Abstract
MenBvac and Menjugate are safe and efficacious vaccines. The purpose of this study was to evaluate safety and immunogenicity of the combination (MenB/C) of the lyophilized active components of the conjugated group C vaccine Menjugate when reconstituted with the full liquid group B outer membrane vesicle vaccine MenBvac compared to MenBvac and Menjugate given separately. At 6-week intervals, healthy adults were given one dose of MenB/C followed by two doses of MenBvac (MenB/C group), three doses of MenBvac (MenB group), or one dose of Menjugate and two doses of placebo (MenC group). Injection site reactions were frequent in all groups. However, most reactions were short lasting and mild or moderate in intensity, and the vaccines were found to be well tolerated, with no vaccine-related serious adverse events. MenB/C was immunogenic with regard to both serogroup B and C meningococci. Both the serum bactericidal assay and the enzyme-linked immunosorbent assay analyses showed that the immune responses of the combination vaccine were similar to the immune responses of its separate components MenBvac and Menjugate for both serogroup B and C. In conclusion, the combined MenB/C vaccine is safe and immunogenic. The two vaccines do not interact negatively with each other and can easily be administered in the same syringe. The induced immune responses suggest that the combined vaccine is likely to confer protection against systemic group B disease caused by the vaccine strain as well as against group C meningococcal disease.
Systemic meningococcal disease may strike all ages but is most prevalent in infants and young children and, in some countries, also in teenagers. The clinical manifestations of systemic meningococcal disease are diverse and range from asymptomatic bacteremia to fulminant disease (cerebrospinal meningitis and/or septicemia). Septicemic cases are characterized by a rapid course and not infrequently, despite immediate treatment with effective antibiotics, a fatal outcome. Both systemic serogroup B disease and serogroup C disease have constituted a health problem in many industrialized countries during the last 50 years, with epidemics in, e.g., Norway and New Zealand and outbreaks in the United States, Canada, the United Kingdom, The Netherlands, Belgium, and Spain (1, 3, 5, 6, 11, 22). More than 80% of the cases of meningococcal diseases in developed countries are caused by serogroup B or C.
Menjugate, which is one of the new generation of safe meningococcal serogroup C protein-polysaccharide conjugate vaccines (a diphtheria toxoid mutant is used as a carrier protein for the polysaccharide), offers the advantages of a serologic response from as early as 2 months of age, higher antibody titers, long-term immunity, and induction of immunological memory (23). In the UK, virtually all serogroup C disease in childhood has been eliminated as a result of the introduction of the protein-polysaccharide conjugate group C vaccines into the infant immunization schedule (1). However, cases of serogroup B meningococcal disease remain.
MenBvac, an outer membrane vesicle (OMV) vaccine, based on a serogroup B strain (B:15:P1.7,16) representative for the epidemic starting in Norway in 1974, has been shown to be safe and immunogenic and to confer protection against group B meningococcal disease (2, 14, 15). Currently, a similar serogroup B OMV vaccine (MeNZB) produced using a strain (B:4:P1.7b,4) representative of the epidemic in New Zealand is used to control the ongoing epidemic in that country (20).
Serogroup B strains similar to that responsible for the Norwegian epidemic are still causing disease in many countries. Thus, a significant proportion of serogroup B and C cases might be prevented by using a combination of MenBvac and a serogroup C conjugate vaccine (4). MenBvac and Menjugate are easy to mix. MenBvac is a liquid formulation, and the active components of Menjugate are in lyophilized presentation, which can be reconstituted with MenBvac prior to administration. The aim of this study was to compare the immunogenicity and reactogenicity of a combination of MenBvac and lyophilized group C-conjugated vaccine (MenB/C) with that of MenBvac only or Menjugate only.
MATERIALS AND METHODS
Vaccines.
MenBvac, manufactured by the Norwegian Institute of Public Health (NIPH), is prepared from a B:15:P1.7,16 meningococcal strain (44/76) by fermentor growth and extraction of the OMVs with the detergent deoxycholate. OMVs are purified by ultracentrifugation and adsorbed to aluminum hydroxide. One dose (0.5 ml) of MenBvac contains 25 μg outer membrane protein and 1.67 mg aluminum hydroxide (8).
Menjugate, manufactured by Chiron Vaccines, consists of meningococcal group C oligosaccharides conjugated to diphtheria toxin cross-reacting material (CRM197). One dose (0.5 ml) of Menjugate, when reconstituted with aluminum hydroxide adjuvant, contained 10 μg of meningococcal C oligosaccharide, 12.5 to 25.0 μg diphtheria toxoid (CRM197), and 1 mg aluminum hydroxide.
Aluminum hydroxide was used as a placebo to minimize differences in appearance. One dose (0.5 ml) of placebo contained 1.67 mg aluminum hydroxide in vaccine solvent (same as for MenBvac).
When the vaccines were combined, the lyophilized active components of Menjugate were reconstituted with the complete MenBvac immediately before administration, and the combined MenB/C vaccine was administered in one syringe.
Administration.
Three injections were given to healthy adults, at weeks 0, 6, and 12. The subjects were randomly assigned 2:1:1 to receive one dose of the combined MenB/C vaccine followed by two doses of MenBvac (MenB/C group), three doses of MenBvac (MenB group), or one dose of Menjugate (as in conventional clinical use in adults) and two doses of placebo (MenC group). All vaccines were administered intramuscularly in the deltoid region of the nondominant arm.
Vaccinees.
Healthy adults, 18 to 35 years old, who had given written informed consent prior to study entry were eligible for participation in the study. Participants were excluded if they had experienced a hypersensitivity reaction following previous vaccinations, had an acute or chronic systemic illness, or had received immunosuppressive therapy or blood products within 3 months prior to vaccination. Earlier immunization with meningococcal B or C vaccine of any kind, previous suspected disease caused by Neisseria meningitidis, or close contact with a patient with meningococcal disease were also reasons for exclusions.
Immunogenicity.
Blood samples (10 ml whole blood) for serological assays were collected by venipuncture at the time of the first, second, and third dose, 6 weeks after the third dose, and about 1 year after vaccination. The blood samples were fractionated, and the serum was aliquoted and stored at −20°C.
SBA.
The serum bactericidal assay (SBA) measures functional antibodies (antibodies with bactericidal activity) acquired through vaccination against or through carriage of N. meningitidis.
The serogroup B SBA analyses were performed at NIPH, using 25% human complement from one donor without detectable antibodies against group B. The microtiter plates were incubated for 60 min at 37°C. The strain 44/76-SL (B:15:P1.7,16) was used as the target strain (15). The number of CFU was counted by visual inspection of the wells, using a magnifying glass. Twofold serum dilutions starting at 1:2 were tested, and titers were reported as the reciprocal value of the highest serum dilution resulting in ≥50% killing of bacteria (10, 15).
In the serogroup C SBA, performed at Chiron Vaccines, sera were tested at twofold dilutions starting at 1:4. The meningococcal serogroup C strain C11 was used as the target strain (a C:16:P1.7-1,1 strain). Human serum without detectable antibodies against meningococci group C was used as the complement source as described before (12). Bacterial survival in the final reaction mixture was measured after incubation for 30 min at 37°C (18).
ELISA.
The IgG antibodies against serogroup B OMVs and against C polysaccharide were analyzed by enzyme-linked immunosorbent assay (ELISA). The OMV-ELISA was performed as described by Rosenqvist et al. (17). The antigen for coating of the microtiter plates was OMVs from strain 44/76 prepared as for vaccine production (8). In the C polysaccharide ELISA, sera were diluted in thiocyanate buffer to favor detection of high-avidity antibodies (9).
Safety monitoring.
After each vaccination, the subjects were asked to report if they felt unwell or experienced any local or systemic adverse event. The subjects were instructed to complete a diary card to describe local reactions at the injection site (pain, redness, swelling, other) and systemic reactions (headache, malaise, fever [i.e., axillary temperature ≥ 38°C], other) during the first and second 24-hour period after each dose and 7 days after each dose. The vaccinees were also asked to record the axillary temperature if they felt feverish. Local pain and other reactions after the first week and medication taken during the study were recorded in interviews performed at the planned visit days. All serious adverse events occurring throughout the study were collected.
Ethics.
This study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization guideline for Good Clinical Practice and other local legal and regulatory requirements. The trial was approved by the Regional Committee for Ethics and Medical Research and the Norwegian Medicine Agency. Before enrollment, all subjects signed informed consent.
Statistical methods.
Analyses were done descriptively. The primary immunogenicity variables were the geometric mean titers (GMTs) of antibodies against serogroup B and C meningococci as measured by SBA. At each time point, GMTs and 95% confidence intervals (CIs) were calculated using log-transformed (base 10) titers. These were obtained from a one-way analysis of variance.
The proportion of subjects with bactericidal titers ≥ 4 against serogroup B and the proportion of subjects with bactericidal titers ≥ 8 against serogroup C and associated 95% Clopper-Pearson CIs were computed for each vaccine group.
The incidence of prespecified and unspecified local and systemic reactions as well as other adverse events was calculated by maximum severity after each vaccination. All statistical analyses were performed using SAS version 6.12 or higher (SAS Institute, Cary, NC).
RESULTS
This study was designed as a single-blinded, randomized, and prospective investigation of immunogenicity and reactogenicity of MenBvac and Menjugate given in combination or separately to healthy adults.
A total of 73 subjects were given the first vaccine dose, 35 in the MenB/C group, 19 in the MenB group, and 19 in the MenC group. Of these, 66 received the second dose, 31 in the MenB/C group, 16 in the MenB group, and 19 in the MenC group. The third dose was given to 64 subjects, 30 in the MenB/C group, 15 in the MenB group, and 19 in the MenC group.
Two subjects in the MenB/C group withdrew consent (one subject due to “problems with the vaccine” and one subject due to pain), and three were excluded after the first dose since they were not fulfilling the inclusion criteria. In the MenB group, one subject withdrew consent (not related to study vaccine) and three were excluded (two due to having previously received meningococcal vaccine and one due to problems with blood collection at the first blood sampling).
The treatment groups were similar regarding age; the mean age was 23.3 years (range, 20 to 32) in the MenB/C group, 22.1 (range, 18 to 29) in the MenB group, and 21.8 (range, 20 to 28) in the MenC group. Also, the distributions of gender were similar between the treatment groups: 71% in the MenB/C group, 79% in the MenB group, and 74% in the MenC group were females.
Immunogenicity. (i) SBA geometric mean antibody titers (GMTs), serogroup B.
At baseline, the SBA GMT against serogroup B ranged from 2.9 in the MenB group to 4.8 in the MenB/C group. The SBA GMTs against serogroup B increased after the first, second, and third injection in both the MenB and the MenB/C groups, reaching the highest level at week 18 (6 weeks after the third dose) (Table 1). At week 18, the GMT had increased to 29.1 (95% CI, 17.3 to 49.0) for MenB/C and to 14.5 for MenB (95% CI, 6.8 to 30.7). At week 52, the GMT was 16.4 (95% CI, 9.8 to 27.5) for MenB/C and 7.3 (95% CI, 3.6 to 14.6) for MenB (Table 1). Although the SBA levels were approximately twice as high in the MenB/C group as in the MenB group, the difference was not statistically significant, as indicated by the overlapping confidence intervals. The GMT in the MenC group remained stable during the study.
TABLE 1.
SBA geometric mean antibody titers (GMT) against serogroup B before and after immunization with meningococcal vaccinea
| Study time point | No. of subjects in indicated group; GMT (95% CI)
|
||
|---|---|---|---|
| MenB/C | MenB | MenC | |
| Prestudy | 32; 4.8 (2.9-7.9) | 15; 2.9 (1.4-6.1) | 19; 3.1 (1.9-5.2) |
| 6 weeks after 1st dose | 32; 10.8 (6.3-18.5) | 15; 8.8 (4.0-19.2) | 19; 3.1 (1.8-5.2) |
| 6 weeks after 2nd dose | 30; 14.6 (8.9-23.9) | 15; 10.6 (5.2-21.2) | 19; 2.4 (1.5-3.7) |
| 6 weeks after 3rd dose | 29; 29.1 (17.3-49.0) | 14; 14.5 (6.8-30.7) | 19; 2.2 (1.4-3.2) |
| 1 year after 1st dose | 27; 16.4 (9.8-27.5) | 15; 7.3 (3.6-14.6) | 17; 4.2 (2.8-6.1) |
Subjects in the MenB/C group received one dose of MenB/C followed by two doses of MenBvac, subjects in the MenB group received three doses of MenBvac, and subjects in the MenC group received one dose of Menjugate followed by two doses of placebo (aluminum hydroxide).
(ii) SBA geometric mean antibody titers, serogroup C.
At baseline, the SBA GMTs against serogroup C were comparable in the MenB/C group (7.3) and the MenC group (6.9), while GMT was somewhat lower in the MenB group (4.9). The highest level of SBA GMTs against serogroup C was obtained at week 6 (Table 2). At this time point, the GMTs had increased to 99.5 (95% CI, 52.3 to 189.4) for MenB/C and to 119.6 (95% CI, 51.9 to 275.7) for MenC. Also, in the MenB group a small increase to 7.3 (95% CI, 4.7 to 11.6) was observed at week 6. At week 52, the GMT was 38.8 (95% CI, 20.2 to 74.3) for MenB/C and 44.0 (95% CI, 19.4 to 100.0) for MenC (Table 2). The differences between the MenB/C group and the MenC group were not statistically significant, as indicated by the overlapping confidence intervals.
TABLE 2.
SBA geometric mean antibody titers (GMT) against serogroup C before and after immunization with meningococcal vaccinea
| Study time point | No. of subjects in indicated group; GMT (95% CI)
|
||
|---|---|---|---|
| Men B/C | Men B | Men C | |
| Prestudy | 32; 7.3 (4.7-11.4) | 15; 4.9 (3.2-7.4) | 19; 6.9 (3.9-12.1) |
| 6 weeks after 1st dose | 32; 99.5 (52.3-189.4) | 15; 7.3 (4.7-11.6) | 19; 119.6 (51.9-275.7) |
| 6 weeks after 2nd dose | 30; 73.4 (39.3-137.0) | 15; 6.8 (4.0-11.6) | 19; 93.6 (42.7-205.1) |
| 6 weeks after 3rd dose | 29; 79.3 (44.8-140.3) | 14; 7.2 (4.2-12.3) | 19; 81.9 (40.5-165.8) |
| 1 year after 1st dose | 27; 38.8 (20.2-74.3) | 15; 4.2 (2.7-6.6) | 17; 44.0 (19.4-100.0) |
Subjects in the MenB/C group received one dose of MenB/C followed by two doses of MenBvac, subjects in the MenB group received three doses of MenBvac, and subjects in the MenC group received one dose of Menjugate followed by two doses of placebo (aluminum hydroxide).
(iii) Proportion of subjects with an SBA titer ≥ 4 against serogroup B.
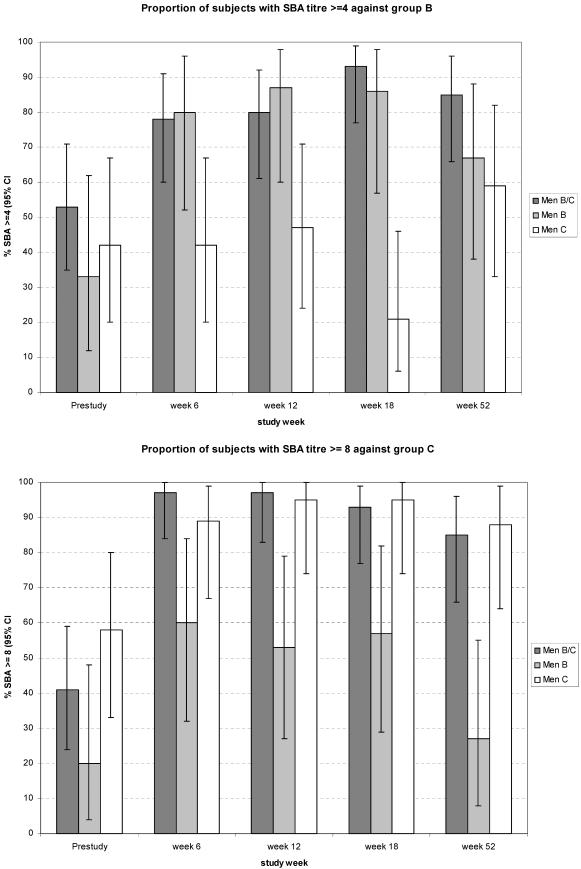
Before vaccination the proportion of subjects with SBA titers ≥ 4 against the serogroup B strain was as high as 53% (95% CI, 35 to 71) for MenB/C, 33% (95% CI, 12 to 62) for MenB, and 42% (95% CI, 20 to 67) for MenC. By week 18, the corresponding figures were 93% (95% CI, 77 to 99), 86% (95% CI, 57 to 98), and 21% (95% CI, 6 to 46), respectively. At week 52, the proportions remained high, being 85% (95% CI, 66 to 96) for MenB/C, 67% (95% CI, 38 to 88) for MenB, and 59% (95% CI, 33 to 82) for MenC (Fig. 1, top panel).
FIG. 1.
Proportion of subjects with an SBA titer ≥ 4 against serogroup B (top) and proportion of subjects with an SBA titer ≥ 8 against serogroup C (bottom). Subjects in the MenB/C group received one dose of MenB/C at week 0 followed by two doses of MenBvac (one at week 6 and one at week 12). Subjects in the MenB group received three doses of MenBvac (at weeks 0, 6, and 12). Subjects in the MenC group received one dose of Menjugate at week 0 and placebo (aluminum hydroxide) at weeks 6 and 12. At weeks 0 and 6, serum samples were available from 32 subjects in the MenB/C group, 15 subjects in the MenB group, and 19 subjects in the MenC group. At week 12 the corresponding figures were 30, 15, and 19, and at week 18 they were 29, 14, and 19, respectively. At week 52 the corresponding figures were 27, 15, and 17, respectively.
(iv) Proportion of subjects with an SBA titer ≥ 8 against serogroup C.
Before vaccination, the proportion of subjects with SBA titers ≥ 8 against serogroup C was 41% (95% CI, 24 to 59) for MenB/C, 20% (95% CI, 4 to 48) for MenB, and 58% (95% CI, 33 to 80) for MenC. At week 6, the corresponding figures were 97% (95% CI, 84 to 100), 60% (95% CI, 32 to 84), and 89% (95% CI, 67 to 99), respectively, and they remained at this level at weeks 12 and 18. At week 52, the proportions were 85% (95% CI, 66 to 96) for MenB/C, 27% (95% CI, 8 to 55) for MenB, and 88% (95% CI, 64 to 99) for MenC (Fig. 1, bottom panel).
(v) ELISA geometric mean antibody concentrations (GMCs).
The analyses of ELISA GMCs confirmed that the combined MenB/C vaccine was as immunogenic regarding responses to serogroup B and to serogroup C, respectively, as when the respective vaccine (MenBvac or Menjugate) was given. At baseline GMCs against serogroup B were similar in the three vaccine groups and ranged from 30 to 45 U/ml. At week 18, the GMT had increased to 1,214 in the MenB/C group (95% CI, 960 to 1,535) and to 565 (95% CI, 401 to 797) in the MenB group while no increase was observed in the MenC group. The difference between the MenB/C group and the MenB group was statistically significant, as indicated by the nonoverlapping confidence intervals. Also, baseline GMCs against serogroup C were similar in the three vaccine groups and ranged from 0.3 to 0.4 U/ml. At week 6, the GMT had increased to 10 in the MenB/C group (95% CI, 6.04 to 17) and to 17 (95% CI, 8.91 to 32) in the MenC group while no increase was observed in the MenB group. The difference between the MenB/C group and the MenC group was not statistically significant, as indicated by the overlapping confidence intervals.
Safety.
The occurrence of local and systemic reactions as well as other adverse events was highest in the group receiving MenB/C. In comparisons of the MenB group with the MenC group, the incidence of local and systemic reactions was higher in the MenB group, which may in part be explained by the fact that the subjects in the MenC group received placebo at the second and third vaccination. The incidences of reactions, both local and systemic, after the first, second, and third dose in the MenB/C and the MenB group were similar. In the MenC group, the incidence of reactions was lower after the second and third vaccination when these subjects received placebo. No serious adverse event related to the study treatment was reported.
Injection site reactions.
Injection site reactions (pain, redness, and swelling) were very common. Pain was reported by all subjects in all three treatment groups. Pain of severe intensity was reported by 20% of the subjects in the MenB/C group (compared with 11% in the MenB group and 0% in the MenC group) (Table 3). However, in all groups, the intensity of pain was lower after the second and third vaccination compared with after the first. Seven days after the first vaccination, pain was reported in 29%, 44%, and 11% of the subjects in the MenB/C, MenB, and MenC groups, respectively. After the third vaccination the corresponding proportions had decreased to 13%, 7%, and 0%, respectively (data not shown). Local redness or local swelling was also reported by a majority (56 to 66%) of the subjects receiving MenB/C or MenB compared with 11 to 32% of the subjects receiving MenC. For redness and swelling, most of the reactions (89 to 100% in the three groups) were of mild or moderate intensity (Table 3).
TABLE 3.
Local (injection site) reactions
| Reaction | Intensityd | No. (%) of subjects reporting the specified reaction
|
||
|---|---|---|---|---|
| Men B/C (n = 35) | MenB (n = 18) | Men C (n = 19) | ||
| Pain | 1st inj.—all | 35 (100) | 18 (100) | 18 (95) |
| 1st inj.—severe | 4 (11) | 0 | 0 | |
| 2nd inj.—all | 31 (100)b | 16 (100) | 17 (89)c | |
| 2nd inj.—severe | 3 (10) | 1 (6) | 0 | |
| 3rd inj.—all | 30 (100)b | 15 (100) | 14 (74)c | |
| 3rd inj.—severe | 1 (3) | 1 (7) | 0 | |
| Total any inj.—all | 35 (100) | 18 (100) | 19 (100) | |
| Total any inj.—severea | 7 (20) | 2 (11) | 0 | |
| Redness | 1st inj.—all | 13 (37) | 8 (44) | 1 (5) |
| 1st inj.—severe | 1 (3) | 0 | 0 | |
| 2nd inj.—all | 9 (29)b | 8 (50) | 1 (5)c | |
| 2nd inj.—severe | 0 | 0 | 0 | |
| 3rd inj.—all | 10 (33)b | 5 (33) | 0c | |
| 3rd inj.—severe | 2 (7) | 0 | 0 | |
| Total any inj.—all | 20 (57) | 11 (61) | 2 (11) | |
| Total any inj.—severea | 3 (9) | 0 | 0 | |
| Swelling | 1st inj.—all | 16 (46) | 7 (39) | 5 (26) |
| 1st inj.—severe | 3 (9) | 0 | 0 | |
| 2nd inj.—all | 18 (58)b | 4 (25) | 3 (16)c | |
| 2nd inj.—severe | 1 (3) | 1 (6) | 0 | |
| 3rd inj.—all | 14 (47)b | 6 (40) | 1 (5)c | |
| 3rd inj.—severe | 1 (3) | 0 | 0 | |
| Total any inj.—all | 23 (66) | 10 (56) | 6 (32) | |
| Total any inj.—severea | 4 (11) | 1 (6) | 0 | |
Most severe across all injections.
Subjects in the MenB/C group received MenBvac only at the 2nd and 3rd dose.
Subjects in the MenC group received placebo [Al(OH)3] at the 2nd and 3rd dose.
inj., injection; all, all intensity levels.
Systemic reactions.
Most systemic reactions were mild or moderate in severity and resolved within a few days. In the three groups, headache was reported by 16 to 29% of the subjects and malaise by 26 to 50% (Table 4). Fever was reported by 2 of 35 vaccinees in the MenB/C group, by none in the MenB group, and by 1 of 19 in the MenC group (Table 4).
TABLE 4.
Systemic reactions
| Reaction | Temperature or intensityd | No. (%) of subjects reporting the specified reaction
|
||
|---|---|---|---|---|
| MenB/C (n = 35) | MenB (n = 18) | MenC (n = 19) | ||
| Headache | 1st inj.—all | 7 (20) | 3 (17) | 2 (11) |
| 1st inj.—severe | 1 (3) | 0 | 0 | |
| 2nd inj.—all | 2 (6)b | 2 (13) | 1 (5)c | |
| 2nd inj.—severe | 0 | 0 | 0 | |
| 3rd inj.—all | 6 (20)b | 3 (20) | 1 (5)c | |
| 3rd inj.—severe | 0 | 0 | 0 | |
| Total any inj.—all | 10 (29) | 5 (28) | 3 (16) | |
| Total any inj.—severea | 1 (3) | 0 | 0 | |
| Malaise | 1st inj.—all | 6 (17) | 4 (22) | 4 (21) |
| 1st inj.—severe | 0 | 1 (6) | 1 (5) | |
| 2nd inj.—all | 8 (26)b | 5 (31) | 1 (5)c | |
| 2nd inj.—severe | 0 | 0 | 0 | |
| 3rd inj.—all | 7 (23)b | 3 (20) | 1 (5)c | |
| 3rd inj.—severe | 2 (7) | 1 (7) | 0 | |
| Total any inj.—all | 11 (31) | 9 (50) | 5 (26) | |
| Total any inj.—severea | 2 (6) | 1 (6) | 1 (5) | |
| Axillary temp | 1st inj. 38-≤39°C | 1 (3) | 0 | 1 (5) |
| ≥38°C | 2nd inj. 38-≤39°C | 0b | 0 | 0c |
| 3rd inj. 38-≤39°C | 1 (3)b | 0 | 0c | |
| Total any inj. 38-≤39°C | 2 (6) | 0 | 1 (5) | |
| Total any inj. >39a | 0 | 0 | 0 | |
Most severe across all injections.
Subjects in the MenB/C group received MenBvac only at the 2nd and 3rd dose.
Subjects in the MenC group received placebo [Al(OH)3] at the 2nd and 3rd dose.
inj., injection; all, all intensity levels.
Other adverse events.
In addition to the prespecified reactions recorded in the diary, a total of 10 subjects reported 15 adverse events (6 subjects in the MenB/C group, 3 subjects in the MenB group, and 1 subject in the MenC group) which were classified by the investigator as possibly, probably, or definitely related to the treatment. These included eye pain, diarrhea, nausea, influenza-like illness, pain, nasopharyngitis, neck stiffness, headache, and dizziness. None of the related events was classified as severe.
DISCUSSION
A combination of the two safe and efficacious vaccines, the licensed Menjugate group C vaccine and MenBvac shown to confer protection in an efficacy trial performed during the Norwegian group B epidemic (2), is one way to meet the need for simultaneous protection against both group B and C meningococcal disease. It has earlier been demonstrated that MenBvac induces immune responses to different serogroup B strains (7, 21). Results from mouse studies indicate that an even broader cross-reactivity may be elicited by including OMVs from different group B strains in the vaccine (13).
In contrast to earlier B/C combination vaccines, in which the group C component had been based on plain polysaccharides, this combination vaccine was based on a protein-conjugated group C vaccine conferring the advantage of higher and longer-lasting antibody titers. The immune response elicited by MenB/C was evaluated by two serological methods, SBA and ELISA. SBA is currently the best test to indicate protective immunity against meningococci. The results from this study showed that combined MenB/C vaccination was immunogenic with regard to both serogroup B and C and that the immune responses of the combination vaccine were similar to the immune responses of its separate components MenBvac and Menjugate for both serogroup B and C.
No negative interaction with regard to immunogenicity or safety was observed when the combined vaccine was given. Instead, our ELISA results showed that the combination of these two vaccines enhanced the elicited immune responses. Such a trend, although not being statistically significant, was also observed for the SBA analyses.
Adults are known to have higher prevaccination titers than younger individuals due to natural exposure to the antigens. Thus, the proportion of subjects with SBA titers ≥ 4 against serogroup B and the proportion of subjects with SBA titers ≥ 8 against serogroup C were already high before vaccination (33 to 53% for serogroup B and 20 to 58% for serogroup C). After vaccination with MenB/C or MenBvac, approximately 90% of the subjects achieved this antibody titer against serogroup B and the same was the case for group C among those receiving MenB/C or Menjugate.
The increase in the proportion of subjects in the MenB group with SBA titers ≥ 8 against serogroup C (from 20% at baseline to 60% 6 weeks after the first dose, returning to 27% after 1 year) may indicate that MenBvac immunization results in cross-reactivity by inducing antibodies against protein antigens in the group C strain used in the SBA.
This study was conducted among adults, since it was the first time this vaccine combination (given in one syringe) was tested. Since the licensed vaccine Menjugate is widely used and approximately 180,000 subjects have received MenBvac in clinical trials, the proposed schedule for the combination might be used down to toddler age. MenBvac has been shown to be safe and immunogenic also in infants and toddlers (19, 21). It is reasonable to believe that in younger age groups the safety and reactogenicity profile, as well as the immune response of this combination, will be similar to that observed among adults in this study. In infants, more than one dose is needed not only for the serogroup B vaccine but also for serogroup C-conjugated vaccines. Furthermore, the combination vaccine could be given at the first or at any subsequent dose of the OMV immunization schedule. Thus, it is a great advantage to be able to combine these components and thereby induce broader protection with the same number of injections.
Occurrence of local and systemic reactions is very common for intramuscularly administered aluminum hydroxide-containing vaccines, and therefore MenBvac was expected to have such a profile (14). The most common adverse reaction observed in this study was local pain, which is consistent with earlier data (16). Most of the local and systemic reactions were of mild or moderate intensity. Among those subjects who experienced local reactions of severe intensity after the first vaccine dose, local reactions of severe intensity were not frequently reported after the subsequent doses. Adverse events other than local and systemic reactions were not commonly reported, and no serious adverse events were assessed by the investigators as possibly related to the administration of MenBvac or Menjugate. Although all three vaccine regimens were reactogenic, with MenBvac being more reactogenic than Menjugate, and with the highest occurrence of reactions and adverse events in the group receiving combined MenB/C vaccination, it can be concluded that, considering the severity of systemic meningococcal disease, the benefits of immunization with combined MenB/C vaccine by far override the risks in an epidemic setting or a local outbreak situation if both serogroup B and C are included in the clinical picture.
In conclusion, this study showed that combined immunization with MenB/C vaccine is safe and likely to confer protection against meningococcal disease in epidemics and outbreaks caused by serogroup C strains and serogroup B strains similar to the vaccine strain. By using different serogroup B OMV vaccines in combination with the C conjugate vaccine, protection against other serogroup B strains may also be obtained.
Acknowledgments
This study was sponsored by NIPH and Chiron Vaccines.
We thank Marit Meum, Aslaug Flydal, and Kirsten Konsmo at NIPH, Annette Karsten and Mark Wakefield at Chiron Vaccines and Karin Gewert at Clinical Data Care for skillful assistance.
REFERENCES
- 1.Bethell, D., and A. J. Pollard. 2002. Meningococcal vaccines. Expert Rev. Vaccines 1:75-84. [DOI] [PubMed] [Google Scholar]
- 2.Bjune, G., E. A. Høiby, J. K. Grønnesby, O. Arnesen, J. H. Fredriksen, A. Halstensen, et al. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. [DOI] [PubMed] [Google Scholar]
- 3.Cano, R., A. Larrauri, S. Mateo, B. Alcalá, C. Salcedo, and J. A. Vázquez. 2004. Impact of the meningococcal C conjugate vaccine in Spain: an epidemiological and microbiological decision. Euro Surveill. 9:5-6. [DOI] [PubMed] [Google Scholar]
- 4.Connolly, M., N. Noah, and the European Meningitis Surveillance Group. 1999. Is group C meningococcal disease increasing in Europe? A report of surveillance of meningococcal infection in Europe 1993-6. Epidemiol. Infect. 122:41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Wals, P., L. Hertoghe, I. Borlee-Grimee, S. De Maeyer-Cleempoel, G. Reginster-Haneuse, A. Dachy, et al. 1981. Meningococcal disease in Belgium. Secondary attack rate among household, day-care nursery and pre-elementary school contacts. J. Infect. 3(Suppl. 1):53-61. [DOI] [PubMed] [Google Scholar]
- 6.Dyet, K., A. Devoy, R. McDowell, and D. Martin. 2005. New Zealand's epidemic of meningococcal disease described using molecular analysis: implications for vaccine delivery. Vaccine 23:2228-2230. [DOI] [PubMed] [Google Scholar]
- 7.Feiring, B., L. M. Næss, J. Fuglesang, E. Rosenqvist, M. A. R. Bergsaker, A. Haugan, et al. 2004. Antibody responses against homologous and heterologous meningococcal serogroup B strains after a fourth dose of a meningococcal serogroup B OMV vaccine (MenBvac). Abstracts of the 14th International Pathogenic Neisseria Conference, Milwaukee, Wis., p. 154. (www.neisseria.org.)
- 8.Fredriksen, J. H., E. Rosenqvist, E. Wedege, K. Bryn, G. Bjune, L. O. Frøholm, et al. 1991. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH (Natl. Inst. Public Health) Ann. 14:67-79. [PubMed] [Google Scholar]
- 9.Granoff, D. M., S. E. Maslanka, G. M. Carlone, B. D. Plikaytis, G. F. Santos, A. Mokatrin, et al. 1998. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin. Diagn. Lab. Immunol. 5:479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Høiby, E. A., E. Rosenqvist, L. O. Frøholm, G. Bjune, B. Feiring, H. Nøkleby, et al. 1991. Bactericidal antibodies after vaccination with the Norwegian meningococcal serogroup B outer membrane vesicle vaccine: a brief survey. NIPH (Natl. Inst. Public Health) Ann. 14:147-155. [PubMed] [Google Scholar]
- 11.Martin, D. R., S. J. Walker, M. G. Baker, and D. R. Lennon. 1998. New Zealand epidemic of meningococcal disease identified by a strain with phenotype B:4:P1.4. J. Infect. Dis. 177:497-500. [DOI] [PubMed] [Google Scholar]
- 12.Maslanka, S. E., L. L. Gheesling, D. E. Libutti, K. B. J. Donaldson, H. S. Harakeh, J. K. Dykes, et al. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin. Diagn. Lab. Immunol. 4:156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Næss, L. M., T. Tangen, A. C. Kristoffersen, I. S. Aaberge, E. A. Høiby, and E. Rosenqvist. 2002. Immunogenicity of a combination of two different outer membrane protein based meningococcal group B vaccines. In D. A. Caugant and E. Wedege (ed.), Abstracts of the 13th International Pathogenic Neisseria Conference, Oslo, Nordberg Aksidenstrykkeri AS, Oslo, Norway, p. 284.
- 14.Nøkleby, H., and B. Feiring. 1991. The Norwegian meningococcal group B outer membrane vesicle vaccine: side effects in phase II trials. NIPH (Natl. Inst. Public Health) Ann. 14:95-102. [PubMed] [Google Scholar]
- 15.Rosenqvist, E., E. A. Høiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, et al. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenqvist, E., E. A. Høiby, G. Bjune, A. Aase, A. Halstensen, A. K. Lehmann, et al. 1998. Effect of aluminium hydroxide and meningococcal serogroup C capsular polysaccharide on the immunogenicity and reactogenicity of a group B Neisseria meningitidis outer membrane vesicle vaccine. Dev. Biol. Stand. 92:323-333. [PubMed] [Google Scholar]
- 17.Rosenqvist, E., S. Harthug, L. O. Frøholm, E. A. Høiby, K. Bøvre, and W. D. Zollinger. 1988. Antibody responses to serogroup B meningococcal outer membrane antigens after vaccination and infection. J. Clin. Microbiol. 26:1543-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos, G. F., R. R. Deck, J. Donnelly, W. Blackwelder, and D. M. Granoff. 2001. Importance of complement source in measuring meningococcal bactericidal titers. Clin. Diagn. Lab. Immunol. 8:616-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sexton, K., D. Lennon, P. Oster, I. Aaberge, D. Martin, S. Reid, et al. 2004. Proceedings of the Meningococcal Vaccine Strategy World Health Organization Satellite Meeting, 10 March 2004, Auckland, New Zealand. N. Z. Med. J., vol. 117, no. 1200, ISSN 1175 8716. [PubMed]
- 20.Sexton, K., D. Lennon, P. Oster, S. Crengle, D. Martin, K. Mulholland, et al. 2004. The New Zealand meningococcal vaccine strategy: a tailor-made vaccine to combat a devastating epidemic. N. Z. Med. J., vol. 117, no. 1200, ISSN 1175 8716. [PubMed]
- 21.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, et al. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines. A randomized controlled trial in Chile. JAMA 281:1520-1527. [DOI] [PubMed] [Google Scholar]
- 22.Wenger, J. D., and B. A. Perkins. 1998. Patterns in the emergence of epidemic meningococcal disease, p. 125-136. In W. M. Scheld, D. Armstrong, and J. M. Hughes (ed.), Emerging infectious diseases, vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 23.Zhang, Q., E. Pettitt, R. Burkinshaw, G. Race, L. Shaw, and A. Finn. 2002. Mucosal immune responses to meningococcal conjugate polysaccharide vaccines in infants. Pediatr. Infect. Dis. J. 21:209-213. [DOI] [PubMed] [Google Scholar]