Abstract
Two herpes simplex virus type 1 (HSV-1) entry pathways have been described: direct fusion between the virion envelope and the plasma membrane, as seen on Vero cells, and low-pH-dependent endocytosis, as seen on CHO nectin-1 and HeLa cells. In this paper, we studied HSV entry into C10 murine melanoma cells and identified a third entry pathway for this virus. During entry into C10 cells, virion envelope glycoproteins rapidly became protected from the membrane-impermeable chemical cross-linker BS3 and from proteinase K. Protection was gD receptor dependent, and the time taken to detect protected protein was proportional to the rate of virus entry. Ultrastructural examination revealed that virions attached to the surface of C10 cells were localized to membrane invaginations, whereas those on the surface of receptor-negative B78 cells were peripherally attached. Virus entry into C10 cells was energy dependent, and intracellular enveloped virions were seen within membrane-bound vesicles consistent with endocytic entry. Entry was not inhibited by bafilomycin A1 or ammonium chloride, showing that passage of the virion through a low-pH environment was not required for infection. Resistance to similar reagents should therefore not be taken as proof of HSV entry by a nonendosomal pathway. These data define a novel gD receptor-dependent acid-independent endocytic entry pathway for HSV.
A prerequisite for productive entry of enveloped viruses into cells is fusion between the viral envelope and a cellular membrane. This can occur either on the cell surface, such as in the case of human immunodeficiency virus, or within an endosome, such as with influenza virus (10, 46). Typically, during fusion on the cell surface, receptor binding induces conformational changes in viral glycoproteins that expose a hydrophobic fusion peptide. During endosomal entry, functionally equivalent conformational changes are usually induced in the viral fusion glycoprotein by the low-pH environment of the endosomal lumen (5). Thus, two broad mechanisms exist for the delivery of virion contents to the cytoplasm.
Early studies using Vero or Hep2 cells suggested that herpes simplex virus (HSV) entry occurred by direct fusion of the virion envelope with the plasma membrane (15-17, 50). It was assumed that this entry pathway was applicable to all cell types infected by HSV. It is now clear that, on some cells, such as CHO and HeLa, HSV entry occurs by endocytosis (36, 37).
Both routes of HSV entry are mediated by four essential virion envelope glycoproteins: gB, gD, gH, and gL, and a fifth, gC, that plays a nonessential role. The process is initiated by interactions between gB and/or gC and cell surface proteoglycans (26). Binding of gD to a cellular receptor, such as nectin-1 or HVEM (18, 33), likely initiates the fusion process, which involves an ordered series of interactions between the essential glycoproteins and the target membrane.
In common with many viral endocytic entry pathways, endocytic entry of HSV into receptor-expressing CHO and HeLa cells is inhibited by reagents that raise endosomal pH, suggesting that passage through an acidic compartment is required (36). Since the HSV fusion machinery is functional at neutral pH, for example, during entry to Vero cells or in cell fusion assays (34, 40, 48), it is not clear what role low pH plays in endocytic HSV entry. The role of the gD-receptor interaction in endocytic entry is also uncertain. In CHO cells, HSV is internalized even in the absence of gD receptor, although viral gene expression is initiated only when a functional receptor is present (37).
HSV entry is a rapid process: 50% of inoculum virions become internalized within 10 to 15 min (49). Few studies have analyzed the fate and function of the viral entry glycoproteins during this time window. Therefore, the initial aim of the present study was to monitor the fate of the glycoproteins during the first 10 min of entry. We used B78H1 murine melanoma cells (51) and their human nectin-1-expressing derivative, C10 cells (29). B78H1 cells do not express a functional gD receptor and do not support HSV entry. C10 cells are permissive for HSV entry and production of progeny virions and are thus suitable for studies of HSV entry and the role of gD receptors in that process.
It is clear from a variety of studies that multiple endocytic pathways exist, most of which are potentially suitable for virus entry (7, 38, 45). It seems possible that, as a wider range of cell types is examined, other distinct pathways of HSV entry will be identified. Our studies have led us to identify a third entry pathway for HSV: endocytic entry into C10 cells. The pathway is characterized by two distinct features: first, virion internalization is gD receptor dependent and, second, infection does not require endosome acidification.
MATERIALS AND METHODS
Cells and virus.
Vero cells (ATCC CCL-81) are African green monkey kidney epithelial cells. They were maintained in Dulbecco's minimum essential medium (DMEM) supplemented with 5% fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin (5% DMEM).
B78H1 cells are murine melanoma cells (51) that do not express a functional HSV gD receptor and consequently do not support HSV entry. B78C10 cells were derived from B78H1 and stably express the gD receptor human nectin-1 (29). They are called C10 cells in this paper. B78H1 control 16 cells are B78H1 cells stably transfected with pCDNA3 (29). They are called B78 cells in this paper. B78 cells and C10 cells were maintained in 5% DMEM supplemented with 500 μg/ml G418.
CHO K1 cells are Chinese hamster ovary cells. They do not express a functional gD receptor and are resistant to HSV infection. CHO nectin-1 cells are CHO K1 cells that stably express human nectin-1. Both were maintained in Ham's F-12 medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin as above and, in the case of CHO nectin-1 cells, 250 μg/ml G418.
Sucrose gradient-purified HSV-1 strain KOS was used in most experiments. HSV K26-GFP (9) was used in the energy depletion entry assay. Because gD comigrates in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with the immunoglobulin G (IgG) heavy chain HSV-1 gD-GFP was used in some immunoprecipitation experiments. This virus, derived from HSV strain 17, encodes gD with green fluorescent protein (GFP) fused to its C terminus; it replicates normally in vitro and will be described elsewhere. All strains were propagated and titers were determined on Vero cells.
Vesicular stomatitis virus (VSV; Indiana serotype) was obtained from Yan Yuan (University of Pennsylvania) and was propagated and titers were determined on Vero cells.
Antibodies.
Monoclonal antibodies (MAbs) used for immunoprecipitations were as follows: for gB, BD60 (unpublished data); for gH, 53S (43); for gD, DL6 (11). gD MAbs DL6, DL11 (4, 35), 1D3 (14), LP2 (32), and the c-myc MAb 9E10 (13) were used in blocking experiments. Rabbit polyclonal antibodies used for detection of glycoproteins in Western blot assays were as follows: for gB, R69 (12); for gH, R137 (39); for gD, R7 (24).
Cross-linking assay.
A total of 9 × 105 cells were seeded overnight per well of six-well plates. Cells were washed once with 5% DMEM and then refed with chilled 5% DMEM supplemented with 30 mM HEPES (DMEM-HEPES) and chilled to 4°C. Virus, diluted in DMEM-HEPES, was then added at an input multiplicity of 20 PFU/cell and allowed to adsorb for 45 min at 4°C. Entry was then initiated by raising the temperature to 37°C. After the desired time, entry was stopped by placing the plates on ice.
Cells were washed once with chilled Hank's balanced salt solution containing 30 mM HEPES (HBSS-HEPES). The chemical cross-linker bis-[sulfosuccinimidyl] suberate (BS3; obtained from Pierce [catalog number 21580]) was then added. BS3 is membrane impermeable and contains two NHS ester groups joined by an 11.4-Å linker. One milliliter of freshly prepared 1 mM BS3 (in HBSS-HEPES) was added per well, and cross-linking proceeded for 50 min at 4°C. The reaction was quenched by addition of an equal volume of DMEM-HEPES containing 100 mM Tris-HCl (pH 7.5). Cells were lysed with Tris-buffered saline (10 mM Tris-HCl [pH 7.5], 150 mM NaCl) containing 1% NP-40 (TBS-NP-40) and 1× Complete protease inhibitor (Roche).
Viral glycoproteins were then immunoprecipitated and Western blotted, or they were detected directly on Western blots. For immunoprecipitations, cell lysates from one well of a six-well plate were incubated at 4°C overnight with 1 μg of MAb IgG. Immune complexes were collected with protein A-agarose by incubation for 2 h at 4°C. Pellets were washed with TBS-NP-40, then with TBS-NP-40 (containing 1 M NaCl), then again with TBS-NP-40, and then boiled in SDS sample buffer prior to analysis by SDS-PAGE, Western blotting, and chemiluminescent detection.
In blocking experiments, virus in DMEM-HEPES was exposed to gD or control MAbs (100 μg/ml of IgG) for 1 h at 37°C. An aliquot of MAb-treated virus was titrated on Vero cells, and the remainder was used in the cross-linking assay.
Electron microscopy.
Virus was added to chilled monolayers of B78 or C10 cells in six-well plates at an input multiplicity of 50 PFU/cell and allowed to attach for 2 h at 4°C. The temperature was then raised to 37°C for up to 10 min, and then the plates were chilled again on ice. Cells were washed with ice-cold phosphate-buffered saline (PBS) and then fixed at 4°C overnight in 2.5% glutaraldehyde, 2% paraformaldehyde in 0.1 M sodium cacodylate. Cell pellets were rinsed in 0.1 M sodium cacodylate buffer, postfixed with 2% osmium tetroxide, dehydrated in graded ethanol, and embedded in Epon. Thin sections (70 nm) were stained with uranyl acetate and bismuth subnitrite and then examined with a JEOL JEM 1010 electron microscope. Images were captured using a Hamamatsu charge-coupled device camera and AMT 12-HR software.
Proteinase protection assay.
For the proteinase protection assay, virus was added to cell monolayers and entry was initiated and stopped as in the cross-linking assay (see above). Cells were washed once with HBSS-HEPES supplemented with 1 mM CaCl2 (HBSS-HEPES-CaCl2) and then incubated with proteinase K (catalog no. P2308; Sigma) at various concentrations in HBSS-HEPES-CaCl2 for 1 h at 4°C. Control cells were treated with buffer alone. At the end of the incubation, cells were transferred to a 1.5-ml tube, pelleted, and then lysed in TBS-NP-40 containing 1 mM phenylmethylsulfonyl fluoride (PMSF) to stop proteolysis. Glycoproteins were then immunoprecipitated and detected by SDS-PAGE and Western blotting.
Rate-of-entry assay.
The rate of virus entry was determined using a standard acid inactivation assay (22, 23, 31). C10 cells in 35-mm dishes were chilled on ice, and then 200 PFU of virus was added. Entry was initiated by floating the plates in water baths set to the appropriate temperatures. At intervals after the temperature shift, the cells were washed with acid-citrate buffer (pH 3.0) to inactivate extracellular virus. The plates were then overlaid with 5% DMEM containing 1% carboxymethyl cellulose and incubated at 37°C to allow plaque formation.
Energy depletion.
HSV K26-GFP was bound to cells at 4°C, and then the inoculum was replaced with glucose-free DMEM containing 2% bovine serum albumin, 0.05% sodium azide, and 0.3% 2-deoxy-d-glucose, or with control medium, and held at 4° for 15 min. Cells were then washed, refed with the appropriate medium, and incubated at 37°C for 45 min. Extracellular virus was acid inactivated, the cells were incubated for a further 7 h, and then entry was measured by counting GFP-expressing cells (36, 37). The number of green cells in samples treated with the control medium was taken as 100%.
Drug inhibition experiments.
Bafilomycin A1 (BFLA; Sigma) was prepared as a 100 μM stock solution in dimethyl sulfoxide and stored at −20°C. A 1.5 M stock of ammonium chloride was freshly prepared at the start of each experiment, and the pH was adjusted to 7.4 by the addition of NaOH. Dilutions of each reagent were prepared in growth medium. For BFLA, medium also contained 0.1% dimethyl sulfoxide. Cells were preincubated with drug-containing medium for 1 h at 37°C. Dilutions of virus (HSV or VSV) in drug-containing medium were then added and incubated for 1 h. The inoculum was removed, and the cells were refed with drug-containing medium for a further 4 h and then washed with 5% DMEM and overlaid with 5% DMEM containing 1% carboxymethyl cellulose. VSV plates were fixed (5% formaldehyde in PBS for 2 h at room temperature) at 24 h postinfection and then stained with crystal violet (1% in PBS). HSV plates were fixed at 48 h postinfection and immunostained.
RESULTS
Protection of gB from cross-linking during HSV entry into C10 cells.
The aim of this work was to study the essential HSV envelope glycoproteins during virus entry. Previous studies, using the membrane-impermeable cross-linker BS3, showed changes in the associations of the glycoproteins during the first hour of virus entry (21). Here, we extended these observations using receptor-negative B78 murine melanoma cells, which do not support HSV entry, and their nectin-1-expressing derivative, C10, which is permissive for virus entry and replication.
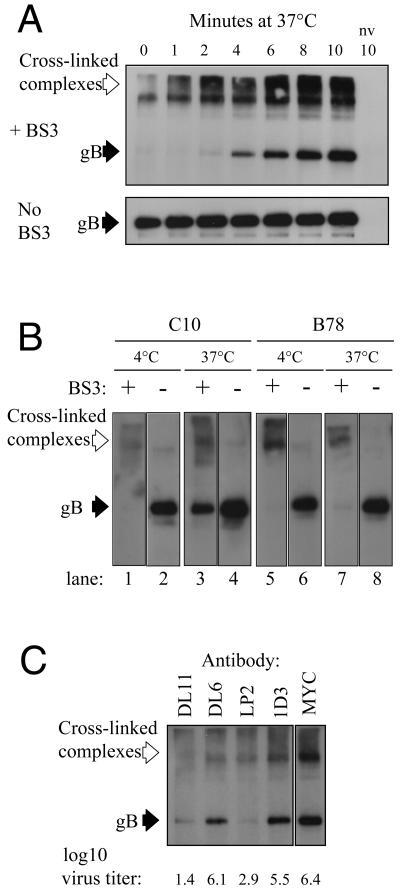
In the first set of experiments, HSV-1 was added to C10 cells at 4°C. At this temperature, virus entry does not progress beyond attachment. Entry was initiated by incubation at 37°C for up to 10 min, then the cells were chilled on ice, cross-linked with BS3, and lysed, and gB was immunoprecipitated and detected by Western blotting.
As expected (21), high-molecular-weight cross-linked complexes containing gB were present at all time points (Fig. 1A). There was one striking change after entry was initiated. At t = 0 min, no unit-length gB was detected, suggesting that all accessible virion gB was cross-linked. By t = 2 min, unit-length gB could be detected, and it became more abundant over the rest of the time course. Numerous other gB MAbs gave the same result (data not shown). Immunoprecipitation of gB from non-cross-linked control samples showed that the amount of gB accessible to the MAb was constant (Fig. 1A).
FIG. 1.
gD receptor-dependent protection of glycoprotein B from BS3 cross-linking during virus entry. A. Virus was attached to C10 cells at 4°C. Entry was then initiated by incubation at 37°C for the indicated times and then stopped by placing the cells on ice. Cells were then cross-linked with BS3 or mock treated and then lysed. gB was detected by immunoprecipitation with MAb BD60 and then Western blotting with polyclonal antibody R69. B. The cross-linking assay was carried out on C10 cells or receptor-negative B78 cells. After virus attachment, samples were held at 4°C (lanes 1, 2, 5, and 6) or placed at 37°C for 10 min (lanes 3, 4, 7, and 8) and then chilled on ice. Samples were then cross-linked with BS3 (lanes 1, 3, 5, and 7) or mock treated (lanes 2, 4, 6, and 8). C. The assay was carried out using virus that was pretreated with the indicated gD-specific MAb. MAb-treated virus was also titrated on Vero cells to measure the degree of neutralization. Titers (log10 PFU/ml) are shown below the panel. Open arrows indicate high-molecular-weight gB-containing complexes formed by cross-linking. Solid arrows point to unit-length gB. nv, no virus.
Thus, a proportion of virion gB became protected from cross-linker concomitant with the initiation of virus entry into C10 cells. We next carried out a similar experiment with C10 cells and receptor-negative B78 cells to ask whether protection of gB was gD receptor dependent.
When cells were maintained at 4°C, no unit-length gB was detected in the cross-linked samples (Fig. 1B, lanes 1 and 5). Equivalent amounts were seen in non-cross-linked samples from both cell types (Fig. 1B, lanes 2 and 6). After incubation at 37°C for 10 min prior to cross-linking, unit-length gB was detected in C10 cells (Fig. 1B, lane 3) but not in B78 cells (Fig. 1B, lane 7). When the cross-linker was omitted, equivalent amounts of unit-length gB were detected (Fig. 1B, lanes 4 and 8). This result showed that protection of virion gB from BS3 cross-linking is gD receptor dependent. In support of this conclusion, when the experiment was carried out in the presence of neutralizing anti-gD MAbs, no protected gB was detected, whereas control, nonneutralizing MAbs had no effect (Fig. 1C).
Since our data suggested that protection of gB required virus entry, we reasoned that if the rate of virus entry were reduced the time taken for gB to become protected should be similarly affected. To test this directly we examined HSV entry at temperatures below 37°C. Figure 2A shows the effect of reduced temperature on the rate of HSV entry into C10 cells. At 37°C, 50% of virions entered the cell within 10 min of the temperature shift. As expected, the rate of entry was reduced at the lower temperatures. At 30°C, the 50% plaque count was not reached until 15 min after the temperature shift. At 22°C, entry was very inefficient, although a few plaques were seen. Next, we carried out a cross-linking experiment under these conditions. As before, at 37°C, protected gB was detected at 2 min and reached maximum abundance at 8 to 12 min after the temperature shift (Fig. 2B). At 30°C, maximum abundance of protected gB was not reached until 16 to 30 min after the temperature shift and, at 22°C, 45 min were required. Thus, when the rate of virus entry was reduced, more time was required for gB to become protected from cross-linking.
FIG. 2.
Protection of gB from cross-linking occurs with kinetics comparable to the rate of virus entry. A. Rate of virus entry into C10 cells at 37°C, 30°C, and 22°C. A standard acid inactivation entry assay was carried out (see Materials and Methods). B. After attachment of virus to C10 cells at 4°C, entry was initiated by incubation at the indicated temperatures for the indicated times. Cross-linking was then carried out as before. For clarity, only the unit-length gB band is shown. nx, no cross-linking; nv, no virus.
These experiments showed that protection of virion gB from cross-linking was gD receptor dependent and occurred with kinetics comparable to the rate of virus entry. To escape cross-linking, a protein must be localized to a site that is inaccessible to the cross-linker. BS3 is membrane impermeable, and so a likely explanation for our results was rapid internalization of virions by endocytosis.
Protection of HSV glycoproteins from proteinase K correlates with endocytic entry.
If, as our gB protection data suggested, virus entry to C10 cells occurred by endocytosis, the other virion glycoproteins should be similarly protected. To test this, we examined the fate of virion gB, gH, and gD in our next experiments in which we used proteinase K protection as an alternative measure of endocytic internalization to complement our cross-linking studies. In this assay, cells are exposed to proteinase K at different times after the initiation of virus entry. Glycoproteins on the cell surface, either in virions that have not entered the cell or from virions that have fused directly with the plasma membrane, remain accessible to proteinase K and are digested. Full-length glycoproteins are detected only if they have become protected from digestion by internalization.
Protection of gB from proteinase K was rapid and gD receptor dependent (Fig. 3A) with kinetics identical to those seen in the cross-linking assay (compare Fig. 3A with 1A). gD also became protected rapidly after the initiation of virus entry into C10 cells (Fig. 3B). Although gD was detected slightly sooner than gB, we attribute this to differences in the sensitivity of the antibodies. We looked for protection of gH at 10 min after the initiation of entry. At this time significant amounts of gB and gD were protected. As for gB and gD, protection of gH from proteinase K digestion was temperature and gD receptor dependent (Fig. 3C, lanes 5 to 8). Equivalent amounts of full-length gB and gH were detected in control undigested samples (Fig. 3C, lanes 1 to 4).
FIG. 3.
Rapid, gD receptor-dependent protection of viral glycoproteins from proteinase K digestion. A. Virus was attached to B78 and C10 cells for 45 min at 4°C. Entry was then initiated by incubation at 37°C for the indicated times. Cells were treated with proteinase K, and gB was detected by immunoprecipitation and Western blotting. B. C10 cells were infected with HSV gD-GFP virus as for panel A. Samples were proteinase K treated and then lysed. gD-GFP was immunoprecipitated with MAb DL6 and detected by Western blotting with polyclonal antibody R7. nv, no virus. C. Virus was attached to B78 (lanes 1, 2, 5, and 6) or C10 (lanes 3, 4, 7, and 8) cells for 45 min at 4°C. Cells were then incubated at 37°C for 10 min (lanes 2, 4, 6, and 8) or held at 4°C (lanes 1, 3, 5, and 7), treated with proteinase K or mock treated for 1 h at 4°C, and lysed in the presence of PMSF, and glycoproteins were detected by immunoprecipitation and Western blotting.
These experiments show that virion glycoproteins gB, gD, and gH are rapidly protected from proteinase K digestion in a gD receptor-dependent manner consistent with rapid gD receptor-dependent endocytosis of virions. To confirm that protection of virion glycoproteins from proteinase K is a valid marker of endocytic entry, we looked for protection of gB during entry into CHO K1 cells, CHO nectin-1 cells, and Vero cells. We expected that our assay would distinguish between the rapid endocytic internalization of HSV seen on CHO K1 and CHO nectin-1 cells (36, 37) and entry into Vero cells that occurs by direct fusion at the cell surface.
Full-length gB was present in mock-treated samples from all cell types (Fig. 4, lanes 1 and 2). After digestion, no protected gB was detected in samples that had been maintained at 4°C, regardless of the cell type (Fig. 4, lanes 3). When cells were incubated at 37°C prior to digestion, protected gB was seen in the CHO K1 and CHO nectin-1 samples but not in the Vero samples (Fig. 4, lanes 4). Thus, protection of virion gB from digestion correlated with use of an endocytic pathway for virus entry.
FIG. 4.
Protection of viral gB from proteinase K correlates with endocytic entry. After virus attachment for 45 min at 4°C, cells were either held at 4°C (lanes 1 and 3) or incubated at 37°C for 10 min (lanes 2 and 4) and then chilled on ice. Cells were then digested (lanes 3 and 4) or mock treated (lanes 1 and 2) with proteinase K and lysed in the presence of PMSF. gB was immunoprecipitated from the lysates and detected by Western blotting.
Protection of gB from proteolysis during entry into receptor-negative CHO K1 cells is consistent with previous data (36) and stands in contrast to the results we obtained with B78 cells (Fig. 3). In the latter cells, which also lack a gD receptor, virion gB was not protected from proteolysis. This highlights a novel aspect of the B78 cell system: internalization of HSV is gD receptor dependent.
Inhibition of ATP synthesis prevents HSV entry to C10 cells.
Endocytic uptake is an active, energy-dependent process and is therefore sensitive to inhibitors of ATP synthesis. Previous work showed that endocytic entry of HSV into CHO nectin-1 cells is inhibited by treatment with energy depletion medium containing sodium azide and 2-deoxy-d-glucose, but that this does not affect entry into Vero cells. Therefore, we tested the effect of this treatment on HSV entry into C10 cells. Entry of a fluorescent reporter virus into C10 cells was almost completely blocked when the cells were incubated with the energy depletion medium for 15 min prior to the initiation of entry (Fig. 5). These data support an endocytic entry pathway for HSV into C10 cells.
FIG. 5.
Energy dependence of HSV entry into C10 cells. C10 cells were treated with energy depletion medium, and then the effect on virus entry was determined using a GFP reporter virus. The number of infected, GFP-expressing cells in the mock-treated sample was set to 100%.
Ultrastructural examination of HSV entry into C10 cells.
Because all of the above experiments provided indirect evidence of endocytic entry, we used electron microscopy (EM) for direct confirmation. C10 and B78 cells were incubated with virus for 45 min at 4°C, and then the temperature was raised to 37°C for up to 10 min or it was held at 4°C. Cells were then placed on ice, fixed, and processed for EM analysis.
At 4°C, virions on the surface of C10 cells were present in almost every case in small plasma membrane invaginations (Fig. 6A to C). The plasma membrane and virion envelope were clearly distinguished and lay apposed to each other (Fig. 6B and C). After entry was initiated by shifting the temperature to 37°C, many virions were intracellular yet were clearly enveloped and contained within a vesicle, consistent with endocytosis of intact particles (Fig. 6D to F). On receptor-negative B78 cells, virions were seen only on the cell surface. They were not present in membrane invaginations, but were peripherally associated with minimal membrane contact (Fig. 6G and H). These images confirm that HSV enters C10 cells by a gD receptor-dependent endocytic pathway.
FIG. 6.
EM analysis of HSV localization on C10 and B78 cells. The localization of HSV on C10 cells (A to F) and B78 cells (G and H) was examined by EM after virus attachment at 4°C (A to C, G, and H) or after initiation of entry by incubation at 37°C for 4 (D and F) or 10 min (E). Original magnification: ×25,000 (A); ×100,000 (B, C, and F); ×50,000 (D, E, G, and H). Bars, 100 nm.
HSV entry into C10 cells is not affected by ammonium chloride or BFLA.
Endocytic entry of HSV into CHO nectin-1 and HeLa cells is characterized by sensitivity to reagents that prevent endosome acidification. Accordingly, we asked whether entry into C10 cells requires a low endosomal pH. We used two mechanistically different reagents: BFLA and ammonium chloride. BFLA is an inhibitor of the vacuolar ATPase and prevents endosome acidification; ammonium chloride is a weak base that neutralizes the acidic endosomal environment. VSV was used as a control in these experiments. This virus enters cells by endocytosis and requires a low-pH environment within the endosome (47).
VSV infection of C10 cells was inhibited by both reagents in a dose-dependent manner, confirming that endosomal pH was raised (Fig. 7). Surprisingly and unlike the case with VSV, neither BFLA (Fig. 7A) nor ammonium chloride (Fig. 7B) affected HSV plaque formation on C10 cells at any concentration tested. Thus, in contrast to entry into CHO nectin-1 and HeLa cells, endocytic entry of HSV into C10 cells does not require a low-pH step.
FIG. 7.
Entry of HSV into C10 cells is not affected by bafilomycin A1 or ammonium chloride. C10 cells were treated with the indicated concentrations of bafilomycin A1 (A) or ammonium chloride (B) for 1 h. Virus was added in the presence of fresh inhibitor. After 90 min the cells were refed with medium containing the inhibitor. After a further 4 h, the inhibitor was washed away and a semisolid overlay was added. Plaques were counted at 24 h (for VSV) or 48 h (for HSV) postinfection.
DISCUSSION
In this paper, we describe a novel endocytic entry pathway for HSV into murine C10 cells. Initially, we used the membrane-impermeable chemical cross-linker BS3 to study the viral glycoproteins during virus entry. We found that, as a consequence of virus entry, virion gB became protected from the cross-linker. The most obvious explanation for this was rapid internalization of intact virions. Accordingly, we set out to test this hypothesis. Cumulatively, our data show that HSV entry into C10 cells occurs by an endocytic pathway with a number of novel features that distinguish it from the established endocytic entry pathway for this virus (see the model in Fig. 8).
FIG. 8.
Model of entry pathways. The figure depicts four pathways by which HSV can enter cells. Pathways A, C, and E result in viral gene expression, and pathway B does not. However, all four pathways are rapid and specific. A. Vero cells: receptor-dependent fusion between virus envelope and plasma membrane. B. CHO K1 cells: gD receptor-independent endocytic internalization of virions leading to degradation of virions. C. CHO nectin-1 cells: involvement of gD receptor in virus internalization is unknown. Acidification is required for successful egress of virions from endosome. D. B78 cells: no rapid internalization. Virions remain on the cell surface and are peripherally attached, without any cell surface alterations at point of contact. (Note that, by EM, virions remain on the surface of B78 cells after 10 min of incubation at 37°C and that no protection of viral glycoproteins from cross-linker is seen on these cells for as long as 30 min at 37°C. However, the ultimate fate of these cell surface virions is unknown.) E. C10 cells: gD receptor-dependent internalization preceded by enwrapment of virions by plasma membrane invaginations. Release of virions from endocytic vesicle does not require endosome acidification.
(i) Virus attachment.
EM analysis showed that virions attached to C10 cells are localized to plasma membrane invaginations that partially wrap the virion, seemingly as a prelude to endocytosis (Fig. 6A to C and 8E). This contrasts with the appearance of virions on B78 cells, which are peripherally associated with the cell, with no distortion of the apposed plasma membrane (Fig. 6G and H and 8D). The distinctive localization of virions on the C10 cell surface suggests that gD is functionally engaged with receptor under these conditions. We did not see any similarly localized virions on Vero cells (data not shown), and they have not been seen in published EM studies of entry of HSV and other herpesviruses by direct fusion (6, 15, 20). More significantly, the appearance of these virions is distinct from that seen during endocytic internalization of HSV into CHO nectin-1 and HeLa cells (A. Nicola, unpublished data), suggesting that HSV internalization on C10 cells occurs by a distinct process.
The peripheral attachment of virions to the B78 cell surface is most likely mediated by the well-characterized interactions between cell surface proteoglycans and the virion glycoproteins gB and gC (26, 42, 44). It probably accounts for the glycoproteins detected in control lanes of the proteinase K protection assays (Fig. 1B and 3A). The more intimate interaction between the virion and C10 cell surface is gD receptor dependent. Our data are consistent with the suggestion that HSV attachment occurs in two distinct stages: an initial weak attachment and a subsequent, rate-limiting, stable attachment (28).
(ii) Virus internalization.
Entry into C10 cells was initiated in our experiments by shifting the temperature from 4°C to 37°C. The consequence of this was protection of viral glycoproteins from cross-linking and proteinase K digestion within 2 to 4 min. This is consistent with rapid endocytic internalization of intact virions (Fig. 8E). Comparable results were obtained with B78A10 cells that express the gD receptor HVEM (data not shown), indicating that protection is not a nectin-1-specific phenomenon. No protection was seen in either assay on B78 cells that lack gD receptors, consistent with the peripheral attachment of virions to these cells and with their inability to support virus entry (Fig. 8D). Additionally, protection from cross-linking (Fig. 1C) and from proteinase K digestion (data not shown) was blocked by gD-specific neutralizing MAbs, but not by control MAbs. Thus, protection is strictly gD receptor dependent (Fig. 8D and E).
Protection from cross-linking required virus entry to occur. When we reduced the rate of entry by lowering the temperature, it took longer for virion gB to become protected. Presumably, at lower temperatures it takes longer for enough virions to be internalized to reach the limit of detection of the assay. Simultaneous reduction in the rate of entry and the rate of protection argues that the protected gB is derived from virions destined to initiate infection and not from a dead-end internalization.
To show that protection of the envelope glycoproteins from proteinase K digestion was a measure of endocytic internalization of virions, we used CHO K1, CHO nectin-1, and Vero cells in a protection assay. We saw protection only on CHO K1 and CHO nectin-1 cells, confirming that protection is a marker of virion endocytosis.
As with other endocytic processes, HSV internalization into C10 cells is energy dependent. Treatment of C10 cells with sodium azide and 2-deoxy-d-glucose almost completely blocked HSV entry. Similar treatment does not prevent HSV entry into Vero cells (36), ruling out any general inhibitory effects. As final confirmation that HSV enters C10 cells by endocytosis, we saw enveloped virions in vesicles within 10 min of the temperature shift. Few virions were seen on the C10 cell surface at this time. Consistent with the proteinase and cross-linking protection assays, we seldom saw intracellular virions in B78 cells. Detection of internalized particles only in C10 cells argues that virion endocytosis into these cells leads to productive entry. Together, these data show that internalization of HSV into C10 cells is a rapid, energy-dependent gD receptor-dependent endocytic process.
(iii) Postinternalization events.
A crucial step in endocytic entry of enveloped viruses is fusion of viral and cellular membranes leading to release of the nucleocapsid into the cytoplasm. In common with most other viral endocytic entry pathways, HSV entry into CHO nectin-1 and HeLa cells requires passage of the virion through an acidic environment (Fig. 8C) (36, 37). However, in contrast to this, neither BFLA nor ammonium chloride inhibited HSV infection of C10 cells. Thus, passage through an acidic compartment is not required for fusion of the HSV envelope with the endosomal membrane in C10 cells (Fig. 8E). Endocytic entry of enveloped viruses without a need for low pH is not unprecedented. Duck hepatitis B virus enters cells by receptor-dependent low-pH-independent endocytosis (3, 25, 41), and Epstein-Barr virus can enter cells via an endocytic route that is not affected by ammonium chloride (30). The low-pH independence of HSV entry into C10 cells may be explained by the occurrence of membrane fusion prior to acidification. Alternatively, the virus may pass through an acidified compartment even though low pH is not required for fusion. We do not know why HSV requires low pH in one cell type but not in another. One possibility is that the requirement for low pH is influenced by the repertoire of cellular receptors available.
We do not yet know which endocytic pathway is used by HSV on C10 cells. Entry into these cells can be inhibited by the cholesterol-sequestering reagent methyl-cyclodextrin, implying involvement of lipid rafts (1), and this treatment also inhibits protection of glycoproteins from BS3 (unpublished data). However, methyl-cyclodextrin also inhibits HSV entry into Vero cells, and there is no evidence of nectin-1 accumulation in rafts on C10 cells. Thus, the role of lipid rafts in the endocytic entry pathway of HSV into C10 cells remains uncertain, and further studies are required.
How is the choice made between endocytosis and direct fusion in the presence of receptor?
One fascinating question is how the choice is made between entry via direct fusion at the plasma membrane and by endocytosis via one of at least two pathways. In principle this might be host or virally determined. It has been suggested that a dense cortical cytoskeleton might prevent entry by direct fusion and favor endocytosis to circumvent this barrier (27, 46).
Transfection of C10 cells with plasmids encoding gB, gD, gH, and gL causes cell fusion (8), suggesting that all the cellular components necessary for the core viral fusion machinery to function are present on the C10 cell surface. This being the case, why doesn't the virus fuse with the plasma membrane on these cells? We know that on C10 cells endocytosis requires binding of gD to its receptor nectin-1. However, the gD-receptor interaction, though necessary, may not be sufficient to initiate endocytic internalization. Perhaps a subsequent virus-cell interaction, triggered by the binding of gD to its receptor, is required. In principle this might involve a second cellular receptor that actively directs virus to the endocytic entry pathway. The viral envelope glycoproteins are candidate viral ligands for such an interaction.
Multiple HSV entry pathways.
The ability of HSV to enter cells by multiple pathways maximizes the chances of achieving productive infection of a given cell type. The ability to enter cells by both endocytosis and direct fusion is not limited to HSV. Human cytomegalovirus enters fibroblasts by direct fusion with the plasma membrane (6) but enters retinal pigment epithelial and endothelial cells by endocytosis (2). Epstein-Barr virus enters normal B cells by endocytosis but enters epithelial cells by direct fusion at the cell surface (30).
An important question is whether the HSV entry pathway we have defined on C10 cells is seen on other cell types. Gianni et al. have identified numerous cell types, including Hep2 and BHK, which HSV enters via a pathway that is not affected by treatment with BFLA or ammonium chloride (19). In preliminary experiments, we have seen protection of virion gB from proteinase K during entry into BHK cells (unpublished data). Our results emphasize that pH independence, as defined by insensitivity to drugs such as BFLA and ammonium chloride, is not proof of entry by direct fusion and that additional methods should be employed to determine the HSV entry pathway on different cell types.
In summary, we have defined a third entry pathway for HSV, characterized by gD receptor-dependent endocytic internalization of virions that does not require passage of the virion through an acidic compartment for establishment of a productive infection. The identification of this pathway provides new opportunities for studying virus-cell interactions and emphasizes the remarkable adaptability of HSV.
Acknowledgments
This work was supported by Public Health Service grant AI-18289 from the National Institute of Allergy and Infectious Diseases (to G.H.C.), grant NS-36731 (to R.J.E.) from the National Institute of Neurological Disorders and Stroke, and grant AI-056045 (to R.J.E.) from the National Institute of Allergy and Infectious Diseases.
We thank Neelima Shah of the Biomedical Imaging Core at the University of Pennsylvania for highly skilled electron microscopic analysis and Claude Krummenacher for helpful comments on the project and the manuscript.
REFERENCES
- 1.Bender, F. C., J. C. Whitbeck, M. P. de. Leon, H. Lou, R. J. Eisenberg, and G. H. Cohen. 2003. Specific associations of glycoprotein B with lipid rafts during herpes simplex virus entry. J. Virol. 77:9542-9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodaghi, B., M. E. Slobbe-van Drunen, A. Topilko, E. Perret, R. C. Vossen, M. C. van Dam-Mieras, D. Zipeto, J. L. Virelizier, P. LeHoang, C. A. Bruggeman, and S. Michelson. 1999. Entry of human cytomegalovirus into retinal pigment epithelial and endothelial cells by endocytosis. Investig. Ophthalmol. Vis. Sci. 40:2598-2607. [PubMed] [Google Scholar]
- 3.Breiner, K. M., and H. Schaller. 2000. Cellular receptor traffic is essential for productive duck hepatitis B virus infection. J. Virol. 74:2203-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen, G. H., V. J. Isola, J. Kuhns, P. W. Berman, and R. J. Eisenberg. 1986. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J. Virol. 60:157-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colman, P. M., and M. C. Lawrence. 2003. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 4:309-319. [DOI] [PubMed] [Google Scholar]
- 6.Compton, T., R. R. Nepomuceno, and D. M. Nowlin. 1992. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191:387-395. [DOI] [PubMed] [Google Scholar]
- 7.Conner, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the cell. Nature 422:37-44. [DOI] [PubMed] [Google Scholar]
- 8.Connolly, S. A., D. J. Landsburg, A. Carfi, D. C. Wiley, G. H. Cohen, and R. J. Eisenberg. 2003. Structure-based mutagenesis of herpes simplex virus glycoprotein D defines three critical regions at the gD/HveA interface. J. Virol. 77:8127-8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai, P., and S. Person. 1998. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. 72:7563-7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimitrov, D. S. 2004. Virus entry: molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2:109-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenberg, R. J., D. Long, M. Ponce de Leon, J. T. Matthews, P. G. Spear, M. G. Gibson, L. A. Lasky, P. Berman, E. Golub, and G. H. Cohen. 1985. Localization of epitopes of herpes simplex virus type 1 glycoprotein D. J. Virol. 53:634-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenberg, R. J., M. Ponce de Leon, H. M. Friedman, L. F. Fries, M. M. Frank, J. C. Hastings, and G. H. Cohen. 1987. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb. Pathog. 3:423-435. [DOI] [PubMed] [Google Scholar]
- 13.Evan, G. I., G. K. Lewis, G. Ramsay, and J. M. Bishop. 1985. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 5:3610-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman, H. M., G. H. Cohen, R. J. Eisenberg, C. A. Seidel, and D. B. Cines. 1984. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b component of complement on infected cells. Nature 309:633-635. [DOI] [PubMed] [Google Scholar]
- 15.Fuller, A. O., and W. C. Lee. 1992. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J. Virol. 66:5002-5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuller, A. O., R. E. Santos, and P. G. Spear. 1989. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J. Virol. 63:3435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller, A. O., and P. G. Spear. 1987. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc. Natl. Acad. Sci. USA 84:5454-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geraghty, R. J., C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 19.Gianni, T., G. Campadelli-Fiume, and L. Menotti. 2004. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J. Virol. 78:12268-12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granzow, H., F. Weiland, A. Jons, B. G. Klupp, A. Karger, and T. C. Mettenleiter. 1997. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J. Virol. 71:2072-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handler, C. G., G. H. Cohen, and R. J. Eisenberg. 1996. Cross-linking of glycoprotein oligomers during herpes simplex virus type 1 entry. J. Virol. 70:6076-6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Highlander, S. L., D. J. Dorney, P. J. Gage, T. C. Holland, W. Cai, S. Person, M. Levine, and J. C. Glorioso. 1989. Identification of mar mutations in herpes simplex virus type 1 glycoprotein B which alter antigenic structure and function in virus penetration. J. Virol. 63:730-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, A., and R. Wagner. 1964. Penetration of herpes simplex virus into human epidermoid cells. Proc. Soc. Exp. Biol. Med. 116:863-869. [DOI] [PubMed] [Google Scholar]
- 24.Isola, V. J., R. J. Eisenberg, G. R. Siebert, C. J. Heilman, W. C. Wilcox, and G. H. Cohen. 1989. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J. Virol. 63:2325-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kock, J., E. M. Borst, and H. J. Schlicht. 1996. Uptake of duck hepatitis B virus into hepatocytes occurs by endocytosis but does not require passage of the virus through an acidic intracellular compartment. J. Virol. 70:5827-5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laquerre, S., R. Argnani, D. B. Anderson, S. Zucchini, R. Manservigi, and J. C. Glorioso. 1998. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J. Virol. 72:6119-6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsh, M., and R. Bron. 1997. SFV infection in CHO cells: cell-type specific restrictions to productive virus entry at the cell surface. J. Cell Sci. 110:95-103. [DOI] [PubMed] [Google Scholar]
- 28.McClain, D. S., and A. O. Fuller. 1994. Cell-specific kinetics and efficiency of herpes simplex virus type 1 entry are determined by two distinct phases of attachment. Virology 198:690-702. [DOI] [PubMed] [Google Scholar]
- 29.Miller, C. G., C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and N. W. Fraser. 2001. Development of a syngeneic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol. Ther. 3:160-168. [DOI] [PubMed] [Google Scholar]
- 30.Miller, N., and L. M. Hutt-Fletcher. 1992. Epstein-Barr virus enters B cells and epithelial cells by different routes. J. Virol. 66:3409-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milne, R. S. B., S. L. Hanna, A. H. Rux, S. H. Willis, G. H. Cohen, and R. J. Eisenberg. 2003. Function of herpes simplex virus type 1 gD mutants with different receptor-binding affinities in virus entry and fusion. J. Virol. 77:8962-8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minson, A. C., T. C. Hodgman, P. Digard, D. C. Hancock, S. E. Bell, and E. A. Buckmaster. 1986. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J. Gen. Virol. 67:1001-1013. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 34.Muggeridge, M. I. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J. Gen. Virol. 81:2017-2027. [DOI] [PubMed] [Google Scholar]
- 35.Muggeridge, M. I., V. J. Isola, R. A. Byrn, T. J. Tucker, A. C. Minson, J. C. Glorioso, G. H. Cohen, and R. J. Eisenberg. 1988. Antigenic analysis of a major neutralization site of herpes simplex virus glycoprotein D, using deletion mutants and monoclonal antibody-resistant mutants. J. Virol. 62:3274-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 77:5324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicola, A. V., and S. E. Straus. 2004. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J. Virol. 78:7508-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelkmans, L., and A. Helenius. 2003. Insider information: what viruses tell us about endocytosis. Curr. Opin. Cell Biol. 15:414-422. [DOI] [PubMed] [Google Scholar]
- 39.Peng, T., M. Ponce de Leon, M. J. Novotny, H. Jiang, J. D. Lambris, G. Dubin, P. G. Spear, R. J. Eisenberg, and G. H. Cohen. 1998. Structural and antigenic analysis of a truncated form of the herpes simplex virus glycoprotein gH-gL complex. J. Virol. 72:6092-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 41.Rigg, R. J., and H. Schaller. 1992. Duck hepatitis B virus infection of hepatocytes is not dependent on low pH. J. Virol. 66:2829-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rux, A. H., H. Lou, J. D. Lambris, H. M. Friedman, R. J. Eisenberg, and G. H. Cohen. 2002. Kinetic analysis of glycoprotein C of herpes simplex virus types 1 and 2 binding to heparin, heparan sulfate, and complement component C3b. Virology 294:324-332. [DOI] [PubMed] [Google Scholar]
- 43.Showalter, S. D., M. Zweig, and B. Hampar. 1981. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect. Immun. 34:684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shukla, D., and P. G. Spear. 2001. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Investig. 108:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535-1545. [DOI] [PubMed] [Google Scholar]
- 46.Smith, A. E., and A. Helenius. 2004. How viruses enter animal cells. Science 304:237-242. [DOI] [PubMed] [Google Scholar]
- 47.Superti, F., L. Seganti, F. M. Ruggeri, A. Tinari, G. Donelli, and N. Orsi. 1987. Entry pathway of vesicular stomatitis virus into different host cells. J. Gen. Virol. 68:387-399. [DOI] [PubMed] [Google Scholar]
- 48.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wanas, E., S. Efler, K. Ghosh, and H. P. Ghosh. 1999. Mutations in the conserved carboxy-terminal hydrophobic region of glycoprotein gB affect infectivity of herpes simplex virus. J. Gen. Virol. 80:3189-3198. [DOI] [PubMed] [Google Scholar]
- 50.Wittels, M., and P. G. Spear. 1990. Penetration of cells by herpes simplex virus does not require a low pH-dependent endocytic pathway. Virus Res. 18:271-290. [DOI] [PubMed] [Google Scholar]
- 51.Yasamura, Y., A. H. Tashjian, Jr., and G. H. Sato. 1966. Establishment of four functional, clonal strains of animal cells in culture. Science 154:1186-1189. [DOI] [PubMed] [Google Scholar]