Abstract
Infection by Chlamydia pneumoniae or Chlamydia pecorum commonly causes chronic, fibrotic disease of the urogenital tracts of female koalas. Studies of humans have associated titers of serum immunoglobulin G (IgG) against chlamydial hsp60 and hsp10 antigens with chronic infection, salpingeal fibrosis, and tubal infertility. To determine whether a similar relationship exists in Chlamydia-infected koalas, samples were collected opportunistically from 34 wild female koalas and examined by gross pathology and histopathology, PCR, and immunohistochemistry for Chlamydia spp. and enzyme-linked immunosorbent assay for serological responses to chlamydial hsp10 and hsp60 antigens. Greater anti-hsp titers occurred in Chlamydia-infected koalas with fibrous occlusion of the uterus or uterine tube than in other Chlamydia-infected koalas (for hsp10 IgG, P = 0.005; for hsp60 IgG, P = 0.001; for hsp10 IgA, P = 0.04; for hsp60 IgA, P = 0.09). However, as in humans, some koalas with tubal occlusion had low titers. Among Chlamydia-infected koalas with tubal occlusion, those with low titers were more likely to have an active component to their ongoing uterine or salpingeal inflammation (P = 0.007), such that the assay predicted, with 79% sensitivity and 92% specificity, tubal occlusion where an active component of inflammation was absent. Findings of this study permit advancement of clinical and epidemiological studies of host-pathogen-environment interactions and pose intriguing questions regarding the significance of the Th1/Th2 paradigm and antigen-presenting and inflammation-regulating capabilities of uterine epithelial cells and the roles of latency and reactivation of chlamydial infections in pathogenesis of upper reproductive tract disease of koalas.
The koala (Phascolarctos cinereus) is an iconic Australian marsupial. With a natural life span of approximately 15 to 18 years, it is highly adapted to a relatively low-nutrient, high-toxin diet of eucalypt leaves. It is widespread in eucalypt forests of eastern Australia but is subject to a range of potentially threatening processes, such as inbreeding from historical hunting and population management (11, 29), effects of ongoing habitat fragmentation on social structure and food quality and availability (7, 15), and increased mortality due to predation by domestic dogs, road trauma, and disease (5, 30). Infection by Chlamydia pneumoniae or Chlamydia pecorum commonly causes proliferative conjunctivitis and chronic, fibrotic disease of the urogenital tracts of koalas, often resulting in infertility and death (3, 32). It is the most commonly documented disease of koalas, and among infected populations, its prevalence is often higher in those living in habitats affected more by humans than in habitats affected less by humans (10, 16, 20). To identify factors that influence the susceptibility of koalas to this disease, there is a need to understand the mechanisms governing elimination or persistence of the organism and the induction of subclinical or clinical disease states. However, as in studies of chlamydial disease of humans, interpretation of epidemiological surveys of chlamydial disease in koala populations is made difficult because significant disease can occur without overt signs (17). Better understanding of the pathogenesis of this disease and more-reliable techniques of determining disease status would benefit epidemiological modeling of the disease and its subsequent management in wild populations.
Several studies of women have identified a relationship between titers of serum immunoglobulin G (IgG) against the 60- and 10-kDa chlamydial heat shock proteins (c-hsp60 and c-hsp10) and chronic infection, salpingeal fibrosis, and tubal infertility (9, 23, 40, 43, 46). We hypothesized that this might be a useful assay to predict the extent of disease in the upper female reproductive tracts (uFRT) of live koalas. Grossly, the koala female reproductive tract is bipartite; two lateral vaginae converge with the urethra into a common urogenital sinus, which leads to a cloaca, or common urogenital and rectal opening. The young are born through a median vagina, which forms at parturition (31). The two lateral vaginae and single median vagina constitute the vaginal complex. Despite these differences in gross anatomy, chlamydial lesions of koalas are similar grossly and histologically to those caused by Chlamydia trachomatis in humans (17). Girjes et al. (13) described koala IgG responses to chlamydial lipopolysaccharide (LPS) and 39-kDa (major outer membrane protein), 31-kDa and 18-kDa chlamydial antigens but did not detect serological response to antigens of 10 kDa or 60 kDa by Western blotting. The present cross-sectional study aimed to determine whether koalas recognize the chlamydial antigens c-hsp60 and c-hsp10; whether a relationship exists between disease status and IgG, IgA, and IgE titers against these antigens; and whether, if such a relationship exists, it is applicable to epidemiological surveys of wild koalas.
Development of an assay for hsp-specific antibody is also expected to assist the study of pathogenesis. Hypotheses for the association of c-hsp-specific antibodies with tubal fibrosis in women include the following: restriction of antibody expression to chronically diseased patients due to low immunogenicity of the antigens (23); and eventual development of immunoreactivity to highly conserved epitopes of hsp60, thus perpetuating pathogenesis by autoimmune pathways or by reaction to similar epitopes on other bacterial species (24, 25, 27, 45). In addition, measurement of serological response might be useful in future studies of mechanisms through which host, pathogen, and environmental factors interact to promote disease. In simplistic terms, a serological response can be thought of as representing a move away from a Th1-dominated immune response toward a Th2-dominated response. The Th1 response is characterized by a predominance of cytotoxic T lymphocytes, macrophages, neutrophils, and the cytokine gamma interferon (IFN-γ) and is associated with production of IgG2a. It is considered essential for elimination of the infection but can cause severe tissue damage if excessive (6, 8). In contrast, the Th2 response is characterized by production of IgE, IgA, and IgG isotypes other than IgG2a and a cytokine profile that encourages chlamydial persistence, low grade, chronic inflammation and fibrosis (8). Many factors, such as genetic characteristics (44), low dietary protein (26), and exposure to corticosteroids (14), or reproductive hormones (1), can direct the immune response towards Th2 dominance in some species, thus providing a potential mechanism through which host and environmental factors could influence susceptibility to this disease.
MATERIALS AND METHODS
Sample collection.
Normal plasma samples were obtained from five healthy koalas in the collection of Taronga Zoo (Sydney, New South Wales, Australia), a quarantined, Chlamydia-free population (noninfected captive [NC]). Wild subjects were adult female, sick or injured koalas brought to a koala hospital from an area encompassing a 100-km radius of Brisbane, Australia. During March and October of 2001 and 2002, koalas were anaesthetized (Zoletil; Virbac, Australia) and examined by hospital staff. If the koalas were unsuitable for treatment and release, blood was collected, and euthanasia was performed by injection of barbiturate (Lethabarb; Arnolds, Australia). The resulting sample of 34 koalas was not intended to represent admissions to the hospital but represented as wide a range of pathological states as possible. A detailed postmortem examination was performed within 1 hour of euthanasia, and tissues from all major organs were fixed in 10% neutral buffered formalin. Because it was considered possible that severe debilitation or old age might confound results by restricting immunoglobulin production, condition score, age, and plasma proteins were assessed for correlation with antibody titers. Condition score was assessed by palpation of the infra- and supraspinatus muscles and graded from 1 (emaciated) to 10 (excellent condition), and age was estimated from tooth wear (28). Total plasma protein was measured by the biuret method, albumin was measured by the bromocresyl green method, and globulin was derived by subtraction. In addition, Beagley and Gockel suggest that reproductive state might influence antibody production (1). Therefore, animals were also classified as luteal (corpus luteum exceeding 4-mm diameter), estrous (follicle exceeding 4-mm diameter or ruptured, nonluteinized follicle) or reproductively inactive (ovary necrosed or replaced by fibrosis or without structures exceeding 4-mm diameter) (21).
Histopathology.
Histological examination was performed on hematoxylin-and-eosin-stained sections of all tissues collected. For the urogenital tract, these included the following: transverse sections of the urogenital sinus, vaginal complex, cervices, and uteri; ovary through the largest ovarian structure; uterine tube sectioned along its axis to include several transverse sections of the tortuous duct; and bladder.
PCR for detection of chlamydial DNA from swabs.
DNA was extracted from swabs of urogenital sinus and conjunctiva using the QiaAmp Mini kit (QIAGEN Pty Ltd., Australia) according to the manufacturer's recommendations, and the 50-μl eluate was stored at −20°C. The PCR method was a modification of the genus-specific method described by Girjes et al. (12). Reaction mixtures of 49 μl contained 200 μM of each deoxynucleoside triphosphate, 500 μM of each primer, 2.5 mM MgCl2 and 1, 2, and 5 μl DNA template in PCR buffer. These were denatured (95°C, 8 min) and then cooled to 62°C before 1 U Taq DNA polymerase was added. A touch-down PCR was performed, comprising denaturation (95°C, 60 s), annealing (starting with 60 s at 66°C and reducing the temperature by 2°C every two cycles to 56°C, then 60 s at 56°C for 35 cycles), and extension (72°C, 60 s); then final extension (72°C, 5 min). PCR product was separated by electrophoresis on a 4% high-resolution agarose gel (J234-100G; Amresco, Solon, Ohio) in Tris-acetate-EDTA (TAE) buffer at 90 V/90 mA for 45 min and visualized by staining with ethidium bromide. All samples were assayed in duplicate, and concurrent with DNA extracts of plain phosphate-buffered saline (PBS) and a dilution of C. pneumoniae DNA that approximated the sensitivity limit of the reaction.
Immunohistochemistry (IHC) for detection of Chlamydia spp. in situ.
Tissue sections (4 μm) were fixed to glass slides with horse serum, dried, dewaxed in xylene, and rehydrated through an ethanol gradient. Sections for immunoperoxidase labeling were incubated with 3% hydrogen peroxide for 5 min. Duplicate sections were then equilibrated in Tris-buffered saline (pH 7.6) with 0.05% Tween 20 (TTBS), before incubation for 40 min at room temperature with either (i) polyclonal antibody to Chlamydia trachomatis elementary bodies (anti-elementary body 1611; Virostat, Portland, ME) diluted 1 in 200, (ii) the same concentration of rabbit immunoglobulin (X0903; Dako, Carpinteria, CA) as a negative control, (iii) monoclonal antibody to chlamydial lipopolysaccharide (LPS) (anti-LPS 512F; Cell Labs Diagnostics, Brookvale, Australia) diluted 1 in 200, or (iv) TTBS as a negative control for the anti-LPS, in view of the unknown Ig concentration of the latter. Primary antibodies were detected with a 1 in 100 dilution of alkaline phosphatase-conjugated secondary antibody (AP322A; Chemicon, Melbourne, Australia) with nitroblue tetrazolium/5-bromo-4-chloro-3-indolylphosphate (BCIP) chromogen (catalog no. 00-2211; Zymed, San Francisco, CA), or by the biotin-streptavidin-immunoperoxidase method (K0492; Dako) and diaminobenzidine chromogen (K346; Dako). TTBS was used as the diluent and for all washes, and 10% goat serum was added to antibody diluents. At least one negative and one positive koala tissue was included in each batch.
Labeling of chlamydial antigen was defined by disrupted or clearly defined, single or multiple cytoplasmic inclusions that contained a positive-staining mixture of densely packed, approximately 300-nm to 1.2-μm diameter structures, which are typical of chlamydial elementary and reticular bodies (38). For each complete transverse section of the reproductive tract, the number of infected epithelial cells was counted. Mean values were calculated from at least two sections from each side for each part of the reproductive tract. An overall score for epithelial chlamydial infection was represented by the mean of those values from urogenital sinus and vaginal complex (lower female reproductive tract); or endocervices, uteri, uterine tubes, and ovarian bursae (uFRT).
ELISA for detection of hsp60- and hsp10-specific antibodies.
A three-stage indirect enzyme-linked immunosorbent assay (ELISA) technique was used. Reagent concentrations were optimized by checkerboard titrations and trials of a range of blocking agents and incubation conditions. Standard sera were 10 doubling dilutions of a patient plasma sample that reacted strongly to the relevant antigen in a preliminary survey of sera. c-hsp10 and c-hsp60 recombinant peptide antigens were those used by LaVerda et al. (23). Anti-koala IgG was unpurified rabbit serum raised against affinity-purified (Gamma-bind Plus; Pharmacia Biotech, Boronia, Australia) koala IgG. Cross-reactivity of affinity-purified sheep anti-marsupial IgA (35) and IgE (Department of Biological Sciences, Macquarie University, Australia), with quantities of affinity-purified koala IgG approximating 90, 50, and 20% binding were 4, 1, and 0%, respectively, compared with anti-koala IgG.
Test (antigen and patient sera), control (patient sera, no antigen), and blank (no antigen, no sera) wells of a 96-well plate (Immulon 2HB) (catalog no. 3455; Thermo Labsystems, Franklin, MA) were incubated with 0.1 μg recombinant antigen or no antigen, respectively, in 100 μl bicarbonate buffer (pH 9.6) at 4°C for 48 h, then washed four times with PBS (pH 7.6) containing 0.01% Tween 20 (TPBS). Wells were then incubated on a 37°C water bath, in turn, with 200 μl 1% casein (catalog no. C8654; Sigma, Castle Hill, Australia) in TPBS (120 min); 100 μl patient sera diluted 1/100 in TPBS, 100 μl standard sera, or 100 μl plain TPBS (blank wells) (60 min); 100 μl rabbit anti-koala IgG diluted 1/100 in TPBS, sheep anti-marsupial IgA diluted 1/10 in TTBS, or sheep anti-marsupial IgE diluted 1/10 in TTBS (60 min); and 100 μl of the relevant alkaline phosphatase-conjugated, sheep anti-rabbit Ig or donkey anti-sheep Ig antibody (AP322A, UAP; Chemicon) diluted 1/500 in TPBS (60 min). Between incubations and after the final incubation, wells were washed four times with TPBS or TBS, respectively. Signal was created by incubation with 100 μl p-nitrophenylphosphate (catalog no. 172-1063; Bio-Rad, Hercules, CA) at room temperature. The reaction was stopped at 6 min by adding 50 μl 2 M NaOH, and the absorbance, relative to blank wells, was read at 405 nm on an automated plate reader (Spectromax 250; Molecular Devices, Sunnyvale, CA). IgG assays were performed on all samples across triplicate plates, as indicated by convergence of cumulative means. Due to limited reagents, IgA and IgE assays were performed in duplicate and only on sera from Chlamydia-positive koalas. Control wells for IgA and IgE were assayed once, as these assays detected minimal nonspecific binding.
Intra-assay coefficients of variation of the IgG assay system, as estimated from the concurrent assay of 13 replicates of three standards representing 80, 50, and 20% binding were 4, 7, and 4%, respectively. Coefficients of variation for the IgA and IgE assays were assumed to be similar to those of the IgG assay but could not be calculated, as quantities of reagents were limited.
Classification of disease status of koalas.
Chlamydial infection was defined as positive response to PCR of urogenital or conjunctival swabs, and/or positive immunohistochemical staining of formalin-fixed conjunctiva or urogenital tract. Tubal occlusion was defined as one or more sites where the lumen and epithelium of one or both uteri or uterine tubes had been replaced by fibrosis. Thus, wild koalas were classified into the following pathology groups: noninfected (NW; n = 7); infected, patent (IP; n = 7); or infected, occluded (IO; n = 20). A subset of koalas were selected for further investigation to determine whether an association existed between anti-hsp antibody titers and aspects of urogenital tract pathology other than fibrotic occlusion: five koalas from the IP group, all koalas from the IO group with anti-hsp10 and anti-hsp60 IgG titers both below the mean (IO low, n = 8), and all koalas with anti-hsp10 and anti-hsp60 IgG titers both above the mean (IO high, n = 7) were investigated.
Statistical analysis.
Optical densities (OD) presented are mean differences between ELISA test and control wells. Sensitivity, specificity, likelihood ratios and Cohen's kappa values for concordance were calculated for a range of arbitrary cutoff points, and from these, an optimal cutoff point was selected for each assay. To ensure test cutoff values were within the linear portion of the standard curve, OD were applied to a standard curve that was generated using Statistica 5.1 (Statsoft, Inc., Tulsa, OK) from a four-parameter log-logit equation (SOFTmax PRO users manual; Molecular Devices, Sunnyvale, CA).
Log-transformed anti-hsp10 IgG assay results were compared between groups with different pathologies, sampling periods, and reproductive status using analysis of variance with orthogonal contrasts and Student t tests (Genstat 5; VSN International). The data for remaining variables could not be transformed for parametric analysis. Therefore, unless specified, all other analyses were performed using the Kruskal-Wallis or Mann-Whitney U test or Spearman's rank correlation after examining dot plots for potential nonlinear relationships (Minitab 14.1; Minitab, Inc.).
RESULTS
Titers of all Ig classes of the same antigen specificity were significantly correlated (rs > 0.83, P < 0.001). IgA and IgE titers were correlated most strongly (rs > 0.97, P < 0.001); therefore, only IgG and IgA values are presented.
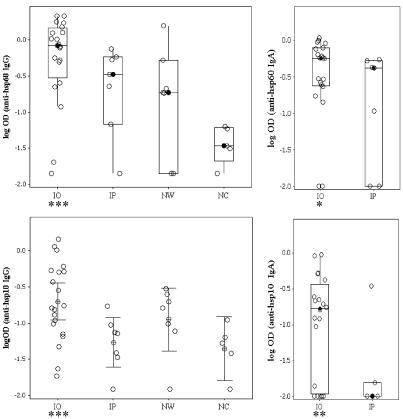
Higher anti-hsp antibody titers were associated with IO koalas than with noninfected (NW and NC) and IP groups combined (for hsp10 IgG, P = 0.005; for hsp60 IgG, P = 0.001; for hsp10 IgA, P = 0.04; for hsp60 IgA, P = 0.09) (Fig. 1). Anti-hsp antibody titers did not differ significantly among NW, IP, and NC groups (for anti-hsp10, P = 0.316; for anti-hsp60, P = 0.1).
FIG. 1.
Comparison of serological responses to chlamydial hsp10 and hsp60 antigens among koalas in four groups: Chlamydia-infected koalas with one or more fibrous occlusions of one or both uteri or uterine tubes (IO), infected koalas with patent uteri and uterine tubes (IP), noninfected wild koalas (NW), and noninfected captive koalas (NC). Box plots indicate individual values (○), medians (•), and interquartile ranges (box) for data analyzed by the Kruskal-Wallis and Mann-Whitney U-tests. The remaining plot (anti-hsp10 IgG) indicates individual values (○) and means and 95% confidence intervals for data analyzed by analysis of variance with orthogonal contrasts. Groups with significantly different values (P values of <0.1 [*], <0.05 [**], and <0.01 [***]) are indicated. Mean anti-hsp antibody titers of IP, NW, and NC groups did not differ significantly but were significantly lower than the IO group.
The sensitivity and specificity of the assays as a diagnostic test for tubal occlusion was limited by a subset of IO koalas that failed to develop titers greater than those of koalas in other groups (Fig. 1 and Table 1). An attempt was made to identify factors associated with these low-titer IO koalas. Factors inherent in disabled wild koalas did not appear likely to affect titers. Among all wild Chlamydia-infected koalas (n = 27) and within the IO group (n = 20), Spearman's rank correlation matrices indicated no association of total serum protein (range, 32 to 69 g/liter), albumin (18 to 38 g/liter), and globulin (13 to 36 g/liter); condition score (1 to 7 out of 10); or age (2 to 12 years) with anti-hsp titers (P > 0.2). Concurrent diseases were randomly distributed across all urogenital pathology groups and, excluding weak evidence for an effect of reproductive status on anti-hsp10 IgG titer (P = 0.08), mean titers did not differ significantly with sampling period (P > 0.7), reproductive status (P > 0.25), or presence of conjunctivitis (P > 0.25).
TABLE 1.
Diagnostic value of anti-c-hsp antibody ELISA for koalasa
| Pathology | Antigen | Ig subclass | Sensitivity (%) | Specificity (%) | LR+b | LR−c | Cohen's kappad | 95% CIe for kappa |
|---|---|---|---|---|---|---|---|---|
| Tubal occlusion | hsp60 | IgG | 60 | 86 | 4.2 | 0.47 | 0.29 | −0.11-0.70 |
| IgA | 55 | 100 | NA | 0.45 | 0.39 | 0.00-0.77 | ||
| hsp10 | IgG | 65 | 86 | 4.6 | 0.41 | 0.39 | 0.02-0.77 | |
| IgA | 70 | 86 | 4.9 | 0.35 | 0.45 | 0.09-0.81 | ||
| Tubal occlusion without active inflammation | hsp60 | IgG | 73 | 84 | 5.1 | 0.25 | 0.63 | 0.33-0.92 |
| IgA | 71 | 92 | 9.2 | 0.30 | 0.63 | 0.34-0.92 | ||
| hsp10 | IgG | 79 | 92 | 10.2 | 0.23 | 0.70 | 0.44-0.97 | |
| IgA | 78 | 69 | 2.6 | 0.30 | 0.48 | 0.14-0.81 |
Sensitivities, specificities, likelihood ratios, and Cohen's kappa values confidence intervals of optimal cutoff values in hsp60- and hsp10-specific IgG and IgA assays, predicting, among Chlamydia-positive koalas, occlusion of uteri or uterine tubes (tubal occlusion) with or without an active component of inflammation, and tubal occlusion without active inflammation of the upper reproductive tract.
LR+, positive likelihood ratio. 2- to 5, fair; 5 to 10, good; >10, excellent; NA, not applicable.
LR−, negative likelihood ratio. 0.5 to 0.2, fair; 0.2 to 0.1, good; <0.1, excellent.
Kappa of >0.4 denotes accurate diagnostic test (22).
95% CI, 95% confidence interval.
To ensure that poor assay sensitivity was not responsible for the low titers, the IgG assay was repeated on plasma samples from six (each) seronegative IO and IP koalas, at 1/50 plasma dilution in wells coated with 0.5 μg hsp60/well. This increased most of these OD values to the linear segment of the standard curve but did not distinguish IO from IP koalas. Also, good concordance of PCR, IHC, and histopathological results indicated accurate classification of animals. Of the 27 koalas examined by both PCR and IHC, inclusions were demonstrated within the urogenital tracts of 19 of 20 PCR-positive koalas, and all PCR-positive koalas showed at least mild plasma cell infiltrates or fibrosis in the urogenital tract. Of the seven PCR-negative koalas, all were negative by IHC, and only one showed histopathological changes.
A subset of koalas were selected for further investigation to determine whether an association existed between anti-hsp antibody titers and other aspects of urogenital tract pathology. All koalas had active, ongoing inflammation of the lower reproductive tract. All uFRT had fibrosis and infiltrates or aggregates of plasma cells and macrophages within the submucosa or myometrium to various degrees. However, those koalas with tubal occlusion and low anti-hsp antibody titers (IO, low titers) were more likely to have foci of active inflammation within the uFRT (P = 0.007 by Fisher's exact test). All six IO koalas that showed this active inflammation of the uFRT (IO, active) occurred within the IO low-titer group (Table 2). Active foci were characterized by edema and hyperemia and submucosal and epithelial infiltrates with a prominent neutrophil component and a variable but usually prominent lymphocyte component. The prevalence of an active phase of inflammation in ongoing cystitis lesions was not significantly greater in the IO low-titer than in the IO high-titer group (Table 2).
TABLE 2.
Comparison of hsp-specific IgG titers, presence/absence of active inflammation, score for epithelial chlamydial infection, and site of macrophages labeled by anti-chlamydial IHC in individual koalas grouped by pathology group and titer
| Pathology group and titera | Anti-hsp IgG (log OD)
|
Active inflammationb
|
Scorec for epithelial chlamydial infection
|
IHC-positive macrophages | ||||
|---|---|---|---|---|---|---|---|---|
| Anti-hsp60 | Anti-hsp10 | uFRT | Bladder | lFRT | uFRT | Bladder | ||
| Infected, patent uFRT | −0.64 | −0.76 | + | + | 0.5 | 0 | 2 | |
| −0.28 | −1.48 | + | + | 104 | 41 | 18 | Liver, spleen | |
| −1.17 | −1.42 | − | + | 0 | 0 | 1 | ||
| −0.24 | −1.13 | + | + | 1 | 1 | 2 | ||
| −0.12 | −1.15 | + | + | 65 | 21 | 60 | Exudate, submucosa | |
| Infected, occluded | ||||||||
| Low titers | −1.7 | −1.74 | + | + | 34 | 55 | 17 | Submucosa |
| −0.11 | −1.63 | + | + | 0 | 2 | 3 | Submucosa | |
| −1.85 | −1.33 | + | − | 3 | 3 | 0 | Exudate | |
| −0.3 | −1.15 | − | + | 1 | 0 | 3 | ||
| −0.6 | −1.01 | + | − | 11 | 0 | 0 | Exudate | |
| −0.93 | −0.96 | − | − | 8 | 0 | 0 | ||
| −0.28 | −0.88 | + | + | 5 | 12 | 0 | Submucosa | |
| −0.65 | −0.79 | + | + | 0 | 2 | 0 | Exudate | |
| Mid-range titers (n = 5) | − | + | ||||||
| High titers | 0.1 | −0.76 | − | − | 20 | 0 | 0 | |
| 0.26 | −0.43 | − | − | 1 | 0 | 0 | ||
| −0.06 | 0.01 | − | + | 0 | 0 | 11 | ||
| 0.24 | −0.29 | − | + | 1 | 0 | 12 | ||
| 0.01 | −0.22 | − | − | 0 | 0 | 0 | ||
| 0.19 | 0.06 | − | − | 1 | 0 | 0 | ||
| 0.34 | 0.16 | − | − | 2 | 0 | 0 | ||
Infected indicates positive by PCR or immunohistochemistry. Occluded indicates one or more uteri or uterine tubes occluded by fibrosis. Low titers indicates both anti-hsp60 and hsp 10 titers below the mean. High titers indicates both anti-hsp60 and hsp 10 titers above the mean.
Presence (+) or absence (−) of active inflammation.
Score for chlamydial infection represents the approximate chlamydial load, calculated from the mean number of chlamydial inclusions detected by IHC in each of the components of the uFRT (cervix, ulerus, uterine tubes, ovarian bursa, and ovary), lower FRT (lFRT) (urogenital sinus and vaginal complex), and urinary bladder. The mean numbers of inclusions/cross-section for each component of the FRT were estimated from at least two sections for each side of the FRT.
Low anti-hsp antibody titers did not appear to result from an absence of Chlamydia. Immunohistochemical stains of all 10 actively inflamed uFRT (6 occluded, 4 patent) demonstrated chlamydial inclusions in epithelial cells or macrophages in 9 cases (Table 2). In the uFRT, epithelial chlamydial inclusions were associated with the active phase of inflammation where epithelium remained and were, therefore, most common in IP koalas, less common in IO low-titer koalas, and not detected in the uFRT of IO high-titer koalas. Chlamydial inclusions were evident in macrophages of IP and IO low-titer koalas but not in IO high-titer koalas.
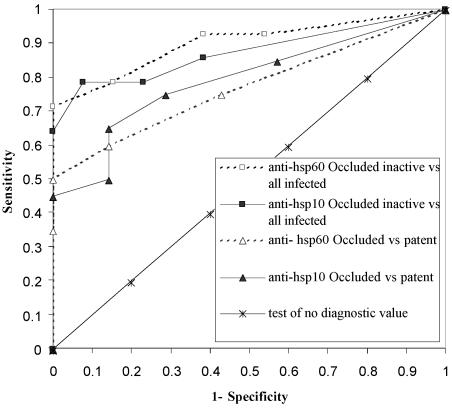
On the basis of these findings, the assays were assessed as a diagnostic test to detect, among Chlamydia-infected koalas (n = 27), those with tubal occlusion where the active component of uFRT inflammation was absent (IO, inactive; n = 14). Comparison of receiver operating characteristic curves of all tests illustrated that assays of anti-hsp60 and anti-hsp10 antibodies were more sensitive and specific in diagnosing koalas with tubal occlusion but without an active component of metritis or salpingitis (for anti-hsp10 IgG, sensitivity of 79% and specificity of 92%) than diagnosing tubal occlusion alone (Fig. 2). This is further illustrated by likelihood ratios and Cohen's kappa values (Table 1). Concordance of the anti-hsp10 IgG assay results was greatest, with the lower 95% confidence interval for the Cohen's kappa value exceeding 0.4.
FIG. 2.
Receiver operating curves, comparing koala anti-hsp60 and anti-hsp10 IgG assays as tests to predict (i) tubal occlusion alone (fibrous occlusion of one or more sites in one or both uteri or uterine tubes) and (ii) tubal occlusion without active inflammation (occluded inactive) among Chlamydia-infected koalas. Curves are generated from sensitivity and specificity values calculated for a range of arbitrary cutoff values. The best assays and cutoff points are those closest to the top left corner (high sensitivity and specificity). Anti-hsp IgG assays were more predictive of koalas with tubal occlusion without an active component of inflammation in the uFRT than of tubal occlusion alone. vs, versus.
DISCUSSION
This study reports, for the first time, IgG, IgA, and IgE responses of koalas to two important chlamydial antigens: c-hsp60, a likely pathogenic antigen of chlamydial disease in other species; and c-hsp10, a prominent T-cell antigen, which stimulates responses that are associated with reproductive tract pathology in women (23). As in women, elevated antibody responses to c-hsp60 and c-hsp10 were associated with fibrosis and occlusion of the uFRT of Chlamydia-infected koalas, and as found by La Verda et al. (23), anti-hsp10 IgG was a more accurate indicator of disease state than anti-hsp60 IgG.
Additional investigation in this study was prompted by the observation that a subset of koalas with tubal occlusion failed to develop hsp-specific antibody titers, thereby limiting the sensitivity of the assays for detecting occlusive fibrosis of the uFRT. This phenomenon has been documented in a study of women affected by chlamydiosis, in which it was suggested that titers diminish with age, as most infections occur in young adulthood (22). The present study detected no effect of age on titers within the life span of koalas, nor did it suggest that the poor body condition and marked hypoproteinemia suffered by many koalas significantly impairs production of hsp-specific antibodies. Rather, we found an association between low antibody titers and the presence of active inflammation in koalas with tubal occlusion: Chlamydia-infected koalas with low titers had more chlamydial inclusions and intense submucosal and epithelial infiltrates of neutrophils and lymphocytes, with or without a background of fibrosis and plasma cell infiltration, whereas those with high titers had occlusive fibrosis of the upper reproductive tract but no detectable chlamydial inclusions and either no inflammatory infiltrate or mild infiltrates of macrophages and plasma cells only.
Though reported for chlamydial metritis of humans and koalas (17), uterine epithelial lymphocytic infiltrates are not considered traditionally to be a characteristic feature of active inflammation, nor are they considered to be characteristic of the disease in humans, as physiological infiltrates also occur at certain phases of the reproductive cycle (33). In the current study, the lymphocytic infiltrates that accompanied infiltrates of neutrophils were much more pronounced than those seen in healthy koala reproductive tracts in a range of reproductive states and were strongly associated with the presence of chlamydial inclusions. The significance of these lymphocytic infiltrates, which can be associated with marked inflammatory damage to tissues, is not understood fully. As the present study uses a novel approach to the study of chlamydial disease in koalas, the relationship between low anti-hsp antibody titers and epithelial neutrophil and lymphocyte infiltrates in koalas is discussed below in the context of two models from studies of chlamydial disease of humans: the Th1/Th2 balance and the epithelial response.
Firstly, intense lymphocytic infiltrates and low antibody titers are considered widely to reflect delayed-type hypersensitivity, or Th1-mediated, response to chlamydial antigens, such as hsp60. As these antigens are well-conserved, this model constitutes a theoretical basis for potential pathogenic interaction between different chlamydial species or serotypes or between chlamydial and nonchlamydial bacteria and for putative autoimmunity to self-hsp60 antigens. This model would be supported by demonstration of antigen-presenting cells expressing major histocompatibility complex class II (MHC-II) (APCII), CD4+ T helper cells, and CD8+ cytotoxic T cells within the uterine epithelium, as the Th1 and Th2 responses are mediated via these cells. In addition, if MHCII expression of uterine epithelial cells were to exceed that of urinary tract epithelium, it might explain the stronger association of anti-hsp titers with uFRT pathology than bladder pathology. MHCII is expressed on uterine epithelial cells of rats and humans in response to IFN-γ, and these cells have been demonstrated to present antigen to lymphocytes (41). While neither CD4- nor CD8-specific antibodies have been identified for marsupials, Hemsley and Canfield (17) identified an antibody cross-reactive to marsupial HLA-DR (an epitope of MHCII) and labeled MHCII in macrophages, urogenital sinus, and prostatic epithelial cells in inflamed tissues of koalas. Therefore, the potential to pursue this avenue in part exists.
In addition, serological assays developed for this study might prove useful to investigate the Th1/Th2 model in koalas. If the prominent lymphocytic infiltrates seen in inflamed tissues do reflect Th1-mediated responses, it could be speculated that antibody titers might decrease during periodic episodes of chlamydial replication and reactivated inflammation, because antibody production is predominantly associated with Th2 responses. Then, as the organism is reduced to below detectable levels in the uFRT, antibody titers might again increase as Th2 dominance resumes and is perpetuated by chronic low-grade stimulation by the remaining hsp antigens, which are preferentially expressed by persistent “aberrant” latent forms of Chlamydia (2). Alternative sources of stimulatory hsp antigens might be cross-reactive epitopes of the host's own hsp antigens or conserved hsp antigens of other microorganisms, as suggested by Domeika et al. (9). Fluctuating antibody titers in longitudinal studies of Chlamydia-infected koalas would support this model. Further, if fluctuating antibody titers correspond (presumably with a lag phase for breakdown of existing antibody) to recurrent bouts of reactivated uFRT infection and inflammation, and thereby indicate movement between Th1 and Th2 dominance, they might correlate with host or environmental factors influencing this balance. The Th1/Th2 paradigm is still contentious (36), especially in veterinary medicine (4, 8), and it is likely that, if taken strictly, it is an oversimplification of a complex system. However, the Th1/Th2 model would provide a mechanism through which the koala might focus its immune response efficiently, conserving energy and protein. If economically balanced between these disease-specific pathways, the koalas' immune system might be vulnerable to the influence of the various changing environmental or genetic factors that influence the balance in other species. If it is shown that antibody titers diminish in association with recurrent bouts of reactivated infection, such assays could be applied in longitudinal studies of koalas in an attempt to identify environmental factors that might promote reactivation of latent infection. In the current study, IgA and IgE titers were measured in addition to IgG, because it was anticipated they might be more-specific indicators of the Th2 response than IgG, as some IgG2a production is associated with the Th1 response. Data in the current study indicated that IgA and IgE responses were similar to IgG responses, but small sample size and the bimodal distribution of data made the assessment of IgA and IgE assays as predictive tests difficult.
Another method by which to identify Th1 or Th2 dominance is by detection of the characteristic cytokines, IFN-γ and interleukin 4 (IL-4), respectively. A commercial bovine anti-IFN-γ antibody labeled, in flow cytometric studies, a product of stimulated koala lymphocytes, which is likely to be IFN-γ (19). Therefore, studies of Th1 cells in marsupials will be possible in the future.
A second model exists to explain these intense epithelial infiltrates of lymphocytes. Stephens (37) proposed that local cellular responses, mediated by epithelial production of IL-1a, IL-8, granulocyte-macrophage colony-stimulating factor, and IL-6 (34), are sufficient to induce a damaging lymphocytic response that continues as long as infected epithelial cells remain. Such a system does not rely on APCII pathways and, therefore, might be expected to be relatively independent of antibody production. Therefore, absence of MHCII expression by uterine epithelial cells, a paucity of CD4+ lymphocytes in epithelial infiltrates, and failure of antibody titers to diminish with recurrent reactivation of infection would be supportive of this model. In this context, the data in the current study might reflect development of titers over time, representing a progression from actively inflamed, patent (IP); to actively inflamed, occluded (IO low); to occluded with reduction of organisms to below detectable levels and disappearance of the active component of inflammation (IO high). LaVerda et al. (23) suggest this to be the case by hypothesizing that hsp10 may be poorly immunogenic, requiring the chronic exposure associated with fibrosis to elicit a response. In this case, one might expect titers to remain elevated during subsequent reactivation of infection and inflammation. If the epithelial response model were to prevail, it would diminish support for a role for conserved antigens in pathogenic interactions between different invading organisms, as it is not based on hypersensitization to conserved antigens.
Regardless of whether changing titers represent progression of the disease, movement between Th1 and Th2 dominance in response to reinfection, or reactivation and regression of persistent infection, an assay to predict the nature of the disease in the reproductive tract has application in assessing the responses of hospitalized koalas to treatment and in epidemiological studies of wild koalas. The findings of such studies might have considerable impact on our management of koalas in the wild and in captivity and better describe mechanisms by which environmental factors influence the emergence of disease.
Confidence in this assay as a predictive test will be improved with additional data. Limited sample sizes are a common feature of studies of koala immunology and pathology in comparison to studies in humans (3, 18, 32, 42), and it was difficult to obtain wild uninfected koalas or Chlamydia-infected koalas without marked urogenital tract fibrosis, as most of these were treated for release. However, it appears that the accumulation of data necessary to validate this assay as a diagnostic test and to better interpret its significance is feasible. Standards were developed in the current study to assist this process. In addition, its predictive value might be improved further by combination with an additional test. For example, acute-phase reactants can be elevated in cats with chlamydial conjunctivitis (39), and future investigation of this aspect might yield an additional indicator of disease state in koalas.
Though not consistent throughout this study, data suggested that reproductive status might influence IgG production. This finding cannot be considered significant at this stage but should be considered in future studies.
Notwithstanding these limitations, the findings of this study do suggest that serological response against chlamydial hsp60 and hsp10 is a useful tool to predict tubal occlusion and the absence of an active component of uFRT inflammation among Chlamydia-infected koalas. These assays are a significant advancement in clinical or epidemiological studies of host-pathogen-environment interactions in chlamydial disease of koalas. These findings also pose intriguing questions regarding the significance of these prominent epithelial neutrophil and, particularly, lymphocyte infiltrates in terms of the Th1/Th2 paradigm, antigen-presenting and inflammation-regulating capabilities of uterine epithelial cells, and the roles of latency and reactivation of chlamydial infections in pathogenesis of upper reproductive tract disease. Future work in this area is expected to shed additional light on pathogenesis of the disease in both koalas and humans.
Acknowledgments
This work was supported by grants from the Australian Research Council, the Winifred Scott Foundation, the Jean Walker Trust, and the University of Sydney UPA scheme.
All activities were performed with permission of the University of Sydney Animal Ethics Committee, Queensland Parks and Wildlife Service, and NSW National Parks and Wildlife Service.
In particular we thank Richard Morrison (Montana State University) and Gerald Byrne (University of Wisconsin—Madison) for their generous donation of chlamydial hsp60 and hsp10 peptides, the staff of Moggill Koala Hospital QPWS for their assistance in sample collection, and Renee Rawson (Macquarie University) for her kind donation of anti-marsupial IgA and IgE antibodies. We also thank Karen Barnes and Elaine Chew (VPDS, University of Sydney) for preparation of tissue sections, Peter Thompson (University of Sydney) for advice in statistics, and the journal referees for their comments on the manuscript.
REFERENCES
- 1.Beagley, K. W., and C. M. Gockel. 2003. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol. Med. Microbiol. 38:13-22. [DOI] [PubMed] [Google Scholar]
- 2.Beatty, W. L., G. I. Byrne, and R. P. Morrison. 1993. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc. Natl. Acad. Sci. USA 90:3998-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, A. S., and R. G. Grice. 1986. Experimental transmission of Chlamydia psittaci in the koala, p. 349-352. In D. Oriel, G. Ridgeway, J. Schachter, D. Taylor-Robinson, and M. Ward (ed.), Chlamydial infections. Cambridge University Press, Cambridge, England.
- 4.Brown, W. C., A. C. Rice-Ficht, and D. M. Estes. 1998. Bovine type 1 and type 2 responses. Vet. Immunol. Immunopathol. 63:45-55. [DOI] [PubMed] [Google Scholar]
- 5.Canfield, P. 1990. Disease studies on New South Wales koalas, p. 249-254. In A. K. Lee, K. A. Handasyde, and G. D. Sanson (ed.), Biology of the koala. Surrey Beatty and Sons, Sydney, Australia.
- 6.Casadevall, A., and L. A. Pirofski. 1999. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect. Immun. 67:3703-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cork, S. J., and L. W. Braithwaite. 1990. Resource availability, eucalypt chemical defences, and habitat quality for leaf eating marsupials, p. 9-16. In G. Gordon (ed.), Koalas: research for management. Proceedings of the Brisbane Koala Symposium. World Koala Research, Inc., Brisbane, Australia.
- 8.Debattista, J., P. Timms, and J. Allan. 2003. Immunopathogenesis of Chlamydia trachomatis infections in women. Fertil. Steril. 79:1273-1287. [DOI] [PubMed] [Google Scholar]
- 9.Domeika, M., K. Domeika, J. Paavonen, P. A. Mardh, and S. S. Witkin. 1998. Humoral immune response to conserved epitopes of Chlamydia trachomatis and human 60-kDa heat-shock protein in women with pelvic inflammatory disease. J. Infect. Dis. 177:714-719. [DOI] [PubMed] [Google Scholar]
- 10.Ellis, W. A., A. A. Girjes, F. N. Carrick, and A. Melzer. 1993. Chlamydial infection in koalas under relatively little alienation pressure. Aust. Vet. J. 70:427-428. [DOI] [PubMed] [Google Scholar]
- 11.Fowler, E. V., B. A. Houlden, P. Hoeben, and P. Timms. 2000. Genetic diversity and gene flow among southeastern Queensland koalas (Phascolarctos cinereus). Mol. Ecol. 9:155-164. [DOI] [PubMed] [Google Scholar]
- 12.Girjes, A. A., F. N. Carrick, and M. F. Lavin. 1999. Single DNA sequence common to all chlamydial species employed for PCR detection of these organisms. Res. Microbiol. 150:483-489. [DOI] [PubMed] [Google Scholar]
- 13.Girjes, A. A., W. A. Ellis, F. N. Carrick, and M. F. Lavin. 1993. Some aspects of the immune response of koalas (Phascolarctos cinereus) and in vitro neutralization of Chlamydia psittaci (koala strains). FEMS Immunol. Med. Microbiol. 6:21-30. [DOI] [PubMed] [Google Scholar]
- 14.Griffin, J. F. T., and A. J. Thomson. 1998. Farmed deer: a large animal model for stress. Domestic Animal Endocrinol. 15:445-456. [DOI] [PubMed] [Google Scholar]
- 15.Handasyde, K. A. 1986. Factors affecting reproduction in the female koala (Phascolarctos cinereus). Ph.D. thesis. Monash University, Clayton, Victoria, Australia.
- 16.Handasyde, K. A., R. W. Martin, and A. K. Lee. 1988. Field investigations into chlamydial disease and infertility in koalas in Victoria, p. 505-515. In Proceedings 104. Post Graduate Committee in Veterinary Science, University of Sydney, Sydney, Australia.
- 17.Hemsley, S., and P. J. Canfield. 1997. Histopathological and immunohistochemical investigation of naturally occurring chlamydial conjunctivitis and urogenital inflammation in koalas (Phascolarctos cinereus). J. Comp. Pathol. 116:273-290. [DOI] [PubMed] [Google Scholar]
- 18.Hemsley, S. W., P. J. Canfield, and A. J. Husband. 1996. Histological and immunohistological investigation of alimentary tract lymphoid tissue in the koala (Phascolarctos cinereus), brushtail possum (Trichosurus vulpecula) and ringtail possum (Pseudocheirus peregrinus). J. Anat. 188:279-288. [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins, D. P., S. Hemsley, and P. J. Canfield. 2004. Assessment of anti-bovine IL4 and IFN gamma antibodies to label IL4 and IFN gamma in lymphocytes of the koala and brushtail possum. Vet. Immunol. Immunopathol. 101:153-160. [DOI] [PubMed] [Google Scholar]
- 20.Jackson, M., N. White, P. Giffard, and P. Timms. 1999. Epizootiology of Chlamydia infections in two free-range koala populations. Vet. Microbiol. 65:255-264. [DOI] [PubMed] [Google Scholar]
- 21.Johnston, S. D. 1999. Studies towards the development of an artificial insemination program in the koala (Phascolarctos cinereus). University of Queensland, Brisbane, Australia.
- 22.Land, J. A., J. L. Evers, and V. J. Goossens. 1998. How to use Chlamydia antibody testing in subfertility patients. Hum. Reprod. 13:1094-1098. [DOI] [PubMed] [Google Scholar]
- 23.LaVerda, D., L. N. Albanese, P. E. Ruther, S. G. Morrison, R. P. Morrison, K. A. Ault, and G. I. Byrne. 2000. Seroreactivity to Chlamydia trachomatis Hsp10 correlates with severity of human genital tract disease. Infect. Immun. 68:303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaVerda, D., M. V. Kalayoglu, and G. I. Byrne. 1999. Chlamydial heat shock proteins and disease pathology: new paradigms for old problems? Infect. Dis. Obst. Gynecol. 7:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, R. H., M. J. Mamula, J. A. Hardin, and C. A. Janeway, Jr. 1991. Induction of autoreactive B cells allows priming of autoreactive T cells. J. Exp. Med. 173:1433-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mainali, E. S., and D. N. McMurray. 1998. Protein deficiency induces alterations in the distribution of T-cell subsets in experimental pulmonary tuberculosis. Infect. Immun. 66:927-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mamula, M. J., R. H. Lin, C. A. Janeway, Jr., and J. A. Hardin. 1992. Breaking T cell tolerance with foreign and self co-immunogens. A study of autoimmune B and T cell epitopes of cytochrome c. J. Immunol. 149:789-795. [PubMed] [Google Scholar]
- 28.Martin, R. W. 1981. Age-specific fertility in 3 populations of the koala, Phascolarctos cinereus, in Victoria, Australia. Aust. Wildl. Res. 8:275-284. [Google Scholar]
- 29.Montgomery, M. E., R. Duckett, B. A. Houlden, and D. A. Taggart. 2001. Inbreeding depression in male koalas, p. 215. In Proceedings of Veterinary Conservation Biology, Wildlife Health and Management in Australasia. Australian Veterinary Association, Kingston, Australia.
- 30.Obendorf, D. L. 1983. Causes of mortality and morbidity of wild koalas, Phascolarctos cinereus, in Victoria, Australia. J. Wildl. Dis. 19:123-131. [DOI] [PubMed] [Google Scholar]
- 31.Obendorf, D. L. 1988. The pathogenesis of urogenital tract disease in the koala, p. 649-655. In Proceedings 104. Post Graduate Committee in Veterinary Science, University of Sydney, Sydney, Australia.
- 32.Obendorf, D. L., and K. A. Handasyde. 1990. Pathology of chlamydial infection in the reproductive tract of the female koala, p. 255-259. In A. K. Lee, K. A. Handasyde, and G. D. Sanson (ed.), Biology of the koala. Surrey Beatty and Sons, Sydney, Australia.
- 33.Paavonen, J., R. Aine, K. Teisala, P. K. Heinonen, R. Punnonen, M. Lehtinen, A. Miettinen, and P. Gronroos. 1985. Chlamydial endometritis. J. Clin. Pathol. 38:726-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasmussen, S. J., L. Eckmann, A. J. Quayle, L. Shen, Y. X. Zhang, D. J. Anderson, J. Fierer, R. S. Stephens, and M. F. Kagnoff. 1997. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Investig. 99:77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rawson, R. R., K. Belov, A. A. Gidley-Baird, and D. W. Cooper. 2002. Characterisation of antisera to recombinant IgA of the common brushtail possum (Trichosurus vulpecula). Vet. Immunol. Immunopathol. 88:89-95. [DOI] [PubMed] [Google Scholar]
- 36.Rook, G. 2001. Th1- or Th2-cell commitment during infectious disease: an oversimplification? Trends Immunol. 22:481-482. [DOI] [PubMed] [Google Scholar]
- 37.Stephens, R. S. 2003. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 11:44-51. [DOI] [PubMed] [Google Scholar]
- 38.Storz, J., and B. Kaltenboeck. 1993. The Chlamydiales, p. 27-64. In Z. Woldehiwet and M. Ristic (ed.), Rickettsial and chlamydial diseases of domestic animals. Pergamon Press, Oxford, England.
- 39.TerWee, J., M. Sabara, K. Kokjohn, J. Sandbulte, P. Frenchick, and K. J. Dreier. 1998. Characterization of the systemic disease and ocular signs induced by experimental infection with Chlamydia psittaci in cats. Vet. Microbiol. 59:259-281. [DOI] [PubMed] [Google Scholar]
- 40.Toye, B., C. Laferriere, P. Claman, P. Jessamine, and R. Peeling. 1993. Association between antibody to the chlamydial heat-shock protein and tubal infertility. J. Infect. Dis. 168:1236-1240. [DOI] [PubMed] [Google Scholar]
- 41.Wallace, P. K., G. R. Yeaman, K. Johnson, J. E. Collins, P. M. Guyre, and C. R. Wira. 2001. MHC class II expression and antigen presentation by human endometrial cells. J. Steroid Biochem. Mol. Biol. 76:203-211. [DOI] [PubMed] [Google Scholar]
- 42.Wilkinson, R., I. Kotlarski, and M. Barton. 1994. Further characterisation of the immune response of the koala. Vet. Immunol. Immunopathol. 40:325-339. [DOI] [PubMed] [Google Scholar]
- 43.Witkin, S. S., M. Askienazy-Elbhar, J. Henry-Suchet, J. Belaisch-Allart, J. Tort-Grumbach, and K. Sarjdine. 1998. Circulating antibodies to a conserved epitope of the Chlamydia trachomatis 60 kDa heat shock protein (hsp60) in infertile couples and its relationship to antibodies to C. trachomatis surface antigens and the Escherichia coli and human Hsp60. Hum. Reprod. 13:1175-1179. [DOI] [PubMed] [Google Scholar]
- 44.Yang, X., K. T. HayGlass, and R. C. Brunham. 1996. Genetically determined differences in IL-10 and IFN-gamma responses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J. Immunol. 156:4338-4344. [PubMed] [Google Scholar]
- 45.Yi, Y., X. Yang, and R. C. Brunham. 1997. Autoimmunity to heat shock protein 60 and antigen-specific production of interleukin-10. Infect. Immun. 65:1669-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong, G., and R. C. Brunham. 1992. Antibody responses to the chlamydial heat shock proteins hsp60 and hsp70 are H-2 linked. Infect. Immun. 60:3143-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]