Abstract
The effectiveness of lipid-lowering therapies may be insufficient in high-risk cardiovascular patients and depends on the genetic variability of drug-metabolizing enzymes. Customizing statin therapy, including treatment with atorvastatin, may improve clinical outcomes. Currently, there is a lack of guidelines allowing the prediction of the therapeutic efficacy of lipid-lowering statin therapy. This study aimed to determine the effects of clinically significant gene variants of CYP2C19 on atorvastatin therapy in patients with acute coronary syndromes. In total, 92 patients with a confirmed diagnosis of ST-elevation myocardial infarction (STEMI) or non-ST-elevation myocardial infarction (NSTEMI) were sequenced for target regions within the CYP2C19 gene on the Illumina Miniseq system. The CYP2C19 poor metabolizer phenotype (carriers of CYP2C19*2, CYP2C19*4, and CYP2C19*8 alleles) was detected in 29% of patients. These patients had significantly lower responses to treatment with atorvastatin than patients with the normal metabolizer phenotype. CYP2C19-metabolizing phenotype, patient age, and smoking increased the odds of undertreatment in patients (∆LDL-C (mmol/L) < 1). These results revealed that the CYP2C19 phenotype may significantly impact atorvastatin therapy personalization in patients requiring LDL lipid-lowering therapy.
Keywords: statins, atorvastatin, LDL cholesterol, CYP2C19, STEMI, NSTEMI
1. Introduction
Hypercholesterolemia, or hyperlipidemia, is characterized by elevated levels of cholesterol in the blood and stands as a major risk factor for cardiovascular diseases, contributing significantly to the global burden of morbidity and mortality [1]. While lifestyle factors and dietary choices significantly impact cholesterol concentrations, genetic factors play a pivotal role in determining a predisposition to hypercholesterolemia [2]. A large study sponsored by the NIH (National Institutes of Health) discovered that a cholesterol-lowering diet and medical treatment decreased heart attacks in males with high cholesterol [3].
The discovery of compounds decreasing cholesterol levels in 1976 [4,5] has paved the way for a new class of drugs, 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoA reductase, HMGCR) inhibitors or statins. Goldstein and Brown were the first to describe that patients with familial hypercholesterolemia who lack low-density lipoprotein receptors (LDLr) and produce increased amounts of cholesterol in response to the absence of these receptors [6]. It was assumed that inhibiting cholesterol through HMG-CoA reductase and the upregulation of LDLr is a key tool in the prevention of atherosclerotic cardiovascular disease [7,8]. In addition, statins can have a positive effect by stabilizing the atherosclerotic plaque and reducing atherothrombotic events through this mechanism [7].
The current European Society of Cardiology guidelines for the management of dyslipidemias [9] and American Heart Association guidelines for the Management of Patients With Chronic Coronary Disease [10] agree that primary prevention with statins, according to the patients’ risk category, should be initiated when lifestyle modification does not work and the low-density lipoprotein–cholesterol (LDL-C) goal is not achieved. For chronic coronary syndrome (CCS), familial hypercholesterolemia (FH), severe diabetes mellitus (DM), and chronic kidney disease (CKD) patients’ treatment should be initiated immediately after the diagnosis [10,11].
The main focus of the current guidelines is on lowering LDL-C through the use of drug combinations along with other lipid-lowering therapies [9,10]. Studies show that lipid-lowering therapies are insufficiently effective in patients, especially in high cardiovascular disease (CVD) risk patients [12,13,14,15,16]. Atorvastatin is among the most commonly prescribed statins. However, studies show it in adherence in patients with dyslipidemia [17].
In this era of precision medicine, better-customized statin therapy can be used. Some authors show that genetic variants can influence statin therapy. According to Cano-Corres, the HMGCR c.1564-106A>G variant reduces the effect of statins [18] Maxwell has examined a number of studies that have analyzed the influence of ABCB1, APOE, KIF6, and TLR4 on the improvement of clinical outcomes during statin therapy [19]. The first gene-based clinical guideline on the use of statins approved by the Clinical Pharmacogenetics Implementation Consortium (CPIC) was published in 2012. The gene-based prescription of simvastatin was based on SLCO1B1 variants. In 2014, the document was updated [20]. The latest “CPIC® Guideline for Statins and SLCO1B1, ABCG2 and CYP2C9“ summarizes data from genotyping studies that demonstrate the influence of SLCO1B1, ABCG2, and CYP2C9 on safer statin therapy that may allow the avoidance of statin-associated effects [21]. The guidelines described here aim to reduce the risk of statin-associated musculoskeletal symptoms (SAMS), as research studies show that statins can cause muscle pain. Still, this pain is rare and usually manifests as mild symptoms [22,23].
Some studies have shown that variants in CYP2C19 may impact treatment effectiveness with statins [24]. Currently, no clear studies show that the effect of atorvastatin may depend on CYP2C19 gene variants. It is worth noting that an individual carrying a non-functional allele such as CYP2C19*2, *3, or *4 will have impaired drug metabolism and is considered an intermediate metabolizer. In contrast, an individual carrying two non-functional alleles will be regarded as a poor metabolizer [25]. Currently, no guidelines allow for the prediction of the therapeutic efficacy of lipid-lowering statin therapy. Various studies show that linoleic acid derivatives may be significant in lipid metabolism [26]. Thus, this study aimed to determine the effects of CYP2C19 gene variants and linoleic acid derivatives on atorvastatin therapy in patients with established cardiovascular disease.
2. Results
Among the patients, 30.4% were using statins before hospitalization, with 39.1% experiencing STEMI and 20.7% having undergone previous PCI (Table 1).
Table 1.
Basic characteristics of the represented patient’s group.
| Variable | n | % |
|---|---|---|
| Sex | ||
| Men | 49 | 53.3 |
| Women | 43 | 46.7 |
| Smoking | 54 | 58.7 |
| STEMI | 36 | 39.1 |
| Hypertension | 47 | 51.1 |
| Diabetes mellitus | 14 | 15.2 |
| Renal insufficiency | 11 | 12 |
| Statins at hospitalization | 28 | 30.4 |
| Family anamnesis of ischemic heart disease | 39 | 42.4 |
| Previous PCI | 19 | 20.7 |
Of the patients with STEMI, 28.6% were on statins, and 43.8% were non-users prior to hospitalization (Table 2). No statistical significance was found between these two groups of patients.
Table 2.
Patients with STEMI vs. NSTEMI according to statin therapy prior to hospitalization.
| STEMI | On Statins | No Statin | Fisher’s Exact Test p |
|---|---|---|---|
| Yes n (%) | 8 (28.6) | 28 (43.8) | 0.245 |
| No n (%) | 20 (71.4) | 36 (56.3) | |
| Total n (%) | 28 (100) | 64 (100) |
Table 3 lists all variants detected in the CYP2C19 gene. Of the variants that impact CYP2C19 functionality, rs4244285 was the most common. Two other variants, rs28399504 and rs41291556, described in the PharmVar database [27], were also detected, leading to reduced enzyme activity. One patient was found to be carrying a rare variant of uncertain significance.
Table 3.
CYP2C19 variants in the represented patient sample.
| dbSNP rs ID | c.DNA Position (NM_000771.4) | AA Change (NP_000762.2) | Pharmvar Allele | Impact on Function | No of Het | No of Hom |
|---|---|---|---|---|---|---|
| rs3758581 | c.991A>G | p.Ile331Val | - | - | 7 | 0 |
| rs17885098 | c.99T>C | p.Pro33Pro | - | - | 8 | 0 |
| rs28399504 | c.1A>G | p.M1V | CYP2C19*4 | No function | 2 | 0 |
| rs58973490 | c.449G>A | p.Arg150His | CYP2C19*11 | Normal function | 1 | 0 |
| rs17878459 | c.276G>C | p.Glu92Asp | - | - | 12 | 0 |
| rs17882744 | c.1059C>T | p.His353= | - | - | 1 | 0 |
| rs3758580 | c.990C>T | p.Val330Val | - | - | 19 | 4 |
| rs142974781 | c.448C>T | p.Arg150Cys | - | - | 1 | 0 |
| rs41291556 | c.358T>C | p.Trp120Arg | CYP2C19*8 | No function | 1 | 0 |
| rs4244285 | c.681G>A | p.Pro227Pro | CYP2C19*2 | No function | 19 | 4 |
Patients were divided into two groups based on their CYP2C19 metabolism: poor metabolizers and normal metabolizers. Carriers of the CYP2C19*2, CYP2C19*4, and CYP2C19*8 alleles were considered poor metabolizers, while all other patients were considered normal metabolizers. Normal metabolizers had a higher reduction in LDL-C (Table 4).
Table 4.
Patient CYP2C19 phenotype in the represented samples.
| CYP2C19 Metabolizer Status | n (%) | LDL-C at Hospitalization (mmol/L) Median (Min-Max) | LDL-C 6 Months after Hospitalization (mmol/L) Median (Min-Max) |
∆LDL-C (mmol/L) Median (Min-Max) | p-Value *** |
|---|---|---|---|---|---|
| Normal metabolizer | 66 (71) | 3.88 (2.87–6.36) | 2.24 (1.17–5.41) ** | 1.69 (0.13–3.57) | 0.006 |
| Poor metabolizer * | 27 (29) | 3.78 (2.79–6.32) | 2.78 (1.19–5.97) ** | 0.49 (0.01–4.09) | |
| Total | 93 (100) | 3.87 (2.79–6.36) | 2.29 (1.17–5.97) |
* CYP2C19 *2, *4, *8; ** the LDL-C values between two medians differed, p = 0.01; *** p-value between medians of ∆LDL-C.
2.1. Regression Model
The multivariable logistic regression model showed (Table 5) that poor CYP2C19 metabolizing phenotype, patient age, and smoking increased the odds of undertreatment in patients (∆LDL-C (mmol/L) < 1) who received standard atorvastatin cholesterol-lowering therapy.
Table 5.
Variables that may decrease the effect of atorvastatin lipid-lowering therapy.
| Variable | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| CYP2C19 poor metabolizer phenotype | 7.027 | (2.287–21.590) | 0.001 |
| Patient age in years | 1.064 | (1.017–1.113) | 0.007 |
| Smoking | 3.396 | (1.167–9.884) | 0.025 |
2.2. Analysis of Metabolite Data
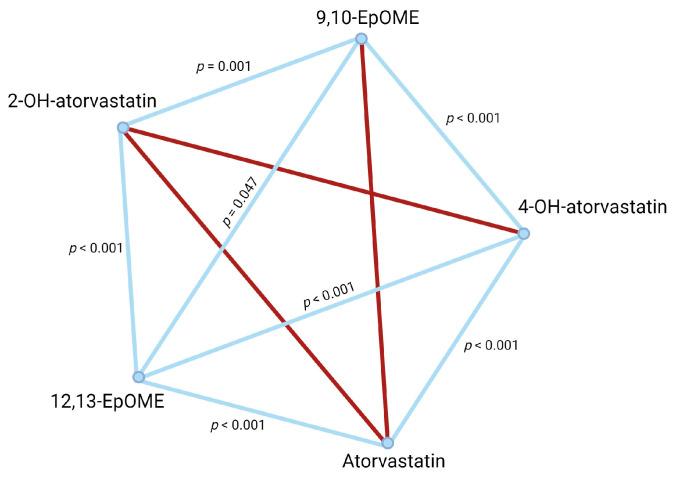
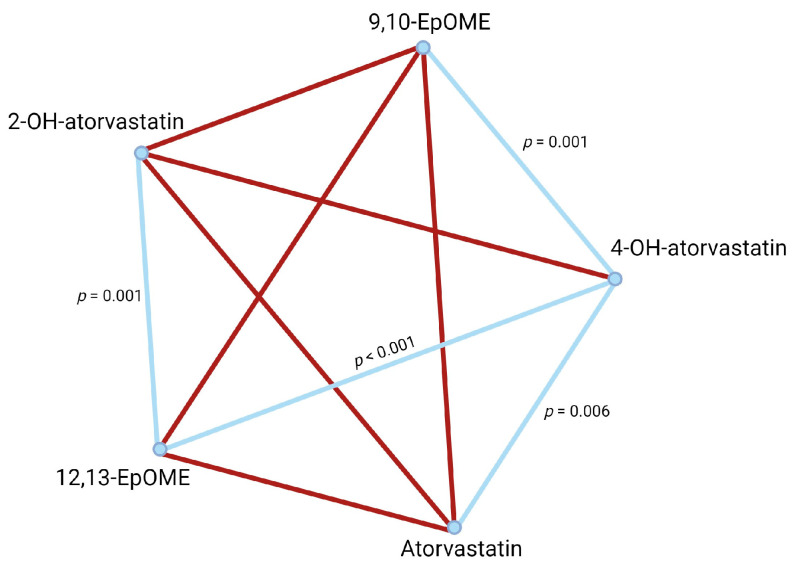
Pairwise analysis of plasma metabolite concentrations (Table 6) of atorvastatin, 4-OH-atorvastatin, 2-OH-atorvastatin, 9(10)-EpOME, and 12(13)-EpOME revealed that for normal metabolizers (Figure 1), 4-OH-atorvastatin concentration was dependent on atorvastatin concentration in blood plasma. Both 9,10-EpOME and 12,13-EpOME concentrations were associated with 4-OH-atorvastatin and 2-OH-atorvastatin plasma concentrations. There were different results in poor metabolizers (Figure 2): 4-OH-atorvastatin concentration was associated with atorvastatin and 9,10-EpOME and 12,13-EpOME concentrations. 12,13-EpOME concentration was associated with 2-OH-atorvastatin plasma concentrations.
Table 6.
Atorvastatin, 4-OH-atorvastatin, 2-OH-atorvastatin, 9,10-EpOME, and 12,13-EpOME concentrations in the represented patient sample.
| CYP2C19 Metabolizer Status |
Atorvastatin in ng, Median (Min-Max) | 4-OH-atorvastatin in ng, Median (Min-Max) | 2-OH-atorvastatin in ng, Median (Min-Max) | 9,10-EpOME, in ng, Median (Min-Max) | 12,13-EpOME in ng, Median (Min-Max) |
|---|---|---|---|---|---|
| Normal metabolizer (n = 42) |
6.5 (0–157.4) | 0.5 (0–32.1) | 4.1 (0–65.2) | 21.3 (6.9–167.3) | 37.4 (13–144.1) |
| Poor metabolizer (n = 21) |
5.3 (0–60.1) | 1.1 (0–8) | 5.9 (0–24.8) | 20 (0–148.7) | 29 (0–180) |
| Total (n = 63) | 5.3 (0–157.4) | 0.6 (0–32.1) | 4.5 (0–65.2) | 20.5 (0–167.3) | 34.8 (0–180) |
Figure 1.
Pairwise analysis of compound concentrations in normal metabolizers. Significant associations are represented as blue lines. Non-significant associations are in red.
Figure 2.
Pairwise analysis of compound concentrations in poor metabolizers. Significant associations are represented as blue lines. Non-significant associations are in red.
3. Discussion
The results of this study have shown for the first time that CYP2C19 variants may significantly affect atorvastatin lipid-lowering therapy. In addition, patient age and smoking were also associated with a decreased effect of atorvastatin therapy.
Statins are the cornerstone of lipid-lowering therapies for patients with cardiovascular disease. The European Society of Cardiology (ESC) guidelines divide patients into four risk categories: low, moderate, high, and very high risk. Lifestyle modification or drug therapy is considered according to the patient’s risk and LDL-C levels. If the primary (or secondary) prevention patient is without DM or FH but at very high risk, LDL-C reduction from baseline ≥ 50% and LDL-C goal of <1.4 mmol/L is recommended (I class, level C), and primary prevention for patients with high-risk LDL-C have a goal of <1.8 mmol/L (I class, level A). For individuals at moderate risk, the LDL-C goal is <2.6 mmol/L (IIa class, level A), and for low risk, <3 mmol/L (IIb class, level A). In most of the primary prevention cases, when lifestyle modification does not work and the desirable LDL-C-lowering effect is not achieved, statin therapy must be initiated [9]. Not only is the statin-induced LDL-C-lowering result relevant for secondary prevention patients, but its pleiotropic nature and their effect on CV morbidity and mortality are also vital for CVD prevention. The American Heart Association recommendations (2023) for chronic coronary syndrome (CCS) patients also support statin therapy to lower LDL-C to reduce the risk of major acute cardiovascular events (MACE) (class I, level A) [10].
Chronic oral statin treatment may have cardioprotective effects before STEMI [28]. Statins can prevent plaque rupture [29]. In a large study representing 5103 patients, 27% of the patients were low-intensity statin therapy users (LIST), and 17% used high-intensity statin therapy (HIST) before index acute coronary syndrome. In total, 56% were non-users (statin-naïve patients). The incidence of STEMI was lower in HIST patients than in LIST patients [30]. Our represented patients cannot confirm this effect because one of the limitations of this study was the relatively low patient number compared with the large-scale studies. However, the aim of this study was also different from the above-mentioned studies and was optimal for analyzing gene variants related to drug metabolism in the liver.
The most common loss-of-function variant in the represented patient group was rs4244285, also known as the *2 variant allele. CYP2C19 loss-of-function alleles are known to affect the effectiveness of clopidogrel antiplatelet therapy, residual platelet aggregation, and a higher rate of stent thrombosis [31]. The natural function of CYP2C19 in the liver is the metabolism of various substrates. Studies have shown that the CYP2C9 and CYP2C19 variant alleles are associated with a higher prevalence of atherosclerosis in cigarette smokers [32]. In patients following PCI, CYP2C19 loss-of-function alleles with peripheral endothelial dysfunction may predict future cardiovascular events [33]. Another study explained that a decline in epoxyeicosatrienoic acid concentrations due to CYP2C19 variants may be associated with coronary microvascular dysfunction [34]. This same study also shows that increased epoxyeicosatrienoic acid concentrations lead to lower inflammation in the microvascular system. Inflammation has been shown to suppress CYP-450, including CYP3A4 [35]. Our data show that patients with normal CYP2C19 function had different atorvastatin metabolites and EpOMEs profiles compared to poor metabolizers. Researchers have shown that EpOMEs (vernolic acid (12,13-EpOME) and coronaric acid (9,10-EpOME)) are produced by activated neutrophils and macrophages during inflammation and are known as leukotoxins [36]. These compounds are linoleic acid derivatives produced by CYP450 enzymes [37]. EpOMEs are further metabolized into 9,10-dihydroxy-octadecamonoenoate (9,10-DiHOME) and 12,13-dihydroxy-octadecamonoenoate (12,13-DiHOME). The later compounds suppress neutrophil respiratory (or oxidative) bursts, impair immunological signaling [38], and probably impact the cholesterol metabolism or cholesterol-lowering effect of statins.
Rosuvastatin undergoes metabolism through CYP2C9 and CYP2C19; however, according to the literature, the main atorvastatin-metabolizing enzyme remains as CYP3A4 [39,40]. One study showed an effect of rosuvastatin but not atorvastatin on P2Y12 receptor reaction units (PRU) in patients with CYP2C19 variant alleles and concomitant use of clopidogrel and statin [41]. A recent study examined the levels of LDL cholesterol in patients who were taking statins. The authors found that CYP2C19 variants were associated with sdLDL-C levels and may predict the efficacy of statin therapy [24]. The results of our study revealed that reduction in LDL-C levels over six months was more effective in patients with normal metabolizer phenotypes of CYP2C19. Poor metabolizers had at least three times lower reduction in LDL-C blood plasma concentrations, despite the exact atorvastatin dosages as normal metabolizers (80 mg once daily). Our pioneering study describes the effect of the CYP2C19 phenotype on atorvastatin therapy in patients with acute coronary syndromes. Thus, the second limitation of this study is that functional studies were not performed to clarify the observations. However, one survey showed that CYP2C19 could metabolize atorvastatin lactone and 2-OH-atorvastatin lactone. The authors provide a more detailed description of how various statins can be metabolized. Atorvastatin is prescribed and used in the active acid form. Acid drugs are metabolized slower by liver cytochromes than more lipophilic lactone drugs. Under the action of certain enzymes, acid forms can be biotransformed into lactone forms and undergo metabolism through liver cytochromes [42]. Regarding the potentially inconsistent data on the impact of CYP2C19 on atorvastatin metabolism based on different studies, more detailed research is required to clarify the effect of atorvastatin metabolism in patients with acute coronary syndromes. The study utilized next-generation sequencing, a robust but time-consuming technique, to analyze variants of interest. However, alternative methods to sequencing can improve turnaround times while still detecting the most common variants in CYP2C19 (CYP2C19 *2, *4, *8 alleles) that have an impact. Real-time PCR or other alternative methods could be used for the fast detection of gene variants that may impact statin therapy, leading to precise cholesterol-reducing therapy with tailored treatments adopted for each patient individually.
4. Materials and Methods
4.1. Study Population and Inclusion Criteria
This study was a prospective, single-center investigation conducted at the cardiac intensive care unit of the Hospital of the Lithuanian University of Health Sciences Kaunas Clinics. The research included 92 consecutive patients admitted between January and November 2021, and all tested negative for COVID-19. These patients presented with either ST-Elevation Myocardial Infarction (STEMI) or Non-ST-Elevation Myocardial Infarction (NSTEMI). Each patient underwent invasive angiography followed by primary percutaneous coronary intervention (PCI). Patients were excluded from enrollment if they had a diagnosis of atrial fibrillation or pericardial diseases; a history of prior coronary artery bypass graft surgery; were pregnant; or had been diagnosed with significant structural heart diseases, including valvular heart diseases. Additionally, exclusion criteria encompassed patients with a documented history of hepatic, oncological, or lung diseases; allergy to contrast media; renal failure; and severe dementia.
Data collection included patient demographics, comorbidities, medications, and clinical course. STEMI, as per the 2023 ESC Guidelines, is marked by ST-segment elevation in two ECG leads, with specific thresholds for chest and limb leads and factoring in age and gender. A new left bundle branch block (LBBB) can also indicate STEMI. Diagnosis requires both ischemic symptoms and obstructive coronary artery disease confirmed by angiography. Without angiographic evidence of obstruction, a STEMI diagnosis is not made, even if ECG and clinical signs are present [43]. NSTEMI is identified through a 12-lead ECG showing a depressed ST-segment or T-wave inversion and a cardiac troponin rise exceeding five times the 99th percentile limit. Confirmation via coronary angiography is essential to diagnose the extent of arterial blockage [43]. All patients received standard treatment, including statins (80 mg of atorvastatin), angiotensin-converting enzyme inhibitors (or angiotensin receptor I blockers), β-adreno-blockers, and dual antiplatelet therapy (DAPT) comprising aspirin and a P2Y12 inhibitor such as ticagrelor or clopidogrel.
4.2. Lipid Profile Assessment
Lipid levels in our study were assessed upon admission and again at six months. For most lipids, enzymatic hydrolysis was used to measure total cholesterol, triglycerides, and HDL cholesterol, with colorimetric analysis indicating their concentrations. LDL cholesterol was estimated using the Friedewald formula (LDL cholesterol = total cholesterol − HDL cholesterol − (triglycerides/2.2)), effective when triglycerides are below 4.5 mmol/L. For higher triglyceride levels, direct LDL measurement was employed.
4.3. DNA Extraction and Sequencing
Genomic DNA extraction was performed employing standard laboratory procedures, and subsequent library preparation utilized Illumina DNA Prep kits (Illumina, San Diego, CA, USA) according to the manufacturer’s protocol. Specifically, a DNA quantity ranging from 200 to 500 ng underwent bead-linked tagmentation. After tagmentation, samples were subjected to amplification through 9 cycles of polymerase chain reaction (PCR). A unique pair of indices was assigned to each during the amplification process to ensure sample distinction. After amplification and a subsequent clean-up step, samples were equitably pooled, each comprising 12 samples.
A customized panel was employed for target region enrichment, utilizing xGen Lock Down probes from Integrated DNA Technologies (IDT, Coralville, IA, USA). This panel comprehensively covered all exons of CYP2C19 included in the canonical transcript NM_000769.4, as per the National Center for Biotechnology Information (NCBI) Reference Sequence Database (https://www.ncbi.nlm.nih.gov/refseq, accessed on 1 October 2021). After enrichment, library pools underwent a sixteen-cycle PCR amplification. Subsequently, the prepared library pools were quantified using the Qubit High Sensitivity assay (Invitrogen, Carlsbad, CA, USA).
Sequencing was executed on an Illumina Miniseq system (Illumina, San Diego, CA, USA) utilizing the medium output 300 cycles kit. Data analysis was conducted with the Genomic Assembly Tool Kit (GATK) version 4.2.6.1, adhering to the best practice guidelines. This comprehensive methodology ensures robust and accurate interrogation of the genetic landscape, particularly focusing on the target region within the CYP2C19 gene.
4.4. Metabolite Analysis: UPLC-ESI-MS/MS Conditions
Analysis of targeted compounds in human plasma samples was carried out with an Acquity H-class UPLC system (Waters, Milford, MA, USA) equipped with a triple quadrupole tandem mass spectrometer (Xevo TQD, Waters, Milford, MA, USA) with an electrospray ionization source (ESI) working in both positive and negative modes. An Acquity UPLC BEH C18 (100 × 2.1 mm 1.7 µm) column was used for the separation of the targeted compounds. The column temperature was maintained at 40 °C. Gradient elution was performed with a mobile phase consisting of 0.1% acetic acid water solution (solvent A) and acetonitrile (solvent B) with the flow rate set to 0.5 mL/min. Linear gradient profile was applied with the following proportions of solvent A: 0 to 1 min–75%, 8.0 to 8.5 min.–5%, 8.51 min; 75% total analysis time—10 min. Electrospray ionization was applied for analysis with the following settings: capillary voltage: 2.5 kV for negative mode and 3.5 kV for positive mode, source temperature: –120 °C, desolvation temperature: –400 °C, desolvation gas flow: 650 L/h, cone gas flow: 10 L/h. Collision energy and cone voltage were optimized for each compound separately (Table 7).
Table 7.
MS condition and MRM transitions for compounds of interest.
| Compound | ESI Mode | Retention Time, min | Cone Voltage | Collision Energy | MRM Transition |
|---|---|---|---|---|---|
| Atorvastatin | Positive | 5.68 | 30 | 40 | 559 > 250 |
| Atorvastatin-d5 (IS) | Positive | 5.68 | 30 | 40 | 564 > 255 |
| 2-Hydroxyatorvastatin | Positive | 5.43 | 30 | 40 | 575 > 250 |
| Parahydroxyatorvastatin, or Atorvastatin-4OH |
Positive | 4.42 | 30 | 40 | 575 > 250 |
| 20-HETE-d6 (IS) | Negative | 6.45 | 50 | 12 | 325 > 307 |
| 9,10-Epoxy-12Z-octadecenoic acid (9(10)-EpOME) |
Negative | 6.79 | 30 | 20 | 295 > 171 |
| 12(13)Epoxy-9Z-octadecenoic acid (12(13)-EpOME) |
Negative | 6.74 | 30 | 20 | 295 > 195 |
4.5. Statistical Analysis
Frequencies are presented in percentages. Quantitative clinical parameters were evaluated using a nonparametric Kruskal–Wallis test. Fisher’s exact test was used to assess the proportions of categorical variables. A p-value < 0.05 was considered statistically significant. All variables were chosen for the multivariable model by backward selection, with the final model containing only those with p < 0.05. According to Wang et al., 1 mmol/L reduction in LDL-C corresponds to a 19% lower risk of major vascular events and is independent of the baseline LDL cholesterol [44]. Therefore, a 1 mmol/L reduction in LDL cholesterol was used in this study to determine the relative efficacy of statin therapy.
Pairwise comparisons were used to evaluate the associations between atorvastatin, its metabolites (4-OH-atorvastatin, 2-OH-atorvastatin), and EPOME (9,10-epoxy-12Z-octadecenoic acid (9(10)-EpOME), as well as 12(13)epoxy-9Z-octadecenoic acid (12(13)-EpOME) concentrations in the represented patient sample.
5. Conclusions
The results revealed that the CYP2C19 phenotype may significantly impact atorvastatin therapy personalization in patients requiring LDL lipid-lowering therapy. However, more detailed studies are needed to show the exact role of CYP2C19 in determining the effect of atorvastatin.
Author Contributions
Conceptualization, D.Č. and V.T.; methodology, A.A., D.Č., K.Z., V.Ž., V.J., V.S., A.G. and V.T.; validation, A.A., K.Z., D.Č., V.Ž., V.J., I.Č., V.S. and V.T.; formal analysis, D.Č. and V.T.; investigation, D.Č., I.Č., V.S. and V.Ž.; resources, V.Z., V.L., D.Ž., V.J., V.T., O.D. and G.Š.; data curation, D.Č., A.A., K.Z., V.R. and V.T.; writing—original draft preparation, D.Č., A.A., K.Z. and V.T.; writing—review and editing, all authors; visualization, D.Č. and I.Č.; supervision, V.T.; funding acquisition, V.L., D.Č. and V.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Regional Bioethics Committee of Kaunas, Lithuania (permission no. BE-2-5).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets and resources generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the funds of Lithuanian University of Health Sciences “Mokslo fondas”.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pirillo A., Norata G.D. The Burden of Hypercholesterolemia and Ischemic Heart Disease in an Ageing World. Pharmacol. Res. 2023;193:106814. doi: 10.1016/j.phrs.2023.106814. [DOI] [PubMed] [Google Scholar]
- 2.Jarauta E., Bea-Sanz A.M., Marco-Benedi V., Lamiquiz-Moneo I. Genetics of Hypercholesterolemia: Comparison Between Familial Hypercholesterolemia and Hypercholesterolemia Nonrelated to LDL Receptor. Front. Genet. 2020;11:554931. doi: 10.3389/fgene.2020.554931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg D. The Cholesterol Wars: The Cholesterol Skeptics vs the Preponderance of Evidence. Academic Press-Elsevier; San Diego, CA, USA: 2007. [Google Scholar]
- 4.Endo A., Kuroda M., Tsujita Y. ML-236A, ML-236B, and ML-236C, New Inhibitors of Cholesterogensis Produced by Penicillium Citrinum. J. Antibiot. 1976;29:1346–1348. doi: 10.7164/antibiotics.29.1346. [DOI] [PubMed] [Google Scholar]
- 5.Endo A., Kuroda M., Tanzawa K. Competitive Inhibition of 3-hydroxy-3-methylglutaryl Coenzyme a Reductase by ML-236A and ML-236B Fungal Metabolites, Having Hypocholesterolemic Activity. FEBS Lett. 1976;72:323–326. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein L.J., Brown S.M. The Low-Density Lipoprotein Pathway and Its Relation to Atherosclerosis. Annu. Rev. Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- 7.Razavi A.C., Mehta A., Sperling L.S. Statin Therapy for the Primary Prevention of Cardiovascular Disease: Pros. Atherosclerosis. 2022;356:41–45. doi: 10.1016/j.atherosclerosis.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Li M., Wang X., Li X., Chen H., Hu Y., Zhang X., Tang X., Miao Y., Tian G., Shang H. Statins for the Primary Prevention of Coronary Heart Disease. BioMed Res. Int. 2019;2019:4870350. doi: 10.1155/2019/4870350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., De Backer G.G., Delgado V., Ference B.A., et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 10.Virani S.S., Newby L.K., Arnold S.V., Bittner V., Brewer L.C., Demeter S.H., Dixon D.L., Fearon W.F., Hess B., Johnson H.M., et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023;148:E9–E119. doi: 10.1161/CIR.0000000000001168. [DOI] [PubMed] [Google Scholar]
- 11.Marx N., Federici M., Schütt K., Müller-Wieland D., A Ajjan R., Antunes M.J., Christodorescu R.M., Crawford C., Di Angelantonio E., Eliasson B., et al. 2023 ESC Guidelines for the Management of Cardiovascular Disease in Patients with Diabetes. Eur. Heart J. 2023;44:4043–4140. doi: 10.1093/eurheartj/ehad192. [DOI] [PubMed] [Google Scholar]
- 12.Gitt A.K., Lautsch D., Ferrières J., De Ferrari G.M., Vyas A., Baxter C.A., Bash L.D., Ashton V., Horack M., Almahmeed W., et al. Cholesterol Target Value Attainment and Lipid-Lowering Therapy in Patients with Stable or Acute Coronary Heart Disease: Results from the Dyslipidemia International Study II. Atherosclerosis. 2017;266:158–166. doi: 10.1016/j.atherosclerosis.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 13.De Backer G., Jankowski P., Kotseva K., Mirrakhimov E., Reiner Ž., Rydén L., Tokgözoğlu L., Wood D., De Bacquer D., EUROASPIRE V collaborators et al. Management of Dyslipidaemia in Patients with Coronary Heart Disease: Results from the ESC-EORP EUROASPIRE V Survey in 27 Countries. Atherosclerosis. 2019;285:135–146. doi: 10.1016/j.atherosclerosis.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Yao X., Shah N.D., Gersh B.J., Lopez-Jimenez F., Noseworthy P.A. Assessment of Trends in Statin Therapy for Secondary Prevention of Atherosclerotic Cardiovascular Disease in US Adults From 2007 to 2016. JAMA Netw. Open. 2020;3:e2025505. doi: 10.1001/jamanetworkopen.2020.25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin S., Shin D.W., Cho I.Y., Jeong S.-M., Jung H. Status of Dyslipidemia Management and Statin Undertreatment in Korean Cancer Survivors: A Korean National Health and Nutrition Examination Survey Study. Eur. J. Prev. Cardiol. 2021;28:864–872. doi: 10.1177/2047487320905722. [DOI] [PubMed] [Google Scholar]
- 16.Danchin N., Almahmeed W., Al-Rasadi K., Azuri J., Berrah A., Cuneo C.A., Karpov Y., Kaul U., Kayıkçıoğlu M., Mitchenko O., et al. Achievement of Low-Density Lipoprotein Cholesterol Goals in 18 Countries Outside Western Europe: The International ChoLesterol Management Practice Study (ICLPS) Eur. J. Prev. Cardiol. 2018;25:1087–1094. doi: 10.1177/2047487318777079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsioufis K., Vázquez J.M.C., Sykara G., Malvestiti F.M., van Vugt J. Real-World Evidence for Adherence and Persistence with Atorvastatin Therapy. Cardiol. Ther. 2021;10:445–464. doi: 10.1007/s40119-021-00240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cano-Corres R., Candás-Estébanez B., Padró-Miquel A., Fanlo-Maresma M., Pintó X., Alía-Ramos P. Influence of 6 Genetic Variants on the Efficacy of Statins in Patients with Dyslipidemia. J. Clin. Lab. Anal. 2018;32:e22566. doi: 10.1002/jcla.22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maxwell W.D., Ramsey L.B., Johnson S.G., Moore K.G., Shtutman M., Schoonover J.H., Kawaguchi-Suzuki M. Impact of Pharmacogenetics on Efficacy and Safety of Statin Therapy for Dyslipidemia. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017;37:1172–1190. doi: 10.1002/phar.1981. [DOI] [PubMed] [Google Scholar]
- 20.Wilke R.A., Ramsey L.B., Johnson S.G., Maxwell W.D., McLeod H.L., Voora D., Krauss R.M., Roden D.M., Feng Q., Cooper-DeHoff R.M., et al. The Clinical Pharmacogenomics Implementation Consortium: CPIC Guideline for SLCO1B1 and Simvastatin-Induced Myopathy. Clin. Pharmacol. Ther. 2012;92:112–117. doi: 10.1038/clpt.2012.57. Updated in Clin. Pharmacol. Ther. 2014, 96, 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper-DeHoff R.M., Niemi M., Ramsey L.B., Luzum J.A., Tarkiainen E.K., Straka R.J., Gong L., Tuteja S., Wilke R.A., Wadelius M., et al. The Clinical Pharmacogenetics Implementation Consortium Guideline for SLCO1B1, ABCG2, and CYP2C9 Genotypes and Statin-Associated Musculoskeletal Symptoms. Clin. Pharmacol. Ther. 2022;111:1007–1021. doi: 10.1002/cpt.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reith C., Baigent C., Blackwell L., Emberson J., Spata E., Davies K., Halls H., Holland L., Wilson K., Armitage J., et al. Effect of Statin Therapy on Muscle Symptoms: An Individual Participant Data Meta-Analysis of Large-Scale, Randomised, Double-Blind Trials. Lancet. 2022;400:832–845. doi: 10.1016/S0140-6736(22)01545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navar A.M., Peterson E.D., Li S., Robinson J.G., Roger V.L., Goldberg A.C., Virani S., Wilson P.W., Nanna M.G., Lee L.V., et al. Prevalence and Management of Symptoms Associated with Statin Therapy in Community Practice. Circ. Cardiovasc. Qual. Outcomes. 2018;11:e004249. doi: 10.1161/CIRCOUTCOMES.117.004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai R., Zhao X., Zhuo H., Wang W., Xu Y., Hu Z., Zhang T., Zhao J. CYP2C19 Metabolizer Phenotypes May Affect the Efficacy of Statins on Lowering Small Dense Low-Density Lipoprotein Cholesterol of Patients with Coronary Artery Disease. Front. Cardiovasc. Med. 2022;9:1016126. doi: 10.3389/fcvm.2022.1016126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caudle K.E., Dunnenberger H.M., Freimuth R.R., Peterson J.F., Burlison J.D., Whirl-Carrillo M., Scott S.A., Rehm H.L., Williams M.S., Klein T.E., et al. Standardizing Terms for Clinical Pharmacogenetic Test Results: Consensus Terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC) Genet. Med. 2017;19:215–223. doi: 10.1038/gim.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hildreth K., Kodani S.D., Hammock B.D., Zhao L. Cytochrome P450-Derived Linoleic Acid Metabolites EpOMEs and DiHOMEs: A Review of Recent Studies. J. Nutr. Biochem. 2020;86:108484. doi: 10.1016/j.jnutbio.2020.108484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.PharmVar Pharmacogene Variation Consortium. [(accessed on 17 January 2024)]. Available online: https://www.pharmvar.org/gene/CYP2C19.
- 28.Badimon G.M., Calvo M., Guzman J., Perez P., Alamar M., Vilahur G., Gavara J., Vargas S., Rello P., Valente F., et al. CMR Analysis of the Cardioprotective Effects of Chronic Statin Therapy Prior to First STEMI: A Propensity Score Analysis. Eur. Heart J. 2021;42((Suppl. 1)):ehab724.1461. doi: 10.1093/eurheartj/ehab724.1461. [DOI] [Google Scholar]
- 29.Otsuka F., Hibi K., Kusama I., Endo M., Kosuge M., Iwahashi N., Okuda J., Tsukahara K., Ebina T., Kojima S., et al. Impact of Statin Pretreatment on the Incidence of Plaque Rupture in ST-Elevation Acute Myocardial Infarction. Atherosclerosis. 2010;213:505–511. doi: 10.1016/j.atherosclerosis.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Dadon Z., Moriel M., Iakobishvili Z., Asher E., Samuel T.Y., Gavish D., Glikson M., Gottlieb S. Association of Contemporary Statin Pretreatment Intensity and LDL-C Levels on the Incidence of STEMI Presentation. Life. 2021;11:1268. doi: 10.3390/life11111268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mega J.L., Close S.L., Wiviott S.D., Shen L., Hockett R.D., Brandt J.T., Walker J.R., Antman E.M., Macias W., Braunwald E., et al. Cytochrome P-450 Polymorphisms and Response to Clopidogrel. N. Engl. J. Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 32.Ercan B., Ayaz L., Çiçek D., Tamer L. Role of CYP2C9 and CYP2C19 Polymorphisms in Patients with Atherosclerosis. Cell Biochem. Funct. 2008;26:309–313. doi: 10.1002/cbf.1437. [DOI] [PubMed] [Google Scholar]
- 33.Tabata N., Hokimoto S., Akasaka T., Arima Y., Sakamoto K., Yamamoto E., Tsujita K., Izumiya Y., Yamamuro M., Kojima S., et al. Patients with Both CYP2C19 Loss-of-Function Allele and Peripheral Endothelial Dysfunction Are Significantly Correlated with Adverse Cardiovascular Events Following Coronary Stent Implantation. J. Cardiol. 2016;67:104–109. doi: 10.1016/j.jjcc.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Akasaka T., Sueta D., Arima Y., Tabata N., Takashio S., Izumiya Y., Yamamoto E., Tsujita K., Kojima S., Kaikita K., et al. CYP2C19 Variants and Epoxyeicosatrienoic Acids in Patients with Microvascular Angina. IJC Heart Vasc. 2017;15:15–20. doi: 10.1016/j.ijcha.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White C.M. Inflammation Suppresses Patients’ Ability to Metabolize Cytochrome P450 Substrate Drugs. Ann. Pharmacother. 2022;56:809–819. doi: 10.1177/10600280211047864. [DOI] [PubMed] [Google Scholar]
- 36.Ozawa T., Sugiyama S., Hayakawa M., Satake T., Taki F., Iwata M., Taki K. Existence of Leukotoxin 9,10-Epoxy-12-0ctadecenoate in Lung Lavages from Rats Breathing Pure Oxygen and from Patients with the Adult Respiratory Distress Syndrome. Am. Rev. Respir. Dis. 1988;137:535–540. doi: 10.1164/ajrccm/137.3.535. [DOI] [PubMed] [Google Scholar]
- 37.Newman J.W., Morisseau C., Hammock B.D. Epoxide Hydrolases: Their Roles and Interactions with Lipid Metabolism. Prog. Lipid Res. 2005;44:1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Thompson D.A., Hammock B.D. Dihydroxyoctadecamonoenoate esters inhibit the neutrophil respiratory burst. J. Biosci. 2007;32:279–291. doi: 10.1007/s12038-007-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palleria C., Roberti R., Iannone L.F., Tallarico M., Barbieri M.A., Vero A., Manti A., De Sarro G., Spina E., Russo E. Clinically Relevant Drug Interactions between Statins and Antidepressants. J. Clin. Pharm. Ther. 2020;45:227–239. doi: 10.1111/jcpt.13058. [DOI] [PubMed] [Google Scholar]
- 40.Kee P.S., Chin P.K.L., Kennedy M.A., Maggo S.D.S. Pharmacogenetics of Statin-Induced Myotoxicity. Front. Genet. 2020;11:575678. doi: 10.3389/fgene.2020.575678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suh J.-W., Cha M.-J., Lee S.-P., Chae I.-H., Kwon T.-G., Bae J.-W., Cho M.-C., Rha S.-W., Kim H.-S. Relationship Between Statin Type and Responsiveness to Clopidogrel in Patients Treated with Percutaneous Coronary Intervention: A Subgroup Analysis of the CILON-T Trial. J. Atheroscler. Thromb. 2014;21:140–150. doi: 10.5551/jat.19265. [DOI] [PubMed] [Google Scholar]
- 42.Filppula A.M., Hirvensalo P., Parviainen H., Ivaska V.E., Lönnberg K.I., Deng F., Viinamäki J., Kurkela M., Neuvonen M., Niemi M. Comparative Hepatic and Intestinal Metabolism and Pharmacodynamics of Statins. Drug Metab. Dispos. 2021;49:658–667. doi: 10.1124/dmd.121.000406. [DOI] [PubMed] [Google Scholar]
- 43.Byrne R.A., Rossello X., Coughlan J.J., Barbato E., Berry C., Chieffo A., Claeys M.J., Dan G.-A., Dweck M.R., Galbraith M., et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023;44:3720–3826. doi: 10.1093/eurheartj/ehad191. [DOI] [PubMed] [Google Scholar]
- 44.Wang N., Fulcher J., Abeysuriya N., Park L., Kumar S., Di Tanna G.L., Wilcox I., Keech A., Rodgers A., Lal S. Intensive LDL Cholesterol-Lowering Treatment beyond Current Recommendations for the Prevention of Major Vascular Events: A Systematic Review and Meta-Analysis of Randomised Trials Including 327 037 Participants. Lancet Diabetes Endocrinol. 2020;8:36–49. doi: 10.1016/S2213-8587(19)30388-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets and resources generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.