Abstract
A commercial assay for detection of measles immunoglobulin G (IgG) in oral fluid was evaluated in a highly vaccinated cohort using serum IgG as gold standard. In contrast to previous studies from cohorts protected by natural immunity, antibody prevalence was significantly underestimated (−7.4%; confidence interval: −1.5 to −13.2%; P = 0.01) due to a reduced sensitivity when antibody levels were low.
Vaccination strategies to control and eliminate measles have been widely monitored by seroepidemiological studies. The prevalence of immunoglobulin G (IgG) antibodies in the blood is considered a solid correlate of population immunity. A major drawback of serum IgG is the need to draw blood with all its risks and ethical implications. Therefore, the detection of IgG in oral fluid, which can be collected with relative ease, has been promoted as a noninvasive alternative for seroprevalence studies (6). Specific IgG of a number of viruses has been found in oral fluid (3, 4, 7, 8). Nigatu et al. have developed an antibody capture enzyme immunoassay (EIA) to detect measles IgG in oral fluid, which was recently commercialized (5). Estimates of antibody prevalence in two cohorts from Ethiopia with low vaccination coverage did not significantly differ when IgG in oral fluid and serum were compared (5, 6). The aim of the present study was to evaluate IgG detection in oral fluid in a highly vaccinated central European community.
Paired serum and oral fluid samples were collected from 283 school-children (age range: 18.1 to 21.9; mean age: 19.6; male: 20.1%) attending three different secondary schools in LuxembourgMost (87.3%) of them had received one or two doses of measles vaccine during early childhood and/or adolescence. The others were not vaccinated (6.0%) or had unreliable vaccination records (6.7%). Seventeen individuals with high serum antibody titers were not vaccinated and are considered convalescent measles patients. Informed consent was obtained from all participants or their guardians. The study was approved by the local ethics committee and the responsible ministries.
Peripheral blood was obtained by venipuncture and incubated for 1 h at 37°C. Serum was collected after centrifugation for 25 min at 1,700 × g, divided into aliquots, and stored at −20°C until testing. Anti-measles virus IgG levels in the serum were assessed by the World Health Organization-recommended EIA (Enzygnost; Dade-Behring, Marburg) with a validated sensitivity and specificity of 99.6 and 100%. Antibody status (positive >0.2 optical densities [OD]; negative <0.1 OD; or equivocal 0.1 to 0.2 OD) and concentrations (mIU/ml) were determined using the alpha method (2) according to the manufacturer's protocol.
Oral fluid was collected as described elsewhere (7) by moving a cylindrical sponge (Oracol; Malvern Medical Developments, Worcester, United Kingdom) around the gums for about 1 min and kept at 4°C until processing. Within a maximum of 4 h postcollection oral fluid was extracted from the sponge by centrifugation (700 × g, 5 min) and immediately stored undiluted at −20°C. Antibody levels were determined using a commercial EIA recommended and optimized for oral fluid (Microimmune Ltd., Middlesex) following strictly the manufacturer's instructions (positive, >1.25× the mean OD of three negative controls [ODnc]); negative, <1.1× ODnc; equivocal, between 1.1 to 1.25× ODnc). Briefly total IgG from undiluted oral fluid was captured in anti-human IgG coated microtiter wells. Recombinant measles nucleoprotein (rMVN), a monoclonal antibody to rMVN conjugated to horseradish peroxidase and the substrate (tetramethylbenzidine) were sequentially added to reveal the presence of measles-specific antibodies. Sensitivity, specificity, and positive and negative predictive values of the oral fluid assay were determined by comparison to serum IgG as described by Nokes et al. (7) using the Enzygnost test as a gold standard. Exact binomial confidence intervals of proportions and differences between proportions (z-test) were determined using SigmaStat version 3.0.1a software (Systat software Inc., Point Richmond).
Measles specific IgG was measured in 283 paired oral fluid and serum samples. Numbers of individuals with positive, negative, or equivocal absorbance values in the two assays are shown in Table 1. When equivocal absorbances in either of the assays were excluded, prevalence estimates obtained from serum (89.0%, confidence interval [CI]: 84.8 to 92.4%) were significantly higher than those for oral fluid (81.6%, CI: 76.6 to 86.0%). Higher values but similar differences were obtained when equivocal values were included as positives (serum, 95.4%, CI: 92.3 to 97.5%; oral fluid, 88.3%, CI: 84.0 to 91.8%). A concordant antibody status (positive, negative, equivocal) was found in 227 individuals (80.2%). When individuals with equivocal absorbances in either test were excluded, a concordance of 90.0% was observed.
TABLE 1.
Numbers of individuals by measles-specific antibody status (positive, negative, equivocal) in serum and oral fluid
| Oral fluid | Serum
|
Total | ||
|---|---|---|---|---|
| Positive n (%) | Negative n (%) | Equivocal n (%) | ||
| Positive n (%) | 217 (76.7) | 2 (0.7) | 12 (4.2) | 231 (81.7) |
| Negative n (%) | 23 (8.1) | 7 (2.5) | 3 (1.1) | 33 (11.6) |
| Equivocal n (%) | 12 (4.2) | 4 (1.4) | 3 (1.1) | 19 (6.7) |
| Total | 252 (89.0) | 13 (4.6) | 18 (6.4) | 283 (100) |
When the serum assay was taken as a gold standard and individuals with equivocal results in either test (12.0%) were excluded, sensitivity and positive predictive values of the oral fluid assay were 90.4% (CI: 86.0 to 93.8%) and 99.1% (CI: 96.7 to 99.9%), respectively. The specificity (77.8%; CI: 40.0 to 97.2%) as well as negative predictive values were lower (23.3%; CI: 9.9 to 42.3%), but the number of seronegative individuals (n = 13) was limited.
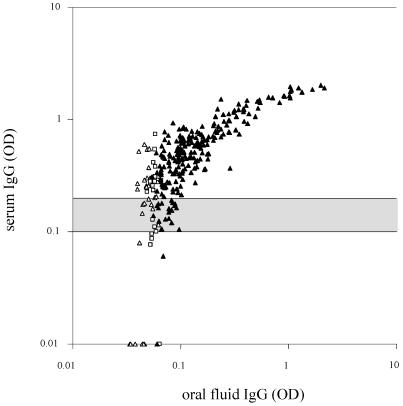
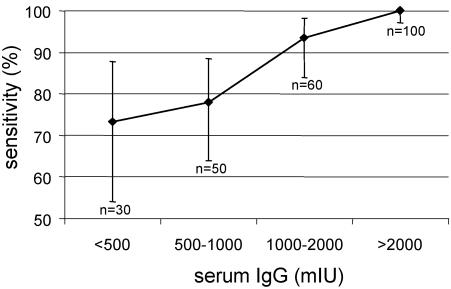
Figure 1 shows that the oral fluid assay misses many of the individuals with low specific serum IgG. Sensitivity was significantly lower in our cohort of central European vaccinees than those determined by Nokes et al. (6) in a cohort from rural Ethiopia (97%). Very low and low vaccination coverage rates (16.6 to 54.8%) were reported in that study from rural and urban communities in Ethiopia. Furthermore, about half of the individuals were >20 years old and unlikely to have been vaccinated. The rural cohort may have consisted of 85% and more of late convalescent measles patients. Natural infection normally induces much higher, by some accounts 5 to 10 times higher, antibodies than vaccination (1), similar to rubella (9). Thus, the discrepancies in sensitivity may be fully explained by the differences in antibody levels in individuals with wild-type virus or vaccine-induced immunity. To compensate for low antibody concentrations, the oral fluid EIA is based on total IgG capture technology (5). In contrast, the serum EIA binds measles-specific antibody only. This may partially account for the difference in sensitivity between both methods. Figure 2 shows that the sensitivity of the oral fluid method increases with higher serum antibody levels. The lowest sensitivity (73.3%, CI: 54.1 to 87.7%) was found among individuals with antibody titers below 500 mIU (0.28 OD) and gradually increased to 100% (CI: 97.1 to 100%) when serum antibodies reached >2,000 mIU (0.65 OD). As a consequence seroprevalence in a highly vaccinated community is underestimated by oral fluid IgG detection due to an insufficient sensitivity of the assay in the low titer range. However, the oral fluid test may be useful to identify individuals with no or low serum antibodies for revaccination. In this case a high specificity must be warranted to avoid that seronegative individuals would be missed. In the present cohort, 2 of 13 seronegative individuals gave weak but false-positive results in the oral fluid assay. Although only weakly positive, these false positive donors could only be excluded at the expense of higher number of false negatives. For instance, if the positive threshold is raised from 1.25× to 1.5× ODnc, the number of greyzone individuals would increase from 19 to 59 in this study. A careful reevaluation of the positive/negative cutoffs even in a cohort of vaccinees including sufficient numbers of seronegative donors may not necessarily be the solution. In general, overvaccination would be preferred to undervaccination.
FIG. 1.
Comparison of measles-specific IgG (OD values) in serum and oral fluid of 283 adolescents. Horizontal lines indicate cutoff values of the serum assay (negative, <0.1 OD; positive, >0.2 OD; greyzone, 0.1 to 0.2 OD). IgG-positive (▴), -negative (▵), and -equivocal (□) oral fluid samples. In contrast to the serum assay, greyzone intervals of the oral fluid assay are defined with respect to negative controls on the same test plate and not in absolute OD values.
FIG. 2.
Sensitivity and CI of the oral fluid test for different levels of serum IgG (mIU). Number of individuals per category are given.
Our results show that characteristics, in particular the sensitivity of measles antibody assays, largely depend on the vaccination status of the cohort and should be evaluated not only in late convalescents but also in vaccinees.
Acknowledgments
We thank all participants for donating blood and oral fluid. We also thank Ulla Muller, Evguenia Pasthukova, and Stephanie Willième for administrative and technical support. We also acknowledge the support of the Centre de Recherche Public-Santé, the Ministère de la Recherche, the Ministère de la Santé, and the participating schools.
J.R.K. is supported by a fellowship BFR of the Ministère de la Recherche, Luxembourg.
REFERENCES
- 1.Damien, B., S. Huiss, F. Schneider, and C. P. Muller. 1998. Estimated susceptibility to asymptomatic secondary immune response against measles in late convalescent and vaccinated persons. J. Med. Virol. 56:85-90. [DOI] [PubMed] [Google Scholar]
- 2.Dopatka, H. D., and B. Giesendorf. 1992. Single point quantification of antibody by ELISA without need of a reference curve. J. Clin. Lab. Anal. 6:417-422. [DOI] [PubMed] [Google Scholar]
- 3.Granade, T. C., S. K. Phillips, B. Parekh, P. Gomez, W. Kitson-Piggott, H. Oleander, B. Mahabir, W. Charles, and S. Lee-Thomas. 1998. Detection of antibodies to human immunodeficiency virus type 1 in oral fluids: a large-scale evaluation of immunoassay performance. Clin. Diagn. Lab. Immunol. 5:171-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moe, C. L., A. Sair, L. Lindesmith, M. K. Estes, and L. A. Jaykus. 2004. Diagnosis of Norwalk virus infection by indirect enzyme immunoassay detection of salivary antibodies to recombinant Norwalk virus antigen. Clin. Diagn. Lab. Immunol. 11:1028-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nigatu, W., D. J. Nokes, F. Enquselassie, D. W. Brown, B. J. Cohen, A. J. Vyse, and F. T. Cutts. 1999. Detection of measles specific IgG in oral fluid using an FITC/anti-FITC IgG capture enzyme linked immunosorbent assay (GACELISA). J. Virol. Methods 83:135-144. [DOI] [PubMed] [Google Scholar]
- 6.Nokes, D. J., F. Enquselassie, W. Nigatu, A. J. Vyse, B. J. Cohen, D. W. Brown, and F. T. Cutts. 2001. Has oral fluid the potential to replace serum for the evaluation of population immunity levels? A study of measles, rubella and hepatitis B in rural Ethiopia. Bull. W. H. O. 79:588-595. [PMC free article] [PubMed] [Google Scholar]
- 7.Nokes, D. J., W. Nigatu, A. Abebe, T. Messele, A. Dejene, F. Enquselassie, A. Vyse, D. Brown, and F. T. Cutts. 1998. A comparison of oral fluid and serum for the detection of rubella-specific antibodies in a community study in Addis Ababa, Ethiopia. Trop. Med. Int. Health 3:258-267. [DOI] [PubMed] [Google Scholar]
- 8.Sheppard, C., B. Cohen, N. Andrews, and H. Surridge. 2001. Development and evaluation of an antibody capture ELISA for detection of IgG to Epstein-Barr virus in oral fluid samples. J. Virol. Methods 93:157-166. [DOI] [PubMed] [Google Scholar]
- 9.Vyse, A. J., B. J. Cohen, and M. E. Ramsay. 2001. A comparison of oral fluid collection devices for use in the surveillance of virus diseases in children. Public Health 115:201-207. [DOI] [PubMed] [Google Scholar]