Abstract
Pyrococcus horikoshii OT3, a hyperthermophilic and anaerobic archaeon, was found to have an open reading frame (PH1938) whose deduced amino acid sequence of the N-terminal and C-terminal halves showed significant similarity to two key enzymes of the ribulose monophosphate pathway for formaldehyde fixation in methylotrophic bacteria, 3-hexulose-6-phosphate synthase (HPS) and 6-phospho-3-hexuloisomerase (PHI), respectively. The organism constitutively produced the encoded protein and exhibited activity of the sequential HPS- and PHI-mediated reactions in a particulate fraction. The full-length gene encoding the hybrid enzyme, the sequence corresponding to the HPS region, and the sequence corresponding to the PHI region were expressed in Escherichia coli and were found to produce active enzymes, rHps-Phi, rHps, or rPhi, respectively. Purified rHps-Phi and rHps were found to be active at the growth temperatures of the parent strain, but purified rPhi exhibited significant susceptibility to heat, suggesting that thermostability of the PHI moiety of the bifunctional enzyme (rHps-Phi) resulted from fusion with HPS. The bifunctional enzyme catalyzed the sequential reaction much more efficiently than a mixture of rHps and rPhi. These and other biochemical characterizations of the PH1938 gene product suggest that the ribulose monophosphate pathway plays a significant role in the archaeon under extreme environmental conditions.
Formaldehyde is ubiquitous in nature, produced through the degradation of compounds containing methyl or methoxyl groups, e.g., lignin and pectin. Many microorganisms have the metabolic ability to detoxify this harmful compound through reaction with C1 carrier molecules, such as tetrahydrofolate, tetrahydromethanopterin, and glutathione (25). The ribulose monophosphate (RuMP) pathway, which was originally found in methylotrophic bacteria, is now recognized as a widespread procaryotic pathway for formaldehyde fixation and detoxification (7, 20). In this pathway, formaldehyde reacts with ribulose 5-phosphate to form d-arabino-3-hexulose 6-phosphate, which is then isomerized to fructose 6-phosphate. These two reactions are catalyzed by 3-hexulose-6-phosphate synthase (HPS) and 6-phospho-3-hexuloisomerase (PHI), respectively. Ribulose 5-phosphate, the acceptor of formaldehyde, is regenerated through the general metabolic pathway for sugar phosphate conversion, which is not specific to the RuMP pathway (7, 20). In most cases, organisms have both HPS and PHI orthologs, implying that these two genes evolved as a pair.
The presence of both HPS and PHI in the organism also suggests that formaldehyde can be fixed and detoxified via the RuMP pathway. Our recent studies have shown that in some nonmethylotrophs the RuMP pathway is indeed involved in detoxification of formaldehyde especially. In Bacillus subtilis for example, yckG and yckF encode enzymatically active forms of HPS and PHI, respectively, and the RuMP operon was found to be induced by the presence of formaldehyde (27). In vanillin-degrading Burkholderia cepacia TM1, HPS and PHI play significant roles in detoxification and assimilation of formaldehyde, which is released through the reaction of vanillic acid demethylase (17).
Homology searches have revealed the presence of HPS and PHI orthologs in archaea (18, 25). Interestingly, some HPS orthologs in archaeal genes have been found to be fused with other genes to form single open reading frames (ORFs), i.e., hps and phi homologs in hyperthermophilic strains of Pyrococcus (13), and two homologous hps sequences each fused with a putative formaldehyde-activating enzyme (AAM30975) and a demethylmenaquinone methyltransferase (AAM30911) in the methanogen Methanosarcina mazei Goe1. These observations suggest that the formaldehyde fixation pathway in an aerobic methylotroph and in an anaerobic archaeon share common genes across the bacterial and archaeal kingdoms, analogous to the case of the C1-converting enzymes of methylotrophs and methanogens (4, 5).
In this report, we show that Pyrococcus horikoshii OT3 produces a fusion protein having both HPS and PHI enzymatic activities encoded by PH1938 (B71209). The entire fusion protein and separate HPS and PHI polypeptides were expressed in Escherichia coli, purified, and biochemically characterized, providing insights into the physiological significance of this bifunctional enzyme.
MATERIALS AND METHODS
Strains, vectors, and culture conditions.
P. horikoshii OT3 JCM9974 (9) was obtained from the Japan Collection of Microorganisms (Saitama, Japan). Cultivation was performed using conditions recommended in the Japan Collection of Microorganisms catalogue, 8th ed. (2002). E. coli JM109 was used as a host for plasmid propagation and was grown in Luria-Bertani (LB, pH 7.0) broth, which contained 1% Bacto tryptone (Difco Laboratories, Detroit, MI), 0.5% Bacto yeast extract (Difco Lab.), 0.5% NaCl, and ampicillin (50 mg/liter). P. horikoshii genes were expressed in E. coli Rosetta (DE3) (Novagen, Madison, WI) grown in LB medium containing ampicillin (50 mg/liter) and chloramphenicol (34 mg/liter). E. coli transformants were grown in LB at 37°C, to which 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added at mid-log phase, followed by an additional 3 h of growth. The T7 expression vector pET23a(+) was purchased from Novagen.
Preparation of cell extracts.
Cells were suspended in 20 mM Tris-HCl (pH 7.5) containing 5 mM MgCl2 and 1 mM dithiothreitol (buffer A) and were disrupted by sonication for 30 min (19 kHz; Insonator model 201 M; Kubota, Tokyo, Japan). For Western blotting, cells in buffer A were disrupted using a French press (Thermo Spectronic, Rochester, NY). Unbroken cells and cell debris were removed by centrifugation at 5,000 × g for 30 min at 4°C. The resulting supernatant was used as a cell extract.
Enzyme assays.
HPS activity was determined by measuring the rate of ribulose 5-phosphate-dependent disappearance of formaldehyde essentially as described previously (11). Conversion of ribose 5-phosphate to ribulose 5-phosphate by phosphoriboisomerase from spinach (Sigma-Aldrich, St. Louis, MO) was performed at 30°C for 5 min, and the following HPS reaction was conducted at 80°C. Activity of PHI and of the coupled HPS and PHI reaction (HPS/PHI) was determined as described previously (1), with the following modifications. Purified rHps was used for synthesis of d-arabino-3-hexulose 6-phosphate when the activity of PHI was assayed. After performance of the PHI reaction at 60°C for 5 min or the HPS/PHI reaction at 80°C for 5 min, each was stopped by transfer to ice, followed by enzymatic determination of the amount of fructose 6-phosphate formed (1). One unit of activity was defined as the amount of enzyme that consumed 1 μmol of formaldehyde for HPS or that produced 1 μmol of fructose 6-phosphate for PHI and HPS/PHI per minute at the aforementioned temperatures.
Cloning and expression of PH1938, hps, and phi.
The entire PH1938 gene and fragments encoding hps and phi were amplified by PCR using chromosomal DNA of P. horikoshii OT3 as templates. PH1938 was amplified using primers 5′-ggaattcCATATGATCCTCCAGGTAGCTCT-3′ and 5′-gGAATTCCTCACTCGAGCGTTGCATGCTT-3′ (added NdeI and EcoRI sites are underlined, and additional sequences for efficient cleavage are shown in lowercase letters). Based on the deduced amino acid sequence of PH1938 using sequences of hps and phi from Bacillus brevis S1 (28), the nucleotide sequences from 1 to 618 and from 636 to 1,221 were determined to include hps and phi, respectively, where 1 is the first A of the PH1938 start codon. hps was amplified using primers 5′-ggaattcCATATGATCCTCAGGTAGCTCT-3′ and 5′-gGAATTCCTCAGAGGTCTATTATCTTCCTA-3′. phi was amplified using primers 5′-ggaattcCATATGAAGACTATTAGGAAGGC-3′ and 5′-gGAATTCCTCACTCGAGCGTTGCATGCTT-3′. PCR was performed using Pyrobest DNA polymerase (Takara Shuzo, Kyoto, Japan). PCR products corresponding to the entire PH1938 gene, the hps sequence, or the phi sequence were digested with NdeI and EcoRI and purified with a Mag Extractor (TOYOBO, Osaka, Japan). Each purified PCR product was ligated into the NdeI/EcoRI site of the T7 expression vector pET23a(+) and introduced into JM109 for plasmid propagation. The resulting plasmids harboring PH1938, hps, and phi were designated pEHPOT3, pEHOT3, and pEPOT3, respectively. E. coli Rosetta (DE3) strains transformed separately with each plasmid were used for overexpression of the corresponding genes. The gene products encoded by PH1938, hps, and phi were designated rHps-Phi, rHps, and rPhi, respectively.
Purification of rHps-Phi.
Protein purification was performed at ambient temperature. Cell extracts of E. coli Rosetta (DE3) [pEHPOT3] were centrifuged at 100,000 × g for 60 min. Most HPS/PHI activity was found in the resulting pellet. The pellet was washed with buffer A containing 0.2 M KCl and then resuspended in buffer A containing 2% CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate), 50 mM EDTA, and 10% glycerol and stirred for 3 h. The supernatant obtained by centrifugation at 100,000 × g for 60 min was applied to a DEAE-Sepharose column (4.5 × 35 cm) preequilibrated with buffer A containing 0.1% CHAPS and 10% glycerol. HPS/PHI activity was found in the fractions eluted with the equilibrated buffer. Active fractions were dialyzed against the same buffer for 18 h. The precipitate formed during dialysis was collected by centrifugation at 20,000 × g for 30 min and dissolved by dialysis against buffer A containing 1% CHAPS and 10% glycerol for 48 h. After centrifugation (20,000 × g for 30 min), the supernatant was used as purified rHps-Phi.
The purified enzyme was soluble as a large micelle in the detergent-containing buffer system and as such was not suitable for a determination of molecular mass by gel filtration. Therefore, the particulate fraction that was dissolved in buffer A containing 50 mM EDTA and 10% glycerol was used for this purpose. The purity of rHps-Phi in this preparation was about 60%.
Purification of rHps and rPhi.
Purifications of rHps and rPhi were performed by the same procedures using a fast-protein liquid chromatography system (Amersham Biosciences K.K., Tokyo, Japan) at 4°C. A cell extract of E. coli Rosetta (DE3) [pEHOT3] or E. coli Rosetta (DE3) [pEPOT3] was centrifuged at 150,000 × g for 60 min. The resulting supernatant was applied to a DEAE-Toyopearl column (2.2 × 20 cm; Tosoh Co., Tokyo, Japan) preequilibrated with buffer A and then eluted with a linear gradient with an increasing concentration of KCl (0 to 0.5 M). The active fraction, to which (NH4)2SO4 (1.0 M) was added, was applied to a butyl-Toyopearl column (2.2 × 20 cm, Tosoh) preequilibrated with buffer A containing 1.0 M (NH4)2SO4 and was eluted with a linear gradient containing (NH4)2SO4 (1.0 to 0 M). The active fractions were collected, dialyzed against buffer A, and used as purified preparations of rHps or rPhi.
Analytical methods.
Protein was measured using a Bio-Rad protein assay kit (Japan Bio-Rad Laboratories, Tokyo, Japan) with bovine serum albumin as the standard (2). Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) for purified rHps-Phi, rHpsi, and rPhi was performed in a 12% polyacrylamide gel (16). Prestained protein markers (low range) for SDS-PAGE (Nacalai Tesque, Kyoto, Japan) were used as size standards. The relative molecular masses of the SDS-denatured proteins and the N-terminal amino acid sequence were determined as described previously (14). The apparent molecular weights of the native enzymes were determined by fast-protein liquid chromatography using a Superose 12 column (1.0 × 30 cm; Amersham Bioscience K.K.) (14). To determine the molecular weight of rHps-Phi, the partially purified preparation was used as described above. Gel filtration was performed with a buffer system containing 50 mM EDTA and 10% glycerol. Proteins were detected by activity. HPS-containing protein was detected by Western blot analysis (23) using rabbit anti-Hps antibody raised against purified rHps (Western Lightning Chemiluminescent Reagent Plus; Perkin-Elmer, Boston, MA).
RESULTS
The PH1938 gene of P. horikoshii OT3 encodes a fused protein consisting of HPS and PHI homologs. A BLAST search of the HPS and PHI sequences (Bacillus subtilis and several methylotrophs) against available databases detected the PH1938 ORF from P. horikoshii. Interestingly, the PH1938 ORF contained both hps and phi sequences within a single ORF. The N-terminal (amino acids 1 to 206) and C-terminal (amino acids 212 to 407) regions of the PH1938 ORF were found to share a high degree of similarity to hps (63%) and to phi (63%) from B. brevis S1, respectively (Fig. 1). These sequences were designated hps and phi, respectively. The predicted molecular mass of the PH1938 gene product is 44,407 Da.
FIG. 1.
Alignment of the deduced amino acid sequence of the PH1938 gene with sequences of hps and phi of B. brevis S1. Asterisks indicate identical amino acid residues; colons and dots indicate conserved amino acid residues. The two hydrophobic regions shown in the boxes were predicted by the use of the SOSUI program (http://sosui.proteome.bio.tuat.ac.jp/sosuimenu0.html).
To determine whether P. horikoshii OT3 indeed produced PH1938 as a single ORF and to discount the possibility that the fused structure in the database sequence is due to a cloning or sequencing artifact, a cell extract prepared from P. horikoshii OT3 was subjected to immunoblot analysis using rabbit polyclonal antibody raised against recombinant HPS from E. coli. As shown in Fig. 2A, the band size detected on the immunoblot was 47 kDa, which agreed well with the theoretical molecular mass of the PH1938 protein. A possible processed HPS fragment (25 kDa) was not detected. These indicated that the organism indeed produced the protein corresponding to the full-length PH1938 ORF.
FIG. 2.
Western blot analyses of cell extracts of P. horikoshii OT3 (A) and E. coli Rosetta (DE3) [pEHOT3] (B). P. horikoshii OT3 was grown in medium with (+ HCHO) or without (− HCHO) 0.5 mM formaldehyde. The E. coli transformant was grown as described under Materials and Methods. Abbreviations: CFE, cell extract; S, soluble fraction; P, particulate fraction.
HPS and PHI activities in P. horikoshii OT3.
When formaldehyde and ribulose 5-phosphate were added to the cell extract from P. horikoshii OT3, fructose 6-phosphate was formed. This observation suggested that the organism exhibited both HPS and PHI activities. Specific activity for the HPS/PHI reaction in cell extracts of cells grown with or without formaldehyde (0.5 mM) was found to be 0.250 and 0.210 units per mg protein, respectively. This suggests that formaldehyde is not an inducer of enzyme activity but that the enzyme is produced constitutively. The enzyme activity in P. horikoshii OT3 under growing conditions was comparable to the induced level of the enzyme in B. subtilis (0.310 units per mg protein) (27).
The PH1938-encoded protein was mostly found in the particulate fraction (Fig. 2A). The hydrophobicity of the protein is presumably due to two hydrophobic regions, occurring in the P. horikoshii OT3 hps sequence, amino acids 148 to 170 and 172 to 192, respectively (Fig. 1). HPSs isolated from methylotrophs have been exclusively found in soluble fractions (1, 11, 28), except for the enzyme from Methylococcus capsulatus, which was found loosely associated with the membrane (8).
Expression of PH1938, hps, and phi in E. coli.
The biochemical properties of the HPS-PHI fusion protein were characterized by overproducing a recombinant protein purified from E. coli. To this purpose, three expression plasmids were constructed containing the entire sequence of the PH1938 gene (pEHPOT3), the hps sequence (pEHOT3), and the phi sequence (pEPOT3), respectively. Each was expressed in E. coli Rosetta (DE3), grown on LB medium, and induced with IPTG. While a small amount of insoluble material was found in each transformant, presumably due to overexpression, cell extracts from the transformants harboring pEHPOT3, pEHOT3, and pEPOT3 exhibited activities of HPS/PHI, HPS, and PHI, respectively. On Western blotting, the cell extract from E. coli Rosetta (DE3) [pEHPOT3] yielded a 47-kDa band from a particulate fraction similar to the PH1938 gene product in the parent strain P. horikoshii OT3 (Fig. 2). SDS-PAGE of the soluble fractions from E. coli Rosetta (DE3) [pEHOT3] and E. coli Rosetta (DE3) [pEPOT3] yielded a major band whose molecular mass was in close agreement with the theoretical values for rHps and rPhi, respectively (data not shown).
The difference in localization or hydrophobicity between rHps-Phi and rHps is striking, because the two hydrophobic motifs are present in the HPS moiety of both proteins as mentioned above. This implies that HPS as a component of the hybrid rHps-Phi protein adopts a different conformation than when it exists as an individual polypeptide.
Purification of rHps-Phi, rHps, and rPhi from E. coli.
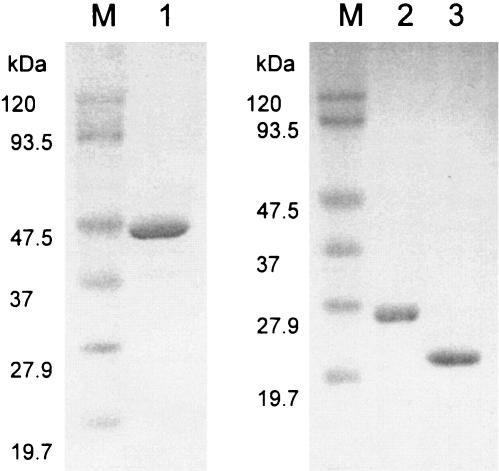
In order to solubilize rHps-Phi from the particulate fraction of E. coli Rosetta (DE3) [pEHPOT3] cell extracts, buffer A containing 1% CHAPS and 50 mM EDTA was selected after evaluation of a number of other detergents. rHps-Phi was purified 10.9-fold from the cell extract (Table 1). The N-terminal sequence (MILQVALDLT) of the purified enzyme was identical to the deduced amino acid sequence. rHps and rPhi were purified 4.14- and 11.8-fold, respectively, from the soluble fraction of the respective E. coli transformants (Table 1). Each purified preparation of rHps-Phi, rHps, and rPhi yielded a single protein band by SDS-PAGE (Fig. 3). The N-terminal sequences of purified rHps and rPhi were determined to be MILQVALDLT and MKTIRKAMKD, respectively, which are identical to the deduced amino acid sequences. These results clearly indicate that the gene product of PH1938 is a bifunctional enzyme responsible for the two sequential reactions catalyzed by HPS and PHI.
TABLE 1.
Purifications of rHps-Phi, rHps, and rPhi
| Enzyme and step | Activity (U) | Protein (mg) | Sp act (U/mg) | Recovery (%) | Purification (fold) |
|---|---|---|---|---|---|
| rHps-Phi | |||||
| Cell extract | 14,400 | 1,020 | 14.1 | 100 | 1.0 |
| Solubilization | 6,580 | 333 | 28.8 | 45.7 | 2.03 |
| DEAE-Sepharose | 3,950 | 86.5 | 45.7 | 27.4 | 3.24 |
| Solubilization of precipitate formed during dialysis | 481 | 3.12 | 154 | 3.3 | 10.9 |
| rHps | |||||
| Cell extract | 17,100 | 302 | 56.7 | 100 | 1.0 |
| DEAE-Toyopearl | 12,600 | 78.3 | 161 | 73.7 | 2.84 |
| Butyl-Toyopearl | 3,490 | 14.9 | 235 | 20.4 | 4.14 |
| rPhi | |||||
| Cell extract | 13,300 | 292 | 45.5 | 100 | 1.0 |
| DEAE-Toyopearl | 2,140 | 12.5 | 171 | 16.1 | 3.76 |
| Butyl-Toyopearl | 802 | 1.48 | 539 | 6.04 | 11.8 |
FIG. 3.
SDS-PAGE of purified rHps-Phi (lane 1), rHps (lane 2), and rPhi (lane 3). Lanes M indicate molecular mass markers.
Subunit structures of purified rHps-Phi, rHps, and rPhi.
The relative molecular mass of purified rHps-Phi was estimated to be 47 kDa by SDS-PAGE, in good agreement with the theoretical molecular mass. The relative molecular mass of the EDTA-solubilized enzyme was estimated to be 162 kDa. Judging from these results, rHps-Phi is a homotetrameric enzyme. The relative molecular masses of purified rHps were estimated to be 27 kDa by SDS-PAGE and 25 kDa by gel filtration. The molecular mass of the subunit was in good agreement with the theoretical value (22,209 Da), indicating that rHps is a monomeric enzyme. The relative molecular mass of purified rPhi was estimated to be 21 kDa by SDS-PAGE and 75 kDa by gel filtration, indicating that rPhi is likely a homotetrameric enzyme. The subunit molecular mass was in good agreement with the theoretical value (21,475 Da).
Effect of temperature on activity and stability of purified rHps-Phi, rHps, and rPhi.
Figure 4 shows the effect of temperature on activity and stability of the purified enzymes. The temperature profiles of rHps-Phi and rHps were similar, with the optimum temperature for activity being 80 to 85°C. Both enzymes were found to be stable against heat treatment. The half-lives of rHps-Phi and rHps at 90°C were >90 min and 45 min, respectively. These temperature profiles are consistent with the growth temperatures of the parent strain, P. horikoshii OT3. On the other hand, the optimum temperature of rPhi activity was 60°C, and the half-life of enzyme activity at 90°C was <5 min. Thus, rPhi was hardly active at all at the growth temperatures of the parent organism. rHps-Phi activity was assayed by detection of fructose 6-phosphate formation from formaldehyde and ribulose 5-phosphate, indicating that both HPS and PHI components of rHps-Phi were active at 90°C. On the other hand, rPhi alone exhibited significant sensitivity to heat. These observations suggest that the thermostability of PHI as a covalent component of the hybrid rHps-Phi protein results from fusion with the thermotolerant HPS moiety.
FIG. 4.
Effect of temperature on activity (A) and stability (B) of rHps-Phi, rHps, and rPhi. (A) Enzyme activity was assayed under standard conditions at the indicated temperatures. (B) rHps-Phi was dissolved in buffer A containing 1% CHAPS, 50 mM EDTA, and 10% glycerol; rHps and rPhi were dissolved in buffer A. The enzyme solution was incubated at 90°C for the indicated period. Activity of rHps-Phi was assayed by the method used for HPS/PHI. Activities of rHps and rPhi were assayed under the standard conditions. Symbols: circles, rHps-Phi; triangles, rHps; squares, rPhi.
Kinetic properties of rHps-Phi and rHps.
Both rHps-Phi and rHps enzyme activities were assayed by a determination of rates of formaldehyde consumption. Kinetic parameters were obtained from double reciprocal plots of enzyme activities versus formaldehyde concentration using a fixed concentration of ribulose 5-phosphate (2.5 mM). Table 2 summarizes the Km for formaldehyde, and Vmax and kcat values for the purified recombinant enzymes. Kms for formaldehyde of rHps-Phi at 60 to 80°C were higher than those of rHps. The affinity of the enzymes for formaldehyde seemed to increase at higher temperatures, implying that the rHps-Phi and rHps proteins adopt more suitable catalytic conformations under such conditions. Molar activities (kcat) of rHps-Phi at both temperatures were significantly higher than those of rHps, while catalytic efficiencies (kcat/Km) for rHps-Phi and rHps were comparable.
TABLE 2.
Kinetic parameters of purified rHps-Phi and rHps
| Enzyme | Temp (°C) | Apparent Kma for formaldehyde (mM) | Vmaxa (U/mg) | kcat (s−1) | kcat/Km (mM−1 · s−1) |
|---|---|---|---|---|---|
| rHps-Phi | 60 | 16.9 | 384 | 281 | 45.0 |
| 80 | 6.25 | 526 | 386 | 61.8 | |
| rHps | 60 | 2.31 | 170 | 62.2 | 26.9 |
| 80 | 1.95 | 435 | 159 | 81.5 |
Km and Vmax were obtained by double reciprocal plots of HPS activity versus formaldehyde concentration.
Efficiency of formaldehyde fixation by the hybrid protein rHps-Phi.
Fusion of the two enzymes to form a hybrid may increase the efficiency of the net reaction, by causing the localized concentration of the product of the HPS-catalyzed reaction, d-arabino-3-hexulose 6-phosphate, to be significantly higher in the vicinity of the PHI component of the hybrid enzyme than would occur in the case of two separate polypeptides. The molar activities of rHps-Phi were compared to a mixture of rHps and rPhi on an equimolar subunit basis (Table 3). The activity catalyzed by rHps-Phi was found to be 1.8 times higher than that catalyzed by the equimolar mixture of rHps and rPhi at 60°C. Activity of the fused enzyme at 80°C was about 50 times higher than that of the enzyme mixture. At this temperature, rPhi exhibited about 30% of its maximum activity at 60°C. Based on these results, the fused enzyme appears to efficiently catalyze the coupled reaction at the normal growth temperatures of the parent organism.
TABLE 3.
Comparison of HPS/PHI activity to activity of rHps-Phi and a mixture of rHps and rPhi
| Protein | Amt of enzyme used (pmol of subunit)a | Activity (U/nmol of subunit)b
|
|
|---|---|---|---|
| 60°C | 80°C | ||
| rHps-Phi | 38 | 2.47 | 6.58 |
| rHps+rPhi | 29 each | 1.36 | 0.119 |
Enzyme was added to 1 ml of reaction mixture.
HPS/PHI activity was determined.
DISCUSSION
We herein demonstrate that the archaeon P. horikoshii OT3 constitutively produces a bifunctional enzyme catalyzing sequential HPS and PHI reactions. Our present studies have revealed that the recombinant bifunctional enzyme rHps-Phi catalyzed the sequential reaction much more efficiently than a mixture of rHps and rPhi. Furthermore, while purified rHps-Phi and rHps were active at the growth temperature of P. horikoshii, purified rPhi was found to lack an equivalent thermostability. These represent the unique features of the hybrid PH1938 protein, i.e., (i) efficient catalytic activity and (ii) extreme thermostability, both of which were conferred by being a fusion protein.
rHps-Phi was highly active at normal growth temperatures for P. horikoshii OT3, although activity of purified rHps or rPhi at moderate temperatures was much lower than that of the corresponding enzymes from methylotrophic bacteria (1, 8, 11, 12, 19). The apparent affinities (Kms) of rHps-Phi for formaldehyde (6.25 mM at 80°C) were also lower than those of HPSs from methylotrophic bacteria, whose apparent Kms range from 0.15 to 1.4 mM (7). With the enzyme from P. horikoshii OT3, the Vmax of rHps-Phi for formaldehyde fixation (526 units per mg protein) was found to be comparable to that of the thermotolerant methylotroph Bacillus strain C1 (480 units per mg protein at 50°C) (1). On the basis of its high catalytic efficiency at normal growth temperature of P. horikoshii OT3, it is evident that this hyperthermophilic and anaerobic archaeon has an enzyme activity sufficient to play a significant physiological role. From an evolutionary standpoint, it is noteworthy that the apparent Km of rHps for formaldehyde (1.95 mM) is comparable to that of some methylotrophs and is significantly lower than that of the fused enzyme. The HPS and PHI orthologs appear also in the genomes of the other hyperthermophilic archaea Pyrodictium abyssi (CAB50617) and Pyrococcus furiosus (AAL63515), suggesting that the fused gene is the genetic trait in Pyrococcus. The biochemical conversion of formaldehyde in the archaea may provide clues to the evolution of aerobic methylotrophy in bacteria.
Xavier et al. proposed a novel glycolytic pathway that involves the cleavage of a six-carbon compound to yield pentose phosphate and formate in Thermococcus zilligii by 13C labeling experiments (26). Recently, Verhees et al. provided an alternative interpretation of the labeling pattern (24). According to them, fructose 6-phosphate is isomerized to d-arabino-3-hexulose 6-phosphate, which then cleaved to formaldehyde and ribulose 5-phosphate. These reactions correspond to the reverse reactions of formaldehyde fixation reaction catalyzed by HPS and PHI. Our experiment showed that the purified rHps-Phi catalyzed the conversion of fructose 6-phosphate to formaldehyde and ribulose 5-phosphate (data not shown). The specific activity of the reverse reaction was approximately one-seventh of that of the forward reaction, however, suggesting that the equilibrium of the reaction was in favor of formaldehyde fixation similar to the case in the methylotroph M. capsulatus (8). Since the enzyme was synthesized constitutively in P. horikoshii, HPS-PHI fused protein seems to be involved not only in the formaldehyde fixation but also in the glycolytic pathway. In the pathway proposed by Verhees et al., the generated formaldehyde is oxidized to formate by formaldehyde ferredoxin oxidoreductase (24). P. furiosus has been shown to have tungsten-containing formaldehyde ferredoxin oxidoreductase, which oxidizes a variety of aldehydes including formaldehyde (10, 21). The genome of P. horikoshii also contains the gene for this enzyme. The apparent Km and Vmax values for formaldehyde of formaldehyde ferredoxin oxidoreductase from P. furiosus are 25 mM and 62 μmol · min−1 · mg protein−1, respectively. The former value suggests that formaldehyde is unlikely to be the physiological substrate (21). On the other hand, the Km value of rHps-Phi for formaldehyde is much lower and the Vmax value at 80°C is much higher than those of formaldehyde ferredoxin oxidoreductase. Thus, formaldehyde-fixation activity is estimated to be much more effective than formaldehyde oxidation in this organism. More-detailed analyses are required to elucidate the physiological function of HPS-PHI fusion protein and the fate of formaldehyde in P. horikoshii.
In methylotrophs and nonmethylotrophs characterized to date, HPS and PHI were both found to be inducible by formaldehyde (18, 22, 27, 28). On the other hand, P. horikoshii OT3, originally isolated from a marine hydrothermal vent, produced the HPS-PHI fusion protein constitutively. Although the presence of formaldehyde has not been demonstrated directly in these extreme environmental conditions, its presence would not be surprising because C1 compounds are known to be present at a variety of redox levels in marine hydrothermal vents (i.e., methane, CO, and CO2) (3, 6, 15). Constitutive expression in P. horikoshii OT3 may indicate functional formaldehyde metabolism in such extreme environmental conditions.
Creation of a fused gene through recombinant DNA technology can be used to improve functional properties for applied purposes. Fusion is also advantageous in that it allows regulation of the two genes as a single ORF and permits enzyme purification in one process. As revealed in this study, fusion of HPS and PHI increased the efficiency of two sequential reactions and the stability of the individual enzymes against thermal denaturation in P. horikoshii OT3. This suggests that HPS-PHI enzymes possessing reaction profiles to meet a variety of objectives may be created through combination of hps and phi genes obtained from various microorganisms.
Acknowledgments
This research was supported in part by Grant-in-Aid for Scientific Research (S) 13854008 to Y.S. This paper is supported in part by COE for Microbial-Process Development Pioneering Future Production System (COE program of the Ministry of Education, Culture, Sports, Science and Technology, Japan).
REFERENCES
- 1.Arfman, N., L. Bystrykh, N. I. Govorukhina, and L. Dijkhuizen. 1990. 3-Hexulose-6-phosphate synthase from thermotolerant methylotroph Bacillus C1. Methods Enzymol. 188:391-397. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Canet, C., R. M. Prol-Ledesma, J.-C. Melgarejo, and A. Reyes. 2003. Methane-related carbonates formed at submarine hydrothermal springs: a new setting for microbially-derived carbonates? Mar. Geol. 199:245-261. [Google Scholar]
- 4.Chistoserdova, L., C. Jenkins, M. G. Kalyuzhnaya, C. J. Marx, A. Lapidus, J. A. Vorholt, J. T. Staley, and M. E. Lidstrom. 2004. The enigmatic planctomycetes may hold a key to the origins of methanogenesis and methylotrophy. Mol. Biol. Evol. 21:1234-1241. [DOI] [PubMed] [Google Scholar]
- 5.Chistoserdova, L., J. A. Vorholt, R. K. Thauer, and M. E. Lidstrom. 1998. C1 transfer enzymes and coenzymes linking methylotrophic and methanogenic Archaea. Science 281:99-102. [DOI] [PubMed] [Google Scholar]
- 6.Damm, E., and G. Budeus. 2003. Fate of vent-derived methane in seawater above the Hakon Mosby mud volcano (Norwegian Sea). Mar. Chem. 82:1-11. [Google Scholar]
- 7.Dijkhuizen, L., P. R. Levering, and K. De Vries. 1992. The physiology and biochemistry of aerobic methanol-utilizing Gram-negative and Gram-positive bacteria, vol. 5. Plenum Publishing Co., New York, N.Y.
- 8.Ferenci, T., T. Strom, and J. R. Quayle. 1974. Purification and properties of 3-hexulose phosphate synthase and phospho-3-hexuloisomerase from Methylococcus capsulatus. Biochem. J. 144:477-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez, J. M., Y. Masuchi, F. T. Robb, J. W. Ammerman, D. L. Maeder, M. Yanagibayashi, J. Tamaoka, and C. Kato. 1998. Pyrococcus horikoshii sp. nov., a hyperthermophilic archaeon isolated from a hydrothermal vent at the Okinawa Trough. Extremophiles 2:123-130. [DOI] [PubMed] [Google Scholar]
- 10.Hu, Y., S. Faham, R. Roy, M. W. W. Adams, and D. C. Rees. 1999. Formaldehyde ferredoxin oxidoreductase from Pyrococcus furiosus: the 1.85 A resolution crystal structure and its mechanistic implications. J. Mol. Biol. 286:899-914. [DOI] [PubMed] [Google Scholar]
- 11.Kato, N. 1990. 3-Hexulose-6-phosphate synthase from Mycobacterium gastri MB19. Methods Enzymol. 188:397-401. [DOI] [PubMed] [Google Scholar]
- 12.Kato, N., H. Ohashi, Y. Tani, and K. Ogata. 1978. 3-Hexulosephosphate synthase from Methylomonas aminofaciens 77a: purification, properties and kinetics. Biochim. Biophys. Acta 523:238-244. [DOI] [PubMed] [Google Scholar]
- 13.Kawarabayasi, Y., M. Sawada, H. Horikawa, Y. Haikawa, Y. Hino, S. Yamamoto, M. Sekine, S. Baba, H. Kosugi, A. Hosoyama, Y. Nagai, M. Sakai, K. Ogura, R. Otsuka, H. Nakazawa, M. Takamiya, Y. Ohfuku, T. Funahashi, T. Tanaka, Y. Kudoh, J. Yamazaki, N. Kushida, A. Oguchi, K. Aoki, and H. Kikuchi. 1998. Complete sequence and gene organization of the genome of a hyper-thermophilic archaebacterium, Pyrococcus horikoshii OT3. DNA Res. 5:55-76. [DOI] [PubMed] [Google Scholar]
- 14.Kotani, T., T. Yamamoto, H. Yurimoto, Y. Sakai, and N. Kato. 2003. Propane monooxygenase and NAD+-dependent secondary alcohol dehydrogenase in propane metabolism by Gordonia sp. strain TY-5. J. Bacteriol. 185:7120-7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotelnikova, S. 2002. Microbial production and oxidation of methane in deep subsurface. Earth-Sci. Rev. 58:367-395. [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Mitsui, R., Y. Kusano, H. Yurimoto, Y. Sakai, N. Kato, and M. Tanaka. 2003. Formaldehyde fixation contributes to detoxification for growth of a nonmethylotroph, Burkholderia cepacia TM1, on vanillic acid. Appl. Environ. Microbiol. 69:6128-6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitsui, R., Y. Sakai, H. Yasueda, and N. Kato. 2000. A novel operon encoding formaldehyde fixation: the ribulose monophosphate pathway in the gram-positive facultative methylotrophic bacterium Mycobacterium gastri MB19. J. Bacteriol. 184:944-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller, R. H., and W. Babel. 1990. 3-Hexulose-6-phosphate synthase from Acetobacter methanolicus MB58. Methods Enzymol. 188:401-405. [DOI] [PubMed] [Google Scholar]
- 20.Quayle, J. R., and T. Ferenci. 1978. Evolutionary aspects of autotrophy. Microbiol. Rev. 42:251-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy, R., S. Mukund, G. J. Schut, D. M. Dunn, R. Weiss, and M. W. W. Adams. 1999. Purification and molecular characterization of the tungsten-containing formaldehyde ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus: the third of a putative five-member tungstoenzyme family. J. Bacteriol. 181:1171-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakai, Y., R. Mitsui, Y. Katayama, H. Yanase, and N. Kato. 1999. Organization of the genes involved in the ribulose monophosphate pathway in an obligate methylotrophic bacterium, Methylomonas aminofaciens 77a. FEMS Microbiol. Lett. 176:125-130. [DOI] [PubMed] [Google Scholar]
- 23.Towbin, H., T. Staehelin, and J. Gordon. 1992. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. 1979. Bio/Technology 24:145-149. [PubMed] [Google Scholar]
- 24.Verhees, C. H., S. W. M. Kengen, J. E. Tuininga, G. J. Schut, M. W. W. Adams, W. M. de Vos, and J. van der Oost. 2003. The unique features of glycolytic pathways in Archaea. Biochem. J. 375:231-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vorholt, J. A., C. J. Marx, M. E. Lidstrom, and R. K. Thauer. 2000. Novel formaldehyde-activating enzyme in Methylobacterium extorquens AM1 required for growth on methanol. J. Bacteriol. 182:6645-6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xavier, K. B., M. S. Da Costa, and H. Santos. 2000. Demonstration of a novel glycolytic pathway in the hyperthermophilic archaeon Thermococcus zilligii by 13C-labeling experiments and nuclear magnetic resonance analysis. J. Bacteriol. 182:4632-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasueda, H., K. Kawahara, and S. Sugimoto. 1999. Bacillus subtilis yckG and yckF encode two key enzymes of the ribulose monophosphate pathway used by methylotrophs, and yckH is required for their expression. J. Bacteriol. 181:7154-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yurimoto, H., R. Hirai, H. Yasueda, R. Mitsui, Y. Sakai, and N. Kato. 2002. The ribulose monophosphate pathway operon encoding formaldehyde fixation in a thermotolerant methylotroph, Bacillus brevis S1. FEMS Microbiol. Lett. 214:189-193. [DOI] [PubMed] [Google Scholar]