Abstract
Diagnostic and therapeutic decision-making in pregnancy with suspected pulmonary embolism (PE) is challenging. European and other international professional societies have proposed various recommendations that are ambiguous, probably due to the unavailability of randomized controlled trials. In the following sections, we discuss the supporting diagnostic steps and treatments. We suggest a standardized diagnostic work-up in pregnant patients presenting with symptoms of PE to make evidence-based diagnostic and therapeutic decisions. We strongly recommend that clinical decisions on treatment in pregnant patients with intermediate- or high-risk pulmonary embolism should include a multidisciplinary team approach involving emergency physicians, pulmonologists, angiologist, cardiologists, thoracic and/or cardiovascular surgeons, radiologists, and obstetricians to choose a tailored management option including an interventional treatment. It is important to be aware of the differences among guidelines and to assess each case individually, considering the specific views of the different specialties. This review summarizes key concepts of the diagnostics and acute management of pregnant women with suspected PE that are supportive for the clinician on duty.
Keywords: pulmonary embolism, pregnancy, multidisciplinary team, management, review
1. Introduction
Pulmonary embolism (PE), a major cause of maternal mortality in pregnant women, occurs in approximately 1 in 1000–3000 pregnancies [1]. Pregnancy is a hypercoagulable state caused by increased levels of fibrinogen, d-dimers, and coagulation factors (VII, VIII, and IX) with decreased protein S activity [2,3]. Not surprisingly, pregnancy-induced physiological adaptations are associated with significant changes in physiological measures, signs and symptoms, and laboratory test results that are comparable to those of pregnant patients with PE. Most clinical risk scores used to assess PE are not validated for pregnant women because of the limited number of relevant prospective studies. In recent years, European and other international societies have proposed various algorithms for the assessment of suspected PE in pregnancy [4,5,6,7,8,9,10,11]. The paucity of concise, evidence-based recommendations leads to different clinical practices [10]. In this review, we propose a practical summary of the important diagnostic steps and suggest a practical management concept of pregnant women with suspected PE at the time of presentation to the emergency department.
2. Pathophysiological Changes Contributing to PE during Pregnancy
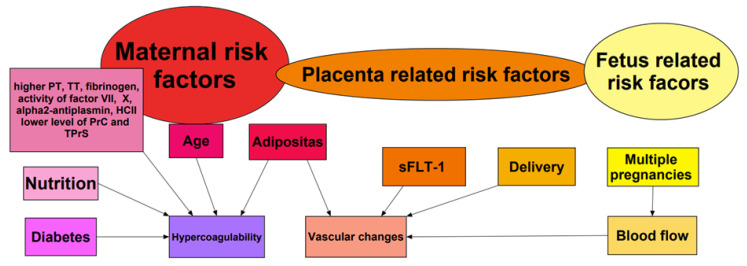
PE is associated with complex changes during pregnancy. Detailed concepts are presented in Figure 1.
Figure 1.
Pathophysiological changes and factors contributing to PE during pregnancy. PT—prothrombin time; TT—thrombin time; HCII—activity of heparin cofactor II; PrC—protein C; TPrS—total protein S; sFLT-1—soluble FMS-like tyrosine kinase-1.
A major factor for increased risk of PE in pregnancy is related to Virchow’s triad: hypercoagulability, venous stasis and turbulence, and endothelial injury and dysfunction [12,13]. Moreover, pregnancy is a state of hypercoagulability due to changes in procoagulatory proteins: factors I, II, VII, VIII, IX and X increase during pregnancy. On the other hand, pregnancy increases resistance to antithrombotic factors such as protein C and protein S [14]. Thrombophilia can exacerbate these changes in clotting proteins, further increasing the patient’s risk of PE [15]. Moreover, excessive weight gain during pregnancy is associated with increased hypercoagulability and vascular changes that contribute to clot formation [16]. Inadequate nutrient intake, maternal age, and gestational diabetes mellitus (GDM) increase the risk of PE due to changes in insulin sensitivity and blood glucose levels, which may affect blood coagulation factors and increase the likelihood of thrombotembolic events [15,16].
Of note, placenta-related factors such as sFLT-1 (soluble FMS-like tyrosine kinase-1) have been suggested to increase the risk of PE. sFLT-1 plays a crucial role in thromboembolic diseases, particularly in the development of pre-eclampsia due to endothelial dysfunction [17]. Most thromboembolic events occur postpartum, because of vascular trauma during childbirth. Twins, triplets, or other multiple pregnancies are associated with a higher risk of PE due to increased blood volume and altered blood flow.
Risk factors modulated by the fetus may be caused by placental abruption and intrauterine growth restriction [16].
3. Clinical Presentation
The clinical manifestations of PE during pregnancy are variable and non-specific. The most common clinical manifestations of PE during pregnancy pertain to the respiratory system, but those respiratory signs and symptoms, such as dyspnea at rest or on exertion, pleuritic chest pain, cough, and tachypnea, are not specific for diagnosis.
Specific risk factors for pulmonary embolism during pregnancy include previous thromboembolism, age > 35 years, body mass index > 30 kg/m2, multiparity, comorbidities, pre-eclampsia, and immobility [18,19,20]. Of note, data supporting risk stratification based only on clinical risk factors are limited and uncertain [6,21,22,23]. The clinical manifestations of PE are comparable among pregnant women with PE, non-pregnant women with PE, and pregnant women without PE. To compare and better understanding we summarize the most of symptoms in mentioned groups in Table 1 [21,24,25,26,27].
Table 1.
Comparison of clinical manifestations of PE in different patient populations.
| Symptoms | Pregnant Women with PE [%] | Pregnant Women without PE [%] | General Population with PE [%] |
|---|---|---|---|
| Dyspnea | 62 | 60–75 | 66–97 |
| Chest pain | 46 (pleuritic) | 93 | 28–45 |
| 19 (nonpleuritic) | |||
| Hemoptysis | 8 | No data | 6–16 |
| Signs or symptoms of lower limb DVT | 7 | 1 | 38–55 |
| Syncope | No data | No data | 7–38 |
PE—pulmonary embolism; DVT—deep vein thrombosis.
There are some established scoring systems that predict PE. The well-established Wells and revised Geneva score can be used for calculating PE risk and guidance to perform a computed tomography pulmonary angiography (CTPA) in general population with suspicion of PE. One of Wells’s criteria reflects the clinical subjective opinion: “PE is the most probable diagnosis or equally likely”. For comparison, the PADUA score includes clinical features from medical history such as active cancer, previous VTE, reduced mobility, known thrombophilic conditions, recent trauma and/or surgery, elderly age, heart and/or respiratory failure, acute myocardial infarction and/or ischemic stroke, acute infection and/or rheumatologic disorder, obesity (BMI ≥ 30), and ongoing hormonal treatment. Each risk factor is assigned a certain number of points, and the total score determines the need for thromboprophylaxis in hospitalized, non-pregnant adult patients and is also an useful tool for stratifying patients before they undergo CTPA [28].
Tools to estimate the pretest probability for PE in the general population (Wells score, revised Geneva score, PADUA score, PERC rule and IMPROVE score) seem not to be valuable for use in pregnant women due to their non-optimal sensitivity, specificity, PPV, and NPV in pregnancy. For example, the Wells score has 40.7%, 81.5%, 44%, and 79.4%, respectively, in these categories, and the revised Geneva score has 62.9%, 59.2%, 35.4%, and 81.8%, respectively [5,7,10,29,30].
In 2021, the pregnancy-adapted Geneva (PAG) score was introduced to estimate the pretest probability of pregnant women with suspected PE. The PAG score appears promising and may facilitate medical decision-making at first contact [31]. The PAG score includes (1) age ≥ 40 years, (2) lower limb surgery or fracture in the last month, (3) previous deep vein thrombosis or PE, (4) unilateral lower limb pain, (5) hemoptysis, (6) tenderness of the lower limb and unilateral edema, and (7) heart rate > 110 bpm [31] (Table 2).
Table 2.
The Pregnancy-Adapted Geneva score (modified from Robert-Ebadi et al. [31]).
| Pregnancy-Adapted Geneva Score | ||
|---|---|---|
| ITEM | POINTS | |
| Age ≥ 40 years | +1 | |
| Surgery (under GA) or lower limb fracture in the past month | +2 | |
| Previous DVT or PE | +3 | |
| Unilateral lower limb pain | +3 | |
| Hemoptysis | +2 | |
| Lower limb tenderness and unilateral edema | +4 | |
| Heart rate > 110 bpm | +5 | |
| Maximum point number | 20 | |
| Points | Category | PE Prevalence |
| 0–1 | Low | 1.0–4.9% |
| 2–6 | Intermediate | 6.9–18.9% |
| ≥7 | High | 35.5–82.2% |
GA, general anesthesia; DVT, deep vein thrombosis; PE, pulmonary embolism.
The PAG score ranges from 0 to 20 points. Patients were categorized as having low (0–1 points), medium (2–6 points), or high (≥7 points) clinical pretest probability [31], which corresponded to prevalence rates of 2.3%, 11.6%, and 61.5% of PE in pregnant patients [31]. This indicates that the PAG score may be valuable in predicting the prevalence of PE in group of pregnant women with suspected PE.
The PAG score shows a high discriminative power to identify patients at low, intermediate, or high pre-test probability (PTP) compared with the Geneva Score (AUC: 0.795 vs. AUC: 0.684 in ROC analysis, respectively). Nevertheless, prospective external validation of the PAG score is required [31]. Of interest, the YEARS algorithm has been prospectively examined, which was used in conjunction with d-dimer measurement and consisted of the following YEARS items: clinical signs of DVT, hemoptysis, or the most likely diagnosis of PE. This approach is discussed in the next section.
4. Laboratory Test Values
Physiological changes during pregnancy influence several laboratory markers [32]; d-dimer levels normally increase during pregnancy as gestation progresses [33,34,35]. In the first trimester, the d-dimer threshold is 50% higher, in the second trimester 100% higher, and in the third trimester, it is 125% higher than the normal threshold [30,36].
In this context, Kovac et al. suggested d-dimer thresholds for the first (286 ng/mL), second (457 ng/mL), and third trimesters of pregnancy (644 ng/mL) to exclude PE in this patient cohort [35]. In 2021, an Iranian research group suggested higher d-dimer cut-off values: a d-dimer cut-off value of 1447 g/L displayed a high specificity and sensitivity for PE diagnosis, irrespective of the gestational age [36]. In addition, the following reference ranges for the d-dimer level have been suggested: 169–1202, 393–3258, and 551–3333 µg/L for the first, second, and third trimesters of pregnancy, respectively [36]. All these data require prospective external validation. Of major interest, the following factors may influence d-dimer levels during pregnancy, regardless of the course of the pregnancy [37]:
Type of pregnancy: in normal twin pregnancies, d-dimer levels are significantly higher during pregnancy than in normal singleton pregnancies [37].
Gestational diabetes mellitus (GDM): the plasma d-dimer values of the GDM group are significantly higher in the third trimester than those of the group with normal singleton pregnancies [37].
Arterial hypertension: hypertensive disorders in pregnancy constitute an independent risk factor for venous thromboembolism, which causes d-dimer level increments [38].
Type of delivery: plasma d-dimer levels are significantly higher 24–48 h after delivery in women who underwent cesarean section than in women who gave birth vaginally [37].
Breastfeeding: women who breastfeed have higher d-dimer levels [39].
An approach using a laboratory test (d-dimer) alone to exclude PE does not provide sufficient negative predictive value in pregnancy. Among other examples, the DiPEP study showed that d-dimer alone could not distinguish between pregnant and postpartum women who have PE and those who do not [22,23,40], particularly in women at high risk and/or in the third trimester of pregnancy (Table 3). Furthermore, it has been discussed not to overuse d-dimer measurements in pregnancy.
Table 3.
Comparison of the DiPEP, ARTEMIS, and CT-PE-Pregnancy studies.
| Study | Study Design |
Used C-PTP | Recommendations and Key Findings |
|---|---|---|---|
| DiPEP | - prospective and retrospective, descriptive - women during and after pregnancy |
- the PERC rule, Well’s PE criteria, and the simplified revised Geneva score | - clinical decision rules and blood tests alone should not be used to determine suspected PE in pregnancy or postpartum. - d-dimer and other biomarkers were not reliable in ruling out PE during pregnancy without a clinical context [23] |
| ARTEMIS | - international, multicenter - prospective management study - pregnant women |
- 3 YEARS criteria: 1. clinical signs of deep vein thrombosis 2. hemoptysis 3. pulmonary embolism is the most likely diagnosis |
- the YEARS algorithm is safe and effective, and it results in less radiation exposure compared with conventional diagnostic methods - d-dimer and other biomarkers were not reliable in ruling out VTE during pregnancy without a clinical context |
| CT-PE- Pregnancy |
- prospective study - pregnant women or postpartum women |
- the revised Geneva score [41] | - the YEARS algorithm safely excludes PE in pregnant women and reduces the need for CTPA [19] |
DiPEP: an observational study of the diagnostic accuracy of clinical assessment, d-dimer, and chest radiography for suspected pulmonary embolism in pregnancy and postpartum; C-PTP: clinical pretest probability; PERC rule: pulmonary embolism rule-out criteria.
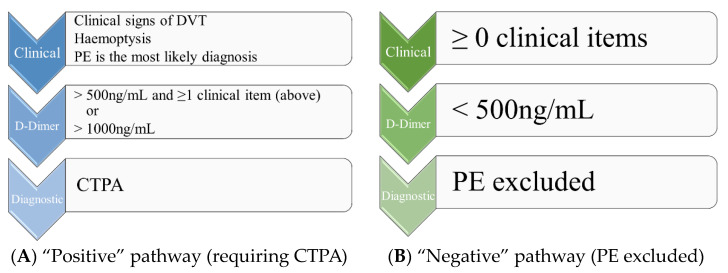
Finally, the combination of the clinical pretest probability (C-PTP) and laboratory tests increased the diagnostic value of risk prediction, as shown in the pregnancy-adapted YEARS approach (Figure 2).
Figure 2.
(A,B) Simplified pregnancy-adapted YEARS algorithm for the management of suspected acute pulmonary embolism in pregnant patients (modified from van der Pol et al. [21]). DVT, deep vein thrombosis; PE, pulmonary embolism; CTPA, computed tomography–pulmonary angiography.
The CT-PE pregnancy and ARTEMIS studies demonstrated the safety of excluding PE during pregnancy by a negative d-dimer test in cases of low or intermediate C-PTP [40,41,42,43] using the Geneva score and a pregnancy-adapted YEARS model. The YEARS algorithm integrates the following clinical findings: (1) signs of deep vein thrombosis, (2) hemoptysis, and (3) PE as the most likely diagnosis, which was evaluated based on the patient’s history and physical examination results. The thresholds for d-dimer levels were a cut-off of 500 µg/L used in patients with at least one item and a cut-off of 1000 µg/L in patients without any of those items [21,23,40,41,42]. The negative predictive value of the YEARS algorithm was highest during the first trimester of pregnancy and lowest during the third trimester. Despite the lower efficiency during this period of pregnancy, CTPA was avoided in 32% of cases [21,44]. In addition to these findings, the study by Kearon et al. found that in patients with a low C-PTP and a d-dimer level of <1000 ng/mL or a moderate C-PTP and a d-dimer level of <500 ng/mL, pulmonary embolism can be ruled out without further testing. Overall, these studies provide significant evidence in favor of the use of clinical probability-adjusted d-dimers in the management of PE [45].
5. Electrocardiogram
The electrocardiogram (ECG) has low sensitivity as a diagnostic test for PE, including pregnant patients [46]. Sometimes, changes may appear on an ECG that raise suspicion and support the diagnosis, such as sinus tachycardia, right bundle branch block, right axis deviation, or the classic S1Q3T3 pattern. However, even in the presence of a massive embolism, these changes may be absent. Moreover, physiological changes can occur during pregnancy that affect the ECG, such as left axial deviation and/or the presence of pronounced Q waves in ECG leads II, III, and aVF [46].
6. Imaging
Current discussions in clinical practice cover the question of which imaging modality should be used to exclude or diagnose PE in pregnancy. To facilitate clinical decisions, the challenges of different modalities will be discussed, such as specificity, sensitivity, negative predictive value, radiation dose, treatment, and diagnostic consequences. It is essential to mention that a d-dimer test, which is elevated in cases of DVT, may not always indicate the presence of PE. For stable patients, especially pregnant ones, avoiding a CT scan can prevent unnecessary exposure to radiation if it does not alter treatment plans.
6.1. Diagnostic Accuracy of Plain Chest Radiography
Chest X-ray is often recommended in the guidelines but appears not to be the imaging modality of choice for the diagnosis of PE in pregnant women. Chest radiographs often appear normal even in patients with PE [18]. The DiPEP study found that the presence of PE and non-PE-related abnormalities in chest X-rays increased the likelihood of a PE diagnosis [22,40]. In some guidelines, chest radiography is used to decide whether lung scintigraphy or CTPA should be used [7,10]. In addition, chest radiography may rule out non-PE-related diagnoses such as pneumothorax or pneumonia.
6.2. Diagnostic Accuracy of Echocardiography
The negative predictive value of bedside echocardiography is about 50%, indicating that echocardiography alone cannot be used to exclude the diagnosis of PE [6]. Notably, echocardiography is commonly available and can be used as a “rule-in” test in the emergency department at the patient’s bedside, supporting the decision for immediate treatment in high-risk PE [47,48,49]. For example, the McConnell sign (akinesia of the midportion of the right ventricular wall with preserved right ventricular function of the apex) and reduced longitudinal systolic function (TAPSE) show 100% specificity [50]. Right ventricular overload signs (D-shaping, dilatation of the right ventricle) have a sensitivity of approximately 80% for the diagnosis of PE [50], supporting the usefulness of echocardiography, especially in hemodynamically unstable patients with suspected PE [6]. The use of speckle-tracking echo (STE)—especially RV strain—appears promising to assess the severity of RV dysfunction also in pregnant women with PE. While there is extensive research on its benefits in non-pregnant individuals, its application among pregnant women, especially those with suspicion of PE, is still an area of ongoing study [51].
6.3. Diagnostic Value of Bilateral Venous Compression Ultrasound of the Lower Extremities
Compression ultrasonography (CUS) is useful in pregnant women with lower limb pain or swelling; therefore, it is incorporated into the pregnancy-adapted algorithms of the YEARS algorithm (ARTEMIS study) [21]. The use of the pregnancy-adapted YEARS algorithm excludes PE in 80% of pregnant women without the need for radiation exposure [19,41]. In the CT-PE-Pregnancy study, CUS was performed on 75% of the overall study population: among patients without leg symptoms, 2% were diagnosed with proximal deep vein thrombosis (DVT), and 9% of patients with leg symptoms had more positive results [41].
However, isolated deep pelvic vein thrombosis is a common cause of false-negative CUS results [52]. This limitation was already considered in the second consensus document on the diagnosis and treatment of acute deep vein thrombosis in pregnant women [53]. Thus, CUS should also include the visualization of the iliac veins or indirect signs of pelvic thrombosis, such as the monophasic flow of the common femoral vein [54,55]. The effectiveness of CUS without considering the clinical context (a sign of thrombosis) is limited [54,56]. It should be interpreted by including C-PTP and/or calf swelling with a diameter ≥ 3 cm greater than that of the asymptomatic calf [53,57]. The more sensitive method is magnetic resonance venography (sensitivity 92%, specificity 99%) and should be performed as the next diagnostic step if an isolated deep vein thrombosis in the pelvis is suspected but cannot be safely ruled out using ultrasonography due to the low sensitivity of 50% (the specificity was 99%) [58]. Some case reports have described the usefulness of magnetic resonance direct imaging (MRDTI) in the diagnosis of isolated venous thrombosis [59].
6.4. Diagnostic Value of Lung Scintigraphy
Several international guidelines and prospective studies [4,60] recommend the following diagnostic steps based on the GRADE (grades of recommendation, assessment, development) system: chest X-ray (CXR) as the first radiation-associated procedure, perfusion scintigraphy of the lung as the preferred test for normal CX, and CTPA over digital subtraction angiography (DSA) for non-diagnostic ventilation-perfusion (V/Q) results [61]. Perfusion scintigraphy of the lung displays a diagnostic accuracy comparable to CTPA, with different rates of non-diagnostic results: 5.9–14% vs. 4–12% respectively [62,63]. This difference is probably because there is no accepted definition of “non-diagnostic results” in the Cochrane database. The median negative predictive value of lung scintigraphy was 100% [62]. To summarize, lung perfusion scintigraphy is suitable for ruling out PE during pregnancy if the chest X-ray (CXR) is normal [4,61].
6.5. Diagnostic Value of Computed Tomographic Pulmonary Angiography
In the Cochrane database, the median negative predictive value for CTPA was 100%, and the median sensitivity was 83% [62]. The negative predictive value in pregnant women is high (100%), probably because of the low prevalence of PE and the low clinical probability of predisposed pregnant patients [64,65]. Additional reviews from 2018 and 2019 confirmed that CTPA is suitable for ruling out PE during pregnancy and that the diagnosis is based on a validated YEARS algorithm [62,63]. However, it is necessary to be aware of some methodological limitations, such as poor pulmonary arterial opacification and artifacts due to respiratory motion, which are more common in pregnant women than in non-pregnant ones [66]. Approximately 4–36% of CTPAs in pregnant women are non-diagnostic [18,62,63,66,67,68]. Thus, modifications of the imaging protocol are required to improve image quality. Some modifications, such as high concentration, high volume, and high rate of contrast injection followed by saline flush or shallow inspiration breathing, appear to be useful [69]; however, they are standard in the meantime with the use of modern CT scanners [70]. Close collaboration between the physician ordering the examination and the radiologist is of major importance.
6.6. Diagnostic Value of Magnetic Resonance Imaging
Recently, new techniques have been developed to improve spatial resolution, reduce the acquisition time, and reduce motion artifacts in magnetic resonance imaging for the diagnosis of pulmonary embolism. Herèdia et al. investigated balanced steady-state free-precession imaging. The protocol visualizes central, lobar, and segmental arteries with sufficient image quality in pregnant women [71]. Perfusion magnetic resonance imaging is the best stand-alone technique for PE diagnosis in pregnant women (sensitivity and specificity of 100% and 91%, respectively) [72]. According to the American College of Obstetricians and Gynecologists (ACOG), the use of gadolinium contrast agents in MRI should be restricted during pregnancy. Gadolinium may only be used as a contrast agent in a pregnant woman if it significantly improves diagnostic performance and is expected to improve fetal or maternal outcomes. Breastfeeding should not be interrupted after gadolinium administration [73].
Studies suggest that non-contrast MRI (with free-breathing arterial spin labeling) is a viable alternative for the diagnosis of pulmonary embolism [74]. A recent study showed that non-contrast magnetic resonance angiography (MRA) has high sensitivity and specificity in the diagnosis of pulmonary embolism, particularly in the proximal pulmonary arteries [75]. Another study discussed the sensitivity and specificity of non-contrast MRI and contrast-enhanced MRA. Non-contrast MRI had 89% sensitivity and 98% specificity, whereas contrast-enhanced MRA had 81% sensitivity and 100% specificity [76]. Mudge et al. reported the feasibility of diagnosing PE using non-contrast MRI. Although their MRI protocol was not optimized for the detection of pulmonary embolism, it was still 69% sensitive per vessel and 82% sensitive per patient [76]. Nevertheless, MRI is not currently the gold standard, and further research and evaluation are needed to validate its clinical utility. MRI techniques cannot be used in routine clinical practice for the diagnostic work-up of suspected pulmonary embolism in pregnant and non-pregnant women because of a lack of outcome studies demonstrating necessary safety and feasibility.
6.7. Radiation- and Contrast-Enhanced Computed Tomographic Pulmonary Angiography versus Pulmonary Scintigraphy
The fetal radiation dose is low in both CTPA and lung scintigraphy, with a mean dose of 0.01–0.66 mGy for CTPA and 0.1–0.8 mGy for V/Q-SPECT protocols [77]. Of interest, the radiation dose is higher in the third trimester than in the first trimester [78]. The radiation doses for 256-slice CTPA are listed in the online Supplement Table S1 [61]. Moreover, CTPA may cause a 0.2–2.2% increased the relative lifetime risk of breast or lung cancer in young mothers [79]. The radiosensitivity of the breast in pregnant women is higher than that in other parts of the body and in non-pregnant women because of several factors, including increased cell division, hormonal changes, and increased blood flow [80].
Strategies to minimize the absorbed radiation dose include the use of bismuth shields, breathing strategies, and automatic exposure controls [79]. An ongoing prospective multicenter study (OPTICA study) will evaluate the efficacy and safety of a low-dose CTPA protocol [81]. Of note, many studies have shown that V/Q scanning produces lower effective doses to the breast and fetus than CTPA [82,83,84]. In CTPA and perfusion scintigraphy, the average doses were estimated to be effective doses of 21 mGy and 1.04 mGy, doses absorbed by the maternal breast of 10–70 and 0.22–0.28 mGy (per breast), and doses absorbed by the uterus and fetus of 0.46 mGy and 0.25 mGy, respectively [84]. Despite these limitations, the risks associated with radiation exposure for both CTPA and V/Q scanning appear to be lower than the risk of a missed PE diagnosis [18], and CT angiography is the preferred method of diagnosis, as suggested by several guidelines [61]. In addition, several techniques exist to reduce the radiation dose (for example, photon-counting computed tomography) by approximately 48% and still have high sensitivity, such as a reduced scan length defined by the upper part of the aortic arch and the upper part of the lower hemidiaphragm [85,86]. It is important to realize that scintigraphy is only available to a limited extent in daily clinical practice. CTPA is readily available in most hospitals. This advantage usually influences decisions in daily clinical practice and outweighs the consideration of radiation exposure in urgent cases. The iodinated contrast agents used during CTPA were classified as pregnancy category B safe drugs by the Food and Drug Administration (FDA) [87].
In summary, the use of contrast media and radiation for diagnostic imaging in pregnant women with suspected pulmonary embolism, is considered safe when used prudently and when the benefits of accurate diagnosis and management outweigh the minimal risks. According to the American College of Obstetricians and Gynecologists (ACOG), the radiation exposure from CT scans is much lower than the dose associated with fetal harm, and the use of iodinated contrast media has not been shown to harm the fetus [88].
6.8. Diagnostic Value of Lung Sonography
Lung ultrasonography (LUS), a point-of-care diagnostic tool, is particularly beneficial in the diagnosis of critically ill patients, for whom transport to a radiology department may entail additional risks. It facilitates the detection of parenchymal changes in the lungs that indicate pulmonary embolism. In addition, an integrated approach with triple point-of-care ultrasonography, which simultaneously examines the lungs, heart, and leg veins, may improve the diagnostic accuracy of PE. This method could be particularly beneficial for pregnant women because it reduces radiation exposure and bed rest [86]. Unfortunately, the use of lung sonography to detect PE in pregnant women is currently not established. Studies performed using the BLUE protocol have shown 81% sensitivity and 99% specificity for the diagnosis of pulmonary embolism in patients with acute dyspnea in the presence of an “A-profile” and a deep vein thrombosis (DVT) [89].
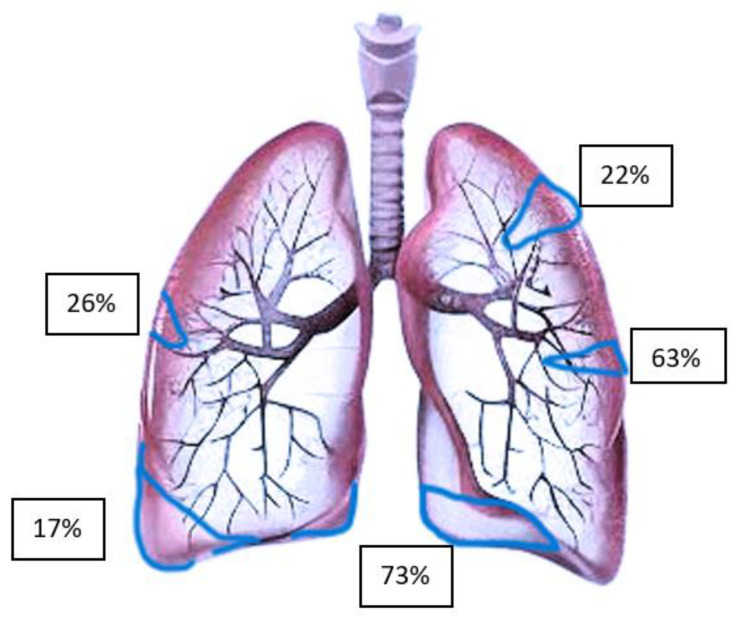
The distribution of lesions detected by LUS is shown in Figure 3 [90]. It is evident that several pulmonary emboli involve the peripheral lung. Furthermore, it is evident that central PE with hemodynamic relevance cannot be detected by LUS with sufficient sensitivity due to air-filled lungs around the central structures, causing total reflection of ultrasound waves. An autopsy study of lethal PE reported multiple pulmonary infarcts (peripheral lesions) of different ages in up to 15% of affected patients [91]. Hence, central PE with hemodynamic relevance could be better diagnosed using echocardiography.
Figure 3.
Distribution of pulmonary embolism lesions detected by transthoracic ultrasonography (Adapted from Comert et al. [90]).
The typical characteristics of suspicious (peripheral) lesions are [92]:
Hypoechoic, pleural-based parenchymal lesion, usually wedge-shaped (>85%), sometimes round or polygonal, may be present.
A central hyperechoic lesion may be present in 20% of cases, indicating an air-filled bronchiole.
Lesions may be associated with pleural effusion.
Color Doppler cannot detect pulmonary arterial flow in pulmonary infarction. This is referred to as “consolidation with low perfusion”.
A congested thromboembolic vessel may be visible, which is referred to as a “vascular sign”.
The posterior lower parts of the lung are affected in most patients (>70%). Although the explanation for this is not clear, it could be due to the anatomical structure of the pulmonary tree. In addition, the posterior lower parts of the lung are one of the easiest to access by transthoracic ultrasonography.
The right lung is more frequently affected than the left one (66.7%).
In a prospective multicenter study based on triangular, hypoechoic, and pleural parenchymal lesions, LUS showed a 74% sensitivity and 95% specificity for the diagnosis of peripheral PE (positive predictive value: 95%, negative predictive value: 75% and accuracy: 84%) [92].
LUS is a non-invasive, widely available, cost-effective method that can be performed rapidly. A negative LUS examination cannot rule out PE, but a positive LUS finding with moderate/high suspicion of PE can prove to be a valuable tool in the diagnosis of PE [90].
In international consensus, LUS is recognized as an alternative diagnostic tool for the diagnosis of PE when CT is contraindicated, not available, or declined by the patient (level of evidence: A) [93]. Multi-organ ultrasonography (heart, lung, and leg veins) increases the accuracy of clinical pre-test probability estimation in patients with suspected PE, and with 90% sensitivity and 86.2% specificity. Thus, use of those multiple ultrasound techniques may reduce the indication of multidetector computed tomography pulmonary angiography (MDCTPA) with associated ionizing radiation exposure [94]. To date, three societies (ATS-STR, ESC, RCOG) recommend deep vein ultrasonography before further imaging if lower limb symptoms are present, while two others recommend ultrasonography regardless of the presence of clinical manifestations of deep vein thrombosis (GTH, SOGC) [10].
In summary, the diagnostic role of LUS is currently not well defined and not recommended to exclude PE. Nevertheless, LUS may contribute specific information in specific groups of patients.
7. Therapy
The acute treatment of PE depends on the risk group of the affected patient. In stable patients (very low/low/and low–intermediate risk groups), systemic anticoagulation is the first line of treatment. In patients with hemodynamic instability, invasive support with thrombolytic therapy or interventional approaches such as catheter-directed therapy or surgical thrombectomy should be considered. We describe the different treatment options for the affected pregnant patient.
7.1. Anticoagulation
Low molecular weight heparin (LMWH) and unfractionated heparin (UFH), which are used as first-line anticoagulation therapies, are safe during pregnancy and breastfeeding because they do not cross the placenta [2,77]. UFH can be administered both intravenously and subcutaneously. PTT in response to UFH is shortened in pregnant women [95]. However, improved monitoring can be achieved by analyzing anti-factor Xa levels during UFH use (target values: 0.5–0.80 IU/mL). Current guidelines do not recommend routine anti-factor Xa level monitoring in pregnant women with normal renal function during LMWH administration [77]. Anti-factor Xa levels should be monitored in patients with mechanical heart valves, impaired renal function, extreme body weight (<50 kg and >90 kg), or recurrent VTE despite anticoagulation [11]. If dose adjustment is required, the peak serum anti-factor Xa level should be measured four hours after injection. No data are available on dose adjustment during pregnancy [96]. The use of new oral anticoagulants is contraindicated during pregnancy because they can cross the placenta and affect the blood coagulation system of the fetus and also exert teratogenic effects [97]. LMWH should be discontinued 12–24 h before lumbar instrumentation (such as epidural placement) or cesarean delivery and at least six hours before vaginal delivery. The restart of LMWH should be discussed in a multidisciplinary team. Usually, anticoagulation is started within six hours after vaginal delivery or within twelve hours after a cesarean section [11,77].
7.2. Thrombolytic Treatment
There is a paucity of evidence for the safety and efficacy of thrombolytic therapy in pregnant women. Systemic thrombolysis is only recommended in unstable high-risk patients [6] and is associated with favorable maternal outcomes in patients with massive PE; the maternal survival rate was approximately 92% [98]. Complications such as cardiac arrest (17.6%) and severe maternal hemorrhage (28.4%) occur during systemic thrombolysis. The fetal mortality rate is up to 20% [98]. Alteplase is usually administered at a dose of 100 mg over two hours, so the likelihood of transplacental transition is low [99]. Intravenous UFH administration should be initiated immediately after thrombolysis. At our institution, pregnant women receiving thrombolytic therapy are closely monitored by our obstetric team, and all treatments are discussed during interdisciplinary rounds.
7.3. Catheter-Directed Therapy (CDT)
CDT is intended for high-risk patients in whom thrombolysis or appropriately dosed anticoagulation has either failed or is contraindicated [100]. The rate of major hemorrhage (including intracranial hemorrhage) is low (18%) [101]. There are already more than ten devices used to treat unstable non-pregnant patients, which are based on aspiration, mechanical fragmentation, and/or thrombolytic infusion [100]. To date, CDT has rarely been used in affected pregnant patients with PE. The maternal survival rate was 100% in reported cases of mechanical percutaneous thrombectomy based on thrombus aspiration, thrombus fragmentation, and rheolytic thrombectomy [102,103]. There is a paucity of randomized trials comparing these treatments with systemic thrombolysis, even in the non-pregnant population. Therefore, the decision to perform percutaneous thrombolysis and/or thrombectomy should be individually discussed, including factors such as a high risk of bleeding, expected radiation exposure, and the availability of the procedure. We recommend that CDT procedures should be performed only in specialized centers [86,99].
7.4. Surgical Thrombectomy
Surgical thrombectomy is more frequently considered for the treatment of PE during pregnancy, especially in cases where anticoagulation is ineffective, or the patient is hemodynamically unstable [104]. Surgical thrombectomy with cardiopulmonary bypass is performed without cardioplegia by removing proximal pulmonary clots through incisions in the two main pulmonary arteries. Hemodynamic improvement and/or the return of spontaneous circulation was documented in 93.8% of patients after surgical thrombectomy and maternal survival occurred in 84–86.1% of patients [98].
7.5. Extracorporeal Membrane Oxygenation
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is considered a lifesaver for patients with high-risk PE [99,105]. Venous and arterial cannulas are placed in the inferior vena cava and common femoral artery, respectively. VA-ECMO is usually combined with fibrinolysis or embolectomy to improve outcomes of affected patients. The 30-day mortality rate for patients with high-risk PE treated with and without ECMO was 62% and 43%, respectively [106]. The most appropriate indication for VA-ECMO is refractory cardiac arrest due to PE. Only a few case reports have been published in which these invasive techniques have been used as rescue strategies [99,105,106,107]. There are studies that recommend ECMO with thrombolysis or surgical thrombectomy. However, it is unclear which trial is superior to another [108] in the general population, even in pregnant women (there are no known studies in this group).
8. Follow-Up
Follow-up care after an acute PE during pregnancy involves several important steps [109]:
Identify optimal anticoagulation strategies after delivery.
Age-appropriate screening for cancer.
Regular exercise and sports activities.
Ensure adherence to anticoagulants and avoid relevant drug interactions.
Exclude chronic thromboembolic pulmonary hypertension in all patients with persistent clinical manifestations of dyspnea or right heart failure.
Assess the overall bleeding risk.
Treatment of pregnancy-associated PE should be continued for at least three months, including six weeks postpartum [96,97]. The discussion about anticoagulant therapy duration in pregnant women is controversial due to the paucity of available data. Hormonal contraceptives (such as birth control pills) can be continued during anticoagulant treatment to prevent pregnancy and mitigate the risk of abnormal uterine bleeding. Current evidence suggests that there is no increased risk of recurrent PE in women receiving combined hormonal or progestin-only contraceptives during anticoagulation [109].
9. Conclusions
Diagnostic and therapeutic decision-making in pregnant patients with suspected PE is challenging. Therefore, we suggest that the treatment of affected patients should involve a multidisciplinary team approach with emergency physicians, pulmonologists, angiologist, cardiologists, thoracic surgeons, radiologists, and/or obstetricians as team members to choose optimal diagnostic and therapeutic approaches. The pulmonary embolism response team (PERT) has been suggested to improve the outcomes of care for high-risk PE patients [110]. Unfortunately, evidence from prospective cohort or interventional studies is rare, and recommendations for the diagnosis and management of PE in this situation are either ambiguous or contradictory in currently available guidelines.
Questions for future research
Diagnostic role of lung ultrasonography for the safe rule-out of PE in pregnant patients.
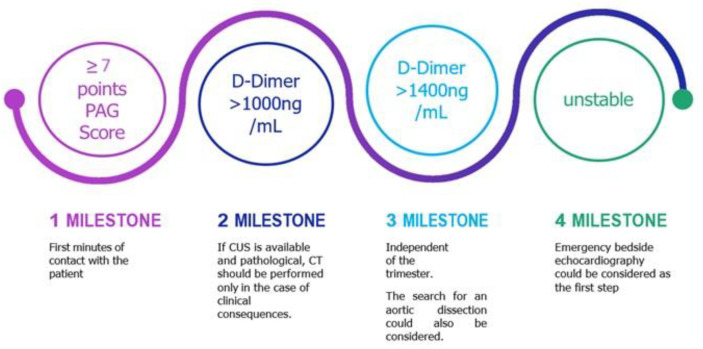
Decision support for the initial diagnosis of PE in the emergency department (Figure 4).
Combinations of laboratory tests (including cardiac biomarkers) to increase the sensitivity of the diagnostic algorithm for PE in pregnancy.
Summarized take home messages are presented in online Supplement Table S2.
Figure 4.
Proposed fast track CTPA evaluations for emergency physicians.
Abbreviations
AAA, abdominal aortic aneurysm; AVF, arteriovenous fistula; IVC, inferior vena cava; CRRT, continuous renal replacement therapy; CT, computed tomography; ICU, intensive care unit; ALT, alanine transaminase; AST, aspartate transaminase; TBIL, total bilirubin; DBIL, direct bilirubin.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13102863/s1, Table S1: Radiation doses for 256-Slice CTPA; Table S2: Take home messages.
Author Contributions
All authors contributed to editorial changes in the manuscript. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Uchikova E.H., Ledjev I.I. Changes in haemostasis during normal pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005;119:185–188. doi: 10.1016/j.ejogrb.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Greer I.A. Clinical practice. Pregnancy complicated by venous thrombosis. N. Engl. J. Med. 2015;373:540–547. doi: 10.1056/NEJMcp1407434. [DOI] [PubMed] [Google Scholar]
- 3.Szecsi P.B., Jørgensen M., Klajnbard A., Andersen M.R., Colov N.P., Stender S. Haemostatic reference intervals in pregnancy. Thromb. Haemost. 2010;103:718–727. doi: 10.1160/TH09-10-0704. [DOI] [PubMed] [Google Scholar]
- 4.Leung A.N., Bull T.M., Jaeschke R., Lockwood C.J., Boiselle P.M., Hurwitz L.M., James A.H., McCullough L.B., Menda Y., Paidas M.J., et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: Evaluation of suspected pulmonary embolism in pregnancy. Am. J. Respir. Crit. Care Med. 2011;184:1200–1208. doi: 10.1164/rccm.201108-1575ST. [DOI] [PubMed] [Google Scholar]
- 5.Shen J.H., Chen H.L., Chen J.R., Xing J.L., Gu P., Zhu B.F. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: A systematic review and meta-analysis. J. Thromb. Thrombolysis. 2016;41:482–492. doi: 10.1007/s11239-015-1250-2. [DOI] [PubMed] [Google Scholar]
- 6.Konstantinides S.V., Meyer G., Becattini C., Bueno H., Geersing G.J., Harjola V.P., Huisman M.V., Humbert M., Jennings C.S., Jiménez D., et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur. Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 7.Wan T., Skeith L., Karovitch A., Rodger M., Le Gal G. Guidance for the diagnosis of pulmonary embolism during pregnancy: Consensus and controversies. Thromb. Res. 2017;157:23–28. doi: 10.1016/j.thromres.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Linnemann B., Bauersachs R., Rott H., Halimeh S., Zotz R., Gerhardt A., Boddenberg-Pätzold B., Toth B., Scholz U. Diagnosis of pregnancy-associated venous thromboembolism–position paper of the working Group in women’s health of the Society of Thrombosis and Haemostasis (GTH) VASA. Z. Fur Gefasskrankh. 2016;45:87–101. doi: 10.1024/0301-1526/a000503. [DOI] [PubMed] [Google Scholar]
- 9.McLintock C., Brighton T., Chunilal S., Dekker G., McDonnell N., McRae S., Muller P., Tran H., Walters B.N.J., Young L. Recommendations for the diagnosis and treatment of deep venous thrombosis and pulmonary embolism in pregnancy and the postpartum period. Aust. N. Z. J. Obstet. Gynaecol. 2012;52:14–22. doi: 10.1111/j.1479-828X.2011.01361.x. [DOI] [PubMed] [Google Scholar]
- 10.Cohen S.L., Feizullayeva C., McCandlish J.A., Sanelli P.C., McGinn T., Brenner B., Spyropoulos A.C. Comparison of international societal guidelines for the diagnosis of suspected pulmonary embolism during pregnancy. Lancet. Haematol. 2020;7:e247–e258. doi: 10.1016/S2352-3026(19)30250-9. [DOI] [PubMed] [Google Scholar]
- 11.Royal College of Obstetricians and Gynaecologists Thrombosis and Embolism during Pregnancy and the Puerperium: Acute Management (Green-Top Guideline No. 37b) 2015. [(accessed on 15 February 2024)]. Available online: https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/thrombosis-and-embolism-during-pregnancy-and-the-puerperium-acute-management-green-top-guideline-no-37b/
- 12.Khan F., Tritschler T., Kahn S.R., Rodger M.A. Venous thromboembolism. Lancet. 2021;398:64–77. doi: 10.1016/s0140-6736(20)32658-1. [DOI] [PubMed] [Google Scholar]
- 13.Kushner A., West W.P., Khan Suheb M.Z., Pillarisetty L.S. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2024. Virchow Triad. [PubMed] [Google Scholar]
- 14.Grouzi E., Pouliakis A., Aktypi A., Christoforidou A., Kotsi P., Anagnostou G., Foifa A., Papadakis E. Pregnancy and thrombosis risk for women without a history of thrombotic events: A retrospective study of the real risks. Thromb. J. 2022;20:60. doi: 10.1186/s12959-022-00419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James A.H. Pregnancy-associated thrombosis. Hematol. Am. Soc. Hematol. Educ. Program. 2009;2009:277–285. doi: 10.1182/asheducation-2009.1.277. [DOI] [PubMed] [Google Scholar]
- 16.Muglia L.J., Benhalima K., Tong S., Ozanne S. Maternal factors during pregnancy influencing maternal, fetal, and childhood outcomes. BMC Med. 2022;20:418. doi: 10.1186/s12916-022-02632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safira A., Tjahjadi A.K., Adytia G.J., Waitupu A., Sutanto H. Peripartum cardiomyopathy unveiled: Etiology, diagnosis, and therapeutic insights. Curr. Probl. Cardiol. 2024;49:102474. doi: 10.1016/j.cpcardiol.2024.102474. [DOI] [PubMed] [Google Scholar]
- 18.Robert-Ebadi H., Le Gal G., Righini M. Diagnostic management of pregnant women with suspected pulmonary embolism. Front. Cardiovasc. Med. 2022;9:851985. doi: 10.3389/fcvm.2022.851985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Righini M., Robert-Ebadi H., Elias A., Sanchez O., Le Moigne E., Schmidt J., Le Gall C., Cornuz J., Aujesky D., Roy P.M. Diagnosis of pulmonary embolism during pregnancy: A multicenter prospective management outcome study. Ann. Intern. Med. 2018;169:766–773. doi: 10.7326/M18-1670. [DOI] [PubMed] [Google Scholar]
- 20.Kahn S.R., de Wit K. Pulmonary embolism. N. Engl. J. Med. 2022;387:45–57. doi: 10.1056/NEJMcp2116489. [DOI] [PubMed] [Google Scholar]
- 21.van der Pol L.M., Tromeur C., Bistervels I.M., Ni Ainle F., van Bemmel T., Bertoletti L., Couturaud F., van Dooren Y.P.A., Elias A., Faber L.M., et al. Pregnancy-adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. N. Engl. J. Med. 2019;380:1139–1149. doi: 10.1056/NEJMoa1813865. [DOI] [PubMed] [Google Scholar]
- 22.Goodacre S., Hunt B.J., Nelson-Piercy C. Diagnosis of suspected pulmonary embolism in pregnancy. N. Engl. J. Med. 2019;380:e49. doi: 10.1056/NEJMc1905283. [DOI] [PubMed] [Google Scholar]
- 23.Hunt B.J., Parmar K., Horspool K., Shephard N., Nelson-Piercy C., Goodacre S. The DiPEP (diagnosis of PE in pregnancy) biomarker study: An observational cohort study augmented with additional cases to determine the diagnostic utility of biomarkers for suspected venous thromboembolism during pregnancy and puerperium. Br. J. Haematol. 2018;180:694–704. doi: 10.1111/bjh.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapinsky S.E., Bourjeily G. Dyspnoea of Pregnancy. In: Lapinsky S.E., Plante L.A., editors. Respiratory Disease in Pregnancy. Cambridge University Press; Cambridge, MA, USA: 2020. pp. 159–162. [Google Scholar]
- 25.Chan W.S., Ray J.G., Murray S., Coady G.E., Coates G., Ginsberg J.S. Suspected Pulmonary Embolism in Pregnancy: Clinical Presentation, Results of Lung Scanning, and Subsequent Maternal and Pediatric Outcomes. Arch. Intern. Med. 2002;162:1170–1175. doi: 10.1001/archinte.162.10.1170. [DOI] [PubMed] [Google Scholar]
- 26.Ružičić D.P., Dzudovic B., Matijasevic J., Benic M., Salinger S., Kos L., Kovacevic-Preradovic T., Mitevska I., Neskovic A., Bozovic B. Signs and symptoms of acute pulmonary embolism and their predictive value for all-cause hospital death in respect of severity of the disease, age, sex and body mass index: Retrospective analysis of the Regional PE Registry (REPER) BMJ Open Respir. Res. 2023;10:e001559. doi: 10.1136/bmjresp-2022-001559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobohm L., Farmakis I.T., Münzel T., Konstantinides S., Keller K. Pulmonary Embolism and Pregnancy—Challenges in Diagnostic and Therapeutic Decisions in High-Risk Patients. Front. Cardiovasc. Med. 2022;9:856594. doi: 10.3389/fcvm.2022.856594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kandagatla P., Goranta S., Antoine H., Marashi S.M., Schmoekel N., Gupta A.H. PADUA score as a predictor for pulmonary embolism: A potential strategy for reducing unnecessary imaging. J. Thromb. Thrombolysis. 2019;47:566–571. doi: 10.1007/s11239-018-01801-w. [DOI] [PubMed] [Google Scholar]
- 29.Tran H.A., Gibbs H., Merriman E., Curnow J.L., Young L., Bennett A., Tan C.W., Chunilal S.D., Ward C.M., Baker R., et al. New guidelines from the Thrombosis and Haemostasis Society of Australia and New Zealand for the diagnosis and management of venous thromboembolism. Med. J. Aust. 2019;210:227–235. doi: 10.5694/mja2.50004. [DOI] [PubMed] [Google Scholar]
- 30.Kline J.A., Mitchell A.M., Kabrhel C., Richman P.B., Courtney D.M. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J. Thromb. Haemost. 2004;2:1247–1255. doi: 10.1111/j.1538-7836.2004.00790.x. [DOI] [PubMed] [Google Scholar]
- 31.Robert-Ebadi H., Elias A., Sanchez O., Le Moigne E., Schmidt J., Le Gall C., Aujesky D., Roy P.-M., Moumneh T., Chauleur C., et al. Assessing the clinical probability of pulmonary embolism during pregnancy: The pregnancy-adapted Geneva (PAG) score. J. Thromb. Haemost. JTH. 2021;19:3044–3050. doi: 10.1111/jth.15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klajnbard A., Szecsi P.B., Colov N.P., Andersen M.R., Jørgensen M., Bjørngaard B., Barfoed A., Haahr K., Stender S. Laboratory reference intervals during pregnancy, delivery and the early postpartum period. Clin. Chem. Lab. Med. 2010;48:237–248. doi: 10.1515/CCLM.2010.033. [DOI] [PubMed] [Google Scholar]
- 33.Chabloz P., Reber G., Boehlen F., Hohlfeld P., De Moerloose P. TAFI antigen and D-dimer levels during normal pregnancy and at delivery. Br. J. Haematol. 2001;115:150–152. doi: 10.1046/j.1365-2141.2001.03082.x. [DOI] [PubMed] [Google Scholar]
- 34.Ercan Ş., Özkan S., Yücel N., Orçun A. Establishing reference intervals for D-dimer to trimesters. J. Matern.-Fetal Neonatal Med. 2015;28:983–987. doi: 10.3109/14767058.2014.940891. [DOI] [PubMed] [Google Scholar]
- 35.Kovac M., Mikovic Z., Rakicevic L., Srzentic S., Mandic V., Djordjevic V., Radojkovic D., Elezovic I. The use of D-dimer with new cutoff can be useful in diagnosis of venous thromboembolism in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010;148:27–30. doi: 10.1016/j.ejogrb.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Sadeghi S., Golshani M., Safaeian B. New cut-off point for D-dimer in the diagnosis of pulmonary embolism during pregnancy. Blood Res. 2021;56:150–155. doi: 10.5045/br.2021.2021069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Q., Dai L., Chen H.Q., Xia W., Wang Q.L., Zhu C.R., Zhou R. Specific changes and clinical significance of plasma D-dimer during pregnancy and puerperium: A prospective study. BMC Pregnancy Childbirth. 2023;23:248. doi: 10.1186/s12884-023-05561-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyamoto K., Komatsu H., Okawa M., Iida Y., Osaku D., Azuma Y., Tsuneto T., Harada T., Taniguchi F., Harada T. D-dimer level significance for deep vein thrombosis screening in the third trimester: A retrospective study. BMC Pregnancy Childbirth. 2022;22:21. doi: 10.1186/s12884-021-04353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamari R., Billett H.H., Cohen H. D-dimer variability in the postpartum period. Blood. 2011;118:3337. doi: 10.1182/blood.V118.21.3337.3337. [DOI] [Google Scholar]
- 40.Okonofua F. The DiPEP (diagnosis of pulmonary embolism in pregnancy) study and the limited accuracy of clinical decision rules and D-dimer: What next? BJOG Int. J. Obstet. Gynaecol. 2019;126:393. doi: 10.1111/1471-0528.15309. [DOI] [PubMed] [Google Scholar]
- 41.Langlois E., Cusson-Dufour C., Moumneh T., Elias A., Meyer G., Lacut K., Schmidt J., Le Gall C., Chauleur C., Glauser F., et al. Could the YEARS algorithm be used to exclude pulmonary embolism during pregnancy? Data from the CT-PE-pregnancy study. J. Thromb. Haemost. 2019;17:1329–1334. doi: 10.1111/jth.14483. [DOI] [PubMed] [Google Scholar]
- 42.Goodacre S., Horspool K., Nelson-Piercy C., Knight M., Shephard N., Lecky F., Thomas S., Hunt B.J., Fuller G. The DiPEP study: An observational study of the diagnostic accuracy of clinical assessment, D-dimer and chest X-ray for suspected pulmonary embolism in pregnancy and postpartum. BJOG Int. J. Obstet. Gynaecol. 2019;126:383–392. doi: 10.1111/1471-0528.15286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viau-Lapointe J., Arsenault M.P. New evidence in diagnosis of pulmonary embolism during pregnancy. Obstet. Med. 2020;13:120–124. doi: 10.1177/1753495X19875589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L., Chen Y., Liu W., Wang X., Zhang S., Zhang W., Zhao S., Zhang M., Zhang S., Jiao G. Predictive value of D-dimer and analysis of risk factors in pregnant women with suspected pulmonary embolism after cesarean section. BMC Pulm. Med. 2021;21:391. doi: 10.1186/s12890-021-01757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kearon C., de Wit K., Parpia S., Schulman S., Afilalo M., Hirsch A., Spencer F.A., Sharma S., D’Aragon F., Deshaies J.-F., et al. Diagnosis of pulmonary embolism with d-dimer adjusted to clinical probability. N. Engl. J. Med. 2019;381:2125–2134. doi: 10.1056/NEJMoa1909159. [DOI] [PubMed] [Google Scholar]
- 46.Thomson D., Kourounis G., Trenear R., Messow C.M., Hrobar P., Mackay A., Isles C. ECG in suspected pulmonary embolism. Postgrad. Med. J. 2019;95:12–17. doi: 10.1136/postgradmedj-2018-136178. [DOI] [PubMed] [Google Scholar]
- 47.Fields J.M., Davis J., Girson L., Au A., Potts J., Morgan C.J., Vetter I., Riesenberg L.A. Transthoracic echocardiography for diagnosing pulmonary embolism: A systematic review and meta-analysis. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2017;30:714–723.e4. doi: 10.1016/j.echo.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Liu S., Elkayam U., Naqvi T.Z. Echocardiography in pregnancy: Part 1. Curr. Cardiol. Rep. 2016;18:92. doi: 10.1007/s11886-016-0760-7. [DOI] [PubMed] [Google Scholar]
- 49.O’Kelly A.C., Sharma G., Vaught A.J., Zakaria S. The use of echocardiography and advanced cardiac ultrasonography during pregnancy. Curr. Treat. Options Cardiovasc. Med. 2019;21:71. doi: 10.1007/s11936-019-0785-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kulka H.C., Zeller A., Fornaro J., Wuillemin W.A., Konstantinides S., Christ M. Acute pulmonary embolism–its diagnosis and treatment from a multidisciplinary viewpoint. Dtsch. Ärzteblatt Int. 2021;118:618–628. doi: 10.3238/arztebl.m2021.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chornenki N.L.J., Poorzargar K., Shanjer M., Mbuagbaw L., Crowther M., Siegal D.M. Detection of Right Ventricular Dysfunction in Acute Pulmonary Embolism By CT Scan: A Systematic Review and Meta-Analysis. Blood. 2020;136((Suppl. S1)):25–26. doi: 10.1182/blood-2020-138464. [DOI] [PubMed] [Google Scholar]
- 52.Bourjeily G., Paidas M., Khalil H., Rosene-Montella K., Rodger M. Pulmonary embolism in pregnancy. Lancet. 2010;375:500–512. doi: 10.1016/S0140-6736(09)60996-X. [DOI] [PubMed] [Google Scholar]
- 53.Mazzolai L., Ageno W., Alatri A., Bauersachs R., Becattini C., Brodmann M., Emmerich J., Konstantinides S., Meyer G., Middeldorp S., et al. Second consensus document on diagnosis and management of acute deep vein thrombosis: Updated document elaborated by the ESC Working Group on aorta and peripheral vascular diseases and the ESC Working Group on pulmonary circulation and right ventricular function. Eur. J. Prev. Cardiol. 2022;29:1248–1263. doi: 10.1093/eurjpc/zwab088. [DOI] [PubMed] [Google Scholar]
- 54.Kayılıoğlu S.I., Köksoy C., Alaçayır İ. Diagnostic value of the femoral vein flow pattern for the detection of an iliocaval venous obstruction. J. Vasc. Surg. Venous Lymphat. Disord. 2016;4:2–8. doi: 10.1016/j.jvsv.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Sloves J., Almeida J.I. Venous duplex ultrasound protocol for iliocaval disease. J. Vasc. Surg. Venous Lymphat. Disord. 2018;6:748–757. doi: 10.1016/j.jvsv.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Stals M.A.M., Moumneh T., Ainle F.N., Aujesky D., van Bemmel T., Bertoletti L., Bistervels I.M., Chauleur C., Couturaud F., van Dooren Y.P.A., et al. Noninvasive diagnostic work-up for suspected acute pulmonary embolism during pregnancy: A systematic review and meta-analysis of individual patient data. J. Thromb. Haemost. JTH. 2023;21:606–615. doi: 10.1016/j.jtha.2022.11.025. [DOI] [PubMed] [Google Scholar]
- 57.Fronas S.G., Jørgensen C.T., Dahm A.E.A., Wik H.S., Gleditsch J., Raouf N., Holst R., Klok F.A., Ghanima W. Safety of a strategy combining D-dimer testing and whole-leg ultrasonography to rule out deep vein thrombosis. Blood Adv. 2020;4:5002–5010. doi: 10.1182/bloodadvances.2020002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allain Wouterlood M.A., Malhamé I., Lévesque K., Dayan N., Mahone M., Côté A.M., Cumyn A., Malick M., Sauvé N. Pregnancy-associated pelvic vein thrombosis: Insights from a multicenter case series. J. Thromb. Haemost. JTH. 2021;19:1926–1931. doi: 10.1111/jth.15333. [DOI] [PubMed] [Google Scholar]
- 59.Dronkers C.E.A., Srámek A., Huisman M.V., Klok F.A. Accurate diagnosis of iliac vein thrombosis in pregnancy with magnetic resonance direct thrombus imaging (MRDTI) BMJ Case Rep. 2016;2016:bcr2016218091. doi: 10.1136/bcr-2016-218091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bajc M., Schümichen C., Grüning T., Lindqvist A., Le Roux P.Y., Alatri A., Bauer R.W., Dilic M., Neilly B., Verberne H.J., et al. EANM guideline for ventilation/perfusion single-photon emission computed tomography (SPECT) for diagnosis of pulmonary embolism and beyond. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:2429–2451. doi: 10.1007/s00259-019-04450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barr R.G., Parr D.G., Vogel-Claussen J. Imaging. European Respiratory Society; Lausanne, Switzerland: 2016. p. 37b. [Google Scholar]
- 62.van Mens T.E., Scheres L.J., de Jong P.G., Leeflang M.M., Nijkeuter M., Middeldorp S. Imaging for the exclusion of pulmonary embolism in pregnancy. Cochrane Database Syst. Rev. 2017;1:CD011053. doi: 10.1002/14651858.CD011053.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tromeur C., van der Pol L.M., Le Roux P.Y., Ende-Verhaar Y., Salaun P.Y., Leroyer C., Couturaud F., Kroft L.J.M., Huisman M.V., Klok F.A. Computed tomography pulmonary angiography versus ventilation-perfusion lung scanning for diagnosing pulmonary embolism during pregnancy: A systematic review and meta-analysis. Haematologica. 2019;104:176–188. doi: 10.3324/haematol.2018.196121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stein P.D., Fowler S.E., Goodman L.R., Gottschalk A., Hales C.A., Hull R.D., Leeper K.V., Jr., Popovich J., Jr., Quinn D.A., Sos T.A., et al. Multidetector computed tomography for acute pulmonary embolism. N. Engl. J. Med. 2006;354:2317–2327. doi: 10.1056/NEJMoa052367. [DOI] [PubMed] [Google Scholar]
- 65.Dronkers C.E.A., van der Hulle T., Le Gal G., Kyrle P.A., Huisman M.V., Cannegieter S.C., Klok F.A., Subcommittee on Predictive and Diagnostic Variables in Thrombotic Disease Towards a tailored diagnostic standard for future diagnostic studies in pulmonary embolism: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2017;15:1040–1043. doi: 10.1111/jth.13654. [DOI] [PubMed] [Google Scholar]
- 66.U-King-Im J.M., Freeman S.J., Boylan T. Cheow H.K. Quality of CT pulmonary angiography for suspected pulmonary embolus in pregnancy. Eur. Radiol. 2008;18:2709–2715. doi: 10.1007/s00330-008-1100-0. [DOI] [PubMed] [Google Scholar]
- 67.Ridge C.A., McDermott S., Freyne B.J., Brennan D.J., Collins C.D., Skehan S.J. Pulmonary embolism in pregnancy: Comparison of pulmonary CT angiography and lung scintigraphy. Am. J. Roentgenol. 2009;193:1223–1227. doi: 10.2214/AJR.09.2360. [DOI] [PubMed] [Google Scholar]
- 68.Andreou A.K., Curtin J.J., Wilde S., Clark A. Does pregnancy affect vascular enhancement in patients undergoing CT pulmonary angiography? Eur. Radiol. 2008;18:2716–2722. doi: 10.1007/s00330-008-1114-7. [DOI] [PubMed] [Google Scholar]
- 69.Ridge C.A., Mhuircheartaigh J.N., Dodd J.D., Skehan S.J. Pulmonary CT angiography protocol adapted to the hemodynamic effects of pregnancy. Am. J. Roentgenol. 2011;197:1058–1063. doi: 10.2214/AJR.10.5385. [DOI] [PubMed] [Google Scholar]
- 70.Shahir K., Goodman L.R., Tali A., Thorsen K.M., Hellman R.S. Pulmonary embolism in pregnancy: CT pulmonary angiography versus perfusion scanning. AJR Am. J. Roentgenol. 2010;195:W214–W220. doi: 10.2214/AJR.09.3506. [DOI] [PubMed] [Google Scholar]
- 71.Herédia V., Altun E., Ramalho M., de Campos R., Azevedo R., Pamuklar E., Semelka R.C. MRI of pregnant patients for suspected pulmonary embolism: Steady-state free precession vs postgadolinium 3D-GRE. Acta Médica Port. 2012;25:359–367. doi: 10.20344/amp.1352. [DOI] [PubMed] [Google Scholar]
- 72.Kluge A., Luboldt W., Bachmann G. Acute pulmonary embolism to the subsegmental level: Diagnostic accuracy of three MRI techniques compared with 16-MDCT. Am. J. Roentgenol. 2006;187:W7–W14. doi: 10.2214/AJR.04.1814. [DOI] [PubMed] [Google Scholar]
- 73.Othman A.E., Liang C., Komma Y., Munz M., Kolb M., Rath D., Gückel B., Pohmann R., Nikolaou K., Schwartz M., et al. Free-breathing arterial spin labeling MRI for the detection of pulmonary embolism. Radiology. 2023;307:e221998. doi: 10.1148/radiol.221998. [DOI] [PubMed] [Google Scholar]
- 74.Mohammad A.H., Mostafa H.M., Megaly H.I., Mohamed M.Z., Taha M.G., Abdelal S.M. Role of MRI in diagnosis of pulmonary embolism. Egypt. J. Bronchology. 2023;17:40. doi: 10.1186/s43168-023-00212-7. [DOI] [Google Scholar]
- 75.Shahrouki P., Jalili M.H., Kooraki S., Rahsepar A.A., Shen J., Hassani C., Bedayat A. MR vascular imaging: Update on new techniques and protocols. Curr. Radiol. Rep. 2023;11:81–95. doi: 10.1007/s40134-023-00413-4. [DOI] [Google Scholar]
- 76.Mudge C.S., Healey T.T., Atalay M.K., Pezzullo J.A. Feasibility of detecting pulmonary embolism using noncontrast MRI. ISRN Radiol. 2013;2013:729271. doi: 10.5402/2013/729271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sadigh G., Kelly A.M., Cronin P. Challenges, controversies, and hot topics in pulmonary embolism imaging. AJR Am. J. Roentgenol. 2011;196:497–515. doi: 10.2214/AJR.10.5830. [DOI] [PubMed] [Google Scholar]
- 78.Hurwitz L.M., Reiman R.E., Yoshizumi T.T., Goodman P.C., Toncheva G., Nguyen G., Lowry C. Radiation dose from contemporary cardiothoracic multidetector CT protocols with an anthropomorphic female phantom: Implications for cancer induction. Radiology. 2007;245:742–750. doi: 10.1148/radiol.2453062046. [DOI] [PubMed] [Google Scholar]
- 79.Arasu V.A., Kannan N., Krishnarao P.M., Kuehner G., Kuan M.C., Kim J.C., Joe B.N., Lee A. Imaging the breast in pregnant or lactating Women. Curr. Radiol. Rep. 2018;6:10. doi: 10.1007/s40134-018-0267-7. [DOI] [Google Scholar]
- 80.Gillespie C., Foley S., Rowan M., Ewins K., NiAinle F., MacMahon P. The optica study (optimised computed tomography pulmonary angiography in pregnancy Quality and safety study): Rationale and design of a prospective trial assessing the quality and safety of an optimised CTPA protocol in pregnancy. Thromb. Res. 2019;177:172–179. doi: 10.1016/j.thromres.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Astani S.A., Davis L.C., Harkness B.A., Supanich M.P., Dalal I. Detection of pulmonary embolism during pregnancy: Comparing radiation doses of CTPA and pulmonary scintigraphy. Nucl. Med. Commun. 2014;35:704–711. doi: 10.1097/MNM.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 82.Perisinakis K., Seimenis I., Tzedakis A., Damilakis J. Perfusion scintigraphy versus 256-slice CT angiography in pregnant patients suspected of pulmonary embolism: Comparison of radiation risks. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2014;55:1273–1280. doi: 10.2967/jnumed.114.137968. [DOI] [PubMed] [Google Scholar]
- 83.Tester J., Rees M., Pascoe D., Earl V., Einsiedel P., Lim W.K., Irving L., Hammerschlag G. Diagnostic imaging for suspected pulmonary embolism during pregnancy and postpartum: A comparative radiation dose study. J. Med. Imaging Radiat. Oncol. 2023;67:223–231. doi: 10.1111/1754-9485.13420. [DOI] [PubMed] [Google Scholar]
- 84.Schmid J., Nagy E., Kaufmann-Bühler A.K., Steiner J., Janisch M., Janek E., Reiter C., Eibisberger M., Softic N., Guss H., et al. Diagnosing pulmonary embolism with computed tomography pulmonary angiography: Diagnostic accuracy of a reduced scan range. J. Thorac. Imaging. 2022;37:323–330. doi: 10.1097/RTI.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pannenbecker P., Huflage H., Grunz J.P., Gruschwitz P., Patzer T.S., Weng A.M., Heidenreich J.F., Bley T.A., Petritsch B. Photon-counting CT for diagnosis of acute pulmonary embolism: Potential for contrast medium and radiation dose reduction. Eur. Radiol. 2023;33:7830–7839. doi: 10.1007/s00330-023-09777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valero E., González-D’Gregorio J.G., Carbonell N., García-Simón M., Ferreres J., Sanchís J. Percutaneous treatment of pulmonary embolism during pregnancy. Rev. Española Cardiol. 2020;73:427–429. doi: 10.1016/j.rec.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 87.Leek J.C., Arif H. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. [Google Scholar]
- 88.Shaw P., Duncan A., Vouyouka A., Ozsvath K. Radiation exposure and pregnancy. J. Vasc. Surg. 2011;53:28S34S. doi: 10.1016/j.jvs.2010.05.140. [DOI] [PubMed] [Google Scholar]
- 89.Lichtenstein D.A. BLUE-protocol and FALLS-protocol: Two applications of lung ultrasound in the critically ill. Chest. 2015;147:1659–1670. doi: 10.1378/chest.14-1313. [DOI] [PubMed] [Google Scholar]
- 90.Comert S.S., Caglayan B., Akturk U., Fidan A., Kıral N., Parmaksız E., Salepci B., Kurtulus B.A.O. The role of thoracic ultrasonography in the diagnosis of pulmonary embolism. Ann. Thorac. Med. 2013;8:99–104. doi: 10.4103/1817-1737.109822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morpurgo M., Schmid C. Clinico-pathological correlations in pulmonary embolism: A posteriori evaluation. Prog. Respir. Dis. 1980;13:8–15. [Google Scholar]
- 92.Reissig A., Heyne J.P., Kroegel C. Sonography of lung and pleura in pulmonary embolism: Sonomorphologic characterization and comparison with spiral CT scanning. Chest. 2001;120:1977–1983. doi: 10.1378/chest.120.6.1977. [DOI] [PubMed] [Google Scholar]
- 93.Volpicelli G., Elbarbary M., Blaivas M., Lichtenstein D.A., Mathis G., Kirkpatrick A.W., Melniker L., Gargani L., Noble V.E., Via G., et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 94.Nazerian P., Vanni S., Volpicelli G., Gigli C., Zanobetti M., Bartolucci M., Ciavattone A., Lamorte A., Veltri A., Fabbri A., et al. Accuracy of point-of-care multiorgan ultrasonography for the diagnosis of pulmonary embolism. Chest. 2014;145:950–957. doi: 10.1378/chest.13-1087. [DOI] [PubMed] [Google Scholar]
- 95.Raschke R.A., Guidry J.R., Foley M.R. Apparent heparin resistance from elevated factor VIII during pregnancy. Obstet. Gynecol. 2000;96:804–806. doi: 10.1016/s0029-7844(00)01053-x. [DOI] [PubMed] [Google Scholar]
- 96.Goland S., Elkayam U. Anticoagulation in pregnancy. Cardiol. Clin. 2012;30:395–405. doi: 10.1016/j.ccl.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 97.Bates S.M. Pulmonary embolism in pregnancy. Semin. Respir. Crit. Care Med. 2021;42:284–298. doi: 10.1055/s-0041-1722867. [DOI] [PubMed] [Google Scholar]
- 98.Martillotti G., Boehlen F., Robert-Ebadi H., Jastrow N., Righini M., Blondon M. Treatment options for severe pulmonary embolism during pregnancy and the postpartum period: A systematic review. J. Thromb. Haemost. 2017;15:1942–1950. doi: 10.1111/jth.13802. [DOI] [PubMed] [Google Scholar]
- 99.Blondon M., Martinez de Tejada B., Glauser F., Righini M., Robert-Ebadi H. Management of high-risk pulmonary embolism in pregnancy. Thromb. Res. 2021;204:57–65. doi: 10.1016/j.thromres.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 100.Pruszczyk P., Klok F.A., Kucher N., Roik M., Meneveau N., Sharp A.S.P., Nielsen-Kudsk J.E., Obradović S., Barco S., Giannini F., et al. Percutaneous treatment options for acute pulmonary embolism: A clinical consensus statement by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function and the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2022;18:e623–e638. doi: 10.4244/EIJ-D-22-00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rodriguez D., Jerjes-Sanchez C., Fonseca S., Garcia-Toto R., Martinez-Alvarado J., Panneflek J., Ortiz-Ledesma C., Nevarez F. Thrombolysis in massive and submassive pulmonary embolism during pregnancy and the puerperium: A systematic review. J. Thromb. Thrombolysis. 2020;50:929–941. doi: 10.1007/s11239-020-02122-7. [DOI] [PubMed] [Google Scholar]
- 102.O’Keeffe S.A., McGrath A., Ryan J.M., Byrne B. Management of a massive pulmonary embolism in a pregnant patient with mechanical fragmentation followed by delayed catheter-directed thrombolysis in the early postpartum period. J. Matern. Fetal Neonatal Med. 2008;21:591–594. doi: 10.1080/14767050802165604. [DOI] [PubMed] [Google Scholar]
- 103.Sofocleous C.T., Hinrichs C., Bahramipour P., Barone A., Abujudeh H.H., Contractor D. Percutaneous management of life-threatening pulmonary embolism complicating early pregnancy. J. Vasc. Interv. Radiol. 2001;12:1355–1356. doi: 10.1016/S1051-0443(07)61566-8. [DOI] [PubMed] [Google Scholar]
- 104.Taenaka H., Ootaki C., Matsuda C., Fujino Y. Successful pulmonary embolectomy for massive pulmonary embolism during pregnancy: A case report. JA Clin. Rep. 2017;3:44. doi: 10.1186/s40981-017-0116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Agerstrand C., Abrams D., Biscotti M., Moroz L., Rosenzweig E.B., D’Alton M., Brodie D., Bacchetta M. Extracorporeal membrane oxygenation for cardiopulmonary failure during pregnancy and postpartum. Ann. Thorac. Surg. 2016;102:774–779. doi: 10.1016/j.athoracsur.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 106.Meneveau N., Guillon B., Planquette B., Piton G., Kimmoun A., Gaide-Chevronnay L., Aissaoui N., Neuschwander A., Zogheib E., Dupont H., et al. Outcomes after extracorporeal membrane oxygenation for the treatment of high-risk pulmonary embolism: A multicentre series of 52 cases. Eur. Heart J. 2018;39:4196–4204. doi: 10.1093/eurheartj/ehy464. [DOI] [PubMed] [Google Scholar]
- 107.Bataillard A., Hébrard A., Gaide-Chevronnay L., Casez M., Dessertaine G., Durand M., Chavanon O., Albaladejo P. Extracorporeal life support for massive pulmonary embolism during pregnancy. Perfusion. 2016;31:169–171. doi: 10.1177/0267659115586578. [DOI] [PubMed] [Google Scholar]
- 108.Ünver S.S., Kalkan A., Demirel A., Kaya N., Uzun Ö. Extracorporeal membrane oxygenation for pulmonary embolism during pregnancy and postpartum. Eur. Arch. Med. Res. 2020;36:1–7. doi: 10.4274/eamr.galenos.2020.94830. [DOI] [Google Scholar]
- 109.Klok F.A., Ageno W., Ay C., Bäck M., Barco S., Bertoletti L., Becattini C., Carlsen J., Delcroix M., van Es N., et al. Optimal follow-up after acute pulmonary embolism: A position paper of the European Society of Cardiology Working Group on Pulmonary Circulation and Right Ventricular Function, in collaboration with the European Society of Cardiology Working Group on Atherosclerosis and Vascular Biology, endorsed by the European Respiratory Society. Eur. Heart J. 2022;43:183–189. doi: 10.1093/eurheartj/ehab816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hobohm L., Farmakis I.T., Keller K., Scibior B., Mavromanoli A.C., Sagoschen I., Münzel T., Ahrens I., Konstantinides S. Pulmonary embolism response team (PERT) implementation and its clinical value across countries: A scoping review and meta-analysis. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2023;112:1351–1361. doi: 10.1007/s00392-022-02077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.