Abstract
We report that late in a simian virus 40 (SV40) infection in CV-1 cells, there are significant decreases in phosphorylations of two mammalian target of rapamycin (mTOR) signaling effectors, the eIF4E-binding protein (4E-BP1) and p70 S6 kinase (p70S6K). The hypophosphorylation of 4E-BP1 results in 4E-BP1 binding to eIF4E, leading to the inhibition of cap-dependent translation. The dephosphorylation of 4E-BP1 is specifically mediated by SV40 small t antigen and requires the protein phosphatase 2A binding domain but not an active DnaJ domain. Serum-starved primary African green monkey kidney (AGMK) cells also showed decreased phosphorylations of mTOR, 4E-BP1, and p70S6K at late times in infection (48 h postinfection [hpi]). However, at earlier times (12 and 24 hpi), in AGMK cells, phosphorylated p70S6K was moderately increased, correlating with a significant increase in phosphorylation of the p70S6K substrate, ribosomal protein S6. Hyperphosphorylation of 4E-BP1 at early times could not be determined, since hyperphosphorylated 4E-BP1 was present in mock-infected AGMK cells. Elevated levels of phosphorylated eIF4G, a third mTOR effector, were detected in both CV-1 and AGMK cells at all times after infection, indicating that eIF4G phosphorylation was induced throughout the infection and unaffected by small t antigen. The data suggest that during SV40 lytic infection in monkey cells, the phosphorylations of p70S6K, S6, and eIF4G are increased early in the infection (12 and 24 hpi), but late in the infection (48 hpi), the phosphorylations of mTOR, p70S6K, and 4E-BP1 are dramatically decreased by a mechanism mediated, at least in part, by small t antigen.
Simian virus 40 (SV40) and other mammalian double-stranded DNA viruses adapt cellular processes to permit the synthesis of viral proteins, the replication of viral DNA, and the production of new virions. These adaptations result in increased metabolic, growth, and synthetic rates, which may induce stress in the cells. Such stress initiates cellular stress responses, which mediate signaling designed to limit cellular activity, while the cells attempt to recover. One consequence of these stress responses is the inhibition of translation (1, 11, 15), an event which would be deleterious to the course of the infection; thus, we hypothesized that viruses have means to adapt or counteract the effects of these stress responses. Indeed, our studies of the human cytomegalovirus have confirmed this (16).
In the present study, we specifically examine the effects of SV40 infection on signaling mediated by mammalian target of rapamycin (mTOR) (3, 25), a major controller of translational initiation (11, 23) and a target for inactivation by (i) cellular stress responses, such as hypoxia (1, 22), which causes mTOR to be maintained in its hypophosphorylated, inactive state, and (ii) the drug rapamycin (32), which blocks active mTOR from phosphorylating its downstream effectors (8, 9, 11). mTOR is believed to be activated by a number of signaling pathways, including PI3K/Akt signaling (11). We and others have previously shown that viral proteins such as SV40 large T and small t antigens, polyomavirus middle T antigen, and the human cytomegalovirus major immediate early proteins can each activate PI3K/Akt signaling (5, 14, 31, 36, 37), thus potentially activating mTOR.
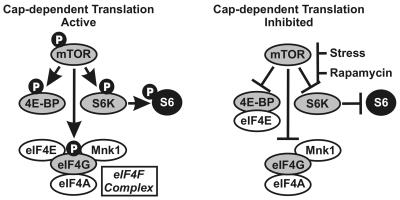
Activated mTOR increases translation by phosphorylating three major effectors (Fig. 1) (11). The phosphorylation of eIF4E-binding protein (4E-BP1) prevents it from binding eIF4E, the cap-binding protein. During active cap-dependent translation, eIF4E is a component of the eIF4F complex, which also contains an RNA helicase, eIF4A, the Mnk1 kinase, and the scaffolding protein eIF4G. As part of eIF4F, eIF4E is maintained in an active state, through phosphorylation by Mnk-1, and is able to mediate cap-dependent translation. Under stressful conditions (e.g., nutrient deprivation or hypoxia), mTOR becomes inactive (hypophosphorylated) and fails to phosphorylate 4E-BP1. This allows 4E-BP1 to bind eIF4E, dissociating it from the eIF4F complex, thus inhibiting cap-dependent translation (11). mTOR also phosphorylates and activates p70 S6 kinase (p70S6K), resulting in the phosphorylation of ribosomal protein S6; this correlates with increased ribosome biogenesis (11). Both p70S6K and 4E-BP1 can be converted from the phosphorylated state to the hypophosphorylated state by protein phosphatase 2A (PP2A), the major phosphatase to counteract mTOR kinase (19, 20). mTOR's third substrate is eIF4G, the scaffolding protein of eIF4F; the functional consequences of eIF4G phosphorylation are unknown (11).
FIG. 1.
The mTOR pathway. See the text for details. “p” indicates protein phosphorylation.
SV40 small t antigen plays a key role in permissive and nonpermissive infections, increasing virus yields in lytically infected primate cells and enhancing the ability of large T antigen to transform rodent and human cells (24). Small t antigen is a 174-amino-acid protein which shares the N-terminal 82 residues with large T antigen. This shared region contains a DnaJ domain which has been implicated in many functions of these proteins (24, 29, 30). The mutations of residues 43 and 45 eliminate the DnaJ activity of small t antigen (21, 24, 29, 30). The remaining 92 amino acids form the small t antigen's unique carboxyl terminal domain, which binds to PP2A (24). The mutation of either cysteine 97 or cysteine 103 severely diminishes PP2A binding (18). Mutations in both the DnaJ and PP2A binding regions decrease SV40's transformation efficiency (24, 29).
PP2A is a major protein phosphatase in eukaryotic cells that counteracts the activity of several protein kinases (17). The heterotrimeric complex contains a catalytic C subunit constitutively bound to scaffold subunit A. The AC core dimer can further complex with an array of regulatory B subunits which modulate catalytic activity, substrate specificity, and subcellular localization (27). Small t antigen can displace some B subunits from the AC core dimer to form a tAC trimer (24). Small t decreases PP2A phosphatase activity for most substrates; consequently, these substrates may become hyperphosphorylated by their cognate kinases.
Here we report that during SV40 lytic infection in monkey cells, the phosphorylations of p70S6K, S6, and eIF4G are increased early in infection (12 and 24 h postinfection [hpi]), but at late times (48 hpi), the phosphorylations of mTOR, p70S6K, and 4E-BP1 are dramatically decreased by a mechanism mediated by small t antigen and requiring small t antigen's PP2A binding domain.
MATERIALS AND METHODS
Cells and viruses.
CV-1 cells, an established African green monkey kidney (AGMK) cell line, were propagated and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM Gluta-Max (all reagents from GIBCO-BRL, Gaithersburg, MD). Primary AGMK cells were purchased from Diagnostics Hybrids, Inc., Athens, Ohio, and grown in DMEM containing 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM Gluta-Max. Rapamycin was added to culture medium at 50 nM. Wild-type SV40, strain 776, and small t antigen deletion mutant dl888 (28) were propagated and their titers were determined on CV-1 cells. Virus stocks were prepared by infecting CV-1 cells at a multiplicity of infection (MOI) of 0.1. When cytopathic effect was seen throughout the culture, the medium was harvested and cleared of cell debris by centrifugation at 5,000 rpm for 30 min at 4°C. The viruses were purified by velocity centrifugation; 25 ml of infected cell supernatant was underlaid with a 10 ml sucrose cushion containing 20% sucrose, 50 mM Tris-HCl (pH 7.2), 1 mM MgCl2, and 100 μg of bacitracin/ml in an Ultra-Clear centrifuge tube. Virions were pelleted onto the cushion by centrifugation at 25,000 rpm for 90 min at 4°C in a Beckman SW28 rotor. Virion pellets were retrieved from the cushion and resuspended in serum-free DMEM, the titers were determined, and the purified virions were stored at −80°C.
293 cells were maintained in DMEM containing 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM Gluta-Max and were used to grow recombinant adenoviruses. All adenovirus infections, except infections for making virus stocks, were performed in serum-free conditions.
Construction of recombinant adenoviruses.
Recombinant adenoviruses expressing large T antigen (Ad-LT) and small t antigen (Ad-st) were constructed using established vectors and procedures (6, 12). cDNAs of SV40 large T and small t antigens were cloned into the adenovirus shuttle plasmid pBHad-CMV. One-half of a microgram of EcoRI-digested shuttle plasmid DNA and 0.5 μg of ClaI-digested adenovirus DNA (derived from an adenovirus which expresses green fluorescent protein [Ad-GFP]) were cotransfected into 293 cells. Plaques produced by recombinant adenoviruses were purified and expanded, and proper protein production was confirmed by a Western blot analysis of expressed protein from infected 293 cells. Each recombinant adenovirus was plaque purified three times. Adenoviruses expressing the P43L/K45N and C103S mutants of small t antigen were gifts from Kathy Rundell (13).
Western analysis.
The procedures for Western analysis and the preparation of cellular extracts for Western analysis have been previously described (36). The vendor catalogue numbers for each purchased antibody are provided in parentheses. The following primary antibodies were purchased from Cell Signaling Technology, Beverly, Mass.: anti-phospho-p70S6K Thr 389 (9205S), anti-phospho-S6 protein Ser235/236 (2211S), anti-S6 protein (2212), anti-4E-BP1 (9452), anti-phospho-eIF4G Ser 1108 (2441S), anti-phospho mTOR Ser2448 (2971S), anti-mTOR (2972), anti-phospho-eIF4E Ser 209 (9741S), and anti-eIF4E (9742). Anti-eIF4G (sc-11373) and antiactin (sc-1615) were purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, Calif. Anti-p70S6K (611260) was purchased from BD Biosciences Pharmingen, San Diego, Calif. The monoclonal antibody PAb419, which reacts with both large T and small t antigens, was produced and purified in this lab.
Metabolic labeling.
CV-1 cells were washed with methionine/cysteine-free DMEM and labeled with [35S]methionine/cysteine (Amersham Biosciences) in methionine/cysteine-free DMEM (100 μCi/ml) for 30 min. The cells were lysed in radioimmunoprecipitation assay (RIPA) buffer. Forty micrograms of total cell lysate protein was separated by 11% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Gels were dried and visualized by autoradiography.
Cap-binding protein affinity purification.
Cells were extracted by use of complete affinity purification (AP) buffer (0.5% Triton X-100, 50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1.5 mM EDTA, 20 mM β-glycerophosphate, 10 mM NaF, 200 μM NaVO4, 2 mM phenylmethylsulfonyl fluoride, 1.5 μg/ml aprotinin, and 1.0 μg/ml leupeptin). Extracts were clarified by centrifugation in a Sorvall MC12C tabletop centrifuge at top speed for 10 min and proteins quantitated by the Bradford method. Two hundred micrograms of total extract protein was incubated with a 10-μl volume of m7-GTP Sepharose beads for 2 h at 4°C. The beads were washed twice with cold AP buffer, boiled in 20 μl of 1 × SDS loading buffer (62.5 mM Tris-HCl, pH 6.8, 2% glycerol, 150 mM 2-mercaptoethanol, 0.01% bromophenol blue), separated by 12% SDS-PAGE, and subjected to Western analysis.
RESULTS
Phosphorylation of p70S6K, S6, and 4E-BP1 is inhibited in CV-1 cells late in an SV40 infection.
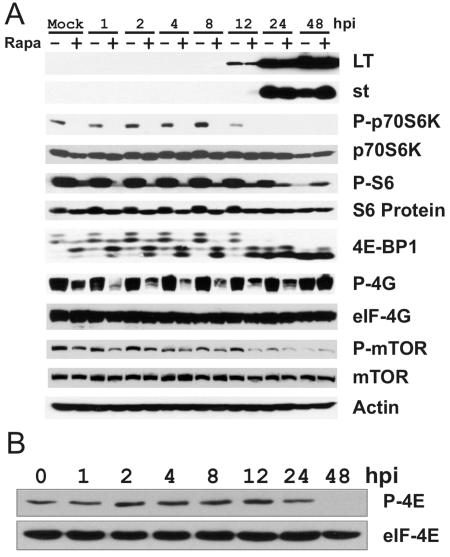
To investigate the relationship between SV40 infection and cellular translational control, confluent CV-1 cells were serum starved for 24 to 48 h. The serum-starved cells were treated with either 50 nM rapamycin (dissolved in dimethyl sulfoxide [DMSO]) or an equal volume of DMSO for 1 h prior to infection. The cells were infected with purified, serum-free SV40 at a multiplicity of infection of 4 PFU per cell, in the presence or absence of 50 nM rapamycin. Cell lysates were prepared at the indicated hours post infection, and proteins of interest were analyzed by Western analysis (Fig. 2A). Detectable levels of SV40 large T antigen substantially accumulate between 8 and 12 hpi; upon longer exposure, large T antigen is clearly present in the 8-h sample (not shown). Small t antigen is clearly detectable by 24 hpi and can be detected at 12 hpi upon longer exposure (not shown). The presence of rapamycin in the cultures made no difference in the appearance or accumulation of the SV40 proteins.
FIG. 2.
SV40 infection affects mTOR signaling in CV-1 cells. (A) Serum-starved CV-1 cells were pretreated with 50 nM rapamycin or the same volume of DMSO (the rapamycin solvent) for 1 h at 37°C and then mock infected or infected with wild-type SV40 strain 776 at an MOI of 4 in the presence or absence of 50 nM rapamycin. Whole-cell lysates were harvested in RIPA buffer at 0 (Mock), 1, 2, 4, 8, 12, 24, and 48 hpi. Fifty micrograms of lysate protein was analyzed by Western analysis for large T and small t antigen expression and the levels of phosphorylated (P) and total p70S6K (Thr389), total ribosome protein S6 (Ser235/236), total eIF4G (Ser1108), and total mTOR (Ser2448). 4E-BP1 was examined using an antibody which detects total 4E-BP1; the hyperphosphorylated forms were detected by mobility differences. The levels of actin protein, which served as the loading control, were determined. (B) The levels of total eIF4E and phosphorylated eIF4E (Ser209) (P-4E) were determined by Western analysis of samples harvested at the indicated times postinfection.
We next examined the levels and phosphorylation status of mTOR effectors by using antibodies specific for the phosphorylated forms and/or antibodies which recognize the total levels of the proteins (Fig. 2A). In mock-infected, serum-starved cells, we noted relatively high levels of phosphorylated p70S6K (P-p70S6K, Fig. 2A), phosphorylated S6 (P-S6), hyperphosphorylated 4E-BP1, and phosphorylated eIF4G (P-4G). The phosphorylation of each of these was inhibited by rapamycin, indicating that, despite serum starvation, mTOR kinase activity remained high in the CV-1 cells. This is not surprising, since CV-1 cells are established and immortalized and are not significantly growth arrested by serum starvation.
In infected cells, the phosphorylation of p70S6K increased moderately up to 8 hpi. In all cases, the phosphorylation was rapamycin sensitive. By 12 and 24 hpi, concomitant with detectable expression of small t antigen, phosphorylated p70S6K decreased and became undetectable regardless of the presence of rapamycin. Examination of total protein (p70S6K) (Fig. 2A) shows a moderate decrease in p70S6K by 48 hpi; however, this cannot account for the total loss of the phosphorylated forms at 24 and 48 hpi.
In agreement with the loss of active, phosphorylated p70S6K, the levels of P-S6 (Fig. 2A) decreased by 24 h postinfection and were very low by 48 h postinfection. Total levels of S6 (Fig. 2A) did not vary significantly over the course of the infection.
A similar loss of hyperphosphorylated forms of 4E-BP1 was noted over the infection time course. In these Western analyses, we used an antibody that recognizes total 4E-BP1; the several hyperphosphorylated forms are indicated by the slower migrating bands which disappear upon rapamycin treatment (Fig. 2A). Significant levels of hyperphosphorylated 4E-BP1 remained through 12 hpi; this phosphorylation is sensitive to rapamycin. Between 12 and 24 hpi, hyperphosphorylated forms of 4E-BP1 became undetectable in the infected cells regardless of the presence of rapamycin.
Unlike with p70S6K and 4E-BP1, the phosphorylation of eIF4G (Fig. 2A) did not decrease during the course of the infection in the absence of rapamycin, remaining as high at 48 hpi as it was in mock-infected cells. Rapamycin treatment lowered the levels of phosphorylated eIF4G but did not completely inhibit the phosphorylation. Resistance to rapamycin appears to increase after 12 hpi, and by 48 hpi, the phosphorylation of eIF4G was repeatedly found to be insensitive to rapamycin. The total levels of eIF4G (Fig. 2A) were stable throughout the infection time course.
The hypophosphorylated 4E-BP1 seen late in infection is predicted to bind eIF4E, the cap-binding protein, and inhibit cap-dependent translation by sequestering eIF4E away from its interaction with eIF4G in the eIF4F complex. When eIF4E is not in the eIF4F complex, its phosphorylation on Ser209 cannot be maintained (2, 10). Thus, we examined the phosphorylation of eIF4E over the infection time course (Fig. 2B). Despite the presence of hypophosphorylated 4E-BP1, by 24 hpi (Fig. 2A), there remains a significant level of phosphorylated eIF4E (P-4E) (Fig. 2B), suggesting that some eIF4E remains in the eIF4F complex at 24 h postinfection. However, by 48 h postinfection, no phosphorylated eIF4E is detected (Fig. 2B), in agreement with the extreme hypophosphorylation of 4E-BP1 at 48 h postinfection (Fig. 2A). Total levels of eIF4E remained constant during the course of the infection; therefore, the loss of phosphorylated eIF4E at 48 h is not due to a loss of total protein.
The loss of the phosphorylations of p70S6K and 4E-BP1 late in infection suggests that the activation of mTOR is lost late in infection. mTOR is activated via phosphorylation on Ser2448. By use of a phospho-specific antibody, it can be seen that phosphorylated mTOR (P-mTOR) does decrease in the infected cells by 24 hpi and is very low by 48 hpi (Fig. 2A). Total levels of mTOR do not change during the course of the infection (Fig. 2A).
Cellular translation is attenuated late in SV40 infection in CV-1 cells.
The effects of SV40 infection on the phosphorylation levels of p70S6K, S6, 4E-BP1, and eIF4E suggest that translation, particularly cap-dependent translation, may be inhibited. To test this, serum-starved CV-1 cells were mock infected or SV40 infected for 24 and 48 h and then pulse labeled for 30 min with [35S]Met/Cys. Equal amounts of labeled extract were displayed by SDS-PAGE. Autoradiographs (Fig. 3) show that there was no apparent loss of incorporation of 35S-amino acids in infected cells at 24 hpi, in agreement with the observation that phosphorylated eIF4E remained at 24 hpi. However, at 48 hpi, there is a significant decrease in incorporation in infected cells compared to that in mock-infected cells (at least a 50% decrease in incorporated 35S), in agreement with the loss of phosphorylation of eIF4E at 48 h (Fig. 2B).
FIG. 3.
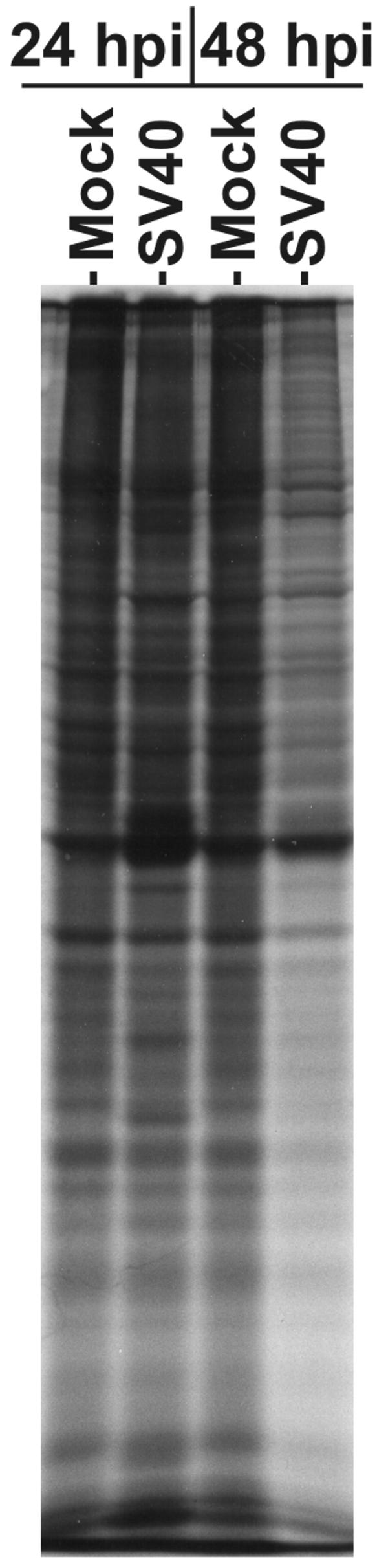
SV40 infection reduces protein synthesis late in infection of CV-1 cells. CV-1 cells were infected with SV40 at an MOI of 4. At 24 and 48 hpi, the cells were labeled with [35S]Met/Cys (150 μCi) for 30 min. Cell lysates were prepared by use of RIPA buffer. Forty micrograms of total protein was separated by SDS-PAGE and labeled protein detected by autoradiography.
SV40 small t antigen mediates the loss of phosphorylated 4E-BP1.
Large T and small t antigens are the major regulatory proteins of SV40; therefore, we asked whether either, or both, could mediate the loss of phosphorylated forms of p70S6K and 4E-BP1 seen late in infection. We focused these studies on the phosphorylation of 4E-BP1, since, as described above, hypophosphorylated 4E-BP1 binding to eIF4E is expected to have a significant inhibitory effect on cap-dependent translation.
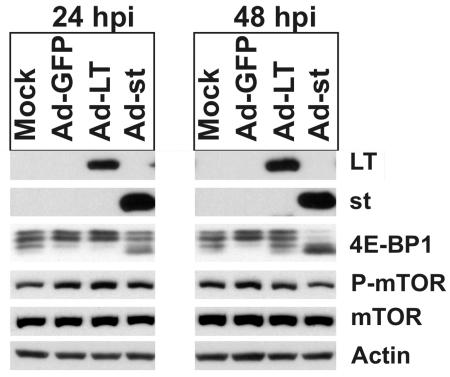
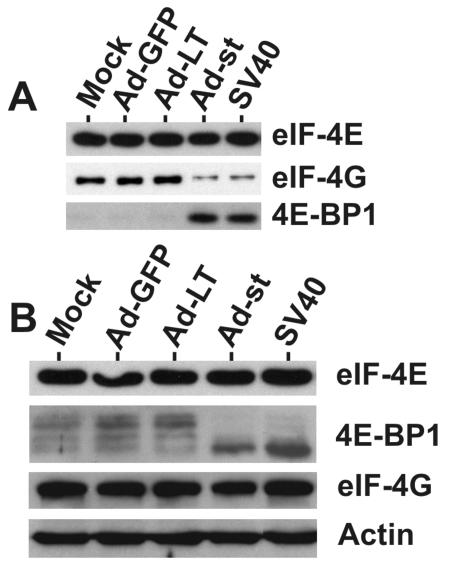
To address this question, adenoviruses expressing either large T (Ad-LT) or small t antigen (Ad-st) were constructed and propagated. Serum-starved CV-1 cells were infected with purified, serum-free adenovirus stocks at an MOI of 5, and cellular extracts were prepared at 24 and 48 hpi. Figure 4 shows that both large T and small t antigens were expressed efficiently from the adenovirus vectors in CV-1 cells. 4E-BP1 shows at least three hyperphosphorylated bands in the 24- and 48-h mock-infected cells. Using the adenovirus infection control vector which expressed green fluorescent protein (Ad-GFP) we noted increased hyperphosphorylation, indicating that adenovirus infection alone can induce hyperphosphorylation of 4E-BP1. There was no difference in 4E-BP1 phosphorylation between Ad-GFP and Ad-LT at 24 h postinfection; however, at 48 h postinfection, Ad-LT did not induce as much hyperphosphorylated 4E-BP1 as Ad-GFP. This modest effect of the large T antigen was overshadowed by the results of infection with Ad-st, for which some hypophosphorylated 4E-BP1 was seen at 24 h, and this dramatically increased by 48 h. These data suggest that small t antigen mediates the loss of phosphorylated forms of 4E-BP1. This effect of small t antigen was also seen when both Ad-LT and Ad-st were coinfected for 24 or 48 h (data not shown); hence, under these experimental conditions, the effects of small t antigen are not altered by the presence of large T antigen. We were unable to test whether large T or small t antigen alters the phosphorylation of p70S6K, because Ad-GFP, the control adenovirus, induced a very strong phosphorylation of p70S6K (data not shown).
FIG. 4.
Small t antigen causes the loss of phosphorylated 4E-BP1. Adenovirus vectors expressing GFP, large T antigen, or small t antigen were used to infect serum-starved CV-1 cells at an MOI of 5. Cell lysates were prepared at 24 and 48 hpi. Twenty micrograms of total protein from each sample was analyzed by Western analysis probing for large T and small t antigens, as well as total and phosphorylated (P) forms of 4E-BP1 and mTOR. The levels of actin, which served as the loading control, were determined.
We also show in Fig. 4 that Ad-GFP, Ad-LT, and Ad-st infections had little effect on mTOR phosphorylation or total mTOR protein levels, suggesting that the loss of 4E-BP1 phosphorylation is not the result of inactivation of mTOR kinase activity. The maintenance of phosphorylated mTOR could be a result of the adenovirus infection, since we noted decreased phosphorylation of mTOR at late times in the SV40 infection (Fig. 2A). The data suggest that small t antigen-mediated dephosphorylation of 4E-BP1 can occur in the presence of activated mTOR.
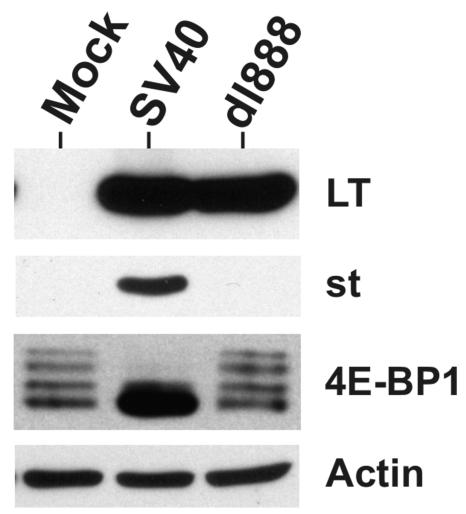
To further confirm the role of small t antigen in the loss of phosphorylated 4E-BP1, serum-starved CV-1 cells were infected for 48 h with purified, serum-free wild-type SV40 or SV40 mutant dl888 (28), which does not produce small t antigen, due to a deletion in the small t antigen unique coding region. Figure 5 shows that the wild-type infection caused the expected loss of hyperphosphorylated forms of 4E-BP1, while the levels of hyperphosphorylated 4E-BP1 were not altered by the small t antigen deletion mutant.
FIG. 5.
SV40 mutant with a small t antigen deletion does not cause 4E-BP1 dephosphorylation late in infection. Serum-starved CV-1 cells were infected for 48 h with wild-type SV40 or small t antigen deletion mutant dl888. Total and phosphorylated forms of 4E-BP1, as well as the levels of large T and small t antigens, were determined by Western analysis as described above. The levels of actin, which served as the loading control, were determined.
Presence of small t antigen causes eIF4E to associate with 4E-BP1 and not with eIF4G.
As discussed for Fig. 1, the eIF4F complex consists of the cap-binding protein eIF4E; an RNA helicase, eIF4A; and a kinase, Mnk1; each bound to the scaffolding protein eIF4G. The binding of eIF4E to eIF4G can be disrupted when hypophosphorylated 4E-BP1 binds to eIF4E and removes it from the eIF4F complex, thus inhibiting cap-dependent translation. eIF4E can bind to the cap structure regardless of whether it is in association with eIF4G or 4E-BP1. Thus, the binding status of eIF4E in cellular extracts can be determined by using Sepharose beads coupled with cap analog, which binds eIF4E and its associated proteins. The associated proteins can be identified by Western analysis.
To determine whether the small t antigen-mediated loss of phosphorylation of 4E-BP1 correlated with dissociation of eIF4E from eIF4G and the concomitant binding to 4E-BP1, we performed a cap-binding experiment using extracts from CV-1 cells infected with Ad-GFP, Ad-LT, Ad-st, or wild-type SV40. Figure 6A shows that equivalent amounts of eIF4E were recovered from all extracts by using the Sepharose beads coupled with cap analog. In the mock-infected cells, Ad-GFP (control)-infected cells, and the cells infected with Ad-LT, the eIF4E is exclusively associated with eIF4G indicating that it is translationally active as part of the eIF4F complex. However, in the cells infected with Ad-st or SV40 for 48 h, there was a significant reduction of eIF4E binding to eIF4G and a concomitant increase in binding to 4E-BP1. These data indicate that the small t antigen-induced loss of phosphorylation of 4E-BP1 (Fig. 4) results in the expected increase in binding of eIF4E to 4E-BP1 and decreased binding to eIF4G.
FIG. 6.
Small t antigen-mediated dephosphorylation of 4E-BP1 results in 4E-BP1 association with eIF4E. Serum-starved CV-1 cells were infected with Ad-GFP, Ad-LT, Ad-st, or wild-type SV40. Whole-cell lysates were prepared by use of cap-binding AP buffer at 48 hpi. (A) Two hundred micrograms of total lysate protein was incubated with m7-GTP Sepharose beads for 2 h at 4°C; see Materials and Methods for details. Proteins bound to the beads were analyzed by Western analysis probing for eIF4E, 4E-BP1, and eIF4G. (B) The total amounts of eIF4E, 4E-BP1, and eIF4G in 20 μg of total lysate protein were determined by Western analysis.
Figure 6B is an examination of the total proteins in the lysates used for the cap-binding experiment, showing that the total levels of eIF4E, 4E-BP1, and eIF4G do not differ between samples; thus, increases or decreases in protein concentrations cannot account for the results of the binding studies.
A PP2A binding mutant of small t antigen, but not a DnaJ domain mutant, failed to mediate the loss of phosphorylation of 4E-BP1.
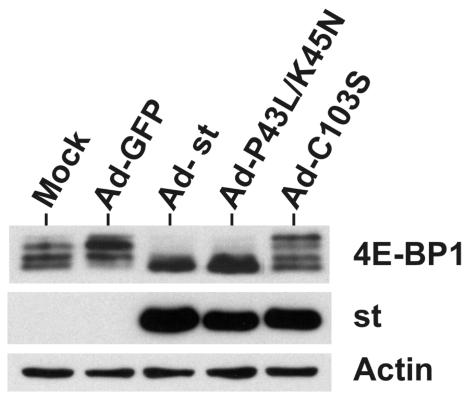
As described in the introduction, small t antigen has two characterized domains, an N-terminal DnaJ domain and a C-terminal PP2A binding domain. To determine which domain of small t antigen mediates the loss of phosphorylated 4E-BP1, we tested recombinant adenoviruses expressing two mutant small t antigens. P43L/K45N is a double mutation located in the DnaJ domain; this mutant is severely impaired in DnaJ function (21, 30). Figure 7 shows that the P43L/K45N mutant was fully competent for meditating the loss of phosphorylation of 4E-BP1.
FIG. 7.
Small t antigen's ability to bind PP2A correlates with its ability to mediate 4E-BP1 dephosphorylation. Serum-starved CV-1 cells were either mock infected or infected with adenovirus vectors expressing GFP (Ad-GFP), wild-type small t antigen (Ad-st), a DnaJ domain mutant of small t antigen (Ad-P43L/K45N), or a PP2A binding mutant of small t antigen (Ad-C103S). Cell lysates were prepared at 48 hpi. 4E-BP1, small t antigen, and actin levels were determined by Western analysis.
The other small t antigen mutation, C103S, significantly reduces the ability of small t antigen to bind PP2A (18). This mutant showed a greatly diminished ability to mediate the loss of phosphorylated 4E-BP1 (Fig. 7), suggesting that the interaction of small t antigen with PP2A may be necessary to mediate the loss of phosphorylations of 4E-BP1 and, possibly, p70S6K (Fig. 2A).
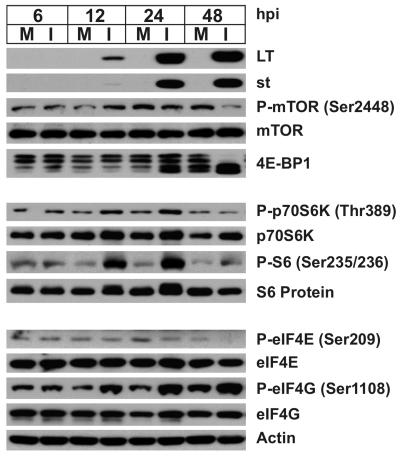
SV40 infection of primary AGMK cells shows additional effects on mTOR effectors.
CV-1 cells are an established and immortalized cell line which cannot be significantly growth arrested by serum starvation. Therefore, we felt that this inability to growth arrest the cells may mask phosphorylation of mTOR or its effectors mediated by SV40 infection. Therefore, we performed an infection time course in primary AGMK cells that had been serum-starved for 24 h and then mock infected or infected with purified, serum-free SV40 and maintained under serum-free conditions (Fig. 8). Western analysis was used to probe the cell lysates as described for Fig. 2. As seen for CV-1 cells, late in the infection (48 hpi), there was a marked decrease in the phosphorylations of mTOR, 4E-BP1, p70S6K, and S6 (P-S6). However, at earlier time points (12 and 24 hpi), we did detect additional effects of the viral infection. Levels of phosphorylated p70S6K were moderately increased, and this correlated with a significant increase in phosphorylated S6. Elevated levels of phosphorylated eIF4G in infected cells were detected at 12, 24, and even 48 hpi. Hyperphosphorylated forms of 4E-BP1 were detected in the serum-starved mock-infected samples; thus, it was not possible to determine whether the viral infection could cause the increased phosphorylation of 4E-BP1. In sum, the data suggest that SV40 infection can maintain or induce phosphorylations of p70S6K, S6, and eIF4G early in the infection (12 and 24 hpi) and then reduce the phosphorylation of mTOR, p70S6K, S6, and 4E-BP1 late in the infection (48 hpi).
FIG. 8.
SV40 infection affects mTOR signaling in AGMK cells. Serum-starved AGMK cells were mock infected (M) or infected (I) with wild-type SV40 strain 776 at an MOI of 4 for 6, 12, 24, or 48 h. Whole-cell lysates were harvested in RIPA buffer, and 20 μg of lysate protein was analyzed by Western analysis probing for the total and phosphorylated (P) forms of the proteins indicated. The levels of actin protein, which served as the loading control, were determined.
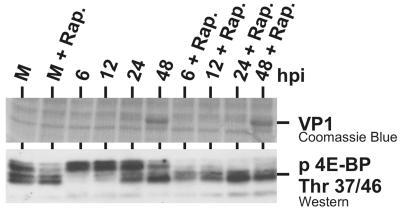
VP1 synthesis does not depend upon 4E-BP1 hyperphosphorylation.
The data presented suggest that 4E-BP1 becomes hypophosphorylated late in SV40 infection and can inhibit cap-dependent translation at a time in the infection when significant levels of virions structural proteins, e.g., VP1, need to be synthesized. Thus, we asked whether VP1 synthesis was affected by the hypophosphorylation of 4E-BP1. Figure 9 shows an infection time course done in the presence or absence of rapamycin. As discussed above, rapamycin inhibits mTOR kinase preventing hyperphosphorylation of 4E-BP1. Western analysis shows that throughout the time course, in rapamycin 4E-BP1 was maintained in its hypophosphorylated state, which would inhibit cap-dependent translation. In the normal infection, hypophosphorylation of 4E-BP1 was noted at 24 hpi and greatly increased by 48 hpi, in agreement with the data in Fig. 2A and 8. VP1 accumulation, shown in a Coomassie-stained gel, is seen modestly at 24 hpi and abundantly at 48 hpi with little difference between normal and rapamycin-treated samples. Thus, VP1 translation and accumulation appear to be relatively resistant to 4E-BP1 hypophosphorylation. Possible explanations for this are discussed below.
FIG. 9.
VP1 synthesis does not depend upon 4E-BP1 hyperphosphorylation. Serum-starved CV-1 cells were either untreated or treated with rapamycin (Rap.) and mock infected (M) or infected with SV40 as described in the legend to Fig. 2A. The SV40 infections were harvested at the times shown. The level of VP1 was determined by Coomassie blue staining after SDS-PAGE. The phosphorylation statue of 4E-BP1 was determined by Western analysis as described in the legend to Fig. 2A.
DISCUSSION
Viruses depend on the host cell protein synthetic machinery for the production of viral proteins. Many viruses have evolved effective means to redirect the host translation machinery to favor viral protein synthesis (7, 26). The majority of control over cellular mRNA translation occurs during initiation. Our studies of AGMK cells demonstrate that during an SV40 infection, the phosphorylations of p70S6K, S6, and eIF4G are induced early in the infection (12 and 24 hpi). It is likely that the phosphorylation of 4E-BP1 can also be upregulated, but this cannot be measured in AGMK or CV-1 cells, since high levels of hyperphosphorylated 4E-BP1 are maintained in these cells even under serum-starved conditions.
The virally induced phosphorylation of these proteins is largely sensitive to rapamycin, suggesting that mTOR kinase is activated by the viral infection, most likely via activated Akt (Fig. 1). The activation of Akt by SV40 large T and small t antigens has previously been described (36, 37). Interestingly, late in the infection (by 48 hpi), the levels of phosphorylated mTOR, p70S6K, S6, and 4E-BP1 dramatically decrease in the infected cell. The only mTOR effector whose phosphorylation is not decreased is eIF4G, which remains highly phosphorylated at 48 hpi in both CV-1 and AGMK cells. In the rapamycin inhibition studies using CV-1 cells, we noted that at 48 hpi, the phosphorylation of eIF4G was rapamycin insensitive, suggesting that late in infection, an mTOR-independent mechanism is induced for the phosphorylation of eIF4G. We have previously reported mTOR-independent phosphorylation of eIF4G during human cytomegalovirus infection (16). At present, the effect of phosphorylation of eIF4G on translation or other cellular processes is unknown. However, the targeting of eIF4G for mTOR-independent phosphorylation by two very different DNA viruses suggests that eIF4G phosphorylation produces a cellular environment which favors viral protein synthesis or replication.
The data presented suggest that SV40 infection induces mTOR signaling early in the infection in order to ensure cap-dependent translation and ribosome biogenesis, situations which would aid the viral infection. In contrast, the dramatic dephosphorylations of mTOR, p70S6K, S6, and 4E-BP1 late in the infection suggest that the virus infection inhibits cap-dependent translation and ribosome biogenesis at late times. The inhibition of cap-dependent translation is supported by our finding that the hypophosphorylation of 4E-BP1, mediated by small t antigen, correlates with the removal of eIF4E from the eIF4F complex. Such inhibition late in the infection may not matter; it is possible that by the time the inhibition occurs, sufficient viral structural proteins have been made and capsid formation can occur regardless of the state of translation. However, this conclusion is not support by the data in Fig. 9, which suggest that VP1 can accumulate in the presence of hypophosphorylated 4E-BP1 caused by the addition of rapamycin. Although the inhibitory effect of rapamycin on mTOR signaling is well established, its presence does not produce a global inhibitory effect on translation in mammalian cells (10); for example, reductions in total cap-dependent translation of no more than 30 to 35% have been reported. However, among specific mRNAs dependent on the cap for translation, there is a wide range in the degree of inhibition elicited by rapamycin treatment (10). Thus, it is possible that SV40 mRNAs are of the type that is more readily translated in the presence of rapamycin.
On the other hand, the inhibition of cap-dependent translation may promote the success of the viral infection by favoring translation using internal ribosome entry sites (IRESs) in order to produce the large amounts of virion structural proteins (VP1, VP2, and VP3) needed for virion formation. The 16S and 19S SV40 late mRNAs are splice variants which are polycistronic; the 16S mRNA encodes the agnoprotein and the major virion structural protein VP1; the 19S mRNA encodes the agnoprotein and the minor virion structural proteins VP2 and VP3, as well as VP1. Although no studies have been done to identify IRESs in the SV40 late mRNAs, utilization of the IRESs could explain how these polycistronic mRNAs can produce sufficient amounts of virion structural proteins. SV40 VP1 must be produced in large amounts late in the infection in order to efficiently produce virions. The translational start codon (AUG) for VP1 in both the 16S and 19S mRNAs is the last utilized AUG. In the best situation, the 16S late mRNA, the VP1 AUG is the third AUG in the message (4). Thus, the utilization of an IRES to facilitate translational initiation at the VP1 AUG would be a reasonable mechanism. For the 19S mRNA, a strong case can be made for IRES-directed utilization of the AUG for VP3, since this AUG is downstream of the VP2 AUG and there is no translational stop codon prior to the VP3 AUG. If IRESs are utilized, then the inhibition of cap-dependent translation late in infection would enhance the translation of virion structural proteins at a time when they are most needed for virion formation.
An additional reason to believe that the cessation of cap-dependent translation may be significant for the success of the viral infection is our finding that the decrease in the phosphorylation of 4E-BP1, and probably that of p70S6K, is a specific function of small t antigen. Many viable mutants of small t antigen have been studied (28, 33). Although the mutants are viable for lytic growth in monkey cells, the rate of growth, virus yield, and plaque size are generally reduced in infections by these deletion mutants. These phenotypes are compatible with the model of IRES-dependent late protein synthesis; specifically, the loss of small t antigen would result in cap-dependent translation being maintained, and IRES utilization would not be enhanced, thus hindering the accumulation of virion structural proteins.
Our data suggest that small t antigen's ability to interact with PP2A is needed to induce hypophosphorylation of 4E-BP1. PP2A is suspected to be the phosphatase which acts on 4E-BP1 and p70S6K (19, 20). The small t antigen-PP2A interaction usually results in inhibition of PP2A function (24), however our data suggest that PP2A's phosphatase activity may be specifically activated and targeted to dephosphorylate 4E-BP1 in the presence of small t antigen. This is not unprecedented; increased PP2A activity in the presence of small t antigen has previously been reported for the dephosphorylation of histone H1 (35); in addition, recent data suggest that small t antigen may mediate the transfer of PP2A to the androgen receptor, thus directing phosphatase activity to this protein (34). It is possible that small t antigen-PP2A directs the dephosphorylation and inactivation of mTOR, resulting in decreased phosphorylations of 4E-BP1 and p70S6K. However, the data in Fig. 4 indicated that small t antigen expressed from the adenovirus vector had little effect on mTOR phosphorylation, while there was significant loss of 4E-BP1 phosphorylation. This suggests that small t antigen can mediate the hypophosphorylation of 4E-BP1 in the presence of activated mTOR. Thus, our data suggest two potential mechanisms: (i) the interaction of small t antigen with PP2A results in PP2A phosphatase activity being specifically targeted to 4E-BP1, or (ii) the interaction of PP2A with small t antigen results in the known inhibition of PP2A, but this inhibition allows the activation of a second phosphatase which actively dephosphorylates 4E-BP1.
Acknowledgments
We thank Kathy Rundell for reagents and helpful discussion and all the members of the Alwine laboratory for support and critical evaluation of the experiments and data.
This work was supported by Public Health Service grant R01 CA28379-25 awarded to J.C.A. by the National Cancer Institute.
REFERENCES
- 1.Arsham, A. M., J. J. Howell, and M. C. Simon. 2003. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J. Biol. Chem. 278:29655-29660. [DOI] [PubMed] [Google Scholar]
- 2.Beretta, L., A. C. Gingras, Y. V. Svitkin, M. N. Hall, and N. Sonenberg. 1996. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 15:658-664. [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, E. J., M. W. Albers, T. B. Shin, K. Ichikawa, C. T. Keith, W. S. Lane, and S. L. Schreiber. 1994. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 369:756-758. [DOI] [PubMed] [Google Scholar]
- 4.Dabrowski, C., and J. C. Alwine. 1988. Translation control of the synthesis of simian virus 40 late proteins from the polycistronic 192 late mRNA. J. Virol. 62:3182-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahl, J., A. Jurczak, L. A. Cheng, D. C. Baker, and T. L. Benjamin. 1998. Evidence of a role for phosphatidylinositol 3-kinase activation in the blocking of apoptosis by polyomavirus middle T antigen. J. Virol. 72:3221-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, A. R., N. A. Wivel, J. L. Palladino, L. Tao, and J. M. Wilson. 2001. Construction of adenoviral vectors. Mol. Biotechnol. 18:63-70. [DOI] [PubMed] [Google Scholar]
- 7.Gale, M., Jr., S. L. Tan, and M. G. Katze. 2000. Translational control of viral gene expression in eukaryotes. Microbiol. Mol. Biol. Rev. 64:239-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gingras, A. C., S. P. Gygi, B. Raught, R. D. Polakiewicz, R. T. Abraham, M. G. Hoekstra, R. Aebersold, and N. Sonernberg. 1999. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 13:1422-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gingras, A. C., B. Raught, S. P. Gygi, A. Niedzwiecka, M. Miron, S. K. Burley, R. D. Polakiewicz, A. Wyslouch-Cieszynska, R. Aebersold, and N. Sonenberg. 2001. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 15:2852-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gingras, A. C., B. Raught, and N. Sonenberg. 2004. mTOR signaling to translation. Curr. Top. Microbiol. Immunol. 279:169-197. [DOI] [PubMed] [Google Scholar]
- 11.Gingras, A. C., B. Raught, and N. Sonernberg. 2001. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 15:807-826. [DOI] [PubMed] [Google Scholar]
- 12.Halford, W. P., C. D. Kemp, J. A. Isler, D. J. Davido, and P. A. Schaffer. 2001. ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J. Virol. 75:6143-6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howe, A. K., S. Gaillard, J. S. Bennett, and K. Rundell. 1998. Cell cycle progression in monkey cells expressing simian virus 40 small t antigen from adenovirus vectors. J. Virol. 72:9637-9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, R. A., X. Wang, X. L. Ma, S. M. Huong, and E. S. Huang. 2001. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: inhibition of PI3-K activity inhibits viral replication and virus-induced signaling. J. Virol. 75:6022-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufman, R. J., D. Scheuner, M. Schroder, X. Shen, K. Lee, C. Y. Liu, and S. M. Arnold. 2002. The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 3:411-421. [DOI] [PubMed] [Google Scholar]
- 16.Kudchodkar, S., Y. Yu, T. Maguire, and J. C. Alwine. 2004. Human cytomegalovirus infection induces rapamycin insensitive phosphorylation of downstream effectors of mTOR kinase. J. Virol. 78:11030-11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millward, T. A., S. Zolnierowicz, and B. A. Hemmings. 1999. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 24:186-191. [DOI] [PubMed] [Google Scholar]
- 18.Mungre, S., K. Enderle, B. Turk, A. Porras, Y. Q. Wu, M. C. Mumby, and K. Rundell. 1994. Mutations which affect the inhibition of protein phosphatase 2A by simian virus 40 small-t antigen in vitro decrease viral transformation. J. Virol. 68:1675-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nanahoshi, M., T. Nishiuma, Y. Tsujishita, K. Hara, S. Inui, N. Sakaguchi, and K. Yonezawa. 1998. Regulation of protein phosphatase 2A catalytic activity by alpha4 protein and its yeast homolog Tap42. Biochem. Biophys. Res. Commun. 251:520-526. [DOI] [PubMed] [Google Scholar]
- 20.Peterson, R. T., B. N. Desai, J. S. Hardwick, and S. L. Schreiber. 1999. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinassociated protein. Proc. Natl. Acad. Sci. USA 96:4438-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porras, A., J. Bennett, A. Howe, K. Tokos, N. Bouck, B. Henglein, S. Sathyamangalam, B. Thimmapaya, and K. Rundell. 1996. A novel simian virus 40 early-region domain mediates transactivation of the cyclin A promoter by small-t antigen and is required for transformation in small-t antigen-dependent assays. J. Virol. 70:6902-6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proud, G. C. 2004. The multifaceted role of mTOR in cellular stress responses. DNA Repair 3:927-934. [DOI] [PubMed] [Google Scholar]
- 23.Raught, B., A. C. Gingras, and N. Sonenberg. 2001. The target of rapamycin (TOR) proteins. Proc. Natl. Acad. Sci. USA 98:7037-7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rundell, K., and R. Parakati. 2001. The role of the SV40 ST antigen in cell growth promotion and transformation. Semin. Cancer Biol. 11:5-13. [DOI] [PubMed] [Google Scholar]
- 25.Sabatini, D. M., H. Erdjument-Bromage, M. Lui, P. Tempst, and S. H. Snyder. 1994. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 78:35-43. [DOI] [PubMed] [Google Scholar]
- 26.Schneider, R. J., and I. Mohr. 2003. Translation initiation and viral tricks. Trends Biochem. Sci. 28:130-136. [DOI] [PubMed] [Google Scholar]
- 27.Schonthal, A. H. 2001. Role of serine/threonine protein phosphatase 2A in cancer. Cancer Lett. 170:1-13. [DOI] [PubMed] [Google Scholar]
- 28.Shenk, T. E., J. Carbon, and P. Berg. 1976. Construction and analysis of viable deletion mutants of simian virus 40. J. Virol. 18:664-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skoczylas, C., K. M. Fahrbach, and K. Rundell. 2004. Cellular targets of the SV40 small-t antigen in human cell transformation. Cell Cycle 3:5606-5610. [PubMed] [Google Scholar]
- 30.Sullivan, C. S., and J. M. Pipas. 2002. T antigens of simian virus 40: molecular chaperones for viral replication and tumorigenesis. Microbiol. Mol. Biol. Rev. 66:179-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Summers, S. A., L. Lipfert, and M. J. Birnbaum. 1998. Polyoma middle T antigen activates the Ser/Thr kinase Akt in a PI3-kinase-dependent manner. Biochem. Biophys. Res. Commun. 246:76-81. [DOI] [PubMed] [Google Scholar]
- 32.Vezina, C., A. Kudelski, and S. N. Sehgal. 1975. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. 28:721-726. [DOI] [PubMed] [Google Scholar]
- 33.Volckaert, G., J. Feunteun, L. V. Crawford, P. Berg, and W. Fiers. 1979. Nucleotide sequence deletions within the coding region for small-t antigen of simian virus 40. J. Virol. 30:674-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang, C.-S., M. J. Vitto, S. A. Busby, B. A. Garcia, C. T. Kesler, D. Gioeli, J. Shabanowitz, D. F. Hunt, K. Rundell, D. L. Brautigan, and B. M. Paschal. 2005. Simian virus 40 small t antigen mediates conformation-dependent transfer of protein phosphatase 2A onto the androgen receptor. Mol. Cell. Biol. 25:1298-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang, S., I., R. L. Lickteig, R. Estes, K. Rundell, G. Walter, and M. C. Mumby. 1991. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol. Cell. Biol. 11:1988-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu, Y., and J. C. Alwine. 2002. Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3′-OH kinase pathway and cellular kinase Akt. J. Virol. 76:3731-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan, H., T. Veldman, K. Rundell, and R. Schlegel. 2002. Simian virus 40 small tumor antigen activates AKT and telomerase and induces anchorage-independent growth of human epithelial cells. J. Virol. 76:10685-10691. [DOI] [PMC free article] [PubMed] [Google Scholar]