Osteoporosis is in part a natural consequence of aging, although endogenous estrogen deficiency is an important pathophysiologic contribution to its occurrence in women. Age-related bone loss is the main cause of hip and vertebral fractures in elderly people. In addition to the morbidity and costs associated with these fractures, there is the nontrivial mortality. About 20% of patients with hip fracture die within the year, and about the same percentage require long-term care.1 Vertebral fractures due to osteoporosis also contribute significantly to increased mortality2,3 and prolonged disability.4 The age-adjusted relative risk of dying from any clinical fracture more than doubles for individuals over the age of 60 years (relative risk [RR] 2.5, 95% confidence interval [CI] 1.37–3.42).5
Osteoporosis can be detected by measurements of bone mineral density (BMD) or inferred from the presence of pre-existing osteoporosis-related fractures such as vertebral or hip fractures. Among patients with osteoporosis (by BMD measurements but without pre-existing fractures), the 3–4-year incidence of new vertebral fractures ranges from 2% to 4%,6,7,8 whereas hip fracture rates range from 1.1% to 5.1%.6,9 The presence of pre-existing osteoporosis-related fractures is markedly associated with an increase in risk for future fractures at many skeletal sites (over a comparable time frame), such that vertebral fracture incidence increases to 15%–29%,7,8,10,11,12,13,14 and hip fracture incidence increases to 2.2%–5.7%.9,12,13,14 These fracture rates were observed in randomized placebo-controlled clinical trials designed to assess the efficacy of new pharmaceutical drugs intended to reduce incident fracture rates. Although the inclusion criteria for enrolment of subjects into these trials were not strictly comparable, the fracture incidence data in placebo-treated patients consistently demonstrate that a pre-existing osteoporosis-related fracture serves as a harbinger of an increased risk of subsequent fracture, even if the patient takes modest calcium and vitamin D supplements.
A number of drugs have been used to slow down the progress of osteoporosis and, most importantly, to reduce the risk of both vertebral and all nonvertebral fractures, including those of the hip. Although practice guidelines published by the Osteoporosis Society of Canada15 recommend estrogen replacement as the therapy of choice for osteoporosis, there are as yet no randomized controlled trials to support this recommendation. Vitamin D and calcium supplementation have been shown to reduce the incidence of nonvertebral fractures,16 but this benefit may be more limited than was once thought.17,18
The bisphosphonates are a new class of compounds that act by selectively inhibiting osteoclast function, and thus bone resorption, during the remodelling cycle of bone turnover. Although the precise mechanism of action has not been defined, the net effect of these compounds appears to be a reversal of the remodelling deficit in patients with osteoporosis, such that bone remodelling leads to gradual increments in bone mass, which are easily detectable by skeletal BMD measurements at either the lumbar spine or the femoral neck.
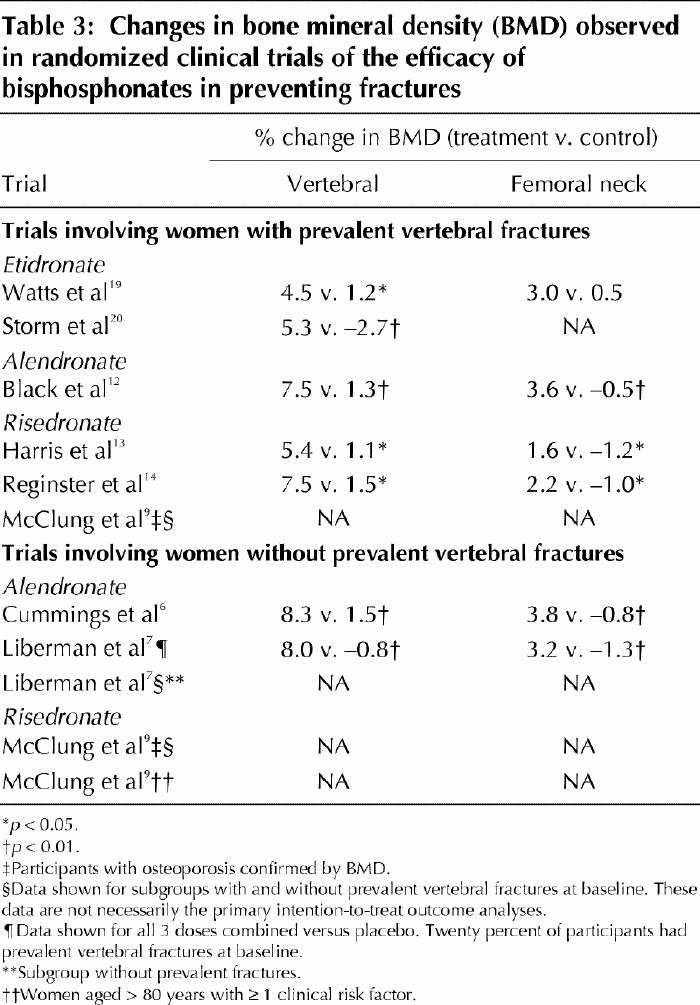
It is now well established that taking bisphosphonates leads to consistent increases in axial BMD, irrespective of the severity of the underlying osteoporosis.6,7,9,12,13,14,19,20 Randomized controlled clinical trials (RCTs) have demonstrated BMD gains of 4.5%–8.3% at the lumbar spine and 1.6%–3.8% at the femoral neck for patients treated for 3–4 years. Patients in the control groups, who are usually treated with calcium and vitamin D supplements, show no clinically significant changes in measured BMD. The increment in BMD during bisphosphonate therapy should translate into a reduction in observed fractures. This hypothesis has now been tested in several RCTs that evaluated the antifracture efficacy of etidronate, alendronate and risedronate. Information about the pivotal RCTs that were designed to assess the efficacy of oral bisphosphonates appears in Tables 1–3. The relative risk of either vertebral or nonvertebral fracture, or hip fracture, is consistently reduced during 3–4 years of therapy, but the clinical and statistically significant reductions of incident fractures are greater among women who have already experienced a vertebral fracture on entry to the trial.
Table 1
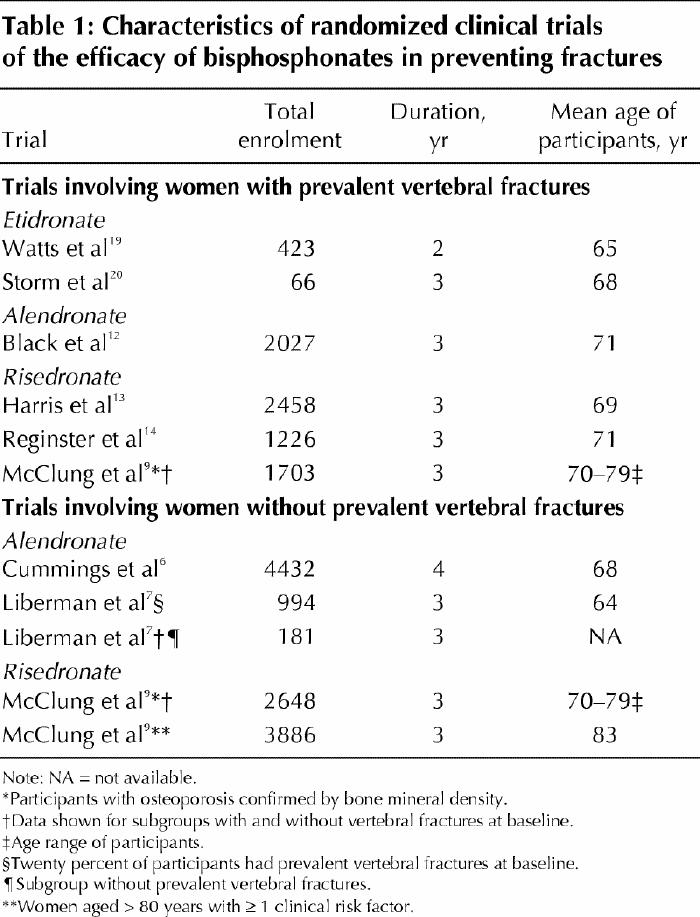
Table 2
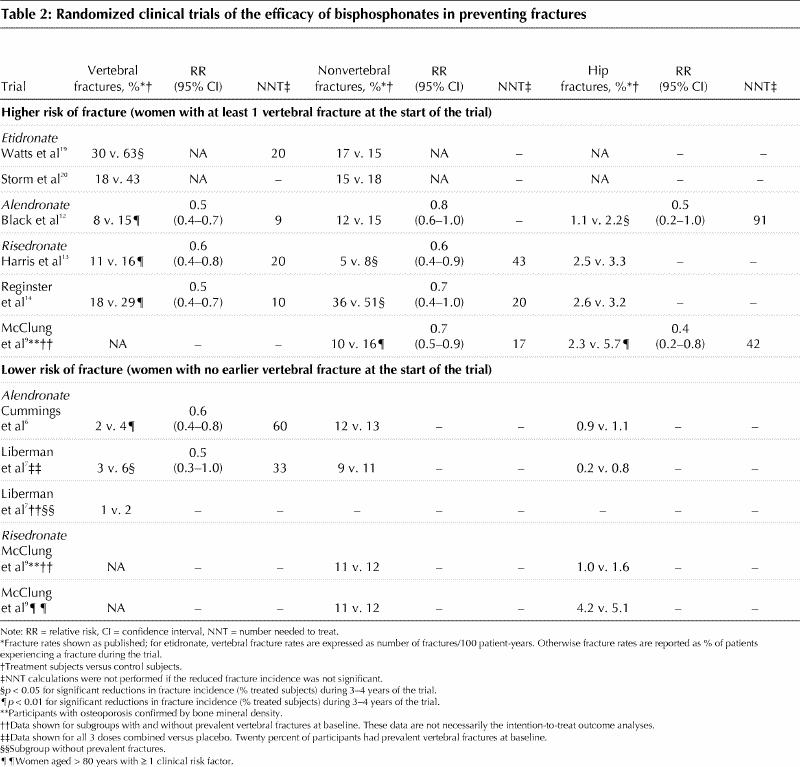
Table 3
Cyclical etidronate has been an approved treatment in Canada for osteoporosis since 1995. However, 2 early randomized clinical trials of etidronate were limited to several hundred subjects and were powered to detect increments in BMD rather than reductions in fractures, which typically require several thousand subjects.19,20 Thus, the RCT evidence for fracture efficacy with etidronate is not available. In a recent meta-analysis of 13 trials that randomized women to etidronate or an alternative (placebo or calcium and/or vitamin D), the data suggested a reduction in vertebral fractures with a pooled relative risk of 0.63 (95% CI 0.44–0.92). There was no effect on nonvertebral fractures.21 However, a large matched cohort study within the General Practitioners Research Database in the United Kingdom has shown a significant reduction in both nonvertebral (RR 0.80, 95% CI 0.70–0.92) and hip fractures (RR 0.66, 95% CI 0.51–0.85) among patients diagnosed with osteoporosis and prescribed cyclical etidronate compared with an apparently similar cohort treated with “other” medication.22
Although cyclical etidronate has been the mainstay of established osteoporosis therapy in Canada for 6 years, phase III RCT data concerning antifracture efficacy during alendronate and risedronate treatment allow for a more critical appraisal of the ability of bisphosphonates to prevent incident fractures. In Table 2, we describe fracture outcomes according to the presence or absence of prevalent vertebral fractures at the time of enrolment. For the sake of consistency, only the subgroups of enrolled patients conforming to this description are detailed.7,9
For patients with prevalent vertebral fractures at enrolment, both alendronate and risedronate consistently reduce the 3–4-year incidence of new vertebral and, in general, nonvertebral fractures (Table 2). Additional risk factors, particularly advanced age, and initial BMD are not strictly comparable for the alendronate and risedronate trials; however, the absolute risk reduction is such that the number of patients who need to be treated with 3–4 years of bisphosphonate therapy is quite consistent: 9–20 to prevent a vertebral fracture and 17–43 to prevent a nonvertebral fracture (Table 2). Only two studies had a sufficiently large sample size to detect the efficacy of alendronate12 and risedronate9 in preventing hip fractures. In these 2 trials, a reduction of about 50% in hip fracture incidence required larger estimates of the number of patients who need to be treated to prevent one hip fracture of 9112 and 429 respectively.
The data for incident fracture prevention in postmenopausal women without prevalent vertebral fractures at enrolment are less convincing (Table 2). RCTs of alendronate therapy demonstrated significant reductions in incident vertebral fractures,6,7 but the incident vertebral fracture rates of the control groups were 25% lower than those documented in other alendronate trials (Table 2). This resulted in estimates of the number of patients who need to be treated to prevent new vertebral fractures that were 3 times higher than those calculated in bisphosphonate trials involving women with pre-existing vertebral fractures (Table 2).
Although the incidence of nonvertebral fracture, and specifically hip fractures, is quite similar in all of the studies outlined in Table 2, bisphosphonates have not been unequivocally shown to reduce the incidence of hip fractures in the absence of documented prevalent vertebral fractures.6,9 The risedronate trial was specifically designed to evaluate hip fracture incidence (Hip fracture Intervention Program, HIP) in elderly women with unequivocally low BMD measurements (femoral neck T-scores ≤ 3.0).9 In the intention-to-treat analysis, an overall reduction in hip fractures was found (RR 0.6, 95% CI 0.4–0.9). However, within the preplanned stratified analysis, the significant reductions in observed incident hip fractures occurred only in the subgroup with prevalent vertebral fractures at the time of enrolment (Table 2). Indeed, the incidence of hip fractures in the HIP trial cohort comprising women aged 80 years and older with at least one nonskeletal risk factor for “frailty” failed to show antifracture efficacy of risedronate, even though the hip fracture incidence in the control group was generally higher than that in other published studies.9 The large Fracture Intervention Trial with alendronate confirmed that it provided protection against hip fractures for women with prevalent vertebral fractures,12 but this was not found in the intention-to-treat analysis of women who had no prevalent vertebral fractures at enrolment.6 However a subgroup analysis of women without prevalent vertebral fractures, but with a confirmed BMD measurement at the femoral neck that was less than or equal to 2.5 standard deviations below peak adult bone mass, did show a significant reduction in the incidence of hip fractures.6 Thus, a low BMD is still seen as an important independent risk factor for hip fracture.
Thus, these RCTs of all 3 of the bisphosphonates in the treatment of osteoporosis clearly show a beneficial effect on BMD at several skeletal sites. The balance of evidence supports a beneficial antifracture efficacy for etidronate, but this agent may be less effective than either alendronate or risedronate. For example, in one study, 80% of patients treated with cyclical etidronate demonstrated an increased BMD at the lumbar spine,23 whereas over 95% responded to alendronate.24 In the absence of a head-to-head comparison, the therapeutic equivalence, or lack of it, of etidronate as compared with alendronate or risedronate in the prevention of fractures must remain an open question. In women with prevalent vertebral fractures, particularly if they have BMD measurements diagnostic of osteoporosis (i.e., a T-score at any site ≤ –2.5), both alendronate and risedronate unequivocally reduce incident fractures at all sites by up to 50% when treatment continues for 3–4 years or longer. Number-needed-to-treat calculations suggest that 40 or fewer women need treatment to prevent either a vertebral or nonvertebral fracture.
In women with a low BMD, but no prevalent fractures, the risk reduction for subsequent fractures is comparable, but more women require treatment to prevent a fracture because fracture rates are much lower in this group. To date, significant reductions in hip fracture incidence have been shown in subjects who have already suffered vertebral compression fractures, although it is probable that similar protection is conferred in elderly women with very low BMD measurements. However, even frail elderly patients over 80 years of age are not significantly protected from hip fracture in the absence of demonstrable “fragility” fractures at other sites.
In conclusion, the evidence to date confirms the effectiveness of the bisphosphonate family in the treatment of women with severe osteoporosis and in protecting them from future fractures. There is little information documenting the impact of therapy on mortality and morbidity. From the known epidemiologic data on the consequence of fractures in elderly women (as discussed earlier), prevention of fractures should reduce both morbidity and mortality. The morbidity experienced by women in the alendronate trials has been partially reported; women in the treatment arm experienced significantly fewer days with back pain and fewer days of bedrest.25 Information regarding admissions to hospital and mortality during bisphosphonate treatment has yet to be made available. Bisphosphonates are well tolerated, but poorly absorbed, requiring a rigorous dosing protocol. The recent finding that weekly dosing, as compared with daily dosing, with alendronate results in comparable increments in BMD measurements should improve the side-effect profile of this drug and enhance compliance.24 This family of therapeutic agents is a potent addition to the treatment of postmenopausal osteoporosis.
Footnotes
This article has been peer reviewed.
Contributors: Dr. Hodsman was responsible for the initial literature review and analysis, the initial text draft and subsequent revisions to the text. Drs. Hanley and Josse were responsible for the critical review of manuscript content. They also directed editorial revisions and made significant intellectual contributions to the final “message” of the article.
Competing interests: All 3 authors have significant professional relationships with Merck Frosst Canada and Procter and Gamble Canada with respect to commercial and developing therapies for osteoporosis. As such, we serve on medical advisory boards convened by these companies. In this capacity, we have received appropriate honoraria and consultancy fees, speaker engagement fees and travel assistance to important national/international conferences held to disseminate research within the field of osteoporosis and related metabolic bone disease. However, the development of this article was entirely at the discretion of the authors; it was not commissioned by any pharmaceutical company or national organization and was not submitted to such agencies for approval or input before submission to CMAJ.
Correspondence to: Dr. A.B. Hodsman, Department of Medicine, St. Joseph's Health Centre, Rm. F215, 268 Grosvenor St., London ON N6A 4V2; fax 519 646-6074; anthony.hodsman@sjhc.london.on.ca
References
- 1.Chrischilles EA, Butler CD, Davis CS, Wallace RB. A model of lifetime osteoporosis impact. Arch Intern Med 1991;151:2026-32. [PubMed]
- 2.Ensrud KE, Thompson DE, Cauley JA, Nevitt MC, Kado DM, Hochberg MC, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc 2000;48:241-9. [DOI] [PubMed]
- 3.Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1999;159:1215-20. [DOI] [PubMed]
- 4.Nevitt MC, Ettinger B, Black DM, Stone K, Jamal SA, Ensrud K, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med 1998;128:793-800. [DOI] [PubMed]
- 5.Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int 2000;11:556-61. [DOI] [PubMed]
- 6.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures. JAMA 1998;280:2077-82. [DOI] [PubMed]
- 7.Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 1995;333:1437-43. [DOI] [PubMed]
- 8.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene. Results from a 3-year randomized clinical trial. JAMA 1999;282:637-45. [DOI] [PubMed]
- 9.McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, et al. Effect of risedronate on hip fracture risk in elderly women. N Engl J Med 2001; 344:333-40. [DOI] [PubMed]
- 10.Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, et al. Risk of new vertebral fractures in the year following a fracture. JAMA 2001; 285: 320-3. [DOI] [PubMed]
- 11.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA III, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 2000;15:721-39. [DOI] [PubMed]
- 12.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996;348:1535-41. [DOI] [PubMed]
- 13.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis. JAMA 1999;282(14):1344-52. [DOI] [PubMed]
- 14.Reginster JY, Minne HW, Sorenson OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporosis Int 2000; 11: 83-91. [DOI] [PubMed]
- 15.Scientific Advisory Board, Osteoporosis Society of Canada. Clinical practice guidelines for the diagnosis and management of osteoporosis. CMAJ 1996; 155(8):1113-33. [PMC free article] [PubMed]
- 16.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992; 327:1637-42. [DOI] [PubMed]
- 17.Gillespie WJ, Henry DA, O'Connell DL, Robertson J. Vitamin D and vitamin D analogues for preventing fracures associated with involutional and post-menopausal osteoporosis [Cochrane review]. In: The Cochrane Library; Issue 3, 2000. Oxford: Update Software. [DOI] [PubMed]
- 18.Cumming RG, Nevitt MC. Calcium for prevention of osteoporotic fractures in postmenopausal women. J Bone Miner Res 1997;12:1321-9. [DOI] [PubMed]
- 19.Watts NB, Harris ST, Genant HK, Wasnich RD, Miller PD, Jackson RD, et al. Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med 1990;323:73-9. [DOI] [PubMed]
- 20.Storm T, Thamsborg G, Steiniche T, Genant HK, Sorensen OH. Effect of intermittent cyclical etidronate therapy on bone mass and fracture rate in women with postmenopausal osteoporosis. N Engl J Med 1990;322:1265-71. [DOI] [PubMed]
- 21.Cranney A, Guyatt G, Krolicki N, Welch V, Griffith L, Adachi JD, et al. A meta-analysis of etidronate for the treatment of postmenopausal osteoporosis. Osteoporos Int 2001;12:140-51. [DOI] [PubMed]
- 22.Van Staa TP, Abenhaim L, Coopers C. Use of cyclical etidronate and prevention of non-vertebral fractures. Br J Rheumatol 1998;37:87-94. [DOI] [PubMed]
- 23.Crilly RG, Sebaldt RJ, Hodsman AB, Adachi JD, Brown JP, Goldsmith CH, et al. Predicting subsequent bone density response to intermittent cyclical therapy with etidronate from initial density response in patients with osteoporosis. Osteoporos Int 2000;11:607-14. [DOI] [PubMed]
- 24.Schnitzer T, Bone HG, Crepaldi G, Adami S, McClung M, Kiel D, et al. Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Aging (Milano) 2000;12(1):1-12. [PubMed]
- 25.Nevitt MC, Thompson DE, Black DM, Rubin SR, Ensrud K, Yates AJ, et al. Effect of alendronate on limited-activity days and bed-disability days caused by back pain in postmenopausal women with existing vertebral fractures. Arch Intern Med 2000;160:77-85. [DOI] [PubMed]


