proposed for the formation of seed oil bodies, from which the budding model is the most accepted (Fig. 4). It is based on the formation of small TAG droplets at special subdomains between the two PL leaflets of the ER (48, 103, 140). The intramembranous accumulation of TAGs was confirmed on isolated sunflower microsomes with active TAG biosynthesis, showing that TAG molecules tumble isotropically between both PL leaflets in an environment similar to that in native oil bodies (79). Progressive lipid accumulation will then lead to a swelling of the ER and subsequently to the budding of a lipid body surrounded by a PL monolayer derived from the outer ER leaflet. The coating of nascent oil bodies with oleosins is mediated by a cotranslational insertion of the oleosins into the ER membrane (59, 86). This hypothesis was also confirmed by the localization of TAG biosynthesis enzymes at special subdomains of the ER (26, 77, 79, 118).
FIG. 4.
Model for the formation of lipid bodies in plant seeds. The model involves the accumulation of lipids between both leaflets of the ER membrane. Budding oil bodies are surrounded by a monolayer of phospholipids, which were derived from the outer leaflet of the ER and in which oleosins are embedded (100, 151).
Mammalian lipid bodies.
The storage of TAGs is fundamental in almost any animal. The main components of intracellular lipid bodies in mammals are TAGs and steryl esters. The main location of long-term TAG storage is white adipose tissue, in which only a limited number of lipid bodies per cell with a maximum diameter of 100 nm can be observed (98). In hibernating animals and fetuses and neonates of many animals, brown adipose tissue also occurs. The quantity of lipid bodies in brown adipocytes is much higher than that in white adipocytes, but their extension is restricted to 2 to 10 nm. In contrast to white adipose tissue, whose main function is TAG storage, the main function of brown adipocytes is thermogenesis through lipolysis and uncoupled respiration. Also in most other cells (for example, in liver, heart, muscle, intestine, and mammary gland), lipid bodies are present (56, 141, 142). Mammalian lipid bodies are spherical structures with a core of neutral lipids coated by a PL monolayer into which specific proteins are embedded. However, only a few of these proteins have been identified, and little is known about their structural properties and functions (Table 1) (34, 151). The main proteins associated with mammalian lipid bodies are proteins of the PAT family, namely perilipins, adipose differentiation-related protein (ADRP) and tail-interacting protein of 47 kDa (TIP47) (96). The best-characterized members of the PAT family are the perilipins, which are highly phosphorylated proteins localized on the surface of lipid bodies of adipocytes and steroidogenic cells and which are involved in lipid body biogenesis and their stabilization. They act as shielding proteins controlling both the storage and lipolysis of TAGs. After phosphorylation by protein kinase A, which is activated by hormonally controlled cyclic AMP, perilipins are released from the lipid bodies, thus enabling hormone-sensitive lipase to bind to the lipid core and to hydrolyze the embedded TAGs. Perilipins are encoded by a single-copy gene in three different forms (A, B, and C) by alternative splicing of the respective mRNA, depending on the tissues in which they occurr. Perilipins A and B from adipocytes have molecular masses of 56 and 46 kDa, both exhibiting a common N-terminal amino acid sequence, which is also very similar to the N termini of ADRP and TIP47 (87). The anchorage of perilipin A is mediated by three hydrophobic regions in the central region of the protein. These regions are flanked by an amphiphilic β-stranded region and the C terminus, which both shield the lipid core from the cytoplasm, and the protein kinase A consensus site (51). In contrast to the restricted occurrence of perilipins in adipocytes and steroidogenic cells, ADRP and TIP47 are expressed in many tissues and cell types. In adipocytes, ADRP seems to play a major role in lipid body differentiation, and during formation of adipocytes from preadipocytes, nascent lipid bodies are surrounded by ADRP (68). The term ADRP is a reference to reflect the early appearance of this protein, which was originally isolated as a strong protein marker in adipocyte differentiation. In a later stage, ADRP is replaced by perilipin as the predominant protein of mammalian lipid bodies (20). ADRP does not exhibit an extended hydrophobic domain like the perilipins; however, a recent study revealed that an amino- and a carboxy-terminal region of ADRP is sufficient for targeting to lipid bodies (102).
Formation of lipid bodies in mammals might occur in a similar way to how it was described for oil body formation in plant seeds; however, the mechanism involved in this process is not well understood. Blanchette-Mackie (18) described a junction between the ER membrane and the lipid body surface, indicating that their formation is associated with the ER. It was discussed whether small lipid bodies in adipocytes undergo coalescence with each other to form one or more large lipid bodies found in matured adipocytes, but the mechanisms involved in controlled coalescence remained unclear (98). This hypothesis was supported by the demonstration that small lipid bodies in adipocytes are associated with cytoskeletal intermediate filaments (e.g., vimentin), which form a cage-like structure around them. This association could prevent fusion and play a role in regulation of controlled coalescence or may play a role in intracellular trafficking of lipid bodies (3, 4, 47).
Yeast and fungi.
Similar to higher eukaryotes and the prokaryotes described above, several yeasts and filamentous fungi, for example, Rhodotorula sp., Saccharomyces cerevisiae, Lipomyces starkeyi, or Mortierella alpina, are able to accumulate large amounts of intracellular lipids after the cells have entered the stationary growth phase (14, 15, 112, 151). Yeast lipid bodies share many properties with those from plants and mammals, and their formation is proposed to rely on the same mechanisms (151). They consist of a hydrophobic core containing TAGs and steryl esters in a similar proportion, surrounded by a PL monolayer accommodating a small amount of specific proteins. In contrast to plants and mammals, no putative structural proteins were identified among them, until now. Similar to their role in higher organisms, yeast lipid bodies serve as a depot for storage lipids, but it is very likely that they also play a major role in biosynthesis, intracellular transport, and channelling of lipid metabolites, because most of the lipid body proteins identified so far are involved in sterol, fatty acid, and TAG metabolism (Table 1) (14, 15, 81, 82, 94, 124, 131, 135).
Plastids.
Lipid bodies in plastids may be similar to prokaryotic lipid bodies but are only poorly characterized. In chloroplasts and chromoplasts, TAGs, sterylesters, and carotenoids are stored in special structures during growth and under stress conditions (40, 111, 130, 136). Furthermore, in many fruits, for example, bell pepper (Capsicum annuum L.), chloroplasts differentiate while ripening into photosynthetically inactive chromoplasts (25). This differentiation involves degradation of chlorophylls and structural reorganizations, including the degradation of thylakoid membranes and accumulation of lipid bodies (40). Plastid lipid bodies are termed plastoglobules, tubules, fibrils, or crystalloids. Their function is not only restricted to lipid storage, but carotenoids also act as attractants to animals in ripened fruits or as light receptors in photosynthesis. Plastoglobules appear as globular osmiophilic structures not surrounded by a boundary membrane in electron microscopy (54, 57), and it was suggested that they are in direct contact with the stromal surface of the thylakoid membranes and the inner envelope of plastids (75). The neutral lipids inside the plastoglobules form a hydrophobic matrix, which is surrounded by PLs, glycolipids, and proteins, which are termed plastid-lipid associated proteins (PAPs) or plastoglobulins. It was suggested that PAPs control carotenoid function on thylakoid membranes and stabilize lipids in plastoglobules, where they may act as an attachment site to the thylakoid membrane or as regulatory proteins mediating lipid transfer between the plastoglobule and the thylakoid membranes (40, 75, 97, 136).
CONCLUSIONS AND OUTLOOK
The cellular substructures referred to as prokaryotic lipid bodys or lipid inclusions discussed here share many similar aspects to lipid bodies in plants, mammals, or yeasts. Both eukaryotic and prokaryotic neutral lipid bodies are composed of a hydropobic core of lipids surrounded by a monolayer of PLs and obviously with no essential need for structural proteins. Formation of eukaryotic and prokaryotic lipid bodies is directly associated with a cellular membrane, the ER membrane, or the plasma membrane, respectively. Formation of eukaryotic lipid bodies occurs by lipid accumulation between the two leaflets of the ER and subsequent budding into the cytoplasm, whereas in prokaryotes lipid bodies are formed at the membrane-docked WS/DGAT, leading to the formation of membrane-associated SLDs and lipid prebodies, which are released into the cytoplasm after reaching a critical size and from which the mature lipid bodies are finally formed.
Although we are still at the very beginning of an understanding of the metabolic roles and structures of lipid bodies in bacteria, several important steps toward progress were recently made. Nevertheless, many questions remain open. (i) How are bacterial lipid and lipid body biosynthesis and mobilization regulated? (ii) Which enzymes catalyze the intracellular mobilization of lipids, and how do they get access to the lipid bodies? (iii) What is the exact metabolic role of neutral lipid accumulation in bacteria, especially in pathogens? (iv) How are PLs exactly inserted into the lipid body surface? (v) How are the shape and size of the lipid bodies controlled? Similarly, important aspects of PHA inclusion formation and PHA mobilization remain to be addressed. (i) What are the functions of the multiple phasin proteins in PHAscl-accumulating bacteria? (ii) How do PHA depolymerases get access to the surface of PHA inclusions and by this to their substrate during mobilization of PHA? (iii) Are PLs present at the surface of the PHA inclusions, and how are they inserted? To obtain answers, basic functions of the enzymes and proteins involved in lipid and PHA metabolism must be investigated. Applications of highly sophisticated techniques and an interdisciplinary approach of biochemistry, molecular genetics, cell biology, and biophysics promise the answers. The knowledge revealed not only will be important to understand the biogenesis and structure of these inclusions. It will be also important to understand the metabolism of the storage compounds because more and more direct and indirect evidence for interactions between structure, proteins, and enzymes involved in the biosynthesis or the mobilization of the storage compounds and its regulation is obtained.
Acknowledgments
The authors are grateful for financial support of the Deutsche Forschungsgemeinschaft (STE 386/7-2).
REFERENCES
- 1.Abell, B. M., L. A. Holbrook, M. Abenes, D. J. Murphy, M. J. Hills, and M. M. Moloney. 1997. Role of the proline knot motif in oleosin endoplasmic reticulum topology and oil body targeting. Plant Cell 9:1481-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akao, T., and T. Kusaka. 1976. Solubilization of diglyceride acyltransferase from membrane of Mycobacterium smegmatis. J. Biochem. (Tokyo) 80:723-728. [DOI] [PubMed] [Google Scholar]
- 3.Almahbobi, G., L. J. Williams, X. G. Han, and P. F. Hall. 1993. Binding of lipid droplets and mitochondria to intermediate filaments in rat Leydig cells. J. Reprod. Fertil. 98:209-217. [DOI] [PubMed] [Google Scholar]
- 4.Almahbobi, G. 1995. Adhesion of intermediate filaments and lipid droplets in adrenal cells studied by field-emission scanning electron-microscopy. Cell Tissue Res. 281:387-390. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez, H. M., F. Mayer, D. Fabritius, and A. Steinbüchel. 1996. Formation of intracytoplasmic lipid inclusions by Rhodococcus opacus strain PD630. Arch. Microbiol. 165:377-386. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez, H. M., R. Kalscheuer, and A. Steinbüchel. 1997. Accumulation of storage lipids in species of Rhodococcus and Nocardia and effect of inhibitors and polyethylene glycol. Fett Lipid 99:239-246. [Google Scholar]
- 7.Alvarez, H. M., O. H. Pucci, and A. Steinbüchel. 1997. Lipid storage compounds in marine bacteria. Appl. Microbiol. Biotechnol. 47:132-139. [Google Scholar]
- 8.Alvarez, H. M., R. Kalscheuer, and A. Steinbüchel. 2000. Accumulation and mobilization of storage lipids by Rhodococcus opacus PD630 and Rhodococcus ruber NCIMB 40126. Appl. Microbiol. Biotechnol. 54:218-223. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez, H. M., M. F. Souto, A. Viale, and O. H. Pucci. 2001. Biosynthesis of fatty acids and triacylglycerols by 2,6,10,14-tetramethyl pentadecane-grown cells of Nocardia globerula 432. FEMS Microbiol. Lett. 200:195-200. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez, H. M., and A. Steinbüchel. 2002. Triacylglycerols in prokaryotic microorganisms. Appl. Microbiol. Biotechnol. 60:367-376. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez, H. M., H. Luftmann, R. A. Silva, A. C. Cesari, A. Viale, M. Wältermann, and A. Steinbüchel. 2002. Identification of phenyldecanoic acid as a constituent of triacylglycerols and wax ester produced by Rhodococcus opacus PD630. Microbiology 148:1407-1412. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez, H. M., R. A. Silva, A. C. Cesari, A. L. Zamit, S. R. Peressutti, R. Reichelt, U. Keller, U. Malkus, C. Rasch, T. Maskow, F. Mayer, and A. Steinbüchel. 2004. Physiological and morphological responses of the soil bacterium Rhodococcus opacus PD630 to water stress. FEMS Microbiol. Ecol. 50:75-86. [DOI] [PubMed]
- 13.Anderson, A. J., and E. A. Dawes. 1990. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 54:450-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Athenstaedt, K., and G. Daum. 1997. Biosynthesis of phosphatidic acid in lipid particles and endoplasmic reticulum of Saccharomyces cerevisiae. J. Bacteriol. 179:7611-7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Athenstaedt, K., and G. Daum. 2003. YMR313c/TGL3 encodes a novel triacylglycerol lipase located in lipid particles of Saccharomyces cerevisiae. J. Biol. Chem. 278:23317-23323. [DOI] [PubMed] [Google Scholar]
- 16.Bacchin, P., A. Robertiello, and A. Viglia. 1974. Identification of n-decane oxidation products in Corynebacterium cultures by combined gas chromatography mass spectrometry. Appl. Microbiol. 28:737-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barksdale, L., and K.-S. Kim. 1977. Mycobacterium. Bacteriol. Rev. 41:217-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanchette-Mackie, E. I., N. K. Dwyer, T. Barber, R. A. Coxey, T. Takeda, C. M. Rondinone, J. L. Theodorakis, A. S. Greenberg, and C. Londos. 1995. Perilipin is located on the surface-layer of intracellular lipid droplets in adipocytes. J. Lipid Res. 36:1211-1226. [PubMed] [Google Scholar]
- 19.Bouvier-Navé, P., P. Benveniste, P. Oelkers, S. L. Sturley, and H. Schaller. 2000. Expression in yeast and tobacco of plant cDNAs encoding acyl CoA: diacylglycerol acyltransferase. Eur. J. Biochem. 267:85-96. [DOI] [PubMed] [Google Scholar]
- 20.Brasaemle, D. L., T. Barber, N. E. Wolins, G. Serrero, E. J. Blanchette-Mackie, and C. Londos. 1997. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J. Lipid Res. 38:2249-2263. [PubMed] [Google Scholar]
- 21.Bredemeier, R., R. Hulsch, J. O. Metzger, and L. Berthe-Corti. 2003. Submersed culture production of extracellular wax esters by the marine bacterium Fundibacter jadensis. Mar. Biotechnol. 5:579-583. [DOI] [PubMed] [Google Scholar]
- 22.Brieger, E. M., and A. M. Glauert. 1956. Spore-like structures in the tubercle bacillus. Nature 178:544. [DOI] [PubMed] [Google Scholar]
- 23.Bryn, K., E. Jantzen, and K. Bovre. 1977. Occurrence and patterns of waxes in Neisseriaceae. J. Gen. Microbiol. 102:33-43. [DOI] [PubMed] [Google Scholar]
- 24.Burdon, K. L. 1946. Fatty material in bacteria and fungi revealed by staining dried, fixed slide preparations. J. Bacteriol. 52:665-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camara, B., J. Bousquet, C. Cheniclet, J. P. Carde, M. Kuntz, J. L. Evrard, and J. H. Weil. 1989. Enzymology and isoprenoid biosynthesis and expression of plastid and nuclear genes during chromoplast differentiation in pepper fruits (Capsicum annum), p. 141-156. In C. D. Boyer, J. C. Shannon, and R. C. Hardison (ed.), Biochemistry and genetics of nongreen plastids. American Society of Plant Physiologists, Rockville, Md.
- 26.Cao, Y. Z., and A. H. C. Huang. 1986. Diacylglycerol acyltransferase in maturing oil seeds of maize and other species. Plant Physiol. 82:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cases, S., S. J. Smith, Y. W. Zheng, H. M. Myers, S. R. Lear, E. Sande, S. Novak, C. Collins, C. B. Welch, A. J. Lusis, S. K. Erickson, and R. V. Farese. 1998. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 95:13018-13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cases, S., S. J. Stone, P. Zhou, E. Yen, B. Tow, K. D. Lardizabal, T. Voelker, and R. V. Farese. 2001. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 276:38870-38876. [DOI] [PubMed] [Google Scholar]
- 29.Chater, K. F., and M. J. Merrick. 1979. Streptomycetes, p. 93-114. In J. H. Parish (ed.), Developmental biology in prokaryotes. University of California Press, Berkeley.
- 30.Chen, J. C. F., R. H. Lin, A. H. C. Huang, and J. T. C. Tzen. 1997. Cloning, expression and isoform classification of a minor oleosin in sesame oil bodies. J. Biochem. (Tokyo) 122:819-824. [DOI] [PubMed] [Google Scholar]
- 31.Chen, J. C. F., and T. C. Tzen. 2001. An in vitro system to examine the effective phospholipids and structural domain for protein targeting to seed oil bodies. Plant Cell Physiol. 42:1245-1252. [DOI] [PubMed] [Google Scholar]
- 32.Chen, M. C. M., C. L. Chyan, T. T. T. Lee, S. H. Huang, and J. T. C. Tzen. 2004. Constitution of stable artificial oil bodies with triacylglycerol, phospholipid and caleosin. J. Agric. Food Chem. 52:3982-3987. [DOI] [PubMed] [Google Scholar]
- 33.Christensen, H., N. J. Garton, R. W. Horobin, D. E. Minnikin, and M. R. Barer. 1999. Lipid domains of mycobacteria studied with fluorescent molecular probes. Mol. Microbiol. 31:1561-1572. [DOI] [PubMed] [Google Scholar]
- 34.Christiansen, K., and P. K. Jensen. 1972. Membrane-bound lipid particles from beef heart—chemical composition and structure. Biochim. Biophys. Acta 260:449-459. [DOI] [PubMed] [Google Scholar]
- 35.Clausen, M. K., K. Christia, P. K. Jensen, and O. Behnke. 1974. Isolation of lipid particles from bakers-yeast. FEBS Lett. 43:176-179. [DOI] [PubMed] [Google Scholar]
- 36.Coleman, J. 1990. Characterization of Escherichia coli cells deficient in 1-acyl-sn-glycerol 3-phosphate acyltransferase activity. J. Biol. Chem. 265:17215-17221. [PubMed] [Google Scholar]
- 37.Daniel, J., C. Deb, V. S. Dubey, T. D. Sirakova, B. Abomoelak, H. R. Morbidoni, and P. E. Kolattakudy. 2004. Induction of a novel class of diacylglycerol acyltransferases in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J. Bacteriol. 186:5017-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.deKoning, G. J. M., and I. A. Maxwell. 1993. Biosynthesis of poly-(R)-3-hydroxyalkanoate: an emulsion polymerization. J. Environ. Degrad. 1:223-226. [Google Scholar]
- 39.Dennis, D., C. Liebig, T. Holley, K. S. Thomas, A. Khosla, D. Wilson, and B. Augustine. 2003. Preliminary analysis of polyhydroxyalkanoate inclusions using atomic force microscopy. FEMS Microbiol. Lett. 226:113-119. [DOI] [PubMed] [Google Scholar]
- 40.Deruere, J., S. Romer, A. Dharlingue, R. A. Backhaus, M. Kuntz, and B. Camara. 1994. Fibril assembly and carotenoid overaccumulation in chromoplasts—a model for supramolecular lipoprotein structures. Plant Cell 6:119-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dewitt, S., J. L. Ervin, D. Howesorchison, D. Dalietos, S. L. Neidleman, and J. Geigert. 1982. Saturated and unsaturated wax esters produced by Acinetobacter sp. HO1-N grown on C16-C20 n-alkanes. J. Am. Oil Chem. Soc. 59:69-74. [Google Scholar]
- 42.Ellar, E., D. G. Lundgren, K. Okamura, and R. M. Marchessault. 1968. Morphology of poly-β-hydroxybutyrate granules. J. Mol. Biol. 35:489-502. [DOI] [PubMed] [Google Scholar]
- 43.Faller, M., M. Niederweis, and G. E. Schulz. 2004. The structure of a mycobacterial outer-membrane channel. Science 303:1189-1192. [DOI] [PubMed] [Google Scholar]
- 44.Fixter, L. M., and C. A. Fewson. 1974. Accumulation of waxes by Acinetobacter calcoaceticus NCIB-8250. Biochem. Soc. Trans. 2:944-945. [Google Scholar]
- 45.Fixter, L. M., and J. G. McCormack. 1976. Effect of growth-conditions on wax content of various strains of Acinetobacter. Biochem. Soc. Trans. 4:504-505. [DOI] [PubMed] [Google Scholar]
- 46.Frandsen, G. I., J. Mundy., and J. T. C. Tzen. 2001. Oil bodies and their associated proteins, oleosin and caleosin. Physiol. Plant 112:301-307. [DOI] [PubMed] [Google Scholar]
- 47.Franke, W. W., M. Hergt, and C. Grund. 1987. Rearrangement of the vimentin cytoskeleton during adipose conversion—formation of an intermediate filament cage around lipid globules. Cell 49:131-141. [DOI] [PubMed] [Google Scholar]
- 48.Frey-Wyssling, A., K. Muhlethaler, and E. Grieshaber. 1963. Origin of spherosomes in plant cells. J. Ultrastruct. Mol. Struct. Res. 8:506-516. [Google Scholar]
- 49.Gale, G. R., and H. H. McLain. 1963. Effect of ethambutol on cytology of Mycobacterium smegmatis. J. Bacteriol. 86:749-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallagher, I. H. C. 1971. Occurrence of waxes in Acinetobacter. J. Gen. Microbiol. 68:245-247. [DOI] [PubMed] [Google Scholar]
- 51.Garcia, A., V. Subramanian, A. Sekowski, and D. L. Brasaemle. 2004. The amino and carboxyl termini of perilipin A facilitate the storage of triacylglycerols. J. Biol. Chem. 279:8409-8416. [DOI] [PubMed] [Google Scholar]
- 52.Garton, N. J., H. Christensen, D. E. Minnikin, R. A. Adegbola, and M. R. Barer. 2002. Intracellular lipophilic inclusions of mycobacteria in vitro and in sputum. Microbiology 148:2951-2958. [DOI] [PubMed] [Google Scholar]
- 53.Gemmrich, A. R. 1981. Ultrastructural and enzymatic studies on the development of microbodies in germinating spores of the fern Anemia phyllitidis. Z. Pflanzenphysiol. 102:69-80. [Google Scholar]
- 54.Greenwood, A. D., R. M. Leech, and J. P. Williams. 1963. Osmiophilic globules of chloroplasts/osmiophilic globules as a normal component of chloroplasts and their isolation and composition in Vicia faba L. Biochim. Biophys. Acta 78:148-162. [Google Scholar]
- 55.Griebel, R., Z. Smith, and J. M. Merrick. 1968. Metabolism of poly-β-hydroxybutyrate: purification, composition and properties of native poly-β-hydroxybutyrate granules from Bacillus megaterium. Biochemistry 7:3676-3681. [DOI] [PubMed] [Google Scholar]
- 56.Hammerson, F. 1985. Histology: color atlas of microscopic anatomy, 2nd ed. Urban and Schwarzenberg, Baltimore, Md.
- 57.Hansmann, P., and P. Sitte. 1982. Composition and molecular structure of chromoplast globules of Viola tricolor. Plant Cell Rep. 1:111-114. [DOI] [PubMed] [Google Scholar]
- 58.Hezayen, F. F., A. Steinbüchel, and B. H. A. Rehm. 2002. Biochemical and enzymological properties of the polyhydroxybutyrate synthase from the extremely halophilic archaeon strain 56. Arch. Biochem. Biophys. 403:284-291. [DOI] [PubMed] [Google Scholar]
- 59.Hills, M. J., M. D. Watson, and D. J. Murphy. 1993. Targeting of oleosins to the oil bodies of oilseed rape (Brassica napus L). Planta 189:24-29. [DOI] [PubMed] [Google Scholar]
- 60.Hobbs, D. H., and M. J. Hills. 1999. Expression and characterization of diacylglycerol acyltransferase from Arabidopsis thaliana in insect cell cultures. FEBS Lett. 452:145-149. [DOI] [PubMed] [Google Scholar]
- 61.Hocking, P. J., and R. H. Marchessault. 1994. Biopolyesters, p. 48-96. In G. Griffin (ed.), Chemistry and technology for biodegradable polymers. Chapman and Hall, London, United Kingdom.
- 62.Hoskisson, P. A., G. Hobbs, and G. P. Sharples. 2001. Antibiotic production, accumulation of intracellular carbon reserves, and sporulation in Micromonospora echinospora (ATCC 15837). Can. J. Microbiol. 47:148-152. [PubMed] [Google Scholar]
- 63.Huang, A. H. C. 1992. Oil bodies and oleosins in seeds. Annu. Rev. Plant Physiol. 43:177-200. [Google Scholar]
- 64.Ishige, T., A. Tani, K. Takabe, K. Kawasaki, Y. Sakai, and N. Kato. 2002. Wax ester production from n-alkanes by Acinetobacter sp. strain M-1: ultrastructure of cellular inclusions and role of acyl coenzyme A reductase. Appl. Environ. Microbiol. 68:1192-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jayaram, S., and A. K. Bal. 1991. Oleosomes (lipid bodies) in nitrogen-fixing peanut nodules. Plant Cell Environ. 14:195-203. [Google Scholar]
- 66.Jensen, T. E., and L. M. Sicko. 1971. Fine structure of poly-β-hydroxybutyric acid granules in a blue-green alga, Chlorogloea fritschii. J. Bacteriol. 106:683-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jensen, T. E., and L. M. Sicko. 1973. Fine-structure of cell-wall of Gloeocapsa alpicola, a blue-green-alga. Cytobiologie 6:439-446. [Google Scholar]
- 68.Jiang, H. P., and G. Serrero. 1992. Isolation and characterization of a full-length cDNA coding for an adipose differentiation-related protein. Proc. Natl. Acad. Sci. USA 89:7856-7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jolivet, P., E. Roux, S. D′Andrea, M. Davanture, L. Negroni, M. Zivy, and T. Chardot. 2004. Protein composition of oil bodies in Arabidopsis thaliana ecotype WS. Plant Physiol. Biochem. 42:501-509. [DOI] [PubMed] [Google Scholar]
- 70.Jurasek, L., and R. H. Marchessault. 2004. Polyhydroxyalkanoate (PHA) granule formation in Ralstonia eutropha cells: a computer simulation. Appl. Microbiol. Biotechnol. 64:611-617. [DOI] [PubMed] [Google Scholar]
- 71.Kalscheuer, R., M. Wältermann, and A. Steinbüchel. 1999. Biosynthese und Speicherung von Triglyceriden und Wachsen in Bakterien, p. 253-261. In Biokonversion nachwachsender Rohstoffe 15. Landwirtschaftsverlag Münster, Münster, Germany.
- 72.Kalscheuer, R., M. Wältermann, H. M. Alvarez, and A. Steinbüchel. 2001. Preparative isolation of lipid inclusions from Rhodococcus opacus and Rhodococcus ruber and identification of granule-associated proteins. Arch. Microbiol. 177:20-28. [DOI] [PubMed] [Google Scholar]
- 73.Kalscheuer, R., and A. Steinbüchel. 2003. A novel bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J. Biol. Chem. 287:8075-8082. [DOI] [PubMed] [Google Scholar]
- 74.Kalscheuer, R., S. Uthoff, H. Luftmann, and A. Steinbuchel. 2003. In vitro and in vivo biosynthesis of wax diesters by an unspecific bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase from Acinetobacter calcoaceticus ADP1. Eur. J. Lipid Sci. Technol. 105:578-584. [Google Scholar]
- 75.Kessler, F., D. Schnell, and G. Blobel. 1999. Identification of proteins associated with plastoglobules isolated from pea (Pisum sativum L.) chloroplasts. Planta 208:107-113. [DOI] [PubMed] [Google Scholar]
- 76.Knaysi, G., J. Hillier, and C. Fabricant. 1950. The cytology of an avian strain of Mycobacterium tuberculosis studied with the electron and light microscopes. J. Bacteriol. 60:423-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lacey, D. J., and M. J. Hills. 1996. Heterogeneity of the endoplasmic reticulum with respect to lipid synthesis in developing seeds of Brassica napus L. Planta 199:545-551. [Google Scholar]
- 78.Lacey, D. J., N. Wellner, F. Beaudoin, J. A. Napier, and P. R. Shewry. 1998. Secondary structure of oleosins in oil bodies isolated from seeds of safflower (Carthamus tinctorius L.) and sunflower (Helianthus annuus L.). Biochem. J. 334:469-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lacey, D. J., F. Beaudoin, C. E. Dempsey, P. R. Shewry, and J. A. Napier. 1999. The accumulation of triacylglycerols within the endoplasmic reticulum of developing seeds of Helianthus annuus. Plant J. 17:397-405. [Google Scholar]
- 80.Lardizabal, K. D., J. G. Metz, T. Sakamoto, W. C. Hutton, M. R. Pollard, and M. W. Lassner. 2000. Purification of a jojoba embryo wax synthase, cloning of its cDNA, and production of high levels of wax in seeds of transgenic Arabidopsis. Plant Physiol. 122:645-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leber, R., E. Zinser, G. Zellnig, F. Paltauf, and G. Daum. 1994. Characterization of lipid particles of the yeast, Saccharomyces cerevisiae. Yeast 10:1421-1428. [DOI] [PubMed] [Google Scholar]
- 82.Leber, R., K. Landl, E. Zinser, H. Ahorn, A. Spok, S. D. Kohlwein, F. Turnowsky, and G. Daum. 1998. Dual localization of squalene epoxidase, Erg1p, in yeast reflects a relationship between the endoplasmic reticulum and lipid particles. Mol. Biol. Cell 9:375-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lemoigne, M. 1926. Produits de deshydration et de polymerisation de lácide β-oxybutyrique. Bull. Soc. Chim. Biol. 8:770-782. [Google Scholar]
- 84.Leprince, O., A. C. van Aelst, H. W. Pritchard, and D. J. Murphy. 1998. Oleosins prevent oil-body coalescence during seed imbibition as suggested by a low-temperature scanning electron microscope study of desiccation-tolerant and -sensitive oilseeds. Planta 204:109-119. [Google Scholar]
- 85.Lin, L. J., S. S. K. Tai, C. C. Peng, and J. T. C. Tzen. 2002. Steroleosin, a sterolbinding dehydrogenase in seed oil bodies. Plant Physiol. 128:1200-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Loer, D. S., and E. M. Herman. 1993. Cotranslational integration of soybean (Glycine max) oil body membrane-protein oleosin into microsomal-membranes. Plant Physiol. 101:993-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Londos, C., D. L. Brasaemle, C. J. Schultz, J. P. Segrest, and A. R. Kimmel. 1999. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin. Cell Dev. Biol. 10:51-58. [DOI] [PubMed] [Google Scholar]
- 88.Lundgren, D. G., J. M. Merrick, and R. M. Pfister. 1964. Structure of poly-β-hydroxybutyric acid granules. J. Gen. Microbiol. 34:441-446. [DOI] [PubMed] [Google Scholar]
- 89.Lütke-Eversloh, T., K. Bergander, H. Luftmann, and A. Steinbüchel. 2001. Identification of a new class of biopolymer: bacterial synthesis of a sulfur analogue to poly(3-hydroxybutyrate) (PHB). Microbiology 147:11-19.11160796 [Google Scholar]
- 90.Lütke-Eversloh, T., A. Fischer, U. Remminghorst, J. Kawada, R. H. Marchessault, A. Bögershausen, M. Kalwei, H. Eckert, R. Reichelt, S. J. Liu, and A. Steinbüchel. 2002. Biosynthesis of novel thermoplastic polythioesters by engineered Escherichia coli. Nat. Mater. 1:236-240. [DOI] [PubMed] [Google Scholar]
- 91.Makula, R. A., P. J. Lockwood, and W. R. Finnerty. 1975. Comparative analysis of lipids of Acinetobacter species grown on hexadecane. J. Bacteriol. 121:250-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Masamune, S., C. T. Walsh, A. J. Sinskey, and O. P. Peoples. 1989. Poly-(R)-3-hydroxybutyrate (PHB) biosynthesis—mechanistic studies on the biological Claisen condensation catalyzed by β-ketoacyl thiolase. Pure Appl. Chem. 61:303-312. [Google Scholar]
- 93.Mayer, F., and M. Hoppert. 1997. Determination of the thickness of the boundary layer surrounding bacterial PHA inclusion bodies, and implications for models describing the molecular architecture of this layer. J. Basic Microbiol. 37:45-52. [Google Scholar]
- 94.Milla, P., K. Athenstaedt, F. Viola, S. Oliaro-Bosso, S. D. Kohlwein, G. Daum, and G. Balliano. 2002. Yeast oxidosqualene cyclase (Erg7p) is a major component of lipid particles. J. Biol. Chem. 277:2406-2412. [DOI] [PubMed] [Google Scholar]
- 95.Millichip, M., A. S. Tatham, F. Jackson, G. Griffiths, P. R. Shewry, and A. K. Stobart. 1996. Purification and characterization of oil-bodies (oleosomes) and oil-body boundary proteins (oleosins) from the developing cotyledons of sunflower (Helianthus annuus L). Biochem. J. 314:333-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miura, S., J. W. Gan, J. Brzostowski, M. J. Parisi, C. J. Schultz, C. Londos, B. Oliver, and A. R. Kimmel. 2002. Functional conservation for lipid storage droplet association among perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J. Biol. Chem. 277:32253-32257. [DOI] [PubMed] [Google Scholar]
- 97.Monte, E., D. Ludevid, and S. Prat. 1999. Leaf C40.4: a carotenoid-associated protein involved in the modulation of photosynthetic efficiency. Plant J. 19:399-410. [DOI] [PubMed] [Google Scholar]
- 98.Murphy, D. J., and J. Vance. 1999. Mechanisms of lipid body formation. Trends Biochem. Sci. 24:109-115.10203758 [Google Scholar]
- 99.Murphy, D. J., and I. Cummins. 1989. Biosynthesis of seed storage products during embryogenesis in rapeseed, Brassica napus. J. Plant Physiol. 135:63-69. [Google Scholar]
- 100.Murphy, D. J. 2001. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog. Lipid Res. 40:325-438. [DOI] [PubMed] [Google Scholar]
- 101.Næsted, H., G. I. Frandsen, G. Y. Jauh, I. Hernandez-Pinzon, H. B. Nielsen, D. J. Murphy, J. C. Rogers, and J. Mundy. 2000. Caleosins: Ca2+-binding proteins associated with lipid-bodies. Plant Mol. Biol. 44:463-476. [DOI] [PubMed] [Google Scholar]
- 102.Nakamura, N., and T. Fujimoto. 2003. Adipose differentiation-related protein has two independent domains for targeting to lipid droplets. Biochem. Biophys. Res. Commun. 306:333-338. [DOI] [PubMed] [Google Scholar]
- 103.Napier, J. A., F. Beaudoin, A. S. Tatham, L. G. Alexander, and P. R. Shrewy. 1999. The seed oleosins: structure, properties and biological role. Adv. Bot. Res. 35:111-138. [Google Scholar]
- 104.Niederweis, M., S. Ehrt, C. Heinz, U. Klocker, S. Karosi, K. M. Swiderek, L. W. Riley, and R. Benz. 1999. Cloning of the mspA gene encoding a porin from Mycobacterium smegmatis. Mol. Microbiol. 33:933-945. [DOI] [PubMed] [Google Scholar]
- 105.Olukoshi, E. R., and N. M. Packter. 1994. Importance of stored triacylglycerols in Streptomyces—possible carbon source for antibiotics. Microbiology 140:931-943. [DOI] [PubMed] [Google Scholar]
- 106.Osafune, T., S. Sumida, T. Ehara, N. Ueno, E. Hase, and J. A. Schiff. 1990. Lipid (wax) and paramylum as sources of carbon and energy for the early development of proplastids in dark-grown Euglena gracilis cells transferred to an inorganic medium. J. Electron Microsc. 39:372-381. [Google Scholar]
- 107.Packter, N. M., and E. R. Olukoshi. 1995. Ultrastructural studies of neutral lipid localisation in Streptomyces. Arch. Microbiol. 164:420-427. [DOI] [PubMed] [Google Scholar]
- 108.Pieper-Fürst, U., M. H. Madkour, F. Mayer, and A. Steinbüchel. 1995. Identification of the region of a 14-kilodalton protein of Rhodococcus ruber that is responsible for the binding of this phasin to polyhydroxyalkanoic acid granules. J. Bacteriol. 177:2513-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pötter, M., M. H. Madkour, F. Mayer, and A. Steinbüchel. 2002. Regulation of phasin expression and polyhydroxyalkanoate (PHA) granule formation in Ralstonia eutropha H16. Microbiology 148:2413-2426. [DOI] [PubMed] [Google Scholar]
- 110.Pötter, M., H. Müller, F. Reinecke, R. Wieczorek, F. Fricke, B. Bowien, B. Friedrich, and A. Steinbüchel. 2004. The complex structure of polyhydroxybutyrate (PHB) granules: four orthologous and paralogous phasins occur in Ralstonia eutropha. Microbiology 150:2301-2311. [DOI] [PubMed] [Google Scholar]
- 111.Rabbani, S., P. Beyer, J. von Lintig, P. Hugueney, and H. Kleinig. 1998. Induced beta-carotene synthesis driven by triacylglycerol deposition in the unicellular alga Dunaliella bardawi L. Plant Physiol. 116:1239-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ratledge, C. 2002. Regulation of lipid accumulation in oleagenous micro-organisms. Biochem. Soc. Trans. 30:1047-1050. [DOI] [PubMed] [Google Scholar]
- 113.Raymond, R. L., and J. B. Davis. 1960. n-Alkane utilization and lipid formation by a Nocardia. Appl. Microbiol. 8:329-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rehm, B. H. A. 2003. Polyester synthases: natural catalysts for plastics. Biochem. J. 376:15-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rehm, B. H. A., and A. Steinbüchel. 1999. Biochemical and genetic analysis of PHA synthases and other proteins required for PHA synthesis. Int. J. Biol. Macromol. 25:3-19. [DOI] [PubMed] [Google Scholar]
- 116.Reiser, S., and C. Somerville. 1997. Isolation of mutants of Acinetobacter calcoaceticus deficient in wax ester synthesis and complementation of one mutation with a gene encoding a fatty acyl coenzyme A reductase. J. Bacteriol. 179:2969-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ross, J. H. E., J. Sanchez, F. Millan, and D. J. Murphy. 1993. Differential presence of oleosins in oleogenic seed and mesocarp tissues in olive (Olea europaea) and avocado (Persea americana). Plant Sci. 93:203-210. [Google Scholar]
- 118.Routaboul, J. M., C. Benning, N. Bechtold, M. Caboche, and L. Lepiniec. 1999. The TAG1 locus of Arabidopsis encodes for a diacylglycerol acyltransferase. Plant Physiol. Biochem. 37:831-840. [DOI] [PubMed] [Google Scholar]
- 119.Russell, N. J., and J. K. Volkman. 1980. The effect of growth temperature and wax ester composition in the psychrophilic bacterium Micrococcus cryophilus ATCC 15174. J. Gen. Microbiol. 118:131-141. [Google Scholar]
- 120.Saegusa, H., M. Shiraki, C. Kanai, and T. Saito. 2002. Cloning of an intracellular poly[d(−)-3-hydroxybutyrate] depolymerase gene from Ralstonia eutropha H16 and characterization of the gene product. J. Bacteriol. 183:94-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sandager, L., M. H. Gustavsson, U. Stahl, A. Dahlqvist, E. Wiberg, A. Banas, M. Lenman, H. Ronne, and S. Stymne. 2002. Storage lipid synthesis is non-essential in yeast. J. Biol. Chem. 277:6478-6482. [DOI] [PubMed] [Google Scholar]
- 122.Scott, C. C. L., and W. R. Finnerty. 1976. Characterization of intracytoplasmic hydrocarbon inclusions from hydrocarbon-oxidizing Acinetobacter species HO1-N. J. Bacteriol. 127:481-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Singer, M. E., S. M. Tyler, and W. R. Finnerty. 1985. Growth of Acinetobacter sp. strain HO1-N on n-hexadecanol: physiological and ultrastructural characteristics. J. Bacteriol. 162:162-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smid, A., M. Riva, F. Bouet, A. Sentenac, and C. Carles. 1995. The association of 3 subunits with yeast RNA-polymerase is stabilized by A14. J. Biol. Chem. 270:13534-13540. [DOI] [PubMed] [Google Scholar]
- 125.Steinbüchel, A. 2001. Perspectives for biotechnological production and utilization of biopolymers: metabolic engineering of polyhydroxyalkanoate biosynthesis pathways as a successful example. Macromol. Biosci. 1:1-24. [Google Scholar]
- 126.Steinbüchel, A., K. Aerts, W. Babel, C. Follner, M. Liebergesell, M. H. Madkour, F. Mayer, U. Pieper-Fürst, A. Pries, H. E. Valentin, and R. Wieczorek. 1995. Considerations on the structure and biochemistry of bacterial polyhydroxyalkanoic acid inclusions. Can. J. Microbiol. 41(Suppl. 1):94-105. [DOI] [PubMed] [Google Scholar]
- 127.Steinbüchel, A., and H. E. Valentin. 1995. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 128:219-228. [Google Scholar]
- 128.Stöveken, T., R. Kalscheuer, U. Malkus, R. Reichelt, and A. Steinbüchel. 2005. The wax ester synthase/acyl-coenzyme A:diacylglycerol acyltransferase from Acinetobacter sp. strain ADP1: characterization of a novel type of acyltransferase. J. Bacteriol. 187:1869-1376. [DOI] [PMC free article] [PubMed]
- 129.Stubbe, J., and J. Tian. 2003. Polyhydroxyalkanoate (PHA) homeostasis: the role of the PHA synthase. Nat. Prod. Rep. 20:445-457. [DOI] [PubMed] [Google Scholar]
- 130.Ting, J. T. L., S. S. H. Wu, C. Ratnayake, and A. H. C. Huang. 1998. Constituents of the tapetosomes and elaioplasts in Brassica campestris tapetum and their degradation and retention during microsporogenesis. Plant J. 16:541-551. [DOI] [PubMed] [Google Scholar]
- 131.Tsitsigiannis, D. I., R. Zarnowski, and N. P. Keller. 2004. The lipid body protein, PpoA, coordinates sexual and asexual sporulation in Aspergillus nidulans. J. Biol. Chem. 279:11344-11353. [DOI] [PubMed] [Google Scholar]
- 132.Tzen, J. T., and A. H. C. Huang. 1992. Surface-structure and properties of plant seed oil bodies. J. Cell Biol. 117:327-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tzen, J. T. C., Y. Z. Cao, P. Laurent, C. Ratnayake, and A. H. C. Huang. 1993. Lipids, proteins, and structure of seed oil bodies from diverse species. Plant Physiol. 101:267-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vance, V. B., and A. H. C. Huang. 1987. The major protein from lipid bodies of maize—characterization and structure based on cDNA cloning. J. Biol. Chem. 262:11275-11279. [PubMed] [Google Scholar]
- 135.Verstrepen, K. J., S. D. M. van Laere, J. Vercammen, G. Derdelinckx, J. P. Dufour, I. S. Pretorius, J. Winderickx, J. M. Thevelein, and F. R. Delvaux. 2004. The Saccharomyces cerevisiae alcohol acetyl transferase Atf1p is localized in lipid particles. Yeast 21:367-376. [DOI] [PubMed] [Google Scholar]
- 136.Vishnevetsky, M., M. Ovadis, H. Itzhaki, and A. Vainstein. 1997. CHRC, encoding a chromoplast-specific carotenoid-associated protein, is an early gibberellic acid-responsive gene. J. Biol. Chem. 272:24747-24750. [DOI] [PubMed] [Google Scholar]
- 137.Wältermann, M., H. Luftmann, D. Baumeister, R. Kalscheuer, and A. Steinbüchel. 2000. Rhodococcus opacus strain PD630 as a new source of high-value single-cell oil? Isolation and characterization of triacylglycerols and other storage lipids. Microbiology 146:1143-1149. [DOI] [PubMed] [Google Scholar]
- 138.Wältermann, M., A. Hinz, H. Robenek, D. Troyer, R. Reichelt, U. Malkus, H. J. Galla, R. Kalscheuer, T. Stöveken, P. von Landenberg, and A. Steinbüchel. 2005. Mechanism of lipid body formation in bacteria: how bacteria fatten up. Mol. Microbiol. 55:750-763. [DOI] [PubMed] [Google Scholar]
- 139.Wang, L., H. K. Schnoes, K. Takayama, and D. S. Goldman. 1972. Synthesis of alcohol and wax ester by a cell-free system in Mycobacterium tuberculosis. Biochim. Biophys. Acta 260:41-48. [DOI] [PubMed] [Google Scholar]
- 140.Wanner, G., and R. R. Theimer. 1978. Membranous appendices of spherosomes (oleosomes)—possible role in fat utilization in germinating oil seeds. Planta 140:163-169. [DOI] [PubMed] [Google Scholar]
- 141.Weather, P. R., H. G. Burkiott, and V. G. Daniels. 1987. Functional histology: a text and color atlas, 2nd ed. Churchill Livingstone, New York, N.Y.
- 142.Weiss, L. 1983. Histology: cell and tissue biology, 5th ed. Elsevier Biomedical, New York, N.Y.
- 143.Wieczorek, R., A. Pries, A. Steinbüchel, and F. Mayer. 1995. Analysis of a 24-kilodalton protein associated with the polyhydroxyalkanoic acid granules in Alcaligenes eutrophus. J. Bacteriol. 177:2425-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wildermut, H., and D. A. Hopwood. 1970. Septation during sporulation in Streptomyces coelicolor. J. Gen. Microbiol. 60:51-59. [DOI] [PubMed] [Google Scholar]
- 145.Wilkinson, W. O., and R. M. Bell. 1997. sn-Glycerol-3-phosphate acyltransferase from Escherichia coli. Biochim. Biophys. Acta 1348:3-9. [DOI] [PubMed] [Google Scholar]
- 146.Yatsu, L. Y., and T. J. Jacks. 1972. Spherosome membranes—half-unit membranes. Plant Physiol. 49:937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yermanos, D. M. 1975. Composition of jojoba seed during development. J. Am. Oil Chem. Soc. 52:115-117. [Google Scholar]
- 148.York, G. M., B. H. Junker, J. Stubbe, and A. J. Sinskey. 2001. Accumulation of the PhaP phasin of Ralstonia eutropha is dependent on production of polyhydroxybutyrate in cells. J. Bacteriol. 183:4217-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.York, G. M., J. Lupberger, J. Tian, A. G. Lawrence, J. Stubbe, and A. J. Sinskey. 2003. Ralstonia eutropha H16 encodes two and possibly three intracellular poly[d-(−)-3-hydroxybutyrate] depolymerase genes. J. Bacteriol. 185:3788-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zou, J. T., Y. D. Wei, C. Jako, A. Kumar, G. Selvaraj, and D. C. Taylor. 1999. The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J. 19:645-653. [DOI] [PubMed] [Google Scholar]
- 151.Zweytick, D., K. Athenstaedt, and G. Daum. 2000. Intracellular lipid particles of eukaryotic cells. Biochim. Biophys. Acta Rev. Biomembranes 1469:101-120. [DOI] [PubMed] [Google Scholar]