Abstract
Simple Summary
Insect-specific viruses (ISVs) are increasingly recognised for their role in causing severe illnesses and mortality in both humans and animals. In this study, the full genome of ISV (Rice thrips ollusvirus 1, RTOV1) was revealed in rice thrips using metatranscriptome sequencing, RT-PCR, and RACE, respectively. RTOV1 has a typical linear G-N-L genome structure of the order Jingchuvirales. Phylogenetic analysis categorised this virus as an Ollusvirus. The infection of host insects with RTOV1 triggered antiviral RNA interference (RNAi), resulting in a significant accumulation of 22-nt virus-derived small interfering RNAs (vsiRNAs), with a notable bias towards the A/U content. Our study provides valuable information on ISVs in thrips that may be useful for pest management.
Abstract
Insects constitute the largest proportion of animals on Earth and act as significant reservoirs and vectors in disease transmission. Rice thrips (Haplothrips aculeatus, family Phlaeothripidae) are one of the most common pests in agriculture. In this study, the full genome sequence of a novel Ollusvirus, provisionally named “Rice thrips ollusvirus 1” (RTOV1), was elucidated using transcriptome sequencing and the rapid amplification of cDNA ends (RACE). A homology search and phylogenetic tree analysis revealed that the newly identified virus is a member of the family Aliusviridae (order Jingchuvirales). The genome of RTOV1 contains four predicted open reading frames (ORFs), including a polymerase protein (L, 7590 nt), a glycoprotein (G, 4206 nt), a nucleocapsid protein (N, 2415 nt) and a small protein of unknown function (291 nt). All of the ORFs are encoded by the complementary genome, suggesting that the virus is a negative-stranded RNA virus. Phylogenetic analysis using polymerase sequences suggested that RTOV1 was closely related to ollusvirus 1. Deep small RNA sequencing analysis reveals a significant accumulation of small RNAs derived from RTOV1, indicating that the virus replicated in the insect. According to our understanding, this is the first report of an Ollusvirus identified in a member of the insect family Phlaeothripidae. The characterisation and discovery of RTOV1 is a significant contribution to the understanding of Ollusvirus diversity in insects.
Keywords: Haplothrips aculeatus, thrips, metatranscriptomic sequencing, Ollusvirus, negative-strand RNA virus, RdRp
1. Introduction
An increasing number of novel ISVs are being discovered using next-generation sequencing (NGS) technology and transcriptome analysis [1,2,3]. The identification of a large number of viral sequences has led to the realisation that the presence of viruses in ecosystems is ecologically and evolutionarily important [4,5]. Some insects have been shown to serve as viral vectors for animals or plants, and the viruses in insects mainly include insect-specific viruses (ISVs), vector-borne pathogenic viruses, and viruses from insect endosymbiotic microorganisms or digestive matter [6,7].
Negative-strand RNA viruses (NSVs) cause various diseases in plants, animals and humans and are broadly classified into segmented and non-segmented viruses [8]. An increasing number of plant- and insect-infecting NSVs are being discovered, and they are considered emerging pathogens. The new NSVs in the family of Aliusviridae belong to the order Jingchuvirale, which was created by the International Committee on Classification of Viruses (ICTV) in 2018 [9,10]. The order Jingchuvirale presents a diverse array of genome organisations, encompassing unsegmented, bi-segmented and circular configurations [11]. The viral genomes encode a glycoprotein (G), a nucleoprotein (N) and a polymerase (L), though some viral genomes may lack the glycoprotein. It has been reported that the loss of the G gene may have occurred during the virus’s long-term evolution [1]. Ollusvirus belongs to the family Aliusviridae, with a genome structure of G-N-L, and it is a genus with relatively few viruses [10].
A growing body of research suggests that arthropods may be important reservoirs for a wide range of viruses and may play an important role in virus evolution [1,12]. Haplothrips aculeatus, belonging to the family Phlaeothripidae and order Thysanoptera, is a rice pest that directly feeds on rice and can transmit plant viruses, causing significant damage to agricultural production [13,14]. H. aculeatus is distributed in all the rice-growing areas of Asia and in most of China. It is widely parasitic on cereal crops and a variety of grass weeds. To further evaluate the potential causes of rice losses caused by H. aculeatus, we analysed the possible insect-transmitted pathogens using RNA-seq. In this paper, we have successfully identified a new ISV from H. aculeatus and classified it as the genus Ollusvirus in the family Aliusviridae. This is of significant importance for expanding our understanding of viruses and provides a theoretical basis for future research on whether the disease caused by this virus will become prevalent.
2. Materials and Methods
2.1. Sample Preparation and RNA Extraction
Insect samples of H. aculeatus were collected from a rice field in Shandong, China, in July 2021. The extraction of total RNA proceeded as previously described, with some modifications for optimisation [15]. Specifically, each sample consisted of 50 adult thrips, and RNA extraction was performed using AG RNAex Pro Reagent (agbio, Changsha, Hunan, China) instead of Trizol reagent (Invitrogen, Carlsbad, CA, USA). The methods used were exactly the same as previously described. The quality of the RNA was confirmed using a NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA, USA). The cDNA synthesis for whole-genome sequence determination, transcriptomic sequencing and small RNA (sRNA) sequencing, respectively, used 2 μg of RNA each, while 500 ng of RNA was used for first-strand cDNA synthesis in RACE.
2.2. Host Insect Identification
The assembled contigs were first compared with the Barcode of Life Data Systems (accessed on 14 December 2019, https://www.boldsystems.org/) to determine the mitochondrial cytochrome coxidase subunit I (COI) sequence of the rice thrips species. The COI sequence was then confirmed via a BLASTn search against the nucleotide database of the National Center for Biotechnology Information (NCBI).
2.3. Transcriptomic and Small RNA (sRNA) Sequencing
Novogene (Tianjin, China) conducted transcriptomic and small RNA (sRNA) sequencing on the aforementioned insect RNA samples. The transcriptome and sRNA libraries were prepared as described previously [16]. In brief, sequencing was performed on the constructed paired-end (150 bp) libraries using the Illumina HiSeq 4000 platform. The raw reads were quality trimmed using Trimmomatic software (version 0.39), and subsequently de novo assembled [17]. The Illumina TruSeq Small RNA Sample Preparation Kit (Illumina, USA) was used to prepare the sRNA libraries for sequencing. Subsequently, sRNA sequencing was carried out on the Illumina HiSeq 2500 platform. The Cutadapt tool was then used to remove the adapters and low-quality sequences from the raw output data [18].
2.4. Virus Discovery and Confirmation by Reverse Transcription-PCR (RT-PCR)
The identification of the viral contig involved the following steps: Initially, the assembled contigs were aligned against a locally downloaded virus database sourced from the NCBI viral reference database (accessed on 9 July 2023, https://www.ncbi.nlm.nih.gov/genome/viruses). Afterward, in order to prevent false positives, the putative viral sequence was subjected to comparison against both the NCBI nucleotide (NT) and non-redundant (NR) protein databases. Finally, the identified viral contigs were validated using RT-PCR, followed by Sanger sequencing using specific primers (Supplementary Table S1).
2.5. Determination of Viral Genome Termini and Transcript Abundance
The full genome sequence of the virus was obtained by rapid amplification using cDNA ends (RACE) technology. Briefly, the 5′-RACE and 3′-RACE cDNA were synthesised using the 5′/3′ RACE kit (agbio, Changsha, China) according to the manufacturer’s instructions. Subsequently, the PCR products were cloned into the pTO vector (Generalbiol, Chuzhou, Anhui, China), and Sanger sequencing was performed on the positive recombinant plasmids. The primers utilised for RACE are detailed in Supplementary Table S1.
To assess the transcript coverage and abundance of the viruses, we utilised both Bowtie2 and Samtools to align the adaptor-trimmed and quality-trimmed reads of the transcriptome back to the complete viral genomes [19,20]. Subsequently, the coverage of the aligned reads on the viral genomes was visualised using the Integrated Genomics Viewer (IGV) [21].
2.6. Small RNA Analysis
Small RNA analysis was carried out as described previously [22]. First, an sRNA library was prepared using the Illumina TruSeq sRNA Sample Preparation Kit (Illumina, San Diego, USA) and sequenced on the Illumina HiSeq 2500 platform. Next, the raw data were processed to obtain clean reads, from which sRNAs with lengths ranging from 18 to 30 nucleotides were extracted for further analysis. Finally, the sRNA reads from the previous step were mapped back to the entire viral genome sequence using Bowtie (allowing for zero mismatches) to identify vsiRNAs. The results were then further analysed using custom Perl scripts and Linux Bash scripts to analyse the obtained vsiRNAs [23].
2.7. Genome Annotation and Phylogenetic Analysis
The ORF Finder program at the NCBI (accessed on 20 January 2024, https://www.ncbi.nlm.nih.gov/orffinder/) was utilised to predict potential open reading frames (ORFs) within the virus genomes. The conserved protein structural domains were predicted using InterProScan (version InterPro 98.0, https://www.ebi.ac.uk/interpro; accessed on 20 January 2024). The amino acid sequence of the predicted Ollusvirus RdRp was obtained from the NCBI. Sequences were aligned using MAFFT (version 7.450) with Gblock [24]. The alternative model was evaluated using ModelTest-NG according to the default parameters [25]. Subsequently, maximum likelihood (ML) trees were constructed using RAxMLNG (version 0.9.0) with 1000 bootstrap replications [26].
3. Results
3.1. Discovery of RNA Virus-Related Sequences in H. aculeatus
To identify the RNA virus-related sequences in H. aculeatus, NGS was used to analyse the total RNA samples by RNA sequencing. The results show that 13.08 Gbp of the raw data from the cDNA library of insect samples were obtained by deep sequencing using the Illumina HiSeq 4000 platform (150 bp paired-end reads) of Novogene. A total of 23,001,625 clean reads were obtained. The assembled contigs were first compared with the Barcode of Life Data Systems to determine the cytochrome coxidase subunit I (COI) sequence of the rice thrips species. Then, the COI sequence was confirmed by a BLASTn search against the nucleotide database of the National Center for Biotechnology Information (NCBI). The result showed that the COI sequence was 99.66% identical to that of H. aculeatus (GenBank: MF716896.1) (Supplementary File S1). A total of 2054 virus-like reads were found in this thrips RNA library. To identify the viral contig, the assembled contigs were searched against the NCBI viral reference database. The result revealed the presence of a new RNA virus belonging to the genus Ollusvirus. The viral sequence was validated using RT-PCR, and the complete genome sequence of Ollusvirus was obtained by RACE.
3.2. Characterisation of Rice Thrips Ollusvirus 1
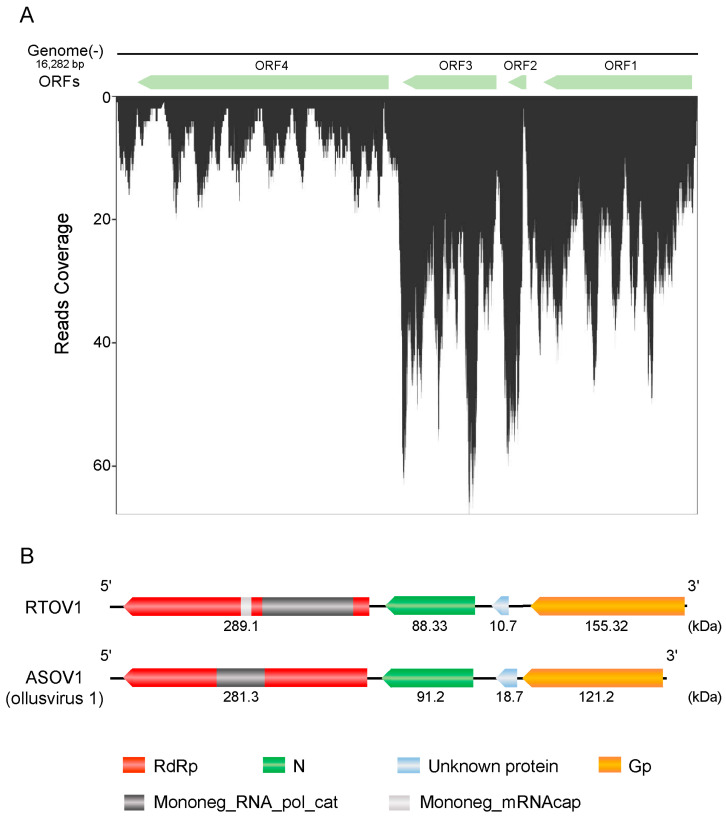
The full genome sequence of RTOV1 is 16,282 nt long, including a 223 nt 5′ untranslated region (UTR), with four non-overlapping predicted ORFs (ORF1, 224–7813 nt; ORF2, 8148–10,562 nt; ORF3, 12,018–16,223 nt; ORF4, 12,018–16,223 nt), and a 59 nt 3′ UTR (Figure 1A). Based on the InterProScan prediction of conserved domains for RTOV1, ORF4 contains a Mononegavirales RNA-directed RNA polymerase catalytic domain (Mo-noneg_RNA_pol_cat) and a Mononegavirales mRNA-capping domain V (Mo-noneg_mRNAcap) (Figure 1A). To characterise the proteins encoded by these ORFs, we used the NCBI Open Reading Frame Finder. Three of these ORFs encode major viral proteins. ORF1 was predicted to encode a 155.32 kDa glycoprotein (Gp), ORF3 was predicted to encode an 88.33 kDa nucleoprotein (N), and ORF4 was predicted to encode a 289.1 kDa polymerase protein (RdRp) (Supplementary File S2). However, no biological function could be attributed to ORF2 on the basis of protein homology (Figure 1A). The virus was tentatively named “Rice thrips ollusvirus 1” (RTOV1), and the full genome sequence was submitted to the GenBank database (GenBank: OR886228) (Supplementary File S3). The genome structure of RTOV1 was highly similar to that of Atractomorpha sinensis ollusvirus 1 (ASOV1, genus: Ollusvirus), which was previously detected by in Pink-Winged Grasshopper, Atractomorpha sinensis (genus: Atractomorpha; family: Pyrgomorphidae) (Figure 1B) [27].
Figure 1.
Characterisation of Rice thrips ollusvirus 1 (RTOV1). (A) High-quality reads were mapped against the validated, near full-length genome sequence of RTOV1. Predicted ORFs were manually annotated (green). The readmap depicts the nucleotide coverage by viral genomes RNA reads. Note the relatively higher RNA fraction towards the 3′ end of the genome. (B) Comparison of the genome organisation (not to scale) of RTOV1 and its closest relative ollusvirus 1 (ASOV1). The genomes of RTOV1 and ASOV1 are unsegmented negative-sense RNA, and RdRp (L), nucleoprotein (N), ORF2 (unknown biological function) and glycoprotein (Gp) have similar positions and mass ranges.
RTOV1 shared 21.61% identity in the capsid protein (CP, also named nucleoprotein in NSVs) region with its closest homologue, ollusvirus 1 (ASOV1, GenBank: WAB51682.1), meeting the delimitation criterion for establishing a new species (less than 90% CP identity). The abundance and coverage of RTOV1 were assessed by realigning RNA-seq reads with the full genome sequence of RTOV1 obtained. A total of 22,701,157 reads were perfectly aligned to the RTOV1 genome, representing 0.0048% of the total RNA-seq reads (Figure 1A). As shown in Figure 1A, the transcripts were distributed across the viral genome, with a particularly high level of abundance at the 3′ terminus, indicating that RTOV1 efficiently replicated in the insects.
3.3. Phylogenetic Analysis of RTOV1 and Related Jingchuvirals
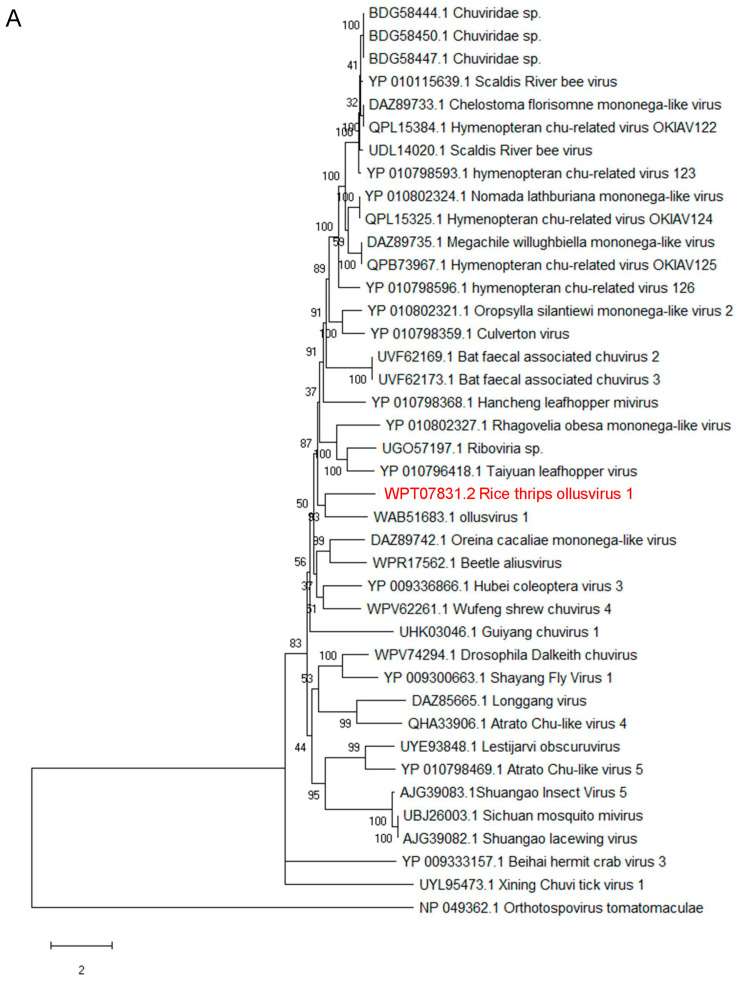
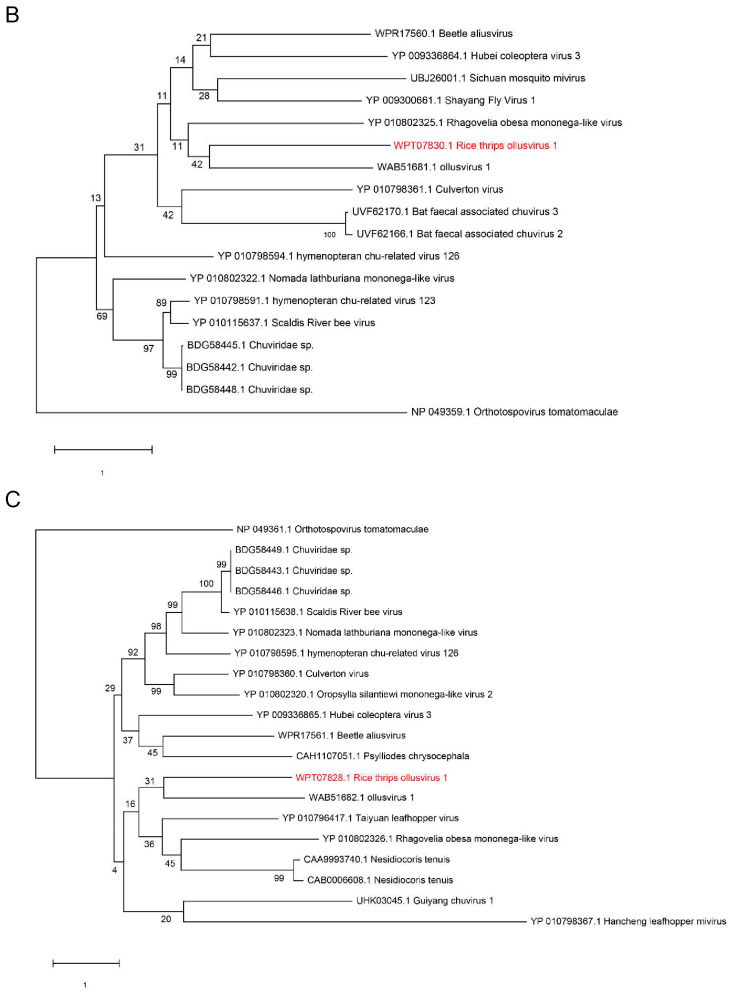
For a full insight into the evolution of RTOV1, phylogenetic trees of the viral Gp, N and RdRp proteins were generated, respectively. As illustrated in Figure 2A, RTOV1 RdRp is on a branch with ollusvirus 1 of the genus Ollusvirus in a phylogenetic tree based on the jingchuviral RdRp protein sequences. The N protein tree indicated that RTOV1 was related to ollusvirus 1 (Figure 2B). Similarly, the RTOV1 GP was found to be closely related to ollusvirus 1 (Figure 2C).
Figure 2.
Phylogenetic analysis of RTOV1 and related jingchuvirals based on the putative amino sequences for RdRp (A), nucleoproteins (B) and glycoproteins (C). Phylogenetic trees were constructed using the maximum likelihood method, with bootstrap values calculated for 1000 replicates. The best-fit models were the LG (Le and Gascual) + G (gamma distributed) + F (Fregs.) model for the RdRp and nucleoprotein tree and the WAG (Whelan and Goldman) + G + F model for the glycoprotein tree. The RTOV1 isolate identified in this study is indicated by red text. RdRp, nucleoproteins and glycoproteins of a typical negative-sense RNA virus, Tomato spotted wilt virus (TSWV), were used as the outgroups. The scale bars represent percentage divergence.
In addition, a BLASTp search of the NCBI reference viral sequence database was performed to identify the viruses related to RTOV1. The results showed that the ORF1 (Gp) sequence shared the highest sequence similarity with Beetle aliusvirus (GenBank: WPR17560.1) of the family Aliusviridae with 21.74% amino acid sequence identity. With 21.61% amino acid sequence identity, the N protein sequence showed the highest sequence similarity to ollusvirus 1. Similarly, the RTOV1 RdRp protein sequences exhibited the highest sequence similarity to Osmia-associated bee chuvirus (OABV-49, also named Chuviridae sp. GenBank: BDG58444.1), a novel Ollusvirus identified in wild bees, O. taurus (32.09% amino acid sequence identity) (Supplementary Table S2) [28].
To investigate the conserved motifs of the viral RdRp, we identified five conserved motifs in the RdRp sequences of RTOV1 and the four homologous Ollusviruses using the default parameters of MEME (Version 5.5.5, https://meme-suite.org/meme/tools/meme; accessed on 20 January 2024) (Supplementary Figure S1). In conclusion, the aforementioned results suggest that the newly identified RTOV1 could be added to the family Aliusviridae.
3.4. Activation of Antiviral RNA Interference Pathway in H. aculeatus Responsive to ISVs
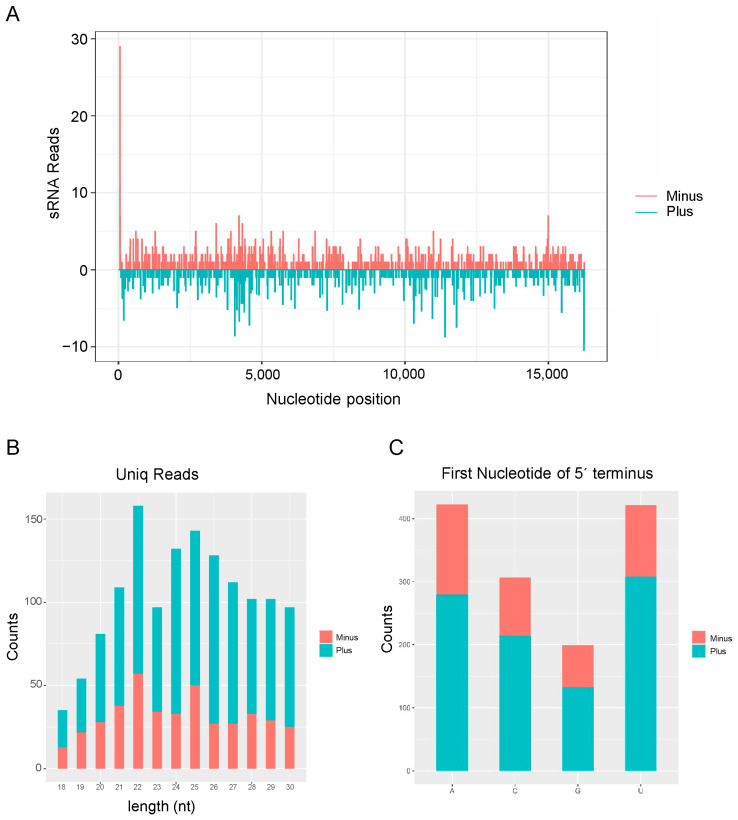
RNA silencing serves as a crucial antiviral immune response in insects, playing a significant role in the elimination of viruses [29,30]. Viral dsRNA is cleaved by the insect RNase III enzyme Dicer-2 (Dcr2), and subsequently generates large amounts of virus-derived siRNAs (vsiRNAs) in the host [31]. To investigate siRNA-based antiviral immunity in response to the RTOV1 infection of H. aculeatus, small RNAs (sRNAs) of the host insect were sequenced, and the vsiRNAs were comprehensively analysed. A total of 2,699,871 vsiRNA reads, including 1350 unique reads, were perfectly mapped to the assembled RTOV1 genome sequence (Figure 3A). Of these vsiRNAs, more were derived from the positive-sense strand than from the negative-sense strand of the viral genome (Figure 3B). To provide further analyses of the types of these vsiRNAs, it was shown that vsiRNAs from the viral positive-sense strand of the viral genome were predominantly 22 nt in length (Figure 3B).
Figure 3.
Virus-derived small interfering RNAs (vsiRNAs) of RTOV1. (A) Distribution of RTOV1 siRNAs alongside the viral genome. Cyan and pink represent siRNAs derived from the sense (plus strand) and antisense (minus strand) genomic strands of RTOV1, respectively. (B) Size distribution of RTOV1 siRNAs. (C) Five’-terminal nucleotide percentage of RTOV1 siRNAs.
The vsiRNAs displayed a clear preference for A/U at their 5′-terminal nucleotides and were evenly spread throughout the viral genome (Figure 3A,C). Notably, although the RTOV1 vsiRNAs were distributed throughout the viral genome, there was an extremely high abundance at the 5′-terminal nucleotides (Figure 3A). These results suggest that RTOV1 replicated in H. aculeatus, and viral RNA was cleaved by the host antiviral RNA interference pathway into predominantly 22 nt-sized small RNAs.
4. Discussion
Many RNA viruses have been discovered through the application of HTS and sophisticated bioinformatics techniques, leading to an enhanced comprehension of insect viromes and the evolutionary dynamics of viruses. As an important agricultural pest, thrips can transmit multiple viruses, leading to the occurrence of viral diseases. Western flower thrips, Frankliniella occidentalis Pergande, primarily cause the spread of viruses belonging to the genus Orthotospovirus (e.g., Tomato spotted wilt virus and Tomato chlorotic spot virus) and Tobacco streak virus (TSV; genus Ilarvirus) [32,33]. Thrips palm Karny can cause the harm of viruses like Watermelon silver mottle virus (WSMoV; genus Orthotospovirus), Peanut bud necrosis virus (PBNV; genus Orthotospovirus) and Muskmelon yellow spot virus (MYSV; genus Orthotospovirus) [34,35]. Thrips tabaci Lindeman play a crucial role in the prevalence of diseases, such as Tobacco ringspot virus (TRSV; genus Nepovirus, family Secoviridae), Sowbane mosaic virus (SoMV; genus Sobemovirus) and Iris yellow spot virus (IYSV; genus Orthotospovirus) [36,37,38]. All the thrips species that transmit plant viruses belong to the family Thripidae and the subfamily Thripinae. In this paper, we identified a new virus belonging to the genus Ollusvirus in family Phlaeothripidae and named it RTOV1. At present, 15 species of thrips have been confirmed to transmit viruses, accounting for just 0.2% of the total number of recorded thrips species [39]. In the future, investigating its virulence in the host, host range, and potential transmission to rice would not only deepen our understanding of the diversity and evolution of ISVs in insects, but also have significant implications for pest management strategies. Additionally, this would help uncover the potential reasons behind the damage caused to rice by thrip species.
The N, Gp and RdRp proteins are conserved throughout negative-sense RNA viruses, except for a few viruses where the Gp protein is lost during long-term evolution [1]. From three phylogenetic tree of these proteins, both of them show close phylogenetic relationships with ollusvirus 1. However, according to the results obtained from BLASTp analysis, only the N protein exhibited more homology with ollusvirus 1. The Gp and RdRp proteins showed strong homology with Beetle aliusvirus and Chuviridae sp, respectively. Due to a recently discovered ISV, there are a few research reports on Beetle aliusvirus [40]. According to the information provided in the GenBank database, this virus belongs to the order Jingchuvirales and the family Aliusviridae. Chuviridae sp, also known as Osmia-associated bee chuvirus (OABV-49), is an ISV belonging to the family Aliusviridae and the genus Ollusvirus [27]. According to the BLASTp data, we added this novel virus into the family Aliusviridae. Considering the evolutionary tree relationships, we further categorised this new virus into the genus Ollusvirus. Notably, the RdRp protein demonstrated a strong phylogenetic affinity and homology with Ollusviruses.
The abundance of virus-induced small RNAs intuitively reflects the strength of the host’s antiviral RNA interference response and, to some extent, indicates the level of viral proliferation in the host. The typical characteristics of vsiRNAs strongly suggest that the host antiviral RNAi plays an active role in responding to RTOV1 infection. More vsiRNAs originate from the positive strand rather than the negative strand (Figure 3B). Such results are likely due to the negative-sense RNA virus genome (minus strand) being protected by the N protein, thereby reducing cleavage by the RNAi pathway. The complementary strand (plus strand), however, may be extensively cleaved due to the lack of protection [41]. Interestingly, the vsiRNAs of TSWV generated from Frankliniella fusca (family Thripidae) show a higher abundance of 22 nt sRNAs compared to 21 nt [42]. As shown in Figure 3B, we also observed a higher quantity of 22 nt vsiRNAs from RTOV1 compared to 21 nt vsiRNAs. Although H. aculeatus and F. fusca do not belong to the same family, they both seem to mediate the abundant production of 22 nt vsiRNAs. We speculate that this may be due to a specific RNA cleavage mechanism in thrips.
5. Conclusions
In conclusion, a novel Ollusvirus was identified in H. aculeatu. The full-length genome of RTOV1 was revealed using metatranscriptome sequencing and RACE technology. To the best of our knowledge and the consulted literature, this is the first Ollusvirus identified in family Phlaeothripidae [3]. RTOV1 is 16,282 nt long and encodes three major viral proteins (RdRp, N and Gp). The host’s antiviral RNAi mechanism can target this virus, leading to the accumulation of abundant 21–24 nt vsiRNAs. The discovery of this new Ollusvirus not only enriches our understanding of insect microbial composition, but also helps reveal the diversity, evolution and ecological significance of insect viruses, which are crucial for agricultural production and ecosystem health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects15050303/s1. Figure S1: Multiple sequence alignment and motifs position of L proteins of RTOV1 and closely related Ollusviruses; Table S1: Primers used in this study; Table S2: Amino acid/nucleotide identity analysis of RTOV1 based on the conserved amino acid and nucleotide sequences; File S1: The COI sequence of Haplothrips aculeatus; File S2: Three ORFs amino acid sequence of RTOV1; File S3: The full genome sequence of RTOV1.
Author Contributions
Conceptualisation, H.H., J.L. and X.X.; methodology, X.Y., H.H. and G.L.; software, Z.H., Z.Y. and K.F.; validation, Z.Y., K.F. and G.L.; formal analysis, H.H., Z.Y., X.S., X.Y. and S.X.; investigation, S.X., H.H., X.S., M.Z., Z.H. and S.J.; resources, B.W., J.L. and X.X.; data curation, H.H., M.Z., Z.Y. and G.L.; writing—original draft preparation, H.H.; writing—review and editing, S.J., H.H. and G.L.; visualisation, H.H., Z.Y. and K.F.; supervision, J.L. and X.X.; project administration, B.W., J.L. and X.X.; funding acquisition, H.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant no. 32202271) and Agricultural scientific and technological innovation project of Shandong Academy of Agricultural Sciences (grant no. CXGC2022E04).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Li C.X., Shi M., Tian J.H., Lin X.D., Kang Y.J., Chen L.J., Qin X.C., Xu J., Holmes E.C., Zhang Y.Z. Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. eLife. 2015;4:1–26. doi: 10.7554/eLife.05378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edgar R.C., Taylor B., Lin V., Altman T., Barbera P., Meleshko D., Lohr D., Novakovsky G., Buchfink B., Al-Shayeb B., et al. Petabase-scale sequence alignment catalyses viral discovery. Nature. 2022;602:142–147. doi: 10.1038/s41586-021-04332-2. [DOI] [PubMed] [Google Scholar]
- 3.Qi Y.H., Ye Z.X., Zhang C.X., Chen J.P., Li J.M. Diversity of RNA viruses in agricultural insects. Comput. Struct. Biotechnol. J. 2023;21:4312–4321. doi: 10.1016/j.csbj.2023.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Almeida J.P., Aguiar E.R., Armache J.N., Olmo R.P., Marques J.T. The virome of vector mosquitoes. Curr. Opin. Virol. 2021;49:7–12. doi: 10.1016/j.coviro.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Koonin E.V., Krupovic M., Dolja V.V. The global virome: How much diversity and how many independent origins? Environ. Microbiol. 2023;25:40–44. doi: 10.1111/1462-2920.16207. [DOI] [PubMed] [Google Scholar]
- 6.Bonning B.C. The Insect Virome: Opportunities and Challenges. Curr. Issues Mol. Biol. 2020;34:1–12. doi: 10.21775/cimb.034.001. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho V.L., Long M.T. Insect-Specific Viruses: An overview and their relationship to arboviruses of concern to humans and animals. Virology. 2021;557:34–43. doi: 10.1016/j.virol.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Kormelink R., Garcia M.L., Goodin M., Sasaya T., Haenni A.L. Negative-strand RNA viruses: The plant-infecting counterparts. Virus Res. 2011;162:184–202. doi: 10.1016/j.virusres.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 9.Wolf Y., Krupovic M., Zhang Y.Z., Maes P., Dolja V., Koonin E.V., Kuhn J.H. Megataxonomy of Negative-Sense RNA Viruses. International Committee for Taxonomy of Viruses; London, UK: 2017. Proposal (Taxoprop). No. 2017.006M. [Google Scholar]
- 10.Kuhn J.H., Dheilly N.M., Junglen S., Paraskevopoulou S., Shi M., Di Paola N. ICTV Virus Taxonomy Profile: Jingchuvirales 2023. J. Gen Virol. 2023;104:001924. doi: 10.1099/jgv.0.001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi M., Lin X.D., Tian J.H., Chen L.J., Chen X., Li C.X., Qin X.C., Li J., Cao J.P., Eden J.S., et al. Redefining the invertebrate RNA virosphere. Nature. 2016;540:539–543. doi: 10.1038/nature20167. [DOI] [PubMed] [Google Scholar]
- 12.Wu H.M., Pang R., Cheng T., Xue L., Zeng H.Y., Lei T., Chen M.T., Wu S., Ding Y., Zhang J.M., et al. Abundant and Diverse RNA Viruses in Insects Revealed by RNA-Seq Analysis: Ecological and Evolutionary Implications. mSystems. 2020;5:1–14. doi: 10.1128/mSystems.00039-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pakrashi A., Patidar A., Singha D., Kumar V., Tyagi K. Comparative analysis of the two suborders of Thysanoptera and characterization of the complete mitochondrial genome of Thrips parvispinus. Arch. Insect Biochem. Physiol. 2023;114:1–15. doi: 10.1002/arch.22010. [DOI] [PubMed] [Google Scholar]
- 14.Mound L.A. Thysanoptera: Diversity and interactions. Annu. Rev. Entomol. 2005;50:247–269. doi: 10.1146/annurev.ento.49.061802.123318. [DOI] [PubMed] [Google Scholar]
- 15.Widana Gamage S.M.K., Rotenberg D., Schneweis D.J., Tsai C.W., Dietzgen R.G. Transcriptome-wide responses of adult melon thrips (Thrips palmi) associated with capsicum chlorosis virus infection. PLoS ONE. 2018;13:e0208538. doi: 10.1371/journal.pone.0208538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu G., Ye Z.X., He Y.J., Zhang Y., Wang X., Huang H.J., Zhuo J.C., Sun Z.T., Yan F., Chen J.P., et al. Discovery of Two Novel Negeviruses in a Dungfly Collected from the Arctic. Viruses. 2020;12:692. doi: 10.3390/v12070692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q., et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. 2011;17:10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 19.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R. 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative genomics viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren P.P., Ye Z.X., Wang S.N., Li J.M., Chen J.P., Zhang C.X., Lu J.B. Complete genome analysis of a novel chuvirus from a southern green stink bug (Nezara viridula) Arch. Virol. 2022;167:2423–2427. doi: 10.1007/s00705-022-05560-1. [DOI] [PubMed] [Google Scholar]
- 23.Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darriba D., Posada D., Kozlov A.M., Stamatakis A., Morel B., Flouri T. ModelTest-NG: A New and Scalable Tool for the Selection of DNA and Protein Evolutionary Models. Mol. Biol. Evol. 2020;37:291–294. doi: 10.1093/molbev/msz189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozlov A.M., Darriba D., Flouri T., Morel B., Stamatakis A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics. 2019;35:4453–4455. doi: 10.1093/bioinformatics/btz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Y.J., Ye Z.X., Zhang C.X., Li J.M., Chen J.P., Lu G. An RNA Virome Analysis of the Pink-Winged Grasshopper Atractomorpha sinensis. Insects. 2022;14:9. doi: 10.3390/insects14010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takemae H., Nunomura Y., Yokota T., Oba M., Mizutani T., Hsu W.L., Sakamoto Y. Novel ollusvirus detected in a solitary wild bee species (Osmia taurus) in Japan. Arch. Virol. 2023;168:183. doi: 10.1007/s00705-023-05805-7. [DOI] [PubMed] [Google Scholar]
- 29.Bonning B.C., Saleh M.C. The Interplay Between Viruses and RNAi Pathways in Insects. Annu. Rev. Entomol. 2021;66:61–79. doi: 10.1146/annurev-ento-033020-090410. [DOI] [PubMed] [Google Scholar]
- 30.Brennecke J., Aravin A.A., Stark A., Dus M., Kellis M., Sachidanandam R., Hannon G.J. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–1103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 31.Naganuma M., Tadakuma H., Tomari Y. Single-molecule analysis of processive double-stranded RNA cleavage by Drosophila Dicer-2. Nat. Commun. 2021;12:4268. doi: 10.1038/s41467-021-24555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rotenberg D., Jacobson A.L., Schneweis D.J., Whitfield A.E. Thrips transmission of tospoviruses. Curr. Opin. Virol. 2015;15:80–89. doi: 10.1016/j.coviro.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Muppudathi S.P., Natarajan G., Varagur Ganesan M., Sevugapperumal N., Subbarayalu M., John Samuel K., Perumal R. Role of Thrips palmi and Parthenium hysterophorus pollen in active spread of tobacco streak virus in the cotton ecosystem. Virus Res. 2020;284:197979. doi: 10.1016/j.virusres.2020.197979. [DOI] [PubMed] [Google Scholar]
- 34.Mou D.F., Chen W.T., Li W.H., Chen T.C., Tseng C.H., Huang L.H., Peng J.C., Yeh S.D., Tsai C.W. Transmission mode of watermelon silver mottle virus by Thrips palmi. PLoS ONE. 2021;16:e0247500. doi: 10.1371/journal.pone.0247500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daimei G., Raina H.S., Devi P.P., Saurav G.K., Renukadevi P., Malathi V.G., Senthilraja C., Mandal B., Rajagopal R. Influence of Groundnut bud necrosis virus on the Life History Traits and Feeding Preference of Its Vector, Thrips palmi. Phytopathology. 2017;107:1440–1445. doi: 10.1094/PHYTO-08-16-0296-R. [DOI] [PubMed] [Google Scholar]
- 36.Messieha M. Transmission of tobacco ringspot virus by thrips. Phytopathology. 1969;59:943–945. [PubMed] [Google Scholar]
- 37.Martin R.R., MacFarlane S., Sabanadzovic S., Quito D., Poudel B., Tzanetakis I.E. Viruses and Virus Diseases of Rubus. Plant Dis. 2013;97:168–182. doi: 10.1094/PDIS-04-12-0362-FE. [DOI] [PubMed] [Google Scholar]
- 38.Leach A., Fuchs M., Harding R., Nault B.A. Iris Yellow Spot Virus Prolongs the Adult Lifespan of Its Primary Vector, Onion Thrips (Thrips tabaci) (Thysanoptera: Thripidae) J. Insect Sci. 2019;19:8. doi: 10.1093/jisesa/iez041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mound L.A., Wang Z., Lima É.F.B., Marullo R. Problems with the Concept of “Pest” among the Diversity of Pestiferous Thrips. Insects. 2022;13:61. doi: 10.3390/insects13010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.French R.K., Anderson S.H., Cain K.E., Greene T.C., Minor M., Miskelly C.M., Montoya J.M., Wille M., Muller C.G., Taylor M.W., et al. Host phylogeny shapes viral transmission networks in an island ecosystem. Nat. Ecol. Evol. 2023;7:1834–1843. doi: 10.1038/s41559-023-02192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo M., Terrell J.R., Mcmanus S.A. Nucleocapsid Structure of Negative Strand RNA Virus. Viruses. 2020;12:835. doi: 10.3390/v12080835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fletcher S.J., Shrestha A., Peters J.R., Carroll B.J., Srinivasan R., Pappu H.R., Mitter N. The Tomato Spotted Wilt Virus Genome Is Processed Differentially in its Plant Host Arachis hypogaea and its Thrips Vector Frankliniella fusca. Front. Plant Sci. 2016;7:1349. doi: 10.3389/fpls.2016.01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available within the article and Supplementary Materials.