Abstract
A systematic analysis of the type III secretion (T3S) genes of Pseudomonas aeruginosa strain PAO1 revealed that they are under quorum-sensing control. This observation was supported by the down-regulation of the T3S regulon in the presence of RhlR-C4HSL and the corresponding advanced secretion of ExoS in a rhlI mutant.
Pseudomonas aeruginosa is an opportunistic bacterial pathogen, responsible for infections in immuno-compromised people and individuals with cystic fibrosis. The type III secretion system (T3S) is a major virulence determinant of P. aeruginosa and is correlated with the severity of human infections (17). It allows direct delivery of several toxic proteins, called effectors, into the cytosol of the eukaryotic target cell. The T3S is induced under low-Ca2+ conditions (8) or upon contact between the bacterium and the eukaryotic cell (24). The T3S regulon is controlled by the transcriptional activator ExsA (8), a member of the AraC/XylS family, which binds a consensus sequence located within the target gene promoter (12). The ExsD protein was shown to be an antiactivator that counteracts the positive effect of ExsA (16), whereas ExsC, which interacts with ExsD, could be an anti-anti-activator (5). Moreover, a novel regulatory pathway, which is dependent on cyclic AMP and the cyclic AMP-binding protein Vfr, activates the T3S regulon (27).
In P. aeruginosa, synthesis and secretion of a number of virulence factors are controlled by quorum sensing (QS). QS is crucial in the pathogenesis of P. aeruginosa infections (18) and controls virulence factor gene expression in the lungs of cystic fibrosis patients (6). QS is a regulatory mechanism whereby bacteria sense the environment and coordinate the expression of various genes within the bacterial population (10, 15). It involves an interaction between a small diffusible molecule, an acylhomoserine lactone, and a transcriptional activator. Two QS systems, LasR/I-3OC12-homoserine lactone (HSL) and RhlR/I-C4-HSL, have been well characterized in P. aeruginosa (9, 14). In the QS hierarchy, the Las system controls expression of rhlR (13). The Las and Rhl systems have been shown to activate the expression of over 200 genes (20, 25). In this report, the activity of T3S gene promoters from the PAO1 strain, whose genome has been sequenced (23), was systematically checked upon standard T3S induction before studying the relationship with QS regulation.
ExsA-dependent and Ca2+-independent expression of exsA and psc secretion genes.
All transcriptional lacZ fusions used in this study (Table 1) were constructed using PCR-amplified promoter regions of PAO1 T3S genes, containing −10/−35 RNA polymerase-binding boxes and the ExsA-binding consensus sequence. The DNA fragments were cloned in pMP220 upstream of the promoterless lacZ gene. The strains containing the pMP220-derived constructs were grown at 37°C under noninducing (LB) or inducing (LB, 5 mM EGTA, 20 mM MgCl2) T3S conditions. The β-galactosidase activity was measured during cell growth as previously described (1).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| P. aeruginosa strains | ||
| PAO1 | Wild-type prototroph | B. Holloway |
| PAO1exsA | exsA mutant of PAO1, Cbr | 19 |
| PAO1pscC | pscC mutant of PAO1, Cbr | 19 |
| PAO1R | lasR mutant of PAO1 | 13 |
| PDO100 | rhlI mutant of PAO1, Hgr | 2 |
| PA103 | Cytotoxic respiratory clinical isolate, Fla− | 7 |
| E. coli strains | ||
| TOP10 F′ | φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 (F′ lacIq Tn10 mcrA Δ(mrr-hsdRMS-mcrBC) | Invitrogen |
| TG1 | supE Δ(lac-proAB) thi hsdRΔ5 (F′ traΔ36 proA+B+ZΔM15) | Laboratory collection |
| Plasmids | ||
| pRK2013 | Tra+ Mob+ Kmr | Laboratory collection |
| pCR2.1 | TA cloning vector, Apr | Invitrogen |
| pMP220 | Broad-host-range lacZ transcriptional fusion, Tcr | Laboratory collection |
| pMMB190 | Broad host range, tac promoter, Apr | Laboratory collection |
| pSBC6 | 1.2-kb DNA fragment carrying exsA, cloned in pMMB190 | This work |
| pSB307 | Broad host range, 275-bp-containing pS, pS-lacZ reporter, Tcr | This work |
| pSB302 | Broad host range, 211-bp-containing pT, pT-lacZ reporter, Tcr | This work |
| pSB303 | Broad host range, 191-bp-containing pY, pY-lacZ reporter, Tcr | This work |
| pSB305 | Broad host range, 219-bp-containing pD, pD-lacZ reporter, Tcr | This work |
| pSB308 | Broad host range, 320-bp-containing pG, pG-lacZ reporter, Tcr | This work |
| pSB313 | Broad host range, 552-bp-containing pN, pN-lacZ reporter, Tcr | This work |
| pPP4 | Broad host range, 249-bp-containing pC, pC-lacZ reporter, Tcr | This work |
| pBAD/Myc-HisA | Expression vector, araBAD promoter, addition of a poly(His) tag at the C-terminal part of the protein | Invitrogen |
| pPP8 | 1,145-bp DNA fragment (SBO25-26) containing exoY from PAO1, digested with PstI and HindIII, cloned in pBAD/Myc-HisA digested with PstI and HindIII | This work |
| pSBC4 | 270-bp DNA fragment (SBO9-10) containing pscF from PAO1, digested with NcoI and HindIII, cloned in pBAD/Myc-HisA digested with NcoI and HindIII | This work |
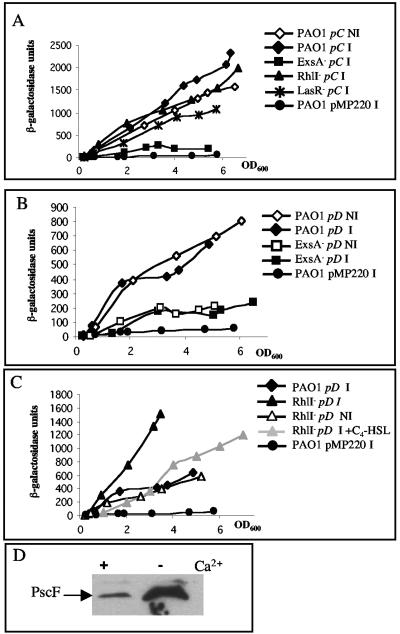
Analyses of the exsCBA operon, encoding regulatory components, and of the exsD-pscA-L operon, encoding the Psc components of the PAO1 T3S machinery, indicated that their respective promoters, pC and pD, were activated in an ExsA-dependent manner (eightfold and threefold decrease in an exsA mutant), independently of Ca2+ limitation (Fig. 1A and B). In an exsA mutant, both pC and pD activities were slightly higher than the control strain carrying the pMP220 empty vector, suggesting a basal level of expression that is ExsA independent.
FIG. 1.
Expression of the transcriptional fusions pC-lacZ from pPP4 (A) and pD-lacZ from pSB305 (B and C) in P. aeruginosa strain PAO1 (diamonds), PAO1exsA (squares), PAO1rhlI (triangles), or PAO1lasR (crosses). Cultures were grown at 37°C in the absence (closed symbols, I) or presence (open symbols, NI) of Ca2+ and with exogenously added 10 μM C4-HSL (grey symbols). The results represent a single experiment, which was repeated three times without variations. The level of β-galactosidase activity from PAO1/pMP220 (circles) was recorded after growth under T3S-inducing conditions. (D) Intracellular immunodetection of PscF in P. aeruginosa strain PA103 grown under T3S-inducing (−Ca2+) or noninducing (+Ca2+) conditions. The equivalent of an OD600 of 0.4 was loaded in each case.
We confirmed the Ca2+-independent expression of psc secretion genes from pD by testing the presence of PscF within cell extracts of P. aeruginosa strain PA103 grown in a medium containing Ca2+, or not, and using anti-PscF antibodies. PscF was produced in the presence of Ca2+, even though at a markedly reduced level compared to a strain grown in a Ca2+-depleted medium (Fig. 1D). This suggests that the secretion apparatus might assemble before the contact with the eukaryotic cell.
ExsA- and Ca2+-dependent expression of effector genes.
The expression analysis of effector genes of PAO1, namely, exoS, -T, and -Y genes, showed that they were all strictly regulated by ExsA in a Ca2+-dependent manner (Table 2). As described by Wolfgang and collaborators (27), we observed that expression of each effector gene was greatly induced upon ExsA overproduction, even in a Ca2+-rich medium (Table 2). To test whether the massive expression of exoY could lead to increased in vitro secretion, the occurrence of extracellular ExoY was monitored using anti-ExoY antibodies. Whereas no ExoY effector was detected in the supernatant of an exsA mutant, ExsA overproduction led to a dramatic increase in the extracellular level of ExoY under T3S-inducing conditions (data not shown). Interestingly, ExoY neither could be found in the supernatant nor was accumulated in the cytoplasm (data not shown) when strains were grown in the presence of Ca2+, indicating that overproduction of ExsA did not override Ca2+ regulation in terms of global function of the T3S. Similar results were obtained for ExoS secretion (data not shown).
TABLE 2.
T3S promoter gene activities
| Promoter operon or gene | Fold induction in Ca2+ depletiona | ExsA dependency | Feedback in secretion mutantb | Fold induction upon ExsA overproductionc | Fold induction in rhlI mutantc |
|---|---|---|---|---|---|
| pS | 8.3 | Strict | 1.9 | 6.5 | 2 |
| pT | 5.6 | Strict | 1.4 | 21.7 | 4.2 |
| pY | 3.9 | Strict | 2.9 | 37.8 | 4.2 |
| pC | Blind | Marginal | No change | ||
| pD | Blind | Marginal | 2.5 | ||
| pG | 3 | Strict | 4.3 | ||
| pN | 3.2 | Strict | 1.5 |
Fold induction corresponds to the β-galactosidase activity ratio observed in early stationary phase and due to T3S induction.
Fold repression in β-galactosidase activity ratio due to pscC mutation.
β-Galactosidase activity ratio.
ExsA- and Ca2+-dependent expression of the “translocation” and the “plug” operons.
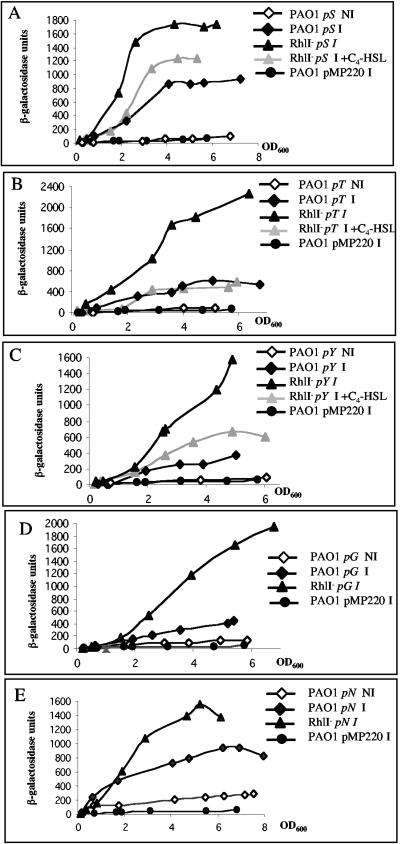
Lastly, we studied activity of the pG and pN promoters. pG controls expression of the pcrGVH-popBD operon, which encodes components required for effector translocation across the eukaryotic cell membrane (4). pN controls expression of the popN-pcr1234DR operon, which encodes PopN and Pcr1, the YopN (also called plug) and TyeA homologues, two proteins involved in the control of Yop effectors release in Yersinia (3). pG and pN activities were observed only under T3S-inducing conditions (without Ca2+) in the PAO1 strain and were dependent on ExsA (Table 2).
Our data revealing the Ca2+ independency and marginal ExsA dependency of T3S regulatory and secretion operons in PAO1 corroborate a DNA microarray study done with the PAK strain (27). The data are partially in disagreement with earlier studies done in other P. aeruginosa backgrounds, which showed a strict ExsA and Ca2+ chelation dependency for all T3S regulons (4, 5, 16, 28). Ca2+ independency and marginal VirF dependency were previously described for Yersinia T3S regulatory and secretion operons (3). The Yersinia T3S and the P. aeruginosa T3S were classified in the same T3S subfamily, according to their conserved genetic organization and homologies. Our observation describing the similarity in T3S regulation between these bacteria supports this classification.
T3S genes are negatively controlled by QS.
We examined the effect of QS on expression of the T3S regulon. Firstly, we verified that expression of the lasR and rhlR genes, encoding the two QS regulators, was not modified under T3S-inducing conditions (data not shown). Secondly, we used the same set of T3S promoter fusions, which were introduced in either a lasR (PAOR) (13) or a rhlI (PDO100) (2) mutant. In the lasR mutant, expression levels of pD (“secretion” operon), pS, pT, pY, pG (“translocation” operon), and pN (“plug” operon) were fairly similar to expression levels obtained in the PAO1 strain (data not shown). Interestingly, each of these promoter fusions was 1.5- to 4.3-fold up-regulated in the rhlI genetic background (Fig. 1C and 2A to E). The inactivation of a gene encoding a homoserine lactone (HSL) synthase, such as rhlI, can be phenotypically restored by addition of the corresponding HSL (26). Addition of C4-HSL in the rhlI mutant culture medium reduced activities of pD, pS, pT, and pY to PAO1 levels (Fig. 1C and 2A to C).
FIG. 2.
Expression of the transcriptional fusions pS-lacZ from pSB307 (A), pT-lacZ from pSB302 (B), pY-lacZ from pSB303 (C), pG-lacZ from pSB308 (D), and pN-lacZ from pSB313 (E) in P. aeruginosa strains PAO1 (diamonds) and PAO1rhlI (triangles). See the legend of Fig. 1 for more information.
Our observation corroborates a preliminary study showing QS-dependent control of exoS (11) and presents this control as a global mechanism on the T3S regulon, since we concluded that pD, pS, pT, pY, pG, and pN are all submitted to a negative RhlR-C4-HSL-dependent control. Interestingly, exsCBA is the only T3S operon that is not controlled by a QS component, since pC activity is affected by neither a lasR nor a rhlI mutation (Fig. 1A).
More interestingly, we noticed that the effect of the rhlI mutation on secretion genes is observed only in Ca2+ limitation. In the presence of Ca2+, these genes are indeed expressed at a wild-type level in the rhlI mutant (Fig. 1C). This suggests that the effect of rhlI on secretion gene expression is probably indirect and goes through an intermediate component whose expression is Ca2+ regulated. This component cannot be encoded by exsA, exsC, or exsD, since we showed that their expression is Ca2+ independent. In agreement with this hypothesis is our observation that exsCBA is not regulated by QS. Previous work indicated that two adenylate cyclases, CyaA and CyaB, are produced in a Ca2+-dependent manner and have been identified as T3S regulators (27). These proteins might be possible candidates for the link between T3S and QS.
Influence of the rhlI mutation on ExoS secretion.
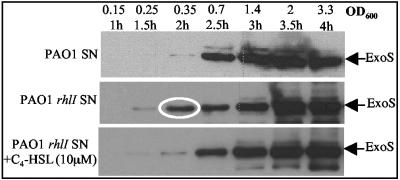
We also studied the effect of the rhlI mutation on the in vitro secretion of ExoS (Fig. 3). At an early stage during the exponential growth phase (optical density at 600 nm [OD600] = 0.35), ExoS was significantly secreted by the rhlI mutant. By contrast, efficient ExoS secretion in the PAO1 supernatant was found only at later growth stages (OD600 = 0.7) (Fig. 3). Thus, ExoS secretion is advanced during the growth of an rhlI mutant. Moreover, the addition of exogenous C4-HSL to the culture medium of a rhlI mutant delayed ExoS secretion and thus mimicked the behavior observed in PAO1 (Fig. 3). These results strictly corroborate our data obtained with the promoter gene fusions.
FIG. 3.
Immunodetection of ExoS in culture supernatants of PAO1 or PAO1rhlI grown at 37°C under T3S-inducing conditions and with exogenously added 10 μM C4-HSL where indicated. Samples were taken each 30 min over a 4-h growth period. The equivalent of an OD600 of 1 was loaded on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel.
Our study clearly identified the T3S regulon, except for the regulatory operon exsCBA, as a negative target for QS in P. aeruginosa. This is the first P. aeruginosa virulence factor for which a negative regulation by RhlR/I-C4-HSL has been demonstrated. The QS repression of the T3S regulon suggests that the associated virulence functions are likely to be required at early stages of bacterial infection (colonization and dissemination), prior to the development of a chronic infection and the establishment of a high-cell-density bacterial population.
A relationship between QS and T3S has previously been proposed for enteropathogenic and enterohemorrhagic Escherichia coli. In those bacteria, T3S is required for the production of attaching and effacing lesions on epithelial cells. However, in this case, and in contrast to the P. aeruginosa T3S, QS activates enteropathogenic and enterohemorrhagic E. coli T3S genes via a LuxS protein and its cognate autoinducer, called AI-3, which is different from the HSL system (21, 22).
Acknowledgments
We thank Y. Brun for the gift of C4-HSL and E. Frithz-Lindsten for the gift of anti-ExoS.
P.N.O. was supported by a grant from Brazil (CNPq). The A.F. laboratory is supported by a grant from the French Cystic Fibrosis Foundation (VLM).
REFERENCES
- 1.Ball, G., E. Durand, A. Lazdunski, and A. Filloux. 2002. A novel type II secretion system in Pseudomonas aeruginosa. Mol. Microbiol. 43:475-485. [DOI] [PubMed] [Google Scholar]
- 2.Brint, J. M., and D. E. Ohman. 1995. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 177:7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dacheux, D., I. Attree, C. Schneider, and B. Toussaint. 1999. Cell death of human polymorphonuclear neutrophils induced by a Pseudomonas aeruginosa cystic fibrosis isolate requires a functional type III secretion system. Infect. Immun. 67:6164-6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasgupta, N., G. L. Lykken, M. C. Wolfgang, and T. L. Yahr. 2004. A novel anti-anti-activator mechanism regulates expression of the Pseudomonas aeruginosa type III secretion system. Mol. Microbiol. 53:297-308. [DOI] [PubMed] [Google Scholar]
- 6.Erickson, D. L., R. Endersby, A. Kirkham, K. Stuber, D. D. Vollman, H. R. Rabin, I. Mitchell, and D. G. Storey. 2002. Pseudomonas aeruginosa quorum-sensing systems may control virulence factor expression in the lungs of patients with cystic fibrosis. Infect. Immun. 70:1783-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleiszig, S. M., J. P. Wiener-Kronish, H. Miyazaki, V. Vallas, K. E. Mostov, D. Kanada, T. Sawa, T. S. Yen, and D. W. Frank. 1997. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect. Immun. 65:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank, D. W. 1997. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 26:621-629. [DOI] [PubMed] [Google Scholar]
- 9.Gambello, M. J., and B. H. Iglewski. 1991. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 173:3000-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg, E. P. 2003. Bacterial communication: tiny teamwork. Nature 424:134. [DOI] [PubMed] [Google Scholar]
- 11.Hogardt, M., M. Roeder, A. M. Schreff, L. Eberl, and J. Heesemann. 2004. Expression of Pseudomonas aeruginosa exoS is controlled by quorum sensing and RpoS. Microbiology 150:843-851. [DOI] [PubMed] [Google Scholar]
- 12.Hovey, A. K., and D. W. Frank. 1995. Analyses of the DNA-binding and transcriptional activation properties of ExsA, the transcriptional activator of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 177:4427-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latifi, A., M. Foglino, K. Tanaka, P. Williams, and A. Lazdunski. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 21:1137-1146. [DOI] [PubMed] [Google Scholar]
- 14.Latifi, A., M. K. Winson, M. Foglino, B. W. Bycroft, G. S. Stewart, A. Lazdunski, and P. Williams. 1995. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 17:333-343. [DOI] [PubMed] [Google Scholar]
- 15.Lazdunski, A. M., I. Ventre, and J. N. Sturgis. 2004. Regulatory circuits and communication in gram-negative bacteria. Nat. Rev. Microbiol. 2:581-592. [DOI] [PubMed] [Google Scholar]
- 16.McCaw, M. L., G. L. Lykken, P. K. Singh, and T. L. Yahr. 2002. ExsD is a negative regulator of the Pseudomonas aeruginosa type III secretion regulon. Mol. Microbiol. 46:1123-1133. [DOI] [PubMed] [Google Scholar]
- 17.Roy-Burman, A., R. H. Savel, S. Racine, B. L. Swanson, N. S. Revadigar, J. Fujimoto, T. Sawa, D. W. Frank, and J. P. Wiener-Kronish. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183:1767-1774. [DOI] [PubMed] [Google Scholar]
- 18.Rumbaugh, K. P., J. A. Griswold, and A. N. Hamood. 2000. The role of quorum sensing in the in vivo virulence of Pseudomonas aeruginosa. Microbes Infect. 2:1721-1731. [DOI] [PubMed] [Google Scholar]
- 19.Saliba, A. M., A. Filloux, G. Ball, A. S. Silva, M. C. Assis, and M. C. Plotkowski. 2002. Type III secretion-mediated killing of endothelial cells by Pseudomonas aeruginosa. Microb. Pathog. 33:153-166. [PubMed] [Google Scholar]
- 20.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sircili, M. P., M. Walters, L. R. Trabulsi, and V. Sperandio. 2004. Modulation of enteropathogenic Escherichia coli virulence by quorum sensing. Infect. Immun. 72:2329-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperandio, V., A. G. Torres, B. Jarvis, J. P. Nataro, and J. B. Kaper. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. USA 100:8951-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 24.Vallis, A. J., T. L. Yahr, J. T. Barbieri, and D. W. Frank. 1999. Regulation of ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions. Infect. Immun. 67:914-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winson, M. K., M. Camara, A. Latifi, M. Foglino, S. R. Chhabra, M. Daykin, M. Bally, V. Chapon, G. P. Salmond, B. W. Bycroft, A. Lazdunski, G. S. Stewart, and P. Williams. 1995. Multiple N-acyl-l-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:9427-9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfgang, M. C., V. T. Lee, M. E. Gilmore, and S. Lory. 2003. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev. Cell 4:253-263. [DOI] [PubMed] [Google Scholar]
- 28.Yahr, T. L., and D. W. Frank. 1994. Transcriptional organization of the trans-regulatory locus which controls exoenzyme S synthesis in Pseudomonas aeruginosa. J. Bacteriol. 176:3832-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]