Abstract
We have recently developed a candidate human immunodeficiency virus type 1 (HIV-1) vaccine model, based on virus-like particles (VLPs) expressing gp120 from a Ugandan HIV-1 isolate of clade A (HIV-VLPAs), which shows the induction of neutralizing antibodies as well as cytotoxic T lymphocytes (CTL) in BALB/c mice by intraperitoneal (i.p.) administration. In the present study, immunization experiments based on a multiple-dose regimen have been performed with BALB/c mice to compare different routes of administration. i.p. and intranasal (i.n.), but not oral, administration induce systemic as well as mucosal (vaginal and intestinal) immunoglobulin G (IgG) and IgA responses. These immune sera exhibit >50% ex vivo neutralizing activity against both autologous and heterologous primary isolates. Furthermore, the administration of HIV-VLPAs by the i.n. immunization route induces a specific CTL activity, although at lower efficiency than the i.p. route. The HIV-VLPAs represent an efficient strategy to stimulate both arms of immunity; furthermore, the induction of specific humoral immunity at mucosal sites, which nowadays represent the main port of entry for HIV-1 infection, is of great interest. All these properties, and the possible cross-clade in vivo protection, could make these HIV-VLPAs a good candidate for a mono- and multicomponent worldwide preventive vaccine approach not restricted to high-priority regions, such as sub-Saharan countries.
The development of an effective, safe, and affordable vaccine strategy represents a crucial goal for both industrialized and developing countries. In fact, although highly active antiretroviral therapy induces dramatic reductions in both human immunodeficiency virus (HIV)-related morbidity and mortality, it fails to eradicate the viral infection and selects resistant viral populations which cannot be controlled with alternative “salvage” strategies (21, 39).
Considering the discordant reports on the role of the humoral and cellular immune responses in the containment of HIV type 1 (HIV-1) disease progression (5, 9, 34, 41, 45, 47), a preventive HIV-1 vaccine should elicit both virus-specific neutralizing antibodies and cytotoxic T lymphocytes (CTLs). In this perspective, we have chosen a vaccine approach based on virus-like particles (VLPs), a model currently under investigation as a potential vaccine for different human viruses, such as hepatitis viruses, papillomavirus, rotavirus, parvovirus, and Norwalk virus (25, 30, 33, 36, 46, 53). The HIV-targeted VLPs (HIV-VLPs) are based on the fact that the HIV-1 Pr55gag precursor protein assembles as immature, nonreplicating, noninfectious VLPs with effective induction of both arms of the immune response (10, 16, 27, 29, 51). Furthermore, HIV-VLPs can be employed to deliver additional antigenic structures, such as whole proteins or specific individual epitopes; in particular, specific HIV-VLPs have been developed to present an entire gp120 molecule, anchored through the transmembrane portion of the Epstein-Barr virus gp220/350 (Env-Gag-hybrid VLPs), and to increase the expression and stability of the glycoprotein on the surfaces of VLPs without affecting its oligomerization (10). The gp120 glycoprotein selected for these HIV-VLPs derives from a Ugandan HIV-1 clade A isolate (11, 12), which represents the second most prevalent HIV-1 subtype worldwide (approximately 25%) and is predominant in many developing countries. These HIV-VLPs (HIV-VLPAs) show strong in vivo immunogenicity in BALB/c mice in the absence of adjuvants, and HIV-1-specific CTLs as well as cross-clade neutralizing antibodies have been detected in immunized animals (13).
The transmission of HIV-1 infection during heterosexual or homosexual intercourse accounts for as much as 80% of AIDS globally (59). Infection can involve both free and cell-associated HIV-1, which, for successful sexual transmission, engages in different strategies to cross the mucosal barriers of both the intestinal and genital tracts in order to infect CD4+ T cells (7, 31, 35, 49, 56). Mucosal secretory immunoglobulin A (sIgA) specific for HIV-1 envelope glycoproteins is consistently detected in seropositive subjects (3, 18) and has been strongly associated with protection from HIV-1 infection in uninfected individuals having unprotected sexual intercourse with HIV-1-seropositive partners (19, 32, 38, 42).
Considering this epidemiological and experimental evidence, it seems reasonable to believe that specific mucosal immunity is extremely relevant for controlling the primary HIV-1 infection. In this perspective, the nasal route of immunization, better than the oral route, appears to elicit both mucosal and systemic immunity with a limited induction of mucosal tolerance, which may compromise local vaccination with soluble antigens (8). These mucosal vaccination strategies, furthermore, would overcome the major problems faced in developing countries related to the necessity of disposable sterile syringes and needles, which not only result in higher costs but also, if not properly handled, may facilitate the spread of HIV-1 infection.
Several mucosal vaccination approaches have been developed in recent years for human pathogens transmitted mainly through the mucosal system, including influenza A virus (57), Vibrio cholerae (62), human papillomavirus (6), rotavirus (15), and herpes simplex virus type 2 (23). For HIV-1, in particular, a number of different mucosal vaccine strategies have been studied, including bacterial vectors (17, 28, 37, 50), proteins (2, 52), live-attenuated vectors (4, 26), inactivated HIV-1 (1, 20), VLPs (61), and naked DNA (40, 55).
We report here the induction of systemic and mucosal humoral responses as well as specific CTL activity in mice after intraperitoneal (i.p.) as well as intranasal (i.n.) immunization with HIV-VLPAs, without any adjuvant. In contrast, in our model, neither a humoral nor a cellular immune response was obtained following oral administration, as previously shown for rotavirus and parvovirus VLPs (46, 54). Both systemic and mucosal antibody responses exhibit >50% ex vivo neutralization activity against autologous as well as heterologous primary isolates, indicating that the HIV-VLP represents an efficient approach to be employed alone or as part of a multicomponent vaccine strategy.
MATERIALS AND METHODS
Production of recombinant baculovirus and HIV-VLPAs.
Recombinant baculoviruses expressing both the subtype A gp120UG-Epstein-Barr virus transmembrane fusion gene and the Pr55gag cDNA were produced as previously described (10). Briefly, the HIV-1 coding sequences were transferred in a single step, by site-specific transposition, from the pFastBac dual-transfer vector (Gibco-BRL) to the baculovirus DNA. The baculovirus DNA was obtained in a DH10Bac Escherichia coli bacterial strain (Gibco-BRL) modified to propagate both a baculovirus shuttle vector (bacmid), which contains mini-attTn7 attachment sites, and the pMON7124 helper plasmid, which provides the Tn7 transposition functions in trans. The recombinant baculovirus DNA was used to transfect SF9 insect cells, and supernatants were collected at 48 to 72 h posttransfection; different multiplicities of infection were then used to infect High Five cells and scale up the production of VLPs. Particles were purified through a continuous 10-to-60% sucrose gradient, and their production was confirmed by standard electron microscopy analysis (10).
Immunization experiments.
Female BALB/c mice, each group consisting of three animals 6 to 8 weeks of age, were administered 20 or 100 μg purified VLPs by the i.p., i.n., or oral route. Control mice were treated with endotoxin-free phosphate-buffered saline (PBS). The immunization schedule was based on a 4-dose regimen, where the booster inoculations were administered at weeks 3, 7, and 9 after the primary vaccine dose. All the immunization protocols were performed in two independent experiments without addition of adjuvants.
(i) i.n. vaccination.
Prior to inoculation, mice were anesthetized by halothane. Animals were treated by placing 200 μl of inoculum, 50 μl at time, into alternating nostrils and allowing mice to inhale. Animals were monitored until consciousness was regained.
(ii) Oral vaccination.
Mice were gavaged with 40 μl of 5% bicarbonate buffer immediately prior to oral inoculation with VLPs, in order to neutralize stomach acidity. Each mouse was then gavaged with VLPs in a final volume of 200 μl. No adverse signs were noted in any mice receiving i.n. or oral inoculation.
Sample collection and processing. (i) Serum samples.
Blood samples were collected from each animal 1 week after each vaccination dose by puncture of the retro-orbital vein. After the last dose, the entire blood volume was collected by tail bleeding; serum was obtained by standard methods and stored at −80°C until use.
(ii) Vaginal secretions.
Vaginal secretions were collected from each animal 1 week after each vaccination dose by pipetting 50 μl of PBS in and out of the vagina gently until a discrete clump of mucus was recovered. This usually took four to eight cycles of pipetting and required cutting the pipette tip back to a diameter of 1 to 2 mm. A second vaginal wash with 50 μl of PBS was then done to ensure more complete recovery of the vaginal secretions, and this material was combined with the first wash. Vaginal washes were centrifuged at 12,000 × g for 10 min shortly after collection to separate the mucus from the PBS wash solution. The mucus and supernatant were then frozen separately at −80°C. The PBS wash solution contained a cocktail of proteinase inhibitors (153.8 nM aprotinin, 3.2 μM bestatin, and 10 μM leupeptin). In order to obtain complete recovery of sIgA and presumably also IgG from the vaginal mucus, samples were thawed, weighed, and extracted twice for 2 h each time in 100 μl of PBS per sample, with rotation at 20 rpm in a 12-ml polystyrene tube at 4°C. The two extracts and the original wash supernatant were pooled, made up to 300 μl per sample, and frozen at −80°C until needed (48).
(iii) Fecal pellets.
Fecal antibody samples were collected from each mouse 1 week after each vaccination dose. Fecal pellets were resuspended at a 10% (wt/vol) concentration in a stool diluent (10 mM Tris, pH 7.5, 100 mM NaCl, 1 mM CaCl2, 0.05% Tween 20, 5 mM sodium azide, supplemented with 1 mg of aprotinin/ml and 10 mg of leupeptin/ml). After incubation on ice for 20 min, suspensions were centrifuged at 18,000 × g for 15 min in order to remove fecal solids. Processed fecal antibody samples were stored at −80°C.
Serological assays.
The presence and the titers of IgG and IgA antibodies, specific for the native conformational HIV-VLPAs used in the immunization protocols as well as for the Env and Gag epitopes, were evaluated in samples obtained from immunized mice by an enzyme-linked immunosorbent assay (ELISA). Ninety-six-well MICROTEST assay plates (Becton Dickinson) were coated with 2.5 μg of HIV-VLPAs; alternatively, plates were coated either with 0.5 μg of the gp120-Env V3 loop peptide (CTRPYNNTRQSTHIGPGQALYTTNIIGDIRQAHC) (catalog no. EVA7017.1), representing the consensus sequence of Ugandan clade A HIV-1 strains, or with 0.5 μg of 20-mer peptides with a 10-amino-acid overlap (derived from the HIV-SF2 isolate) spanning the entire p24gag protein (catalog no. ARP788.1-22). Both the V3 loop and p24 peptides were obtained through the NIBSC Centralised Facility for AIDS Reagents (http://www.nibsc.ac.uk/catalog/aids-reagent/catalog_2002).
Antigens were allowed to adhere to the plates by incubation at +4°C overnight, and nonspecific protein binding to the plates was blocked by incubation with 5% (wt/vol) dry milk in PBS for 1 h at room temperature (RT). Diluted mouse sera or mucosal fluids were added to the wells and incubated for 1 h at RT. After three washes, wells were treated for 1 h at RT with peroxidase-conjugated goat anti-mouse IgG or IgA; after three additional washes, the reaction was visualized with 0.075% 4-chloro-1-naphthol in 0.056% hydrogen peroxide and stopped with 2 N sulfuric acid. Absorbance was determined at 492 nm, and reactions were considered positive when the mean absorbance for immunized animals exceeded the mean absorbance of equal dilutions of sera collected from control animals by a factor of 3. Serum ELISA results are expressed as the log10 geometric mean titer of the last positive dilution point from the animals of each set in the two independent experiments. Results for antibodies in vaginal or fecal samples are expressed as the optical density (OD) obtained with 1:5-diluted fluids (54).
HIV-1 neutralization assay.
The neutralization assay was performed using as a target the U87 human glioma cell line transfected with the CCR5 chemokine receptors (14). The autologous clade A Ugandan and heterologous clade B Italian field isolates, used for the assays, are HIV-1 primary isolates identified in asymptomatic, recently seroconverted individuals by coculture with fresh peripheral blood mononuclear cells and frozen down with no additional passages on lymphocyte cell lines (11). Primary isolates replicate on CCR5-expressing U87 cells, and they were titrated in these cells for the ex vivo neutralization assays. No signs of replication are detectable on CD4- or CD4/CXCR4-expressing U87 cells.
U87 target cells were seeded in 96-well plates at a density of 1 × 104 cells/well. On the following day, 10 50% tissue culture infective doses (TCID50) of both viruses were mixed with serial dilutions of serum (1:20 to 1:80) or with mucosal fluids (1:5) and incubated for 1 h at 37°C. As controls, the viruses were either not treated or mixed with matched serum dilutions from a preimmunized animal. The mixture was added to well-adherent CCR5-expressing U87 cells in triplicate and incubated overnight. After three washes with phosphate-buffered saline, 200 μl of complete medium was added per well and cultures were incubated for 7 days. The levels of HIV-1 p24 antigen in the supernatants were determined at days 4 and 7 postinfection (p.i.) with a commercially available kit (NEN DuPont); the percentage of neutralization was calculated relative to a dilution-matched control. Cell viability was microscopically verified during incubation and at the end of the incubation period in all wells. The test was scored positive when, on day 7 p.i., the p24 concentration was ≥130 pg/ml in control wells and the tested serum dilution exhibited a >50% neutralization effect.
CTL assay.
Single-cell suspensions of spleens were obtained from immunized and control mice and cocultured in the presence of interleukin-2 (5 IU/ml) for 7 to 14 days with irradiated Env- or Gag-expressing syngeneic splenocytes (stimulators) in order to propagate specific effector T cells. In particular, stimulator cells were induced for 24 h with 5 μg/ml of phytohemagglutinin (PHA; Sigma), infected with 1 PFU/103 cells of Env- or Gag-recombinant vaccinia virus, and, after an additional 16 to 24 h, γ-irradiated with 2,000 rads. For cytolytic assays, target syngeneic H-2d P815 cells were infected with 1 PFU/103 cells of either Gag- or Env-vaccinia virus 16 to 24 h prior to the start of the assay. Both Gag- and Env-vaccinia viruses were obtained from the NIBSC Centralised Facility for AIDS Reagents; the gag sequence derives from the clade B HIV-1BH10 isolate (14), and the env sequence derives from a Ugandan clade A HIV-1 isolate (24). Infected target cells were pulsed for 1 h at 37°C with 51Cr (Amersham Pharmacia Biotech) (30 μCi/106 cells) and washed three times with culture medium before the addition of effector cells. Specific lysis was measured in a triplicate assay performed with 5 × 103 target cells mixed with different ratios of effector cells (effector to target cell [E:T] ratio, 25:1 to 3.125:1) in a total volume of 200 μl. After a 5-h incubation at 37°C, 100 μl culture supernatant was collected for counting the amount of 51Cr release. Positive and negative controls, in the absence of effector cells, were treated with or without 1 N HCl, respectively. The percentage of specific lysis was calculated as follows: 100 × [(specific release − spontaneous release)/(total release − spontaneous release)].
Statistical analyses.
Intergroup comparisons were performed with the Mann-Whitney U test (for univariate nonparametric group analysis). All P values were two tailed and considered significant if less than 0.05.
RESULTS
Induction of serum antibody response by HIV-VLPAs injected by different routes.
The humoral immune efficacy of HIV-VLPAs, produced in insect cells and purified on a 10-to-60% continuous sucrose gradient (10), was evaluated for 18 BALB/c mice, divided into six groups, by following a 4-dose immunization schedule and comparing different routes of administration. In particular, one animal group was treated with an i.p. injection of 20 μg purified VLPs, which has been shown previously to induce specific antibody as well as CTL responses (13); two animal groups received i.n. administration of 20 or 100 μg purified VLPs; two animal groups received oral administration of 20 or 100 μg purified VLPs; and the control group was i.p. injected with PBS alone. At weeks 3, 7, and 9, each group was treated with the same amount of antigen received in the primary vaccination dose, and a week later, blood samples were drawn from the retro-orbital vein. A second experiment with the same group distribution and modality was later performed independently, starting 2 weeks after the end of the first experiment. No animals showed any signs of toxicity, and all remained healthy up to the end of the vaccination protocol.
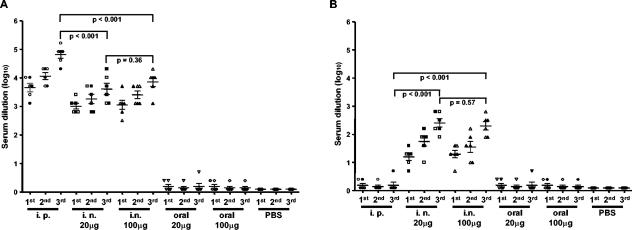
In order to evaluate specific HIV-VLPA-induced serum antibody titers, ELISAs were performed on microwell plates coated with HIV-VLPAs. The data showed that serum IgG titers increased after each vaccination dose administered by the i.p. or i.n. route (up to 1:81,920 for the i.p. and 1:10,240 for the i.n. route), while the oral route did not induce a detectable antibody titer (Fig. 1A). The i.p. immunization protocol induced statistically significantly higher systemic IgG antibody titers (P < 0.001) than both i.n. immunization does (20 and 100 μg) (Fig. 1A) but failed to induce detectable IgA titers (Fig. 1B). IgA antibody titers (1:250, on average), instead, were detected in sera of mice immunized by the i.n. route, and no statistically significant difference was observed between the two HIV-VLPAs doses (20 and 100 μg) (P > 0.5) (Fig. 1B).
FIG. 1.
Levels of specific humoral response induced in mice immunized with HIV-VLPAs. Geometric mean titers of specific serum antibodies induced in each BALB/c mouse group by administration of different doses of HIV-VLPAs via parenteral and mucosal routes in two independent experiments. At 3, 7, and 9 weeks following the primary immunization, each group was administered the same amount of antigen, and a week later, blood samples were drawn from the retro-orbital vein. Serum samples were analyzed for specific IgG (A) and IgA (B) antibodies by ELISA, performed in triplicate on 96-well plates coated with 2.5 μg of HIV-VLPAs. In each panel, results are expressed as the individual average reciprocal last dilution with an OD at 492 nm threefold that of the preimmune sera. Data from the two independent experiments, with three mice per group per experiment, are indicated by open and full symbols. Bars represent the cumulative mean of each group ± the standard deviation. P values are indicated for intergroup comparisons.
ELISAs performed on microwell plates coated with HIV-1 peptides confirmed the same antibody titers for Gag-specific epitopes but showed 1- to 2-dilution-lower titers for Env-specific epitopes. Reactions were considered positive when the absorbance at 492 nm exceeded the average absorbance of equal dilutions of sera collected from control animals by a factor of 3 (data not shown). Within each group, the standard deviations of the antibody responses were always <10% of the mean value. In addition, a standard deviation <10% was observed between the same groups in the two independent experiments performed.
Induction of mucosal antibody responses by HIV-VLPAs injected by different routes.
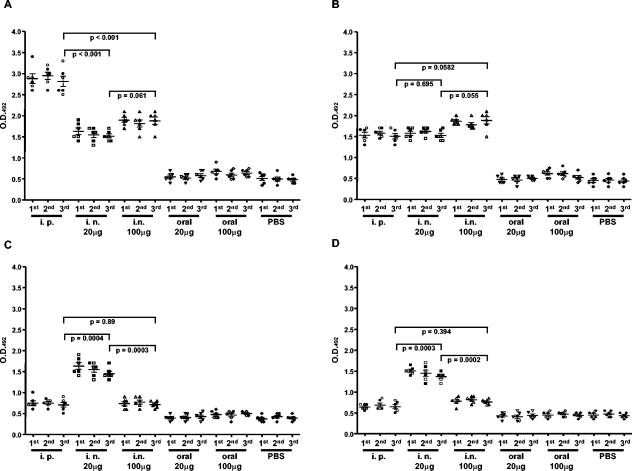
In order to evaluate the antibody response at the mucosal level, vaginal washes and fecal pellets were collected from each animal a week after each HIV-VLPA vaccination dose and evaluated in an ELISA system. Given the low antibody titers at mucosal sites reported by several authors (54, 48, 60), ELISAs were performed at a single dilution, 1:5, and results were expressed as OD values (54). Reactions were considered positive when the absorbance at 492 nm exceeded the average absorbance of samples from control animals by a factor of 2. The results showed that, at the intestinal level, the strongest IgG response was induced by the i.p. immunization route (P < 0.001), whereas the IgA response was equally induced by the i.p and i.n. routes (P > 0.05) (Fig. 2A). In contrast, at the vaginal level, the 20-μg dose administered by the i.n. route induced the strongest IgG and IgA responses, compared to both the i.p. route and the 100-μg i.n. dose (P < 0.001) (Fig. 2B). Consistent with the systemic results, oral administration of HIV-VLPAs did not result in a detectable antibody response at either the intestinal or the vaginal level.
FIG. 2.
Levels of specific antibodies in the feces and vaginal washes of mice immunized with HIV-VLPAs. At 3, 7, and 9 weeks following the primary immunization, each group of mice was administered the same amount of antigen, and a week later, fecal samples and vaginal washes were obtained for each animal. Levels of specific IgG and IgA antibodies in the feces (A and B) and in the vaginal washes (C and D) were evaluated by ELISA, performed in triplicate on 96-well plates coated with 2.5 μg of HIV-VLPAs. In each panel, results are represented as the average OD at 492 nm observed in individual animals at a 1:5 dilution of mucosal samples. Data from the two independent experiments, with three mice per group per experiment, are indicated by open and full symbols. Reactions were considered positive when the absorbance exceeded the average absorbance of samples from control animals by a factor of 2. Bars represent the cumulative mean of each group ± the standard deviation. P values are indicated for intergroup comparisons.
Also for the mucosal antibody response, the standard deviations within each group were always <10% of the mean value, and a standard deviation <10% was observed between the same groups in the two independent experiments performed.
Neutralization activity against autologous and heterologous primary field isolates.
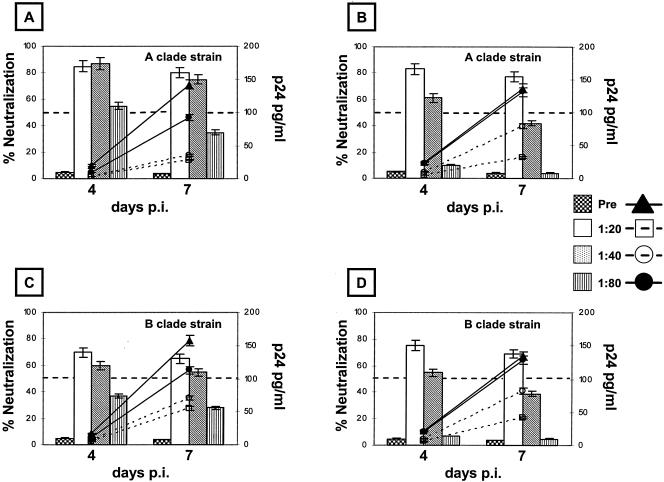
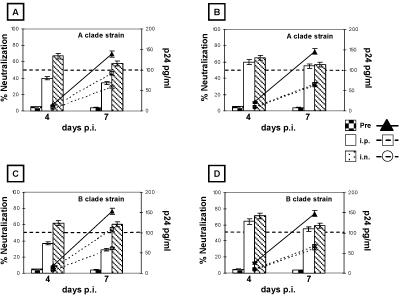
The neutralization activities of antibodies induced by HIV-VLPAs in mice immunized via different routes were verified ex vivo against primary field isolates characterized in our laboratory: the autologous Ugandan clade A isolate, whose gp120 was expressed on the HIV-VLPAs (10), and a heterologous clade B strain identified from an Italian intravenous drug user. The results show that immune sera derived from mice immunized via the i.p. or i.n. route are able to inhibit the replication of both autologous and heterologous isolates in CCR5-expressing U87 cells, up to a final dilution of 1:40 (i.p.) or 1:20 (i.n.). In particular, such serum dilutions show an average neutralization capacity >75% against the autologous isolate for the entire period of observation. In contrast, the next dilutions (1:80 i.p. and 1:40 i.n.) show a transient 55% neutralization on day 4 p.i. which is no longer detectable on day 7 p.i. (Fig. 3A and B). In parallel, the neutralizing serum dilutions are able to inhibit the replication kinetics of the heterologous clade B primary isolate, showing an average 64% neutralization on days 4 and 7 p.i. (Fig. 3C and D). Furthermore, moderate neutralizing activity (approximately 57%) against both autologous and heterologous primary isolates was detected, at a 1:5 dilution, in both vaginal and intestinal fluids from mice immunized by the i.n. route (Fig. 4A to D) and only in vaginal fluids from mice immunized by the i.p. route (Fig. 4B and D). In all the neutralization assays, the mean standard deviation of the samples' triplicate cultures, in both control and immunized groups, was always lower than 5% of the mean value. The mean standard deviation of the combined results from the corresponding groups of the two independent experiments was always lower than 10% of the mean value.
FIG. 3.
Autologous and heterologous neutralization efficiencies of sera from mice immunized with HIV-VLPAs. The HIV-1 neutralization assay was performed on CCR5-expressing U87 cells against autologous and heterologous primary isolates, clade A Ugandan and clade B Italian HIV-1 isolates, respectively. Ten TCID50 of each isolate were pretreated with serial dilutions of the immune sera, collected at the end of the complete immunization schedule, from the mouse groups immunized with 20 μg of HIV-VLPAs via the i.p. or the i.n. route. The neutralization activities of serum antibodies, at different dilutions, induced via the i.p. (A and C) or the i.n. (B and D) route are represented as percentages of the virus replication in control samples (vertical bars). Fifty percent neutralization has been considered the lowest limit for scoring the test positive. In all the panels the kinetics of virus growth during the 7-day neutralization assay is shown (lines). Each serum dilution was evaluated in triplicate, and the reported results include the cumulative data from two independent experiments with three mice per group per experiment.
FIG. 4.
Autologous and heterologous neutralization efficiencies of mucosal antibodies from mice immunized with HIV-VLPAs. The HIV-1 neutralization assay was performed on CCR5-expressing U87 cells against autologous and heterologous primary isolates, clade A Ugandan and clade B Italian HIV-1 isolates, respectively. Ten TCID50 of each isolate were pretreated with a 1:5 dilution of fecal (A and C) or vaginal (B and D) extracts, collected from the mouse groups immunized with 20 μg of HIV-VLPAs via the i.p. or the i.n. route. Neutralization activity is represented as a percentage of the virus replication in control samples (vertical bars). Fifty percent neutralization has been considered the lowest limit for scoring the test positive. In all the panels the kinetics of virus growth during the 7-day neutralization assay is shown (lines). Each dilution was evaluated in triplicate, and the reported results include the cumulative data from two independent experiments with three mice per group per experiment.
Both primary field isolates, treated with preimmune sera or untreated, showed similar effective kinetics of replication in the 7-day-p.i. experiment, characterized by a progressive increase in virus production (by measurement of p24 or reverse transcriptase activity) and a final p24 concentration ≥130 pg/ml.
CTL response to HIV-1 epitopes.
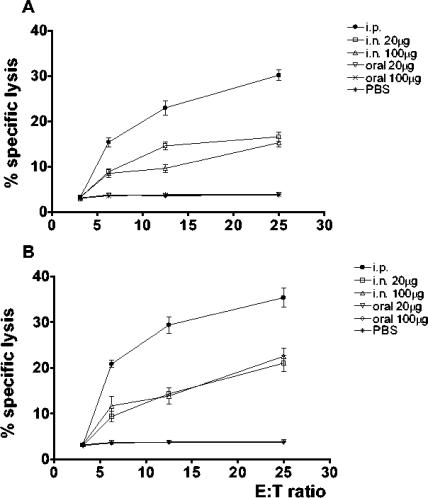
To assess the induction of Env- and Gag-specific CTLs, spleens were removed from immunized as well as control mice. Purified splenocytes (effector cells) were restimulated ex vivo for 2 weeks with γ-irradiated autologous splenocytes infected with either Env- or Gag-vaccinia virus recombinants. The vaccinia virus recombinants express the env sequence derived from a Ugandan clade A HIV-1 isolate (44) and the gag sequence from the clade B HIV-1BH10 isolate (24). The cytotoxic activities of effectors were then evaluated in a 51Cr release assay against syngeneic (H-2d) P815 cells (targets) infected with the same Env- or Gag-vaccinia virus recombinants by using an E:T ratio ranging from 25:1 to 3.125:1. As previously reported (13), a 30 to 35% CTL response was detected at the E:T ratio of 25:1 in each paired experiment performed with splenocytes from animals immunized via the i.p. route. Significantly lower CTL activity (16 to 20%) (P < 0.001) was detected at the same E:T ratio when the experiment was performed with splenocytes from animals immunized via the i.n. route. No CTL activity was induced by oral administration of HIV-VLPAs (Fig. 5A and B). The HIV-1 Env and Gag epitopes were used independently for both the ex vivo restimulation and the lysis assay. In contrast, <5% lysis was observed when the effectors were restimulated ex vivo with Env and mixed with Gag-expressing targets (and vice versa), indicating that the reaction was specifically driven ex vivo by antigen-restimulated CTL clones (data not shown). The correlation between the percentage of observed lysis and the E:T ratio was approximately linear within the range analyzed. As expected, no cytotoxic activity was detected against parental P815 cells. Each end point for each animal was evaluated in triplicate, and the standard deviation was always lower than 5% of the mean value. The standard deviation of the mean was always lower than 8% in each experimental group of three animals and 10% between the two corresponding groups of the two independent experiments.
FIG. 5.
Cytotoxic T-cell activation (CTL analysis) in mice immunized with HIV-VLPAs. CTL response was evaluated in splenocytes from mice immunized with HIV-VLPAs. Splenocyte cultures were restimulated in vitro with γ-irradiated splenocytes infected with either Env (A)- or Gag (B)-expressing vaccinia virus recombinants. Effector cells were then assayed for lysis of P815 target cells presenting either HIV-1 gp160A or GagB epitopes, at an E:T ratio ranging from 25:1 to 3.125:1. Parental P815 cells were used as a negative control in both experiments. Specific cell lysis was measured as 51Cr release and expressed as the percentage of total lysis from acid-disrupted target cells. Data are means of triplicate cultures from all animals included in the two independent experiments with three mice per group per experiment.
DISCUSSION
It has been reported previously that a candidate HIV-1 preventive/therapeutic vaccine recently developed in our laboratory, based on VLPs expressing a gp120 glycoprotein derived from an HIV-1 clade A isolate (HIV-VLPAs) (10, 12), shows strong in vivo immunogenicity in BALB/c mice and induces both a CTL response and cross-clade neutralizing antibodies, in the absence of adjuvants (13).
In the present study, it is shown that the recombinant HIV-VLPAs, administered by the i.p. or i.n. route in the absence of adjuvants, stimulate IgA and/or IgG antibody responses in sera as well as at vaginal and intestinal mucosal sites. Moreover, as already reported for the i.p. route, the i.n. immunization route also provides a way to induce CTL responses, although to a much lower extent.
An immunization protocol based on a 4-dose regimen was performed in parallel on four animal groups with two different doses of HIV-VLPAs (20 and 100 μg, respectively) administered by the i.n. or oral route. A group was immunized under standard conditions (20 μg by the i.p. route) (13), and the control group was i.p. injected with PBS alone. The booster doses elicited increasing serum levels of specific IgG antibodies in animals immunized by the i.p. or i.n. route, while IgA antibodies were induced only by the i.n. route of administration. The i.n. 20-μg dose appeared to be optimal, given that a fivefold-higher dose (100 μg) does not correlate with a higher antibody titer, probably due to a tolerance effect. Local production of specific antibodies was demonstrated at the intestinal and vaginal levels, showing that the i.p. administration route is able to stimulate an IgG response preferentially, whereas the i.n. administration route induces both IgG and IgA responses. In the present study, oral administration of HIV-VLPAs failed to stimulate systemic as well as mucosal immune responses. In this regard, although mice were gavaged with bicarbonate buffer immediately prior to each oral inoculation with VLPs, this treatment could have been insufficient to neutralize stomach acidity, resulting in significant antigen degradation.
Immune sera were tested for ex vivo neutralization activity against primary field isolates, the autologous Ugandan clade-A isolate and a heterologous Italian clade B isolate, using the CR-expressing U87 human glioma cell line as a target (14). Sera from mice immunized with 20 μg of HIV-VLPAs by the i.p. route showed neutralization activity against both the autologous and heterologous field isolates at a final dilution of 1:40. Equivalent neutralization activity against both HIV-1 field isolates was obtained also with a 1:20 dilution of sera from animals immunized by the i.n. administration route. Furthermore, moderate neutralizing activity (approximately 57%) against both autologous and heterologous primary isolates was detected in vaginal and intestinal fluids from mice immunized by the i.n. route and only in intestinal samples from mice immunized by the i.p. route. The breadth of this cross-clade neutralization activity is currently being evaluated using an expanded panel of primary HIV-1 isolates of different subtypes. The neutralization efficacy against HIV-1 primary isolates observed in this study is extremely significant, considering the poor neutralization activity against primary isolates in comparison to T-cell-adapted strains consistently reported in the literature (43, 58), and strongly suggests the potential applications of the VPL as a vaccine approach in countries characterized by multiclade HIV-1 epidemics.
Furthermore, the immunization of mice with HIV-VLPAs by the i.p. and, to a lesser extent, the i.n. administration route was able to induce in vivo CD8+ CTLs with specific cytolytic activity against target cells expressing either Env or Gag molecules derived from HIV-1 isolates of clade A or B, respectively.
All the results were obtained in two independent immunization experiments, where the standard deviation was between 5 and 10% of the mean value, and the standard deviation of the mean was <5% of the mean value, indicating significant consistency of the independent observations.
The data confirm that these HIV-VLPAs are able to induce a strong humoral as well as a cellular immune response, likely due to efficient presentation in a major histocompatibility complex class II context with a resulting stimulation of T-helper-cell responses, as previously reported (13). In particular, the induction of immune responses in mice immunized by the i.n. administration route, without adjuvants, shows that nonreplicating VLPs are immunologically more effective than monomeric proteins or linear peptides, probably due to their particulate structure (6, 30, 54). Most proteins administered alone by mucosal routes, in fact, induce poor if measurable immune responses, and only a few natural antigens, including bacterial toxins such as cholera toxin or E. coli labile toxin, consistently stimulate strong mucosal responses (22). Furthermore, the lower serum antibody titers and CTL activity detected in animals immunized by the i.n. route compared to those in animals immunized by the i.p. route, as well as the lack of HIV-VLPA immunogenicity following oral administration, suggest the need to optimize the immunization protocols. This goal will be pursued by exploring prime/boost strategies (i.e., DNA/HIV-VLP) as well as introducing nontoxic adjuvants in order to enhance the immunogenicity of mucosal immunizations and reduce the dose administered, which should also result in lower potential toxicity.
The results obtained with our HIV-VLPAs, in particular the induction of antibody responses with ex vivo cross-clade neutralization activity, suggest a possible broad in vivo protection which would make this strategy a good candidate for a mono- as well as a multicomponent worldwide vaccine approach not limited to sub-Saharan countries with high incidences of HIV-1 clade A isolates.
Acknowledgments
Recombinant vaccinia virus, expressing gp160 of HIV-1 clade A (EVA 289.1-4) and Pr55gag of HIV-1 clade B (ARP 253), the gp120 V3 peptide (EVA7017.1), and overlapping peptides spanning the entire p24gag protein (ARP788.1-22) were obtained through the NIBSC Centralised Facility for AIDS Reagents, supported by EU Program EVA (contract BMH4 97/2515) and the UK Medical Research Council. The original donors of the reagents were M. Esteban (EVA 289.1-4) and D. Nixon (ARP 253). We thank John McKnight for revising the English style of the manuscript.
This study was supported by grants from the Ministero Italiano della Sanità (Ricerca Corrente and Progetto Finalizzato AIDS 2000) and the ICSC-World Lab, Lausanne, Switzerland (project MCD-2/7).
REFERENCES
- 1.Akagi, T., M. Kawamura, M. Ueno, K. Hiraishi, M. Adachi, T. Serizawa, M. Akashi, and M. Baba. 2003. Mucosal immunization with inactivated HIV-1-capturing nanospheres induces a significant HIV-1-specific vaginal antibody response in mice. J. Med. Virol. 69:163-172. [DOI] [PubMed] [Google Scholar]
- 2.Albu, D. I., A. Jones-Trower, A. M. Woron, K. Stellrecht, C. C. Broder, and D. W. Metzger. 2003. Intranasal vaccination using interleukin-12 and cholera toxin subunit B as adjuvants to enhance mucosal and systemic immunity to human immunodeficiency virus type 1 glycoproteins. J. Virol. 77:5589-5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfsen, A., P. Iniguez, E. Bouguyon, and M. Bomsel. 2001. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J. Immunol. 166:6257-6265. [DOI] [PubMed] [Google Scholar]
- 4.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2002. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Vaccine 20:1949-1955. [DOI] [PubMed] [Google Scholar]
- 5.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian/human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 6.Balmelli, C., S. Demotz, H. Acha-Orbea, P. De Grandi, and D. Nardelli-Haefliger. 2002. Trachea, lung, and tracheobronchial lymph nodes are the major sites where antigen-presenting cells are detected after nasal vaccination of mice with human papillomavirus type 16 virus-like particles. J. Virol. 76:12596-12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bomsel, M. 1997. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 3:42-47. [DOI] [PubMed] [Google Scholar]
- 8.Brandtzaeg, P. 1996. History of oral tolerance and mucosal immunity. Ann. N. Y. Acad. Sci. 778:1-27. [DOI] [PubMed] [Google Scholar]
- 9.Bruck, C., C. Thiriart, L. Fabry, M. Francotte, P. Pala, O. Van Opstal, J. Culp, M. Rosenberg, M. De Wilde, P. Heidt, and J. L. Heeney. 1994. HIV-1 envelope-elicited neutralizing antibody titers correlate with protection and virus load in chimpanzees. Vaccine 12:1141-1148. [DOI] [PubMed] [Google Scholar]
- 10.Buonaguro, L., F. M. Buonaguro, M. L. Tornesello, D. Mantas, E. Beth-Giraldo, R. Wagner, S. Michelson, M.-C. Prevost, H. Wolf, and G. Giraldo. 2001. High efficient production of Pr55gag virus-like particles expressing multiple HIV-1 epitopes, including a gp120 protein derived from an Ugandan HIV-1 isolate of subtype A. Antivir. Res. 49:35-47. [DOI] [PubMed] [Google Scholar]
- 11.Buonaguro, L., E. Del Gaudio, M. Monaco, D. Greco, P. Corti, E. Beth-Giraldo, F. M. Buonaguro, and G. Giraldo. 1995. Heteroduplex mobility assay and phylogenetic analysis of V3 region sequences of human immunodeficiency virus type 1 isolates from Gulu, Northern Uganda. J. Virol. 69:7971-7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buonaguro, L., F. M. Buonaguro, F. Russo, M. L. Tornesello, E. Beth-Giraldo, R. Wagner, H. Wolf, and G. Giraldo. 1998. A novel gp120 sequence from an HIV-1 isolate of the A clade identified in North Uganda. AIDS Res. Hum. Retrovir. 14:1287-1289. [DOI] [PubMed] [Google Scholar]
- 13.Buonaguro, L., L. Racioppi, M. L. Tornesello, C. Arra, M. L. Visciano, B. Biryahwaho, S. D. K. Sempala, G. Giraldo, and F. M. Buonaguro. 2002. Induction of neutralizing antibodies and CTLs in BALB/c mice immunized with virus-like particles presenting a gp120 molecule from a HIV-1 isolate of clade A (HIV-VLPAs). Antivir. Res. 54:189-201. [DOI] [PubMed] [Google Scholar]
- 14.Cecilia, D., V. N. Kewalramani, J. O'Leary, B. Volsky, P. Nyambi, S. Burda, S. Xu, D. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 72:6988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi, A. H., M. M. McNeal, M. Basu, J. A. Flint, S. C. Stone, J. D. Clements, J. A. Bean, S. A. Poe, J. L. VanCott, and R. L. Ward. 2002. Intranasal or oral immunization of inbred and outbred mice with murine or human rotavirus VP6 proteins protects against viral shedding after challenge with murine rotaviruses. Vaccine 20:3310-3321. [DOI] [PubMed] [Google Scholar]
- 16.Deml, L., G. Kratochwil, N. Osterrieder, R. Knuchel, H. Wolf, and R. Wagner. 1997. Increased incorporation of chimeric human immunodeficiency virus type 1 gp120 proteins into Pr55gag virus-like particles by an Epstein-Barr virus gp220/350-derived transmembrane domain. Virology 235:10-25. [DOI] [PubMed] [Google Scholar]
- 17.DeVico, A. L., T. R. Fouts, M. T. Shata, R. Kamin-Lewis, G. K. Lewis, and D. M. Hone. 2002. Development of an oral prime-boost strategy to elicit broadly neutralizing antibodies against HIV-1. Vaccine 20:1968-1974. [DOI] [PubMed] [Google Scholar]
- 18.Devito, C., J. Hinkula, R. Kaul, L. Lopalco, J. J. Bwayo, F. Plummer, M. Clerici, and K. Broliden. 2000. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS 14:1917-1920. [DOI] [PubMed] [Google Scholar]
- 19.Devito, C., J. Hinkula, R. Kaul, J. Kimani, P. Kiama, L. Lopalco, C. Barass, S. Piconi, D. Trabattoni, J. J. Bwayo, F. Plummer, M. Clerici, and K. Broliden. 2002. Cross-clade HIV-1-specific neutralizing IgA in mucosal and systemic compartments of HIV-1-exposed, persistently seronegative subjects. J. Acquir. Immune Defic. Syndr. 30:413-420. [DOI] [PubMed] [Google Scholar]
- 20.Dumais, N., A. Patrick, R. B. Moss, H. L. Davis, and K. L. Rosenthal. 2002. Mucosal immunization with inactivated human immunodeficiency virus plus CpG oligodeoxynucleotides induces genital immune responses and protection against intravaginal challenge. J. Infect. Dis. 186:1098-1105. [DOI] [PubMed] [Google Scholar]
- 21.Falloon, J. 2000. Salvage antiretroviral therapy. AIDS 14(Suppl. 3):S209-S217. [PubMed] [Google Scholar]
- 22.Freytag, L. C., and J. D. Clements. 1999. Bacterial toxins as mucosal adjuvants. Curr. Top. Microbiol. Immunol. 236:215-236. [DOI] [PubMed] [Google Scholar]
- 23.Gallichan, W. S., R. N. Woolstencroft, T. Guarasci, M. J. McCluskie, H. L. Davis, and K. L. Rosenthal. 2001. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166:3451-3457. [DOI] [PubMed] [Google Scholar]
- 24.Gao, F., L. Yue, S. Craig, C. L. Thornton, D. L. Robertson, F. E. McCutchan, J. A. Bradac, P. M. Sharp, B. H. Hahn, and WHO Network for HIV Isolation and Characterization. 1994. Genetic variation of HIV type 1 in four World Health Organization-sponsored vaccine evaluation sites: generation of functional envelope (glycoprotein 160) clones representative of sequence subtypes A, B, C, and E. AIDS Res. Hum. Retrovir. 10:1359-1368. [DOI] [PubMed] [Google Scholar]
- 25.Garnier, L., M. Ravallec, P. Blanchard, H. Chaabihi, J.-P. Bossy, G. Devauchelle, A. Jestin, and M. Cerutti. 1995. Incorporation of pseudorabies virus gD into human immunodeficiency virus type 1 Gag particles produced in baculovirus-infected cells. J. Virol. 69:4060-4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gherardi, M. M., J. L. Najera, E. Perez-Jimenez, S. Guerra, A. Garcia-Sastre, and M. Esteban. 2003. Prime-boost immunization schedules based on influenza virus and vaccinia virus vectors potentiate cellular immune responses against human immunodeficiency virus Env protein systemically and in the genitorectal draining lymph nodes. J. Virol. 77:7048-7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gheysen, D., E. Jacobs, F. de Foresta, C. Thiriart, M. Francotte, D. Thines, and M. De Wilde. 1989. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell 59:103-112. [DOI] [PubMed] [Google Scholar]
- 28.Golding, B., N. Eller, L. Levy, P. Beining, J. Inman, N. Matthews, D. E. Scott, and H. Golding. 2002. Mucosal immunity in mice immunized with HIV-1 peptide conjugated to Brucella abortus. Vaccine 20:1445-1450. [DOI] [PubMed] [Google Scholar]
- 29.Griffiths, J. C., S. J. Harris, G. T. Layton, E. L. Berrie, T. J. French, N. R. Burns, S. E. Adams, and A. J. Kingsman. 1993. Hybrid human immunodeficiency virus Gag particles as an antigen carrier system: induction of cytotoxic T-cell and humoral responses by a Gag:V3 fusion. J. Virol. 67:3191-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerrero, R. A., J. M. Ball, S. S. Krater, S. E. Pacheco, J. D. Clements, and M. K. Estes. 2001. Recombinant Norwalk virus-like particles administered intranasally to mice induce systemic and mucosal (fecal and vaginal) immune responses. J. Virol. 75:9713-9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaul, R., D. Trabattoni, J. J. Bwayo, D. Arienti, A. Zagliani, F. M. Mwangi, C. Kariuki, E. N. Ngugi, K. S. MacDonald, T. B. Ball, M. Clerici, and F. A. Plummer. 1999. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS 13:23-29. [DOI] [PubMed] [Google Scholar]
- 33.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 89:12180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreiss, J. K., and S. G. Hopkins. 1993. The association between circumcision status and human immunodeficiency virus infection among homosexual men. J. Infect. Dis. 168:1404-1408. [DOI] [PubMed] [Google Scholar]
- 36.Li, T. C., Y. Yamakawa, K. Suzuki, M. Tatsumi, M. A. Razak, T. Uchida, N. Takeda, and T. Miyamura. 1997. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J. Virol. 71:7207-7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lieberman, J., and F. R. Frankel. 2002. Engineered Listeria monocytogenes as an AIDS vaccine. Vaccine 20:2007-2010. [DOI] [PubMed] [Google Scholar]
- 38.Lo Caputo, S., D. Trabattoni, F. Vichi, S. Piconi, L. Lopalco, M. L. Villa, F. Mazzotta, and M. Clerici. 2003. Mucosal and systemic HIV-1-specific immunity in HIV-1-exposed but uninfected heterosexual men. AIDS 17:531-539. [DOI] [PubMed] [Google Scholar]
- 39.Loutfy, M. R., and S. L. Walmsley. 2002. Salvage antiretroviral therapy in HIV infection. Expert Opin. Pharmacother. 3:81-90. [DOI] [PubMed] [Google Scholar]
- 40.Lundholm, P., A. C. Leandersson, B. Christensson, G. Bratt, E. Sandstrom, and B. Wahren. 2002. DNA mucosal HIV vaccine in humans. Virus Res. 82:141-145. [DOI] [PubMed] [Google Scholar]
- 41.Mascola, J., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazzoli, S., D. Trabattoni, S. Lo Caputo, S. Piconi, C. Ble, F. Meacci, S. Ruzzante, A. Salvi, F. Semplici, R. Longhi, M. L. Fusi, N. Tofani, M. Biasin, M. L. Villa, F. Mazzotta, and M. Clerici. 1997. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat. Med. 3:1250-1257. [DOI] [PubMed] [Google Scholar]
- 43.Montefiori, D. C., K. A. Reimann, M. S. Wyand, K. Manson, M. G. Lewis, R. G. Collma, J. G. Sodroski, D. P. Bolognesi, and N. L. Letvin. 1998. Neutralizing antibodies in sera from macaques infected with chimeric simian-human immunodeficiency virus containing the envelope glycoproteins of either a laboratory-adapted variant or a primary isolate of human immunodeficiency virus type 1. J. Virol. 72:3427-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nixon, D. F., A. R. Townsend, J. G. Elvin, C. R. Rizza, J. Gallwey, and A. J. McMichael. 1988. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature 336:484-487. [DOI] [PubMed] [Google Scholar]
- 45.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 46.O'Neal, C. M., S. E. Crawford, M. K. Estes, and M. E. Conner. 1997. Rotavirus virus-like particles administered mucosally induce protective immunity. J. Virol. 71:8707-8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pantaleo, G., J. F. Demarest, H. Soudeyns, C. Graziosi, F. Denis, J. W. Adelsberger, P. Borrow, M. S. Saag, G. M. Shaw, and R. P. Sekaly. 1994. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature 370:463-467. [DOI] [PubMed] [Google Scholar]
- 48.Parr, E. L., and M. B. Parr. 1998. Immunoglobulin G, plasma cells, and lymphocytes in the murine vagina after vaginal or parenteral immunization with attenuated herpes simplex virus type 2. J. Virol. 72:5137-5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phillips, D. M., and A. S. Bourinbaiar. 1992. Mechanism of HIV spread from lymphocytes to epithelia. Virology 186:261-273. [DOI] [PubMed] [Google Scholar]
- 50.Rayevskaya, M. V., and F. R. Frankel. 2001. Systemic immunity and mucosal immunity are induced against human immunodeficiency virus Gag protein in mice by a new hyperattenuated strain of Listeria monocytogenes. J. Virol. 75:2786-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rovinski, B., J. R. Haynes, S. X. Cao, O. James, C. Sia, S. Zolla-Pazner, T. J. Matthews, and M. H. Klein. 1992. Expression and characterization of genetically engineered human immunodeficiency virus-like particles containing modified envelope glycoproteins: implications for development of a cross-protective AIDS vaccine. J. Virol. 66:4003-4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakaue, G., T. Hiroi, Y. Nakagawa, K. Someya, K. Iwatani, Y. Sawa, H. Takahashi, M. Honda, J. Kunisawa, and H. Kiyono. 2003. HIV mucosal vaccine: nasal immunization with gp160-encapsulated hemagglutinating virus of Japan-liposome induces antigen-specific CTLs and neutralizing antibody responses. J. Immunol. 170:495-502. [DOI] [PubMed] [Google Scholar]
- 53.Sedlik, C., M.-F. Saron, J. Sarraseca, I. Casal, and C. Leclerc. 1997. Recombinant parvovirus-like particles as an antigen carrier: a novel nonreplicative exogenous antigen to elicit protective antiviral cytotoxic T cells. Proc. Natl. Acad. Sci. USA 94:7503-7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sedlik, C., A. Dridi, E. Deriaud, M.-F. Saron, P. Rueda, J. Sarraseca, I. Casal, and C. Leclerc. 1999. Intranasal delivery of recombinant parvovirus-like particles elicits cytotoxic T-cell and neutralizing antibody responses. J. Virol. 73:2739-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh, M., M. Vajdy, J. Gardner, M. Briones, and D. O'Hagan. 2001. Mucosal immunization with HIV-1 gag DNA on cationic microparticles prolongs gene expression and enhances local and systemic immunity. Vaccine 20:594-602. [DOI] [PubMed] [Google Scholar]
- 56.Stahl-Henning, C., R. M. Steinman, K. Tenner-Racz, M. Pope, N. Stolte, K. Matz-Rensing, G. Grobschupff, B. Raschdorff, G. Hunsmann, and P. Racz. 1999. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science 285:1261-1265. [DOI] [PubMed] [Google Scholar]
- 57.Takada, A., S. Matsushita, A. Ninomiya, Y. Kawaoka, and H. Kida. 2003. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine 21:3212-3218. [DOI] [PubMed] [Google Scholar]
- 58.Trkola, A., T. Ketas, V. N. Kewalramani, F. Endorf, J. M. Binley, H. Katinger, J. Robinson, D. R. Littman, and J. P. Moore. 1998. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of coreceptor usage. J. Virol. 72:1876-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.UNAIDS/WHO. 2002. Epidemiological fact sheets on HIV/AIDS and sexually transmitted infections—2002 update. (Abstract.) UNAIDS, Geneva, Switzerland.
- 60.VanCott, T. C., R. W. Kaminski, J. R. Mascola, V. S. Kalyanaraman, N. M. Wassef, C. R. Alving, J. T. Ulrich, G. H. Lowell, and D. L. Birx. 1998. HIV-1 neutralizing antibodies in the genital and respiratory tracts of mice intranasally immunized with oligomeric gp160. J. Immunol. 160:2000-2012. [PubMed] [Google Scholar]
- 61.Yao, Q., Z. Bu, A. Vzorov, C. Yang, and R. W. Compans. 2003. Virus-like particle and DNA-based candidate AIDS vaccines. Vaccine 21:638-643. [DOI] [PubMed] [Google Scholar]
- 62.Yasuda, Y., M. Isaka, T. Taniguchi, Y. Zhao, K. Matano, H. Matsui, K. Morokuma, J. Maeyama, K. Ohkuma, N. Goto, and K. Tochikubo. 2003. Frequent nasal administrations of recombinant cholera toxin B subunit (rCTB)-containing tetanus and diphtheria toxoid vaccines induced antigen-specific serum and mucosal immune responses in the presence of anti-rCTB antibodies. Vaccine 21:2954-2963. [DOI] [PubMed] [Google Scholar]