Abstract
Viral glycoproteins gB and gD of the swine alphaherpesvirus pseudorabies virus (PRV), which is closely related to human herpes simplex virus and varicella-zoster virus, are able to drive internalization of antibody-antigen complexes that may form at the cell surface of infected monocytes, thereby protecting these cells from efficient antibody-mediated lysis. We found earlier that gB relies on an endocytosis motif in its cytoplasmic domain for its function during this internalization process. Here, we report that the PRV gD protein also contains a functional endocytosis motif (YRLL) in its cytoplasmic domain that drives spontaneous endocytosis of gD from the cell surface early in infection and that acts in concert with the endocytosis motif in gB to contribute to efficient internalization of antibody-antigen complexes in PRV-infected monocytes.
Alphaherpesviruses have developed numerous strategies to delay or avoid recognition and elimination by different components of the immune system (11, 21, 45). The swine alphaherpesvirus pseudorabies virus (PRV) especially excels at circumventing antibody-dependent immunity, which allows it to replicate and sometimes spread in pigs that have been vaccinated with an inactivated vaccine (26, 48). PRV-infected blood monocytes play a pivotal role in spread of PRV in the presence of virus-neutralizing antibodies and carry the virus via the blood throughout the body (26).
In PRV-infected blood monocytes, like in other PRV-infected cells, newly produced viral envelope proteins are incorporated in the plasma membrane (12, 24), thereby rendering the cell recognizable for antibody-dependent immunity (13).
However, we found earlier that binding of virus-specific antibodies to viral cell surface proteins in PRV-infected blood monocytes leads to rapid internalization of the antibody-antigen complexes (12), thereby lowering the susceptibility of the infected cell towards antibody-mediated cell lysis (41). This internalization process was found to be clathrin mediated and to depend on two of the PRV proteins at the cell surface, gB and gD (42).
Clathrin-mediated endocytosis of cellular transmembrane proteins typically depends on so-called endocytosis motifs in their cytoplasmic domain, most notably YXXL and LL motifs (Y standing for tyrosine, L for leucine, and X for any amino acid). These motifs initiate endocytosis by establishing an interaction with the clathrin-associated AP-2 adaptor complex as a first step in the formation of clathrin-coated vesicles (3, 4, 20, 36).
We found that the function of gB in internalization of antibody-antigen complexes from the surface of PRV-infected monocytes depends on a functional tyrosine-based endocytosis motif (YQRL) in its cytoplasmic domain (10), and this motif was found to allow an interaction between gB and the AP-2 complex (43).
How PRV gD is involved in internalization of antibody-antigen complexes, on the other hand, is unknown. PRV gD is a type I membrane glycoprotein of 402 amino acids, consisting of an extracellular domain, transmembrane region, and a short carboxy-terminal domain of 26 amino acids. PRV gD, like gD of many other alphaherpesviruses, is crucial in establishing stable binding of virions with host cell receptors and subsequent virus entry (32, 34, 47). The cytoplasmic domain of gD contains a putative endocytosis sequence, YRLL (located at amino acid positions 384 to 387), where R stands for arginine. The aim of the present study was to reveal if the YRLL motif in PRV gD is a functional endocytosis motif and, if so, if it is involved in internalization of antibody-antigen complexes from the surface of PRV-infected monocytes.
To this end, we introduced defined point mutations in the open reading frame (ORF) of PRV gD (Fig. 1), replacing different amino acids in the YRLL motif with alanine (A), resulting in the following mutated motifs: ARLL, YRAL, YRLA, YRAA, and ARAA. In addition, a mutated gD ORF was constructed in which the lysine codon at position 382 was replaced by a premature translation termination codon (gDtrunc), resulting in a truncation of almost the entire cytosolic domain.
FIG. 1.
(A) Carboxy-terminal amino acid sequence of the PRV gD protein. The transmembrane domain is indicated by the shaded box, and the YRLL endocytosis motif is underlined. (B) Carboxy-terminal amino acid sequence of the PRV gD protein with alanine point mutations introduced in the YRLL motif (in bold and italic) and the mutation resulting in a premature translation termination codon.
Mutated gD ORFs were constructed as follows. The pT7-5 plasmid containing the PRV Becker 6.61-kb Bam7 restriction fragment was NotI-NcoI digested to release a 2.8-kb fragment, containing the PRV gD ORF and 900-bp upstream and 700-bp downstream flanking sequences, which was cloned into a NotI-NcoI-cut pALTER-Ex2 vector (Promega, Madison, Wisconsin) and subsequently subjected to oligonucleotide mutagenesis using the Altered Sites II kit (Promega). The oligonucleotides used were 5′-CGCGGGACCGCCAAGGAGGCGAGCCCCCTTCGCCCC-3′ (ARLL mutation, introducing a StyI site), 5′-CGCGGGACCGCCCAGAGCTCGATACCCCTTCGCGGG-3′ (YRAL mutation, introducing a SacI site), 5′-CGCGGGACCGCCCGCGAGGCGGTACCCCTTCGCCCC-3′ (YRLA mutation, introducing a KpnI site), 5′CGCGGGACCGCCCGCGGCGCGGTACCCCTTCGCCCC-3′ (YRAA mutation, also introducing a KpnI site), 5′-CGCGGGACCGCCAGCTGCGCGAGCCCCCTTCGCCCC-3′ (ARAA mutation, introducing a PvuII site), and 5′-GCCCAGGAGGCGATATCCCTACGCCCC CCTCAGGCG-3′ (gDtrunc mutation, introducing an EcoRV site). All introduced restriction sites facilitated the screening of correct clones. Plasmids (pALTER-Ex2) containing mutated PRV gD ORFs were digested with BstXI and StuI to generate 1.6-kb fragments encompassing mutated gD ORFs, which were ligated into BstXI-PmeI-digested pBudCE4.1 (Invitrogen) mammalian expression vector (blunt/sticky-end ligation). This resulted in eukaryotic expression plasmids encoding the different mutated gD ORFs under control of the human EF-1α promoter.
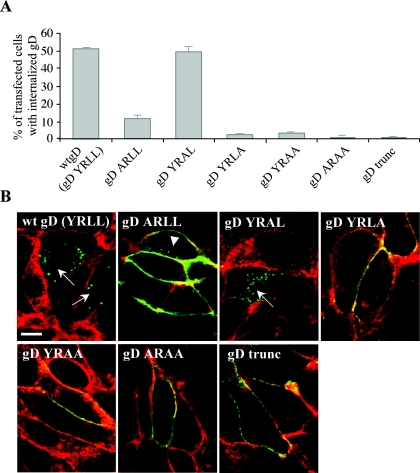
A first aim was to determine whether the YRLL motif in gD constitutes a functional endocytosis signal. Therefore, PK15 cells, grown to 70 to 80% confluency on glass coverslips, were transfected with 0.8 μg of each gD construct using Lipofectamine according to the manufacturer's protocol (Invitrogen) and subjected to an endocytosis assay, essentially as described before (39). Briefly, at 15 h posttransfection, cells were incubated on ice with monoclonal anti-gD antibodies (13D12) (25), washed, and subsequently shifted to 37°C for 30 min. Afterwards, cells were paraformaldehyde fixed, permeabilized with 0.1% Triton X-100, incubated with fluorescein isothiocyanate (FITC)-labeled goat anti-mouse antibodies (Molecular Probes, Eugene, Oregon), counterstained with the actin-labeling phalloidin-Texas Red (Molecular Probes), and examined for gD internalization by fluorescence and confocal microscopy (Leica TCS SP2 confocal system; Leica Microsystems GmbH, Heidelberg, Germany). For each slide, 200 cells were scored for the presence or absence of internalization of gD. Wild-type gD (YRLL motif) was found to undergo internalization from the plasma membrane in 51% of transfected cells (Fig. 2). The Y384A mutation significantly reduced, but did not completely inhibit, gD internalization (11% internalization). The YRAL mutation had no obvious effect on internalization efficiency (50% internalization), whereas the YRLA mutation, the YRAA mutation, the ARAA mutation, and the gDtrunc mutation almost completely abolished internalization (2%, 3%, 1%, and 1% internalization, respectively) (Fig. 2). Thus, the YRLL motif in the PRV gD tail is a functional endocytosis motif.
FIG. 2.
Efficiency of retrieval of PRV gD from the plasma membrane of PK15 cells transfected with plasmids carrying the different mutant gD ORFs. At 15 h posttransfection, cells were subjected to an internalization assay using gD-specific monoclonal antibodies. Afterwards, cells were fixed and permeabilized and localization of gD-specific antibodies was visualized using FITC-labeled goat anti-mouse antibodies (green); cells were counterstained using actin-labeling phalloidin-Texas Red (red) to detect contours of the cells. (A) Percentage of cells with internalization as determined by fluorescence microscopy; data represent means ± standard deviations of triplicate assays. (B) Representative confocal laser scanning microscopy images using the different mutated gD ORFs. Images shown are sections through the centers of the cells. Arrows indicate cells with strong endocytosis of gD, showing no detectable gD remaining at the cell surface. The arrowhead indicates a cell with few endocytic vesicles. Bar, 10 μm.
We found that mutation of the tyrosine residue in the YRLL sequence led to a strong decrease in endocytosis efficiency of gD, suggesting that the YRLL motif belongs to the earlier-mentioned YXXL endocytosis motifs. However, complete abrogation of gD endocytosis could only be observed when mutating the N-terminal residue in the YRLL motif (YRLA), but not when mutating the tyrosine residue (ARLL). This is somewhat surprising, since it is generally accepted that the tyrosine residue in YXXL motifs is a very critical residue in the endocytosis sequence (3, 4). Addition of amantadine-HCl (Sigma), an inhibitor of invagination of clathrin-coated pits, at a concentration of 0.5 mM, as we used before (43), reduced the efficiency of endocytosis of the ARLL-containing gD protein from 12.6 ± 0.6% to 6.5 ± 2.1%. This indicates that the weak endocytosis observed when mutating the tyrosine in the YRLL motif is clathrin mediated. Although speculative, a possible explanation for these observations may be that the YRLL motif not only functions as a YXXL endocytosis signal, but also as an LL endocytosis signal, which is generally believed to be a relatively weak endocytosis signal compared to YXXL motifs (4, 22). Mutation of the tyrosine residue only destroys the YXXL endocytosis motif in the YRLL sequence but not the LL motif, whereas mutation of the N-terminal leucine residue destroys both motifs. However, the LL motif in PRV gD is not preceded by an acidic amino acid at position −4 or −5 and, therefore, does not contain the characteristics of most typical functional LL endocytosis signals (33). Clearly, more research is needed to clarify this issue.
In a next step, we investigated whether PRV gD depends on the YRLL endocytosis signal to drive internalization of antibody-antigen complexes from the cell surface of PRV-infected monocytes, a process that protects these cells from efficient antibody-dependent lysis and that relies on gD and on a YXXL endocytosis motif in gB (10, 12, 41, 42).
To assess this, three mutations that were introduced in the YRLL motif (YRAL, YRLA, and gDtrunc) were selected and introduced in the PRV genome using the self-recombining bacterial artificial chromosome (BAC) containing the entire PRV Becker genome (37). First, a gDnull PRV screening BAC was constructed that contains a kanamycin resistance (KanR) cassette in place of the gD ORF. Therefore, pALTER-Ex2 containing the wild-type gD ORF with flanking sequences (as described earlier) was digested with BstXI-StuI, releasing a 1.6-kb fragment encompassing the entire gD ORF, and Klenow blunt ended. A 1.2-kb KanR cassette (released from pUC4K plasmid [Promega] with BamHI and Klenow blunt ended) was ligated into this plasmid, creating a plasmid containing a KanR cassette with 641-bp downstream and 540-bp upstream flanking sequences of PRV gD. A 3.5-kb fragment encompassing the KanR cassette with PRV gD flanking sequences was released from this plasmid by NotI-XbaI digestion, Klenow blunt ended, and ligated into NotI-digested and Klenow blunt-ended pGS284 BAC transfer plasmid (37). The resulting plasmid was used as a donor plasmid for allelic exchange with wild-type PRV BAC pGS469 (37) to generate the gDnull KanR PRV screening BAC. Subsequently, 60 to 70% confluent PK15 cells were cotransfected with gDnull KanR PRV BAC DNA and DNA containing point-mutated gD ORFs with flanking sequences (described earlier) to allow recombination. Recombinant viruses formed much larger plaques than gD null virus and were positive on immunoperoxidase monolayer assay staining using anti-gD monoclonal antibodies (14). Recombinant virus (PRV-gD-YRAL, PRV-gD-YRLA, or PRV-gDtrunc) was harvested and subjected to three sequential rounds of plaque purification with careful confirmation of the presence of gD. All recombinant viruses were found to display normal growth efficiencies based on one-step growth kinetics, and all mutants expressed gD on the cell surface during infection as assessed by immunofluorescence staining using the gD-specific monoclonal antibody 13D12 (data not shown).
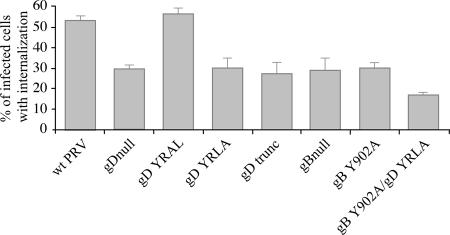
The abilities of the different constructed PRV gDs to induce internalization of antibody-antigen complexes in PRV-infected monocytes were tested as described before (42). Briefly, porcine blood monocytes were isolated and inoculated with the different PRV gD mutants at a multiplicity of infection of 10. At 13 h postinfection (p.i.), when there is abundant expression of viral envelope proteins on the surface of the infected monocytes, cells were incubated with FITC-labeled, PRV-specific porcine polyclonal antibodies for 1 h at 37°C to induce formation and internalization of antibody-antigen complexes. Afterwards, cells were paraformaldehyde fixed and the percentage of monocytes with internalized viral cell surface antigens was determined by fluorescence microscopy. Figure 3 shows that 53% of the wild-type PRV-infected monocytes showed internalization of antibody-antigen complexes. The YRAL mutation, which had no effect on internalization of gD in transfection assays (Fig. 2), had no significant effect on the efficiency of internalization of antibody-antigen complexes (56%). However, PRV strains that contained the YRLA or gDtrunc mutation, which destroy or remove the endocytosis signal in gD, showed a marked decrease in endocytosis of antibody-antigen complexes (30% and 27%, respectively, which is similar to that observed when using a gDnull PRV strain [29%]). Hence, the YRLL endocytosis signal identified in the cytoplasmic domain of gD is of critical importance for the gD protein to fulfill its function during internalization of antibody-antigen complexes in PRV-infected monocytes.
FIG. 3.
Efficiency of internalization of antibody-antigen complexes in monocytes infected with different PRV gD and gB mutant strains. Isolated porcine blood monocytes were infected with the different PRV gD and gB mutants at a multiplicity of infection of 10. At 13 h p.i., monocytes were incubated with FITC-labeled porcine polyclonal PRV-specific antibodies for 1 h at 37°C. Afterwards, the percentage of infected monocytes with internalized viral cell surface proteins was determined using fluorescence microscopy. Data represent means ± standard deviations of triplicate assays.
Our current data together with former data show that endocytosis signals in the cytoplasmic domains of both gB and gD are involved in internalization of antibody-antigen complexes in PRV-infected monocytes. However, mutating the endocytosis motif in either gB and gD, or removing either gB or gD completely, only results in an approximately 40 to 50% reduction in efficiency of antibody-antigen complex internalization (reference 10 and the current study). It is possible that the endocytosis motifs in PRV gB and gD cooperate in initiating internalization of antibody-antigen complexes. In this scenario, a PRV strain carrying mutations in both endocytosis motifs would be expected to be more impaired in driving internalization of antibody-antigen complexes than the PRV strains carrying just one of the mutations.
To investigate this, the YRLA mutation in gD was introduced in a PRV strain that already harbored a mutation in the critical tyrosine residue of the YQRL endocytosis motif in the cytoplasmic domain of gB (10). Construction of this mutant virus was done as described earlier, but using gB Y902A PRV BAC instead of wild-type PRV BAC (10). This virus was significantly more impaired in its capacity to initiate internalization of antibody-antigen complexes compared to PRV strains that harbor mutations in either the gD YRLL motif or the gB YQRL motif alone (68% reduction in internalization compared to wild type, versus 43% and 44% reduction) (Fig. 3). Hence, the endocytosis motifs in PRV gB and gD cooperate to efficiently initiate internalization of antibody-antigen complexes from the surface of PRV-infected monocytes.
A PRV strain with mutations in the endocytosis motifs of both gB and gD still shows some capability to initiate internalization of antibody-antigen complexes. Functional endocytosis motifs have been reported to exist in two other PRV membrane proteins as well: gE and US9 (5, 6, 40). Although we found earlier that removing PRV gE did not significantly affect the efficiency of internalization of antibody-antigen complexes (12, 42), we found recently that removal of US9 results in a significant reduction of internalization of antibody-antigen complexes (unpublished observations). Further research will determine whether the reported endocytosis motif in US9 is responsible for its function in internalization of antibody-antigen complexes and, if so, whether simultaneous mutation of endocytosis motifs in gB, gD, and US9 results in complete inhibition of antibody-antigen complex internalization.
The endocytosis signals in gB and gE have been shown to result in spontaneous, antibody-independent retrieval of these glycoproteins from the cell surface during early stages of infection (<6 h p.i.) (27, 39). We assessed whether, similarly, PRV gD undergoes spontaneous endocytosis early in infection and, if so, whether this relies on the YRLL endocytosis signal. To this end, we used a biotinylation assay, essentially as described before (39). In this assay, cell surface proteins are biotinylated using EZ-Link Sulfo-NHS-SS-biotin (Pierce). Addition of glutathione to these cells removes the biotin label from the cell surface proteins. However, if the biotinylated cell surface proteins are endocytosed, they become insensitive to subsequent glutathione treatment. Analysis of efficiency of spontaneous gD endocytosis occurs as follows: early in infection (5 h p.i.), cells are cooled on ice, biotinylated using EZ-Link Sulfo-NHS-SS-biotin, and shifted to 37°C for 1 h to allow endocytosis to occur. Afterwards, cells are treated for 30 min with 60 mg/ml glutathione (Sigma). Cells are subsequently lysed, and biotinylated proteins are precipitated using streptavidin-agarose (Pharmacia). If gD is spontaneously endocytosed, a fraction of biotinylated gD should become glutathione resistant during the incubation at 37°C and should be present in the precipitate. Precipitates are subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting and analyzed for the presence of gD using monoclonal anti-gD antibodies (13D12). The total amount of initially biotinylated gD is analyzed by immediate lysis of cells after biotinylation without either temperature shift or glutathione treatment. Efficiency of biotin removal by glutathione is checked by immediate glutathione treatment after biotinylation, without temperature shift. Figure 4 shows the results of these experiments using either wild-type PRV (A) or PRV-gD-YRLA (B), with lane T indicating the total amount of biotinylated gD, lane 0′ indicating the amount of biotinylated gD after glutathione treatment immediately following biotinylation (control for efficiency of biotin removal), and lane 60′ indicating the amount of glutathione-resistant biotinylated gD at 60 min post-temperature shift to 37°C. For both viruses, biotinylation of cell surface gD was very efficient (lane T), and addition of glutathione immediately after biotinylation resulted in complete removal of biotin from gD, resulting in no detectable gD band (lane 0′). In cells infected with wild-type PRV, a significant fraction of gD became glutathione resistant (i.e., internalized) by incubation of the cells for 1 h at 37°C after biotinylation (lane 60′). However, this fraction of glutathione-resistant gD was dramatically reduced in cells infected with PRV-gD-YRLA. Hence, PRV gD, like gE and gB, undergoes spontaneous, antibody-independent endocytosis during early stages of infection of a cell, and this endocytosis relies on the YRLL endocytosis signal.
FIG. 4.
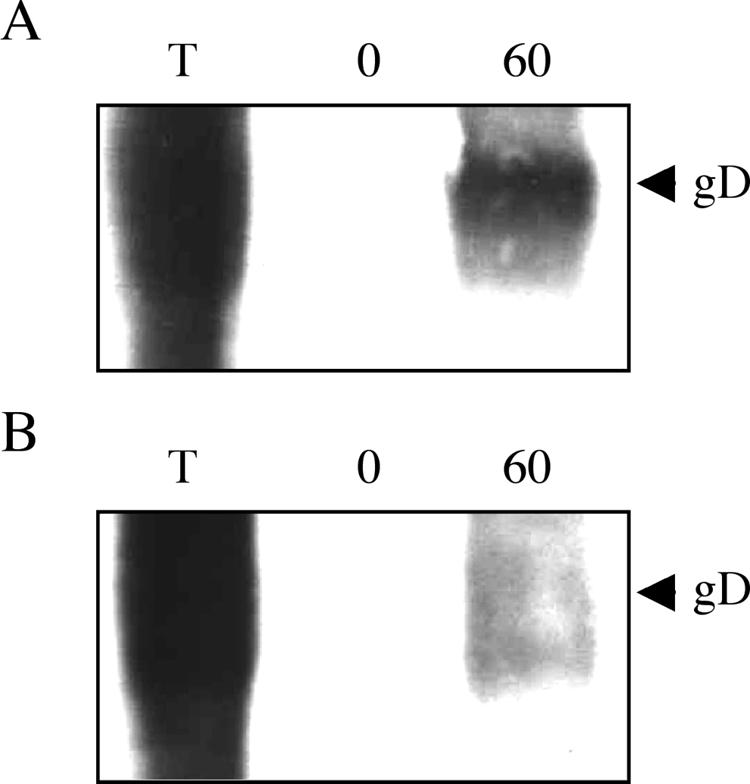
The YRLL motif in PRV gD drives antibody-independent, spontaneous endocytosis of gD in infected PK15 cells. At 5 h postinoculation with wild-type PRV (A) or PRV-gD-YRLA (B), PK15 cells were subjected to a biotinylation assay as described in the text. Lane T, total amount of biotinylated cell surface gD; lane 0′, remaining biotinylated gD after immediate glutathione treatment; lane 60′, amount of glutathione-resistant gD (i.e., endocytosed gD) after 1 h of incubation at 37°C.
Besides PRV gB and gE and, as we have shown here, gD, spontaneous endocytosis has been described for glycoproteins of other alphaherpesviruses as well, including gB, gE, gI, and gH of varicella-zoster virus and gB of herpes simplex virus (HSV), all depending on YXXL and LL motifs (1, 9, 19, 28, 29, 31), indicating that endocytosis of major glycoproteins may serve important roles during the virus life cycle. Our current finding that the gD of an alphaherpesvirus is spontaneously endocytosed from the cell surface during early stages of infection may have important consequences for the functions of this protein during the virus life cycle and will be explored further in future research.
The sequence of the endocytosis signal in gD shows some conservation among alphaherpesviruses, albeit to a lesser extent than the endocytosis signal in gB. The gD orthologues of bovine herpesvirus 1, equine herpesvirus 1, and Marek's disease virus contain a YXXL motif (2, 18, 38) similar to that of the PRV gD sequence, but HSV type 1 (HSV-1) gD does not seem to contain a potential endocytosis motif (46). Future research will show whether these YXXL motifs in other gD orthologues also constitute functional endocytosis motifs.
Recently, endocytosis of the human immunodeficiency virus (HIV) envelope glycoprotein has also been reported to possibly be of importance for immune evasion of the virus (7, 16). Endocytosis of the HIV envelope protein from the cell surface of an HIV-infected cell also relies on a YXXL motif in its cytoplasmic domain, which is very strongly conserved among retroviruses (23). Interestingly, immunization with a DNA vaccine encoding an HIV envelope protein with a mutated endocytosis motif resulted in enhancement of immunogenicity of the vaccine, most notably by the induction of very strong antibody responses (7). This supports the idea that HIV employs this endocytosis motif to evade the immune system and, importantly, this shows that mutation of these motifs may be a useful strategy to improve immunogenicity of vaccines. Therefore, our current finding that PRV gD encodes a functional endocytosis motif that, together with an endocytosis motif in gB, may reduce antibody-mediated elimination of the virus, may also be of significant importance for the construction of DNA vaccines encoding either gB or gD, which are generally considered to be potentially important vaccination strategies for many alphaherpesviruses, including PRV, bovine herpesvirus 1, equine herpesvirus 1, Marek's disease virus, and HSV (8, 15, 17, 18, 30, 35, 44, 49).
In conclusion, our results show that the cytoplasmic domain of the PRV glycoprotein gD contains a functional endocytosis motif, that this motif drives antibody-independent spontaneous endocytosis of gD at early stages of infection, and that this motif acts in concert with an endocytosis motif in PRV gB to contribute to efficient internalization of antibody-antigen complexes from the cell surface of infected monocytes, a process that protects these cells from efficient antibody-mediated lysis.
Acknowledgments
We thank Lynn Enquist and Greg Smith for kindly providing the PRV BAC system and Carine Boonen and Chantal Vanmaercke for excellent technical assistance.
This research was supported by a bilateral scientific cooperation of the Government of Flanders with Poland and by a Concerted Research Action of the Research Council of Ghent University.
REFERENCES
- 1.Alconada, A., U. Bauer, and B. Hoflack. 1996. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 15:6096-6110. [PMC free article] [PubMed] [Google Scholar]
- 2.Audonnet, J. C., J. Winslow, G. Allen, and E. Paoletti. 1990. Equine herpesvirus type 1 unique short fragment encodes glycoproteins with homology to herpes simplex virus type 1 gD, gI, and gE. J. Gen. Virol. 71:2969-2978. [DOI] [PubMed] [Google Scholar]
- 3.Bonifacino, J. S., and E. C. Dell' Angelica. 1999. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 5:923-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonifacino, J. S., and L. M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395-447. [DOI] [PubMed] [Google Scholar]
- 5.Brideau, A. D., M. G. Eldridge, and L. W. Enquist. 2000. Directional transneuronal infection by pseudorabies virus is dependent on an acidic internalization motif in the Us9 cytoplasmic tail. J. Virol. 74:4549-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brideau, A. D., T. Del Rio, E. J. Wolffe, and L. W. Enquist. 1999. Intracellular trafficking and localization of the pseudorabies virus Us9 type II envelope protein to host and viral membranes. J. Virol. 73:4372-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bu, Z., L. Ye, A. Vzorov, D. Taylor, R. W. Compans, and C. Yang. 2004. Enhancement of immunogenicity of an HIV Env DNA vaccine by mutation of the Tyr-based endocytosis motif in the cytoplasmic domain. Virology 328:62-73. [DOI] [PubMed] [Google Scholar]
- 8.Cui, F. D., H. Asada, T. Kishida, Y. Itokawa, T. Nakaya, Y. Ueda, H. Yamagishi, S. Gojo, M. Kita, J. Imanishi, and O. Mazda. 2003. Intravascular naked DNA vaccine encoding glycoprotein B induces protective humoral and cellular immunity against herpes simplex virus type 1 infection in mice. Gene Ther. 10:2059-2066. [DOI] [PubMed] [Google Scholar]
- 9.Fan, Z., M. L. Grantham, M. S. Smith, E. S. Anderson, J. A. Cardelli, and M. I. Muggeridge. 2002. Truncation of herpes simplex virus type 2 glycoprotein B increases its cell surface expression and activity in cell-cell fusion, but these properties are unrelated. J. Virol. 76:9271-9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Favoreel, H. W., G. Van Minnebruggen, H. J. Nauwynck, L. W. Enquist, and M. B. Pensaert. 2002. A tyrosine-based motif in the cytoplasmic tail of pseudorabies virus glycoprotein B is important for both antibody-induced internalization of viral glycoproteins and efficient cell-to-cell spread. J. Virol. 76:6845-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favoreel, H. W., H. J. Nauwynck, and M. B. Pensaert. 2000. Immunological hiding of herpesvirus-infected cells. Arch. Virol. 145:1269-1290. [DOI] [PubMed] [Google Scholar]
- 12.Favoreel, H. W., H. J. Nauwynck, P. Van Oostveldt, T. C. Mettenleiter, and M. B. Pensaert. 1999. Antibody-induced endocytosis of viral glycoproteins and major histocompatibility complex class I on pseudorabies virus-infected monocytes. J. Gen. Virol. 80:1283-1291. [DOI] [PubMed] [Google Scholar]
- 13.Favoreel, H. W., G. R. Van de Walle, H. J. Nauwynck, and M. B. Pensaert. 2003. Virus complement evasion strategies. J. Gen. Virol. 84:1-15. [DOI] [PubMed] [Google Scholar]
- 14.Ficinska, J., K. Bienkowska-Szewczyk, L. Jacobs, G. Plucienniczak, A. Plucienniczak, and B. Szewczyk. 2003. Characterization of changes in the short unique segment of pseudorabies virus BUK-TK900 (Suivac A) vaccine strain. Arch. Virol. 148:1593-1612. [DOI] [PubMed] [Google Scholar]
- 15.Fischer, L., S. Barzu, C. Andreoni, N. Buisson, A. Brun, and J. C. Audonnet. 2003. DNA vaccination of neonate piglets in the face of maternal immunity induces humoral memory and protection against a virulent pseudorabies virus challenge. Vaccine 21:1732-1741. [DOI] [PubMed] [Google Scholar]
- 16.Fultz, P. N., P. J. Vance, M. J. Endres, B. Tao, J. D. Dvorin, I. C. Davis, J. D. Lifson, D. C. Montefiori, M. Marsh, M. H. Malim, and J. A. Hoxie. 2001. In vivo attenuation of simian immunodeficiency virus by disruption of a tyrosine-dependent sorting signal in the envelope glycoprotein cytoplasmic tail. J. Virol. 75:278-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haagmans, B. L., E. M. van Rooij, M. Dubelaar, T. G. Kimman, M. C. Horzinek, V. E. Schijns, and A. T. Bianchi. 1999. Vaccination of pigs against pseudorabies virus with plasmid DNA encoding glycoprotein D. Vaccine 17:1264-1271. [DOI] [PubMed] [Google Scholar]
- 18.Heine, H. G., A. J. Foord, P. L. Young, P. T. Hooper, P. R. Lehrbach, and D. B. Boyle. 1997. Recombinant fowlpox virus vaccines against Australian virulent Marek's disease virus: gene sequence analysis and comparison of vaccine efficacy in specific pathogen free and production chickens. Virus Res. 50:23-33. [DOI] [PubMed] [Google Scholar]
- 19.Heineman, T. C., and S. L. Hall. 2001. VZV gB endocytosis and Golgi localization are mediated by YXXΦ motifs in its cytoplasmic domain. Virology 285:42-49. [DOI] [PubMed] [Google Scholar]
- 20.Kirchhausen, T., J. S. Bonifacino, and H. Riezman. 1997. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr. Opin. Cell Biol. 9:488-495. [DOI] [PubMed] [Google Scholar]
- 21.Leib, D. A. 2002. Counteraction of interferon-induced antiviral responses by herpes simplex viruses. Curr. Top. Microbiol. Immunol. 269:171-185. [DOI] [PubMed] [Google Scholar]
- 22.Marks, M. S., H. Ohno, T. Kirchhausen, and J. S. Bonifacino. 1997. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 7:124-127. [DOI] [PubMed] [Google Scholar]
- 23.Marsh, M., and A. Pelchen-Matthews. 2000. Endocytosis in viral replication. Traffic 1:525-532. [DOI] [PubMed] [Google Scholar]
- 24.Mettenleiter, T. C. 2000. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis—state of art, June 1999. Vet. Res. 31:99-115. [DOI] [PubMed] [Google Scholar]
- 25.Nauwynck, H. J., and M. B. Pensaert. 1995. Effect of specific antibodies on the cell-associated spread of pseudorabies virus in monolayers of different cell types. Arch. Virol. 140:1137-1146. [DOI] [PubMed] [Google Scholar]
- 26.Nauwynck, H. J., and M. B. Pensaert. 1992. Abortion induced by cell-associated Aujeszky's disease virus in vaccinated sows. Am. J. Vet. Res. 53:489-493. [PubMed] [Google Scholar]
- 27.Nixdorf, R., G. Klupp, A. Karger, and T. C. Mettenleiter. 2000. Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity. J. Virol. 74:7137-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson, J. K., and C. Grose. 1997. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J. Virol. 71:4042-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olson, J. K., and C. Grose. 1998. Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor. J. Virol. 72:1542-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osterrieder, N., R. Wagner, C. Brandmuller, P. Schmidt, H. Wolf, and O. R. Kaaden. 1995. Protection against EHV-1 challenge infection in the murine model after vaccination with various formulations of recombinant glycoprotein gp14 (gB). Virology 208:500-510. [DOI] [PubMed] [Google Scholar]
- 31.Pasieka, T. J., L. Maresova, and C. Grose. 2003. A functional YNKI motif in the short cytoplasmic tail of varicella-zoster virus glycoprotein gH mediates clathrin-dependent and antibody-independent endocytosis. J. Virol. 77:4191-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peeters, B., N. de Wind, M. Hooisma, F., Wagenaar, A. Gielkens, and R. Moorman. 1992. Pseudorabies virus envelope glycoprotein gp50 and gII are essential for virus penetration, but only gB is involved in membrane fusion. J. Virol. 66:894-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pond, L., L. A. Kuhn, L. Teyton, M. P. Schutze, J. A. Tainer, M. R. Jackson, and P. A. Peterson. 1995. A role for acidic residues in di-leucine motif-based targeting to the endocytic pathway. J. Biol. Chem. 270:19989-19997. [DOI] [PubMed] [Google Scholar]
- 34.Rauh, I., and T. C. Mettenleiter. 1991. Pseudorabies virus glycoprotein gII and gp50 are essential for virus penetration. J. Virol. 65:5348-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruitenberg, K. M., C. Walker, J. E. Wellington, D. N. Love, and J. M. Whalley. 1999. Potential of DNA-mediated vaccination for equine herpesvirus 1. Vet. Microbiol. 68:35-48. [DOI] [PubMed] [Google Scholar]
- 36.Schmid, S. L. 1997. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu. Rev. Biochem. 66:511-548. [DOI] [PubMed] [Google Scholar]
- 37.Smith, G. A., and L. W. Enquist. 2000. A self-recombining bacterial artificial chromosome and its application for analysis of herpesvirus pathogenesis. Proc. Natl. Acad. Sci. USA 97:4873-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tikoo, S. K., D. R. Fitzpatrick, L. A. Babiuk, and T. J. Zamb. 1990. Molecular cloning, sequencing, and expression of functional bovine herpesvirus 1 glycoprotein gIV in transfected bovine cells. J. Virol. 64:5132-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tirabassi, R. S., and L. W. Enquist. 1998. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tirabassi, R. S., and L. W. Enquist. 1999. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J. Virol. 73:2771-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van de Walle, G. R., H. W. Favoreel, H. J. Nauwynck, and M. B. Pensaert. 2003. Antibody-induced internalization of viral glycoproteins and gE-gI Fc receptor activity protects pseudorabies virus-infected monocytes from efficient complement-mediated lysis. J. Gen. Virol. 84:939-948. [DOI] [PubMed] [Google Scholar]
- 42.Van de Walle, G. R., H. W. Favoreel, H. J. Nauwynck, P. Van Oostveldt, and M. B. Pensaert. 2001. Involvement of cellular cytoskeleton proteins in antibody-induced internalization of viral glycoproteins in pseudorabies virus-infected monocytes. Virology 288:129-138. [DOI] [PubMed] [Google Scholar]
- 43.Van Minnebruggen, G., H. W. Favoreel, and H. J. Nauwynck. 2004. Internalization of pseudorabies virus glycoprotein B is mediated by an interaction between the YQRL motif in its cytoplasmic domain and the clathrin-associated AP-2 adaptor complex. J. Virol. 78:8852-8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Rooij, E. M., B. L. Haagmans, H. L. Glansbeek, Y. E. de Visser, M. G. de Bruin, W. Boersma, and A. T. Bianchi. 2000. A DNA vaccine coding for glycoprotein B of pseudorabies virus induces cell-mediated immunity in pigs and reduces virus excretion early after infection. Vet. Immunol. Immunopathol. 74:121-136. [DOI] [PubMed] [Google Scholar]
- 45.Vossen, M. T., E. M. Westerhout, C. Soderberg-Naucler, and E. J. Wiertz. 2002. Viral immune evasion: a masterpiece of evolution. Immunogenetics 54:527-542. [DOI] [PubMed] [Google Scholar]
- 46.Watson, R. J., J. H. Weis, J. S. Salstrom, and L. W. Enquist. 1982. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science 218:381-384. [DOI] [PubMed] [Google Scholar]
- 47.Whitbeck, J. C., C. Peng, H. Lou, R. Xu, S. H. Willis, M. Ponce de Leon, T. Peng, A. V. Nicola, R. I. Montgomery, M. S. Warner, A. M. Soulika, L. A. Spruce, W. T. Moore, J. D. Lambris, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1997. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator to HSV entry. J. Virol. 71:6083-6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wittmann, G., J. Jakubik, and R. Ahl. 1980. Multiplication and distribution of Aujeszky's disease (pseudorabies) virus in vaccinated and nonvaccinated pigs after intranasal infection. Arch. Virol. 66:227-240. [DOI] [PubMed] [Google Scholar]
- 49.Zamorano, P., O. Taboga, M. Dominguez, A. Romera, M. Puntel, C. Tami, C. Mongini, C. Waldner, E. Palma, and A. Sadir. 2002. BHV-1 DNA vaccination: effect of the adjuvant RN-205 on the modulation of the immune response in mice. Vaccine 20:2656-2664. [DOI] [PubMed] [Google Scholar]