Abstract
PESTICIDE EXPOSURE CAN CAUSE MANY DIFFERENT HEALTH EFFECTS, from acute problems such as dermatitis and asthma exacerbation to chronic problems such as chronic obstructive pulmonary disease and cancer. The resulting clinical presentations are undifferentiated, and specific knowledge of the links to environmental exposures is often required for effective diagnosis. In this article we illustrate the use of the CH2OPD2 mnemonic (Community, Home, Hobbies, Occupation, Personal habits, Drugs and Diet), a history-taking tool that assists physicians in quickly identifying possible environmental exposures. We also provide clinical information on the epidemiology, clinical presentations, treatment and prevention of pesticide exposures.
Case
On a sunny, hot day in June a 4-year-old girl and her mother arrive at the emergency department after the girl experienced 3 brief episodes, each lasting 2–3 seconds, of shaking, staring and verbal unresponsiveness during the hour before arrival. There was no loss of consciousness or incontinence. The girl has been vomiting and complaining of abdominal discomfort for 2 hours and has a rash on her arms and face that began 4 hours before presentation. She has no history of head injury or seizure disorder and has been previously healthy. Direct questioning reveals no likely exposure to amphetamines, cocaine, isoniazid, lidocaine, lithium, MDMA (3,4-methylenedioxymethamphetamine [“ecstasy”]), PCP (phencyclidine), phenytoin or tricyclic antidepressants. The child is afebrile, her pulse 120 beats/min, her blood pressure 80/60 mm Hg and her respiratory rate 26 breaths/min. She is awake but not talking spontaneously. Neurological examination and examination of the ears, nose and throat, the chest and the abdomen reveal no abnormalities. A fine maculopapular rash is apparent on the face, neck, arms and lower legs. After arriving in the emergency department the child has a further episode of shaking and verbal unresponsiveness. Knowing that a toxic exposure is one possible cause, the physician quickly takes an environmental exposure history using the CH2OPD2 mnemonic (Community, Home, Hobbies, Occupation, Personal habits, Diet and Drugs; for children the occupation question refers to workplace contaminants brought into the child's environment)1 to identify possible exposures (Table 1).
Table 1

Questions surrounding this case: Could any of the exposures have caused the child's symptoms? What investigations should be ordered? Who should be consulted for advice if needed?
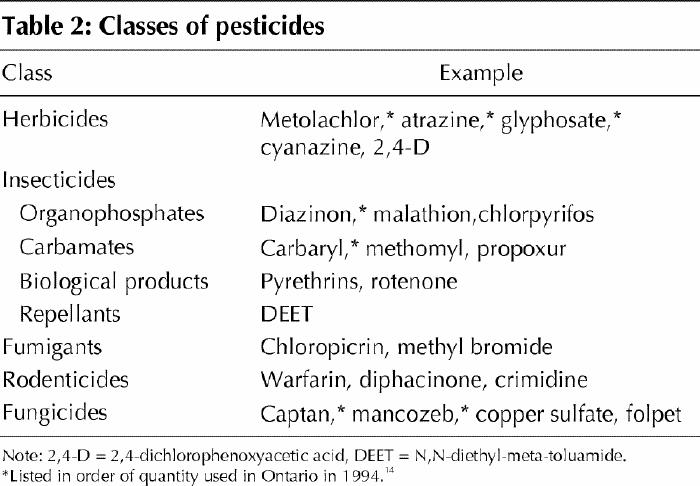
Pesticides are chemical substances used to kill animal, insect, plant and fungal pests in agricultural, domestic and institutional settings.2 The main groups of commonly used pesticides include herbicides, insecticides, fungicides, fumigants and rodenticides (Table 2). The organochlorine, organophosphate and carbamate insecticides are of particular concern because of their toxicity and persistence in the environment.2,3 Organochlorine insecticides were banned for agricultural and domestic use in Europe and North America because of environmental effects, but they are still used in developing countries and continue to linger in the environment because of their chemical stability. Studies in the Canadian Arctic have shown that insecticides and herbicides persist 3 to 8 times longer in cold temperatures than in temperate climates.3
Table 2
Pesticide toxicity can result from ingestion, inhalation or dermal absorption. Outdoor spraying of pesticides poses the obvious risk of inhalation, but pesticide exposure by inhalation also occurs indoors.
The primary routes of exposure for most Canadians, however, are by ingestion of small quantities found in most of the foods we eat,4,5 or by skin absorption through direct contact with surfaces that accumulate pesticide particles.2 Imported foods are noteworthy because they receive pesticide treatment not only during growth, but also during storage and shipment. One example is citrus fruit that is protected against rots and moulds with fungicides; otherwise long distance transport would be more difficult. The Pest Management Regulatory Agency of Health Canada reviews and registers products under the authority of the Pest Control Products Act and determines the amount of pesticide residue that is allowed on a food commodity. About 250–300 pesticide chemicals are registered in Canada for use in production or handling of various foods.5 Maximum residue limits have been established for 100 of these. The remaining chemicals include those too toxic for any residues on foods to be permissible, those unlikely to leave any residue because of their chemical nature and those of such low toxicity that no residue limits need be established for them.
Selected agricultural food commodities in Canada are monitored for pesticide residues. Between 1994 and 1998, 1.2% of domestic foods and 2% of imported fresh products had levels exceeding the maximum residue limits established in the Pest Control Products Act.4 In an Ontario study that tested pesticide residues from 1991 to 1995, 4% of both domestic and imported fruits and vegetables exceeded the maximum allowable residue.4
These percentages are small and on the surface seem acceptable. However, concerns have been raised by physicians and scientists4,6,7 as to the adequacy of the methodology and standards used by the Canadian and US governments both for the release of pesticides and for the acceptance of pesticides used on foods. Setting standards for single chemicals may not reflect the true biological impact of multiple exposures and cumulative effects. For example, a recent study of cumulative dietary pesticide intake in children living on farms and those not living on farms in Washington State found that acceptable chronic dietary doses were exceeded in 56% and 44% of the children respectively.8 Although one insecticide or herbicide may be deemed safe at a particular level, the average Canadian-grown peach may contain residues of several of these pesticides.6 Most of these pesticides interfere in some way with neurotransmission, resulting in an aggregate, if not synergistic, effect. When setting standards, the combined effects of multiple residues plus coincident exposures from other food, water and air sources must somehow be considered. The child dietary pesticide study8 provides a promising model for the use of biological monitoring of exposure to achieve this goal. The Canadian Pest Management Regulatory Agency is currently revising its risk assessment process to account for current shortcomings in risk assessment pertaining to aggregate exposures, cumulative effects and the specific risks of pediatric exposures, among others. Until then, it is advisable to practise precautionary medicine by recognizing groups of patients at increased risk of health effects from pesticide exposure.6
Epidemiology
Children are at increased risk because of exposure patterns and biological vulnerability.2,3,7,8,9,10 This is related to behavioural factors such as hand–mouth behaviour and play patterns and to biological factors such as the immature blood–brain barrier, large skin-surface:body-mass ratio and increased sensitivity of cholinergic receptors to pesticides.9,10 An analysis of all reported pesticide poisonings in the United States showed that 57% of all cases involved children under the age of 6 years.11 Occupationally exposed workers such as pesticide applicators and farmers (and their families) are also at high risk, as are health care workers who have surface contact with exposed patients. Pesticide exposure during pregnancy is associated with an increased risk of spontaneous abortion,12,13 fetal death14,15 and early childhood cancers such as acute lymphocytic leukemia (ALL).16 Indoor use of pesticides increases the risk of pediatric brain tumours, ALL and birth defects.16,17,18,19 Agricultural exposure to pesticides increases the risk of non-Hodgkins lymphoma.20 Moreover, evidence is beginning to accumulate to suggest that specific chronic pesticide effects may develop in elderly people because of the long latency period between exposure and disease. Trends in these data suggest that pesticides exert toxic effects on bone marrow and may be associated with hematopoetic cancers after a latency of 10–25 years.20,21 Pesticides may also have cumulative neurotoxic effects contributing to diseases such as Parkinson's and amyotrophic lateral sclerosis among people who are genetically susceptible.22,23 In mice exposed to 2 extensively used pesticides — paraquat and maneb (a contact herbicide and a water-soluble fungicide respectively) — clinical symptoms and brain lesions of Parkinson's disease developed.24
Table 3 summarizes the common sources of pesticide exposure for the general public and for specific risk groups.
Table 3

Clinical management
The first step in managing a pesticide exposure is to consider it in the differential diagnosis and to take a thorough environmental exposure history using the CH2OPD2 mnemonic.1 This is done with a brief series of questions to elicit possible environmental exposures in the patient's community, home, hobbies, occupational setting, personal habits, diet and drugs. The clinical spectrum of signs and symptoms of acute and chronic pesticide exposure is very broad, which is why incorporating the CH2OPD2 mnemonic routinely into history taking can provide clues about environmental exposures otherwise overlooked. According to one case series, the diagnosis of pesticide poisoning in children was initially missed in 80% of the cases presenting to a major teaching hospital,25 and in another series a history of pesticide exposure was volunteered in only 3 of 25 confirmed cases of pesticide toxicity in children aged 3 months to 7 years.26
The systemic effects of pesticide exposure range from acute poisoning, with neurological effects, diarrhea and bronchial hypersecretion, to an intermediate syndrome of delayed neurological symptoms, to chronic effects including dermatitis, neurobehavioural symptoms and cancer.27 These effects vary according to the toxicological properties of the pesticide. Seizures are characteristic of poisoning with 19 different pesticides and may occur with 12 others, including 2,4-D (2,4-dichlorophenoxyacetic acid), and the organophosphate and carbamate insecticides commonly used on lawns and gardens.27 Seizures as a symptom vary with age, occurring as a presenting symptom in 25% of cases involving children but only in 2%–3% of cases involving adults.27 The signs of acute cholinergic poisoning (salivation, lacrimation, urination, diarrhea and productive cough) suggest insecticide poisoning resulting in cholinesterase inhibition. There are several helpful mnemonics for remembering the clinical effects of organophosphate and carbamate poisoning (Table 4).
Table 4
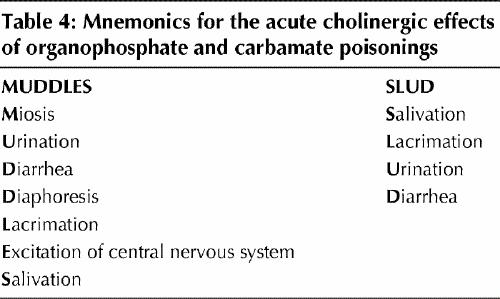
Health care workers are at risk of significant exposure when treating cases of pesticide poisoning and should wear rubber gloves during decontamination. Latex and vinyl surgical gloves may not provide protection against skin absorption of pesticides and should not be used.27 Physicians should proceed with treatment on the basis of the patient's exposure history and clinical findings and in consultation with a poison control centre, rather than await confirmation by laboratory analysis. As always, attending to the “ABCs” of airway, breathing and circulation is the first priority. “D” (decontamination) — the removal of clothing and vigorous, repeated washing of skin, hair and nails with soap and water — is indicated for exposure to all classes of pesticides. If cholinergic signs are present (Table 4) an organophosphate or N–methyl carbamate poisoning should be suspected.27 Blood and serum cholinesterase levels should be measured to confirm the diagnosis; these tests are readily available, but the results are not returned in time to be useful in the initial management. Organophosphate and carbamate insecticides are cholinesterase inhibitors, which cause acute overstimulation of the neuromuscular junction.28 An initial result of a depressed cholinesterase level is diagnostic, but the test is useful even if the first result is normal, because it provides a baseline against which the clinical significance of follow-up results can be assessed. The red blood cell cholinesterase activity may take several days to reach its minimum and 1–3 months to recover.27,28 Because the serum cholinesterase level has a wide normal range, follow-up testing in several weeks will often show a rebound increase of 25% or more, which is diagnostic of recent pesticide toxicity.
When acute pesticide poisoning is suspected, transient improvement in signs and symptoms, in particular a reversal of central nervous system effects, after administration of atropine helps to confirm that the clinical signs are related to poisoning with a cholinesterase-inhibiting (organophosphate or carbamate) insecticide.27 The dose of atropine in children is 0.05–0.1 mg/kg intravenously every 15 minutes; it may be administered intramuscularly or through the endotracheal tube if necessary. More severe insecticide poisonings, if caused by organophosphates, may also require intravenous administration of the specific antidote pralidoxime chloride, a selective antagonist that allows reactivation of cholinesterase by competing with the poison. The dose in children under 12 years of age is 20–50 mg/kg intravenously, mixed in 100 mL of normal saline and infused slowly over at least 30 minutes. A repeat dose in 1–2 hours is usually required. Blood pressure monitoring is required, because the drug may occasionally cause hypertensive crisis.27
Prevention
Physicians have an important role in preventing pesticide exposure by discussing with patients at routine visits the extent of their exposure. High-risk groups that are either occupationally or biologically vulnerable (Table 3) should be targeted for these messages.
Advice for parents
· Cosmetic spraying of lawns and gardens may pose health risks, especially for young children, and should be avoided. Although insecticides (e.g., malathion) are the most toxic, herbicides and fungicides also have subtle neurobehavioural and central nervous system effects as well as nonspecific effects such as fatigue, nausea, rashes and diarrhea.
· Agricultural spraying of pesticides should be done with adequate warning to minimize incidental exposure of children. Spraying in windy conditions must be avoided to prevent accidental spray drift onto residential areas, parks and school playgrounds.
· Young children receive some of their most severe pesticide exposures from indoor spraying of cockroach, flea and tick pesticides.17,18 Such exposure can be avoided with the use of other flea control products, such as injections or pills, on house pets.
· The removal of children from living quarters for an extended period may be necessary for the safe fumigation of an infested building.
· Insect repellants with DEET (N,N-diethyl-meta-toluamide) as the active ingredient should not be used on children under 2 years of age. In older children use repellants on exposed clothing or skin; do not cover the areas with outerwear, because this enhances systemic absorption.10
Advice for adults
· Essential spraying in agricultural settings should be done with adequate protection (respirator with pesticide filter and chemical-resistant gauntlet gloves and overalls). Clothing used during spraying should be washed separately from other laundry. Pesticides are readily absorbed through the skin and should be washed off with soap or shampoo and warm water within 30 minutes after use.
· People should be restricted from work areas for 24–48 hours after indoor spraying with several common pesticides. Adequate ventilation and cleaning of surfaces before the return of workers also reduce exposure.29
Advice for prospective parents
· Agricultural spraying should be done with adequate warning to minimize incidental exposure of pregnant women. An increased risk of fetal death due to congenital anomalies has been found within 2.6 km2 of agricultural spraying.15 Exposure to insecticides sprayed indoors, including in greenhouses, should be avoided by pregnant women because of potential fetal effects.16,18,19
· Because of the apparent link between male pesticide exposure and negative pregnancy outcomes, such as miscarriage and preterm delivery,30 it may be prudent for couples in which the male partner has high seasonal pesticide exposure to avoid conception during the time of high exposure and for 3 subsequent months.
The questions answered
Could any of the exposures have caused the child's symptoms?
In the case described earlier, symptoms began after the child played outside (Table 1), which suggests a pesticide exposure. Exposure to the herbicide 2,4-D may cause dermatitis, because the acid causes skin irritation, especially in combination with heat exposure. The child's other symptoms may be related to exposure to a carbamate or organophosphate insecticide.
What investigations should be ordered?
If the child's history suggests significant dermal exposure, a urine sample should be taken when symptoms are acute and be refrigerated for subsequent analysis of the herbicide level. If further history taking reveals that insecticides had been sprayed on the properties adjacent to the child's play area, the appropriate diagnostic test would be to measure the blood and serum cholinesterase levels. However, although the results of these tests would confirm the diagnosis, they would not arrive quickly enough to provide the treating physician with timely information.
Who should be consulted for advice if needed?
Information on the specific pesticides sprayed on the adjacent properties is important for treatment. In some municipalities, the lawn care company would be required to post a sign on the area sprayed that names all the chemicals applied. Otherwise the parents or emergency staff should obtain this information from the lawn care company, specifically requesting Material Safety Data Sheets (MSDS [www.hc-sc.gc.ca/ehp/ehd/psb/whmis/msds.htm]) for all pesticides applied, while treatment of the child proceeds.
In this case the poison control centre incorrectly advised the treating physician that there was no risk of the unknown pesticides producing such symptoms. The child underwent extensive investigation including measurement of drug and alcohol levels, venous gases, complete blood count, electrolyte levels, urinalysis and a CT scan of the head, all of which gave normal results. After several hours of observation, with no fever or further seizures, the child was released from the emergency department without undergoing tests specific to pesticide poisoning. A later chart audit led to the conclusion that the child's symptoms were probably related to an aggregate pesticide exposure, given the timing of the exposure to symptom onset in a previously healthy child. Information on the specific pesticides sprayed on the adjacent lawns was never collected. There was no record of the child requiring subsequent readmission.
Additional resources .
Environment Canada: www.ec.ca
Pest Management Regulatory Agency: a national Web site (www.hc-sc.gc.ca/pmra) and toll-free service (800 267-6315) for all Canadians on issues related to the use of pesticides
Agency for Toxic Substances and Disease Registry: www.atsdr.cdc.gov
National Institute for Occupational Safety and Health: www.cdc.gov/niosh/homepage.html
US Environmental Protection Agency, comprehensive health information on reregistered pesticides: www.epa.gov/pesticides/reregistration/status.htm
Articles to date in this series .
Weir E. Identifying and managing adverse environmental health effects: a new series. CMAJ 2002;166(8):1041-3.
Marshall L, Weir E, Abelsohn A, Sanborn MD. Identifying and managing adverse environmental health effects: 1. Taking an exposure history. CMAJ 2002;166(8):1049-55.
Abelsohn A, Stieb D, Sanborn MD, Weir E. Identifying and managing adverse environmental health effects: 2. Outdoor air pollution. CMAJ 2002;166(9):1161-7.
Sanborn MD, Abelsohn A, Campbell M, Weir E. Identifying and managing adverse environmental health effects: 3. Lead exposure. CMAJ 2002;166(10):1287-92.
Footnotes
[More background information on this case of pesticide exposure can be found on the Web site of the International Joint Commission (www .ijc .org/boards/hptf/modules/content.html, click on pesticide module).
A detailed exposure history questonnaire is available on the Ontario College of Family Physicians Web site (www.cfpc.ca/ocfp/index.html — click on “Exposure History Sheets in MS Word” in the scrolling menu located in the middle of the page). The different components (Community, Home and Hobbies, Occupation, Personal habits, Diet and Drugs) can be printed on coloured paper for easy identification in patient charts. The questionnaire may be given to a patient to complete at home and bring to the next appointment for review and interpretation.]
This article has been peer reviewed.
Contributors: Drs. Sanborn and Cole wrote the module on which this article is based; the article was drafted by Drs. Sanborn and Weir. Drs. Cole and Abelsohn contributed substantially to the conception and design, and analysis and interpretation of data. All authors contributed to the revising of the manuscript and approved the final version.
Competing interests: None declared.
Correspondence to: Dr. Margaret D. Sanborn, Chesley Medical Clinic, Box 459, 33 Second St. SE, Chesley ON N0G 1L0; msanborn@sbghc.on.ca
References
- 1.Marshall L, Weir E, Abelsohn A, Sanborn MD. Identifying and managing adverse environmental health effects: 1. Taking an exposure history. CMAJ 2002;166(8):1049-55. Available: www.cmaj.ca/cgi/content/full/166/8/1049. [PMC free article] [PubMed]
- 2.Health and the environment. The health and environment handbook for health professionals. Ottawa: Health Canada; 1998. Cat no H46-2/98-2111. Available: www.hc-sc.gc.ca/ehp/ehd/catalogue/bch_pubs/98ehd211/98ehd211.htm (accessed 2002 Apr 22).
- 3.The health of Canada's children: a CICH profile. 3rd ed. Ottawa: Canadian Institute of Child Health; 2000.
- 4.Neidert E, Havelock G. Report on levels and incidences of pesticide residues in selected agricultural food commodities available in Canada during 1994–1998. Ottawa: Canadian Food Inspection Agency; 1998 Nov 6.
- 5.Ripley BD, Lissemore LI, Leishman PD, Denomme MA, Ritter L. Pesticide residues on fruits and vegetables from Ontario, Canada, 1991–1995. J AOAC Int 2000;83(1):196-213. [PubMed]
- 6.Why Canadian physicians are concerned about the policies regulating pesticide use: presentation by Kelly Martin, MD, to the Standing Committee on the Environment. Ottawa: Canadian Association for Physicians for the Environment. Available: www.cape.ca/resources/documents/pesticides.html (accessed 2002 Apr 22).
- 7.National Research Council. Pesticides in the diet of infants and children. Washington: National Academy of Science; 1993.
- 8.Fenske RA, Kissel JC, Lu C, Kalman DA, Simcox NJ, Allen EH, et al. Biologically based dose estimates for children in an agricultural community. Environ Health Perspect 2000;108(6):515-20. [DOI] [PMC free article] [PubMed]
- 9.Faustman EM, Silbernagel SM, Fenske RA, Burbacher TM, Ponce RA. Mechanisms underlying children's susceptibility to environmental toxicants. Environ Health Perspect 2000;108(Suppl 1):13-21. [DOI] [PMC free article] [PubMed]
- 10.Etzel RA, Balk SJ, editors. Handbook of pediatric environmental health. Washington: American Academy of Pediatrics; 1999.
- 11.Klein-Schwartz W, Smith GS. Agricultural and horticultural chemical poisoning: mortality and morbidity in the U.S. Ann Emerg Med 1997;29(2):232-38. [DOI] [PubMed]
- 12.Nurminen T. Maternal pesticide exposure and pregnancy outcome. J Occup Environ Med 1995;37(8):935-40. [DOI] [PubMed]
- 13.Sever LE, Arbuckle TE, Sweeney A. Reproductive and developmental effects of occupational pesticide exposure: the epidemiologic evidence. Occup Med 1997;12(2):305-25. [PubMed]
- 14.Arbuckle TE, Sever LE. Pesticide exposures and fetal death: a review of the epidemiologic literature. Crit Rev Toxicol 1998;28(3):229-70. [DOI] [PubMed]
- 15.Bell EM, Hertz-Picciotto I, Beaumont JJ. A case–control study of fetal death due to congenital anomalies. Epidemiology 2001;12(2):148-56. [DOI] [PubMed]
- 16.Infante-Rivard C, Labuda D, Krajinovic M, Sinnett D. Risk of childhood leukemia associated with exposure to pesticides and with gene polymorphisms. Epidemiology 1999;10:481-7. [PubMed]
- 17.Daniels J, Olshan A, Savitz D. Pesticides and childhood cancers. Environ Health Perspect 1997;105(10):1069-77. [DOI] [PMC free article] [PubMed]
- 18.Pogoda J, Preston-Martin S. Household pesticides and the risk of pediatric brain tumors. Environ Health Perspect 1997;105:1214-20. [DOI] [PMC free article] [PubMed]
- 19.Kristensen P, Irgens LM, Anderson A, Snellingen Bye A, Sundheim L. Birth defects among offspring of Norwegian farmers, 1967–1991. Epidemiology 1997;8(5):537-44. [DOI] [PubMed]
- 20.Hardell L, Eriksson M. A case–control study of non-Hodgkins lymphoma and exposure to pesticides. Cancer 1999;85(6):1353-60. [DOI] [PubMed]
- 21.Rigolin G, Cuneo A, Roberti M, Bardi A, Bigoni R, Piva N, et al. Exposure to myelotoxic agents and myelodysplasia: a case–control study and correlation with clinicobiological findings. Br J Haematol 1998; 103:189-97. [DOI] [PubMed]
- 22.Hubble J, Kuth J, Glatt S, Kurth M, Schellenberg G, Hassanein R, et al. Gene–toxin interaction as a putative risk factor for Parkinson's disease with dementia. Neuroepidemiology 1998;17:96-104. [DOI] [PubMed]
- 23.McGuire V, Longstreth WT, Nelson LM, Koepsell TD, Checkoway H, Morgan MS, et al. Occupational exposures and amyotrophic lateral sclerosis. Am J Epidemiol 1997;145(12):1076-88. [DOI] [PubMed]
- 24.Thiruchelvam M, Brokel EK, Richfield EK, Baggs RB, Task AW, Cory-Slechta DA. Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: Environmental risk factors for Parkinson's disease? Brain Res 2000;873(2):225-34. [DOI] [PubMed]
- 25.Zweiner RJ, Ginsgurg CM. Organophosphate and carbamate poisoning in infants and children. Pediatrics 1988;81:121-6. [PubMed]
- 26.Sofer S, Tal A, Shahak E. Carbamate and organophosphate poisoning in early childhood. Pediatr Emerg Care 1989;5:222-5. [DOI] [PubMed]
- 27.Reigart R, Roberts J. Recognition and management of pesticide poisonings. 5th ed. Arlington (VA): Office of Pesticide Programs, US Environmental Protection Agency; 1999.
- 28.De Bleecker JL, De Rueck JL, Willems JL. Neurologic aspects of organophosphate poisoning. Clin Neurol Neurosurg 1992;94:93-103. [DOI] [PubMed]
- 29.Greenberg MI, Hamilton RJ, Phillips SD, editors. Occupational, industrial, and environmental toxicology. St Louis: Mosby; 1997. p. 620.
- 30.Savitz, D, Arbuckle T, Kaczor D, Curtis K. Male pesticide exposure and pregnancy outcome. Am J Epidemiol 1997;146(12):1025-36. [DOI] [PubMed]


