Abstract
An immunochromatographic test for rapid detection of IgM antibodies in patients with acute hepatitis E infection was developed utilizing the well-characterized recombinant protein EP2.1 and monoclonal antibody 4B2. The new rapid test based on a novel reverse-flow technology was able to generate a positive result within 2 to 3 min. Our study showed that this test was able to detect anti-HEV IgM antibodies in 96.7% of the patient samples tested (n = 151) while maintaining an excellent specificity of 98.6% with samples from various patient or healthy control groups (total n = 208). Furthermore, this rapid test gave a good specificity of 90.9% when tested with rheumatoid factor (RF)-positive sera (RF value of ≤850 IU/ml; n = 11) although a higher concentration of RF in samples might cause cross-reactivity. The new test has a good agreement of 97.2% with a kappa value of 0.943 when compared with a reference enzyme-linked immunosorbent assay. The positive predictive value and the negative predictive value for the rapid test thus reached 98.0 and 97.6%, respectively. This is the first rapid, point-of-care test for hepatitis E and will be especially useful for the diagnosis of acute hepatitis E virus infection in field and emergency settings and in resource-poor countries.
Hepatitis E is known as enterically transmitted non-A non-B hepatitis, and the etiological agent for this disease has been well established as an nonenveloped, positive-sense, single-stranded RNA virus named hepatitis E virus (HEV) (16, 18, 9). Although the disease is a self-limited one with a mortality rate of 1 to 3% in general adult populations, hepatitis E infection in pregnant women can take more severe forms, with a case fatality rate up to 20%, especially during the third trimester (10). Because the causative HEV is excreted in the feces of infected individuals, contaminated water and food supplies can provoke major outbreaks and are assumed to be the primary source for infections. It is therefore not surprising that epidemics of this waterborne hepatitis have occurred frequently in Central and South Asia, North and West Africa, the Middle East, and Mexico, in geographic areas where fecal contamination of drinking water is common. However, increasing evidence suggests that sporadic infections have occurred in areas where traditionally the disease is nonendemic, including the Untied States, Japan, and Europe, and thus the disease might be more widespread than previously recognized (3). Furthermore, existing studies showed that the prevalence of immunoglobulin G (IgG) antibodies to HEV were much lower than expected in areas of endemicity but higher than anticipated in regions where the disease is nonendemic (4). Many individuals are therefore susceptible to the infection. In this respect, hepatitis E is increasingly an important public health concern of global significance (20).
The recent outbreaks of HEV in Chad and Sudan provide a reminder of this concern (http://www.who.int/csr/don/2004_09_28/en/print.html and http://www.who.int/csr/don/2004_09_27a/en/). Over a 4-month period, 6,861 suspected hepatitis E cases and 87 deaths occurred in Sudan, and 1,442 cases and 46 deaths occurred in Chad, with the highest incidence in overcrowded refugee camps. These figures highlight the need not only for sensitive and specific tests but also for rapid diagnostic tools to enable decision making at the point of care. In a situation where early warning is critical, rapid point-of-care tests will greatly aid the prompt identification of patients and the source of infections, which can, in turn, facilitate outbreak management in remote areas where laboratory facilities are not readily available.
There are currently four major recognized genotypes of HEV, but all appear to fall into one single serotype, regardless of the country of origin or genotype of the virus (3). Serological detection of antibodies to the virus even of different origins is thus possible by relying on major epitopes derived from the open reading frames (ORFs) of the virus. For example, recombinant proteins derived from ORF2 and ORF3 are currently being used for this purpose in commercial kits (Genelabs Diagnostics, Singapore, Republic of Singapore). However, ORF2-expressed proteins are believed to be more sensitive in detecting anti-HEV IgM and IgG antibodies. In particular, the C-terminal end of the ORF2 region (ORF2.1; amino acids 394 to 660) was previously found to contain a highly conserved conformational epitope (17) and to be suitable for the specific and sensitive detection of anti-HEV IgG antibody in enzyme-linked immunosorbent assay (ELISA) (1). In addition, a previous study identified murine monoclonal antibodies (MAbs) that reacted exclusively with the conformational epitope of ORF2.1 (17). Such MAbs may provide useful reagents for the development of diagnostic tests. In this study, we thus investigate the utility of a recombinant form of ORF2.1 (ET2.1) expressed in Escherichia coli together with the ORF2.1-specific MAb 4B2 in developing a rapid immunochromatographic test with the aim of providing a novel tool for the control and management of the disease, especially in remote areas.
MATERIALS AND METHODS
Recombinant protein and MAbs.
The materials and methods used for obtaining the recombinant protein ET2.1 have been described in detail elsewhere (12, 1, 17). Briefly, ET2.1 was constructed as a fusion protein containing the ORF2.1 fragment (amino acids 394 to 660) from the capsid protein of HEV (Chinese strain) and a six-histidine tag. This fusion protein was expressed in E. coli, subsequently solubilized in 5 M urea, and purified in the presence of 5 M urea using TALON resin (Clontech Laboratories, Palo Alto, Calif.) (1). The purified protein was refolded by a serial twofold dilution in carbonate buffer (pH 9.6) at 4°C prior to application (1).
The MAb 4B2 has been described in detail elsewhere (17). Briefly, this MAb was isolated following the immunization of mice with ET2.1 in Hunter's Titremax adjuvant, with hybridomas being screened for HEV-specific IgG by ELISA using GST-ORF2.1 (where GST is glutathione transferase) antigen. MAb 4B2 is of IgG1 isotype and reacts against the conformational, immunodominant epitope found within the ORF2.1 fragment (17). Purified 4B2 was prepared from serum-free culture supernatants by protein G affinity column chromatography (Institute of Medical and Veterinary Sciences, Adelaide, Australia) and stored at −70°C.
Serum specimens.
Sera from patients with presumed acute-phase hepatitis E belonged to three groups. The group Nepal 1 (n = 80) represents samples from patients with symptoms of acute viral hepatitis during an institutional outbreak of HEV infection in the Kathmandu Valley, Nepal (1). The group China (n = 41) represents samples from patients with sporadic infections in Shanghai, China, confirmed by a commercial test for anti-HEV IgM antibodies (Genelabs Diagnostic). The group Nepal 2 (n = 30) represents samples with Walter Reed antibody U/ml of >800 (11) for anti-HEV IgM antibodies and confirmed as PCR positive for HEV; samples were originally obtained from the Walter Reed Army Institute of Research through collaboration. Furthermore, 12 samples from patients with past or ongoing hepatitis E infection were also obtained from China. These included samples that were confirmed to have various titers for specific IgG antibodies to HEV but not with IgM (n = 7) or samples that had disproportionately higher amounts of IgG than IgM anti-HEV (n = 5). For normal controls, sera from healthy individuals in Australia (n = 30) or Nepal (n = 30) were collected with consent. In addition, 35 sera from healthy donors of U.S. origin were included in the study, and these were purchased from BioClinical Partners Inc. (Franklin, MA). For the evaluation of the specificity of the new test in various patient controls, serum samples positive for anti-hepatitis A (n = 65) or anti-hepatitis C (n = 10) or hepatitis B surface antigen (n = 13) from either the archive of Genelabs Diagnostics (Singapore, Republic of Singapore) or Burnet Institute (Melbourne, Australia) were used. In addition, serum specimens with specific characteristics were also included in the study for the specificity evaluation. These included sera with elevated levels of IgM antibodies to herpes simplex virus (n = 10) or Toxoplasma gondii (n = 5) or samples associated with rheumatoid factors (RFs) from patients with systemic lupus erythematosus (n = 5) or rheumatoid arthritis (n = 5). Furthermore, 19 samples with known specific amounts of RF were used for additional verifications. All these samples for the specificity study were purchased from BioClinical Partner, Inc. All serum specimens were stored at −70°C until use.
Reference HEV IgM ELISA.
The reference ELISA for IgM anti-HEV is based on the antigen ET2.1 (6xHis-ORF2.1) and is a modification of the IgG anti-HEV ELISA based on the antigen GST-ORF2.1-6xHis (1). Details of this modification will be reported elsewhere. Briefly, the ET2.1 protein was diluted in carbonate buffer (pH 9.6) at a final concentration of 6 μg/ml prior to plate coating. The 96-well polystyrene microtiter plates (MaxiSorp, NUNC, Denmark) were coated with the protein (ET2.1) at a volume of 100 μl per well by incubation overnight (16 to 18 h) at 4°C. The plates were washed five times with phosphate-buffered saline-Tween 20 (PBST), and nonspecific binding sites were blocked with 200 μl per well of a Tris-based diluent for 1 h at room temperature. The plates were further washed another five times before 5 μl of serum in 100 μl of Tris-based diluent (containing 1% each of bovine serum albumin and skim milk powder) was added. Subsequently, the plates were incubated for 30 min at 37°C, followed by six washes with PBST. Horseradish peroxidase (HRP)-conjugated mouse anti-human IgM (1:200 dilution) was added at 100 μl per well and incubated for 30 min at 37°C. The plates were then washed six times in PBST, and the color was developed by the addition of 100 μl per well of enzyme substrate TMB (tetramethylbenzidine). After a 15-min incubation in the dark at 37°C, the reaction was stopped by adding 100 μl of 1N HCl per well. The optical densities (ODs) were measured at 450 nm with a 620-nm reference filter. Results were considered positive if individual ODs of samples were greater than or equal to the cutoff value (COV) of 0.4 plus the mean ODs of negative controls (OD/COV of ≥1).
Rapid immunochromatographic test.
The membrane-based test device consisted of a chromatography strip, a separator, and an absorbent pad, all housed in a cassette as described previously (6).
The chromatography strip was prepared separately as previously described (5) with modifications before assembly into a device. Briefly, a nitrocellulose membrane with an average pore size of 8 μm (Whatman, Ann Arbor, MI) was sprayed with a monoclonal mouse anti-human IgM antibody (Cellabs Pty. Ltd., Australia) at a concentration of 0.4 mg/ml using a BioDot (Irvine, Calif.) striping machine. A second (assay control) line was sprayed with rabbit immunoglobulin (Zymed Laboratories Inc., Calif) at a concentration of 0.2 mg/ml. The membrane was dried for 30 min before being immersed for 1 min in a blocking buffer (Milli-Q-purified water with 6.7% of StabilCoat [SurModics, Inc.], 0.05% Triton, and 0.05% casein). The blocked membrane was then dried at 37°C for 60 min before being affixed to a membrane backing card.
The reagent-bearing (conjugate) pad was prepared using a porous matrix. For each pad, a mixture of 5 μl of colloidal gold (25- to 30-nm)-labeled MAb 4B2 (OD of 10), 1 μl of colloidal gold-labeled goat anti-rabbit IgG (OD of 5), and 2.5 μl of ET2.1 antigen (25 μg/ml) was prepared and incubated for 30 min at room temperature prior to application. The porous matrix was then sprayed with the mixture of the premixed ET2.1 antigen and the gold-labeled monoclonal antibody. This reagent-bearing pad was subsequently dried at 37°C for 2 h prior to incorporation into the device.
A chromatographic card membrane was prepared by affixing a heterophilic blocking reagent (Scantibodies Lab. Inc., Santee, Calif.)-treated porous matrix (sample pad) to one end of the nitrocellulose strip and the reagent-bearing pad (conjugate pad) to the other end on the same membrane backing. This assembly was then cut into a strip approximately 4 by 56 mm.
An assay device was assembled by placing first an absorbent pad at the bottom half of the cassette, then a separator, and, last, one unit of the chromatographic strip before the top half of the cassette was closed. In addition, a reagent-releasing washing buffer was also prepared using Milli-Q-purified water with 1% Triton in 150 mM NaCl.
For testing, 25 μl of undiluted sample was added to the specimen window of the assay device. The sample was allowed to migrate laterally and cover part of the membrane. When the sample reached the indicator line in the viewing window (approximately 30 s), 3 drops of reagent-releasing washing buffer was added to the buffer window, releasing the premixed ET2.1 and 4B2-labeled colloidal gold. The separator was then removed by pulling the protruding end, and 1 drop of wash buffer was added to the sample addition window to allow the chromatographic element and the absorbent pad to come into contact. The mixture of ET2.1 and colloidal gold-labeled 4B2 was then allowed to migrate across the chromatographic strip completely.
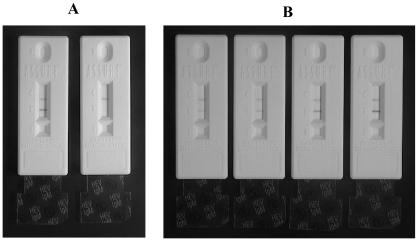
The result can be read in typically 2 to 15 min through the viewing window. However, for the purpose of consistency, all presented data were obtained at 15 min after the final addition of wash buffer. A sample was considered positive for IgM anti-HEV if two colored lines appeared in the viewing window, one at the test line and one at the control line (Fig. 1A). A sample was considered negative for IgM anti-HEV if a colored line appeared only at the control line (Fig. 1A). Positive results were assigned numbers representing the different intensities of the colored test line produced by different samples (Fig. 1B).
FIG. 1.
Examples of the assembled rapid immunochromatographic test devices with their separators (transparent tabs) at the “removed” (after assay) position. (A) To the left is a device after an assay with a sample from an infected patient and to the right is another after an assay with a sample from a healthy control. The two lines in the viewing window for the positive represent the control (top) and the test (bottom) lines. The negative sample generated the control line only. (B) Examples of intensity scores for the test line (from left to right): 1, 2, 3, and 4, respectively.
Statistical analysis.
The kappa statistic was used to measure the strength of agreement between the results by the new rapid test and the reference ELISA. A kappa statistic value of >0.75, 0.40 to 0.75, or <0.40 represents excellent agreement, good to fair agreement, and poor agreement, respectively (15).
RESULTS
Rapid immunochromatographic test.
When tested with sera from patients with acute hepatitis E infection, the rapid test detected IgM antibodies in 96.2% (77 of 80) and in 100% (30 of 30) and 95.1% (39 of 41) of the samples from an outbreak (Nepal 1) or various sporadic infections (Nepal 2 and China), respectively (Table 1). The overall detection rate by the new test was 96.7% (146 of 151) (Table 1). No cross-reactivity was observed when the new test was evaluated using samples (n = 88) from patients with other hepatitis infections (Table 1). The test also presented an excellent specificity of 97.9% with cross-reactivity only to 2 of 95 samples from healthy individuals of different origins (Table 1). When the rapid test was further subjected to testing with samples with elevated levels of IgM antibodies or known to contain RF, a good specificity of 96.0% (24 of 25) was also obtained (Table 1, other patient controls). Because 1 of the 5 samples from patients with rheumatoid arthritis was detected falsely positive by the new test, further verifications were also carried out with additional samples known to contain certain levels of RF. The rapid test yielded a specificity of 90.9% with samples having RF units less than 850 IU/ml but 62% when the RF units were greater than this (Table 2). The test was therefore shown to have an overall specificity of 98.6% (205 of 208) when results of the RF samples were not considered but 96.9% (220 of 227) when they were included. The positive predictive value and negative predictive value of the rapid test were shown to be 98.0% and 97.6%, respectively, with the tested populations (Table 1). In addition, possible interference of anti-HEV-specific IgG antibodies on the new rapid test were examined using serum samples with different levels of IgG or IgM antibodies as indicated by absorbent value (OD) generated by commercially available ELISAs (Genelabs Diagnositics). Different levels of specific IgG antibodies to HEV in the 12 sera tested appeared to have no adverse effect on the performance of the rapid test (Table 3). The results obtained with the five samples with disproportionately higher amounts of IgG to IgM antibodies showed little interference on the detection of the low level of the IgM by the new test (Table 3).
TABLE 1.
Performance of rapid immunochromatographic test and the reference ELISA with sera from hepatitis E patients, other patient controls, and healthy donors
| Serum group and patient status | No. of serum samples | No. of samples positive by the method of:
|
|
|---|---|---|---|
| Reference ELISA | Rapid test | ||
| Patients with: | |||
| Acute hepatitis E (Nepal 1) | 80 | 79 | 77 |
| Acute hepatitis E (Nepal 2) | 30 | 30 | 30 |
| Acute hepatitis E (China) | 41 | 41 | 39 |
| Total for group | 151 | 150 | 146 |
| Other hepatitis patients: | |||
| HAV antibody positive | 65 | 2 | 0 |
| HBsAg positive | 13 | 1 | 0 |
| HCV antibody positive | 10 | 0 | 0 |
| Total for group | 88 | 3 | 0 |
| Other patient controls: | |||
| HSV IgM positive | 10 | 0 | 0 |
| Toxo IgM Positive | 5 | 0 | 0 |
| Rheumatoid arthritis | 5 | 1 | 1 |
| Systemic lupus | 5 | 0 | 0 |
| Total for group | 25 | 1 | 1 |
| Healthy controls: | |||
| Blood donors (Australia) | 30 | 0 | 0 |
| Healthy individuals (Nepal) | 30 | 1 | 0 |
| Blood donors (United States) | 35 | 0 | 2 |
| Total for group | 95 | 1 | 2 |
TABLE 2.
Performance of rapid immunochromatographic test and the reference ELISA with sera with quantified levels of RF units
| Serum group | RF value (IU/mg) | Reference ELISA (OD [interpretation]) | Rapid test (score [inter- pretation]) |
|---|---|---|---|
| RF 0 to 850 (IU/mg) | 20 | 0.136 (negative) | 0 (negative) |
| 596 | 0.157 (negative) | 3 (positive) | |
| 653 | 0.041 (negative) | 0 (negative) | |
| 660 | 0.071 (negative) | 0 (negative) | |
| 667 | 0.072 (negative) | 0 (negative) | |
| 670 | 0.018 (negative) | 0 (negative) | |
| 685 | 0.079 (negative) | 0 (negative) | |
| 690 | 0.163 (negative) | 0 (negative) | |
| 821 | 0.158 (negative) | 0 (negative) | |
| 825 | 0.048 (negative) | 0 (negative) | |
| 845 | 0.037 (negative) | 0 (negative) | |
| RF 850 to 1,500 (IU/mg) | 869 | 0.066 (negative) | 0 (negative) |
| 879 | 0.066 (negative) | 0 (negative) | |
| 882 | 0.204 (negative) | 0 (negative) | |
| 895 | 0.163 (negative) | 1 (positive) | |
| 900 | 0.169 (negative) | 1 (positive) | |
| 928 | 0.088 (negative) | 0 (negative) | |
| 1270 | 0.138 (negative) | 1.5 (positive) | |
| 1350 | 0.051 (negative) | 0 (negative) |
TABLE 3.
Performance of rapid immunochromatographic test with sera containing various levels of anti-HEV IgG antibodiesa
| Serum no. | HEV ELISA for IgG
|
HEV ELISA for IgM
|
Rapid test IgM score (interpretation) | ||
|---|---|---|---|---|---|
| OD | OD/COV | OD | OD/COV | ||
| 22 | 0.657 | 1.2 | 0.127 | 0.3 | 0 (negative) |
| 6 | 0.673 | 1.2 | 0.028 | 0.1 | 0 (negative) |
| 8 | 0.731 | 1.3 | 0.149 | 0.3 | 0 (negative) |
| 10 | 0.845 | 1.6 | 0.070 | 0.2 | 0 (negative) |
| 9 | 1.669 | 3.1 | 0.005 | 0.0 | 0 (negative) |
| 2 | 3.000 | 5.5 | 0.018 | 0.0 | 0 (negative) |
| 17 | 3.000 | 5.5 | 0.052 | 0.1 | 0 (negative) |
| 35 | 3.000 | 5.5 | 0.583 | 1.3 | 2.5 (positive) |
| 37 | 3.000 | 5.5 | 0.753 | 1.7 | 1 (positive) |
| 41 | 3.000 | 5.5 | 0.786 | 1.8 | 2 (positive) |
| 48 | 3.000 | 5.5 | 0.798 | 1.8 | 2 (positive) |
| 47 | 3.000 | 5.5 | 0.948 | 2.2 | 4 (positive) |
Commercial kits from Genelabs Diagnostics (Singapore) were used following the manufacturer's instructions. The COVs for the IgG and IgM test were 0.5 plus the mean OD of the nonreactive control and 0.4 plus the mean OD of the nonreactive control, respectively. A result was considered positive for IgG or IgM anti-HEV only if the OD/COV was equal to or greater than 1.
Comparison of rapid test with a reference ELISA.
The same sets of samples from patients with hepatitis E infection or from healthy or patient control groups were tested in parallel using the reference ELISA, based on the same recombinant HEV antigen (ET2.1). The reference ELISA test generated an overall sensitivity and specificity of 99.3% (150 of 151) and 97.6% (203 of 208), respectively (Table 1). These enabled a comparison between the new testing platform with the conventional approach of ELISA. When the results of the rapid test and the reference ELISA were compared, the two tests gave an agreement of 97.1% with the control samples (HEV negative) but 97.3% with the samples from patients with acute hepatitis E infection. Overall, the results of the two tests provided an excellent agreement of 97.2% with a kappa statistic of 0.943 (Table 4).
TABLE 4.
Agreement between the new rapid test and the reference ELISA with sera from hepatitis E patients or from healthy or patient control groups
| Rapid test | ELISA test result
|
Agreement (%) | Kappa statistica | |
|---|---|---|---|---|
| Positive | Negative | |||
| Positive | 147 | 6 | 97.3 | 0.943 |
| Negative | 4 | 201 | 97.1 | |
Calculated for the total agreement of 97.2%. A kappa statistic of ≥0.75 represents excellent agreement, 0.40 to 0.75 represents good to fair agreement, and <0.40 represents poor agreement (14).
DISCUSSION
Although it has been understood for more than a decade that hepatitis E is a waterborne disease and that major outbreaks occur most frequently in developing nations in areas with poor sanitation, currently available tools for the detection of the disease remain mostly laboratory based, requiring trained personnel and equipment. A simple rapid test that enables early detection at the point of care where laboratory facilities are not readily accessible is, therefore, an unmet need for the management of hepatitis E. For example, the status of patient specimens from a recent outbreak in the Greater Darfur region in Sudan was confirmed using ELISA and PCR in a laboratory in Cairo, Egypt (http://www.who.int/csr/don/2004_08_10/en/print.html). Obviously, unnecessary delays would be unavoidable should all outbreaks occurring in remote areas be managed in a similar manner. Because the detection of antibodies is fundamental for our understanding of the prevalence of hepatitis E infection, reliable serological tests are indispensable tools for studying the epidemiology of the disease. Furthermore, as the IgM class of antibody to the virus is the specific marker for differentiating the acute from the convalescent phase of an infection, recent emphasis has been placed on the development of immunoassays for the detection of this specific marker (19). In the present study, we thus focused on developing a rapid test for the detection of IgM antibody to HEV for use in remote areas without the need for laboratory equipment.
The newly developed test is a reverse-flow immunochromatographic test (6) and uses immobilized mouse anti-human IgM antibodies for capturing the IgM antibodies in the tested samples. The presence of the captured IgM antibody specific to HEV is detected by the colloidal gold-labeled anti-HEV monoclonal antibody 4B2 premixed with the recombinant protein of ET2.1. The results of our study showed that the new, rapid test detected a dominant proportion of patient samples collected either from outbreaks or sporadic infections, regardless of their origins. The rapid test detected IgM antibodies in 96.2% (77 of 80), 100% (30 of 30), and 95.1% (39 of 41) of the serum samples collected from an outbreak in Nepal (Nepal 1) or from sporadic infections Nepal (Nepal 2) China, respectively(Table 1). Thus, the finding of the utility of ET2.1 in the new platform for the detection of IgM antibody is consistent with what had been demonstrated in the ELISA or Western immunoblot formats with related ORF2.1 recombinant proteins (1, 12, 13). ET2.1 is a well-defined recombinant antigen containing a major conformational epitope (12, 13). Furthermore, the results of our study with the new 15-min test are consistent with the recent findings of Yu et al. (19), even though they used a method requiring prolonged (overnight) incubation. These investigators showed that a class-capture enzyme immunoassay using a recombinant protein from the ORF2 region expressed in the baculovirus system had the advantage of distinguishing IgM anti-HEV in the presence of high titers of IgG anti-HEV (19).
The specificity of the rapid test was evaluated using samples from various patients as well as healthy controls. The results obtained with serum samples from patients with hepatitis A, B, or C infection showed an excellent specificity (Table 1), suggesting the likely utility of the new rapid test in detecting hepatitis E infection among other hepatitis infections. This capability of differentiation is an essential feature for the new test because the acute disease syndrome produced by each of the viruses can be quite similar, even though the viruses responsible for different types of hepatitis infections are unrelated to each other in structure and mode of replication (3, 16). In particular, hepatitis A virus (HAV) and HEV share similarities not only in clinical manifestation but also with routes of transmission. Obviously, differentiating an infection with HAV from one with HEV will be critical for the management of the two distinct diseases.
Because the rapid test is a capture-based immunoassay for the detection of IgM antibodies to HEV, it was also important to verify if any cross-reactivity can be generated by samples with elevated amounts of disease-specific IgM class antibodies. Our studies thus included two sets of sera that were confirmed to be positive for IgM but from patients with other infections rather than with hepatitis E. In addition, RFs were known to cause cross-reactivity in ELISAs for IgM antibody regardless of whether tests were capture based or indirect sandwich assays (2, 8). Hence, the present study also included sera from patients with rheumatoid arthritis or systemic lupus, conditions that are known to be associated with high levels of RF. The results obtained with samples positive for IgM antibodies to herpes simplex virus or T. gondii showed no cross-reactivity, and thus the interference of IgM specific to other pathogens is not a real concern with the rapid test (Table 1). However, the result obtained with the serum samples from patients with autoimmune diseases did indicate to a certain extent a possible interference by RFs. To further clarify this concern, serum samples with known RF levels were also introduced in the study. Our results showed a specificity of 90.9% with samples having an RF concentration of less than 850 IU/ml but a specificity of 62% when the RF concentrations were greater (Table 2). RFs at concentrations as low as 0.5 IU/ml coupled with specific IgG antibody were reported to cause significant cross-reactivity in an Epstein-Barr virus ELISA for IgM antibody (8). In the present case, the cross-reactivity generated by the extremely high concentration of RF (IgM class of antibodies) in the tested samples appeared to be unlike the results of previous studies, and thus it warrants further investigation.
Conventional indirect sandwich ELISAs for the detection of an IgM-specific class of antibodies can produce false-negative results and, hence, lose sensitivity when disproportionately higher amounts of the corresponding IgG are present in samples and competing for the same epitopes on antigens (14). Capture-based ELISAs are thus often used as an alternative to overcome this inherent problem (7, 19), in addition to procedures of sample IgG removal (14). To verify that our design of the new capture-based reverse-flow test is adequately free from the interference of higher proportions of HEV-specific IgG to the corresponding IgM, patient samples with known amounts of IgG and IgM were also included in our studies. As expected, disproportional amounts of HEV-specific IgG did not produce any false positives, nor did they result in any false negatives even with samples with borderline amounts of IgM antibodies (Table 3). The new design thus appeared to be adequate and at least in good agreement with current commercial tests in detecting specifically the IgM class of specific antibodies.
Although the rapid test is a simple and membrane-based immunochromatographic test, a strong agreement of 97.2% with a kappa statistic of 0.943 was obtained in the present study between the new test and the reference ELISA on the wide range of samples tested. The slight differences in the sensitivity and specificity observed between the two approaches are not beyond the variations that might be expected between the two very different assay designs. The strong agreement and the overall performance of the new test thus suggested not only that the new test is compatible with currently established methods but also that it is an alternative tool for the routine detection and management of hepatitis E especially in remote areas where the disease most frequently occurs.
REFERENCES
- 1.Anderson, D. A., F. Li, M. Riddell, T. Howard, H.-F. Seow, J. Torresi, G. Perry, D. Sumarisidi, S. M. Shrestha, and I. L. Shrestha. 1999. ELISA for IgG-class antibody to hepatitis E virus based on a highly conserved, conformational epitope expressed in Escherichia coli. J. Virol. Methods 81:131-142. [DOI] [PubMed] [Google Scholar]
- 2.Briantais, M. J., L. Grangeot-Keros, and J. Pillot. 1984. Specificity and sensitivity of the IgM capture immunoassay: studies of possible factors inducing false positive or false negative results. J. Virol. Methods 9:15-26. [DOI] [PubMed] [Google Scholar]
- 3.Emerson, S. U., and R. H. Purcell. 2003. Hepatitis E virus. Rev. Med. Virol. 13:145-154. [DOI] [PubMed] [Google Scholar]
- 4.Emerson, S. U., and R. H. Purcell. 2004. Running like water: the omnipresence of hepatitis E. N. Engl. J. Med. 351:2367-2368. [DOI] [PubMed] [Google Scholar]
- 5.Guan, M., H. Y. Chen, S. Y. Foo, Y. J. Tan, P. Y. Goh, and S. H. Wee. 2004. Recombinant protein-based enzyme-linked immunosorbent assay and immunochromatographic tests for detection of immunoglobulin G antibodies to severe acute respiratory syndrome (SARS) coronavirus in SARS patients. Clin. Diagn. Lab. Immunol. 11:287-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan, M., H. Y. Chen, T. P. Chow, A. R. Pereira, and P. K. Mun. November 2001. Assay devices and methods of analyte detection. U.S. patent 6,316,205.
- 7.Hansen, K., K. Pii, and A.-M. Lebech. 1991. Improved immunoglobulin M serodiagnosis in Lyme borreliosis by using a μ-capture enzyme-linked immunosorbent assay with biotinylated Borrelia burgdorferi flagella. J. Clin. Microbiol. 29:166-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho, D. W. T., P. R. Field, and A. L. Cunningham. 1989. Rapid diagnosis of acute Epstein-Barr virus infection by an indirect enzyme-linked immunosorbent assay for specific immunoglobulin M (IgM) antibody without rheumatoid factor and specific IgG interference. J. Clin. Microbiol. 27:952-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang, C. C., D. Nguyen, J. Fernandez, K. Y. Yun, K. E. Fry, D. W. Bradley, A. W. Tam, and G. R. Reyes. 1992. Molecular cloning and sequencing of the Mexico isolate of hepatitis E virus (HEV). Virology 191:550-558. [DOI] [PubMed] [Google Scholar]
- 10.Hussani, S. H., S. J. Skidmore, P. Richardson, L. M. Sherratt, B. T. Cooper, and J. G. O'Grady. 1997. Severe hepatitis E infection during pregnancy. J. Viral Hepat. 4:51-54. [DOI] [PubMed] [Google Scholar]
- 11.Innes, B. L., J. Seriwatana, R. A. Robinson, M. P. Shrestha, P. O. Yarbough, C. F. Longer, R. M. Scott, D. W. Vaughn, and K. S. A. Myint. 2002. Quantitation of immunoglobulin to hepatitis E virus by enzyme immunoassay. Clin. Diagn. Lab. Immunol. 9:639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, F., H. Zhuang, S. Kolivas, S. A. Locarnini, and D. A. Anderson. 1994. Persistent and transient antibody responses to hepatitis E virus detected by Western immunoblot using open reading frame 2 and 3 and glutathione S-transferase fusion proteins. J. Clin. Microbiol. 32:2060-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, F., J. Torresi, S. A. Locarnini, H. Zhuang, W. F. Zhu, X. X. Guo, and D. A. Anderson. 1997. Amino-terminal epitopes are exposed when full-length open reading frame 2 of hepatitis E virus is expressed in Escherichia coli, but carboxy-terminal epitopes are masked. J. Med. Virol. 52:289-300. [DOI] [PubMed] [Google Scholar]
- 14.Martin, T. B., T. D. Jaskowski, C. L. Mouritsen, and H. R. Hill. 1995. An evaluation of the effectiveness of three immunoglobulin G (IgG) removal procedures for routine IgM serological testing. Clin. Diagn. Lab. Immunol. 2:98-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pottumarthy, S., A. J. Morris, A. C. Harrison, and V. C. Wells. 1999. Evaluation of the tuberculin gamma interferon assay: potential to replace the Mantoux skin test. J. Clin. Microbiol. 37:3229-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reyes, G. R., M. A. Purdy, J. P. Kim, K. C. Luk, L. M. Young, K. E. Fry, and D. W. Bradley. 1990. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science 274:1335-1339. [DOI] [PubMed] [Google Scholar]
- 17.Riddell, M. A., F. Li, and D. A. Anderson. 2000. Identification of immunodominant and conformational epitopes in the capsid protein of hepatitis E virus by using monoclonal antibodies. J. Virol. 74:8011-8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tam, A. W., M. M. Smith, M. E. Guerra, C. C. Huang, D. W. Bradley, K. E. Fry, and G. R. Reyes. 1991. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology 185:120-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu, C., R. E. Engle, J. P. Bryan, S. U. Emerson, and R. H. Purcell. 2003. Detection of immunoglobulin M antibodies to hepatitis E virus by class capture enzyme immunoassay. Clin. Diagn. Lab. Immunol. 10:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Worm, H. C., and G. Wirnsberger. 2004. Hepatitis E vaccines: progress and prospects. Drug 64:1517-1531. [DOI] [PubMed] [Google Scholar]