Abstract
Induction of the heat shock response in Escherichia coli requires the alternative sigma factor σ32 (RpoH). The cellular concentration of σ32 is controlled by proteolysis involving FtsH, other proteases, and the DnaKJ chaperone system. To identify individual σ32 residues critical for degradation, we used a recently developed bacterial one-hybrid system and screened for stabilized versions of σ32. The five single point mutations that rendered the sigma factor more stable mapped to positions L47, A50, and I54 in region 2.1. Strains expressing the stabilized σ32 variants exhibited elevated transcriptional activity, as determined by a groE-lacZ fusion. Structure calculations predicted that the three mutated residues line up on the same face of an α-helix in region 2.1, suggesting that they are positioned to interact with proteins of the degradation machinery.
The heat shock response is a universal reaction in nature to defend cells against temperature-induced damage. Heat stress causes proteins to unfold and aggregate. In the gram-negative bacterium Escherichia coli, a heat shock regulon comprised of more than 30 heat shock genes is induced after a temperature upshift. Most of the encoded heat shock proteins are molecular chaperones (e.g., DnaK, DnaJ, GroEL, and GroES) or proteases (e.g., FtsH, Lon, and HslVU) (reviewed in references 8 and 32).
Transcription of the heat shock regulon is initiated by the alternative sigma factor σ32 (RpoH). The σ32 level is tightly controlled, mainly at the translational and posttranslational levels. Translational regulation requires a secondary structure in the coding region of the rpoH mRNA, which blocks the ribosomal binding site at physiological temperatures (33, 20). At heat shock temperatures (e.g., 42°C), the secondary structure melts, allowing the ribosome to interact with the ribosomal binding site of rpoH mRNA to initiate translation (19).
At the posttranslational level, the activity and stability of σ32 are regulated. About 50 σ32 molecules are found per cell at 30°C (26). This number increases immediately after heat shock to approximately 1,000 molecules. Controlled proteolysis of σ32 involves different proteases (e.g., FtsH, Lon, and HslVU) (11, 13, 29) and a network of molecular chaperones, including DnaKJ and GroESL (6, 10).
DnaK and its cochaperone DnaJ interact with σ32 at physiological temperatures, depleting the freely available sigma factor pool. This interaction renders σ32 susceptible to FtsH-mediated proteolysis. During heat stress, DnaK and DnaJ are titrated away from σ32 by unfolded or aggregated proteins. Free σ32 interacts with core RNA polymerase and induces transcription of the heat shock genes (3, 7). Shutoff of the heat shock response starts after 4 to 6 min at a heat stress temperature. As soon as sufficient amounts of chaperones are synthesized, the heat shock sigma factor is redirected to degradation, reducing the amount of σ32 to the normal level (25, 26).
The exact mechanism of σ32 degradation is unknown. Aiming at isolation of σ32 derivatives that are stable against proteolysis, we used a recently established one-hybrid system. In this system the adenylate cyclase (AC) of Bordetella pertussis is used to complement an AC-deficient (cya) E. coli strain. The catalytic AC fragment, which synthesizes cAMP, comprises two subdomains called T18 and T25, which are connected via a flexible linker. This linker can be replaced by a protein of interest. If the linker is susceptible to proteolysis, the two subdomains are separated and no cAMP is produced. A stable hybrid construct produces cAMP, which can be easily detected (e.g., by a lacZ reporter system) (5, 15).
To isolate stabilized σ32 derivatives, we performed random mutagenesis on rpoH by error-prone PCR. The mutagenized PCR fragments were introduced between the AC subdomains, and the constructs were transformed in cya E. coli cells. Cells expressing stabilized σ32-AC hybrids produced red colonies (Cya+) on MacConkey agar plates, whereas constructs susceptible to proteolysis produced white colonies (Cya−). All single-point mutations in rpoH that resulted in stabilization of σ32 clustered in region 2.1 of the sigma factor.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains used in this study are listed in Table 1. All strains were grown in Luria-Bertani medium (24). Antibiotics and chemicals were added as follows: ampicillin, 200 μg/ml; chloramphenicol, 20 μg/ml; kanamycin, 30 μg/ml; nalidixic acid, 15 μg/ml; and isopropyl-thio-β-d-galactoside (IPTG), 1 mM (final concentration), unless indicated otherwise). E. coli DH5α was grown at 37°C, E. coli DHM1 and C600 and the ftsH1 mutant were grown at 30°C, and the E. coli ΔrpoH mutant was grown at 25°C.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotypea | Reference or source |
|---|---|---|
| E. coli strains | ||
| DH5α | supE44 ΔlacU169 (ψ80lacZΔM15) hsdR17 recA1 gyrA96 thi1 relA1 | 24 |
| DHM1 | cya-854 recA1 gyrA96 (NaI) thi1 hsdR17 spoT1 rfbD1 glnV44(AS) | D. Ladant |
| C600 | thr-1 leu-6 thi-1 supE44 lacY1 tonA21 λ− | Laboratory collection |
| ftsH1 | thr-1 leu-6 thi-1 supE44 lacY1 tonA21 λ−ftsH1(Ts) fts1372(Ts) | 30 |
| ΔrpoH | MG1655 rpoH120::kan zhg-21::Tn10 | 35 |
| Plasmids | ||
| pKACp5 | pKAC derivative carrying p5, an HIV protease cleavage site, between codons 224 and 225 of the catalytic domain of the B. pertussis adenylate cyclase, Kmr | 5 |
| pCD951 | pKAC carrying rpoH N-terminal fragment M1-M156, Kmr | 4 |
| pCD953 | pKAC carrying rpoH C-terminal fragment T142-A284, Kmr | 4 |
| pEC5352 | pKACp5 derivative, p5 replaced by E. coli rpoH, Kmr | This study |
| pEC5378 | pEC5352 derivative coding for AC-RpoH (I54F), Kmr | This study |
| pEC5379 | pEC5352 derivative coding for AC-RpoH (A50), Kmr | This study |
| pEC5380 | pEC5352 derivative coding for AC-RpoH (L47Q), Kmr | This study |
| pEC5381 | pEC5352 derivative coding for AC-RpoH (N83D), Kmr | This study |
| pEC5382 | pEC5352 derivative coding for AC-RpoH (H107L), Kmr | This study |
| pEC5383 | pEC5352 derivative coding for AC-RpoH (I54T), Kmr | This study |
| pAR145 | pHSG575 vector carrying ftsH, Cmr | 30 |
| pAR145b | pAR145 lacking ftsH, Cmr | This study |
| pEC5217 | pUC18 carrying E. coli rpoH, Apr | 21 |
| pEC5356 | pEC5217 derivative coding for RpoH (A50), Apr | This study |
| pEC5357 | pEC5217 derivative coding for RpoH (L47Q), Apr | This study |
| pEC5358 | pEC5217 derivative coding for RpoH (N83D), Apr | This study |
| pEC5359 | pEC5217 derivative coding for RpoH (H107L), Apr | This study |
| pEC5360 | pEC5217 derivative coding for RpoH (I54F), Apr | This study |
HIV, human immunodeficiency virus.
Plasmids and recombinant DNA techniques.
Plasmids used in this study are shown in Table 1. DNA manipulations were performed by using standard protocols (24). To construct pEC5352 encoding the AC-RpoH fusion downstream of a lac promoter, an 870-bp fragment containing E. coli rpoH was amplified from pEC5217 using primers MO3 (5′-ACTCCGCTAGCCATATGACTGACAAAATGCAAAGTTTAGC-3′; restriction site underlined) and MO4 (5′-CACCAGGTACCCTCGAGCGCTTCAATGGCAGCACGC-3′). The PCR fragment was digested with NheI and KpnI and cloned into pKACp5, replacing the p5 fragment. Selected rpoH point mutations were transferred from pEC5352 derivatives into pEC5217, replacing the wild-type MluI-PstI fragment. To construct pAR145b, the ftsH gene was deleted from pAR145 by NdeI restriction and religation.
One-hybrid screening.
Error-prone PCR of pEC5352 was performed with primers MO17 (5′-GACATGTTCGCCATTATGC-3′) and MO18 (5′-GATTTTCCACAACAAGTCG-3′) using Taq polymerase and (i) standard reaction conditions, (ii) an elevated dATP concentration (16 mM instead of 2 mM), and (iii) addition of 0.2 mM MnCl2 (24, 34). NheI/KpnI-digested PCR fragments were cloned into pKACp5 cut with the same enzymes, replacing the p5 fragment. Transformed E. coli DHM1 cells were screened on MacConkey agar plates with maltose as the only carbon source as described by Karimova et al.(14) and in a user manual provided by D. Ladant. Plasmids from red colonies potentially expressing stabilized RpoH constructs were sequenced using primers MO17 and MO18.
In vivo degradation assay and immunoblot analysis.
E. coli C600 and E. coli ftsH1 cells carrying pEC5352 were grown to an optical density at 600 nm of 0.4 to 0.6 at 30°C. The cultures were shifted to 42°C, and expression of AC-RpoH-WT was induced with IPTG for 15 min. Chloramphenicol (200 μg/ml) was added, and samples were taken and frozen in liquid nitrogen. E. coli ΔrpoH cells carrying a pEC5217 derivative were grown to an optical density at 600 nm of 0.4 to 0.6 at 25°C prior to rpoH induction with IPTG for 15 min. Chloramphenicol was added, and samples were taken and frozen in liquid nitrogen. Cells were harvested, washed with 0.9% NaCl, treated with 2 mg/ml lysozyme in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8) for 5 min before sodium dodecyl sulfate (SDS) loading dye was added, and boiled for 10 min. Equivalent amounts of protein (10 μg) were separated on 12% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Hybond-C; Amersham). RpoH proteins were detected using an anti-RpoH antibody (polyclonal rabbit anti-RpoH antibody; 1:3,000 dilution) and a secondary antibody [goat anti-rabbit immunoglobulin G(H+L)-horseradish peroxidase conjugate; Bio-Rad; 1:3,000 dilution], followed by chemiluminescence detection (SuperSignal; Pierce). X-ray films were scanned with a Molecular Dynamics PhosphorImager, and RpoH bands were quantified using the Quantity One program (version 4.5.2; Bio-Rad).
Other methods.
SDS-polyacrylamide gel electrophoresis (24) and β-galactosidase assays (18) were performed as described previously. E. coli DHM1 and E. coli ΔrpoH cultures for β-galactosidase assays were grown in the presence of 0.5 mM IPTG.
RESULTS
Genetic screen to study σ32 degradation.
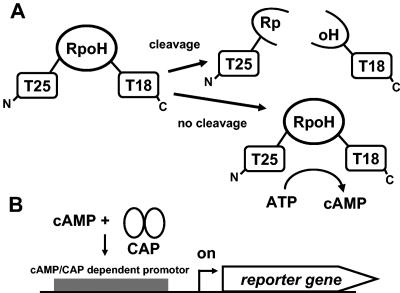
A recently developed one-hybrid system to study protease activity (5) was adapted to explore σ32 degradation. The principle is illustrated in Fig. 1. The reporter system relies on the concept of monitoring cAMP production in an adenylate cyclase-negative (cya) E. coli strain that is provided with a plasmid encoding both catalytic subdomains of the AC from B. pertussis. The two subdomains, called T25 and T18, are connected via a flexible linker region, which can be replaced by the protein of interest, in our case σ32 (RpoH) (Fig. 1A). Intact hybrid constructs produce cAMP, which can be detected easily (e.g., by monitoring cAMP-dependent lacZ expression) (Fig. 1B). If the hybrid construct carries a protease-sensitive linker, the separated subdomains are unable to catalyze cAMP production.
FIG. 1.
Principle of the one-hybrid system. (A) cAMP is produced only when a stable linker is present between T25 and T18. (B) Detection of cAMP by a catabolite gene activator protein (CAP)-controlled reporter gene.
To provide proof of principle, E. coli rpoH was cloned between T25 and T18 as described in Materials and Methods, leading to AC-RpoH-WT. ACp5, a construct carrying a proteolytically stable peptide of the human immunodeficiency virus Gag/Pol protein (5), and two recently described constructs carrying either the N-terminal region (AC-RpoH-NT) or the C-terminal region (AC-RpoH-CT) of RpoH (4) were used as controls.
The lac operon served as a reporter system, and β-galactosidase assays were performed (Fig. 2). The negative control (DHM1 without plasmid; Cya− phenotype) showed some residual activity, which was stimulated 20- to 30-fold by the presence of an intact AC hybrid (ACp5; Cya+ phenotype). β-Galactosidase activity was significantly reduced when the p5 linker was replaced by RpoH, suggesting susceptibility to proteolysis. The presence of the N-terminal half of RpoH reduced β-galactosidase activity even further, whereas the construct carrying the C-terminal half of RpoH was highly active, supporting the proposal that degradation occurs via the N-terminal region (2, 1, 4).
FIG. 2.
β-Galactosidase activity resulting from different AC hybrids in E. coli DHM1. The error bars indicate standard deviations from three independent assays.
To demonstrate proper expression of the hybrid proteins, the presence of AC-RpoH-WT, AC-RpoH-NT, and AC-RpoH-CT was examined by immunoblot analysis using anti-RpoH sera (data not shown). Two different approaches were used to examine whether the AC-RpoH-WT fusion was a substrate of the FtsH protease. First, the fusion was expressed in E. coli DHM1 in the absence or presence of plasmid pAR145 expressing FtsH. Production of additional FtsH in the cell reduced the β-galactosidase activity (Fig. 3A), suggesting that the fusion was cleaved. Second, expression of the fusion protein was monitored by immunoblot analysis in the temperature-sensitive ftsH1 strain. When protein synthesis was inhibited by addition of chloramphenicol and the protease was inactivated by a shift to 42°C for 10 min, the AC-RpoH-WT levels remained constant (Fig. 3B). In contrast, the fusion was degraded in the presence of normal FtsH in E. coli C600.
FIG. 3.
Turnover of the AC-RpoH-WT fusion by FtsH. (A) AC-RpoH-WT-derived β-galactosidase activity in E. coli DHM1 in the presence of control plasmid pAR145b (normal FtsH levels are indicated by one plus sign) or FtsH expression plasmid pAR145 (elevated FtsH levels are indicated by two plus signs). (B) Immunodetection of the AC-RpoH-WT fusion (indicated by the arrow) in E. coli C600 and the ftsH1 strain by use of anti-E. coli RpoH serum. Protein synthesis was blocked, and samples were taken before (30°C) and 10 min after a shift to 42°C.
In summary, we concluded (i) that the AC-RpoH fusion is degraded by FtsH, (ii) that degradation does not proceed from either end of RpoH as the ends are blocked by T25 and T18, and (iii) that the degradation signal resides in the N-terminal region of RpoH.
Screening for stabilized mutants of σ32.
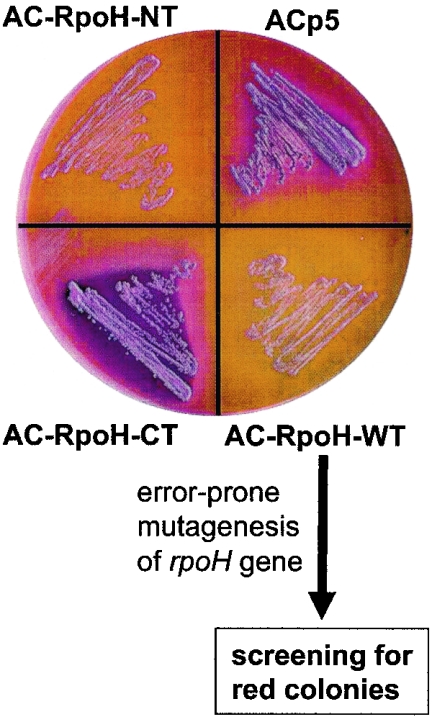
As an additional readout system to facilitate screening on solid media, we chose the cAMP-dependent mal operon and provided maltose as the only carbon source in MacConkey agar plates. In line with the β-galactosidase results, colonies harboring stable AC hybrids turned red, whereas unstable fusions led to white colonies (Fig. 4).
FIG. 4.
Phenotypic appearance of E. coli DHM1 strains expressing AC hybrids. Transformants were plated on MacConkey agar plates containing maltose as the only carbon source and incubated at 30°C for 36 h. The mutagenesis and screening strategy starting from the AC-RpoH-WT protein is shown at the bottom.
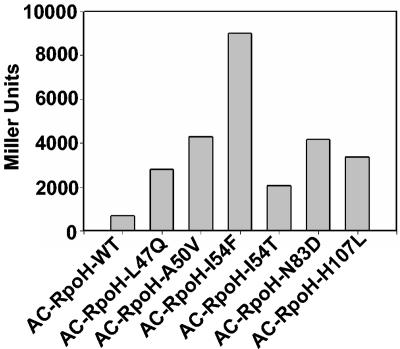
To narrow down the region responsible for degradation in σ32, we designed a random mutagenesis approach, as described in Materials and Methods (Fig. 4). Of approximately 2,000 colonies, 43 had a deep red color. Sequence analysis revealed that most rpoH variants carried multiple point mutations or deletions. Seven candidates, all derived from the standard PCR, carried single point mutations. Remarkably, the mutations clustered in region 2, and five of them were located in region 2.1 from residues 47 to 54 (Fig. 5). In a β-galactosidase assay, all point mutation AC-RpoH variants showed 3- to 13-fold-elevated activity compared to AC-RpoH-WT (Fig. 6), thus indicating enhanced stability.
FIG. 5.
All single point mutations obtained in this study cluster in region 2 of E. coli RpoH. AC-RpoH-L47Q was obtained independently twice. The secondary structure prediction was adapted from the study of Malhotra et al. (16).
FIG. 6.
cAMP-dependent lacZ expression mediated by AC-RpoH constructs with single point mutations in E. coli DHM1 cells.
Stabilized σ32 proteins are functional.
To test sigma factor activity and stability of the mutated RpoH proteins devoid of the AC subdomains, selected single point mutations were transferred into the wild-type rpoH gene as described in Materials and Methods. Five rpoH alleles expressing RpoH-L47Q, -A50V, -I54F, -N83D, and -H107L were constructed. The RpoH derivatives were first tested for sigma factor function. Plasmids carrying the respective point mutated rpoH genes were transformed into an E. coli ΔrpoH strain carrying an RpoH-dependent groE-lacZ fusion. β-Galactosidase assays demonstrated that all RpoH variants were fully active sigma factors (Fig. 7). RpoH-L47Q, -A50V, -I54F, and -N83D showed elevated activities that were between 130 and 170% of the wild-type RpoH activity.
FIG. 7.
Sigma factor activity of RpoH derivatives carrying single point mutations in E. coli ΔrpoH β-galactosidase activity which originated from a groE-lacZ promoter.
To assess the predicted enhanced stability of the RpoH derivatives, degradation assays with plasmid-encoded rpoH variants were performed in the ΔrpoH strain as outlined in Materials and Methods. RpoH-WT displayed a half-life of approximately 2 min under these conditions (Fig. 8). Unexpectedly, RpoH-N83D and RpoH-H107L were not stabilized significantly and showed wild-type-like or only slightly enhanced stability. RpoH-A50V and RpoH-L47Q had half-lives that were two- to three-fold longer than that of RpoH-WT. The half-life of RpoH-I54F was estimated to be increased at least 10-fold.
FIG. 8.
Stability of RpoH derivatives carrying single point mutations. (A) Degradation of RpoH proteins as monitored by immunoblot analysis after protein synthesis was stopped by chloramphenicol (Cm) addition. (B) Quantification of RpoH degradation. Average values from three independent experiments are shown. WT, wild type.
DISCUSSION
Application of the one-hybrid system.
It was demonstrated that degradation of σ32 by the FtsH protease occurs via the N-terminal half of the sigma factor (see below). In order to pin down individual σ32 residues important for this process, we decided to adapt a recently established one-hybrid system (5). A hybrid between full-length RpoH and B. pertussis AC fragments T25 and T18 resulted in low β-galactosidase activity and white colonies on MacConkey agar plates containing maltose, presumably because the sigma factor was accessible to proteolysis. Degradation of the hybrid was shown to be mediated by the FtsH protease. Assuming that stabilized RpoH-AC hybrids would be red on MacConkey agar plates containing maltose, we mutagenized the entire rpoH gene. Approximately 2% of all colonies obtained were red, but most of them contained multiple mutations. Interestingly, the mutations in all seven candidates carrying only single nucleotide exchanges clustered in region 2 of the sigma factor. Five of these seven mutations indeed rendered RpoH stable. Why the N83D and H107L variants showed elevated β-galactosidase activity in the one-hybrid systems but were normally degraded in the absence of the AC fragments remains unclear. Despite these two false-positive candidates it is evident that the one-hybrid screen is a powerful approach for identification of protein regions susceptible to proteolysis.
The usefulness of the one-hybrid system is underlined by the fact that another entirely different screening procedure resulted in the same set of stabilized σ32 variants. Very recently, Horikoshi et al. (12) screened for hyperactive σ32 proteins, arguing that stabilized sigma factors would increase transcription of heat shock genes. The initial screening involved approximately 10 times more clones (20,000 versus 2,000) than our approach. Candidates with apparently higher σ32 activity indeed were stabilized and contained the mutations L47Q, A50S, and L55Q. An additional screening using a different genetic background revealed that mutations in K51 and I54 also stabilized the sigma factor. The importance of region 2.1 for σ32 stability was thus confirmed by two independent approaches, one directly aiming at stabilized variants and the other screening for enhanced sigma factor activity.
Region 2.1 of RpoH.
Prior to our analysis, region 2 had already been implicated in σ32 degradation. Swapping of fragments between E. coli σ32 and the comparatively stable Bradyrhizobium japonicum RpoH1 protein showed that an element between residues 36 and 122 of E. coli RpoH is critical for proteolysis (2). Proteolysis did not require the N-terminal end since truncated versions were degraded normally. The one-hybrid results entirely agree with these results. AC-RpoH-WT was susceptible to proteolysis, although both termini were blocked by T25 and T18. The possibility of a role for the C terminus of σ32 in proteolysis has been excluded (28). Experiments with AC fusions to RpoH-NT and RpoH-CT further supported the hypothesis that the N-terminal region of σ32 is the target for degradation (4) (Fig. 2). Measuring Cy3-σ32 degradation by a fluorescence polarization assay demonstrated that σ32 degradation proceeds from the N terminus to the C terminus (22).
The one-hybrid approach narrowed down an important turnover element in RpoH to a few amino acids in region 2.1. Secondary structure predictions and modeling of the σ32 structure on the basis of the solved crystal structure of a fragment of the housekeeping sigma factor suggest that region 2.1 comprises two α-helices (16, 21). The mutations L47Q, A50V, and I54F are located in the first α-helix and line up on the same face of the helix (Fig. 9A).
FIG. 9.
Structure model and sequence alignment of region 2 from different proteobacteria. (A) Backbone ribbon diagram of a subfragment of E. coli σ32 constructed with the Swiss PDB viewer (9) and adapted from a previous study (21). The colors indicate the degree of sequence conservation according to the alignment in panel B. Variable residues are yellow, poorly conserved residues (pale gray background in panel B) are orange, highly conserved residues (dark gray background in panel B) are red, and entirely conserved residues (black background in panel B) are magenta. The five residues identified by two-hybrid screening are blue. (B) Alignment of RpoH region 2 from 19 bacterial species performed with CLUSTAL W (27). Members of different proteobacterial subdivisions are grouped as indicated on the left. The numbering refers to the numbering for the E. coli RpoH protein. Mutated residues are indicated by asterisks.
Stabilization of the mutated σ32 factors might have various reasons. Enhanced affinity for the RNA polymerase might sequester the sigma factor away from the degradation pathway. However, this possibility was discarded by Horikoshi et al. after they transferred two stabilizing mutations into an rpoH allele known to confer reduced affinity to RNA polymerase (12). Combination of the mutations did not change the apparently low affinity to RNA polymerase (as determined by DnaK and GroEL levels).
Another reason might be an altered interaction with components of the degradation machinery (i.e., either the DnaKJ chaperones or FtsH itself). Both reduced and increased affinity for DnaK or DnaJ might stabilize the sigma factor. A reduced interaction with the targeting factors would prevent delivery of σ32 to the FtsH protease. On the other hand, a stronger interaction with the chaperones would probably protect it from further processing. A previous peptide scanning approach demonstrated that σ32 displays multiple contact sites for DnaK (17). Strikingly, a region from H44 to P74 was found to have high affinity for DnaK. In particular, three 13-mers (L47 to R59, A50 to V62, and L53 to A65), each including at least one of our mutated amino acids, bound DnaK tightly.
Further screening of peptide libraries revealed a characteristic DnaK recognition motif comprised of a hydrophobic core of four or five residues flanked by basic residues. The most frequently occurring core residues are Leu, Ile, Val, Phe, and Tyr (23). The LIL sequence around residue 54 of σ32 (Fig. 5 and 9B) clearly resembles such a characteristic core region. It is tempting to speculate that one reason for the 10-fold-elevated stability of the B. japonicum RpoH1 protein (31) is that the equivalent region is LVT (Fig. 9B). It is interesting that mutations in residues 48, 49, 51, 52, and 53 of E. coli σ32 did not affect proteolysis (12). We propose that residues 47, 50, and 54, which line up on the same face of an α-helix in region 2.1, function as important turnover elements in the heat shock sigma factor and might be critically involved in the interaction with DnaK.
Acknowledgments
We are grateful to Daniel Ladant for providing strains and plasmids for the bacterial one-hybrid system. We thank Bernd Bukau for antisera and Amos Oppenheim and Satish Raina for plasmids. The technical help of Sylvia Balsiger and the support of Hauke Hennecke are appreciated.
This study was supported by grants from the Swiss National Foundation for Scientific Research (grant 3100A0-100713/1) and the German Research Foundation (DFG priority program SPP 1132).
REFERENCES
- 1.Arsene, F., T. Tomoyasu, A. Mogk, C. Schirra, A. Schulze-Specking, and B. Bukau. 1999. Role of region C in regulation of the heat shock gene-specific sigma factor of Escherichia coli, σ32. J. Bacteriol. 181:3552-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertani, D., A. B. Oppenheim, and F. Narberhaus. 2001. An internal region of the RpoH heat shock transcription factor is critical for rapid degradation by the FtsH protease. FEBS Lett. 493:17-20. [DOI] [PubMed] [Google Scholar]
- 3.Blaszczak, A., M. Zylicz, C. Georgopoulos, and K. Liberek. 1995. Both ambient temperature and the DnaK chaperone machine modulate the heat shock response in Escherichia coli by regulating the switch between σ70 and σ32 factors assembled with RNA polymerase. EMBO J. 14:5085-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dartigalongue, C., H. Loferer, and S. Raina. 2001. EcfE, a new essential inner membrane protease: its role in the regulation of heat shock response in Escherichia coli. EMBO J. 20:5908-5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dautin, N., G. Karimova, A. Ullmann, and D. Ladant. 2000. Sensitive genetic screen for protease activity based on a cyclic AMP signaling cascade in Escherichia coli. J. Bacteriol. 182:7060-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamer, J., H. Bujard, and B. Bukau. 1992. Physical interaction between heat shock proteins DnaK, DnaJ, and GrpE and the bacterial heat shock transcription factor σ32. Cell 69:833-842. [DOI] [PubMed] [Google Scholar]
- 7.Gamer, J., G. Multhaup, T. Tomoyasu, J. S. McCarty, S. Rüdiger, H. J. Schönfeld, C. Schirra, H. Bujard, and B. Bukau. 1996. A cycle of binding and release of the DnaK, DnaJ and GrpE chaperones regulates activity of the Escherichia coli heat shock transcription factor σ32. EMBO J. 15:607-617. [PMC free article] [PubMed] [Google Scholar]
- 8.Gross, C. A. 1996. Function and regulation of the heat shock proteins, p. 1382-1399. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 9.Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714-2723. [DOI] [PubMed] [Google Scholar]
- 10.Guisbert, E., C. Herman, C. Z. Lu, and C. A. Gross. 2004. A chaperone network controls the heat shock response in E. coli. Genes Dev. 18:2812-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herman, C., D. Thevenet, R. D'Ari, and P. Bouloc. 1995. Degradation of σ32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc. Natl. Acad. Sci. USA 92:3516-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horikoshi, M., T. Yura, S. Tsuchimoto, Y. Fukumori, and M. Kanemori. 2004. Conserved region 2.1 of Escherichia coli heat shock transcription factor σ32 is required for modulating both metabolic stability and transcriptional activity. J. Bacteriol. 186:7474-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanemori, M., K. Nishihara, H. Yanagi, and T. Yura. 1997. Synergistic roles of HslVU and other ATP-dependent proteases in controlling in vivo turnover of σ32 and abnormal proteins in Escherichia coli. J. Bacteriol. 179:7219-7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karimova, G., A. Ullmann, and D. Ladant. 2000. A bacterial two-hybrid system that exploits a cAMP signaling cascade in Escherichia coli. Methods Enzymol. 328:59-73. [DOI] [PubMed] [Google Scholar]
- 15.Ladant, D., and A. Ullmann. 1999. Bordetella pertussis adenylate cyclase: a toxin with multiple talents. Trends Microbiol. 7:172-176. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra, A., E. Severinova, and S. A. Darst. 1996. Crystal structure of a sigma 70 subunit fragment from E. coli RNA polymerase. Cell 87:127-136. [DOI] [PubMed] [Google Scholar]
- 17.McCarty, J. S., S. Rüdiger, H. J. Schönfeld, J. Schneider-Mergener, K. Nakahigashi, T. Yura, and B. Bukau. 1996. Regulatory region C of the E. coli heat shock transcription factor, σ32, constitutes a DnaK binding site and is conserved among eubacteria. J. Mol. Biol. 256:829-837. [DOI] [PubMed] [Google Scholar]
- 18.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Morita, M. T., Y. Tanaka, T. S. Kodama, Y. Kyogoku, H. Yanagi, and T. Yura. 1999. Translational induction of heat shock transcription factor σ32: evidence for a built-in RNA thermosensor. Genes Dev. 13:655-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagai, H., H. Yuzawa, and T. Yura. 1991. Interplay of two cis-acting mRNA regions in translational control of σ32 synthesis during the heat shock response of Escherichia coli. Proc. Natl. Acad. Sci. USA 88:10515-10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narberhaus, F., and S. Balsiger. 2003. Structure-function studies of Escherichia coli RpoH (σ32) by in vitro linker insertion mutagenesis. J. Bacteriol. 185:2731-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okuno, T., T. Yamada-Inagawa, K. Karata, K. Yamanaka, and T. Ogura. 2004. Spectrometric analysis of degradation of a physiological substrate σ32 by Escherichia coli AAA protease FtsH. J. Struct. Biol. 146:148-154. [DOI] [PubMed] [Google Scholar]
- 23.Rüdiger, S., L. Germeroth, J. Schneider-Mergener, and B. Bukau. 1997. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 16:1501-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Straus, D., W. Walter, and C. A. Gross. 1990. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of σ32. Genes Dev. 4:2202-2209. [DOI] [PubMed] [Google Scholar]
- 26.Straus, D. B., W. A. Walter, and C. A. Gross. 1987. The heat shock response of E. coli is regulated by changes in the concentration of σ32. Nature 329:348-351. [DOI] [PubMed] [Google Scholar]
- 27.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomoyasu, T., F. Arsene, T. Ogura, and B. Bukau. 2001. The C terminus of σ32 is not essential for degradation by FtsH. J. Bacteriol. 183:5911-5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomoyasu, T., J. Gamer, B. Bukau, M. Kanemori, H. Mori, A. J. Rutman, A. B. Oppenheim, T. Yura, K. Yamanaka, H. Niki, S. Hiraga, and T. Ogura. 1995. Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor σ32. EMBO J. 14:2551-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomoyasu, T., T. Yuki, S. Morimura, H. Mori, K. Yamanaka, H. Niki, S. Hiraga, and T. Ogura. 1993. The Escherichia coli FtsH protein is a prokaryotic member of a protein family of putative ATPases involved in membrane functions, cell cycle control, and gene expression. J. Bacteriol. 175:1344-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urech, C., S. Koby, A. B. Oppenheim, M. Münchbach, H. Hennecke, and F. Narberhaus. 2000. Differential degradation of Escherichia coli σ32 and Bradyrhizobium japonicum RpoH factors by the FtsH protease. Eur. J. Biochem. 267:4831-4839. [DOI] [PubMed] [Google Scholar]
- 32.Yura, T., M. Kanemori, and M. T. Morita. 2000. The heat shock response: regulation and function, p. 3-18. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 33.Yuzawa, H., H. Nagai, H. Mori, and T. Yura. 1993. Heat induction of σ32 synthesis mediated by mRNA secondary structure: a primary step of the heat shock response in Escherichia coli. Nucleic Acids Res. 21:5449-5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou, Y. H., X. P. Zhang, and R. H. Ebright. 1991. Random mutagenesis of gene-sized DNA molecules by use of PCR with Taq DNA polymerase. Nucleic Acids Res. 19:6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou, Y. N., N. Kusukawa, J. W. Erickson, C. A. Gross, and T. Yura. 1988. Isolation and characterization of Escherichia coli mutants that lack the heat shock sigma factor σ32. J. Bacteriol. 170:3640-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]