Abstract
Poor wound healing on the face and neck can lead to significant morbidity and dissatisfaction in facial plastic surgery. With current advances in wound healing management and commercially available biologic and tissue engineered products, there are several options available to optimize acute wound healing and treat delayed or chronic wounds. This article summarizes some of the key principals and recent developments in wound healing research in addition to potential future advancements in the field of soft tissue wound healing. Unique adjuncts available to facial plastic surgeons for management of poorly healing or chronic wounds include modern and smart dressings, skin substitutes, negative pressure wound therapy, recombinant growth factor products and stem cells, platelet rich plasma, hair follicle transplantation, exogenous wound bed electrical stimulation, hyperbaric oxygen therapy, and technology and dressings for early detection and prevention of wound infections.
Keywords: Wound healing, Delayed healing, Chronic wounds, Hypertrophic Scar, Tissue Engineered Skin
Introduction
Wound healing is an important concept for the facial plastic surgeon. In order to optimize wound healing, one must understand the normal sequence of acute wound healing in addition to factors that contribute to delayed and chronic healing. In this review, we highlight current techniques for minimizing surgical scars and advances in dressings to optimize wound healing. We also discuss recent discoveries in wound healing research pertaining to tissue engineering, biologic products, and stem cell therapy as well as adjuncts and technology available to the facial plastic surgeon to promote healing of chronic wounds.
Principles of Wound Healing
Overview of Normal Healing
The normal sequence of acute wound healing involves a complex overlapping cascade of biological events orchestrated by cytokines, growth factors, various cell types, and structural elements. The first stage called hemostasis is triggered immediately by tissue injury. This is followed by the inflammatory phase which combats infection. Next, the proliferation stage promotes wound repair through granulation tissue formation, neovascularization, and reepithelialization. The final stage of wound healing is the maturation or remodeling phase during which scar remodeling occurs. Scar maturation begins about 3 weeks after tissue injury and continues up to 12 months later. It is imperative that this normal sequence of events occur in order for wounds to properly heal. Delays in any one of the core phases of acute healing will ultimately pause all subsequent phases and result in delayed or chronic healing wounds.
Approaching a Delayed or Chronic Wound
A delayed wound results when there is cellular dysregulation of the acute healing phase resulting in persistent inflammation. Clinical signs of a poorly healing wound include persistent erythema and inflammation for longer than 7 days, increased exudate, odor, delayed epithelialization, and surrounding skin maceration.1 Wounds remaining in a delayed or poorly healing state for longer than 3 weeks are at risk of transforming into a chronic wound and may exhibit persistent inflammation and infection, necrosis, and wound dehiscence.
The first step in treating a poorly healing wound is systematic wound assessment including identification and removal of risk factors for poor wound healing (Table 1) and determining which clinical state the wound is in: normal acute healing, delayed healing, or chronic healing. After adequate assessment, a comprehensive strategy for wound management1 includes: 1) adequate debridement and cleansing, 2) effective exudate management, 3) restoration of proper moisture and bacterial balance, 4) promotion of granulation tissue formation with a moist environment and re-epithelialization by maintaining fresh wound edges, 5) selection of appropriate dressings, and 6) periodic reassessment with dressing modification as indicated. With proper wound care, chronic wounds should begin to show signs of improvement within 2 to 4 weeks. If the wound does not improve in 4 weeks, the patient should be re-evaluated to ensure proper nutrition, adequate blood supply, appropriate wound care and dressings, protection from injury, sufficient control of wound bacterial contamination, and the need to rule out a neoplastic process.
Table 1:
Risk factors for poor wound healing
| Extrinsic factors | Intrinsic factors |
|---|---|
| Previous irradiation | Malnutrition |
| Chronic steroid use | Obesity |
| Cigarette smoking | Diabetes |
| Vaping | Hypothyroidisn |
| Medications (ex. NSAIDS, aspirin, chemotherapeutic drugs, methotrexate, cocaine, heparin, warfarin, penicillamine) | Ischemic conditions (anemia, COPD, vascular disease, CHF, renal failure) |
| Alcoholism | |
| Immunodeficiency |
Current and Developing Therapies for Optimizing Wound Healing
Minimizing Surgical Scars
Although scarring is an inevitable natural consequence of wound healing, any prolongation in the wound healing process, particularly the inflammatory phase, increases the risk of unfavorable scarring. It is also important to emphasize that scars, especially on the face and neck can be very debilitating, both functionally, aesthetically, and psychosocially. For patients, the aesthetic appearance of a scar is often the most important factor in judging surgical success; therefore, optimization of scar appearance is critical when making and closing incisions and during the acute healing process.
Traditional approaches used by surgeons to optimize scar appearance involve appropriate preoperative planning including placement of incisions along Langer’s lines or relaxed skin tension lines on the head and neck as shown in Figure 1 and carrying those incisions perpendicularly through epidermis, dermis, and subcutaneous tissue. One of the main triggers for decreased collagen extracellular matrix organization during the inflammatory phase of wound healing, delaying wound regeneration and resulting in hypertrophic scar, is wound tension.2 Therefore, it is also important when closing skin incisions to place deep anchor sutures to bring skin edges closer together and minimize tension across the wound as well as superficial sutures with adequate wound edge eversion. Tension offloading is also accomplished by using simple dressings such as paper or adhesive tape. A comprehensive review by O’Reilly et al3 reported that early application of non-stretch tapes directly over linear scars as early as one week postoperatively is effective in prevention of hypertrophic or abnormal scar development with significantly reduced scar characteristics such as height, color, and itch, and overall improved scar rating. Most studies utilized tape therapy for 12 weeks; however longer duration of therapy may further enhance scar appearance. Scar thickness, pliability/softness, and color change were also superiorly rated with the use of non-stretch tapes on mature scars present from one month to 5 years, with one study even reporting a scar age of 20 years.
Figure 1:
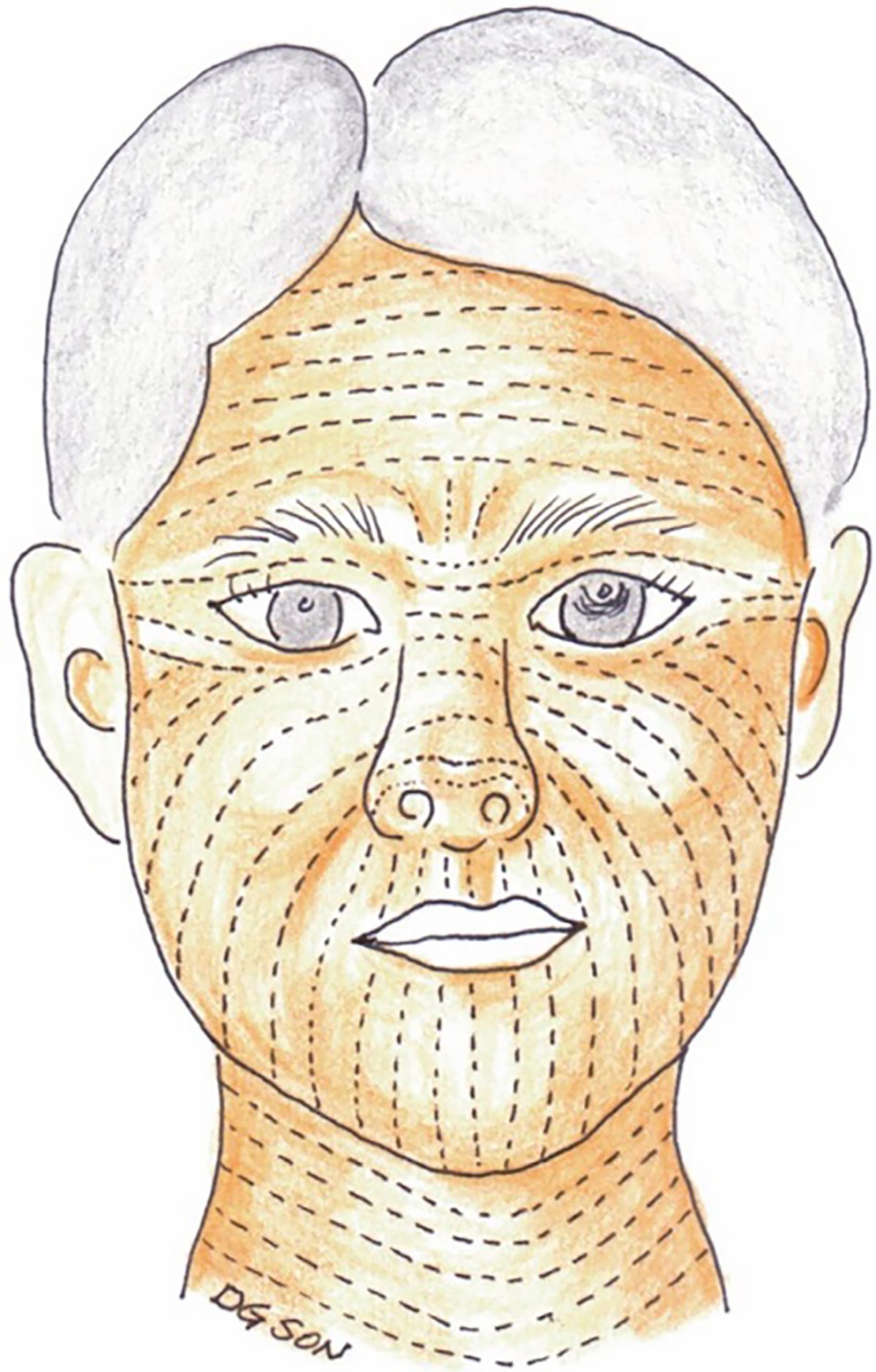
Relaxed skin tension lines (RSTLs) of the head and neck. These lines represent furrows created in the skin when the skin is pinched and relaxed without tension. Skin tension can be reduced when incisions are made parallel to RSTLs resulting in improved cosmesis and decreased risk of hypertrophic scarring. Langer’s lines (not shown) represent the direction of orientation of the underlying collagen fibers and run in the same direction as RSTLs in many areas of the body with the exception of the temple, lateral canthal area, and oral commissure.
(Reprinted with permission from Son D, Harijan A. Overview of surgical scar prevention and management. J Korean Med Sci. 2014 Jun;29(6):751–7. doi: 10.3346/jkms.2014.29.6.751. Epub 2014 May 30. PMID: 24932073; PMCID: PMC4055805.)
Another key concept which was originally discovered in the 1960s was that dry scabs retard epithelialization and thus, keeping wounds moist accelerates re-epithelialization two fold.4 In addition to promoting wound healing, a moist wound environment also facilitates autolytic debridement, reduces pain, activates collagen synthesis, and supports the presence and function of growth factors and other soluble mediators in the wound microenvironment.5 These findings have led to the development of moisture retentive dressings to aid in wound healing. One example is the use of silicone gel sheeting as a method of controlling dermal hydration and improving scar outcome. Silicone sheeting does not rely on pressure but instead provides a moist environment that mimics the function of the stratum corneum and is therefore thought to aide in homeostasis of the scar. Current practical guidelines recommend silicone sheets and gels as the gold standard, first-line, non-invasive therapy to begin about 1 month after incision closure to reduce scarring, especially in individuals prone to scarring.6,7
Additional recommendations for optimal scar management include avoidance of direct sun exposure, use of daily sunscreen of at least 30 SPF, compression therapy, scar massage, and the use of moisturizers.
Topical Treatments for Hypertrophic Scar Management
Non-invasive management of cutaneous scarring includes topical treatments, many of which are over the counter. Silicone-based topicals are widely accepted and effective in prevention of dryness and improving scar cosmesis. Examples of natural plant based topicals which have proven to be effective in some studies, but remain controversial are aloe vera, vitamin E, green tea extract, and onion extract. Partial thickness burns treated with aloe vera resulted in faster rate of reepithelialization and faster healing compared to those treated with silver sulfadiazine8,9 or petroleum jelly gauze.10 Topical aloe vera gel has also been shown to accelerate split-thickness skin graft donor site healing in a randomized, double-blinded, controlled trial.11 Green tea and onion extract have both been shown to have potent anti-oxidant as well as anti-inflammatory properties to prevent overproduction of collagen in clinical trials.12 Despite several studies reporting positive outcomes with onion extract products, clinical efficacy is still debatable as Mederma Skin Care gel (Mers Pharmaceuticals, Greensboro, NC, USA), whose active ingredient is an onion extract, failed to show clinical benefit in scarring in an rabbit ear model and human study involving Mohs microsurgical patients.13 Vitamin E is another natural ingredient commonly used in topical moisturizers and creams for its antioxidant properties and benefit in improving photo-aged skin. It is commonly recommended by clinicians to aid in wound healing; however, evidence in the literature for its efficacy in scar appearance is scarce.14 Similarly, vitamin D has not been shown to be effective in reducing scaring. Moist exposure burn ointment (MEBO®, Julphar Gulf Pharmaceutical Industries, Ras Al- Khaimah, UAE) which is commonly used for burn wounds, has been shown to be effective in improving facial scar formation when applied to wounds closed primarily compared to those receiving either an antibiotic ointment or no treatment.15 The same authors also demonstrated improved healing of split thickness skin graft donors sites following treatment with MEBO.16
Injection Treatments for Hypertrophic Scar Management
Corticosteroid injections are highly effective in the management of hypertrophic scars and keloids. The mechanism of action is believed to be related to reduced fibroblast collagen production and inhibition of inflammation. Typically triamcinolone acetonide (concentration of 5–40 mg/mL depending on the location) is injected directly into the fibrous portion of the scar with care to avoid direct injection in to the subcutaneous fat or surrounding skin to prevent unwanted side effects such as skin atrophy, depigmentation, and telangiectasias.17 In regards to the facial plastic surgeon, recent literature has shown improved outcomes when using intralesional steroid to treat microstomia from perioral scarring in burn patients18 and post-surgical “pincushioning” or early hypertrophic scarring in the nasal alar region following transposition flap.19
Botulinum toxin type A (BTXA) is well known for its muscle chemodenervation properties and is commonly used in facial rejuvenation and aesthetic procedures to treat dynamic rhytids and the aging face. When applied to wound healing, botulinum toxin has been used to immobilize mobile facial areas and subsequently decrease tension across the wound. Use of BTXA has been associated with improved scar appearance compared to placebo after injection adjacent to forehead excisional wounds on monkeys;20 however, efficacy was not replicated in a human trial during which patients with forehead lacerations received either botulinum toxin or placebo at the time of suture repair.21 One possible explanation for these results, is that due to delayed onset of muscle inactivity, BTXA injections must be performed 1 to 2 weeks prior to anticipated incision or laceration in order to have effect at the time of acute healing.22 In addition to BTXA induced wound immobility, in vitro studies and animal studies have also shown that BTXA may promote wound healing through inhibition of neurotransmitters which ultimately result in downstream inhibition of inflammatory mediators, decreased fibroblast proliferation, and decreased expression of growth factors mediating fibrosis, resulting in a decrease in hypertrophic scarring.22–24 A prospective clinical study of 19 patients undergoing monthly BTXA injections for 3 months showed significant improvement in patient and physician rated erythema, pruritus, and pliability scores.25
Other injectable scar modulators such as 5-fluorouracil (5-FU) and bleomycin which have been used for non-surgical treatment of keloid lesions and surgical excision or scar revision are beyond the scope of this chapter.
Advances in Wound Dressings
With numerous commercially available dressings now on the market, one may ask “what is the ideal wound dressing?” Well, the ideal dressing is one that maintains a moist wound environment, allows for adequate gas exchange, absorbs excess exudate, provides proper mechanical strength and protects the surrounding skin. Another ideal property of wound dressings is that they are nonadherent and easily removable to prevent increased pain associated with removing intertwined sections of the dressing from wounds which results in increased inflammation and disturbs the process of healing.26
Gauze dressings made out of woven or nonwoven cotton, rayon, and polyesters comprise traditional wound dressings and are capable of absorbing exudates and fluid in addition to some limited protection against infection. Disadvantages of gauze dressings include need for frequent replacement and adherence to the wound bed. Other types of traditional dressings include cotton or synthetic bandages which can be useful for wounds requiring compression and non-occlusive petroleum-based dressings (ex. Xeroform™). When used, traditional dressings are typically indicated for clean, dry wounds with mild exudate or as secondary dressings. Since traditional dressings fail to provide a moist wound environment, they have been largely replaced with modern dressings types which are capable of actively promoting healing through moisture retention.27
Modern dressings are interactive dressings, usually derived from synthetic based products that prevent dehydration of a wound. With current advances in modern wound dressings, classification of available products and indications for selection are provided (Table 2). Dressings are now enhanced with silver and zinc molecules in addition to polysaccharides such as chitosan, chitin, alginate, hyaluronic acid, and cellulose to provide better water absorbency and anti-microbial properties.28 For delayed or chronic wounds, appropriate dressings are selected based on the wound characteristics and can be modified as the wound progresses through various stages of healing. Foam dressings have been shown to reduce the risk of hospital acquired pressure ulcers29 and have been used to treat chronic wounds in head and neck cancer patients with severe radiation dermatitis in combination with recombinant human epidermal growth factor spray.30
Table 2:
Classification of current FDA-approved moisture retentive wound dressings
| Type of dressing | Description | Characteristics | Indications | Examples |
|---|---|---|---|---|
| Film | Thin, transparent, self-adhesive, porous polyurethane | Elastic, conforms to shape, autolytic debridement properties, moderate water vapor transmission rate, no absorptive capacity, impermeable to liquids and bacteria | Wounds with complex shape, superficial and shallow wounds with low-level exudate, epithelializing wounds | Tegaderm™, Opsite™ |
| Hydrogel | Three-dimensional structure composed of insoluble hydrophilic smaterials made from synthetic polymers | Rehydrate and maintain moist environment, high water vapor transmission rate, low to no absorptive capacity | Full and partial-thickness, dry, non-exudative wounds, necrotic wounds, pressure ulcers and burn wounds | Intrasite™, Nu-gel™, Aquaform™ Curagel |
| Hydrocolloid | Inner hydrophilic gel layer made of colloidal particles and outer polyurethane impermeable layer | Low water vapor transmission rate, moderate absorption, impermeable to bacteria, debridement properties | Shallow wounds, low to medium exudating wounds, pediatric wound management (do not cause pain on removal) | Comfeel™, Tegasorb™, Granuflex™ Aquacel |
| Hydrofiber | Contain sodium carboxymethyl cellulose woven into textile fibers | Aids in autolytic debridement, high absorption capacity | Heavy exuding wounds | Aquacel Hydrofiber |
| Foam | Hydrophobic polyurethane outer film and hydrophilic wound-facing foam | Moderate water vaper transmission rate, semipermeable, moderate to high absorption, thermal insulation, antimicrobial activity | Moderate exuding wounds, infected wounds | Allevyn™, Mepilex™, Lyofoam™ |
| Alginate | Fibrous products derived from brown seaweed that form a gel after binding to wound exudate | High water vapor transmission rate, moderate to high absorption capacity, hemostatic | Highly exudative wounds | Algisite™, Kaltostat™, Sorbsan™ Tegagen |
The future of wound dressings is the transition from passive to smart dressings that can not only protect a wound but actively monitor the status of the wound and provide real time feedback. Advances in microfabrication technologies aim to develop products that closely mimic the native skin tissue environment and have temperature, oxygen, CO2, pH, moisture, and enzymatic sensing capabilities. Smart dressings with such microsensors could be beneficial to healthcare providers and patients by allowing close wound monitoring without the need of removing dressings or in person clinic visits and could also be used for drug application. Future smart bioactive dressings made of smart materials may also have the ability to alter their structure/physiochemical properties in response to environmental change providing in vivo treatment of wounds.26 Pang et al. developed a smart flexible electronics integrated dressing capable of temperature monitoring and UV light emittance allowing for early detection of infection and on-demand treatment via a UV-responsive antibacterial hydrogel in an animal model.31 Similarly, Qiao et al. employed pH-responsive fluorescent nanoprobes into a smart hydrogel dressing to diagnose bacterial infection.32
Tissue Engineered Skin
Numerous bioengineered skin products, also known as bioactive wound dressings, are now available for treatment of acute and chronic partial and full thickness wounds, including burn wounds, surgical wounds, and diabetic wounds. Table 3 lists examples of currently available skin substitutes, their composition, limitations, and current indications for use. Overall, these bioengineered skin substitutes can be expensive, limiting widespread use, but can be cost effective when considering reduced length of hospital stay and the potential for reduced time to complete wound closure. Bioengineered skin substitutes have also been increasingly used to overcome the disadvantages of skin grafting and associated donor site morbidity. Current applications for tissue substitutes in the head and neck literature include reconstruction of large scalp33,34 and face/temporal region defects, radial forearm free flap donor site coverage,35 nasal reconstruction,36–38 and intraoral mucosal (buccal mucosa, floor of mouth, and lateral/ventral tongue) reconstruction39,40 after oncologic resection. Use of Integra® bilayer wound matrix (Integra LifeSciences, Plainsboro, New Jersey) has been described as a reasonable reconstructive solution for denuded avascular wounds including irradiated tissue, bare calvarial bone (Figure 2), and cartilage, either combined with subsequent epidermal autografting or as a single staged procedure.33,36 When used as a staged reconstruction in conjunction with skin grafting, one may accomplish enhanced skin tone matching and improved cosmesis; however, single staged procedures may be beneficial for elderly patients, patients with multiple medical comorbidities, or those who prefer to avoid a second surgery.36,38
Table 3:
FDA approved bioengineered skin equivalents
| Product | Description | FDA Indication | Limitations |
|---|---|---|---|
|
| |||
| Acellular Dermal Matrix | |||
| AlloDerm® | Freeze dried cadaver skin with preserved basement membrane | Considered “banked human tissue” and does not require FDA approval | Uncertain vascularization rates, no cells present in scaffold, safety concerns of allogeneic disease transfer |
| AlloMax™ | Non-crosslinked human dermis | Considered “banked human tissue” and does not require FDA approval | No cells present in scaffold, safety concerns of allogeneic disease transfer |
| FlexHD® | Hydrated acellular human skin | Considered “banked human tissue” and does not require FDA approval, marketed as hernia mesh | No cells present in scaffold, safety concerns of allogeneic disease transfer |
| DermaMatrix™ | Allograft derived from human cadaveric skin | Considered “banked human tissue” and does not require FDA approval | No cells present in scaffold, safety concerns of allogeneic disease transfer |
| Graftjacket® | Pre-meshed acellular human dermis | Considered “banked human tissue” and does not require FDA approval | No cells present in scaffold, safety concerns of allogeneic disease transfer |
| Amniotic Membrane | |||
| EpiFix® | Dehydrated amniotic membrane in sheets | Considered “banked human tissue” and does not require FDA approval | Safety concerns of allogeneic disease transfer, unclear effect on angiogenesis |
| Amniofix® | Injectable micronized dehydrated amniotic membrane | Considered “banked human tissue” and does not require FDA approval | Safety concerns of allogeneic disease transfer, unclear effect on angiogenesis |
| Collagen Scaffold | |||
| OASIS® Wound Matrix | Xenogeneic collagen scaffold derived from porcine small intestine | Partial and full thickness wounds; diabetic, pressure, and venous stasis ulcers; surgical wounds, traumatic wounds | Requires multiple applications |
| Living Cell Therapy | |||
| Apligraf® | Bilayered construct w/ human neonatal fibroblasts cultured in bovine type 1 collagen matrix and human neonatal keratinocytes forming the superficial layer of the graft-mimics normal structure of skin | Non-infected, partial and full thickness skin ulcers due to venous insufficiency; nonresponsive full thickness neuropathic diabetic ulcers | Shelf life of 5 days, risk of disease transfer from allogeneic cells, cannot be used for definitive wound closure in full thickness wounds, requires co-grafting with an autologous epithelium |
| Dermagraft® | Cryopreserved polyglactin mesh seeded w/ human neonatal foreskin fibroblasts | Diabetic foot ulcers | Requires multiple applications, safety concerns with allogeneic cells |
| Epicel | Cultured epithelial autograft | Deep dermal or full thickness burns | Requires long culture times, short shelf-life, variable take rate |
| OrCel® | Bilayered construct w/ human neonatal foreskin fibroblasts and keratinocytes seeded in bovine type 1 collagen sponge | Donor site wounds in bum victims; dystrophic epidermolysis bullosa reconstructive surgery | Allogeneic cell source risk of disease transfer |
| Biosynthetic | |||
| Biobrane® | Temporary wound dressing consisting of nylon imbedded silicon film | Partial thickness burn wounds, donor sites, dressing over meshed autografts | Non-degradable/synthetic and must be removed after 7–14 days |
| Integra | Bovine type I collagen and shark chondroitin-6-sulphate glycosaminoglycan dermal component and a silicone epidermal layer |
Post-excisional treatment of deep partial thickness or full thickness due to thermal injury | Requires about 10–14 days for appropriate vascularization and require second procedure for complete wound closure |
| Trans Cyte® | Temporary wound dressing consisting of human dermal fibroblasts grown on nylon mesh containing porcine collagen | Temporary covering over burns until autografting procedure or healed. | Non-degradable/synthetic and must be removed after 7–14 days |
(Reprinted with permission from K.J. Miller, et al., MicroRNAs in skin tissue engineering, Adv. Drug Deliv. Rev. (2015), http://dx.doi.org/10.1016/j.addr.2015.04.018.)
Figure 2:
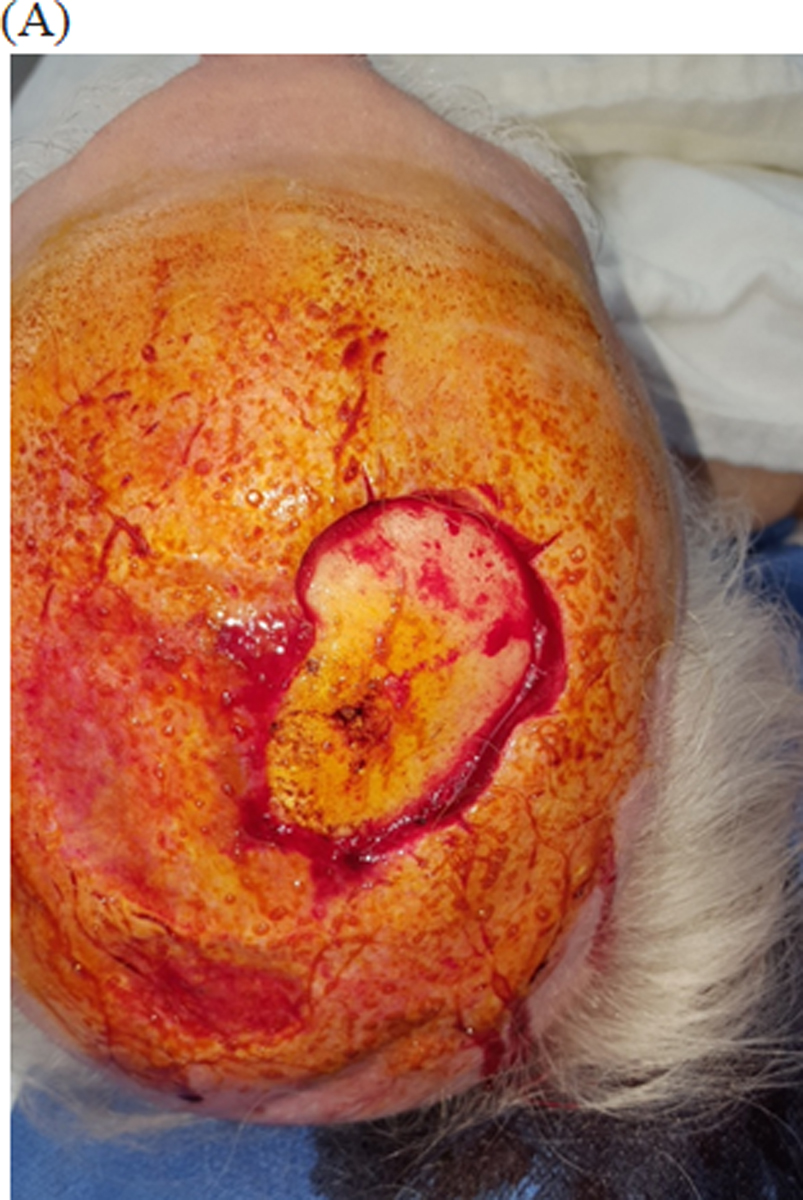
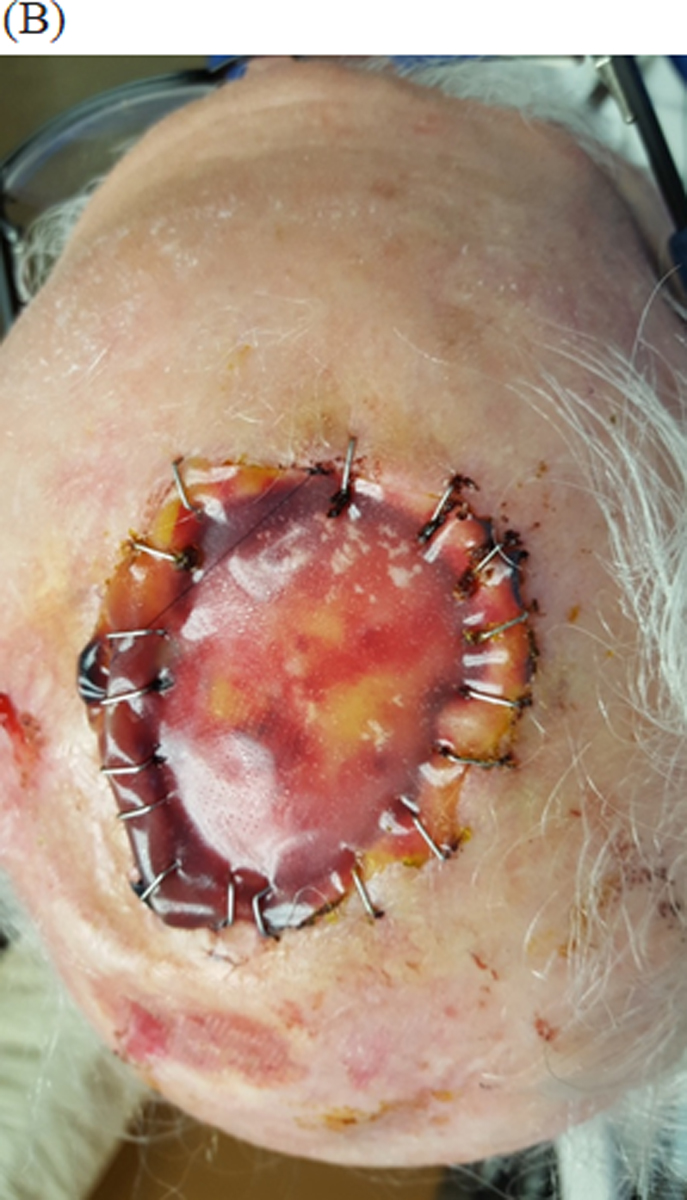
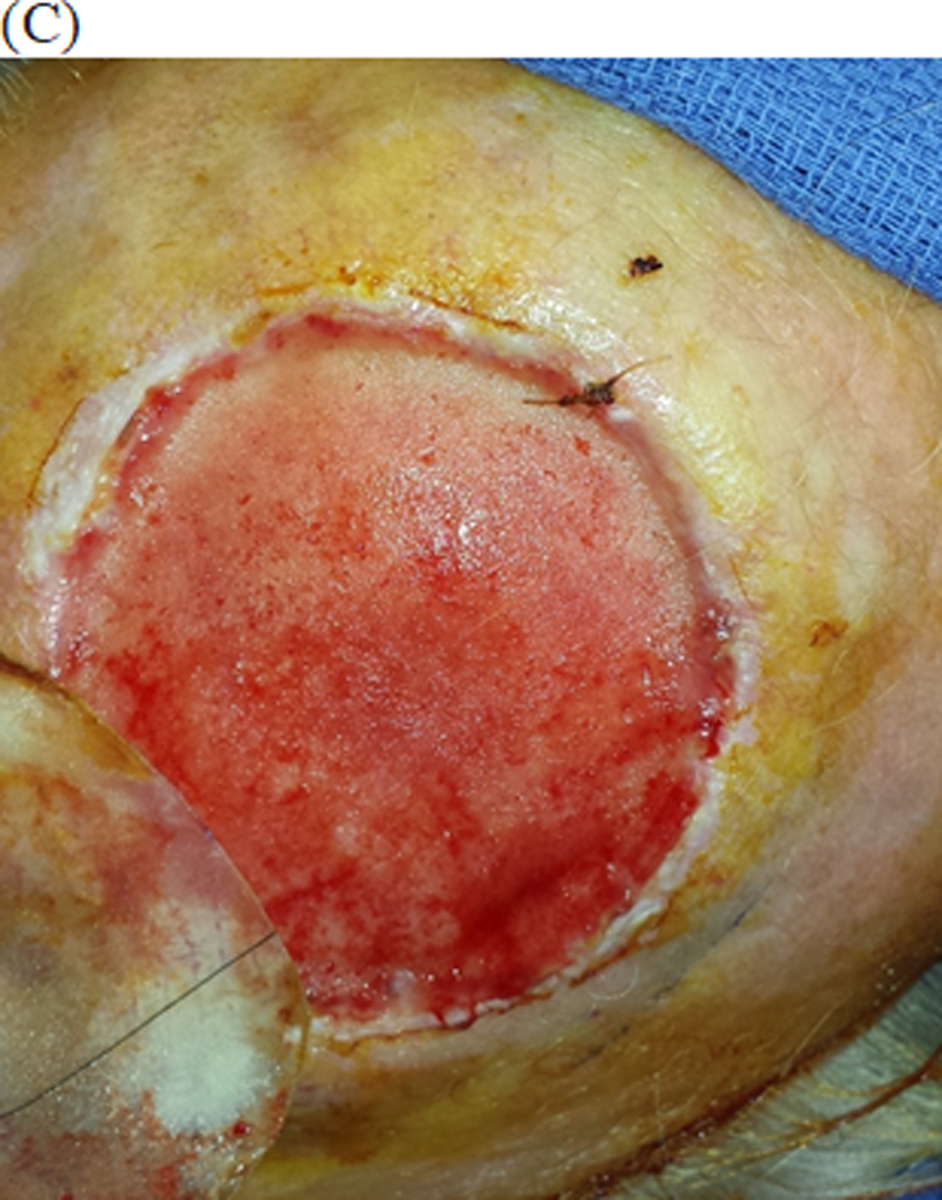
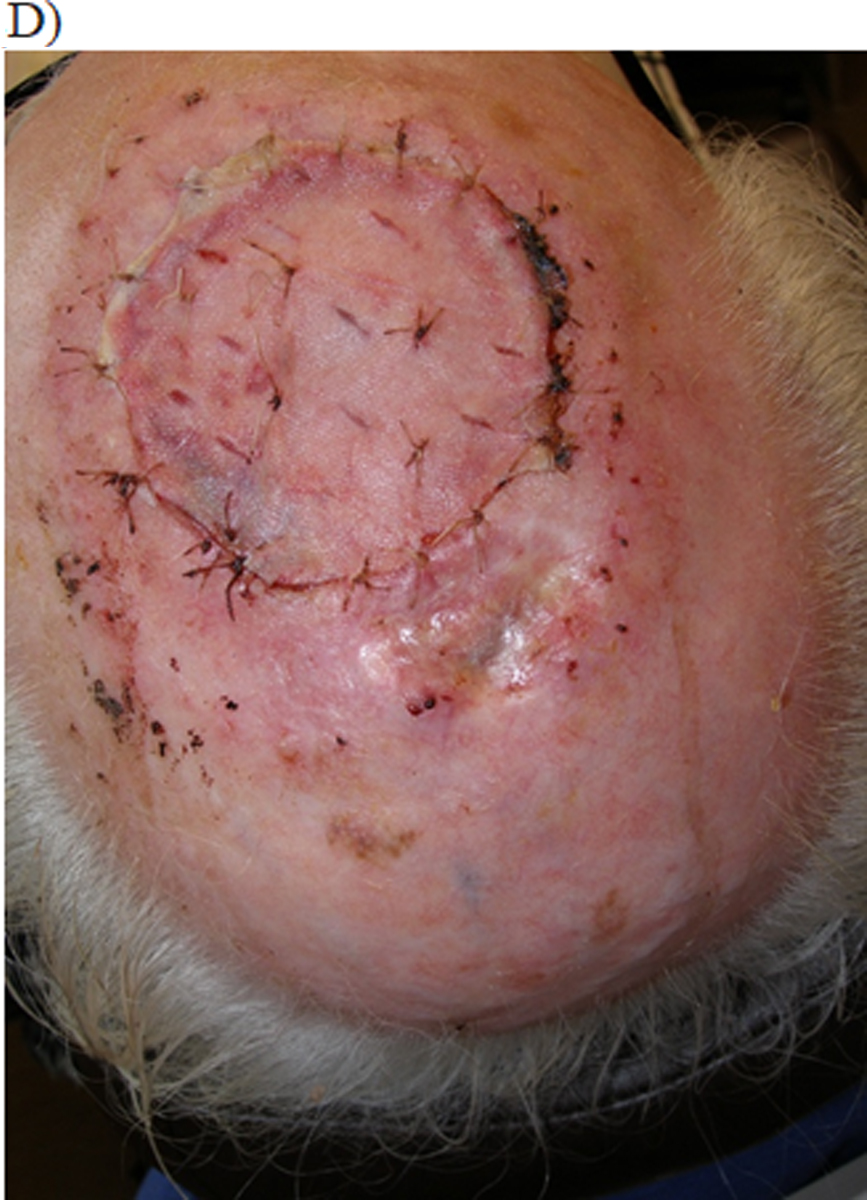
Integra bilayer used for reconstruction of scalp defect
(A) 72 year male with bare calvarial bone defect after Mohs excision of basal cell carcinoma. (No periosteum present)
(B) In a staged fashion Integra dressing was applied and secured for 1 month to induce granulation.
(C) After 1 month, enough granulation tissue growth to receive a split thickness skin graft.
(D) Two weeks after split thickness skin graft placement with good take of skin graft.
Negative pressure wound therapy
Negative pressure wound therapy (NPWT) has been shown to be an effective treatment in accelerating wound healing and has been implemented in the management of infected wounds, chronic wounds, open staged wounds, and post irradiated wounds. Its widespread use in the head and neck however has been limited due to the challenge of securely placing the device and maintaining an appropriate seal along various surface contours on the head and neck.1 Recent studies, however, suggest that application of NPWT reduces donor site morbidity following radial forearm free flap reconstruction41 and may aid in skin graft fixation,42 pharyngocutaneous fistula following total laryngectomy,43 and length of treatment of deep neck abscesses.44
NPWT consists of placing a polyurethane foam sponge into the depth of a wound and sealing the wound with an adherent, occlusive outer dressing that is connected to a vacuum pump to deliver controlled negative pressure to the wound bed. Proposed mechanisms of its role in accelerating the healing process include removal of excess fluid from the wound bed, decreased bacterial burden, decreased interstitial edema, increased vascularity and promotion of granulation tissue, stimulation of fibroblast and endothelial cell proliferation, and mechanical contracture of the wound bed.45 Traditional contraindications to using NPWT in the head and neck region include avoidance of direct contact over vessels, application to the nasal, oral, or tracheal airways, and use over neoplastic sites. Asher et al. proposes that NPWT can be successfully used even in complex wounds including those with salivary contamination, exposed bone, great vessel exposure, vascular pedicle exposure after free tissue transfer, and peristomal application after total laryngectomy under close supervision. From their experience, the authors recommend initial negative pressure dressing placement in the operating room after wound inspection and debridement with subsequent dressing changes either in the operating room or at bedside depending on patient preference.45
Growth factors and stem cells
Growth factors are endogenous molecules involved in cell growth, proliferation, migration, and differentiation and are essential to normal wound healing. Growth factor deficiencies, including platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF) have been reported in non-healing acute and chronic wounds.46 With the advances in genetic engineering and biotechnology, exogenous or recombinant growth factors have been studied as a potential adjunct to improving non-healing wounds. The first and only US Food and Drug Administration (FDA) approved recombinant growth factor product, becaplermin (recombinant human platelet-derived growth factor-BB [rhPDGF]; Regranex, 0.01% gel; Ortho-McNeil Pharmaceutical Inc, Raritan, New Jersey) was introduced in 1998 for the treatment of nonischemic diabetic neuropathic ulcers.47 RhPDGF is believed to increase fibroblast replication, induce fibroblast collagenase production, stimulate development of granulation tissue and extracellular matrix components (glycosaminoglycans and proteoglycans), and aid in angiogenesis.48 Since its approval, recombinant human PDGF has been used as an off label treatment to induce granulation tissue formation in refractory, poorly healing wounds such as irradiated dermal wounds49 and pharyngocutaneous fistulas50 with some success. A systematic review and meta-analysis by Li et al51 examined the use of human recombinant growth factors in orofacial bone regeneration and periodontal wound healing and suggested that 0.3 mg/ml rhPDGF-BB was safe and effective for the healing of tooth extraction sockets, but was insufficient in sinus augmentation or alveolar reconstruction in cleft lip/palate patients. Other commercially available growth factor products include Trafermin (recombinant human basic fibroblast growth factor [rhbFGF]; Fiblast® Spray; PSEU-Kaken Pharmaceutical, Japan) and recombinant human EGF products (Heberprot-P®, Regen-D™ 150 gel, and Easyef® spray) which are all commercialized overseas.
One of the main limitations in growth factor therapy to influence wound healing is the short half-life and challenge of developing the best mode of delivery to achieve the proper therapeutic effect. To mitigate this, rhPDGF-BB has been encapsulated in microspheres52 and several different drug delivery systems including particulate systems, scaffolds, and hydrogels have also been developed to provide improved stability and controlled growth factor release.53 In addition, it is important to note the unknown theoretical risk of malignancy recurrence or transformation with off label use of growth factor therapy, especially in patients with a previous history of malignancy. Although, there was no increased incidence of malignancy seen at the primary treatment site, an increased risk of malignancy distant from the site of application (3% becaplermin group compared to 1% control group) and association of mortality secondary to malignant neoplasm was found in a retrospective review of patients treated with rhPDGF (Regranex gel).54 As such, one of the contraindications to using this therapeutic is known neoplasm at the site of application and caution should be used in patients with any known malignancy.
Stem cells have the capacity for unlimited self-renewal, replication, and differentiation into tissue specific cells and their contribution to wound healing lies in their ability to secrete regenerative growth factors and cytokines. Various stem cell populations such embryonic stem cells (ESCs; pluripotent) and adult mesenchymal stem cells (MSCs; multipotent) have been investigated as potential wound healing agents in both preclinical and clinical studies. The successful transplantation of human embryonic stem cells into nude mice to facilitate wound healing was described by Guenou et al.55 Despite its potential, use of ESCs has been criticized due to the potential for immunogenicity and tumorigenicity in addition to the ethical and legal concerns over ESC derived cells.56
Mesenchymal stem cells are multipotent stromal cells found in bone marrow, adipose tissue, nerve tissue, amniotic fluid, umbilical cord, placenta, menstrual blood, and dental pulp that have the potential to enhance dermal regeneration via differentiation into various cell types such as fibroblasts, osteoblasts, chondrocytes, myocytes, and adipocytes.28 Clinical use of bone marrow derived stem cells (BM-MSCs) is limited due to invasiveness during harvest and difficulty culturing cells, but has been successful in decreasing wound size in a randomized control trial of 24 patients with chronic nonhealing lower extremity ulcers.57 Adipose derived mesenchymal stem cells (ADMSCs) are particularly important in wound healing due to relatively easy acquisition from liposuction waste, ability to differentiate into keratinocytes, fibroblasts, and endothelial cells, and promoting the proliferation phase of wound healing through signal transduction. The use of human adipose derived mesenchymal stem cells was recently studied in over 300 burn or crush injury patients. Patients in this clinical trial received either twice daily topical human derived mesenchymal stem cells or saline. They found that granulation tissue formation rate and thickness of granulation tissue was significantly higher compared to controls after only 10 days of treatment.58 Additionally, the experimental group had a significant decrease in occurrence of wound complications such as bleeding and suppuration. Simultaneous application of ASCs has also been shown to promote fat tissue survival after fat transplantation in the face59 and in breast augmentation.60 Stem cells undoubtedly have tremendous therapeutic potential, however, discerning the optimal source, method of processing, and standard application of stem cells for clinical use remains a challenge. Future investigations evaluating MSC exosomes and integration of stem cells, growth factors, and exosomes into scaffold-based delivery systems may further advance the effectiveness of stem cell therapy in wound healing and enable promising future developments.
Platelet Rich Plasma
Autologous platelet-rich plasma (PRP) is a blood-derived product that contains a high concentration of platelets in plasma and has been shown to be effective in wound healing. Activated platelets in PRP release multiple growth factors and cytokines promoting the wound healing cascade. Preparation of PRP involves centrifugation of autologous whole blood to separate plasma followed by centrifugation of plasma to separate platelet-rich from platelet-poor plasma. The final product is further activated by the addition of thrombin or calcium, resulting in a platelet gel.53 Several small human case series and prospective studies61 support the use of autologous PRP both on its own and in combination with hydrogels as an activated platelet gel, and it is currently used to improve tissue healing in several specialty areas including plastic surgery, oral maxillofacial surgery, orthopedics, cardiovascular surgery, and dermatology. In a single-blinded pilot study Hom et al62 found that 4mm full thickness skin punch wounds healed significantly faster over a 42 day study period in autologous platelet rich gel treated wounds compared to control sites. On histologic analysis, they found that when the platelet concentration of the PRP was more than 6 times the baseline intravascular levels, autologous platelet gel treated wounds demonstrated epithelialization and granulation formation 3 days earlier. A systemic meta-analysis including 20 randomized controlled trials concluded that autologous PRP may increase complete wound closure, shorten healing time, and reduce wound size in patients with chronic diabetic wounds.63 The benefit of using autologous PRP to improve wound healing in acute wounds is more controversial but favors reduced risk of infections.64 Additionally, promising future applications in otolaryngology for autologous PRP include enhanced wound healing following total laryngectomy,65 improved vocal quality in treatment of vocal fold scar and atrophy,66,67 and improved healing of chronic tympanic membrane perforations following tympanoplasty.68,69
Hair Follicle Transplantation
Brown and McDowell were the first to observe that wounds in areas without hair follicles, such as the palms and soles, heal slower than wounds in hair bearing areas and proposed that hair follicles play a role in wound healing.70 It was later discovered that the hair follicle contains epidermal stem cells that become activated after skin injury, differentiate into epidermal cells and migrate into the wound to aid in reepithelialization.71,72 This is of clinical significance because hair follicle harvest and transplantation could be a promising future source of autologous stem cells to enhance wound healing using current well established techniques that are quick, safe, and eliminate the need for large donor site grafting. There are few clinical studies evaluating the efficacy of hair follicle transplantation compared to standard wound management in chronic wounds. Yang et al73 performed a clinical study of 40 patients with chronic full-thickness traumatic or surgical wounds and reported significantly higher skin quality of the recipient site, scar appearance of the donor site, and overall satisfaction in patients receiving hair follicle therapy compared to split thickness skin grafting 12 weeks after intervention. A multicenter, randomized, prospective clinical trial investigating EpiDex™, a tissue engineered autologous epidermal skin equivalent derived from hair follicle stem cells, demonstrated it to be as effective as split thickness skin autografting in promotion of healing and complete closure of refractory lower extremity ulcers.74 EpiDex™ has also been shown to reduce wound pain in the majority of patients and promote complete wound healing in 74% of patients.75 Hair follicle containing dermal grafts from the scalp in addition to tissue engineered dermal grafts containing transplanted hair follicles have also been utilized to enhance reepithelialization in burn wounds;76,77 however, it is difficult to make accurate conclusions regarding the utility of hair follicle transplantation in improving wound healing outcomes and as a true alternative to split thickness skin grafting given the lack of randomized, controlled clinical studies on this topic.
Electrical Stimulation and Wound Healing
After soft tissue injury, an endogenous electric field generated from complex movement of ions exists within the wound and promotes many phases of acute healing. This transcutaneous voltage across a wound has been termed skin battery and must be preserved for normal skin wound healing.78 Studies have investigated the effects of electrical stimulation on fibroblasts during wound healing and found that fibroblast migration is voltage dependent and direct electric current increases DNA and collagen synthesis in fibroblasts by 20% and 100% respectively in pig skin.79,80 The endogenous electrodynamic field also plays a vital role in keratinocyte, macrophage, and monocyte migration in addition to other immune system functions.78 Electrical stimulation has also been found to increase blood flow and VEGF production in stimulated cultured skeletal muscle.81 For chronic wounds, several types of exogenous electrical stimulation modalities such as pulsed electromagnetic field therapy, monophasic high-voltage electric stimulation, and low level laser therapy have been described to improve and accelerate wound healing.82–86
Acupuncture is believed to generate an electrical field due to the temperature gradients between various metals in the needles used. In addition, electric current can be directly applied to acupuncture needles in a procedure called electroacupuncture.78 Acupuncture has been reported to improve wound healing in animal and burn patient human studies by decreasing wound size, decreasing inflammation, promoting epidermal regeneration, and improving microcirculation.
Electric field based wound dressings have also been investigated to determine if weak continuous electric fields applied to the wound can also improve wound healing. Some novel prototypes include application of a wireless electroceutical dressing (WED) in conjunction with negative pressure wound therapy87 to reduce the number of wound dressing changes and WED associated with silver and zinc to prevent and disrupt biofilm formation in chronic wounds.88 Exogenous electric field have also been applied to nanoparticles in hydrogels as a potential method for electrostimulated drug delivery.89
Hyperbaric Oxygen Therapy (HBO)
Chronic wounds are characterized by poor oxygen supply and vascularization impairing wound healing. As such, adjuvant treatment with hyperbaric oxygen therapy (HBOT) has been proposed to “jump start” refractory wounds back into the healing cascade on the basis of increasing tissue oxygenation. By enhancing diffusion of oxygen into hypoxic tissues, HBOT promotes fibroblast proliferation, stimulates angiogenesis and promotes a healthy immune response. Patients are placed in hyperbaric oxygen chambers in which they are exposed to elevated oxygen concentrations at supra-atmospheric pressures to increase the partial pressures of oxygen in their blood.90 Some randomized controlled trials have demonstrated the efficacy of HBOT on the rate of healing in diabetic foot ulcers. Hisamuddin et al91 reported a 44 times higher odds of achieving at least 30% wound size reduction in patients diabetic patients who underwent HBOT. Other applications for HBOT were reviewed in a systematic review by Goldman92 which concluded that HBOT can reduce the risk of amputation in the diabetic foot ulcer population by promoting partial and complete wound healing in addition to promote healing of arterial ulcers, refractory osteomyelitis, and to a lesser extent be useful in salvage of compromised surgical flaps and grafts. HBOT has also been traditionally used for the treatment of osteoradionecrosis in the head and neck; however, recent best practice guidelines highlight the lack of clear evidence and no longer support its routine use in the management nor prevention of ostoradionecrosis.93 For the facial plastic surgeon, the possible use of HBOT is expanding but remains limited to small patient series and case reports. Some of the developing applications of HBOT described in recent literature include the treatment of ischemic soft tissue wounds following facial filler injectables and cutaneous flap compromise following Mohs surgery.94–96
New Developments for Preventing Infection
One of the principles of acute and chronic wound management is prevention and adequate treatment of infection as infection is one of the common causes of delayed wound healing. Current advances to improve the healing process have aimed to address this issue by incorporating antimicrobial agents into wound care dressings. Common antibiotics applied to wound dressings include amoxicillin, ciprofloxacin, tetracycline, doxycycline, gentamicin, and cefuroxime or cefepime. Due to increased resistance among bacterial pathogens involved in wound infections, natural antimicrobials such as essential oils (EOs) and manuka honey have been studied for their antimicrobial properties and ability to accelerate wound healing. Essential oils such as thyme, cinnamon, lavender, tea tree, peppermint, lemongrass, oregano, and eucalyptus have been found to have antibacterial and/or antifungal properties and various EOs have been encapsulated in sodium alginate and chitosan films and even hydrogels such as commercially available Burnaid® (Mundipharma Pty Limited, Sydney, Australia) to treat chronic wounds and burn wounds.97 In vitro studies have shown EO containing products to be effective against common wound infection pathogens including Escheria coli, Staph aureus, and Candida albicans.97 Novel developments in nanotechnology have allowed metal nanoparticles such as silver, gold, and zinc to be utilized as promising future therapeutics to deliver antimicrobial molecules to the wound site. Incorporation of antimicrobial nanoparticles may be used to treat antibiotic resistant bacteria and avoid systemic toxicity of conventional therapeutics.
Several innovative techniques for detecting wound infections are also being developed. The gold standard for diagnosing wound infection is wound culture to identify specific pathogens; however, other diagnostic methods include clinical observation of the wound for signs of infection (i.e. purulent drainage, foul odor, edema, erythema, induration, increasing warmth, or abnormal granulation tissue or breakdown), patient pain assessment, and correlation with elevated laboratory markers such as C-Reactive Protein (CRP), procalcitonin (PCT), presepsin, microbial DNA, and bacterial protease activity (BPA).98 Drawbacks to standard swab wound culture include potential false negative detection of pathogens invading deeper tissue and unreliability in the context of biofilm detection. Other methods to obtain would culture are needle aspiration of wound fluid and tissue biopsy; however, both of these are techniques are invasive, painful, and can be time-consuming.
New non-invasive imaging modalities being employed for cutaneous wound infection diagnosis include spatial frequency domain imaging (SFDI), thermography, and fluorescence imaging. SFDI, a wide-field diffuse optical imaging technique that quantifies volume fraction of tissue chromophores such as oxyhemoglobin, deoxyhemoglobin, and water, has been used to noninvasively measure structural changes in the wound bed during the healing process and detect infection in burn wounds.98,99 Thermography detects infrared radiation emitted from wound tissue and is capable of diagnosing infection based on elevated temperatures. Recent advances include smart phone applications that enable mobile thermography, affording patients the opportunity to monitor wound infections remotely.100,101 Autofluorescence imaging is based on the light absorbing properties of endogenously produced bacterial fluorophores- porphyrins, a red fluorescing heme by-product produced by the majority of bacterial pathogens, and pyoverdines, a cyan fluorescing product specific to pseudomonas species. The MolecuLight i:X is a fluorescent imaging device that can identify the presence of red and cyan fluorescence signals with high positive predictive value and sensitivity for determining the presence or absence of bacteria in chronic, burn, and surgical wounds.98,102,103 Capabilities of non-invasive real time image interpretation of a wound environment could aid in early identification and treatment of wound infections and possibly prevent wounds from transforming into chronic, poorly healing wounds.
| Product | Description | FDA Indication | Limitations |
|---|---|---|---|
| Acellular Dermal Matrix | |||
| AlloDerm® | Freeze dried cadaver skin with preserved basement membrane | *___ | Uncertain vascularization rates, no cells present in scaffold, safety concerns of allogenic disease transfer |
| AlloMax™ | Non-crosslinked human dermis | *___ | No cells present in scaffold, safety concerns of allogenic disease transfer |
| FlexHD® | Hydrated acellular human skin | *___ | No cells present in scaffold, safety concerns of allogenic disease transfer |
| DermaMatrix™ | Allograft derived from human cadaveric skin | *___ | No cells present in scaffold, safety concerns of allogenic disease transfer |
| GraftJacket® | Pre-meshed acellular human dermis | *___ | No cells present in scaffold, safety concerns of allogenic disease transfer |
| Amniotic Membrane | |||
| EpiFix® | Dehydrated amniotic membrane in sheets | *___ | Safety concerns of allogenic disease transfer, unclear effect on angiogenesis |
| Amniofix® | Injectable micronized dehydrated amniotic membrane | *___ | Safety concerns of allogenic disease transfer, unclear effect on angiogenesis |
| Collagen Scaffold | |||
| OASIS® Wound Matrix | Xenogeneic collagen scaffold derived from porcine small intestine | Partial and full thickness wounds; diabetic, pressure, and venous stasis ulcers; surgical wounds, traumatic wounds | Requires multiple applications |
| Living Cell Therapy | |||
| Apligraf® | Bilayered construct w/ human neonatal fibroblasts cultured in bovine type 1 collagen matrix and human neonatal keratinocytes forming the superficial layer of the graft- mimics normal structure of skin | Non-infected, partial and full thickness skin ulcers due to venous insufficiency; nonresponsive full thickness neuropathic diabetic ulcers | Shelf life of 5 days, risk of disease transfer from allogeneic cells, cannot be used for definitive wound closure in full thickness wounds, requires co-grafting with autologous epithelium |
| Dermagraft® | Cryopreserved polyglactin mesh seeded w/ human neonatal foreskin fibroblasts | Diabetic foot ulcers | Requires multiple applications, safety concerns with allogeneic cells |
| Epicel | Cultured epithelial autograft | Deep dermal or full thickness burns | Requires long culture times, short shelf life, variable take rate |
| OrCel® | Bilayered construct w/ human neonatal foreskin fibroblasts and keratinocytes seeded in bovine type 1 collagen sponge | Donor site wounds in burn victims; dystrophic epidermolysis bullosa reconstructive surgery | Allogeneic cell source risk of disease transfer |
| Biosynthetic | |||
| Biobrane® | Temporary wound dressing consisting of nylon imbedded silicon film | Partial thickness burn wounds, donor sites, dressing over meshed autografts | Non-degradable/synthetic and must be removed after 7–14 days |
| Integra | Bovine type 1 collagen and shark chondroitin-6-sulphate glycosaminoglycan dermal component and silicone epidermal layer | Post-excisional treatment of deep partial thickness or full thickness due to thermal injury | Requires about 10–14 days for appropriate vascularization and requires second procedure for complete wound closure |
| TransCyte® | Temporary wound dressing consisting of human dermal fibroblasts grown on nylon mesh containing porcine collagen | Temporary covering over burns until autografting procedure or healed | Non-degradable/synthetic and must be removed after 7–14 days |
Considered “banked human tissue” and does not require FDA approval
Footnotes
Conflict of Interest: None declared
Disclosure Statement: The authors have nothing to disclose
References
- 1.Hom DB, Sun GH, Elluru RG. A contemporary review of wound healing in otolaryngology: Current state and future promise. Laryngoscope. 2009;119(11):2099–2110. doi: 10.1002/lary.20561 [DOI] [PubMed] [Google Scholar]
- 2.Costa AMA, Peyrol S, Pôrto LC, Comparin J-P, Foyatier J-L, Desmoulière A. Mechanical Forces Induce Scar Remodeling. Am J Pathol. 1999;155(5):1671–1679. doi: 10.1016/S0002-9440(10)65482-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Reilly S, Crofton E, Brown J, Strong J, Ziviani J. Use of tape for the management of hypertrophic scar development: A comprehensive review. Scars, Burn Heal. 2021;7:205951312110292. doi: 10.1177/20595131211029206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WINTER GD. Formation of the Scab and the Rate of Epithelization of Superficial Wounds in the Skin of the Young Domestic Pig. Nature. 1962;193(4812):293–294. doi: 10.1038/193293a0 [DOI] [PubMed] [Google Scholar]
- 5.Nuutila K, Eriksson E. Moist Wound Healing with Commonly Available Dressings. Adv Wound Care. 2021;10(12):685–698. doi: 10.1089/wound.2020.1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meaume S, Le Pillouer-Prost A, Richert B, Roseeuw D, Vadoud J. Management of scars: updated practical guidelines and use of silicones. Eur J Dermatology. 2014;24(4):435–443. doi: 10.1684/ejd.2014.2356 [DOI] [PubMed] [Google Scholar]
- 7.Monstrey S, Middelkoop E, Vranckx JJ, et al. Updated Scar Management Practical Guidelines: Non-invasive and invasive measures. J Plast Reconstr Aesthetic Surg. 2014;67(8):1017–1025. doi: 10.1016/j.bjps.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 8.Khorasani G, Hosseinimehr SJ, Azadbakht M, Zamani A, Mahdavi MR. Aloe versus silver sulfadiazine creams for second-degree burns: A randomized controlled study. Surg Today. 2009;39(7):587–591. doi: 10.1007/s00595-008-3944-y [DOI] [PubMed] [Google Scholar]
- 9.Shahzad MN, Ahmed N. Effectiveness of Aloe Vera gel compared with 1% silver sulphadiazine cream as burn wound dressing in second degree burns. J Pak Med Assoc. 2013;63(2):225–230. http://www.ncbi.nlm.nih.gov/pubmed/23894900 [PubMed] [Google Scholar]
- 10.Visuthikosol V, Chowchuen B, Sukwanarat Y, Sriurairatana S, Boonpucknavig V. Effect of aloe vera gel to healing of burn wound a clinical and histologic study. J Med Assoc Thai. 1995;78(8):403–409. http://www.ncbi.nlm.nih.gov/pubmed/7561562 [PubMed] [Google Scholar]
- 11.Burusapat C, Supawan M, Pruksapong C, Pitiseree A, Suwantemee C. Topical Aloe Vera Gel for Accelerated Wound Healing of Split-Thickness Skin Graft Donor Sites. Plast Reconstr Surg. 2018;142(1):217–226. doi: 10.1097/PRS.0000000000004515 [DOI] [PubMed] [Google Scholar]
- 12.Basson R, Bayat A. Skin scarring: Latest update on objective assessment and optimal management. Front Med. 2022;9. doi: 10.3389/fmed.2022.942756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall CD, Hu MS, Leavitt T, Barnes LA, Lorenz HP, Longaker MT. Cutaneous Scarring: Basic Science, Current Treatments, and Future Directions. Adv Wound Care. 2018;7(2):29–45. doi: 10.1089/wound.2016.0696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sidgwick GP, McGeorge D, Bayat A. A comprehensive evidence-based review on the role of topicals and dressings in the management of skin scarring. Arch Dermatol Res. 2015;307(6):461–477. doi: 10.1007/s00403-015-1572-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atiyeh BS, Ioannovich J, Al-Amm CA, El-Musa KA, Dham R. Improving Scar Quality: A Prospective Clinical Study. Aesthetic Plast Surg. 2002;26(6):470–476. doi: 10.1007/s00266-002-2019-5 [DOI] [PubMed] [Google Scholar]
- 16.Atiyeh BS, Amm CA, El Musa KA. Improved Scar Quality Following Primary and Secondary Healing of Cutaneous Wounds. Aesthetic Plast Surg. 2003;27(5):411–417. doi: 10.1007/s00266-003-3049-3 [DOI] [PubMed] [Google Scholar]
- 17.Son D, Harijan A. Overview of Surgical Scar Prevention and Management. J Korean Med Sci. 2014;29(6):751. doi: 10.3346/jkms.2014.29.6.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S, Gupta SH, Viswambaran M, Sachdeva A, Panda BP. Management of postburn perioral contracture using a customized static commissural splint and intralesional injections of triamcinolone. J Prosthet Dent. 2018;119(3):488–491. doi: 10.1016/j.prosdent.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 19.Amici J-M. Hypertrophie cicatricielle précoce post-chirurgicale de la région nasale : intérêt des injections de corticoïde retard. Ann Dermatol Venereol. 2014;141(1):7–13. doi: 10.1016/j.annder.2013.09.167 [DOI] [PubMed] [Google Scholar]
- 20.Gassner HG, Sherris DA, Otley CC. Treatment of Facial Wounds with Botulinum Toxin A Improves Cosmetic Outcome in Primates. Plast Reconstr Surg. 2000;105(6):1948–1953. doi: 10.1097/00006534-200005000-00005 [DOI] [PubMed] [Google Scholar]
- 21.Ziade M, Domergue S, Batifol D, et al. Use of botulinum toxin type A to improve treatment of facial wounds: A prospective randomised study. J Plast Reconstr Aesthetic Surg. 2013;66(2):209–214. doi: 10.1016/j.bjps.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 22.Shokri T, Smith J, Ducic Y. Paradigms in Complex Facial Scar Management. Semin Plast Surg. 2020;34(04):305–313. doi: 10.1055/s-0040-1721768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao Z, Zhang F, Lin W, Zhang M, Liu Y. Effect of Botulinum Toxin Type A on Transforming Growth Factor β1 in Fibroblasts Derived from Hypertrophic Scar: A Preliminary Report. Aesthetic Plast Surg. 2010;34(4):424–427. doi: 10.1007/s00266-009-9423-z [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Tai N, Fan Z. [Effect of botulinum toxin type A on the expression of substance P, calcitonin gene-related peptide, transforming growth factor beta-1 and alpha smooth muscle actin A in wound healing in rats]. Zhonghua Zheng Xing Wai Ke Za Zhi. 2009;25(1):50–53. http://www.ncbi.nlm.nih.gov/pubmed/19408727 [PubMed] [Google Scholar]
- 25.Xiao Z, Zhang F, Cui Z. Treatment of Hypertrophic Scars With Intralesional Botulinum Toxin Type A Injections: A Preliminary Report. Aesthetic Plast Surg. 2009;33(3):409–412. doi: 10.1007/s00266-009-9334-z [DOI] [PubMed] [Google Scholar]
- 26.Farahani M, Shafiee A. Wound Healing: From Passive to Smart Dressings. Adv Healthc Mater. 2021;10(16):2100477. doi: 10.1002/adhm.202100477 [DOI] [PubMed] [Google Scholar]
- 27.Dhivya S, Padma VV, Santhini E. Wound dressings – a review. BioMedicine. 2015;5(4):22. doi: 10.7603/s40681-015-0022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng R, Lin C, Lin Z, et al. Approaches to cutaneous wound healing: basics and future directions. Cell Tissue Res. 2018;374(2):217–232. doi: 10.1007/s00441-018-2830-1 [DOI] [PubMed] [Google Scholar]
- 29.Santamaria N, Gerdtz M, Sage S, et al. A randomised controlled trial of the effectiveness of soft silicone multi-layered foam dressings in the prevention of sacral and heel pressure ulcers in trauma and critically ill patients: the border trial. Int Wound J. 2015;12(3):302–308. doi: 10.1111/iwj.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Lee S, Hong JP, Shon MW, Ryu S-H, Ahn S Do. Foam dressing with epidermal growth factor for severe radiation dermatitis in head and neck cancer patients. Int Wound J. 2016;13(3):390–393. doi: 10.1111/iwj.12317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pang Q, Lou D, Li S, et al. Smart Flexible Electronics-Integrated Wound Dressing for Real-Time Monitoring and On-Demand Treatment of Infected Wounds. Adv Sci. 2020;7(6):1902673. doi: 10.1002/advs.201902673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiao B, Pang Q, Yuan P, Luo Y, Ma L. Smart wound dressing for infection monitoring and NIR-triggered antibacterial treatment. Biomater Sci. 2020;8(6):1649–1657. doi: 10.1039/C9BM02060H [DOI] [PubMed] [Google Scholar]
- 33.Vithlani G, Santos Jorge P, Brizman E, Mitsimponas K. Integra ® as a single-stage dermal regeneration template in reconstruction of large defects of the scalp. Br J Oral Maxillofac Surg. 2017;55(8):844–846. doi: 10.1016/j.bjoms.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 34.Johnson MB, Wong AK. Integra-based Reconstruction of Large Scalp Wounds. Plast Reconstr Surg - Glob Open. 2016;4(10):e1074. doi: 10.1097/GOX.0000000000001074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wirthmann A, Finke JC, Giovanoli P, Lindenblatt N. Long-term follow-up of donor site morbidity after defect coverage with Integra following radial forearm flap elevation. Eur J Plast Surg. 2014;37(3):159–166. doi: 10.1007/s00238-013-0918-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seth AK, Ratanshi I, Dayan JH, Disa JJ, Mehrara BJ. Nasal Reconstruction Using the Integra Dermal Regeneration Template. Plast Reconstr Surg. 2019;144(4):966–970. doi: 10.1097/PRS.0000000000006072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Applebaum MA, Daggett JD, Carter WL. Nasal Tip Reconstruction Using Integra Bilayer Wound Matrix: An Alternative to the Forehead Flap. Eplasty. 2015;15:e52. http://www.ncbi.nlm.nih.gov/pubmed/26681994 [PMC free article] [PubMed] [Google Scholar]
- 38.Depani M, Grush AE, Parham MJ, Jones LM, Thornton JF. Use of Biologic Agents in Nasal and Scalp Reconstruction. Semin Plast Surg. 2022;36(01):017–025. doi: 10.1055/s-0042-1742750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srivastava A, Maniakas A, Myers J, Chambers MS, Cardoso R. Reconstruction of intraoral oncologic surgical defects with Integra ® bilayer wound matrix. Clin Case Reports. 2021;9(1):213–219. doi: 10.1002/ccr3.3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deganello A, Bosio P, Giannini L, et al. Matrix for Mucosal Regeneration in Transoral Glossectomy for Squamous Cell Carcinoma: Objective and Subjective Functional Evaluation. Curr Oncol. 2023;30(2):1354–1362. doi: 10.3390/curroncol30020104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark JM, Rychlik S, Harris J, Seikaly H, Biron VL, O’Connell DA. Donor site morbidity following radial forearm free flap reconstruction with split thickness skin grafts using negative pressure wound therapy. J Otolaryngol - Head Neck Surg. 2019;48(1):21. doi: 10.1186/s40463-019-0344-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avery C, Pereira J, Moody A, Whitworth I. Clinical experience with the negative pressure wound dressing. Br J Oral Maxillofac Surg. 2000;38(4):343–345. doi: 10.1054/bjom.1999.0453 [DOI] [PubMed] [Google Scholar]
- 43.Maleki Delarestaghi M, Ahmadi A, Dehghani Firouzabadi F, Roomiani M, Dehghani Firouzabadi M, Faham Z. Effect of Low-Pressure Drainage Suction on Pharyngocutaneous Fistula After Total Laryngectomy. Ann Otol Rhinol Laryngol. 2021;130(1):32–37. doi: 10.1177/0003489420934506 [DOI] [PubMed] [Google Scholar]
- 44.Govea-Camacho LH, Astudillo-Carrera A, Hermosillo-Sandoval JM, Rodríguez-Reynoso S, González-Ojeda A, Fuentes-Orozco C. Impacto del manejo con cierre asistido al vacío en abscesos profundos de cuello. Cir Cir. 2016;84(4):275–281. doi: 10.1016/j.circir.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 45.Asher SA, White HN, Golden JB, Magnuson JS, Carroll WR, Rosenthal EL. Negative Pressure Wound Therapy in Head and Neck Surgery. JAMA Facial Plast Surg. 2014;16(2):120–126. doi: 10.1001/jamafacial.2013.2163 [DOI] [PubMed] [Google Scholar]
- 46.Yamakawa S, Hayashida K. Advances in surgical applications of growth factors for wound healing. Burn Trauma. 2019;7. doi: 10.1186/s41038-019-0148-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wieman TJ, Smiell JM, Su Y. Efficacy and Safely of a Topical Gel Formulation of Recombinant Human Platelet-Derived Growth Factor-BB (Becaplermin) in Patients With Chronic Neuropathic Diabetic Ulcers: A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21(5):822–827. doi: 10.2337/diacare.21.5.822 [DOI] [PubMed] [Google Scholar]
- 48.Hom DB. New Developments in Wound Healing Relevant to Facial Plastic Surgery. Arch Facial Plast Surg. 2008;10(6):402–406. doi: 10.1001/archfaci.10.6.402 [DOI] [PubMed] [Google Scholar]
- 49.Hom DB, Manivel JC. Promoting Healing With Recombinant Human Platelet-Derived Growth Factor???BB in a Previously Irradiated Problem Wound. Laryngoscope. 2003;113(9):1566–1571. doi: 10.1097/00005537-200309000-00029 [DOI] [PubMed] [Google Scholar]
- 50.Jakubowicz DM, Smith RV. Use of becaplermin in the closure of pharyngocutaneous fistulas. Head Neck. 2005;27(5):433–438. doi: 10.1002/hed.20182 [DOI] [PubMed] [Google Scholar]
- 51.Li F, Yu F, Liao X, et al. Efficacy of Recombinant Human BMP2 and PDGF-BB in Orofacial Bone Regeneration: A Systematic Review and Meta-analysis. Sci Rep. 2019;9(1):8073. doi: 10.1038/s41598-019-44368-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bae YJ, Cho CH, Lee WJ, Huh JS, Lim JO. Optimization of recombinant human platelet-derived growth factor-BB encapsulated in Poly (lactic-co-glycolic acid) microspheres for applications in wound healing. Tissue Eng Regen Med. 2016;13(1):13–20. doi: 10.1007/s13770-015-0029-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park J, Hwang S, Yoon I-S. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules. 2017;22(8):1259. doi: 10.3390/molecules22081259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Company O-M. Regranex [Package Insert].; 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/103691s5074lbl.pdf
- 55.Guenou H, Nissan X, Larcher F, et al. Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: a preclinical study. Lancet. 2009;374(9703):1745–1753. doi: 10.1016/S0140-6736(09)61496-3 [DOI] [PubMed] [Google Scholar]
- 56.Nourian Dehkordi A, Mirahmadi Babaheydari F, Chehelgerdi M, Raeisi Dehkordi S. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther. 2019;10(1):111. doi: 10.1186/s13287-019-1212-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC. Targeting Nonhealing Ulcers of Lower Extremity in Human Through Autologous Bone Marrow-Derived Mesenchymal Stem Cells. Rejuvenation Res. 2009;12(5):359–366. doi: 10.1089/rej.2009.0872 [DOI] [PubMed] [Google Scholar]
- 58.Zhou L, Wang H, Yao S, Li L, Kuang X. Efficacy of Human Adipose Derived Mesenchymal Stem Cells in Promoting Skin Wound Healing. L Ali, ed. J Healthc Eng. 2022;2022:1–5. doi: 10.1155/2022/6590025 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Koh KS, Oh TS, Kim H, et al. Clinical Application of Human Adipose Tissue–Derived Mesenchymal Stem Cells in Progressive Hemifacial Atrophy (Parry-Romberg Disease) With Microfat Grafting Techniques Using 3-Dimensional Computed Tomography and 3-Dimensional Camera. Ann Plast Surg. 2012;69(3):331–337. doi: 10.1097/SAP.0b013e31826239f0 [DOI] [PubMed] [Google Scholar]
- 60.Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-Assisted Lipotransfer for Cosmetic Breast Augmentation: Supportive Use of Adipose-Derived Stem/Stromal Cells. Aesthetic Plast Surg. 2008;32(1):48–55. doi: 10.1007/s00266-007-9019-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sclafani AP, Azzi J. Platelet Preparations for Use in Facial Rejuvenation and Wound Healing: A Critical Review of Current Literature. Aesthetic Plast Surg. 2015;39(4):495–505. doi: 10.1007/s00266-015-0504-x [DOI] [PubMed] [Google Scholar]
- 62.Hom DB, Linzie BM, Huang TC. The Healing Effects of Autologous Platelet Gel on Acute Human Skin Wounds. Arch Facial Plast Surg. 2007;9(3):174–183. doi: 10.1001/archfaci.9.3.174 [DOI] [PubMed] [Google Scholar]
- 63.Qu W, Wang Z, Hunt C, et al. The Effectiveness and Safety of Platelet-Rich Plasma for Chronic Wounds. Mayo Clin Proc. 2021;96(9):2407–2417. doi: 10.1016/j.mayocp.2021.01.030 [DOI] [PubMed] [Google Scholar]
- 64.Carter MJ, Fylling CP, Parnell LKS. Use of platelet rich plasma gel on wound healing: a systematic review and meta-analysis. Eplasty. 2011;11:e38. http://www.ncbi.nlm.nih.gov/pubmed/22028946 [PMC free article] [PubMed] [Google Scholar]
- 65.Reksodiputro MH, Hutauruk SM, Widodo DW, Fardizza F, Mutia D. Platelet-Rich Fibrin Enhances Surgical Wound Healing in Total Laryngectomy. Facial Plast Surg. 2021;37(03):325–332. doi: 10.1055/s-0040-1717083 [DOI] [PubMed] [Google Scholar]
- 66.van der Woerd B, O’Dell K, Castellanos CX, et al. Safety of Platelet-Rich Plasma Subepithelial Infusion for Vocal Fold Scar, Sulcus, and Atrophy. Laryngoscope. 2023;133(3):647–653. doi: 10.1002/lary.30288 [DOI] [PubMed] [Google Scholar]
- 67.Woo P, Murry T. Short-Term Voice Improvement after Repeated Office-Based Platelet-Rich Plasma PRP Injection in Patients with Vocal Fold Scar, Sulcus, and Atrophy. J Voice. Published online March 2021. doi: 10.1016/j.jvoice.2021.02.022 [DOI] [PubMed] [Google Scholar]
- 68.El-Anwar MW, El-Ahl MAS, Zidan AA, Yacoup MA-RA-S. Topical use of autologous platelet rich plasma in myringoplasty. Auris Nasus Larynx. 2015;42(5):365–368. doi: 10.1016/j.anl.2015.02.016 [DOI] [PubMed] [Google Scholar]
- 69.Erkilet E, Koyuncu M, Atmaca S, Yarim M. Platelet-rich plasma improves healing of tympanic membrane perforations: experimental study. J Laryngol Otol. 2009;123(5):482–487. doi: 10.1017/S0022215108003848 [DOI] [PubMed] [Google Scholar]
- 70.Brown JB, McDowell F. EPITHELIAL HEALING AND THE TRANSPLANTATION OF SKIN. Ann Surg. 1942;115(6):1166–1181???1181. doi: 10.1097/00000658-194206000-00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nuutila K. Hair Follicle Transplantation for Wound Repair. Adv Wound Care. 2021;10(3):153–163. doi: 10.1089/wound.2019.1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amoh Y, Hoffman RM. Hair follicle-associated-pluripotent (HAP) stem cells. Cell Cycle. 2017;16(22):2169–2175. doi: 10.1080/15384101.2017.1356513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang Z, Liu J, Zhu N, Qi F. Comparison between hair follicles and split-thickness skin grafts in cutaneous wound repair. Int J Clin Exp Med. 2015;8(9):15822–15827. http://www.ncbi.nlm.nih.gov/pubmed/26629082 [PMC free article] [PubMed] [Google Scholar]
- 74.Tausche A-K, Skaria M, Bohlen L, et al. An autologous epidermal equivalent tissue-engineered from follicular outer root sheath keratinocytes is as effective as split-thickness skin autograft in recalcitrant vascular leg ulcers. Wound Repair Regen. 2003;11(4):248–252. doi: 10.1046/j.1524-475X.2003.11403.x [DOI] [PubMed] [Google Scholar]
- 75.Ortega-Zilic N, Hunziker T, Läuchli S, et al. EpiDex® Swiss Field Trial 2004–2008. Dermatology. 2010;221(4):365–372. doi: 10.1159/000321333 [DOI] [PubMed] [Google Scholar]
- 76.Zakine G, Mimoun M, Pham J, Chaouat M. Reepithelialization from Stem Cells of Hair Follicles of Dermal Graft of the Scalp in Acute Treatment of Third-Degree Burns. Plast Reconstr Surg. 2012;130(1):42e–50e. doi: 10.1097/PRS.0b013e318254fa21 [DOI] [PubMed] [Google Scholar]
- 77.Navsaria HA, Ojeh NO, Moiemen N, Griffiths MA, Frame JD. Reepithelialization of a Full-Thickness Burn from Stem Cells of Hair Follicles Micrografted into a Tissue-Engineered Dermal Template (Integra). Plast Reconstr Surg. 2004;113(3):978–981. doi: 10.1097/01.PRS.0000105632.86651.EF [DOI] [PubMed] [Google Scholar]
- 78.Farber PL, Isoldi FC, Ferreira LM. Electric Factors in Wound Healing. Adv Wound Care. 2021;10(8):461–476. doi: 10.1089/wound.2019.1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alvarez OM, Mertz PM, Smerbeck RV., Eaglstein WH. The Healing of Superficial Skin Wounds Is Stimulated by External Electrical Current. J Invest Dermatol. 1983;81(2):144–148. doi: 10.1111/1523-1747.ep12543498 [DOI] [PubMed] [Google Scholar]
- 80.Guo A, Song B, Reid B, et al. Effects of Physiological Electric Fields on Migration of Human Dermal Fibroblasts. J Invest Dermatol. 2010;130(9):2320–2327. doi: 10.1038/jid.2010.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanno S, Oda N, Abe M, et al. Establishment of a Simple and Practical Procedure Applicable to Therapeutic Angiogenesis. Circulation. 1999;99(20):2682–2687. doi: 10.1161/01.CIR.99.20.2682 [DOI] [PubMed] [Google Scholar]
- 82.Strauch B, Herman C, Dabb R, Ignarro LJ, Pilla AA. Evidence-Based Use of Pulsed Electromagnetic Field Therapy in Clinical Plastic Surgery. Aesthetic Surg J. 2009;29(2):135–143. doi: 10.1016/j.asj.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 83.Kwan RL-C, Wong W-C, Yip S-L, Chan K-L, Zheng Y-P, Cheing GL-Y. Pulsed Electromagnetic Field Therapy Promotes Healing and Microcirculation of Chronic Diabetic Foot Ulcers. Adv Skin Wound Care. 2015;28(5):212–219. doi: 10.1097/01.ASW.0000462012.58911.53 [DOI] [PubMed] [Google Scholar]
- 84.Gomes RC, Guirro ECO, Gonçalves AC, Farina Junior JA, Murta Junior LO, Guirro RRJ. High-voltage electric stimulation of the donor site of skin grafts accelerates the healing process. A randomized blinded clinical trial. Burns. 2018;44(3):636–645. doi: 10.1016/j.burns.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 85.Vaghardoost R, Momeni M, Kazemikhoo N, et al. Effect of low-level laser therapy on the healing process of donor site in patients with grade 3 burn ulcer after skin graft surgery (a randomized clinical trial). Lasers Med Sci. 2018;33(3):603–607. doi: 10.1007/s10103-017-2430-4 [DOI] [PubMed] [Google Scholar]
- 86.Pinfildi CE, Liebano RE, Hochman BS, Ferreira LM. Helium-neon laser in viability of random skin flap in rats. Lasers Surg Med. 2005;37(1):74–77. doi: 10.1002/lsm.20190 [DOI] [PubMed] [Google Scholar]
- 87.Ghatak P Das, Schlanger R, Ganesh K, et al. A Wireless Electroceutical Dressing Lowers Cost of Negative Pressure Wound Therapy. Adv Wound Care. 2015;4(5):302–311. doi: 10.1089/wound.2014.0615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barki KG, Das A, Dixith S, et al. Electric Field Based Dressing Disrupts Mixed-Species Bacterial Biofilm Infection and Restores Functional Wound Healing. Ann Surg. 2019;269(4):756–766. doi: 10.1097/SLA.0000000000002504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kolosnjaj-Tabi J, Gibot L, Fourquaux I, Golzio M, Rols M-P. Electric field-responsive nanoparticles and electric fields: physical, chemical, biological mechanisms and therapeutic prospects. Adv Drug Deliv Rev. 2019;138:56–67. doi: 10.1016/j.addr.2018.10.017 [DOI] [PubMed] [Google Scholar]
- 90.Hershcovitch MD, Hom DB. Update in Wound Healing in Facial Plastic Surgery. Arch Facial Plast Surg. 2012;14(6):387. doi: 10.1001/2013.jamafacial.33 [DOI] [PubMed] [Google Scholar]
- 91.Nik Hisamuddin NAR, Wan Mohd Zahiruddin WN, Mohd Yazid B, Rahmah S. Use of hyperbaric oxygen therapy (HBOT) in chronic diabetic wound - A randomised trial. Med J Malaysia. 2019;74(5):418–424. http://www.ncbi.nlm.nih.gov/pubmed/31649219 [PubMed] [Google Scholar]
- 92.Goldman RJ. Hyperbaric Oxygen Therapy for Wound Healing and Limb Salvage: A Systematic Review. PM&R. 2009;1(5):471–489. doi: 10.1016/j.pmrj.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 93.Lubek JE, Hancock MK, Strome SE. What is the value of hyperbaric oxygen therapy in management of osteoradionecrosis of the head and neck? Laryngoscope. 2013;123(3):555–556. doi: 10.1002/lary.23496 [DOI] [PubMed] [Google Scholar]
- 94.Simman R, Bach K. Role of Hyperbaric Oxygen Therapy in Cosmetic and Reconstructive Surgery in Ischemic Soft Tissue Wounds: A Case Series. Eplasty. 2022;22:e61. doi:36545638 [PMC free article] [PubMed] [Google Scholar]
- 95.Henderson R, Reilly DA, Cooper JS. Hyperbaric Oxygen for Ischemia due to Injection of Cosmetic Fillers. Plast Reconstr Surg - Glob Open. 2018;6(1):e1618. doi: 10.1097/GOX.0000000000001618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Uittenbogaard D, Lansdorp CA, Bauland CG, Boonstra O. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 46(2):207–210. http://www.ncbi.nlm.nih.gov/pubmed/31051067 [PubMed] [Google Scholar]
- 97.Negut I, Grumezescu V, Grumezescu A. Treatment Strategies for Infected Wounds. Molecules. 2018;23(9):2392. doi: 10.3390/molecules23092392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li S, Renick P, Senkowsky J, Nair A, Tang L. Diagnostics for Wound Infections. Adv Wound Care. 2021;10(6):317–327. doi: 10.1089/wound.2019.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kennedy GT, Stone R, Kowalczewski AC, et al. Spatial frequency domain imaging: a quantitative, noninvasive tool for in vivo monitoring of burn wound and skin graft healing. J Biomed Opt. 2019;24(07):1. doi: 10.1117/1.JBO.24.7.071615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu M, Yee A, Meng F, Harmon J, Hinduja S, Yi S. Enhance wound healing monitoring through a thermal imaging based smartphone app. In: Zhang J, Chen P-H, eds. Medical Imaging 2018: Imaging Informatics for Healthcare, Research, and Applications. SPIE; 2018:60. doi: 10.1117/12.2293674 [DOI] [Google Scholar]
- 101.Fraiwan L, AlKhodari M, Ninan J, Mustafa B, Saleh A, Ghazal M. Diabetic foot ulcer mobile detection system using smart phone thermal camera: a feasibility study. Biomed Eng Online. 2017;16(1):117. doi: 10.1186/s12938-017-0408-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ottolino-Perry K, Chamma E, Blackmore KM, et al. Improved detection of clinically relevant wound bacteria using autofluorescence image-guided sampling in diabetic foot ulcers. Int Wound J. 2017;14(5):833–841. doi: 10.1111/iwj.12717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blackshaw EL, Jeffery SLA. Efficacy of an imaging device at identifying the presence of bacteria in wounds at a plastic surgery outpatients clinic. J Wound Care. 2018;27(1):20–26. doi: 10.12968/jowc.2018.27.1.20 [DOI] [PubMed] [Google Scholar]


