Abstract
The genital mucosa is the main site of initial human immunodeficiency virus type 1 (HIV-1) contact with its host. In spite of repeated sexual exposure, some individuals remain seronegative, and a small fraction of them produce immunoglobulin G (IgG) and IgA autoantibodies directed against CCR5, which is probably the cause of the CCR5-minus phenotype observed in the peripheral blood mononuclear cells of these subjects. These antibodies recognize the 89-to-102 extracellular loop of CCR5 in its native conformation. The aim of this study was to induce infection-preventing mucosal anti-CCR5 autoantibodies in individuals at high risk of HIV infection. Thus, we generated chimeric immunogens containing the relevant CCR5 peptide in the context of the capsid protein of Flock House virus, a presentation system in which it is possible to engineer conformationally constrained peptide in a highly immunogenic form. Administered in mice via the systemic or mucosal route, the immunogens elicited anti-CCR5 IgG and IgA (in sera and vaginal fluids). Analogous to exposed seronegative individuals, mice producing anti-CCR5 autoantibodies express significantly reduced levels of CCR5 on the surfaces of CD4+ cells from peripheral blood and vaginal washes. In vitro studies have shown that murine IgG and IgA (i) specifically bind human and mouse CD4+ lymphocytes and the CCR5-transfected U87 cell line, (ii) down-regulate CCR5 expression of CD4+ cells from both humans and untreated mice, (iii) inhibit Mip-1β chemotaxis of CD4+ CCR5+ lymphocytes, and (iv) neutralize HIV R5 strains. These data suggest that immune strategies aimed at generating anti-CCR5 antibodies at the level of the genital mucosa might be feasible and represent a strategy to induce mucosal HIV-protective immunity.
More than 40 million people are currently human immunodeficiency virus (HIV) infected worldwide, with a high prevalence among women and children. Both horizontal and vertical transmission of virus infection mainly occur by mucosal contacts (sexual intercourse and baby delivery) (29, 33). Therefore, in the majority of new HIV infections, the genital mucosa is the environment where the first HIV-cell contacts take place and the region where immune protective mechanisms should be activated or promptly recruited (15).
Several studies have reported that a small fraction of sexual partners of infected individuals and of newborns of HIV-infected mothers escape horizontal or vertical HIV transmission (21). Many different factors have been considered to explain HIV resistance, such as mutations in coreceptor genes (14, 19), local cytotoxic-T-lymphocyte activation and recruitment, cytokine production, and a strong mucosal immune response (17, 20). Other humoral responses, such as high Mip-1α, Mip-1β, and RANTES chemokine levels, have been reported (39). Specific immunoglobulin G (IgG) and IgA antibodies (Abs) able to neutralize HIV infectivity were also found (1, 2, 9, 25, 26). Similar to chemokines, some HIV-neutralizing antibodies have been shown to exert their protective effects by specific binding and down-regulation of CCR5 molecules on the cell surface (22, 30, 39).
CCR5 is the main HIV coreceptor involved in virus entry and cell-to-cell spread. Although not the only coreceptor used by HIV, CCR5 undoubtedly mediates most initial contacts, because CCR5-tropic HIV strains are generally those infecting new hosts (37, 38). Hence, an anti-CCR5 humoral response at mucosal sites could prove protective by preventing HIV binding and entry. The design and setting of new adjuvants and immunogens aimed at eliciting both systemic and mucosal responses to limit HIV spread are highly desirable and anticipated goals worldwide. An immunization strategy aimed at obtaining strong and long-lasting mucosal immunity could significantly limit HIV spread, especially in sub-Saharan Africa, East Asia, and other areas where sexually transmitted diseases are heavy health and social burdens (16, 24).
This work reports the biological activities of anti-CCR5 antibodies elicited in mice by peptide and chimeric protein immunogens via the systemic or mucosal immunization route.
MATERIALS AND METHODS
Synthesis of peptides and preparation of peptide-coupled beads.
The CCR589-102 peptide (kcgYAAAQWDFGNTMCQgk; lowercase letters represent extrasequence amino acids) (22) was synthesized by the solid-phase method based on 9-fluorenyl-methoxycarbonyl amino acids (10), using Applied Biosystems peptide synthesizer 433 A. After peptide assembly, resin-bound peptides were deprotected as previously described (13) and purified to homogeneity by overnight reverse-phase chromatography. In order to obtain the cyclic oxidized form, the procedure was performed in the presence of a fivefold excess of oxidized glutathione.
Peptide coupling to tosyl-activated Dynabeads M280 (Dynal, Oslo, Norway) was obtained following standard procedures. Briefly, 3 × 107 beads were incubated overnight with 9 μg of peptide (amino acids 89 to 102) in 50 mM borate buffer, pH 9.5, at 37°C. After four washes in phosphate-buffered saline (PBS) to remove unbound peptides, the peptide-bound beads were ready to be used.
Cloning and expression of a chimeric FHV-CCR5 immunogen.
The FHV Epitope Presenting System (6, 7, 32, 35), based on a modified capsid precursor protein of the Flock House virus (FHV), was obtained through the courtesy of F. Baralle (ICGEB, Trieste, Italy). The system is composed of a set of five modified capsid gene sequences (cloned in the expression vector pET-3 [Novagen, Madison, WI]), in each of which the nucleotide sequence corresponding to a peptide loop was replaced by a single Bsu36I restriction site to allow the oriented insertion of a synthetic polynucleotide coding for a foreign epitope in five distinct sites (L1, L2, L3, I2, and I3) (Fig. 1 shows the numbers of the original amino acid residues, which are replaced by the epitope of choice). Each of the corresponding DNA sequences has been deleted and replaced with a Bsu36I site, which allows the directional insertion of an epitope-coding oligonucleotide (32, 33). The two synthetic oligonucleotides (CCR5Se, 5′TGAGTGTTACGCTGCAGCTCAGTGGGATTTCGGTAACACTATGTGTCAGCC-3′, and CCR5-As, 5′TCAGGCTGACACATAGTGTTACCGAAATCCCACTGAGCTGCAGCGTAACAC-3′) were obtained from PRIMM (Milan, Italy), denatured at 95°C, and reannealed by letting the temperature return to room temperature. The resulting double-stranded molecules bear 5′ overhangs suitable for ligation into the Bsu36I restriction site. Inclusion bodies were purified from plasmid-transformed BL21(DE3) bacteria (Novagen, Madison, WI) by enzymatic and detergent-mediated lysis (12). Recombinant proteins were obtained by electroelution from sodium dodecyl sulfate-polyacrylamide gel electrophoresis (31).
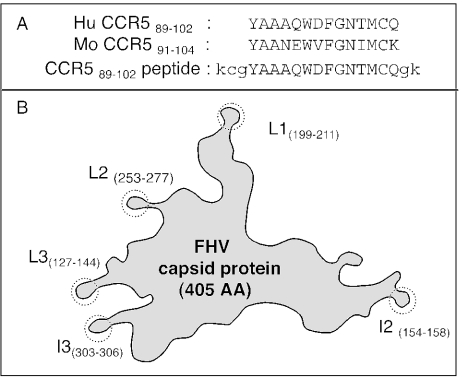
FIG. 1.
(A) Homology between human and mouse CCR5 sequences and sequence of the synthetic peptide. (B) Schematic representation of the FHV capsid protein. The structure displays five different superficial peptide loops (L1, L2, L3, I2, and I3), in each of which a foreign peptide sequence can be inserted and presented on the protein surface in a stable, structurally constrained form. Numbers in parentheses refer to the original amino acid residues replaced by the foreign epitope.
Animal inoculation, collection of fluids, and estrous staging.
BALB/c mice were immunized by two different routes: systemic (intraperitoneal) and mucosal (intranasal). Different groups of mice were immunized with wild-type FHV capsid protein (FHV-WT; negative control) or with engineered CCR5-FHV chimeric proteins (typically FHV-I2; see below). Groups of three mice were injected intraperitoneally with 20 μg of each chimeric FHV immunogen in 200 μl of sterile PBS. Groups of 10 mice were immunized intranasally with the same doses of FHV antigens in 10 μl of sterile PBS. Groups of three BALB/c mice were also immunized with 20 μg of the synthetic cyclic CCR589-102 peptide by the intraperitoneal or intranasal route. Anti-CCR5 responses were evaluated in pooled sera from animal groups by CCR5 enzyme-linked immunosorbent assay (ELISA) carried out on solid-phase synthetic cyclic CCR589-102 peptide.
Seven immunizations were carried out at weekly intervals from day 0 to day 49. For only intraperitoneal administrations, the first antigen doses were associated with Freund's complete adjuvant (day 0), the second and third doses were associated with Freund's incomplete adjuvant (days 7 and 14), and subsequent immunizations (days 21 to 49) did not include any adjuvant. In order to ensure the optimal health maintenance of immunized mice, peripheral blood mononuclear cells (PBMC) could not be sampled at time intervals shorter than 1 day postimmunization. Blood samples were collected weekly from mouse tails and centrifuged at 1,500 rpm. After each immunization, plasma samples were pooled, heat inactivated at 56°C, and stored at −20°C before analysis. Anti-CCR5 responses were monitored by CCR5 ELISA.
To evaluate the long-term kinetics of CCR5 down-regulation in vivo, we monitored CCR5 expression at 1, 2, and 4 weeks after the seventh immunization. The mice were boosted once again and monitored 1 week later. Samples were treated as reported above.
Vaginal washes for estrous staging and antibody determinations were collected daily by pipetting 50 μl of PBS in and out of the vagina six to eight times. The staging of the estrous cycle (estrus, metestrus, diestrus, or proestrus) for each mouse was based on smears from these washings. The smears were stained with Diff-Quik (Baxter Scientific Products) and staged by examining the cells present in the smears. The washes corresponding to the estrus stage were collected twice a week. The cells were separated by centrifugation, while the supernatants were 10-fold concentrated on Ultrafree-15 Biomax 30 membranes with a cutoff of 30 kDa (Millipore, Bedford, MA). Vaginal secretions were pooled and stored at −20°C before analysis.
Affinity purification of Ig and antibodies.
Agarose beads, coupled with goat anti-mouse total Ig, IgA, or IgG antibodies (Sigma-Aldrich, Milan, Italy), were used to purify total Ig and IgA- and IgG-enriched fractions from pre- and postimmune sera and vaginal washes. Igs were obtained by elution with 0.2 M glycine-HCl buffer (pH 2); eluates were neutralized with 1 M Tris buffer, pH 11. Purified Ig fractions were concentrated on Ultrafree-15 Biomax 30 membranes with a cutoff of 30 kDa (Millipore, Bedford, MA). The procedure also excluded low-molecular-weight molecules (which might interfere with chemotaxis and neutralization assays). The eluted Igs from immune sera and from normal mouse sera (NMS) and the non-Ig fractions of all sera were dialyzed in PBS buffer and tested by Mouse Ig ELISA (see below).
Anti-CCR5 antibodies were affinity-purified by incubating 9 μg Igs to 9 μg of peptide-coupled beads for 1 h at room temperature. Abs were eluted in 0.5 M acetic acid; neutralized with 1 M Tris buffer, pH 11; dialyzed against RPMI medium; and tested by CCR5 ELISA (see below).
Ig ELISA.
To quantify serum and mucosal Igs, Microwell plates were coated with dilutions of sera or Ig-containing fractions (up to 1:128 by twofold dilutions) in 50 mM carbonate buffer, pH 9.5, for 1 h at 37°C. Commercial preparations of mouse Ig (total Ig, IgA, or IgG; Sigma Aldrich, Milan, Italy) were used as standards at concentrations ranging from 4 to 0.06 μg/ml (twofold serial dilutions) to generate a calibration curve. The plates were saturated for 1 h with 1% milk powder (Humana 3, Germany) in PBS. Then, peroxidase-conjugated goat anti-mouse total Ig, IgA, or IgG (Sigma) was added and incubated for 30 min at 37°C. The enzymatic reaction was developed with the TMB Microwell Peroxidase Substrate System (KPL, Gaithersburg, Maryland) and read at 492 nm.
CCR5 ELISA.
To quantify the fraction of anti-CCR5 Abs, Microwell plates coated with synthetic cyclic CCR589-102 peptide were used, and the rest of the assay was exactly as for the Ig ELISA. The relative percentage of CCR5-specific Abs was calculated for each sample by comparison with Ig ELISA values.
Cell lines.
Untransfected U87 and transfected U87-CD4-CXCR4 and U87-CD4-CCR5 glioma cell lines were obtained from the AIDS Research and Reference Reagent Program (National Institutes of Health, Bethesda, MD).
Purification of human CD4+ cells.
Human PBMC from healthy blood donors were isolated by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). CD4+ cells were purified from resting PBMC by immune adsorption to anti-CD4 magnetic beads (Oxoid, Hampshire, United Kingdom). Purified CD4+ cells were stimulated for 3 days with phytohemagglutinin (PHA) (1 μg/ml; Sigma-Aldrich, Steinheim, Denmark) and recombinant interleukin 2 (IL-2) (100 U/ml; Amersham, Buckinghamshire, United Kingdom). In order to ensure high-level expression of CCR5 surface receptors, only PBMC from donors not carrying CCR5-Δ32 alleles were used.
Purification of mouse CD4+ cells and proliferation assay.
Mouse PBMC were isolated with Ficoll-Hypaque. CD4+ cells were purified from resting PBMC by immune adsorption to anti-mouse CD4 magnetic beads (Oxoid, Hampshire, United Kingdom). Cells were stimulated for 3 days with PHA (1 μg/ml; Sigma-Aldrich, Steinheim, Denmark) and mouse recombinant IL-12 (100 U/ml; Amersham, Buckinghamshire, United Kingdom) before flow cytometry analysis. The proliferation assay was performed by stimulation of splenic cells from both preimmune and postimmune mice by several mitogens. Briefly 4 × 105 cells/well were plated in 96-well flat-bottom plates and activated with staphylococcal enterotoxinn B (0.02 μM), concanavalin A (10 UI/ml), or PHA (1 μg/ml) (Sigma-Aldrich, Steinheim, Denmark) plus mouse recombinant IL-12 (100 U/ml) and incubated for 3 days. Then, 10 μCi of [3H]thymidine/well (Amersham) was added, and the mixture was incubated for 18 h. The PBMC were washed, and proliferative responses were evaluated by Beta Counter (Top Count; Packard).
Anti-CCR5 radio-binding assay.
A cellural suspension of CCR5-transfected U87 cell monolayers and human purified CD4+ cells pretreated with mouse sera diluted 1/50 or Ig-enriched fractions (20 ng/ml) was processed as described above. A pool of three untreated mouse sera was used as a negative control, while the anti-CCR5 mouse monoclonal Ab (MAb) 2D7 (0.3 μg/ml) was used as a positive control. Anti-CCR5 Abs bound to CCR5-positive cells were revealed by 0.5 μCi/ml 125I-labeled sheep anti-mouse Ig F(ab′)2 fragments (Amersham, Buckinghamshire, United Kingdom). The reaction was read by automatic gamma counter (Wizard; Perkin-Elmer).
CCR5 down-regulation assay and flow cytometry analysis.
Untransfected U87 and CCR5-transfected U87 cell lines and either mouse or human CD4+ lymphocytes were incubated with serum-free medium for 2 h at 37°C, washed with 2 mM EDTA-PBS, and resuspended in serum-free medium, and 105 cells were incubated with affinity-purified anti-CCR5 Abs (20 ng/assay) at 37°C for 1, 24, or 48 h. The cells were washed with ice-cold PBS with additions of 1% fetal calf serum and 1% NaN3 before flow cytometry analysis was performed. Surface-expressed CCR5 molecules were detected with anti-CCR5 MAb 2D7 (1 μg/ml; supplied by the AIDS Research and Reference Reagent Program) and a secondary anti-mouse Ab conjugated with fluorescein isothiocyanate (FITC) according to a published protocol (28). A control medium containing Igs (20 ng/assay) from NMS was also tested. In order to detect CCR5 surface expression on mouse CD4+ lymphocytes, a goat polyclonal IgG to mouse CCR5, M20 (10 μg/ml; Santa Cruz Biotechnology, Santa Cruz, California), and a secondary anti-goat Ab conjugated with FITC (Sigma-Aldrich) were used. To verify CCR5 down-regulation, cells were also incubated with 50 nM RANTES for 1 h and then processed as described above. Relative CCR5 surface expression was calculated according to the following formula: 100 × MCFstimulated − MCFneg-control/MCmedium − MCFneg control, where MCF is mean channel fluorescence. A cell line not expressing CCR5 (untransfected U87) was used as a negative control. All experiments were repeated three times, and the results were expressed as means with range values. CCR5-CD4 double labeling was also performed on mouse CD4+ lymphocytes. In brief, the cells were incubated first with goat IgG to mouse CCR5 (M20), then with FITC-conjugated rabbit anti-goat IgG, and finally, with a phycoerythrin-conjugated rat MAb to mouse CD4 antigen (GK1.5; Santa Cruz Biotechnology). All antibodies were employed at 4 μg/assay for 30 min on ice.
Chemotaxis.
PBMC from one healthy donor were activated with PHA and IL-2 for 3 days. Then, 3 × 105 activated PBMC in 50 μl of RPMI 1640 medium containing 0.3% human serum albumin were placed in the upper chambers of 5-μm-pore-size Transwell plates (Costar Europe, Amsterdam, The Netherlands) and incubated with mouse affinity-purified anti-CCR5 Igs (20 ng/ml). A control medium containing 20 ng pooled total Igs from NMS was also tested. Chemotaxis was conducted in the presence of 1.5 μg/ml of Mip-1β (placed in the lower chambers). The Transwells were incubated for 2 h at 37°C; cells that migrated from the upper to the lower chamber were then quantified by flow cytometry analysis. The results were expressed as the chemotaxis index, which represents the increase in the number of cells migrating in response to Mip-1β over the spontaneous cell migration in control medium normalized by the value obtained in the absence of antibodies.
Virus isolation and titration.
Clade B virus isolates (HIV no. 36, 40, and 45), were obtained as previously described (22). In brief, HIV was isolated from PBMC of HIV-seropositive individuals by cocultivation with PHA-stimulated PBMC of two healthy seronegative donors; the cultures were maintained until increasing levels of HIV-p24 antigen were detected in two consecutive determinations. The infectivity (50% infectious dose [ID50]) of each virus isolate was determined on PBMC from a single donor as follows: six replicates (150 μl) of fivefold serial dilutions (from 1:5 to 1:3,125) of virus were added to six wells of a round-bottom microtiter plate (Nunc) containing 105 resting PBMC in 75 μl of medium, incubated for 2 h, washed, and resuspended in RPMI 1640 medium containing PHA (3 μg/ml) and recombinant IL-2 (10 U/ml). HIV type 1 (HIV-1) p24 antigen was titrated after 5, 7, and 9 days of culture by standard assays (Aalto Bio Reagents Ltd., Dublin, Ireland). ID50 titers were defined as the reciprocal of the virus dilution yielding 50% positive wells (Reed-Muench calculation).
Neutralization of virus infectivity.
The neutralizing activities of anti-CCR5 Abs were evaluated by using activated PBMC as target cells. Briefly, PBMC were cultured in medium containing PHA and IL-2. Affinity purified CCR5-specific IgG and IgA from mice immunized with FHV-I2 or FHV-WT were used at different concentrations (20, 10, 5, and 2 ng/ml). An anti-CD4 IgG, SIM 4 (8 ng/ml), was used as a positive control. PBMC (1.5 × 106) were incubated with antibodies for 48 h prior to the neutralization assay. CCR5 expression was evaluated prior to and after incubation with specific antibodies. The cells were washed and incubated again with 100 μl of specific antibodies. After 1 h of incubation, 100 μl of a virus dilution (adjusted to 20 ID50) was added. The cultures were incubated for an additional 14 h and washed in PBS; then, cell pellets (1 × 106 cells) were frozen at −80°C.
A modified version of the Cobas Amplicor HIV-1 Monitor Test, version 1.5 (Roche, Pleasanton, CA), was used to amplify proviral DNA in cell pellets, as previously described (18). In particular, we removed the reverse transcription step from the amplification cycles and replaced the RNA quantitation standard (QS-RNA) with an analogous QS-DNA retrotranscribed from QS-RNA using the Reverse Transcription System (PROMEGA Bioscience Inc., Madison, WI). Reverse transcription was obtained using a PE Gene Amp PCR System 2400 apparatus and the following cycling conditions: 37°C for 15 min, 48°C for 45 min, and 95°C for 5 min. Then, cDNA was purified using a Wizard PCR Preps DNA Purification System (PROMEGA Bioscience Inc., Madison, WI) and quantified using spectrophotometric reading at 260/280 nm. In each test run, a low positive control (10 HIV DNA copies/105 PBMC) and a high positive control (3 × 103 HIV DNA copies/105 PBMC) were included. DNA was extracted from all of the samples using the QIAamp DNA minikit (QIAGEN GmbH, Hilden, Germany). The DNA was suspended in 100 μl of buffer or H2O, and 10 μl (corresponding to 2 × 105 PBMC plus 30 QS-DNA copies) was used for each test. Samples, in a final reaction volume of 50 μl, were processed for amplification and detection using the automated Cobas Amplicor analyzer following the manufacturer's instructions (Roche Molecular Systems, Pleasanton, CA).
Relative percentages of neutralization were calculated by comparison of the number of DNA copies/106 PBMC in each sample to those obtained in HIV-infected PBMC not treated with antibodies. The numbers of DNA copies in the samples (positive controls) ranged from 320 to 470. Each experiment was repeated three times using PBMC from different healthy blood donors.
RESULTS
Engineered FHV capsid protein enhances both IgG and IgA responses to the CCR589-102 loop in its native conformation.
Anti-CCR5 antibodies were detected in some exposed seronegative (ESN) individuals (22) and in some HIV-positive long-term nonprogressor individuals (unpublished data). These Abs bind CCR5 on the surfaces of CD4+ lymphocytes, protecting them from infection by HIV R5 strains in vitro, and possibly mediate the observed CCR5 down-regulation in vivo, which might in turn limit HIV infection and cell-to-cell viral spread. These putatively protective antibodies recognize a single protein domain spanning the CCR589-102 extracellular loop. The amino acid sequence of this loop presents a good homology between human and mouse CCR5s (Fig. 1A). In preliminary experiments, the synthetic cyclic CCR589-102 peptide (oxidized form) induced specific antibodies in mice, while the linear (reduced) form of the same peptide was ineffective (data not shown). In order to evaluate the antibody response to a structurally constrained CCR589-102 immunogen, we constructed four CCR5-FHV recombinant proteins (FHV-L1, FHV-L2, FHV-I2, and FHV-I3), using the FHV Epitope Presenting System. This system is based on a modified capsid precursor protein of the Flock House virus (7, 32) and is a versatile expression system in which it is possible to engineer peptide epitopes in a highly immunogenic form. The structure of the FHV capsid protein has been solved by X-ray crystallography (11). Five different solvent-exposed loops have been identified (Fig. 1B), and all have been conveniently exploited to insert and present foreign peptides. In the case of the V3 loop of HIV gp120, it was shown that the FHV capsid protein can be a suitable and efficient carrier, able to strengthen the immunogenicity of protein loops (32), possibly by mimicking the conformation in the original protein and presenting it in a different context (35).
In our study, the immunogenicities in mice of CCR5-FHV recombinant proteins were compared to those of negative (FHV-WT) and positive (synthetic cyclic CCR589-102 peptide) controls. The recombinant protein with the CCR5 peptide inserted in position I2 proved to be the best immunogen, in terms of both CCR5 ELISA-reactive antibodies (Fig. 2A) and the abilities of such antibodies to down-regulate CCR5 on the surfaces of human lymphocytes (Fig. 2B). Recombinant proteins FHV-I3, -L1, and -L2 also elicited CCR5-specific antibodies, yet FHV-I2-induced antibodies were more efficient. Therefore, only FHV-I2 recombinant protein (where FHV residues 154 to 158 were replaced by CCR5 residues 89 to 102) was used in all subsequent experiments. The very high serum antibody levels observed (up to 30% of total Igs) (Fig. 2C) are much higher than those observed in ESN subjects (less than 0.1% of total Igs [unpublished data]).
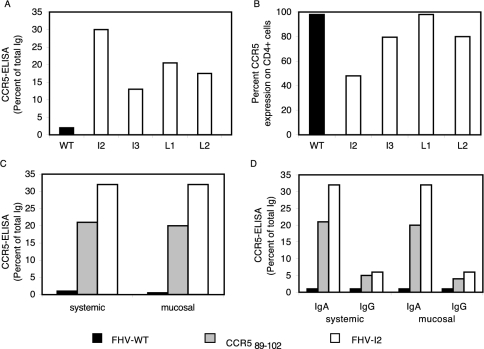
FIG. 2.
Comparison between the immune responses elicited by the four different CCR5-FHV recombinant immunogens (FHV-L1, -L2, -I2, and -I3) and negative (FHV-WT) and positive (synthetic cyclic CCR589-102 peptide) controls. (A) Fractions of CCR5-binding total Igs elicited by FHV recombinant immunogens versus FHV-WT negative control. (B) CCR5 down-regulation on human CD4+ lymphocytes by purified Igs (20 ng/assay) derived from antisera elicited by FHV recombinant immunogens or FHV-WT. (C) Fractions of CCR5-binding total Igs elicited by FHV-I2, FHV-WT, and the synthetic cyclic CCR589-102 peptide administered either via systemic or mucosal route. (D) Fractions of CCR5-binding IgA and IgG purified from the total Ig fractions of panel C.
Because of the major role of mucosal immunity in protection against HIV, the efficacy of the FHV-I2 immunogen was also assessed in the context of mucosal immunization and mucosal response. In order to determine if the mucosal immunization route was a feasible means to induce HIV-protective anti-CCR5 antibodies, two sets of immunization experiments were carried out. Groups of three mice were immunized with 20 μg of FHV-I2, FHV-WT, or synthetic cyclic CCR589-102 peptide via the intraperitoneal route; at the same time, groups of 10 mice were immunized with the same antigen doses via the intranasal route. Serum samples and vaginal washes were collected (once and twice a week, respectively), pooled, and tested. Maximal responses were achieved after the third immunization and remained stable throughout the immunization period (not shown). Most of the data presented refer to samples drawn 1 week after the third immunization.
Both FHV-I2 and the synthetic cyclic CCR589-102 peptide elicited specific immune responses, although the response was higher in mice immunized with FHV-I2 via either the systemic or mucosal immunization route (Fig. 2C).
CCR5-specific IgG and IgA antibodies were elicited by either immunogen, but responses to FHV-I2 were markedly higher than those to the synthetic cyclic CCR589-102 peptide, in spite of the 20-fold-higher molar concentration used for the peptide.
Both IgG and IgA fractions, obtained by affinity purification from pooled sera, contained CCR5-reactive antibodies (Fig. 2D). Very similar reactivities in ELISA testing were observed with sera obtained by either systemic or mucosal immunization. In both cases, the reactivity of IgA was much higher than that of IgG. This unexpected result might be due to the intrinsically higher avidity of dimeric IgA.
Anti-CCR5 antibodies bind and down-regulate cell surface CCR5 on both mouse and human CD4+ lymphocytes.
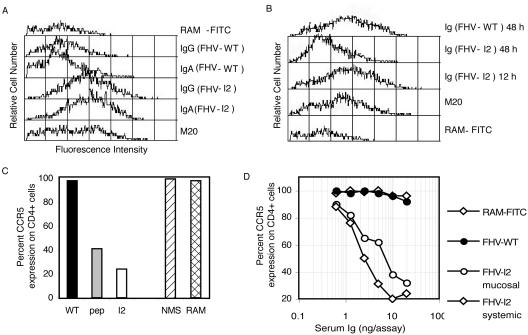
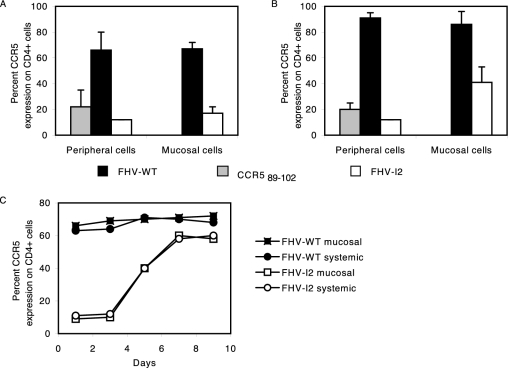
Affinity-purified anti-CCR5 IgG and IgA antibodies, derived from sera of FHV-I2-immunized mice, were shown to bind cell surface CCR5 expressed on murine CD4+ lymphocytes (Fig. 3A). Moreover, purified total Igs from sera of animals immunized with either FHV-I2 or the synthetic cyclic CCR589-102 peptide induced a marked down-regulation of CCR5 on murine CD4+ lymphocytes, with a stronger effect elicited by Igs from FHV-I2-immunized animals (Fig. 3C). Samples from FHV-WTimmunized mice were unable to bind CD4+ cells and consequently did not induce CCR5 down-regulation (Fig. 3A and C). Pronounced CCR5 down-regulation on mouse CD4+ lymphocytes was achieved after 48 h of treatment with Igs from FHV-I2-immunized animals. Conversely, CCR5 down-regulation was not evident after 12 h of incubation (Fig. 3B), while at 24 h an intermediate result was observed (data not shown). At 48 h, the dose dependence of CCR5 down-regulation was studied for the Ig fractions derived from sera of animals immunized by either immunization route. No significant difference between the two routes of immunization could be observed (Fig. 3D).
FIG. 3.
Flow cytometry analysis of the specificities and biological activities of anti-CCR5 total Igs or IgG- or IgA-enriched fractions from antisera elicited by the recombinant immunogen FHV-I2. (A) Binding of either IgA or IgG fractions (20 ng/assay) to CCR5 receptors on mouse CD4+ lymphocytes. Positive controls were lymphocytes treated with an anti-mouse CCR5 MAb (M20), and negative controls were lymphocytes treated only with the secondary Ab (RAM-FITC). (B) Kinetics of CCR5 down-regulation on mouse CD4+ lymphocytes of untreated mice by total Ig fractions (20 ng/assay) purified from FHV-I2 antisera. Lymphocytes were incubated with purified Igs for 12 and 48 h before flow analysis. Positive and negative controls were as in panel A. (C) CCR5 down-regulation obtained by incubating mouse CD4+ lymphocytes with purified Igs (20 ng/assay) for 48 h before flow analysis. As negative controls, target cells were incubated either with Igs purified from NMS or with only the secondary Ab (RAM-FITC). (D) Dose-response curves of CCR5 down-regulation obtained by incubating mouse CD4+ lymphocytes for 48 h with the indicated concentrations of Igs purified from pools of antisera obtained by either systemic or mucosal immunizations. As negative controls, mouse CD4+ lymphocytes were also incubated with Igs purified from FHV-WT antisera or with only the secondary Ab (RAM-FITC). These data were obtained with serum samples drawn 1 week after the third immunization.
The specificity of antibodies elicited by FHV-I2 or the synthetic cyclic CCR589-102 peptide was assessed by radio binding to CCR5- or CXCR4-transfected U87 cells. Both antisera exhibited binding to U87-CCR5, but not to U87-CXCR4 cells (data not shown).
In a radio-binding assay, sera raised by either systemic or mucosal immunization were shown to contain CCR5-specific antibodies able to bind CD4+ human cells isolated from PBMC of healthy donors (previously assessed for polymorphisms affecting CCR5 surface expression).
Interestingly, Igs from mice immunized by FHV-I2 via the mucosal route exhibited a higher binding activity to human cells than Igs from mice immunized via the systemic route. Conversely, the synthetic CCR589-102 peptide induced less intense binding activities, yet slightly stronger in mice immunized via the systemic route than in those immunized via the mucosal route (Fig. 4A).
FIG. 4.
Radio-binding assay analysis of the specificities and biological activities of anti-CCR5 mouse Abs on human CD4+ lymphocytes. (A) CCR5-binding activities of antibodies elicited by systemic or mucosal immunizations with FHV-WT, synthetic cyclic CCR589-102 peptide, or FHV-I2. (B) Radio binding to human CCR5 of serum (Se) IgG, serum IgA, or mucosal (Mu) IgA elicited by mucosal immunization with FHV-WT or FHV-I2. The negative control (Cntr) was an Ig fraction from NMS (20 ng/assay). The positive control was the anti-human CCR5 MAb 2D7. (C) Dose-response curves of CCR5 down-regulation obtained by incubating human CD4+ lymphocytes for 48 h with the indicated dilutions of FHV-I2 or FHV-WT antisera. Negative controls were target cells incubated only with the secondary Ab (RAM-FITC).
IgG and IgA fractions from FHV-I2 sera showed comparable binding to human cells. Serum IgG binding activity was comparable to that of the commercial anti-CCR5 MAb 2D7. Serum IgA, and especially mucosal IgA, showed a higher binding activity (Fig. 4B). Serial dilutions of FHV-I2 antisera were shown to down-regulate CCR5 on CD4+ cells in a dose-dependent manner (Fig. 4C).
Anti-CCR5 antibodies inhibit chemotactic response to Mip-1β.
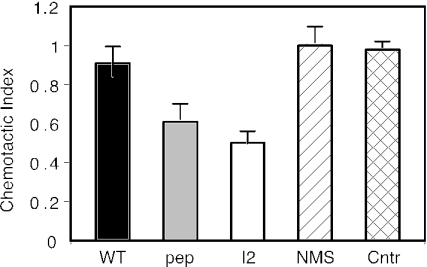
In order to evaluate whether FHV-I2-induced mouse anti-CCR5 antibodies affect the biological functions of human CCR5, purified Ig fractions from sera of mice immunized with FHV-I2, FHV-WT, or synthetic cyclic CCR589-102 peptide were used to inhibit the chemotactic response induced by Mip-1β on human CD4+ T lymphocytes purified from healthy blood donors. Ig fractions from FHV-I2 antisera were able to reduce CD4+ cell chemotaxis to 50%, while antisera raised against the synthetic peptide showed a slightly smaller effect (Fig. 5). CD4+ lymphocytes treated with Ig fractions from FHV-WT antisera or from NMS retained full migration efficiency in response to Mip-1β. These effects suggest that anti-CCR5 antibodies might also be able to interfere with CCR5-mediated biological activities in vivo.
FIG. 5.
Inhibition of Mip-1β-induced chemotaxis of human CD4+ T lymphocytes by anti-CCR5 mouse antibodies. Purified lymphocytes were incubated with Ig fractions (20 ng/ml) from antisera elicited by FHV-I2, FHV-WT, or the synthetic cyclic CCR589-102 peptide. Controls (Cntr) included lymphocytes treated with Ig fractions from NMS and lymphocytes without added antibodies. Means and standard errors from three replicates.
Anti-CCR5 antibodies neutralize the infectivity of primary HIV-1 isolates.
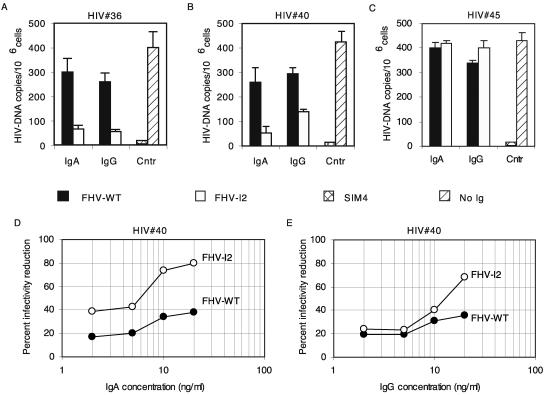
Given the crucial role played by CCR5 in HIV cell entry, we investigated whether mouse anti-CCR5 antibodies could inhibit HIV infectivity. Mucosal IgA and IgG fractions (affinity purified on peptide-coupled beads) derived from samples drawn after the fourth and the fifth immunizations of mice immunized with FHV-WT or FHV-I2 were added to PBMC for 48 h in order to induce CCR5 down-regulation (as shown in Fig. 3). Samples were then tested for the ability to neutralize the infectivity of HIV-1 subtype B primary isolates (the R5-tropic viruses HIV no. 36 and HIV no. 40 or the R5-, R3-, X4-tropic virus HIV no. 45). Commercial anti-CD4 MAb, SIM4, and mucosal IgA and IgG from mice immunized with FHV-WT were used as positive and negative controls, respectively.
The anti-CCR5 IgA and IgG antibodies and SIM4 were able to reduce the infectivity of HIV no. 36 and HIV no. 40 isolates, while IgA and IgG from FHV-WT-immunized mice were not (Fig. 6A and B). Viral neutralization was also confirmed to be R5 specific, because FHV-I2 failed in blocking the infectivity of the R5-, R3-, and X4-tropic virus HIV no. 45 (Fig. 6C). In a dose dependence experiment, mucosal IgA antibodies showed a stronger effect than IgG antibodies, reaching more than 80% inhibition at 20 ng/ml (Fig. 6D and E). In the same experimental setup, control IgA and IgG from FHV-WTimmunized mice showed a low, nonspecific level of HIV infectivity reduction (Fig. 6D and E), possibly due to hyperimmune stimulation by the FHV antigenic system, which is likely to disappear at lower doses of immunogen. These results indicate that, when CCR5 is down-regulated by anti-CCR5 IgA elicited by FHV-I2, strong HIV neutralization is achieved.
FIG. 6.
Neutralization of the infectivity of HIV-1 primary isolates by anti-CCR5 mouse antibodies. Isolates HIV no. 36 and HIV no. 40 were subtype B, R5 tropic; isolate HIV no. 45 was subtype B, R5, R3, X4 tropic. Antibodies were affinity purified on peptide-coupled beads from mucosal IgA- and IgG-enriched fractions derived from FHV-I2 or FHV-WT antisera. In panels A, B, and C, 8 ng/ml of Igs were used. The positive control (Cntr) was the anti-human CD4 MAb SIM4. In the negative control, no antibody was added. The error bars indicate standard deviations. (D and E) Dose-response curves of infectivity reduction obtained by treating lymphocytes with the indicated concentrations of IgA or IgG antibodies from FHV-I2 or FHV-WT antisera. Since only small serum samples could be collected, the antibodies for the neutralization assay were purified from a pool of samples drawn after the fourth and fifth immunizations.
Antibody-mediated CCR5 down-regulation in vivo.
In order to evaluate whether anti-CCR5 antibodies affect the biological functions of CCR5 in vivo, mouse CD4+ lymphocytes were sorted from PBMC and vaginal washes from mice immunized via the systemic or mucosal route. Flow cytometry analysis revealed that the fraction of CCR5+ cells from PBMC and vaginal washes was highly reduced in mice immunized with FHV-I2 and, to a lesser extent, with the synthetic cyclic CCR589-102 peptide (Fig. 7A and B). Systemic and mucosal immunizations down-regulated cell surface CCR5 on peripheral PBMC. However, CCR5 down-regulation in mucosal cells appeared to be stronger after mucosal immunization. When PBMC from mice immunized with FHV-I2 by either route were cultured in the absence of anti-CCR5 Abs, CCR5 remained fully down-regulated for 3 days, slowly reappearing in the following 4 days (Fig. 7C).
FIG. 7.
Antibody-mediated CCR5 down-regulation in vivo. The CCR5 phenotype of CD4+ cells was evaluated by two-color flow cytometry of cells derived from peripheral blood or from vaginal fluids of mice immunized by mucosal or systemic immunizations with FHV-WT, the synthetic cyclic CCR589-102 peptide, or FHV-I2. The double staining was obtained using M20 (anti-mouse CCR5 polyclonal antibody) and GK1.5 (anti-mouse CD4 MAb). (A) CCR5 expression by CD4+ cells of mice subjected to mucosal immunizations. The error bars indicate standard deviations. (B) CCR5 expression by CD4+ cells of mice subjected to systemic immunizations. (C) Kinetics of CCR5 reappearance on the surfaces of cultured PBMC obtained from mice immunized by either route with FHV-WT or FHV-I2.
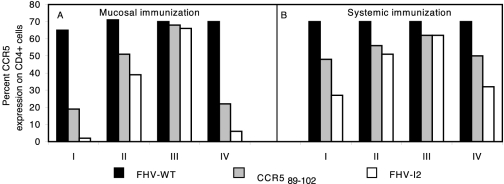
To evaluate the long-term CCR5 down-regulation kinetics in vivo, we monitored CCR5 expression at 1, 2, and 4 weeks after the seventh immunization (Fig. 8, groups I, II, and III, respectively). Thereafter, the mice were boosted once again and monitored 1 week later (Fig. 8, groups IV). Clearly, when administered by the mucosal route, FHV-I2 caused full CCR5 down-regulation on peripheral CD4+ lymphocytes in vivo, while FHV-I2 by the systemic route exhibited a less potent effect (Fig. 8, groups I). Similar differences were observed in mice immunized with the synthetic cyclic CCR589-102 peptide. Expression of CCR5 was gradually recovered in the samples obtained after 2 and 4 weeks of rest (Fig. 8, groups II and III). However, a single further immunization induced a new, marked CCR5 down-regulation (Fig. 8, groups IV). Again, the systemic immunization route was less efficient than the mucosal route.
FIG. 8.
Long-term kinetic assays of in vivo CCR5 down-regulation by FHV-I2 immunization. CCR5 expression by CD4+ cells of mice subjected to mucosal (panel A) or systemic (panel B) immunization with FHV-I2, FHV-WT, or the synthetic cyclic CCR589-102 peptide. The lowest levels of CCR5 expression are seen in the samples drawn 1 week after the seventh immunization (groups I). Expression of CCR5 receptors was gradually recovered in the samples obtained after 2 and 4 weeks of rest (groups II and III). The samples of groups IV were obtained 1 week after a further immunization.
FHV-I2 immunizations do not interfere with T-cell function.
In order to verify that the observed antibody response does not interfere with T-cell function, a splenic-cell proliferation assay was performed after activation by several mitogens (concanavalin A, staphylococcal enterotoxinn B, and PHA in the presence of mouse IL-12). We tested pooled splenic cells from immunized (seventh systemic immunization) and from preimmune mice. No difference was found between the two groups. DNA incorporation values ranged between 3,700 and 4,880 cpm for immunized mice and between 3,950 and 4,500 cpm for preimmune animals.
DISCUSSION
This study focused on the production and the potential role of anti-CCR5 autoantibodies in preventing HIV infection and cell-to-cell viral spread. CCR5 is a member of the chemokine receptor family, and it is well known as a major HIV coreceptor (27). Although CCR5 is a self-antigen, anti-CCR5 antibodies have been found in some cohorts, such as polytransfused subjects and ESN (4, 23, 34, 39) and HIV-positive long-term nonprogressor subjects (unpublished data). CCR5-defective individuals have normal inflammatory function and T-cell immunity. Moreover, the presence of anti-CCR5 autoantibodies did not play any evident pathological role. For these reasons, CCR5 has been interpreted as a redundant molecule in adults. Conversely, the presence of anti-CCR5 autoantibodies has been associated with partial or complete protection from HIV infection, acting via receptor down-regulation (36). Strikingly, anti-CCR5 antibodies found in some ESN individuals predominantly recognize a single CCR5 domain, the extracellular loop spanning residues 89 to 102 (22). Equally noteworthy, ESN individuals with anti-CCR5 Abs also presented the same Abs in mucosal secretions (1). It would be of major relevance if anti-CCR5 Abs could also (or predominantly) exert a protective role at the mucosal level. Currently, mucosal tissues are considered the major HIV entry sites; in fact, sexual intercourse and mother-to-child transmission are the main routes of HIV infection worldwide (5).
The major questions addressed in this study concern (i) the possibility of deliberately raising anti-CCR5 antibodies in mice by means of vaccination and (ii) the capacities of these antibodies to down-regulate cell surface CCR5 (on mouse and human cells) and thus block HIV infection (in human cells). Indeed, we demonstrated that it is possible to induce anti-CCR5 antibodies at serum levels 300-fold greater than those previously found in ESN individuals. This difference is likely attributable to the use of an appropriate carrier adequately stimulating the mouse immune system.
Since linear peptides are generally unable to mimic structured epitopes, it is usual practice to cyclize them in a cysteine loop, providing a constraint that reduces the number of possible conformations. In preliminary experiments, we demonstrated that immunization with synthetic cyclic human CCR589-102 peptide (but not the corresponding linear peptide) could elicit antibodies reactive with native CCR5 in mice (in spite of some amino acid differences between human and murine CCR5 sequences). Interestingly, the use of a recombinant protein, such as FHV-I2, even at a 20-fold-lower molar concentration, yielded higher antibody levels in mice than the synthetic cyclic CCR589-102 peptide. The FHV Epitope Presentation System (7, 11, 33) provided a good probability that, in at least one of the FHV loop positions (Fig. 1), the foreign epitope would be presented as an immunogen able to elicit antibodies reactive to the native epitope. This system, when used with the V3 loop epitope (35) and the ERDRD conformational epitope of gp41 (6), has yielded results that indicate the important role of the backbone structure of the virus both in fixing a conformation similar to the natural one and in promoting a vigorous antibody response through the heterologous context. Hence, we constructed four recombinant FHV capsid proteins expressing the CCR589-102 epitope. The recombinant protein with the epitope in position I2 proved to be the best immunogen in mice, in terms of both CCR5-reactive antibodies and the abilities of these antibodies to down-regulate CCR5 on the surfaces of human lymphocytes (Fig. 2).
Two different immunization routes (intraperitoneal, i.e., systemic, and intranasal, i.e., mucosal) were adopted to assess the capacities of the immunogens to elicit systemic and/or mucosal immune responses. In fact, specific anti-CCR5 antibodies of IgG and IgA isotypes were obtained by both immunization strategies. It is noteworthy that the mucosal route proved to be more efficient (Fig. 2). Therefore, the mucosal immunization route seems to be more promising in terms of efficacy and convenience, since it is less invasive and does not require any adjuvant.
The abilities of the antibodies to bind CCR5 in its native conformation were also confirmed by the inhibition of CD4+ lymphocyte chemotaxis, a response specifically mediated by Mip-1β via CCR5 (Fig. 5). Further confirmation of this ability came from virus neutralization experiments (Fig. 6). In fact, antibodies (especially IgAs) inhibited the infectivities of R5-tropic virus isolates but not those of multitropic strains. Since the affinity of polyclonal antibodies cannot be easily determined, the higher neutralizing efficacies of IgA antibodies (with respect to IgG antibodies at comparable titers) were tentatively explained by their dimeric status.
A major point to be addressed was the biological role of anti-CCR5 antibodies at the mucosal level. IgG and (especially) IgA were able to bind and down-regulate CCR5 expressed on CD4+ CCR5+ cells isolated from mouse and human PBMC, but also receptors expressed on CD4+ CCR5+ mouse cells from vaginal washings. Strikingly, mucosal immunization caused a stronger in vivo down-regulation of CCR5 than systemic immunization (Fig. 7). The remarkable in vivo CCR5 down-regulation and the subsequent restoration of receptor levels follow slow kinetics compared to previously reported in vitro data (28). It would be of great interest to further investigate the molecular pathways involved in CCR5 down-regulation, recycling, and/or degradation. The findings presented here are noteworthy and promising features for clinical implementation of anti-CCR5 prophylactic vaccination.
In vivo, down-regulation of CCR5 persisted for the first 2 weeks after recall immunization (the seventh). However, CCR5 reappeared on the majority of CD4+ cells at 4 weeks after immunization, disappearing again following a new recall immunization (the eighth) (Fig. 8). The surface reappearance of CCR5 did not exactly reproduce the situation occurring in humans, because in ESN individuals presenting anti-CCR5 autoantibodies, a fraction of CD4+ cells are permanently CCR5 negative (22). The reason for this difference is unclear, and its understanding could be critical for potential therapeutic applications of CCR5 vaccination.
Two recent experiments have investigated the in vivo protection conferred by anti-CCR5 autoantibodies raised to the N-terminal domain of CCR5 (3, 8). (i) Chackerian et al. (8) used the N-terminal domain of pigtailed macaque CCR5 fused to streptavidin, which (when conjugated at high density to bovine papillomavirus major capsid protein L1 virus-like particles) induced high-titer anti-CCR5 IgG that blocked infection by CCR5-tropic simian-human immunodeficiency virus (SHIV) in vitro. No decline in the number of CCR5-expressing T cells was detected in immunized animals. In SHIV-challenged animals, viral loads and time to control of viremia were significantly decreased (relative to controls), indicating the possibility that CCR5 autoantibodies contributed to the control of viral replication. (ii) Bogers et al. (3) prepared a vaccine consisting of three extracellular peptides of CCR5, an N-terminal HIV gp120 fragment generated in transgenic plants, and recombinant simian immunodeficiency virus p27. They were linked to the microbial heat shock protein (HSP70) carrier, and the vaccine was administered by mucosal and systemic routes. Vaginal challenge with SHIV infected all macaques but showed a significant variation in viral loads between the animals, and the virus was cleared in five of nine immunized animals. The major difference between these interesting reports and our approach is that they used linear peptides and focused on the N-terminal domain. In these reports, the CCR589-102 was tested as a linear peptide and found to be poorly immunogenic.
Conversely, we studied the possibility of raising antibodies restricted to the 89-to-102 external loop, because of our initial findings that anti-CCR5 autoantibodies from ESN individuals predominantly or uniquely displayed specificity for this CCR5 domain. Having shown that the linear synthetic peptide CCR589-102 is poorly immunogenic, we turned our attention to its cyclic form and to the FHV Epitope Presentation System. Analogous to our results, a recent report has shown that it is possible to select anti-CCR5 single-chain variable fragments from a phage display antibody library and that only those selected by binding to cyclic constrained peptides inhibited CCR5-mediated (but not CXCR4-mediated) HIV infection (40).
Further experiments will be required to explore the possibility of eliciting protective and long-lasting mucosal immunity with a limited number of strong immunogens and to find the best antigens or epitopes that may confer protection in vivo. However, our results demonstrate that a single CCR5 loop, conveniently presented to the immune system, may break immune tolerance and induce a protective response. In conclusion, we established a system that mimics the putatively protective response already observed in vivo in different cohorts of individuals all over the world.
Acknowledgments
This study was supported by Istituto Superiore di Sanità grants 40D50 to L.L. and 40B90 to A.G.S.
We thank Francisco Baralle for reagents and critical reading of the manuscript and Silvia Russo for editorial help.
REFERENCES
- 1.Barassi, C., A. Lazzarin, and L. Lopalco. 2004. CCR5-specific mucosal IgA in saliva and genital fluids of HIV-exposed seronegative subjects. Blood 104:2205-2206. [DOI] [PubMed] [Google Scholar]
- 2.Belec, L., P. D. Ghys, H. Hocini, J. N. Nkengasong, J. Tranchot-Diallo, M. O. Diallo, V. Ettiegne-Traore, C. Maurice, P. Becquart, M. Matta, A. Si-Mohamed, N. Chomont, I. M. Coulibaly, S. Z. Wiktor, and M. D. Kazatchkine. 2001. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1-seronegative African women. J. Infect. Dis. 184:1412-1422. [DOI] [PubMed] [Google Scholar]
- 3.Bogers, W. M. J. M., L. A. Bergmeier, J. Ma, H. Oostermeijer, Y. Wang, C. G. Kelly, P. ten Haaft, M. Singh, J. L. Heeney, and T. Lehner. 2004. A novel HIV-CCR5 receptor vaccine strategy in the control of mucosal SIV/HIV infection. AIDS 18:25-36. [DOI] [PubMed] [Google Scholar]
- 4.Bouhlal, H., H. Hocini, C. Quillent-Gregoire, V. Donkova, S. Rose, A. Amara, R. Longhi, N. Haeffner-Cavaillon, A. Beretta, S. V. Kaveri, and M. D. Kazatchkine. 2001. Antibodies to C-C chemokine receptor 5 in normal human IgG block infection of macrophages and lymphocytes with primary R5-tropic strains of HIV-1. J. Immunol. 166:7606-7611. [DOI] [PubMed] [Google Scholar]
- 5.Broliden, K., J. Hinkula, C. Devito, P. Kiama, J. Kimani, D. Trabbatoni, J. J. Bwayo, M. Clerici, F. Plummer, and R. Kaul. 2001. Functional HIV-1 specific IgA antibodies in HIV-1 exposed, persistently IgG seronegative female sex workers. Immunol. Lett. 79:29-36. [DOI] [PubMed] [Google Scholar]
- 6.Buratti, E., L. McLain, S. Tisminetzky, S. M. Cleveland, N. J. Dimmock, and F. E. Baralle. 1998. The neutralizing antibody response against a conserved region of human immunodeficiency virus type 1 gp41 (amino acid residues 731-752) is uniquely directed against a conformational epitope. J. Gen. Virol. 79:2709-2716. [DOI] [PubMed] [Google Scholar]
- 7.Buratti, E., S. G. Tisminetzky, E. S. Scodeller, and F. E. Baralle. 1996. Conformational display of two neutralizing epitopes of HIV-1 gp41 on the Flock House virus capsid protein. J. Immunol Methods 197:7-18. [DOI] [PubMed] [Google Scholar]
- 8.Chackerian, B., L. Briglio, P. S. Albert, D. R. Lowy, and J. T. Schiller. 2004. Induction of autoantibodies to CCR5 in macaques and subsequent effects upon challenge with an R5-tropic simian/human immunodeficiency virus. J. Virol. 78:4037-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clerici, M., C. Barassi, C. Devito, C. Pastori, S. Piconi, D. Trabattoni, R. Longhi, J. Hinkula, K. Broliden, and L. Lopalco. 2002. Serum IgA of HIV-exposed uninfected individuals inhibit HIV through recognition of a region within the alpha-helix of gp41. AIDS 16:1731-1741. [DOI] [PubMed] [Google Scholar]
- 10.Fields, G. B., and R. L. Noble. 1990. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 35:161-214. [DOI] [PubMed] [Google Scholar]
- 11.Fisher, A. J., and J. E. Johnson. 1993. Ordered duplex RNA controls capsid architecture in an icosahedral animal virus. Nature 361:176-179. [DOI] [PubMed] [Google Scholar]
- 12.Garboczi, D. N., P. Ghosh, U. Utz, Q. R. Fan, W. E. Biddison, and D. C. Wiley. 1996. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature 384:134-141. [DOI] [PubMed] [Google Scholar]
- 13.King, D. S., C. G. Fields, and G. B. Fields. 1990. A cleavage method which minimizes side reactions following Fmoc solid phase peptide synthesis. Int. J. Pept. Protein Res. 36:255-266. [DOI] [PubMed] [Google Scholar]
- 14.Kostrikis, L. G., Y. Huang, J. P. Moore, S. M. Wolinsky, L. Zhang, Y. Guo, L. Deutsch, J. Phair, A. U. Neumann, and D. D. Ho. 1998. A chemokine receptor CCR2 allele delays HIV-1 disease progression and is associated with a CCR5 promoter mutation. Nat. Med. 4:350-353. [DOI] [PubMed] [Google Scholar]
- 15.Kozlowski, P. A., and M. R. Neutra. 2003. The role of mucosal immunity in prevention of HIV transmission. Curr. Mol. Med. 3:217-228. [DOI] [PubMed] [Google Scholar]
- 16.Krupitsky, E., E. Zvartau, G. Karandashova, N. J. Horton, K. R. Schoolwerth, K. Bryant, and J. H. Samet. 2004. The onset of HIV infection in the Leningrad region of Russia: a focus on drug and alcohol dependence. HIV Med. 5:30-33. [DOI] [PubMed] [Google Scholar]
- 17.Lehner, T. 2003. Innate and adaptive mucosal immunity in protection against HIV infection. Vaccine 21(Suppl. 2):S68-S76. [DOI] [PubMed] [Google Scholar]
- 18.Lillo, F. B., M. A. Grasso, S. Lodini, M. G. Bellotti, and, G. Colucci. 2004. Few modifications of the Cobas Amplicor HIV Monitor 1.5 test allow reliable quantitation of HIV-1 proviral load in peripheral blood mononuclear cells. J. Virol. Methods 120:201-205. [DOI] [PubMed] [Google Scholar]
- 19.Liu, R., W. Paxton, S. Choe, D. Ceradini, S. Martin, R. Horuk, M. MacDonald, H. Stuhlman, R. Koup, and N. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:1-11. [DOI] [PubMed] [Google Scholar]
- 20.Lo Caputo, S., D. Trabattoni, F. Vichi, S. Piconi, L. Lopalco, M. L. Villa, F. Mazzotta, and M. Clerici. 2003. Mucosal and systemic HIV-1-specific immunity in HIV-1-exposed but uninfected heterosexual men. AIDS 17:531-539. [DOI] [PubMed] [Google Scholar]
- 21.Lopalco, L. 2004. Humoral immunity in HIV-1 exposure: cause or effect of HIV resistance? Curr. HIV Res. 2:79-92. [DOI] [PubMed] [Google Scholar]
- 22.Lopalco, L., C. Barassi, C. Pastori, R. Longhi, S. E. Burastero, G. Tambussi, F. Mazzotta, A. Lazzarin, M. Clerici, and A. G. Siccardi. 2000. CCR5-reactive antibodies in seronegative partners of HIV-seropositive individuals down-modulate surface CCR5 in vivo and neutralize the infectivity of R5 strains of HIV-1 in vitro. J. Immunol. 164:3426-3433. [DOI] [PubMed] [Google Scholar]
- 23.Lopalco, L., Z. Magnani, C. Confetti, M. Brianza, A. Saracco, G. Ferraris, F. Lillo, C. Vegni, A. Lazzarin, A. G. Siccardi, and S. E. Burastero. 1999. Anti-CD4 antibodies in exposed seronegative adults and in newborns of HIV type 1-seropositive mothers: a follow-up study. AIDS Res. Hum. Retrovir. 15:1079-1085. [DOI] [PubMed] [Google Scholar]
- 24.Matsika-Claquin, M. D., M. Massanga, D. Menard, J. Mazi-Nzapako, J. P. Tenegbia, M. J. Mandeng, J. Willybiro-Sacko, A. Fontanet, and A. Talarmin. 2004. HIV epidemic in Central African Republic: high prevalence rates in both rural and urban areas. J. Med. Virol. 72:358-362. [DOI] [PubMed] [Google Scholar]
- 25.Mazzoli, S., L. Lopalco, A. Salvi, D. Trabattoni, S. Lo Caputo, F. Semplici, M. Biasin, C. Bl, A. Cosma, C. Pastori, F. Meacci, F. Mazzotta, M. L. Villa, A. G. Siccardi, and M. Clerici. 1999. Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J. Infect. Dis. 180:871-875. [DOI] [PubMed] [Google Scholar]
- 26.Mazzoli, S., D. Trabattoni, S. Lo Caputo, S. Piconi, C. Ble, F. Meacci, S. Ruzzante, A. Salvi, F. Semplici, R. Longhi, M. L. Fusi, N. Tofani, M. Biasin, M. L. Villa, F. Mazzotta, and M. Clerici. 1997. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat. Med. 3:1250-1257. [DOI] [PubMed] [Google Scholar]
- 27.Moore, J. P., A. Trkola, and T. Dragic. 1997. Co-receptors for HIV-1 entry. Curr. Opin. Immunol. 9:551-562. [DOI] [PubMed] [Google Scholar]
- 28.Mueller, A., E. Kelly, and P. G. Strange. 2002. Pathways for internalization and recycling of the chemokine receptor CCR5. Blood 99:785-791. [DOI] [PubMed] [Google Scholar]
- 29.Newell, M. L. 2003. Antenatal and perinatal strategies to prevent mother-to-child transmission of HIV infection. Trans. R. Soc. Trop. Med. Hyg. 97:22-24. [DOI] [PubMed] [Google Scholar]
- 30.Olson, W. C., G. E. Rabut, K. A. Nagashima, D. N. Tran, D. J. Anselma, S. P. Monard, J. P. Segal, D. A. Thompson, F. Kajumo, Y. Guo, J. P. Moore, P. J. Maddon, and T. Dragic. 1999. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J. Virol. 73:4145-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiappacassi, M., E. Buratti, P. D'Agaro, L. Ciani, E. S. Scodeller, S. G. Tisminetzky, and F. E. Baralle. 1997. V3 loop core region serotyping of HIV-1 infected patients using the FHV epitope presenting system. J. Virol. Methods 63:121-127. [DOI] [PubMed] [Google Scholar]
- 32.Scodeller, E. A., S. G. Tisminetzky, F. Porro, M. Schiappacassi, A. De Rossi, L. Chiecco-Bianchi, and F. E. Baralle. 1995. A new epitope presenting system displays a HIV-1 V3 loop sequence and induces neutralizing antibodies. Vaccine 13:1233-1239. [DOI] [PubMed] [Google Scholar]
- 33.Sibanda, E. N., G. Stanczuk, and F. Kasolo. 2003. HIV/AIDS in Central Africa: pathogenesis, immunological and medical issues. Int. Arch. Allergy Immunol. 132:183-195. [DOI] [PubMed] [Google Scholar]
- 34.Spruth, M., H. Stoiber, L. Kacani, D. Schonitzer, and M. P. Dierich. 1999. Neutralization of HIV type 1 by alloimmune sera derived from polytransfused patients. AIDS Res. Hum. Retrovir. 15:533-543. [DOI] [PubMed] [Google Scholar]
- 35.Tisminetzky, S. G., E. A. Scodeller, P. Evangelisti, Y. Chen, M. Schiappacassi, F. Porro, F. Bizik, T. Zacchi, G. Lunazzi, S. Miertus, and F. E. Baralle. 1994. Immunoreactivity of chimeric proteins carrying the HIV-1 epitope IGPGRAF. Correlation between predicted conformation and antigenicity. FEBS Lett. 353:1-4. [DOI] [PubMed] [Google Scholar]
- 36.Trkola, A., T. J. Ketas, K. A. Nagashima, L. Zhao, T. Cilliers, L. Morris, J. P. Moore, P. J. Maddon, and W. C. Olson. 2001. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J. Virol. 75:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van't Wout, A. B., N. A. Kootstra, G. A. Mulder-Kampinga, N. Albrecht-van Lent, H. J. Scherpbier, J. Veenstra, K. Boer, R. A. Coutinho, F. Miedema, and H. Schuitemaker. 1994. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J. Clin. Investig. 94:2060-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verani, A., and P. Lusso. 2002. Chemokines as natural HIV antagonists. Curr. Mol. Med. 2:691-702. [DOI] [PubMed] [Google Scholar]
- 39.Wang, Y., J. Underwood, R. Vaughan, A. Harmer, C. Doyle, and T. Lehner. 2002. Allo-immunization elicits CCR5 antibodies, SDF-1 chemokines, and CD8-suppressor factors that inhibit transmission of R5 and X4 HIV-1 in women. Clin. Exp. Immunol. 129:493-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, Y., C. Pool, K. Sadler, H. P. Yan, J. Edl, X. Wang, J. G. Boyd, and J. P. Tam. 2004. Selection of active ScFv to G-protein-coupled receptor CCR5 using surface antigen-mimicking peptides. Biochemistry 43:12575-12584. [DOI] [PubMed] [Google Scholar]