Abstract
CCR5 is the major coreceptor for human immunodeficiency virus (HIV) infection. The murine monoclonal antibody (MAb) 2D7, which recognizes a conformation-dependent epitope in the second extracellular loop of CCR5, is one of the most potent inhibitors of R5 virus cell entry. However, attempts to humanize 2D7 for in vivo human use have been unsuccessful so far. A filamentous phage library expressing random peptides was used to identify a peptide mimitope that is recognized by MAb 2D7. A synthetic peptide containing this sequence (2D7-2SK) bound to MAb 2D7 with high affinity and reversed its HIV type 1 (HIV-1) fusion inhibitory activity. The peptide contains sequence homologies to two distal regions of the second extracellular loop of human CCR5, both of which are required for MAb 2D7 binding. Rabbit anti-2D7-mimitope antibodies competed with MAb 2D7 for binding to the 2D7-2SK peptide in Biacore biosensor testing. Importantly, the rabbit anti-2D7-2SK antibodies bound to CCR5 on cells and specifically inhibited R5 (but not X4) envelope-mediated syncytium formation. These antibodies also neutralized infection of human peripheral blood mononuclear cells with R5 HIV isolates comparably to MAb 2D7. In summary, we have identified a novel peptide that closely mimics the MAb 2D7 epitope on CCR5. This peptide could be included as a potential vaccine candidate or to isolate 2D7-like human antibodies as entry inhibitors for R5 viruses.
The human immunodeficiency virus type 1 (HIV-1) coreceptor CCR5 has been identified as a major target for HIV-1 entry inhibitors, since most of the viruses responsible for person-to-person transmission have been typed as CCR5-using (R5) strains. The naturally occurring Δ32ccr5 allele (9, 21), when homozygous, is associated with resistance to in vitro infection of CD4+ cells with R5 viruses (6, 17). Moreover, Δ32ccr5 homozygosity confers considerable protection against HIV infection in vivo (9, 14). Yet this genotype is not associated with abnormal immune function and may be dispensable due to redundancy in chemokine receptor usage (14, 15).
There are three main classes of CCR5-targeting inhibitors: CC-chemokine analogues, small molecules, and monoclonal antibodies (MAb) (19, 23). One of the most active monoclonal antibodies targeting CCR5 is MAb 2D7, which was generated from the spleen of C57BL/6 mice immunized with the murine pre-B-cell lymphoma line L1.2, which expresses high levels of transfected CCR5 (22). This murine antibody was shown to inhibit in vitro infections of CD4+ CCR5+ human cells by most R5-tropic viruses at a 50% inhibitory dose (ID50) of 2 to 10 μg/ml, making it a good candidate for generating humanized antibodies. Yet no success in humanizing this MAb has been reported. The epitope recognized by MAb 2D7 on CCR5 has been partially mapped to the first half of the second extracellular loop (ECL-2) by mutagenesis studies (16, 22). Amino acids 171-KE-172 were found to be critical for MAb 2D7 binding. But the epitope was determined to be conformation dependent, and the binding is lost in CCR5 mutants lacking the disulfide bridge between ECL-1 and ECL-2, as well as in reduced forms of CCR5 extracted from cells with various detergents (12, 16). The identification of the epitope recognized by MAb 2D7 may be important for the elucidation of the mechanisms for CCR5-based inhibitors, and this could lead to the development of a potential vaccine candidate and HIV-1 therapeutics. Previous attempts to identify a linear sequence recognized by 2D7, using a random peptide phage display library, led to identification of a 15-mer mimitope that was only functional as a phage g3p-fusion protein. It bore no sequence homologies to CCR5, and no immunization potential was shown in terms of generating neutralizing antibodies like the 2D7 (11).
In this study, we have used a random peptide phage display library to identify a 12-amino-acid linear peptide sequence that binds to MAb 2D7 with high affinity and can significantly reduce 2D7's ability to bind to CCR5 and block HIV-1 fusion. This peptide conjugated to keyhole limpet hemocyanin (KLH) was used to immunize rabbits. The rabbit polyclonal antibodies thus generated demonstrate 2D7-like reactivity, including inhibition of HIV-1 fusion and infection of peripheral blood mononuclear cells.
MATERIALS AND METHODS
Materials.
A random linear dodecapeptide phage display library (Ph.D-12), wherein the displayed peptide (12-mer) is expressed fused to the N terminus of gIII protein, was purchased from New England Biolabs (Beverly, MA). Monoclonal antibody (MAb) 2D7 was purchased from BD Pharmingen (San Diego, CA). Immunoglobulin (Ig) and HRP (horseradish peroxidase)-conjugated secondary antibodies used for enzyme-linked immunosorbent assay (ELISA) were obtained from Jackson Immuno Research Laboratories (West Grove, PA). Buffers and substrates for ELISA were purchased from KPL Biotech (Gaithersburg, MD). New Zealand rabbits were procured from Charles River (Wilmington, MA).
Epitope mapping using phage display library.
A random, linear, dodecapeptide-phage display library (Ph.D-12; New England Biolabs) was used for MAb 2D7 epitope mapping. Affinity selection of the phage clones from the random peptide library was carried out per the manufacturer's instructions with minor modifications. Microtiter wells were coated with 200 ng MAb 2D7 in 100 μl phosphate-buffered saline (PBS), pH 7.4, at 4°C for 12 h followed by 1 h at room temperature (RT). The wells were washed with PBS containing 0.05% Tween 20 (PBST) and blocked with EMEM containing 5% fetal bovine serum (FBS) at RT for 2 h. The phage library (1 × 1010 phages/100 μl) was added to the blocked wells and incubated at RT for 1 h. Unbound phages were washed off with 10 washes of PBST followed by three washes with PBS. The bound phages were eluted at low pH by incubation with elution buffer (0.1 N Gly·HCl, pH 2.2, containing 1 mg/ml bovine serum albumin) at 37°C for 10 min. Eluent phages were neutralized with 6 μl of 2 M Tris (pH unadjusted) per 100 μl phage eluate. After four rounds of biopanning, the phage clones were analyzed by DNA sequencing and affinity-capture phage ELISA.
Phage ELISA.
Phages used for ELISA were derived from clones selected after the fourth round of panning. Microtiter plates coated with 200 ng of MAb 2D7 were blocked with EMEM containing 5% FBS (blocking solution). The phages (1010/100 μl/well) diluted in blocking solution were then added and incubated for 1 h at room temperature (RT). The plates were washed three times with PBST prior to addition of HRP-conjugated anti-phage antibody (GE Healthcare, Piscataway, NJ), and the reaction was quantified using ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate. Absorbance was measured at 405 nm.
Peptide ELISA.
Peptides were biotinylated using maleimide PEO2-Biotin reagent per the manufacturer's instructions (Pierce Biotech, Rockford, IL). The biotinylated 2D7-2SK peptide or a scrambled version of this peptide (2D7-2SK-SCR) (1 μg/well) was captured onto wells coated with 200 ng of streptavidin. After blocking with EMEM containing 5% FBS, serial dilutions of MAb 2D7 in blocking solution were added to each well and incubated for 1 h at RT, followed by addition of peroxidase-conjugated goat anti-mouse IgG. The reaction was quantified using ABTS substrate. Absorbance was measured at 405 nm. The synthetic peptides that were used in the study are outlined in Table 1.
TABLE 1.
Sequences of peptides used in the study
| Peptide identity | Peptide sequence |
|---|---|
| 2D7-2SK | E-W-Q-K-E-G-L-V-T-L-W-L-G-G-G-G-S-C |
| 2D7-2SK-T9A | E-W-Q-K-E-G-L-V-A-L-W-L-G-G-G-G-S-C |
| 2D7-2SK-L10A | E-W-Q-K-E-G-L-V-T-A-W-L-G-G-G-G-S-C |
| 2D7-2SK-T9A,L10A | E-W-Q-K-E-G-L-V-A-A-W-L-G-G-G-G-S-C |
| 2D7-2SK-K4R | E-W-Q-R-E-G-L-V-T-L-W-L-G-G-G-G-S-C |
| 2D7-2SK-SCR | Q-L-W-L-G-W-T-E-L-E-G-V-K-G-G-G-S-C |
| M23 | F-C-A-L-D-G-D-F-G-W-L-A-P-A-C |
Competition phage ELISA using synthetic peptides.
Competition of peptides with selected phage clones for binding to MAb 2D7 was performed by phage ELISA. Synthetic 2D7-2SK peptide (or its biotinylated derivative), or a control peptide (CGRAARIGFPGAYTTKNG), was added to MAb 2D7-coated wells for 30 min at RT. The phages (109/100 μl/well) were added to MAb 2D7-coated wells, which were preincubated in the absence or presence of various concentrations of competing peptides. The reaction was developed as described above for phage ELISA.
Cell-ELISA.
The Cf2Th canine cell line and the hCCR5-transfected Cf2Th/synCCR5 derivative were obtained through the AIDS Research and Reference Reagent Program (catalog no. 4662; contributed by Tajib Mirzabekov and Joseph Sodroski [12], McKesson HBOC BioServices, Rockville, MD). At 48 h before the ELISA, 200 μl of 105 cells/ml was plated in 96-well plates and incubated at 5% CO2 and 37°C. The plates were gently washed with Dulbecco's PBS supplemented with Ca2+ and Mg2+ after each incubation step during ELISA. Serial dilutions of control or anti-CCR5 antibodies in EMEM containing 5% FBS were added to each well followed by addition of peroxidase-conjugated goat anti-mouse or anti-rabbit IgG. The reaction was quantified using ABTS substrate. Absorbance was measured at 405 nm.
Flow cytometry.
CEM.NKR.CCR5 cells (a generous gift from John Moore, Rockefeller University) were stained with 10 μg/ml MAb 2D7 (or isotype-matched control mouse IgG) or with 25 μg/ml rabbit anti-2D7-2SK IgG (or with preimmune rabbit IgG) for 60 min on ice, followed by extensive washing and staining with fluorescein isothiocyanate (FITC) goat anti-mouse or with FITC goat anti-rabbit antibodies (Sigma). Flow cytometry was performed and analyzed with Cell Quest Software on FACSCalibur (BD Biosciences).
Rabbit immunization and antibody purification.
New Zealand rabbits were immunized subcutaneously with 500 μg of KLH-conjugated peptide emulsified in complete Freund's adjuvant. Two booster doses at 21-day intervals, with 500 μg antigen emulsified in incomplete Freund's adjuvant, were injected subcutaneously. The rabbits were bled 8 days after the second boost, and the isolated serum was titrated for peptide binding by ELISA. The polyclonal rabbit IgG was affinity purified on a peptide-coupled gel using a sulfolink kit (Pierce Biotech, Rockford, IL).
Affinity measurements by Biacore.
Steady-state equilibrium binding of MAb 2D7 and rabbit antibodies raised against the 2D7-2SK peptide was monitored at 25°C using a Biacore 3000 surface plasmon resonance biosensor (Biacore AB, Uppsala, Sweden). The 2D7-2SK peptide was coupled to an F1 sensor chip using sulfhydryl coupling with 60 resonance units (RU), 120 RU, and 400 RU in the three test flow cells. Samples of 100 μl of freshly prepared antibody at various concentrations were injected at a flow rate of 25 μl/min (240-s contact time). Flow was directed over a mock surface to which no protein was bound, followed by the 2D7-2SK peptide-coupled surface. Responses from the peptide surface were corrected for the response from the mock surface and for responses from a separate, buffer only, injection. Binding surfaces in the flow cells were regenerated by the injection of two 30-s injections of 10 mM glycine at pH 2.5. All injections were done three times. Rabbit anti-anthrax protective antigen or anti-ovalbumin antibodies were used as control nonspecific antibodies in various binding experiments.
For the competition experiments, various concentrations of rabbit anti-2D7-2SK peptide (diluted with nonimmune rabbit IgG so that the rabbit immunoglobulin concentration was constant) were mixed 1:1 with MAb 2D7 at a concentration of 12.5 μg/ml, and 25 μl samples were injected at a flow rate of 10 μl/min through 2D7-2SK peptide-coated chips. In these experiments, MAb 2D7-specific binding in the presence of rabbit antibodies was quantified by injection of 25 μl of goat anti-mouse IgG (25 μg/ml). The experimental conditions were identical in the reverse competition analysis of the rabbit anti-2D7-2SK binding to 2D7-2SK peptide in the presence of MAb 2D7. In that case, MAb 2D7 was diluted with nonimmune mouse IgG so that the mouse immunoglobulin concentration was kept constant. Bound rabbit antibodies were quantified by a goat anti-rabbit IgG antibody (25 μg/ml) to determine the association of rabbit anti-2D7-2SK to the 2D7-2SK peptide-coated chip in the presence of increasing concentrations of MAb 2D7.
Fusion inhibition assays.
The CD4− T-cell line 12E1 was infected with HIV-1 envelope (env)-expressing recombinant vaccinia viruses at 10 PFU/cell overnight. Envelope-expressing 12E1 cells or TF228 (stably transfected cells expressing IIIB/BH10 envelope) were mixed (1:1) with the human PM1 cell line (a CD4+ CXCR4+ CCR5+ derivative of the Hut 78 cell line that is susceptible to infection by both X4 and R5 strains) (10) or with phytohemagglutinin-activated-interleukin-2-activated rhesus peripheral blood mononuclear cells (PBMC). Various antibodies were added at different concentrations to these target cells and incubated for 1 h at 37°C. The envelope-expressing 12E1 effector cells were then added (at a 1:1 effector/target ratio) as previously described (7). The numbers of multinucleated syncytia were scored at various times after initiation of cocultures. Peak syncytium formation was usually observed between 3 and 5 h. All groups were plated at two or three replicates, and all experiments were done at least three times.
HIV-1 strains and virus neutralization assays.
HIV-1 strains IIIB (X4 virus) and BaL, JR-CSF, or primary isolate 92US657 (R5 viruses) were obtained from the AIDS Research and Reference Reagent Program (McKesson HBOC BioServices, Rockville, MD). Viral stocks were propagated, and their titers were determined in phytohemagglutinin-activated peripheral blood mononuclear cells (PBMC). The CCR5-expressing PM1 cells or human PBMCs (2.5 × 104 cells/well in 96-well plates) were preincubated with the different antibodies (twofold dilutions, starting at 50 or 17.0 μg/ml final concentration) for 120 min at 37°C, followed by addition of viruses at 50 to 100 50% tissue culture infective dose (TCID50)/well (five replicates per group). The plates were washed extensively after 24 h to remove residual virions and antibodies. Every second day thereafter, the supernatants were removed and the cultures were supplemented with fresh medium (without inhibitors). Virus production was determined by measuring p24 in the supernatants with an ELISA kit (NEN Life Sciences Products Inc., Boston, MA). Virus neutralization by the different antibodies is expressed as percent inhibition of p24 production at a given concentration.
RESULTS
MAb 2D7 recognizes a linear peptide containing two distant sequences in the ECL-2 of human CCR5.
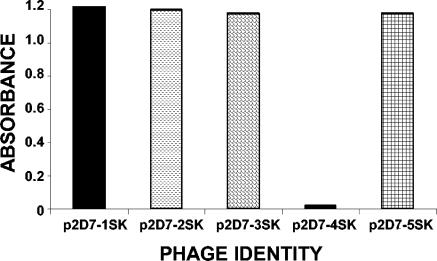
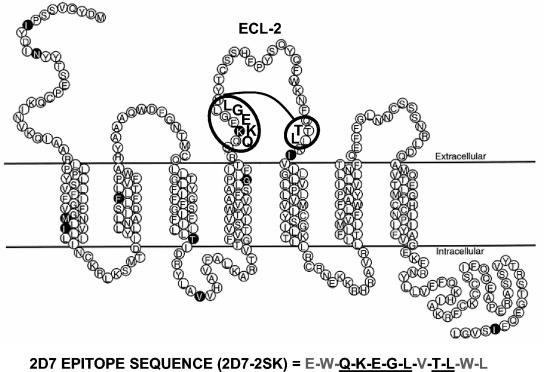
The 2D7 MAb is one of the murine monoclonal antibodies demonstrating a potent inhibition of HIV-1 cell entry. This MAb recognizes an epitope in the first half of ECL-2, which is affected by local conformation and may involve several nonlinear regions (8, 22). Our goal was to identify a linear peptide that binds with high affinity to MAb 2D7 and can be used as an immunogen to elicit 2D7-like antibodies in other species. For that purpose we used a random dodecamer peptide phage-display library to perform biopanning in microtiter wells coated with MAb 2D7. Four rounds of affinity selection led to the isolation of phage clones that showed strong specific reactivity to MAb 2D7 in phage ELISA (Fig. 1). Sequencing of the DNA inserts in four 2D7-binding phages revealed identical peptide sequences with a deduced amino acid sequence, E-W-Q-K-E-G-L-V-T-L-W-L. Alignment of this peptide with the human CCR5 sequence (8, 18) revealed homologies to two distal regions in the ECL-2 of human CCR5 molecule. One located in the amino terminus of ECL-2 (170-QKEGL-174) and the second one at the carboxy terminus of the ECL-2 (195-TL-196) (Fig. 2). The identified peptide insert, termed 2D7-2SK, was chemically synthesized and was used in subsequent binding and functional assays.
FIG. 1.
Selected phage clones react specifically with 2D7 in phage ELISA. Selection of phages expressing random 12-mer peptides on MAb 2D7-coated plates is shown. Clones p2D7-1SK through p2D7-5SK were eluted from panning round IV and were analyzed for their binding to MAb 2D7 by phage ELISA as described in Materials and Methods.
FIG. 2.
Conformational structure of 2D7 epitope on CCR5. The peptide sequences of four specific 2D7-binding phage clones (Fig. 1) from panning round IV were identical. Alignment of the phage-displayed peptide motif with CCR5 ECL-2 identified two regions of homology underlined in the depicted 2D7-2SK peptide sequence. The thread model of human CCR5 is adapted from Siciliano et al. (20). The highlighted amino acids are positions that differ between the human and primate CCR5 proteins.
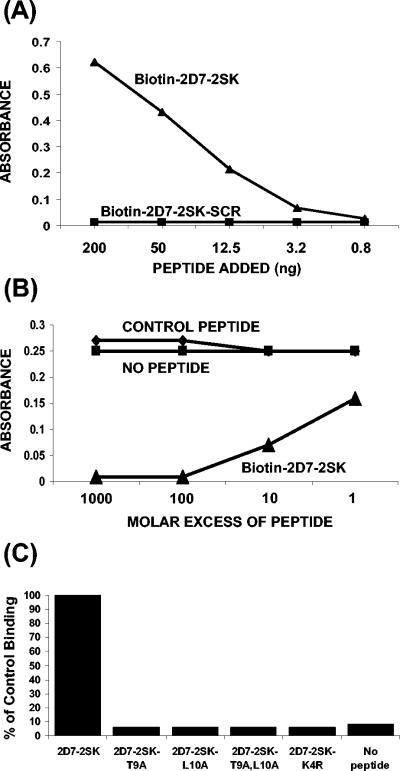
In ELISA, MAb 2D7 showed a concentration-dependent specific reactivity with biotinylated 2D7-2SK peptide (but not with biotinylated scrambled 2D7-2SK peptide) captured by streptavidin (Fig. 3A). Furthermore, binding of phage p2D7-2SK to MAb 2D7-coated plates was inhibited by free 2D7-2SK peptide, but not by a control peptide, in a dose-dependent manner (Fig. 3B).
FIG. 3.
Characterization of MAb 2D7 binding to synthetic peptide 2D7-2SK peptide by ELISA. (A) Direct binding of MAb 2D7 to different concentrations of biotinylated-2D7-2SK or to biotinylated-scrambled 2D7-2SK peptide (2D7-2SK-SCR) captured by streptavidin-coated microtiter plates. The bound antibodies were quantified by HRP-conjugated goat anti-mouse IgG antibody. (B) Competition of binding of phages displaying peptide 2D7-2SK sequence (p2D7-2SK) to MAb 2D7 by soluble 2D7-2SK synthetic peptide. Microtiter wells, coated with MAb 2D7 at 200 ng/well, were incubated with serially diluted synthetic 2D7-2SK peptide or with a control peptide (CGRAARIGFPGAYTTKNG) for 30 min at room temperature. p2D7-2SK phages were then added to all wells (109 phages/well), followed by addition of HRP-conjugated anti-phage antibodies. (C) Identification of critical residues in the 2D7-2SK peptide sequence required for MAb 2D7 binding. MAb 2D7 (50 ng/100 μl/well) was added to plates coated with either unmodified biotin-2D7-2SK peptide or mutated biotin-2D7-2SK peptide derivatives (Table 1), all captured on streptavidin-coated wells. The bound 2D7 antibodies were quantified by HRP-conjugated goat anti-mouse IgG antibody. The absorbance value (0.49) of MAb 2D7 binding to 2D7-2SK peptide (unmodified) is represented as 100% control binding.
The first region of homology with CCR5 includes three amino acids that were previously shown to play a critical role in MAb 2D7 binding (170-QKE-172) (16). However, the two amino acids in the 3′ part of ECL-2 had not been implicated in MAb 2D7 binding. To address their role in the binding of the MAb to the linear peptide, Thr or Leu or both were replaced by alanines. All the alanine substitutions completely abolished binding of MAb 2D7 to the 2D7-2SK peptide in ELISA (Fig. 3C), thus establishing the crucial role played by these amino acids in this peptide for 2D7 binding. The importance of previously reported Lys-171 residue for MAb 2D7 binding was also confirmed in our peptide ELISA, wherein MAb 2D7 completely lost binding to a 2D7-2SK derivative (2D7-2SK-K4R) (Table 1) containing a lysine-to-arginine substitution at the fourth position of the 2D7-2SK peptide. In these experiments, the amounts of different 2D7-2SK peptide derivatives captured by the streptavidin-coated wells were found to be equivalent, as determined by the similar reactivity of rabbit polyclonal antibodies generated against the 2D7-2SK peptide (data not shown).
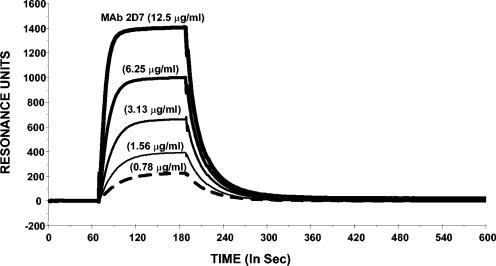
Binding affinity of MAb 2D7 for the 2D7-2SK peptide was determined using surface plasmon resonance analysis in a Biacore system (Fig. 4). The pattern of binding to peptide-coated chips was typical of bivalent antibody molecules. The affinity constant for the binding of MAb 2D7 to 2D7-2SK peptide thus calculated was 0.18 nM.
FIG. 4.
Biacore sensogram for affinity measurements of MAb 2D7 binding to 2D7-2SK peptide. The sensogram shows MAb 2D7 binding to 2D7-2SK peptide immobilized on a CM5 sensor chip through the free thiol group. Several concentrations of 2D7 antibody were injected simultaneously onto both 2D7-2SK peptide and a third flow cell, which was free of peptide and used as a blank. As a control, anti-ovalbumin antibodies were also injected at the same concentrations on the 2D7-2SK peptide-coupled chip, which showed no binding to this peptide (data not shown).
Binding of peptide 2D7-2SK to MAb 2D7 blocks its viral fusion inhibition activity.
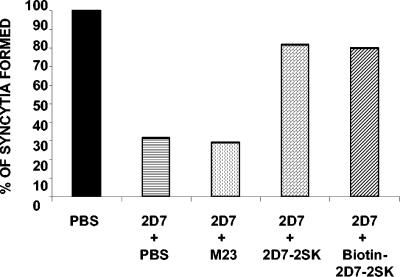
If the linear peptide 2D7-2SK mimics the epitope in the native CCR5 coreceptor recognized by MAb 2D7, then binding of the peptide should interfere with the ability of MAb 2D7 to block HIV-1 envelope-mediated cell fusion. As can be seen in Fig. 5, MAb 2D7 (at 1 μg/ml) blocked fusion between PM1 (CD4+/CCR5+) cells and 12E1 (CD4−) cells expressing JR-FL envelope by 60%, as measured in our syncytium formation assay. The 2D7-2SK peptide by itself did not demonstrate any inhibitory activity in the fusion assay (not shown). Preincubation of MAb 2D7 with 2D7-2SK peptide (either biotinylated or nonbiotinylated) resulted in significant reduction of its fusion-inhibiting activity. Similar results were obtained with 12E1 cells expressing the BaL envelope. In contrast, a previously reported mimitope sequence for MAb 2D7 identified using phage display library (MD23) that contains no homology to CCR5 sequence (11) did not bind to MAb 2D7 (data not shown) and also did not block the fusion inhibiting activity of MAb 2D7 (Fig. 5).
FIG. 5.
Preincubation with soluble 2D7-2SK peptide reduces the fusion inhibition activity of MAb 2D7. 2D7 antibody (at 10 μg/ml) was incubated for 1 h at 37°C with 100-fold molar excess of different peptides before addition to CCR5-expressing PM1 cells for an additional hour at 37°C. Effector 12E1 cells expressing BaL envelope were added, and syncytia were scored after 3 h. The number of syncytia (598/well) observed between effector/target cells in absence of 2D7 antibody is represented as 100%. Fusion inhibition was compared with that of a control culture without IgG additives (PBS). Data shown are representative of four independent experiments. Standard deviations did not exceed 10% of the means for all groups. The 2D7-2SK peptide itself did not demonstrate any inhibitory activity in the fusion assay (not shown).
2D7-2SK peptide can elicit polyclonal antibodies with 2D7-like specificity in rabbits.
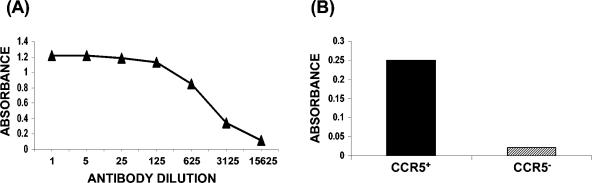
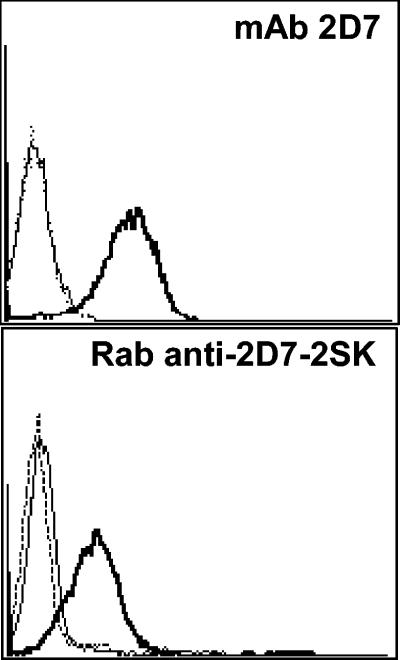
Since 2D7-2SK peptide bound with high affinity to MAb 2D7 and reversed its antiviral activity, it was predicted that this linear peptide acquires a conformation that resembles the epitope recognized by MAb 2D7 in the native form of human CCR5 molecule. To test this hypothesis, we determined whether this peptide can be used as an immunogen to elicit 2D7-like antibodies in animals. To that end, white New Zealand rabbits were immunized thrice with KLH-conjugated 2D7-2SK peptide, and the IgG fraction of the hyperimmune sera was tested in binding assays. The 2D7-2SK-specific antibodies bound in a concentration-dependent fashion to biotinylated 2D7-2SK peptide, captured on streptavidin-coated wells in ELISA (Fig. 6A). No reactivity with control streptavidin-coated wells, or biotinylated-bovine serum albumin captured by streptavidin, was observed, and the preimmune rabbit serum did not react with any of the peptide-coated plates (data not shown). To check whether these rabbit anti-2D7-2SK peptide antibodies recognize native CCR5 molecules expressed on the cell surface, a cell ELISA was performed using the stably transfected CCR5+ Cf2Th cell line (12). The rabbit anti-2D7-2SK antibodies bound CCR5+ Cf2Th cells but only minimally to the CCR5− parental Cf2Th cell line (Fig. 6B). To further confirm the specificity of these rabbit anti-2D7-2SK antibodies to the cell surface native CCR5 molecules, flow cytometry was performed that showed us specific staining of only CCR5-expressing CEM cells by rabbit anti-2D7-2SK antibodies and not by preimmunized rabbit IgG (Fig. 7). No staining of parental CEM cells, not expressing CCR5, was observed.
FIG. 6.
Characterization of rabbit anti-2D7-2SK antibodies. IgG antibodies from rabbit immunized subcutaneously with KLH-conjugated 2D7-2SK peptide were used in peptide and cell ELISA. (A) Serial dilutions of rabbit anti-2D7-2SK antibodies (the starting concentration of antibodies was 283 ng/ml) were added to biotinylated 2D7-2SK captured on streptavidin-coated wells, and the binding was quantified by HRP-conjugated goat anti-rabbit IgG antibody. (B) Rabbit anti-2D7-2SK IgG (28.3 ng/ml) was added to the wells with cultured adherent control Cf2Th canine cells (CCR5−) or Cf2Th cell line expressing CCR5 (CCR5+). Specific antibody binding was measured by HRP-conjugated goat anti-rabbit IgG antibody.
FIG. 7.
Detection of surface CCR5 expression by Rabbit anti-2D7-2SK antibodies. Flow cytometry was used to detect binding of MAb 2D7 and Rab-anti-2D7-2SK to cell surface CCR5 expressed on CEM.NKR.CCR5 cells. Cells were incubated with 25 μg/ml concentrations of each antibody, which were detected with a FITC-labeled goat anti-mouse (top panel) or FITC-labeled goat anti-rabbit IgG (bottom panel). Flow cytometry histograms from a representative experiment are shown: staining of CEM.NKR (thin lines) and CEM.NKR.CCR5 (thick lines) cells with anti-CCR5 MAb 2D7 (top panel) or rabbit anti-2D7-2SK (bottom panel) or with isotype matched controls (dotted lines). Top panel, mouse IgG; bottom panel, preimmune rabbit IgG.
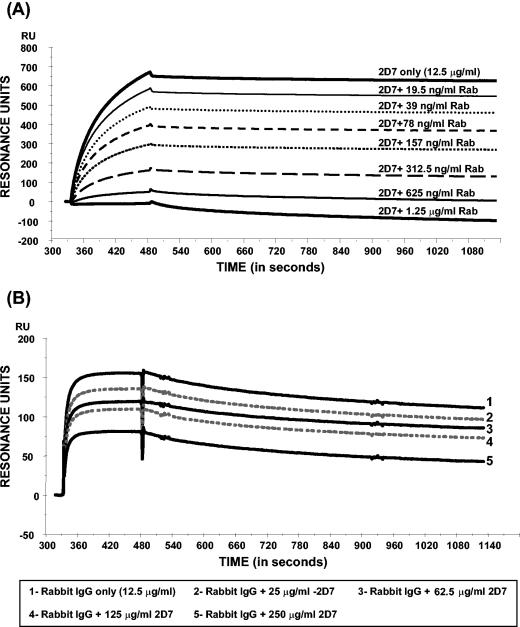
In surface plasmon resonance analysis, the rabbit anti-2D7-2SK antibodies displayed antigen-specific and concentration-dependent binding kinetics to 2D7-2SK peptide. The observed pattern is typical of polyclonal antibodies. The fast on rate, strong binding, and very slow off rate suggest that the rabbit polyclonal IgG contain antibodies with high affinity for 2D7-2SK peptide (Fig. 8). Furthermore, the rabbit antibodies competed with MAb 2D7 for binding to the 2D7-2SK peptide coupled to Biacore CM5 chip (Fig. 9A). This competition was very effective, as even at a 10-fold-lower concentration the rabbit anti-2D7-2SK antibodies (1.25 μg/ml) blocked binding of MAb 2D7 binding (at 12.5 μg/ml) to the 2D7-2SK peptide (Fig. 9A; lowest curve). In a corresponding reverse competitive Biacore analysis, even a 20-fold excess of MAb 2D7 (rabbit IgG-250 μg/ml 2D7) was unable to completely block the binding of rabbit anti-2D7-2SK antibodies to the 2D7-2SK peptide-coupled chip (Fig. 9B).
FIG. 8.
Steady-state equilibrium analysis of rabbit IgG binding to 2D7-2SK peptide by Biacore. Various concentrations of rabbit anti-2D7-2SK IgG antibody were injected simultaneously onto both 2D7-2SK peptide immobilized on a CM5 sensor chip through the free thiol group and a third flow cell, which was free of peptide and used as a blank. Binding was recorded using a Biacore 3000 surface plasmon resonance biosensor instrument. As a control, rabbit anti-anthrax PA antibodies were injected at the same concentrations on a 2D7-2SK peptide-coupled chip.
FIG. 9.
Dose-dependent reciprocal inhibition of MAb 2D7 and rabbit anti-2D7-2SK antibody binding to 2D7-2SK-coated biacore chips. (A) Surface plasmon resonance sensogram depicting binding of MAb 2D7 mixed with increasing concentrations of rabbit anti-2D7-2SK IgG (Rab). Bound MAb 2D7 was detected by goat anti-mouse IgG. The baseline for the sensogram was normalized after injection of rabbit anti-2D7-2SK antibodies and 2D7 through the 2D7-2SK peptide-coated chip. Therefore, the resonance units depicted are only due to the specific binding of anti-mouse IgG antibody to 2D7 to determine the association of MAb 2D7 to the 2D7-2SK peptide-coated chip in the presence of increasing concentrations of rabbit anti-2D7-2SK antibodies. (B) Surface plasmon resonance sensogram depicting binding of rabbit anti-2D7-2SK peptide antibodies mixed with increasing concentrations of 2D7 MAb. Bound rabbit antibodies were detected with goat anti-rabbit IgG. The baseline for the sensogram was normalized as described for Fig. 8A, but here the resonance units depicted are only due to the specific binding of anti-rabbit IgG antibody to rabbit anti-2D7-2SK antibodies to determine the association of rabbit anti-2D7-2SK antibodies to the 2D7-2SK peptide-coated chip in the presence of increasing concentrations of 2D7.
Blocking of HIV fusion and infectivity by rabbit anti-2D7-2SK peptide antibodies.
As similar binding specificities were observed for MAb 2D7 and the rabbit anti-2D7-2SK peptide antibodies, it was important to determine whether they also share the ability to inhibit fusion between R5 HIV-1 envelope-expressing effector cells and CD4+ CCR5+ target cells. As depicted in Fig. 10A, while MAb 2D7 caused 98% fusion inhibition at 10 μg/ml, 77% inhibition was observed for an equivalent concentration of the polyclonal rabbit anti-2D7-2SK antibodies. Similar results were obtained using 12E1-expressing HIV-1 envelopes derived either from BaL or JR-FL strains.
FIG. 10.
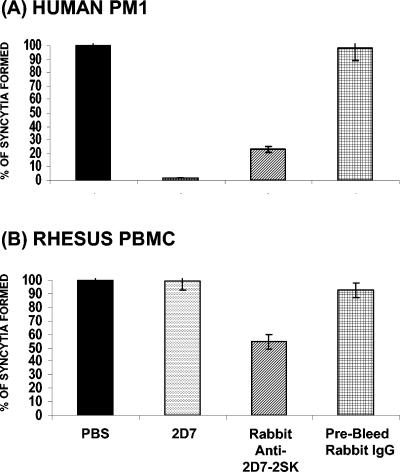
Inhibition of HIV-1-env- or SIV-env-mediated cell fusion by rabbit anti-2D7-2SK antibodies. PM1 cells (A) or activated rhesus PBMCs (B) were incubated for 1 h in the absence (PBS) or presence of 10 μg/ml of 2D7 antibody (2D7), rabbit anti-2D7-2SK IgG (affinity purified on peptide column), or control prebleed rabbit IgG, followed by addition of 12E1 cells expressing HIV-1 BaL envelope (A) or expressing SIVmac239 envelope (B). Fusion inhibition was compared to the number of syncytia observed between E/T cells in absence of antibody (PBS) represented as 100%. The numbers of syncytia observed for fusion of 12E1 cells expressing HIV-1 BaL envelope and PM1 cells were 632/well and for the fusion between rhesus PBMCs and 12E1 cells expressing SIVmac239 envelope were 270/well. Data shown are representative of one of three independent experiments. Standard deviations ranged between 5 to 10% of the means for all groups.
It was previously reported that MAb 2D7 does not interact with rhesus CCR5 and does not block SIV infection. This was attributed to a single substitution (K171R) within the MAb 2D7 putative epitope in rhesus CCR5 (20). The importance of this mutation in MAb 2D7 epitope was confirmed in our peptide ELISA, as MAb 2D7 completely lost binding to a 2D7-2SK derivative (2D7-2SK-K4R) (Fig. 3C) containing a lysine-to-arginine substitution at the fourth position of the 2D7-2SK peptide (Table 1). In further agreement with the above-described finding, MAb 2D7 did not inhibit syncytium formation between of simian immunodeficiency virus (SIV) envelope-expressing cells and the rhesus PBMCs (Fig. 10B). Surprisingly, the rabbit anti-2D7-2SK antibodies showed a moderate but specific inhibition of rhesus PBMC fusion with 12E1 cells expressing SIV envelope (derived either from SIV239 or SIV316), suggesting that a fraction of the polyclonal antibodies recognize CCR5 epitopes that are conserved between human and rhesus CCR5 molecules. Their binding is not entirely dependent on lysine 171, which is crucial for 2D7 binding.
The activity of the rabbit antibodies was further evaluated in cell-free viral infection assays with either PM1 or human PBMC target cells. Using either HIV-1 BaL or JR-CSF R5 viruses (but not X4 virus), significant reduction in p24 was observed in the presence of either MAb 2D7 or rabbit anti-2D7-2SK antibodies at 50 μg/ml final concentration (Fig. 11A). This inhibition of virus entry by rabbit anti-2D7-2SK peptide antibodies was specific, as control rabbit IgGs at similar antibody concentrations did not show any decrease in HIV-1 infection. The CCR5-tropic virus neutralization by polyclonal rabbit anti-2D7-2SK antibodies (72 to 80%) was comparatively lower than that observed for equivalent amounts of 2D7 monoclonal antibody (82 to 93%) in multiple experiments. Antibody titration in PBMC infectivity experiments with the purified rabbit anti-2D7-2SK antibodies (or preimmune antibodies) was performed using BaL, JR-CSF, and primary isolate 92US657 (Fig. 11B; data shown for 92US657). The calculated 50% inhibitory concentration values were between 10 to 20 μg/ml for all the viruses tested. This is in comparison to 50% inhibitory concentration values of 2.5 to 10 μg/ml with MAb 2D7 observed for these isolates in these and previously conducted studies.
FIG. 11.
Neutralization of HIV-1 infection by MAb 2D7 and rabbit anti-2D7-2SK peptide antibodies. (A) Antibody preparations (at 50 μg/ml) were added to CCR5-expressing PM1 cells or activated human PBMCs for 1 h prior to addition of R5 tropic HIV-1 BaL (or JR-CSF) virus at 50 TCID50/well (5 replicates per group). After 24 h, plates were washed extensively to remove unbound virus and antibodies. Virus production was determined by measuring p24 in the supernatants every 2 days. Virus neutralization by the different antibodies is expressed as percent inhibition of p24 production (data represent average p24 values in 5 wells of a 96-well plate). Data shown are for day 5 (PM1) or day 7 (PBMC). Infection inhibition was compared with that of a control culture with no antibody added (PBS), which was defined as 100% infection (equivalent to p24 values of 64,484 pg/ml for HIV-1 BaL infection of PM1 cells and 40,567 pg/ml for infection of human PBMCs). Data shown are representative of one of four independent experiments for infection of PM1 cells and three independent experiments with human PBMCs. (B) Dose-dependent inhibition of infection by R5-tropic primary isolate 92US657 in human PBMC. The experiment was performed as described for panel A but with lower starting concentrations of rabbit anti-2D7-2SK antibodies, as shown on the x axis, with infection by virus at 50 TCID50/well (four replicates per group). No inhibition was observed with preimmune rabbit IgG.
Together these data suggest that the linear 12-mer peptide, which was identified by affinity selection of random peptide phage-display library on immobilized MAb 2D7, behaves similarly to the CCR5 conformational epitope recognized by this potent monoclonal antibody. Furthermore, this peptide mimitope is capable of eliciting 2D7-like HIV-1-neutralizing antibodies in rabbits.
DISCUSSION
MAb 2D7, generated by Wu et al. (22), remains one of the most potent inhibitors of HIV-1 entry, targeting the CCR5 coreceptor. It was shown to bind to the 5′ half of CCR5 ECL-2, in a region that includes residues 171-KE-172 (16). It was further demonstrated that MAb 2D7 recognizes a conformation-sensitive epitope and interacts with a greater proportion of CCR5 molecules on the cell surface compared with other CCR5-specific monoclonal antibodies (3, 8). Thus, the identification of a linear sequence resembling the native conformational epitope could facilitate the isolation of 2D7-like human monoclonal antibodies, which may have therapeutic and transmission-prevention uses. It may also be considered for active vaccination, as recently proposed by other groups (4, 5, 13).
A random-peptide, phage-display library approach was employed to identify sequences that are recognized by MAb 2D7 with high affinity and are capable of blocking its binding to CCR5 and reversing its HIV-1 fusion-inhibiting activity. To improve the chance for success, the screening of the phage library was conducted in culture medium containing EMEM and FCS to mimic the natural condition for 2D7-mediated neutralizing activity in cell culture. Moreover, all high-affinity binders were tested early in our MAb 2D7-mediated fusion inhibition assay. This approach resulted in a panel of phage clones displaying identical peptide sequences. Interestingly, the 12-amino-acid insert shared homology with two nonlinear regions in CCR5, namely, residues QKEGL, located in the 5′ of ECL-2, and residues TL, located in the 3′ of CCR5 ECL-2 close to the predicted TM5. Importantly, a soluble synthetic peptide containing this sequence bound strongly to MAb 2D7. Thus, the identified linear peptide is capable of acquiring a conformational structure in solution similar to that recognized by MAb 2D7 in the native surface CCR5 molecules. The first five amino acids include the 171-KE-172 motif, previously shown in mutagenesis studies to play a critical role in MAb 2D7 binding to human CCR5 (16). The 3′ ECL-2 amino acids TL have not been previously reported to contribute to MAb 2D7 binding. However, our peptide mutagenesis data demonstrate that these two amino acids play a critical role in the binding of MAb 2D7 to the linear peptide, suggesting that this peptide has a conformational structure that strongly mimics the spatial contributions of two distal sequences in the membrane-proximal regions of ECL-2 loop. We plan to investigate whether these residues in the native structure of CCR5 are required contact residues for this monoclonal antibody. This may not be supported by the current predicted theoretical structure model of CCR5 based on the available model for bacteriorhodopsin (1). It is conceivable that the 3′ TL residues play an important role in a secondary structure assumed by the free peptide in solution, which mimics the native 2D7 epitope in CCR5, thus contributing indirectly to its high-affinity binding to MAb 2D7. The Biacore sensogram confirmed the specificity of MAb 2D7 strong binding to the 2D7-SK peptide, and the calculated association constant of 0.18 nM is within the predicted affinity values for binding of MAb 2D7 to native CCR5. More importantly, preincubation of MAb 2D7 with the soluble peptide significantly reduced its ability to block HIV-1 R5 env-mediated cell fusion. A similar approach by another laboratory had identified an unrelated phage-g3p fusion peptide (MD23) with an insert that bore no sequence homology to CCR5 (11). A synthetic peptide containing this sequence (Table 1) was tested in the current study and had no biological activity in our assays. The different outcomes may relate to our improved screening procedures, coupled with testing for desired functional activity in a relevant fusion assay.
An important proof of concept for the potential use of this peptide for vaccination came from immunizing rabbits with KLH-conjugated 2D7-SK peptide. The purified rabbit IgG bound specifically to the 2D7 peptide in ELISA and Biacore sensogram and displaced MAb 2D7 in a dose-dependent fashion. Moreover, these rabbit anti-2D7-2SK antibodies displayed CCR5-specific cell surface staining similar to MAb 2D7. Importantly, the polyclonal antibodies displayed MAb 2D7-like HIV-1 entry-inhibitory activities. They blocked HIV-1 R5 (BaL, JR-FL, ADA) env-mediated fusion of PM1 cells and neutralized infection of activated human PBMC with BaL-, JR-CSF-, and R5-dependent primary 92US657 viruses with a reasonable potency. Unexpectedly, the rabbit anti-2D7-SK antibodies demonstrated a modest but consistent inhibition of SIV env-mediated fusion with activated rhesus PBMC. No such inhibition was ever demonstrated with MAb 2D7. The lack of 2D7 cross-species reactivity was explained by a critical K→R substitution in residue 171 in the rhesus CCR5 molecules (20). This was confirmed in our study, where a single-point mutation of K→R in the 2D7-2SK peptide sequence obliterated the binding of MAb 2D7 to the 2D7-2SK-K4R peptide, thus suggesting that the immune sera generated by 2D7-SK peptide in rabbits contain some cross-reactive antibodies that recognize epitopes common to human and rhesus CCR5 molecules, which are not dependent on the presence of lysine at amino acid 171.
The 2D7-SK peptide was recently used to screen a synthetic human scFv immunoglobulin library. Thus far, we have isolated few scFv monoclonal antibodies with HIV-1 neutralization activity. Construction of complete Fc-containing human antibody is under way. Such human monoclonal antibodies may provide additional therapeutic approaches to the treatment of chronic HIV infections as well as for prevention of HIV transmission. Another anti-CCR5 murine MAb (PRO140) that was humanized recently (19, 21) is entering human clinical trials. In addition to passive immunity, the 2D7-2SK sequence may also be incorporated into a vaccine construct to be used for active immunization of high-risk populations, as previously demonstrated in the primate model by others (4, 5).
REFERENCES
- 1.Abdulaev, N. G., T. T. Strassmaier, T. Ngo, R. Chen, H. Luecke, D. D. Oprian, and K. D. Ridge. 2002. Grafting segments from the extracellular surface of CCR5 onto a bacteriorhodopsin transmembrane scaffold confers HIV-1 coreceptor activity. Structure (Cambridge) 10:515-525. [DOI] [PubMed] [Google Scholar]
- 2.Balotta, C., P. Bagnarelli, M. Violin, A. L. Ridolfo, D. Zhou, A. Berlusconi, S. Corvasce, M. Corbellino, M. Clementi, M. Clerici, M. Moroni, and M. Galli. 1997. Homozygous delta 32 deletion of the CCR-5 chemokine receptor gene in an HIV-1-infected patient. AIDS 11:F67-F71. [DOI] [PubMed] [Google Scholar]
- 3.Blanpain, C., B. Lee, J. Vakili, B. J. Doranz, C. Govaerts, I. Migeotte, M. Sharron, V. Dupriez, G. Vassart, R. W. Doms, and M. Parmentier. 1999. Extracellular cysteines of CCR5 are required for chemokine binding, but dispensable for HIV-1 coreceptor activity. J. Biol. Chem. 274:18902-18908. [DOI] [PubMed] [Google Scholar]
- 4.Bogers, W. M., L. A. Bergmeier, J. Ma, H. Oostermeijer, Y. Wang, C. G. Kelly, P. Ten Haaft, M. Singh, J. L. Heeney, and T. Lehner. 2004. A novel HIV-CCR5 receptor vaccine strategy in the control of mucosal SIV/HIV infection. AIDS 18:25-36. [DOI] [PubMed] [Google Scholar]
- 5.Chackerian, B., L. Briglio, P. S. Albert, D. R. Lowy, and J. T. Schiller. 2004. Induction of autoantibodies to CCR5 in macaques and subsequent effects upon challenge with an R5-tropic simian/human immunodeficiency virus. J. Virol. 78:4037-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 7.Golding, H., M. Zaitseva, E. de Rosny, L. R. King, J. Manischewitz, I. Sidorov, M. K. Gorny, S. Zolla-Pazner, D. S. Dimitrov, and C. D. Weiss. 2002. Dissection of human immunodeficiency virus type 1 entry with neutralizing antibodies to gp41 fusion intermediates. J. Virol. 76:6780-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee, B., M. Sharron, C. Blanpain, B. J. Doranz, J. Vakili, P. Setoh, E. Berg, G. Liu, H. R. Guy, S. R. Durell, M. Parmentier, C. N. Chang, K. Price, M. Tsang, and R. W. Doms. 1999. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J. Biol. Chem. 274:9617-9626. [DOI] [PubMed] [Google Scholar]
- 9.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 10.Lusso, P., F. Cocchi, C. Balotta, P. D. Markham, A. Louie, P. Farci, R. Pal, R. C. Gallo, and M. S. Reitz, Jr. 1995. Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1. J. Virol. 69:3712-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meta, A., N. Torigoe, Y. Ito, R. Arakaki, H. Nakashima, and K. Sugimura. 1999. Inhibition of M-tropic HIV-1 infection by the fd phage-gene 3 protein with MIP-1alpha-binding activity. Mol. Immunol. 36:1249-1254. [DOI] [PubMed] [Google Scholar]
- 12.Mirzabekov, T., N. Bannert, M. Farzan, W. Hofmann, P. Kolchinsky, L. Wu, R. Wyatt, and J. Sodroski. 1999. Enhanced expression, native purification, and characterization of CCR5, a principal HIV-1 coreceptor. J. Biol. Chem. 274:28745-28750. [DOI] [PubMed] [Google Scholar]
- 13.Mooij, P., I. G. Nieuwenhuis, C. J. Knoop, R. W. Doms, W. M. Bogers, P. J. Ten Haaft, H. Niphuis, W. Koornstra, K. Bieler, J. Kostler, B. Morein, A. Cafaro, B. Ensoli, R. Wagner, and J. L. Heeney. 2004. Qualitative T-helper responses to multiple viral antigens correlate with vaccine-induced immunity to simian/human immunodeficiency virus infection. J. Virol. 78:3333-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen, G. T., M. Carrington, J. A. Beeler, M. Dean, L. M. Aledort, P. M. Blatt, A. R. Cohen, D. DiMichele, M. E. Eyster, C. M. Kessler, B. Konkle, C. Leissinger, N. Luban, S. J. O'Brien, J. J. Goedert, and T. R. O'Brien. 1999. Phenotypic expressions of CCR5-delta32/delta32 homozygosity. J. Acquir. Immune Defic. Syndr. 22:75-82. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien, T. R., and J. J. Goedert. 1998. Chemokine receptors and genetic variability: another leap in HIV research. JAMA 279:317-318. [DOI] [PubMed] [Google Scholar]
- 16.Olson, W. C., G. E. Rabut, K. A. Nagashima, D. N. Tran, D. J. Anselma, S. P. Monard, J. P. Segal, D. A. Thompson, F. Kajumo, Y. Guo, J. P. Moore, P. J. Maddon, and T. Dragic. 1999. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J. Virol. 73:4145-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paxton, W. A., T. Dragic, R. A. Koup, and J. P. Moore. 1996. The beta-chemokines, HIV type 1 second receptors, and exposed uninfected persons. AIDS Res. Hum. Retrovir. 12:1203-1207. [DOI] [PubMed] [Google Scholar]
- 18.Samson, M., P. Soularue, G. Vassart, and M. Parmentier. 1996. The genes encoding the human CC-chemokine receptors CC-CKR1 to CC-CKR5 (CMKBR1-CMKBR5) are clustered in the p21.3-p24 region of chromosome 3. Genomics 36:522-526. [DOI] [PubMed] [Google Scholar]
- 19.Shaheen, F., and R. G. Collman. 2004. Co-receptor antagonists as HIV-1 entry inhibitors. Curr. Opin. Infect. Dis. 17:7-16. [DOI] [PubMed] [Google Scholar]
- 20.Siciliano, S. J., S. E. Kuhmann, Y. Weng, N. Madani, M. S. Springer, J. E. Lineberger, R. Danzeisen, M. D. Miller, M. P. Kavanaugh, J. A. DeMartino, and D. Kabat. 1999. A critical site in the core of the CCR5 chemokine receptor required for binding and infectivity of human immunodeficiency virus type 1. J. Biol. Chem. 274:1905-1913. [DOI] [PubMed] [Google Scholar]
- 21.Trkola, A., T. J. Ketas, K. A. Nagashima, L. Zhao, T. Cilliers, L. Morris, J. P. Moore, P. J. Maddon, and W. C. Olson. 2001. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J. Virol. 75:579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu, L., G. LaRosa, N. Kassam, C. J. Gordon, H. Heath, N. Ruffing, H. Chen, J. Humblias, M. Samson, M. Parmentier, J. P. Moore, and C. R. Mackay. 1997. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J. Exp. Med. 186:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaitseva, M., K. Peden, and H. Golding. 2003. HIV coreceptors: role of structure, posttranslational modifications, and internalization in viral-cell fusion and as targets for entry inhibitors. Biochim. Biophys. Acta 1614:51-61. [DOI] [PubMed] [Google Scholar]