Abstract
The filamentous fungus Aspergillus oryzae (A. oryzae) has been extensively used for the biosynthesis of numerous secondary metabolites with significant applications in agriculture and food and medical industries, among others. However, the identification and functional prediction of metabolites through genome mining in A. oryzae are hindered by the complex regulatory mechanisms of secondary metabolite biosynthesis and the inactivity of most of the biosynthetic gene clusters involved. The global regulatory factors, pathway-specific regulatory factors, epigenetics, and environmental signals significantly impact the production of secondary metabolites, indicating that appropriate gene-level modulations are expected to promote the biosynthesis of secondary metabolites in A. oryzae. This review mainly focuses on illuminating the molecular regulatory mechanisms for the activation of potentially unexpressed pathways, possibly revealing the effects of transcriptional, epigenetic, and environmental signal regulation. By gaining a comprehensive understanding of the regulatory mechanisms of secondary metabolite biosynthesis, strategies can be developed to enhance the production and utilization of these metabolites, and potential functions can be fully exploited.
Keywords: Aspergillus oryzae, secondary metabolism, secondary metabolites, regulatory gene
1. Introduction
Aspergillus oryzae is a common aerobic fungus that has been used in the food industry for over a thousand years. Notably, it is the oldest and most widely used microorganism in the brewing industry. In addition, the US Food and Drug Administration has classified A. oryzae as “generally recognized as safe” (GRAS), and the World Health Organization has acknowledged its safety [1,2,3]. The safety of phospholipase A1, a food enzyme produced by the transgenic strain NZYM-LJ/NZYM-PP of A. oryzae, has also been validated [4,5]. Though widely distributed and rapidly multiplying, A. oryzae exhibits slight variations in colony growth among different strains. In China and Japan, A. oryzae plays a crucial role in traditional brewing technology and is even regarded as a “national fungus” in Japan [6].
The A. oryzae RIB40 genome, which measures 37.9 Mb, was first sequenced in 2005 [7]. Compared to other species (Table 1), A. oryzae has a bigger genome, primarily due to the amplification of metabolic genes, including those related to secretory hydrolases, transporters, and primary and secondary metabolism [7,8]. Not only does A. oryzae possess a remarkable protein secretion function, but it also exhibits a closer resemblance to natural eukaryotic genetic products [9,10]. Moreover, with its extensive history of industrial application and capacity for large-scale fermentation, it represents an ideal and efficient system for the expression of foreign genes [11,12,13]. Useful proteins have been successfully generated from animal and plant sources using A. oryzae [14]. Additionally, the successful heterologous expressions of important structural natural products documented in the literature have been achieved through A. oryzae (Table 2). Furthermore, the heterologous expressions of these compounds have resulted in higher yields than those produced using their original strains [15]. For example, the heterogeneous expression of the trili biosynthetic gene clusters of Trichoderma reesei in A. oryzae produced new compounds (two acyl tetramic acids), indicating the fungal host diversity in catalytic reactions [16].
Superbugs constantly emerge and proliferate due to the misuse of antibiotics, inflicting severe damage to human life and health. The filamentous fungi genomes consist of numerous gene clusters responsible for the biosynthesis of secondary metabolites compared to Actinobacteria, indicating their higher potential for producing active metabolites. Notably, secondary metabolites facilitate biological signal transduction throughout an organism’s life cycle and produce “defense compounds” that enable adaptation to environmental changes [17,18,19]. Various microorganisms synthesize diverse secondary metabolites intracellularly or extracellularly during specific temporal windows, which function in signal transduction pathways [20]. In Aspergillus, the biosynthesis pathway of secondary metabolites is regulated by pathway-specific transcription factors and global regulatory factors such as environmental conditions and epigenetic modifications [21,22]. The secondary metabolites of A. oryzae contain numerous physiologically and pharmacologically active compounds and a few mycotoxins [23,24,25]. These products play an integral role in the evolution of A. oryzae and its interactions with other organisms and the environment.
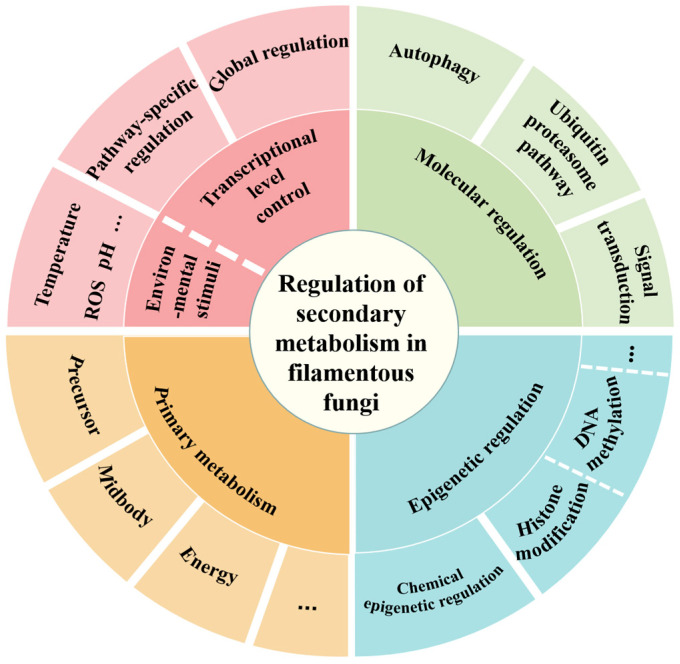
Deciphering the A. oryzae genome, especially with the identification of the many gene clusters responsible for the synthesis of secondary metabolites, has provided a genetic basis for the abundant biosynthesis of secondary metabolites through this microorganism. For example, kojic acid, one of the primary secondary metabolites produced by A. oryzae [26], is widely used in cosmetics as the main component of various whitening products [27]. Kojic acid and its derivatives also serve as a raw material for antibacterial, antifungal, and anti-inflammatory agents in the pharmaceutical industry [28,29,30]. Researchers have also discovered polysaccharides with anti-tumor activity [31,32], active compounds against Alzheimer’s disease, antibacterial compounds [33,34], and enzyme inhibitors [35] from various A. oryzae strains. Although ergosterol, squalene, ceramide, and other common secondary metabolites are synthesized by A. oryzae, there is still limited knowledge on the potential of A. oryzae in producing other secondary metabolites, with reports that the known compounds represent only a minor fraction of the secondary metabolites produced by A. oryzae [3,36,37,38]. Therefore, the identification, validation, and development of more reactive molecules from A. oryzae are still ongoing, highlighting the vast potential of A. oryzae in various fields, such as medicine and agriculture. In this review, we analyzed the secondary metabolism regulation in A. oryzae, with a focus on three key aspects: transcriptional regulation, epigenetic modification, and stimulation through environmental signals. These three regulatory modes converge to form complex regulatory networks (Figure 1) and have been shown to affect the synthesis of secondary metabolites. This review delved into the production of secondary metabolites in A. oryzae at the regulatory level and proposes feasible strategies for mining potentially valuable compounds.
Figure 1.
Regulatory network of secondary metabolism in filamentous fungi.
Table 1.
Comparison of genome characteristics between A. oryzae and other related fungi.
Table 2.
Structural types of natural products heterogeneously expressed by A. oryzae.
2. Transcriptional Regulation of Secondary Metabolism in A. oryzae
Secondary metabolism in filamentous fungi is a complex and multi-level regulatory process involving numerous enzymatic reactions. Specifically, the biosynthesis of secondary metabolites is regulated by pathway-specific transcription factors and global regulatory factors [21].
2.1. Global Regulatory Factors
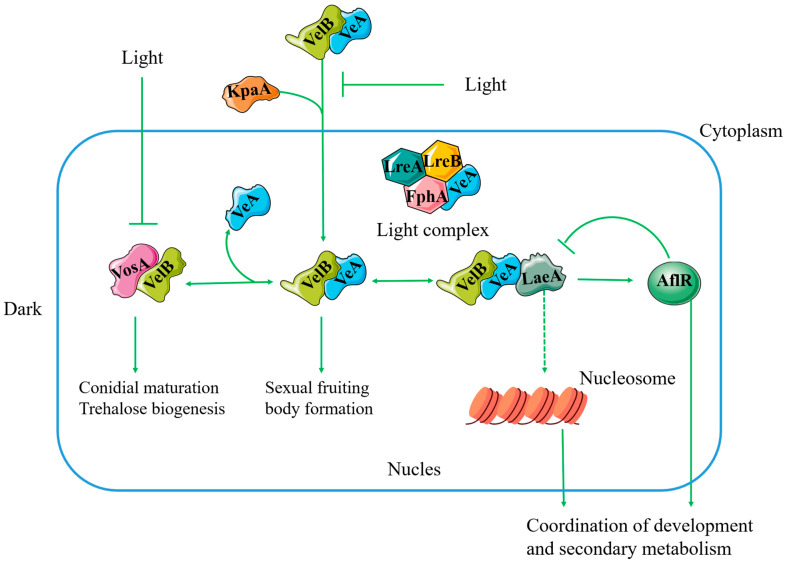
Global regulation refers to the biosynthesis and morphological differentiation regulation of multiple secondary metabolites. Although genes regulating the global transcription factors do not exist in gene clusters, they regulate the transcriptional activation of various genes, thereby influencing the production of secondary metabolites. LaeA is one of the major global regulators of secondary metabolism in filamentous fungi, which was discovered in A. nidulans in 2004 [45,46]. It regulates the expression of multiple secondary metabolic gene clusters and influences the formation of secondary metabolites [47,48]. LaeA forms a protein complex with veA and veB in response to light and regulates the expression of the Velet family members, thus influencing secondary metabolism, growth, and reproduction (Figure 2). It also influences the morphogenesis and development of filamentous fungi by activating the expressions of silent genes to produce new metabolites [49]. For example, fungal-specific sirtuins hstD/Aohst4 interact with laeA, with hstD/Aohst4 acting upstream of laeA [50], altering the production of multiple secondary metabolites. Thus, sirtuins hstD/Aohst4 are essential in the global regulation of the biosynthesis of secondary metabolites. In addition, laeA is highly expressed in hstD/AoHst4-deleted strains, suggesting that hstD/AoHst4 is involved in the suppression of the laeA gene.
Figure 2.
A schematic representation of the global regulation of the LaeA and Velvet family proteins. Importin alpha KapA facilitates the nuclear entry of the VelB-VeA dimer. VelB can assemble into two distinct complexes within the nucleus. The VosA-VelB dimer suppresses asexual spore formation while regulating spore maturation and trehalose synthesis. The VelB-VeA dimer interacts with the LaeA protein to form a trimer, which modulates sexual development and secondary metabolism.
Moreover, the overexpression of Aokap2 under the control of an amyB promoter of A. oryzae inhibits hyphal growth, conidia formation, and biomass yield with increased kojic acid production [36]. The overexpression of Aokap2 elevates the transcription levels of kojA, a key gene involved in kojic acid synthesis, and laeA, a global transcriptional regulator, increasing kojic acid production. Conversely, the expression of Aokap2 is significantly downregulated in laeA mutants, decreasing kojic acid production.
With the rapid development of genetic engineering techniques, many studies are adopting these techniques to select superior strains. Moreover, mutation breeding still plays an important role in obtaining high-yield strains. For example, a kojic acid-producing strain, AR-47, was obtained through the co-mutagenesis of A. oryzae KA-11 [51]. A transcriptional expression analysis of kojic acid biosynthesis-related genes revealed that the expressions of these genes were higher in strain AR-47 compared to those in the original strain. In addition, kojA, kojR, and kojT are involved in kojic acid biosynthesis. KojR is positioned between kojA and kojT, directly regulating kojT and potentially controlling kojA [26,52,53]. LaeA also regulates kojA and kojT by controlling the expression of kojR. Specifically, the upregulation of laeA increases the expression of kojR, kojA, and kojT, implying that laeA positively regulates kojic acid synthesis. As a result, the deletion of the regulatory gene laeA decreases kojic acid production and related gene expression, while its presence restores kojic acid production. These findings suggest that laeA expression is also regulated by other genes. However, what drives the increased expression of kojic acid biosynthesis genes remains unknown. In addition, further research is needed to establish their regulatory relationship with laeA.
The veA gene mediates the response to light and regulates various cellular processes, including asexual and sexual development and secondary metabolism [54]. The beta-lactam antibiotic penicillin is derived from a few filamentous fungi with specialized production ability. In A. oryzae, the positive regulation of the veA gene cluster influences gene transcription, thereby promoting penicillin synthesis [38]. Additionally, the homologous proteins laeA and velB play a role in the regulation of the veA gene. For example, there is a noticeable decrease in penicillin synthesis by A. oryzae after veA deletion. Notably, the expression of ipnA within the penicillin biosynthetic gene clusters is positively regulated by veA in A. oryzae and negatively regulated by veA in A. nidulans [38,54].
The transcription factor brlA, belonging to the C2H2 zinc finger family, plays a crucial role in conidial development and in regulating secondary metabolism [55,56]. KpeA is highly conserved in filamentous fungi and represents a novel Zn(II)2-Cys6 binding protein, exerting global regulatory control. For example, there was a six-fold increase in kojic acid production in the ΔkpeA strain compared to the control strain, which was accompanied by the upregulation of the kojR and kojA genes and downregulation of brlA, abaA, and wetA transcription levels [57]. Therefore, kpeA functions as a Zn(II)2-Cys6 binding protein in the transcriptional regulation of conidiation and the biosynthesis of kojic acid.
In summary, evidence suggests that global regulatory transcription factors play a pivotal role in the secondary metabolism of A. oryzae. While the modulatory mechanisms of some transcription factors remain elusive, it is clear that global transcriptional regulators can effectively control secondary metabolites in A. oryzae.
2.2. Pathway-Specific Regulators
The primary function of gene expression in the secondary metabolic pathway is to regulate the biosynthesis gene cluster through encoding genes. In fungi, the zinc finger protein family is involved in the specific regulation of secondary metabolites. For example, the C2H2 zinc finger regulatory protein encoded by msnA and its cognate genes are the primary transcription factors that regulate fungal cell response to external stress [58,59]. In addition, the growth diameter, spore count, and production of kojic acid are increased in A. nidulans and A. oryzae upon knocking out msn2. Nonetheless, the Aomsn2 gene in A. oryzae regulates kojT expression, impacting kojic acid synthesis. The kpeA mutation decreases the expression of the core regulatory factor brlA and significantly increases the expression of the pathway-specific regulatory factor kojR, increasing the production rate of kojic acid compared to the control strain [57].
Aflatoxins are primarily produced by A. flavus and A. parasiticus. Belonging to the section Flavi, A. oryzae and A. flavus exhibit a 99.5% gene homology. However, domestication may have caused the loss of the toxin-producing ability in A. oryzae [60] due to the significant deletions and multiple mutations in the aflatoxin gene cluster [61]. For example, multiple mutations have occurred in the aflR gene, a pathway-specific regulator of aflatoxin biosynthesis. As a result, some A. oryzae strains do not express aflR, while others exhibit a weak expression [62]. In addition, the deletion of ctnA, a specific transcription factor regulating the citrinin biosynthetic pathway, significantly reduces citrinin production by Monascus purpureus. Conversely, the overexpression of the ctnA gene in A. oryzae leads to an approximately 400-fold increase in citrinin levels [63,64].
Pathway-specific regulators may not only regulate gene transcription within a gene cluster but also outside of it. Additionally, the diverse modes of specific regulatory factors interact to exert control. However, further research is needed to explore specific regulatory factors that affect the A. oryzae secondary metabolism. Understanding these control mechanisms will provide valuable insights into the study and production of secondary metabolites.
3. Epigenetic Regulations of Secondary Metabolism in A. oryzae
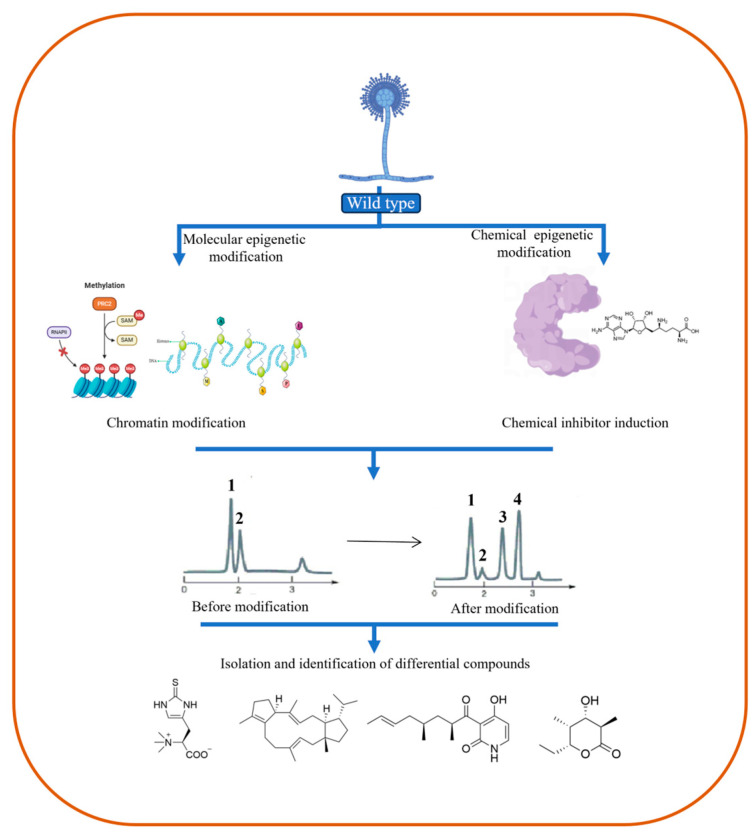
The methodologies employed for the epigenetic modification of secondary metabolism regulation primarily encompass molecular and chemical epigenetic interventions (Figure 3). Molecular epigenetic regulation is based on nucleic acid modifications, including DNA methylation, histone modification, and RNA silencing systems. A significant correlation exists between secondary metabolism and epigenetic states in filamentous fungi [65]. For example, the presence of heterochromatin histone marks silences the secondary metabolism gene clusters. However, in the absence of these marks, the gene clusters are activated, altering the chromatin structure accordingly. Therefore, altering the histone modification state can regulate gene expression and induce numerous changes in metabolite spectra, ultimately activating the recessive secondary metabolite production [66,67].
Figure 3.
Epigenetic regulatory patterns of secondary metabolites in fungi.
3.1. Effect of Epigenetic-Related Genes on Secondary Metabolism
Transcription factor laeA exhibits a sequence similar to those of histone and arginine methyl transferases, suggesting that they may regulate the overall synthesis of secondary metabolites by modulating chromatin structure. In A. oryzae, hstD, a gene homologous to yeast, plays a crucial role in regulating secondary metabolism [50]. In hstD-deficient A. oryzae strains, growth is inhibited, while the yields of kojic acid and penicillin are significantly increased. However, morphogenetic defects and enhanced kojic acid production can be rescued via hstD/Aohst4 gene insertion. Furthermore, genetic interactions between hstD/AoHst4 and laeA suggest that this fungus-specific sirtuin (a member of the NAD (+)-dependent histone deacetylase (HDAC) family) coordinates fungal development and secondary metabolism by regulating laeA in filamentous fungi.
Methylation is a crucial modification that impacts gene expression. Genes homologous to cclA, a component of the histone 3 lysine 4 (H3K4) methyltransferase complex associated with the Set1 complex in other organisms, are present in A. oryzae. A. oryzae also contains genes homologous to sppA in Saccharomyces cerevisiae, an important component of another H3K4 methyltransferase complex [68]. The absence of both cclA and sppA hinders three methylation processes and histone H3K4, which alters the chromosome status. This influences related gene expression, ultimately leading to an improved astellolide yield. However, knowledge of epigenetic genes involved in the secondary metabolism of A. oryzae remains limited. Therefore, further investigations into the regulatory mechanisms governing secondary metabolism are necessary to promote research on A. oryzae.
3.2. Effect of Chemical Epigenetic Agents on Secondary Metabolism
Recent discoveries have revealed several chemical reagents that suppress the activity of enzymes involved in epigenetic modifications, thereby controlling secondary metabolism. For example, HDAC inhibitors induce the production of fungal secondary metabolites by altering histone acetylation on chromatin [69,70]. The modification transforms the silent gene locus from a hypoacetylated state to an actively hyperacetylated state, which activates gene expression and enhances the production of multiple polyketones in fungi. Therefore, modulating fungal gene expression epigenetically with HDAC inhibitors is a potentially powerful method for obtaining recessive biosynthetic natural products.
Moreover, introducing pksCH-1 and pksCH-2 genes from Chaetomium indicum into A. oryzae [69] revealed that pksCH-2 was epigenetically modified to function as the silent non-reducing PKS gene encoding the common precursor 8 for the new compound, which is consistent with ChIP analysis results. However, multiple attempts to express pksCH-1 have been unsuccessful. Nonetheless, given its amino acid sequence and domain similarity to pkeA, it is reasonable to infer that this gene may encode a common precursor for a new compound.
Ergosterol is a crucial pharmaceutical raw material for the production of cortisol and progesterone [71,72]. Most antifungal drugs act as inhibitors of essential enzymes in the ergosterol biosynthesis pathway, which can be categorized into four distinct categories based on their specific roles [73,74,75]. For example, bioinformatic and RNA-seq analyses of the gene expression profiles in A. oryzae treated with tebuconazole and terbinafine revealed that there are many differentially expressed genes associated with ergosterol biosynthesis [76]. In addition, the ergosterol biosynthesis is blocked when tebuconazole inhibits ERG11. In contrast, terbinafine inhibits ERG1, decreasing ergosterol production and squalene accumulation in the plasma membrane, which increases the brittleness of the plasma membrane, impairing its structure and function. These reports demonstrate that tebuconazole and terbinafine have distinct targets and mechanisms of action [76]. Among them, 17 genes (16 downregulated and 1 upregulated) were regulated by both tebuconazole and terbinafine inhibitors, indicating that these genes may be directly targeted by ergosterol.
In summary, epigenetic regulation is a highly convenient and effective strategy for discovering new compounds. Therefore, the use of inhibitors targeting fungal gene expression for epigenetic regulation could be an effective approach to unlocking the recessive biosynthesis of natural products in A. oryzae. Given the limited epigenetics research in A. oryzae, continuous exploration in this field will establish a solid foundation for future research on Aspergillus.
4. Environmental Factor Regulation of Secondary Metabolism in A. oryzae
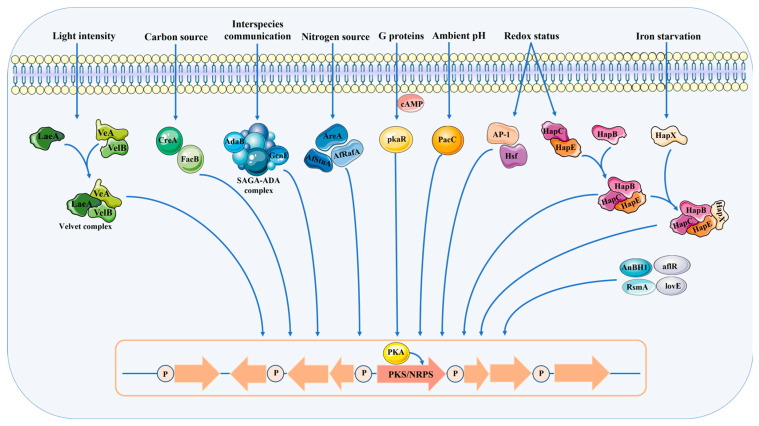
Fungal growth, development, and metabolism are highly susceptible to variations in environmental conditions such as medium composition, reactive oxygen species (ROS), temperature, pH level, light intensity, metal ion concentration, and interspecies interactions (Figure 4) [77,78,79,80]. Changes in these environmental factors alter the enzyme activity, subsequently affecting the diversity of secondary metabolites associated with them. In addition, the transcriptional and epigenetic regulation of genes involved in the biosynthetic pathways of A. oryzae is a response to environmental stimuli, with changes in the secondary metabolites being mediated by intracellular transcription factors or signal transduction pathways. Thus, environmental stimuli are essential for the production of secondary metabolites, like functional proteins, lipids, organic acids, and other secondary metabolites used in the food processing industry [3,81,82].
Figure 4.
Environmental regulation of biosynthetic gene clusters for secondary metabolites in fungi. Environmental signals regulate the expressions of genes in biosynthetic gene clusters, thereby controlling the activities of key enzymes or gene expression levels in secondary metabolic pathways and ultimately affecting the synthesis of secondary metabolites. The intracellular molecule cAMP mediates the internal regulation of biosynthetic gene clusters by modulating camp-dependent protein kinase (PKA).
4.1. The Impact of Carbon Sources
Carbon sources play a pivotal role in fungal growth, where they serve as both carbon skeletons and energy providers. Microbial sources of carbon are classified into two: carbohydrate compounds and organic acids, along with their respective salts. These carbon sources also regulate secondary metabolite synthesis.
In shake-jar cultures of A. oryzae M3B9 supplemented with seven carbon sources (glucose, fructose, sorbitol, sucrose, maltose, lactose, and starch), high concentrations of kojic acid accumulated in the medium with six carbon sources except lactose, significantly inhibiting kojic acid production [83]. This may be attributed to a change in metabolic regulation. Furthermore, A. oryzae M3B9 efficiently utilized fructose to generate elevated levels of kojic acid, contradicting a previous report, which suggested that furanose-derived fructose cannot generate high concentrations of kojic acid [84].
In another study in which glucose was employed as a carbon source, A. oryzae produced 58.2 g/L of malic acid and 4.2 g/L of fumaric acid. However, xylose and glycerol resulted in decreased malic acid production [85]. Furthermore, glycerol had a positive impact on fumaric acid production, while xylose exhibited no significant effect. A comparative analysis of the active growth and lipid accumulation stages of A. oryzae BCC7051 also revealed that this strain exhibited greater efficiency in utilizing carbon sources to produce biomass and lipids in the C5 (xylose) medium compared to a C6 (glucose) medium [86]. Furthermore, when a mixture of glucose and xylose was used as the carbon source in the medium, an increased glucose ratio correspondingly increased the kojic acid yield from A. oryzae BCC7051. Therefore, careful selection of appropriate carbon sources can effectively enhance the production of secondary metabolites.
4.2. The Impact of Nitrogen Sources
Fungi require nitrogen for their growth and reproduction. Nitrogen also regulates the fungal secondary metabolism. Although filamentous fungi are capable of absorbing both ammonium nitrogen (NH4+) and nitrate nitrogen (NO3−), NH4+ is the preferred inorganic nitrogen source for cell growth [87,88]. Aspergillus utilizes various nitrogen-containing compounds as sole nitrogen sources, including ammonia, nitrate, and nitrite. Aspergillus growth is regulated by global transcription factors involved in nitrogen metabolism. The areA gene encodes a nitrogen regulatory protein that activates the transcription of numerous structural genes encoding enzymes that catabolize nitrogen sources under limited nitrogen conditions, thus promoting the availability of favorable nitrogen sources and suppressing the expression of enzymes required for non-favorable nitrogen source catabolism [89,90].
β-lactam biosynthesis is a non-ribosomal peptide synthase system responsible for the production of β-lactam antibiotics, with ACV synthase as the key enzyme in its biosynthetic pathway. The nitrogen source plays a crucial role in determining the ACV yield in A. oryzae after the replacement of the native promoter with AoPgpdA [91]. Among the various nitrogen sources, including yeast extract, mixed nitrogen source, urea, and NaNO3, urea is the most suitable for both cell growth and ACV production. In addition, the amount of chitin varies with changes in nutritional supplements and environmental stress, impacting the glucosamine (GlcN) yield. GlcN is an amino monosaccharide and structural component of chitin and chitosan, with diverse therapeutic effects such as antioxidation, anti-aging, and anti-inflammation [92]. Increasing the nitrogen sources significantly increases the GlcN concentration in A. oryzae NCH-42, with yeast extract being the optimal nitrogen source [93]. Yeast extract and NaNO3 are also the optimal nitrogen sources for the production of anhydromevalonolactone (AMVL), a naturally occurring compound that can be heterogeneously expressed by A. oryzae MTG4 [94]. Increasing the concentration of either yeast extract or NaNO3 enhances AMVL yield. However, excess nitrogen sources may lead to decreased yields. Nonetheless, adding a small amount of NaNO3 to the standard medium results in a complete loss of kojic acid formation, indicating its inhibitory effect on kojic acid formation [52,53]. A similar outcome was observed in the A. oryzae RIB40 strain [95], suggesting that NaNO3 is not the optimal nitrogen source for the production of all secondary metabolites.
These reports suggest that nitrogen sources significantly impact A. oryzae growth, development, and metabolism. Therefore, the selection of the optimal nitrogen source will inevitably influence the yield of secondary metabolites. Moreover, the different nitrogen sources exert varying degrees of influence on the morphology of A. oryzae, impacting subsequent secondary metabolite yields [83].
4.3. The Impact of Temperature
Temperature significantly impacts the growth and metabolism of A. oryzae, with both low and high temperatures hindering mycelial growth and conidia formation [96]. Different culture temperatures primarily impact the activity of the enzymatic system in A. oryzae, subsequently regulating strain growth and metabolism at a molecular level to promote or hinder mold development [97,98]. For example, high temperatures denature and inactivate specific enzymes, decreasing the chemical reaction, metabolism rate, and metabolic products. Conversely, low temperatures hinder the activity of some A. oryzae enzymes, slowing the growth of the fungi. With a temperature growth range of 20–40 °C [99], A. oryzae exhibits optimal growth at 30–35 °C [100]. In addition, fermentation temperatures are typically maintained at 15–45 °C to enhance the relative concentration of flavor compounds [96].
Temperature stress primarily affects glucose, glycerolipid, and linoleic acid metabolism. Low-temperature stress upregulates trehalose synthesis and starch metabolism encoding genes [96]. Conversely, high-temperature stress suppresses the expression of genes regulating fructose, galactose, and glucose metabolism and hinders the normal functioning of the triacylglycerol pathway to decrease the triacylglycerol products [96].
Suppressing AoAur1 gene expression decreases inositol phosphate ceramide (IPC), a signaling molecule that facilitates the adaptation of A. oryzae to diverse environments, but it increases the dihydroceramide and galactoceramide contents [101]. Additionally, inhibiting AoAur1 expression upregulates genes associated with mycelial fusion, enhancing the transduction of stress signals and augmenting cellular adaptability to temperature stress. Therefore, downregulating the AoAur1 gene and reducing IPC accumulation are the underlying mechanisms employed by A. oryzae to adapt to temperature stress.
Mevalonate diphosphate decarboxylase, also known as Erg19, is a crucial enzyme in the mevalonate pathway [102,103]. The AoErg19-overexpressed and RNAi A. oryzae strains exhibit reduced ergosterol content and increased sensitivity to abiotic stress [104]. Moreover, the transcription levels of AoErg19 are decreased with an increasing salt concentration, ethanol concentration, and temperature [105,106]. Therefore, manipulating the temperature and other environmental factors modulates the transcriptional expression of AoErg19 to regulate the biosynthesis of corresponding secondary metabolites. The yield of A. oryzae NCH-42 was low at 20 °C, while the yields of GlcN were high at 25 °C and 30 °C [93]. Additionally, a study using A. oryzae to produce fructo-oligosaccharides revealed that the enhanced activity of mutant V242E is not affected by reaction temperature or other environmental factors [107]. Therefore, not all secondary metabolites are sensitive to temperature, and there are different mechanisms in response to temperature stress.
Overall, there are variations in the impact of temperature stress on the secondary metabolites of A. oryzae, with both positive and negative regulation being observed in metabolite production. Therefore, metabolic gene expression in A. oryzae can be influenced to enhance output by regulating temperature changes to meet the different requirements for various secondary metabolites.
4.4. The Impact of Other Factors
Other factors influencing metabolite production include pH, metal ions, oxidative stress, fermentation time, and so on. Most of these factors exert their influence on the secondary metabolism of A. oryzae by regulating transcription factors and the metabolic gene expression. The effect of pH is mediated by PacC, one of seven genes involved in pH regulation [108,109]. In an acidic environment, PacC remains inactive; however, in an alkaline environment, PacC acts as an activator of alkaline genes and a repressor of acidic genes. Generally, the pal/PacC pathway regulates the synthesis of extracellular hydrolases, including proteases, based on the environmental pH [110]. The pH also influences GlcN yield. At pH 2.5, A. oryzae NCH-42 produces the highest GlcN yield, with GlcN concentration and content being 4.1 and 2.4 times higher than those at pH 4~7, respectively [93]. However, excessive acidity (pH 2.0) significantly reduces the GlcN yield of A. oryzae NCH-42, making it inefficient under acidic conditions.
Among the metal ions influencing metabolite production, Mg2+ is essential for PPTase activity. In the absence of Mg2+, the biomass titer of ACV obtained under neutral pH conditions is significantly reduced. However, when the concentration of Mg2+ is increased to 10 mM, it exerts a strong positive regulatory effect on ACV production [91].
Moreover, oxidative stress responses serve as protective mechanisms against ROS, which induce cellular damage and dyshomeostasis. YAP1 and SKN7 transcription factors are responsible for the expression of crucial genes that encode enzymes essential for ROS detoxification. For example, in A. oryzae, the expression of redox-related genes and YAP1 and SKN7 transcription factors are upregulated under hydrogen peroxide and menadione sodium bisulfite stimulation, resulting in increased glutathione content [111].
The high content of aldehydes significantly contributes to the overall volatile flavor of A. oryzae 100-8. Furthermore, A. oryzae 100-8 produces a notably higher proportion of aldehydes compared to A. oryzae 3.042. Compared with A. oryzae 3.042, A. oryzae 100-8 exhibits faster growth and produces elevated concentrations of aldehydes, esters, and furans [112,113].
Overall, the secondary metabolites of A. oryzae are influenced by environmental factors other than carbon and nitrogen sources and temperature. For example, there are significant variations in 76 secondary metabolites, including volatile components of branched lipids and benzene series produced by A. oryzae exposed to different temperatures and pH levels for varying durations. The high-temperature conditions induced a notable reduction in the composition of linear volatile lipids, while low pH substantially increased the furan compound production [114]. In conclusion, diverse secondary metabolic pathways exhibit distinct responses to various environmental factors. Therefore, modifying environmental conditions is a feasible and effective approach for enhancing the secondary metabolism of A. oryzae.
5. Conclusions and Perspective
In this review, we described how the regulation of biosynthetic gene clusters affects secondary metabolite synthesis in A. oryzae, with a focus on transcriptional regulation, epigenetic regulation, and environmental signal regulation. Compared to primary metabolites, secondary metabolites are greatly diverse and play a more significant role in response to changes in biotic and abiotic factors. By regulating the metabolic pathways and intracellular material transformations, energy transfers can be directed toward the desired pathway, ultimately increasing the secondary metabolite output. The regulation of functional gene expression lies at the heart of A. oryzae secondary metabolism, which is influenced by changes in extracellular environmental factors and intracellular genes. These changes are integrated into a multi-level regulatory network through modifications in chromatin structure, signal transduction pathways, and the activity of transcription factors. Improving the production of secondary metabolites is an important aspect of biological and pharmaceutical research, and mining these compounds can expand their potential applications in various fields.
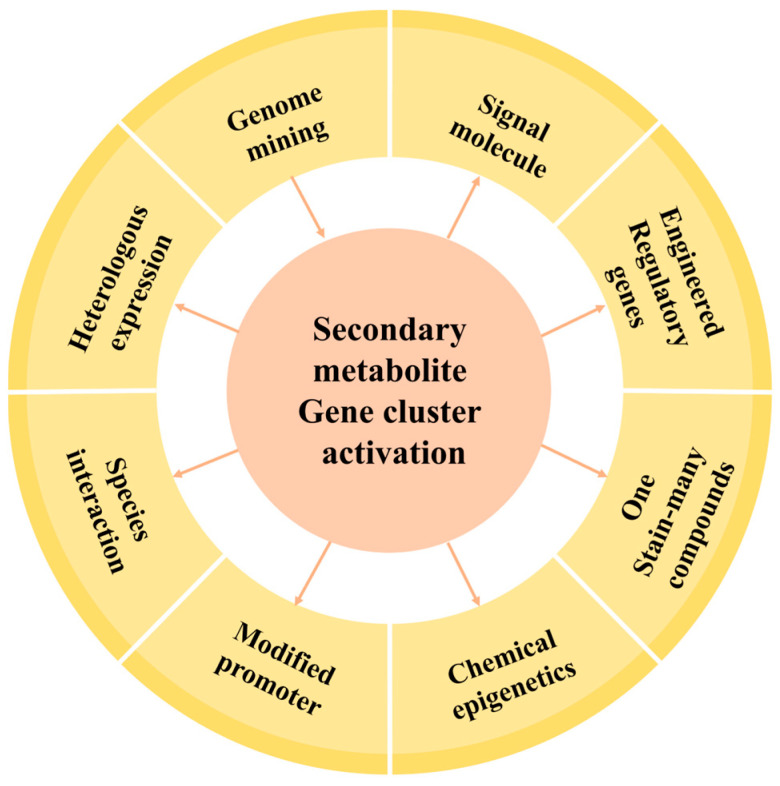
A. oryzae can activate the expression of silent gene clusters and produce new metabolites through heterologous expression, directional modification, and interspecific interaction (Figure 5). Progressive and deeper research on the secondary metabolic regulatory network of A. oryzae can effectively guide us toward a more rational application of its secondary metabolites, thereby expanding the A. oryzae industrial scope. Katayama et al. developed a more efficient genetic engineering technique for A. oryzae based on the CRISPR/Cas9 system and the recycling of autonomously replicating plasmids utilizing AMA1 [115]. This advancement increases the mutagenesis efficiency from 10–20% to 50–100% [115,116]. Utilizing this genetic engineering approach in A. oryzae enables efficient marker-free multi-gene deletion/integration, suitable for molecular breeding aimed at a high-level heterologous production of proteins and secondary metabolites in filamentous fungi [115]. In fully utilizing the CRISPR-Cas system, A. oryzae genome editing can be further enhanced, thus laying the foundation for its application in the increasingly advancing gene editing technology and high-throughput screening technology. Metabolite enhancement should not only focus on metabolic pathways, but also consider the organellar distribution of metabolites and biological reactions. This can be achieved through genome-wide analysis, metabolic network modeling, and metabolomic analysis. In addition, combined with the high-throughput omics data, the application of machine learning and artificial intelligence in the design and remodeling of metabolic pathways of A. oryzae can be explored, so as to formulate metabolic engineering strategies to improve the metabolic capacity and efficiency. Furthermore, whether group-sensitive effects and specific molecular pathways regulate secondary metabolism in A. oryzae is worth investigating. Despite the abundant availability of microbial resources, there are still numerous unexplored functions that can be discovered through ongoing research on Aspergillus. Therefore, ongoing in-depth studies on the metabolic regulation and heterologous expression in A. oryzae, aiming for breakthroughs in its development and application, are necessary.
Figure 5.
Activation of the recessive gene cluster and strategies for mining novel compounds in A. oryzae.
Author Contributions
X.J. conceptualized this paper and wrote it. Y.W. and J.S. participating in revising the manuscript. J.S., Y.H. and M.Y. participated in revising the figures and tables. S.F., Z.S. and R.H. collected the references. B.Z. critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was financially supported by the Project of the Department of Science and Technology of the Jiangxi Province (20213AAG02020), the National Natural Science Foundation of China (32200606), the Key Special Projects of the National Key Research and Development Plan (2021YFA1301302), the Self-made Experimental Instruments and Equipment Project of Shenzhen Technology University (JSZZ202301021), Multiparameter detection analysis Real-time linkage control fermentation system development (20231064010150), and the Self-made Experimental Instruments and Equipment Project of Shenzhen Technology University (JSZZ202301022).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Oda K., Kakizono D., Yamada O., Iefuji H., Akita O., Iwashita K. Proteomic analysis of extracellular proteins from Aspergillus oryzae grown under submerged and solid-state culture conditions. Appl. Environ. Microbiol. 2006;72:3448–3457. doi: 10.1128/AEM.72.5.3448-3457.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobayashi T., Abe K., Asai K., Gomi K., Juvvadi P.R., Kato M., Kitamoto K., Takeuchi M., Machida M. Genomics of Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2007;71:646–670. doi: 10.1271/bbb.60550. [DOI] [PubMed] [Google Scholar]
- 3.He B., Tu Y.Y., Jiang C.M., Zhang Z., Li Y.K., Zeng B. Functional Genomics of Aspergillus oryzae: Strategies and Progress. Microorganisms. 2019;7:103. doi: 10.3390/microorganisms7040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambre C., Baviera J.M.B., Bolognesi C., Cocconcelli P.S., Crebelli R., Gott D.M., Grob K., Lampi E., Mengelers M., Mortensen A., et al. Safety evaluation of the food enzyme phospholipase A1 from the genetically modified Aspergillus oryzae strain NZYM-LJ. Efsa J. 2022;20:e07381. doi: 10.2903/j.efsa.2022.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambree C., Baviera J.M.B., Bolognesi C., Cocconcelli P.S., Crebelli R., Gott D.M., Grob K., Lampi E., Mengelers M., Mortensen A., et al. Safety evaluation of the food enzyme phospholipase A1 from the genetically modified Aspergillus oryzae strain NZYM-PP. Efsa J. 2023;21:e07835. doi: 10.2903/j.efsa.2023.7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichishima E. Development of enzyme technology for Aspergillus oryzae, A. sojae, and A. luchuensis, the national microorganisms of Japan. Biosci. Biotechnol. Biochem. 2016;80:1681–1692. doi: 10.1080/09168451.2016.1177445. [DOI] [PubMed] [Google Scholar]
- 7.Machida M., Asai K., Sano M., Tanaka T., Kumagai T., Terai G., Kusumoto K.I., Arima T., Akita O., Kashiwagi Y., et al. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438:1157–1161. doi: 10.1038/nature04300. [DOI] [PubMed] [Google Scholar]
- 8.Galagan J.E., Calvo S.E., Cuomo C., Ma L.J., Wortman J.R., Batzoglou S., Lee S.I., Basturkmen M., Spevak C.C., Clutterbuck J., et al. Sequencing of Aspergillus nidulans and comparative analysis with A-fumigatus and A-oryzae. Nature. 2005;438:1105–1115. doi: 10.1038/nature04341. [DOI] [PubMed] [Google Scholar]
- 9.Alberti F., Foster G.D., Bailey A.M. Natural products from filamentous fungi and production by heterologous expression. Appl. Microbiol. Biotechnol. 2017;101:493–500. doi: 10.1007/s00253-016-8034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L., Feizi A., Osterlund T., Hjort C., Nielsen J. Genome-scale analysis of the high-efficient protein secretion system of Aspergillus oryzae. BMC Syst. Biol. 2014;8:73. doi: 10.1186/1752-0509-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oikawa H. Reconstitution of biosynthetic machinery of fungal natural products in heterologous hosts. Biosci. Biotechnol. Biochem. 2020;84:433–444. doi: 10.1080/09168451.2019.1690976. [DOI] [PubMed] [Google Scholar]
- 12.Park H.S., Jun S.C., Han K.H., Hong S.B., Yu J.H. Diversity, Application, and Synthetic Biology of Industrially Important Aspergillus Fungi. In: Sariaslani S., Gadd G.M., editors. Advances in Applied Microbiology. Volume 100. Elsevier; Amsterdam, The Netherlands: 2017. pp. 161–202. [DOI] [PubMed] [Google Scholar]
- 13.Oikawa H. Heterologous production of fungal natural products: Reconstitution of biosynthetic gene clusters in model host Aspergillus oryzae. Proc. Jpn. Acad. Ser. B-Phys. Biol. Sci. 2020;96:420–430. doi: 10.2183/pjab.96.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maruyama J. Genome Editing Technology and Its Application Potentials in the Industrial Filamentous Fungus Aspergillus oryzae. J. Fungi. 2021;7:638. doi: 10.3390/jof7080638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamashita N., Komori Y., Okumura Y., Uchiya K., Matsui T., Nishimura A., Ogawa K., Nikai T. High-yields heterologous production of the novel Aspergillus fumigatus elastase inhibitor AFUEI in Aspergillus oryzae. J. Biosci. Bioeng. 2011;112:114–117. doi: 10.1016/j.jbiosc.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Shenouda M.L., Ambilika M., Cox R.J. Trichoderma reesei Contains a Biosynthetic Gene Cluster That Encodes the Antifungal Agent Ilicicolin H. J. Fungi. 2021;7:1034. doi: 10.3390/jof7121034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dufour N., Rao R.P. Secondary metabolites and other small molecules as intercellular pathogenic signals. FEMS Microbiol. Lett. 2011;314:10–17. doi: 10.1111/j.1574-6968.2010.02154.x. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues A.P.D., Carvalho A.S.C., Santos A.S., Alves C.N., do Nascimento J.L.M., Silva E.O. Kojic acid, a secondary metabolite from Aspergillus sp., acts as an inducer of macrophage activation. Cell Biol. Int. 2011;35:335–343. doi: 10.1042/CBI20100083. [DOI] [PubMed] [Google Scholar]
- 19.Rohlfs M., Churchill A.C.L. Fungal secondary metabolites as modulators of interactions with insects and other arthropods. Fungal Genet. Biol. 2011;48:23–34. doi: 10.1016/j.fgb.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Vining L.C. Secondary metabolism, inventive evolution and biochemical diversity—A review. Gene. 1992;115:135–140. doi: 10.1016/0378-1119(92)90551-Y. [DOI] [PubMed] [Google Scholar]
- 21.Keller N.P., Turner G., Bennett J.W. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005;3:937–947. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- 22.Shwab E.K., Keller N.P. Regulation of secondary metabolite production in filamentous ascomycetes. Mycol. Res. 2008;112:225–230. doi: 10.1016/j.mycres.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Tokuoka M., Seshime Y., Fujii I., Kitamoto K., Takahashi T., Koyama Y. Identification of a novel polyketide synthase-nonribosomal peptide synthetase (PKS-NRPS) gene required for the biosynthesis of cyclopiazonic acid in Aspergillus oryzae. Fungal Genet. Biol. 2008;45:1608–1615. doi: 10.1016/j.fgb.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Orth R. Mycotoxins of Aspergillus oryzae strains for use in the food industry as starters and enzyme producing molds. Ann. Nutr. Aliment. 1977;31:617–624. [PubMed] [Google Scholar]
- 25.Iizuka H., Iida M. Maltoryzine, a new toxic metabolite produced by a strain of Aspergillus oryzae var. microsporus isolated from the poisonous malt sprout. Nature. 1962;196:681–682. doi: 10.1038/196681a0. [DOI] [PubMed] [Google Scholar]
- 26.Mizutani T., Oka H., Goto R., Tsurigami R., Maruyama J., Shimizu M., Kato M., Nakano H., Kojima T. The Identification of a Target Gene of the Transcription Factor KojR and Elucidation of Its Role in Carbon Metabolism for Kojic Acid Biosynthesis in Aspergillus oryzae. J. Fungi. 2024;10:113. doi: 10.3390/jof10020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saeedi M., Eslamifar M., Khezri K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019;110:582–593. doi: 10.1016/j.biopha.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Chib S., Jamwal V.L., Kumar V., Gandhi S.G., Saran S. Fungal production of kojic acid and its industrial applications. Appl. Microbiol. Biotechnol. 2023;107:2111–2130. doi: 10.1007/s00253-023-12451-1. [DOI] [PubMed] [Google Scholar]
- 29.Zilles J.C., dos Santos F.L., Kulkamp-Guerreiro I.C., Contri R.V. Biological activities and safety data of kojic acid and its derivatives: A review. Exp. Dermatol. 2022;31:1500–1521. doi: 10.1111/exd.14662. [DOI] [PubMed] [Google Scholar]
- 30.Brtko J. Biological functions of kojic acid and its derivatives in medicine, cosmetics, and food industry: Insights into health aspects. Arch. Pharm. 2022;355:2200215. doi: 10.1002/ardp.202200215. [DOI] [PubMed] [Google Scholar]
- 31.Zhou M., Zhou K., He P., Wang K.M., Zhu R.Z., Wang Y.D., Dong W., Li G.P., Yang H.Y., Ye Y.Q., et al. Antiviral and Cytotoxic Isocoumarin Derivatives from an Endophytic Fungus Aspergillus oryzae. Planta Medica. 2016;82:414–417. doi: 10.1055/s-0035-1558331. [DOI] [PubMed] [Google Scholar]
- 32.Usov A.N., Blanko F.F., Ivanova V.S., Bedrina E.N., Firsova S.A., Sedakova L.A., Funtikova N.S. Structure and antitumor activity of polysaccharides from the micelles of Aspergillus oryzae. Bioorganicheskaia Khimiia. 1991;17:121–125. [PubMed] [Google Scholar]
- 33.Li M.F., Xiao D., Zhu L.C., Liu L., Zheng J.N., Gu X.J., Zhu Y.N., Xie J., Wang X., Dai J.M., et al. Indole Alkaloids from the Cigar Tobacco-Derived Endophytic Fungus Aspergillus oryzae and Their Antibacterial Activity. Chem. Nat. Compd. 2022;58:1093–1097. doi: 10.1007/s10600-022-03872-x. [DOI] [Google Scholar]
- 34.Sakata K., Kuwatsuka T., Sakurai A., Takahashi N., Tamura G. Isolation of Aspirochlorine (=Antibiotic A30641) as a True Antimicrobial Constituent of the Antibiotic, Oryzachlorin, from Aspergillus oryzae. J. Agric. Chem. Soc. Jpn. 1983;47:2673–2674. doi: 10.1271/bbb1961.47.2673. [DOI] [Google Scholar]
- 35.Nonaka N., Asai Y., Nishio M., Takahashi K., Okuda T., Tanaka S., Sugita T., Ohnuki T., Komatsubara S. TMC-2A, -2B and -2C, novel dipeptidyl peptidase IV inhibitors produced by Aspergillus oryzae A374. 1. Taxonomy of producing strain, fermentation, and biochemical properties. J. Antibiot. 1997;50:646–652. doi: 10.7164/antibiotics.50.646. [DOI] [PubMed] [Google Scholar]
- 36.Li Y.Z., Zhang H.X., Chen Z.M., Fan J.X., Chen T.M., Xiao Y., Jie J.Y., Zeng B., Zhang Z. Overexpression of a novel gene Aokap2 affects the growth and kojic acid production in Aspergillus oryzae. Mol. Biol. Rep. 2022;49:2745–2754. doi: 10.1007/s11033-021-07084-4. [DOI] [PubMed] [Google Scholar]
- 37.Tamano K., Kuninaga M., Kojima N., Umemura M., Machida M., Koike H. Use of the kojA promoter, involved in kojic acid biosynthesis, for polyketide production in Aspergillus oryzae: Implications for long-term production. BMC Biotechnol. 2019;19:70. doi: 10.1186/s12896-019-0567-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marui J., Ohashi-Kunihiro S., Ando T., Nishimura M., Koike H., Machida M. Penicillin biosynthesis in Aspergillus oryzae and its overproduction by genetic engineering. J. Biosci. Bioeng. 2010;110:8–11. doi: 10.1016/j.jbiosc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Nierman W.C., Pain A., Anderson M.J., Wortman J.R., Kim H.S., Arroyo J., Berriman M., Abe K., Archer D.B., Bermejo C., et al. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature. 2005;438:1151–1156. doi: 10.1038/nature04332. [DOI] [PubMed] [Google Scholar]
- 40.Goffeau A., Barrell B.G., Bussey H., Davis R.W., Dujon B., Feldmann H., Galibert F., Hoheisel J.D., Jacq C., Johnston M., et al. Life with 6000 genes. Science. 1996;274:563–567. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- 41.Feng J., Hauser M., Cox R.J., Skellam E. Engineering Aspergillus oryzae for the Heterologous Expression of a Bacterial Modular Polyketide Synthase. J. Fungi. 2021;7:1085. doi: 10.3390/jof7121085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang W.Y., Zhong Y., Yu Y., Shi D.F., Huang H.Y., Tang X.L., Wang Y.H., Chen G.D., Zhang H.P., Liu C.L., et al. 4-Hydroxy Pyridones from Heterologous Expression and Cultivation of the Native Host. J. Nat. Prod. 2020;83:3338–3346. doi: 10.1021/acs.jnatprod.0c00675. [DOI] [PubMed] [Google Scholar]
- 43.Guo J.J., Cai Y.S., Cheng F.C., Yang C.J., Zhang W.Q., Yu W.L., Yan J.J., Deng Z.X., Hong K. Genome Mining Reveals a Multiproduct Sesterterpenoid Biosynthetic Gene Cluster in Aspergillus ustus. Org. Lett. 2021;23:1525–1529. doi: 10.1021/acs.orglett.0c03996. [DOI] [PubMed] [Google Scholar]
- 44.Takusagawa S., Satoh Y., Ohtsu I., Dairi T. Ergothioneine production with Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2019;83:181–184. doi: 10.1080/09168451.2018.1527210. [DOI] [PubMed] [Google Scholar]
- 45.Bok J.W., Keller N.P. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot. Cell. 2004;3:527–535. doi: 10.1128/EC.3.2.527-535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bok J.W., Balajee S.A., Marr K.A., Andes D., Nielsen K.F., Frisvad J.C., Keller N.P. LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot. Cell. 2005;4:1574–1582. doi: 10.1128/EC.4.9.1574-1582.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain S., Keller N. Insights to fungal biology through LaeA sleuthing. Fungal Biol. Rev. 2013;27:51–59. doi: 10.1016/j.fbr.2013.05.004. [DOI] [Google Scholar]
- 48.Bayram O., Braus G.H. Coordination of secondary metabolism and development in fungi: The velvet family of regulatory proteins. FEMS Microbiol. Rev. 2012;36:1–24. doi: 10.1111/j.1574-6976.2011.00285.x. [DOI] [PubMed] [Google Scholar]
- 49.Jiang T., Wang M.H., Li L., Si J.G., Song B., Zhou C., Yu M., Wang X.W., Zhang Y.G., Ding G., et al. Overexpression of the Global Regulator LaeA in Chaetomium globosum Leads to the Biosynthesis of Chaetoglobosin Z. J. Nat. Prod. 2016;79:2487–2494. doi: 10.1021/acs.jnatprod.6b00333. [DOI] [PubMed] [Google Scholar]
- 50.Kawauchi M., Nishiura M., Iwashita K. Fungus-Specific Sirtuin HstD Coordinates Secondary Metabolism and Development through Control of LaeA. Eukaryot. Cell. 2013;12:1087–1096. doi: 10.1128/EC.00003-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng W.M., Liang J.R., Wang B.B., Chen J.H. Improvement of kojic acid production in Aspergillus oryzae AR-47 mutant strain by combined mutagenesis. Bioprocess Biosyst. Eng. 2019;42:753–761. doi: 10.1007/s00449-019-02079-9. [DOI] [PubMed] [Google Scholar]
- 52.Terabayashi Y., Sano M., Yamane N., Marui J., Tamano K., Sagara J., Dohmoto M., Oda K., Ohshima E., Tachibana K., et al. Identification and characterization of genes responsible for biosynthesis of kojic acid, an industrially important compound from Aspergillus oryzae. Fungal Genet. Biol. 2010;47:953–961. doi: 10.1016/j.fgb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 53.Marui J., Yamane N., Ohashi-Kunihiro S., Ando T., Terabayashi Y., Sano M., Ohashi S., Ohshima E., Tachibana K., Higa Y. Kojic acid biosynthesis in Aspergillus oryzae is regulated by a Zn(II)(2)Cys(6) transcriptional activator and induced by kojic acid at the transcriptional level. J. Biosci. Bioeng. 2011;112:40–43. doi: 10.1016/j.jbiosc.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 54.Calvo A.M. The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet. Biol. 2008;45:1053–1061. doi: 10.1016/j.fgb.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 55.Shin K.S., Kim Y.H., Yu J.H. Proteomic analyses reveal the key roles of BrlA and AbaA in biogenesis of gliotoxin in Aspergillus fumigatus. Biochem. Biophys. Res. Commun. 2015;463:428–433. doi: 10.1016/j.bbrc.2015.05.090. [DOI] [PubMed] [Google Scholar]
- 56.Jia L.L., Yu J.H., Chen F.S., Chen W.P. Characterization of the asexual developmental genes brlA and wetA in Monascus ruber M7. Fungal Genet. Biol. 2021;151:103564. doi: 10.1016/j.fgb.2021.103564. [DOI] [PubMed] [Google Scholar]
- 57.Arakawa G.Y., Kudo H., Yanase A., Eguchi Y., Kodama H., Ogawa M., Koyama Y., Shindo H., Hosaka M., Tokuoka M. A unique Zn(II)(2)-Cys(6)-type protein, KpeA, is involved in secondary metabolism and conidiation in Aspergillus oryzae. Fungal Genet. Biol. 2019;127:35–44. doi: 10.1016/j.fgb.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Watanabe M., Watanabe D., Akao T., Shimoi H. Overexpression of MSN2 in a sake yeast strain promotes ethanol tolerance and increases ethanol production in sake brewing. J. Biosci. Bioeng. 2009;107:516–518. doi: 10.1016/j.jbiosc.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Chang P.-K., Scharfenstein L.L., Luo M., Mahoney N., Molyneux R.J., Yu J., Brown R.L., Campbell B.C. Loss of msnA, a Putative Stress Regulatory Gene, in Aspergillus parasiticus and Aspergillus flavus Increased Production of Conidia, Aflatoxins and Kojic Acid. Toxins. 2011;3:82–104. doi: 10.3390/toxins3010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim N.Y., Lee J.H., Lee I., Ji G.E. An Evaluation of Aflatoxin and Cyclopiazonic Acid Production in Aspergillus oryzae. J. Food Prot. 2014;77:1010–1016. doi: 10.4315/0362-028X.JFP-13-448. [DOI] [PubMed] [Google Scholar]
- 61.Saha P., Ghosh S., Roy-Barman S. MoLAEA Regulates Secondary Metabolism in Magnaporthe oryzae. mSphere. 2020;5:e00936-19. doi: 10.1128/mSphere.00936-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang B., Guo G.W., Wang C., Lin Y., Wang X.N., Zhao M.M., Guo Y., He M.H., Zhang Y., Pan L. Survey of the transcriptome of Aspergillus oryzae via massively parallel mRNA sequencing. Nucleic Acids Res. 2010;38:5075–5087. doi: 10.1093/nar/gkq256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimizu T., Kinoshita H., Nihira T. Identification and in vivo functional analysis by gene disruption of ctnA, an activator gene involved in citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2007;73:5097–5103. doi: 10.1128/AEM.01979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sakai K., Kinoshita H., Shimizu T., Nihira T. Construction of a Citrinin Gene Cluster Expression System in Heterologous Aspergillus oryzae. J. Biosci. Bioeng. 2008;106:466–472. doi: 10.1263/jbb.106.466. [DOI] [PubMed] [Google Scholar]
- 65.Aghcheh R.K., Kubicek C.P. Epigenetics as an emerging tool for improvement of fungal strains used in biotechnology. Appl. Microbiol. Biotechnol. 2015;99:6167–6181. doi: 10.1007/s00253-015-6763-2. [DOI] [PubMed] [Google Scholar]
- 66.Brakhage A.A., Schroeckh V. Fungal secondary metabolites—Strategies to activate silent gene clusters. Fungal Genet. Biol. 2011;48:15–22. doi: 10.1016/j.fgb.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 67.Xue M.Y., Hou X.W., Fu J.J., Zhang J.Y., Wang J.C., Zhao Z.T., Xu D., Lai D.W., Zhou L.G. Recent Advances in Search of Bioactive Secondary Metabolites from Fungi Triggered by Chemical Epigenetic Modifiers. J. Fungi. 2023;9:172. doi: 10.3390/jof9020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shinohara Y., Kawatani M., Futamura Y., Osada H., Koyama Y. An overproduction of astellolides induced by genetic disruption of chromatin-remodeling factors in Aspergillus oryzae. J. Antibiot. 2016;69:4–8. doi: 10.1038/ja.2015.73. [DOI] [PubMed] [Google Scholar]
- 69.Asai T., Yamamoto T., Shirata N., Taniguchi T., Monde K., Fujii I., Gomi K., Oshima Y. Structurally Diverse Chaetophenol Productions Induced by Chemically Mediated Epigenetic Manipulation of Fungal Gene Expression. Org. Lett. 2013;15:3346–3349. doi: 10.1021/ol401386w. [DOI] [PubMed] [Google Scholar]
- 70.Kawauchi M., Iwashita K. Functional analysis of histone deacetylase and its role in stress response, drug resistance and solid-state cultivation in Aspergillus oryzae. J. Biosci. Bioeng. 2014;118:172–176. doi: 10.1016/j.jbiosc.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 71.Gorog S. Advances in the analysis of steroid hormone drugs in pharmaceuticals and environmental samples (2004–2010) J. Pharm. Biomed. Anal. 2011;55:728–743. doi: 10.1016/j.jpba.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 72.Hu Z.H., He B., Ma L., Sun Y.L., Niu Y.L., Zeng B. Recent Advances in Ergosterol Biosynthesis and Regulation Mechanisms in Saccharomyces cerevisiae. Indian J. Microbiol. 2017;57:270–277. doi: 10.1007/s12088-017-0657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kristan K., Rizner T.L. Steroid-transforming enzymes in fungi. J. Steroid Biochem. Mol. Biol. 2012;129:79–91. doi: 10.1016/j.jsbmb.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 74.Mercer E.I. Inhibitors of sterol biosynthesis and their applications. Prog. Lipid Res. 1993;32:357–416. doi: 10.1016/0163-7827(93)90016-P. [DOI] [PubMed] [Google Scholar]
- 75.Ryder N.S. Terbinafine: Mode of action and properties of the squalene epoxidase inhibition. Br. J. Dermatol. 1992;126((Suppl. 39)):2–7. doi: 10.1111/j.1365-2133.1992.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 76.Hu Z.H., Li G.H., Sun Y.L., Niu Y.L., Ma L., He B., Ai M.Q., Han J.Z., Zeng B. Gene transcription profiling of Aspergillus oryzae 3.042 treated with ergosterol biosynthesis inhibitors. Braz. J. Microbiol. 2019;50:43–52. doi: 10.1007/s42770-018-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng H., Kim J., Liew M., Yan J.K., Herrera O., Bok J.W., Kelleher N.L., Keller N.P., Wang Y. Redox Metabolites Signal Polymicrobial Biofilm Development via the NapA Oxidative Stress Cascade in Aspergillus. Curr. Biol. 2015;25:29–37. doi: 10.1016/j.cub.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caceres I., Al Khoury A., El Khoury R., Lorber S., Oswald I.P., El Khoury A., Atoui A., Puel O., Bailly J.D. Aflatoxin Biosynthesis and Genetic Regulation: A Review. Toxins. 2020;12:150. doi: 10.3390/toxins12030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X., Jiang Y.R., Ma L.X., Ma X.Y., Liu Y., Shan J.H., Ma K., Xing F.G. Comprehensive Transcriptome and Proteome Analyses Reveal the Modulation of Aflatoxin Production byAspergillus flavuson Different Crop Substrates. Front. Microbiol. 2020;11:1497. doi: 10.3389/fmicb.2020.01497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Si P.D., Wang G., Wu W.Q., Hussain S., Guo L., Wu W., Yang Q.L., Xing F.G. SakA Regulates Morphological Development, Ochratoxin A Biosynthesis and Pathogenicity of Aspergillus westerdijkiae and the Response to Different Environmental Stresses. Toxins. 2023;15:292. doi: 10.3390/toxins15040292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Daba G.M., Mostafa F.A., Elkhateeb W.A. The ancient koji mold (Aspergillus oryzae) as a modern biotechnological tool. Bioresour. Bioprocess. 2021;8:52. doi: 10.1186/s40643-021-00408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Vries R.P., Riley R., Wiebenga A., Aguilar-Osorio G., Amillis S., Uchima C.A., Anderluh G., Asadollahi M., Askin M., Barry K., et al. Comparative genomics reveals high biological diversity and specific adaptations in the industrially and medically important fungal genus Aspergillus. Genome Biol. 2017;18:28. doi: 10.1186/s13059-017-1151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wan H.M., Chen C.C., Giridhar R., Chang T.S., Wu W.T. Repeated-batch production of kojic acid in a cell-retention fermenter using Aspergillus oryae M3B9. J. Ind. Microbiol. Biotechnol. 2005;32:227–233. doi: 10.1007/s10295-005-0230-5. [DOI] [PubMed] [Google Scholar]
- 84.Rosfarizan M., Ariff A.B. Kinetics of kojic acid fermentation by Aspergillus flavus using different types and concentrations of carbon and nitrogen sources. J. Ind. Microbiol. Biotechnol. 2000;25:20–24. doi: 10.1038/sj.jim.7000017. [DOI] [Google Scholar]
- 85.Ochsenreither K., Fischer C., Neumann A., Syldatk C. Process characterization and influence of alternative carbon sources and carbon-to-nitrogen ratio on organic acid production by Aspergillus oryzae DSM1863. Appl. Microbiol. Biotechnol. 2014;98:5449–5460. doi: 10.1007/s00253-014-5614-x. [DOI] [PubMed] [Google Scholar]
- 86.Vorapreeda T., Khongto B., Thammarongtham C., Srisuk T., Laoteng K. Metabolic Regulation of Sugar Assimilation for Lipid Production in Aspergillus oryzae BCC7051 through Comparative Transcriptome Perspective. Biology. 2021;10:885. doi: 10.3390/biology10090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang J.P., Zhao J., Duan W.L., Tian S., Wang X.D., Zhuang H., Fu J., Kang Z.S. TaAMT2;3a, a wheat AMT2-type ammonium transporter, facilitates the infection of stripe rust fungus on wheat. BMC Plant Biol. 2019;19:239. doi: 10.1186/s12870-019-1841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chutrakul C., Panchanawaporn S., Vorapreeda T., Jeennor S., Anantayanon J., Laoteng K. The Exploring Functional Role of Ammonium Transporters of Aspergillus oryzae in Nitrogen Metabolism: Challenges towards Cell Biomass Production. Int. J. Mol. Sci. 2022;23:7567. doi: 10.3390/ijms23147567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Christensen T., Hynes M.J., Davis M.A. Role of the regulatory gene areA of Aspergillus oryzae in nitrogen metabolism. Appl. Environ. Microbiol. 1998;64:3232–3237. doi: 10.1128/AEM.64.9.3232-3237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wilson R.A., Arst H.N., Jr. Mutational analysis of AREA, a transcriptional activator mediating nitrogen metabolite repression in Aspergillus nidulans and a member of the “streetwise” GATA family of transcription factors. Microbiol. Mol. Biol. Rev. 1998;62:586–596. doi: 10.1128/MMBR.62.3.586-596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chutrakul C., Panchanawaporn S., Jeennor S., Anantayanon J., Laoteng K. Promoter exchange of the cryptic nonribosomal peptide synthetase gene for oligopeptide production in Aspergillus oryzae. J. Microbiol. 2022;60:47–56. doi: 10.1007/s12275-022-1442-3. [DOI] [PubMed] [Google Scholar]
- 92.Dalirfardouei R., Karimi G., Jamialahmadi K. Molecular mechanisms and biomedical applications of glucosamine as a potential multifunctional therapeutic agent. Life Sci. 2016;152:21–29. doi: 10.1016/j.lfs.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 93.Li J.S., Chew Y.M., Lin M.C., Lau Y.Q., Chen C.S. Enhanced glucosamine production from Aspergillus oryzae NCH-42 via acidic stress under submerged fermentation. Cyta-J. Food. 2021;19:614–624. doi: 10.1080/19476337.2021.1946158. [DOI] [Google Scholar]
- 94.Wattanachaisaereekul S., Tachaleat A., Punya J., Haritakun R., Boonlarppradab C., Cheevadhanarak S. Assessing medium constituents for optimal heterologous production of anhydromevalonolactone in recombinant Aspergillus oryzae. Amb Express. 2014;4:52. doi: 10.1186/s13568-014-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sano M. Aspergillus oryzae nrtA affects kojic acid production. Biosci. Biotechnol. Biochem. 2016;80:1776–1780. doi: 10.1080/09168451.2016.1176517. [DOI] [PubMed] [Google Scholar]
- 96.Jiang C.M., Ge J.X., He B., Zhang Z., Hu Z.H., Li Y.K., Zeng B. Transcriptomic analysis reveals Aspergillus oryzae responds to temperature stress by regulating sugar metabolism and lipid metabolism. PLoS ONE. 2022;17:e0274394. doi: 10.1371/journal.pone.0274394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Machida M., Yamada O., Gomi K. Genomics of Aspergillus oryzae: Learning from the History of Koji Mold and Exploration of Its Future. DNA Res. 2008;15:173–183. doi: 10.1093/dnares/dsn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Umemura M., Koike H., Yamane N., Koyama Y., Satou Y., Kikuzato I., Teruya M., Tsukahara M., Imada Y., Wachi Y., et al. Comparative Genome Analysis Between Aspergillus oryzae Strains Reveals Close Relationship Between Sites of Mutation Localization and Regions of Highly Divergent Genes among Aspergillus Species. DNA Res. 2012;19:375–382. doi: 10.1093/dnares/dss019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sakurai Y., Misawa S., Shiota H. Growth and respiratory activity of aspergillus-oryzae grown on solid-state medium. Agric. Biol. Chem. 1985;49:745–750. doi: 10.1271/bbb1961.49.745. [DOI] [Google Scholar]
- 100.Chen T.T., Xiong S.Q., Jiang S.Y., Wang M.J., Wu Q.L., Wei H. Molecular identification of microbial community in Chinese douchi during post-fermentation process. Food Sci. Biotechnol. 2011;20:1633–1638. doi: 10.1007/s10068-011-0225-0. [DOI] [Google Scholar]
- 101.Ge J.X., Zhang Z., Li Y., Hu Z.H., He B., Li Y.K., Zeng B., Jiang C.M. Inhibition of AoAur1 increases mycelial growth, hyphal fusion and improves physiological adaptation to high-temperature stress in Aspergillus oryzae. Arch. Microbiol. 2022;204:447. doi: 10.1007/s00203-022-03075-6. [DOI] [PubMed] [Google Scholar]
- 102.Hayakawa H., Sobue F., Motoyama K., Yoshimura T., Hemmi H. Identification of enzymes involved in the mevalonate pathway of Flavobacterium johnsoniae. Biochem. Biophys. Res. Commun. 2017;487:702–708. doi: 10.1016/j.bbrc.2017.04.120. [DOI] [PubMed] [Google Scholar]
- 103.Krepkiy D., Miziorko H.M. Identification of active site residues in mevalonate diphosphate decarboxylase: Implications for a family of phosphotransferases. Protein Sci. 2004;13:1875–1881. doi: 10.1110/ps.04725204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu Z.H., Huang H., Sun Y.L., Niu Y.L., Xu W.Z.S., Liu Q.C., Zhang Z., Jiang C.M., Li Y.K., Zeng B. Effects on Gene Transcription Profile and Fatty Acid Composition by Genetic Modification of Mevalonate Diphosphate Decarboxylase MVD/Erg19 in Aspergillus oryzae. Microorganisms. 2019;7:342. doi: 10.3390/microorganisms7090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cordier H., Lacombe C., Karst F., Berges T. The Saccharomyces cerevisiae mevalonate diphosphate decarboxylase (Erg19p) forms homodimers in vivo, and a single substitution in a structurally conserved region impairs dimerization. Curr. Microbiol. 1999;38:290–294. doi: 10.1007/PL00006804. [DOI] [PubMed] [Google Scholar]
- 106.Sun Y.L., Niu Y.L., Huang H., He B., Ma L., Tu Y.Y., Tran V.T., Zeng B., Hu Z.H. Mevalonate Diphosphate Decarboxylase MVD/Erg19 Is Required for Ergosterol Biosynthesis, Growth, Sporulation and Stress Tolerance in Aspergillus oryzae. Front. Microbiol. 2019;10:1074. doi: 10.3389/fmicb.2019.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alvarado-Obando M., Contreras N., Leon D., Botero L., Beltran L., Diaz D., Rodriguez-Lopez A., Reyes L.H., Almeciga-Diaz C.J., Sanchez O.F. Engineering a heterologously expressed fructosyltransferase from Aspergillus oryzae N74 in Komagataella phaffii (Pichia pastoris) for kestose production. New Biotechnol. 2022;69:18–27. doi: 10.1016/j.nbt.2022.02.005. [DOI] [PubMed] [Google Scholar]
- 108.Landraud P., Chuzeville S., Billon-Grande G., Poussereau N., Bruel C. Adaptation to pH and Role of PacC in the Rice Blast Fungus Magnaporthe oryzae. PLoS ONE. 2013;8:e69236. doi: 10.1371/journal.pone.0069236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barda O., Maor U., Sadhasivam S., Bi Y., Zakin V., Prusky D., Sionov E. The pH-Responsive Transcription Factor PacC Governs Pathogenicity and Ochratoxin A Biosynthesis in Aspergillus carbonarius. Front. Microbiol. 2020;11:210. doi: 10.3389/fmicb.2020.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McKelvey S.M., Murphy R.A. Analysis of wide-domain transcriptional regulation in solid-state cultures of Aspergillus oryzae. J. Ind. Microbiol. Biotechnol. 2010;37:455–469. doi: 10.1007/s10295-010-0691-z. [DOI] [PubMed] [Google Scholar]
- 111.Shao H.H., Tu Y.Y., Wang Y.J., Jiang C.M., Ma L., Hu Z.H., Wang J.F., Zeng B. Oxidative Stress Response of Aspergillus oryzae Induced by Hydrogen Peroxide and Menadione Sodium Bisulfite. Microorganisms. 2019;7:225. doi: 10.3390/microorganisms7080225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao G.Z., Hou L.H., Yao Y.P., Wang C.L., Cao X.H. Comparative proteome analysis of Aspergillus oryzae 3.042 and A. oryzae 100-8 strains: Towards the production of different soy sauce flavors. J. Proteom. 2012;75:3914–3924. doi: 10.1016/j.jprot.2012.04.056. [DOI] [PubMed] [Google Scholar]
- 113.Zhao G.Z., Yao Y.P., Hao G.F., Fang D.S., Yin B.X., Cao X.H., Chen W. Gene regulation in Aspergillus oryzae promotes hyphal growth and flavor formation in soy sauce koji. Rsc Adv. 2015;5:24224–24230. doi: 10.1039/C4RA16819D. [DOI] [Google Scholar]
- 114.Park M.K., Seo J.A., Kim Y.S. Comparative study on metabolic changes of Aspergillus oryzae isolated from fermented foods according to culture conditions. Int. J. Food Microbiol. 2019;307:108270. doi: 10.1016/j.ijfoodmicro.2019.108270. [DOI] [PubMed] [Google Scholar]
- 115.Katayama T., Nakamura H., Zhang Y., Pascal A., Fujii W., Maruyama J. Forced Recycling of an AMA1-Based Genome-Editing Plasmid Allows for Efficient Multiple Gene Deletion/Integration in the Industrial Filamentous Fungus Aspergillus oryzae. Appl. Environ. Microbiol. 2019;85:e01896-18. doi: 10.1128/AEM.01896-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Katayama T., Tanaka Y., Okabe T., Nakamura H., Fujii W., Kitamoto K., Maruyama J. Development of a genome editing technique using the CRISPR/Cas9 system in the industrial filamentous fungus Aspergillus oryzae. Biotechnol. Lett. 2016;38:637–642. doi: 10.1007/s10529-015-2015-x. [DOI] [PubMed] [Google Scholar]