Abstract
Background/Objective: Sex-related differences among patients with aneurysmal subarachnoid hemorrhage (aSAH) and their potential clinical implications have been insufficiently investigated. To address this knowledge gap, we conduct a comprehensive systematic review and meta-analysis. Methods: Sex-specific differences in patients with aSAH, including mortality, delayed cerebral ischemia (DCI), and functional outcomes were assessed. The functional outcome was dichotomized into favorable or unfavorable based on the modified Rankin Scale (mRS), Glasgow Outcome Scale (GOS), and Glasgow Outcome Scale Extended (GOSE). Results: Overall, 2823 studies were identified in EMBASE, MEDLINE, PubMed, and by manual search on 14 February 2024. After an initial assessment, 74 studies were included in the meta-analysis. In the analysis of mortality, including 18,534 aSAH patients, no statistically significant differences could be detected (risk ratio (RR) 0.99; 95% CI, 0.90–1.09; p = 0.91). In contrast, the risk analysis for DCI, including 23,864 aSAH patients, showed an 11% relative risk reduction in DCI in males versus females (RR, 0.89; 95% CI, 0.81–0.97; p = 0.01). The functional outcome analysis (favorable vs. unfavorable), including 7739 aSAH patients, showed a tendency towards better functional outcomes in men than women; however, this did not reach statistical significance (RR, 1.02; 95% CI, 0.98–1.07; p = 0.34). Conclusions: In conclusion, the available data suggest that sex/gender may play a significant role in the risk of DCI in patients with aSAH, emphasizing the need for sex-specific management strategies.
Keywords: sex differences, aneurysmal subarachnoid hemorrhage, mortality, delayed cerebral ischemia, functional outcome
1. Introduction
Subarachnoid hemorrhage (SAH) is a type of stroke that accounts for approximately 5% of all strokes [1]. Despite its low frequency, it is associated with high mortality and morbidity rates, including long-term cognitive impairment and reduced quality of life [2]. Aneurysmal subarachnoid hemorrhage (aSAH) is responsible for 85% of nontraumatic SAH cases and occurs when an aneurysm ruptures [1]. Since patients with aSAH are usually younger than patients with ischemic stroke and are still workers at the time of the bleeding, aSAH represents a global economic burden on society and patients [3].
Sex-associated differences in the epidemiology of aSAH are well known. Females suffer more frequently from aSAH than men at all ages [4]. Some possible reasons for this include the vulnerability of the walls of the blood vessels, the interference of collagen and elastin, and hormonal influences that may contribute to the formation of aneurysms in women [5,6]. Additionally, some risk factors, such as smoking, can increase the likelihood of aneurysm rupture more significantly in women [7,8]. Furthermore, differences in aneurysm location have been described. Men are more likely to have aneurysms in the anterior cerebral artery, while in females, aneurysms are mainly located along the internal carotid artery [9,10,11].
On the other hand, less is known about sex differences in the frequency of delayed cerebral ischemia (DCI), functional outcomes, and mortality in aSAH patients. The results of the available literature, in fact, are often contradictory [12,13,14]. Some studies have found that being female is associated with a higher risk of poor outcomes following aSAH, including a higher 30-day case-fatality rate [15] and poorer 2-year outcomes compared to men [16,17,18]. However, other studies have reported that sex is not a determining factor for outcome following aSAH [12,19,20]. DCI is a common complication that occurs in almost 30% of patients after aSAH [21], and it is a significant predictor of unfavorable outcomes [22,23]. While some studies have suggested that women are more likely to experience DCI than men [14,24], others have concluded that there is limited evidence of a sex difference [25].
Understanding the relationship between sex and mortality, DCI, and functional outcomes in patients with aSAH is crucial to developing appropriate and personalized interventions. For example, the identification of patients at higher risk for complications, such as DCI, could have consequences for resource utilization, such as the frequency of clinical and radiological controls to prevent and detect them early.
Hence, we performed a systematic review and meta-analysis of existing data focusing on sex-related differences in mortality, frequency of DCI, and functional outcomes in patients with aSAH.
2. Methods
PRISMA guidelines (Preferred Reporting of Items in Systematic Reviews and Meta-analyses) were employed to guide review processes [26]. This systematic review was registered at The International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42024508960).
2.1. Search Strategy
Studies were identified in the MEDLINE, EMBASE, and PubMed electronic databases. The search was performed on 14 February 2024. The search strategy combined three concepts: (1) gender, sex, sex difference, sex ratio, women, men, female, and male, as well as Medical Subject Heading related; (2) terms related to critical care (i.e., intensive care, intensive care unit, ICU, critically ill patient), and terms related to our outcomes of interest (i.e., mortality, delayed cerebral ischemia, modified Rankin Scale, Glasgow Outcome Scale, Glasgow Outcome Scale Extended), as well as Medical Subject Heading related; and (3) terms related to aneurysmal subarachnoid hemorrhage (i.e., subarachnoid hemorrhage, brain hemorrhage, brain bleeding, brain artery aneurysm rupture, hemorrhagic stroke), as well as Medical Subject Heading related.
The electronic search was supplemented by a manual search of reference lists and recent reviews.
Two reviewers (SB and GB) independently screened titles and abstracts using the platform Covidence. Duplicates were excluded. Secondly, they screened the corresponding publications in full text to assess if the studies met the inclusion criteria. The software notified the reviewers if there were discrepancies, and they were solved through discussion. If consensus could not be reached, a third reviewer mediated to resolve the conflict.
2.2. Inclusion and Exclusion Criteria
The study selection criteria are presented in Table 1 using the PICOS (Population, Interventions, Comparisons, Outcomes, and Study Design) acronym.
Table 1.
Inclusion criteria: Scope of the literature review in the PICOS form. aSAH: aneurysmal subarachnoid hemorrhage; DCI: delayed cerebral ischemia.
| Criteria | Definition |
|---|---|
| Population |
|
| Interventions |
|
| Comparison |
|
| Outcomes |
|
| Study Design |
|
All identified studies were reported using a flowchart according to PRISMA guidelines.
Studies in adult patients with aSAH, which included any of the following outcomes of interest, were considered for eligibility: mortality, DCI, and/or functional outcomes. We assessed the most commonly used functional outcomes in aSAH clinical trials [27], including the modified Rankin Scale (mRS) [28,29], the Glasgow Outcome Scale (GOS) [30], and the Glasgow Outcome Scale Extended (GOSE) [31,32].
Based on the mRS, GOS, and GOSE, the functional outcome was dichotomized into “favorable” or “unfavorable”. A favorable outcome was defined as mRS 0–2, GOS 4–5, and GOSE 5–8. In many clinical trials and in most of the studies included in this analysis (15 of 18 studies), the functional outcome is dichotomized for the analysis into “favorable” and “unfavorable”. Considering the variability in studies regarding the score classified as a favorable or unfavorable outcome in the mRS, we referred to the definition of the European Stroke Organization, where an mRS score of 0–2 is considered favorable [33].
For our analysis, we used only those studies that defined DCI as clinical deterioration (a new focal neurologic deficit or decrease in level of consciousness) deemed secondary to vasospasm and/or a new cerebral infarct after excluding other possible causes [34,35]. The included studies employed various methods to detect vasospasm, such as computerized tomography angiography, a magnetic resonance perfusion scan, transcranial Doppler, or digital subtraction angiography. Cerebral infarction was determined using computed tomography or magnetic resonance imaging. We excluded from our analysis studies that considered asymptomatic vasospasm as a part of their definition of DCI.
Randomized controlled trials (RCTs), prospective observational studies, and retrospective studies with more than 10 patients and clinical registries were eligible. Only studies written in English were included and published from the year 2000 onwards.
Studies that did not distinguish outcomes according to sex or did not include any of the outcomes of interest were not considered eligible. Case reports, case series with less than 10 patients, animal studies, abstracts, and reports with no values in the results were excluded.
2.3. Data Extraction
The trial’s characteristics (first author and publication year); type of study; the number of patients included; the number of females/males; selected outcome results (mortality, DCI, functional outcomes, as assessed with the mRS, GOS, or GOSE); and time of measurement were extracted and summarized using a pre-defined Excel Table.
2.4. Statistical Analysis
We calculated a pooled estimate of the risk ratio (RR) with a 95% confidence interval (95%-CI) for each dichotomous outcome (mortality, DCI, dichotomized functional outcomes (favorable/unfavorable)) by sex. The decision to calculate the RR was driven by the dichotomous nature of the three outcomes and the high proportion of cohort studies in our analysis. Additionally, the RR has the advantage of being easier to interpret compared to the odds ratio [36,37,38]. Each trial-specific effect size was subsequently combined across studies in order to calculate summary estimates and presented as a forest plot. We evaluated heterogeneity by the chi-squared test and calculated I2 [39]. To further estimate the effect size of future studies with similar settings, we applied prediction intervals [40]. The Mantel–Haenszel test was used to construct the random effects model [41].
The presence of publication bias was explored with a funnel plot. All statistical analyses were performed using R, version 4.3.0 [42].
3. Results
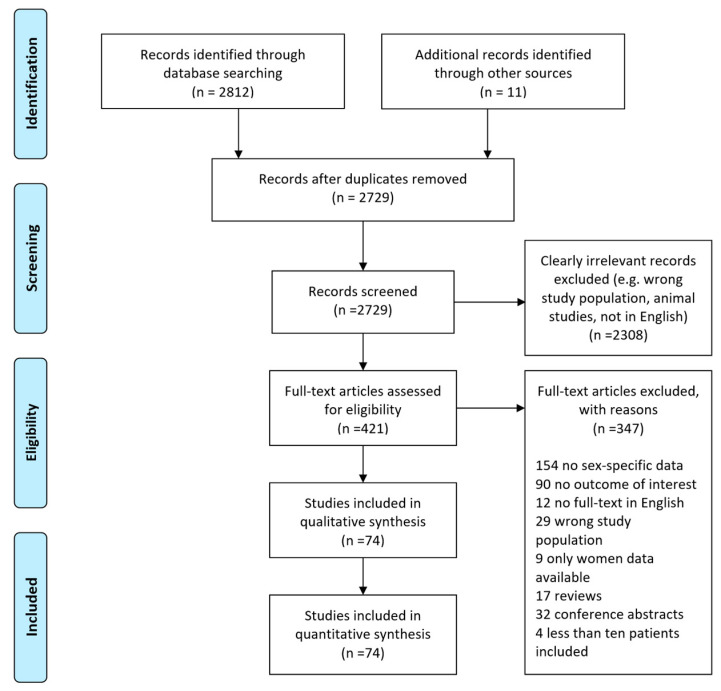
The systematic search identified 2812 studies. By manual search, 11 additional studies were identified. Overall, 2823 abstracts were considered as potentially eligible. After screening based on the inclusion criteria, 421 were selected for full-text review (see flow diagram in Figure 1). Finally, a total of 74 studies were included in the quantitative synthesis (meta-analysis).
Figure 1.
Study selection flow diagram according to PRISMA guidelines.
The list and characteristics of the included studies are presented in Supplementary Tables S1–S3.
3.1. Mortality
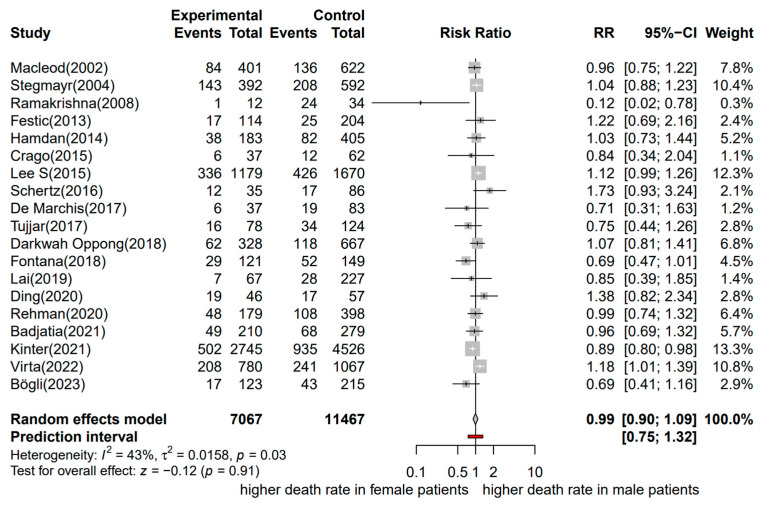
Nineteen studies, referring to 18,534 patients with aSAH, were included in the meta-analysis (with 7067 male and 11,467 female patients) [12,13,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59]. Mortality was evaluated at different time points, from in-hospital mortality/mortality at the intensive care unit (ICU) to 12 months after aSAH. However, most of the studies evaluated mortality as in-hospital mortality or at 30 days (12 of 19 studies). The included studies were published between 2002 and 2023.
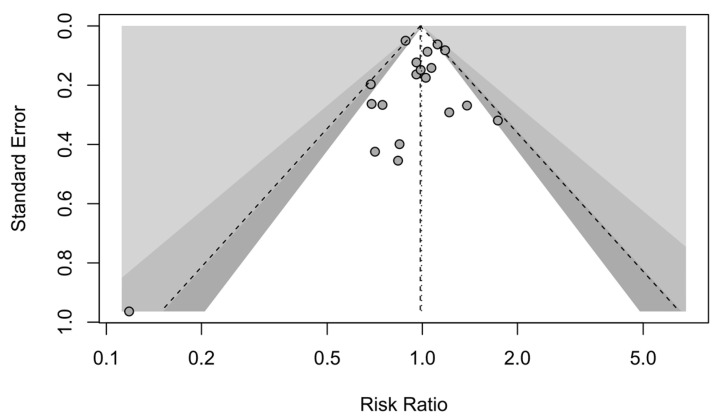
A forest plot of the stratified analysis showed no statistically significant difference in mortality between male and female patients with aSAH (RR, 0.99; 95% CI, 0.90–1.09; p = 0.91). Heterogeneity testing showed I2 = 43% and p = 0.03. The prediction interval ranged from g = 0.75 to 1.32 (see Figure 2). The assessment of publication bias using a contour-enhanced funnel plot indicated symmetry, and most of the data corresponded to points within the 95% CI, as shown in Figure 3.
Figure 2.
Forest plot of mortality in patients with aSAH analyzed by sex. The risk ratio for mortality at the end of follow-up. Comparison between male and female patients with aneurysmal subarachnoid hemorrhage [12,13,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59]. CI, confidence interval; RR, risk ratio.
Figure 3.
Contour-enhanced funnel plot. Mortality studies with contour levels of 0.9, 0.95, and 0.99, respectively.
3.2. Delayed Cerebral Ischemia
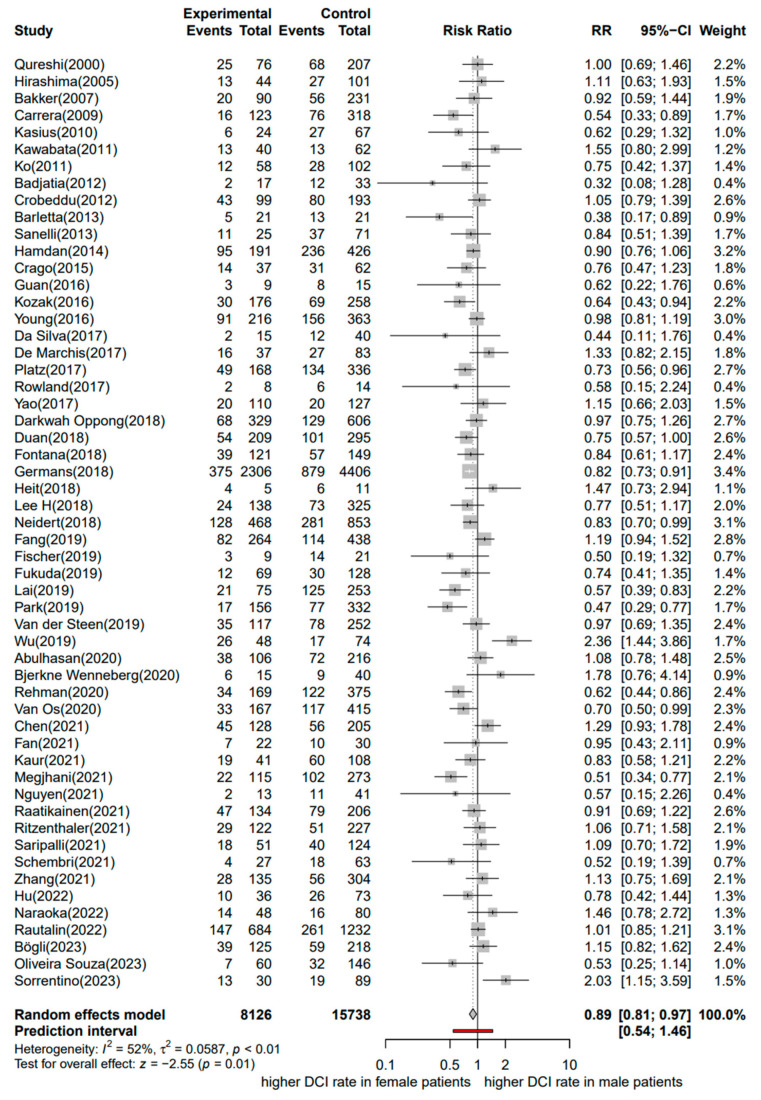
Fifty-five studies including 23,864 patients with aSAH were considered in the meta-analysis with DCI as the outcome of interest (8126 males and 15,738 females) [12,13,14,24,44,45,46,49,54,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104]. These studies were published between 2000 and 2023.
A forest plot of the stratified analysis demonstrated a significantly higher risk for DCI in females than in males after aSAH. Male patients had a 0.89-fold lower risk of DCI than female patients (11% relative risk reduction; RR, 0.89; 95% CI, 0.81–0.97; p = 0.01). Heterogeneity testing showed I2 = 52% and p < 0.01. The prediction interval ranged from g = 0.54 to 1.46 (see Figure 4). The assessment of publication bias using a contour-enhanced funnel plot indicated asymmetry, and most of the data corresponded to points within the 95% CI, as shown in Figure 5. Asymmetry suggests the possibility of either publication bias or a systematic difference between studies of higher and lower precision.
Figure 4.
Forest plot of DCI in patients with aSAH analyzed by sex. The risk ratio for DCI. Comparison between male and female patients with aneurysmal subarachnoid hemorrhage [12,13,14,24,44,45,46,49,54,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104]. CI, confidence interval; DCI, delayed cerebral ischemia; RR, risk ratio.
Figure 5.
Contour-enhanced funnel plot. DCI studies with contour levels of 0.9, 0.95, and 0.99, respectively.
3.3. Functional Outcomes
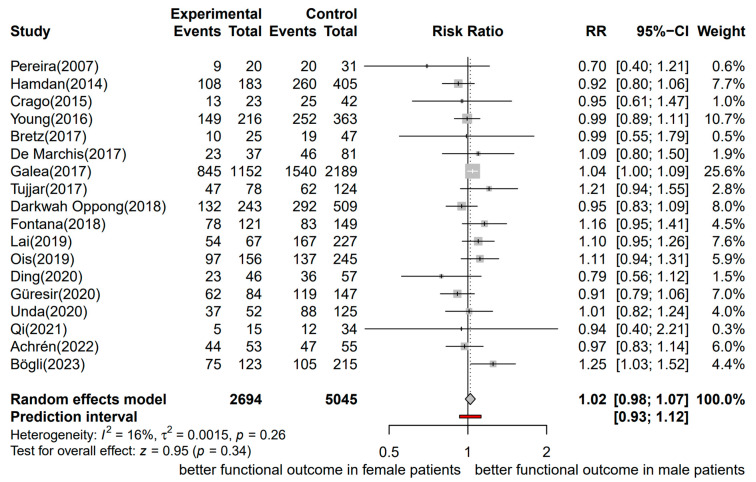
Eighteen studies, including 7739 patients with aSAH, were considered in the meta-analysis with the functional outcome as the outcome of interest (2694 were males and 5045 females) [12,13,44,45,46,47,49,57,59,103,105,106,107,108,109,110,111,112]. The functional outcome was assessed using the mRS in 11 studies, including 3380 patients. The GOS was used in six studies, including 4021 patients, and the GOSE was used in one study, including 338 patients. The time of assessment of the functional outcome varied from hospital discharge to 18 months. However, in most of the studies, the functional outcome was assessed at 3 to 6 months after aSAH (12 of 18 studies). The included studies were published between 2007 and 2023.
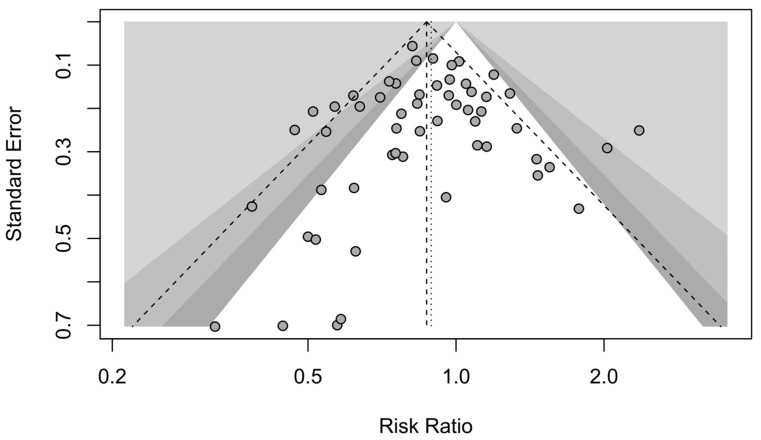
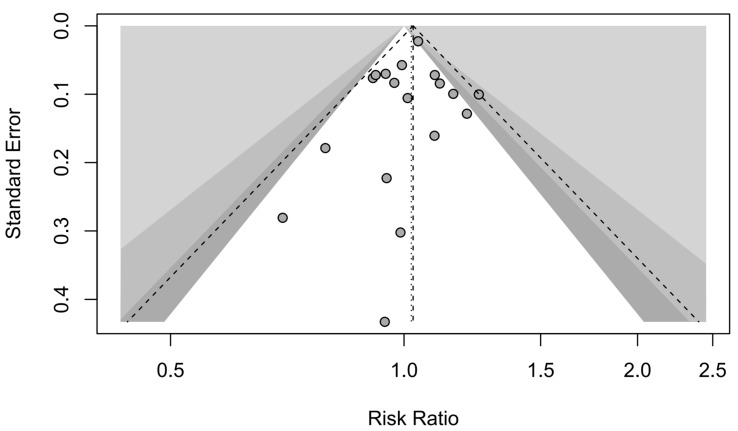
A forest plot of the stratified analysis showed a trend for a better dichotomized functional outcome in male patients versus female patients after aSAH; however, this was not statistically significant (RR, 1.02; 95% CI, 0.98–1.07; p = 0.34). Heterogeneity testing showed I2 = 16% and p = 0.26. The prediction interval ranged from g = 0.93 to 1.12 (see Figure 6). The assessment of publication bias using a funnel plot indicated asymmetry, and all the data corresponded to points within the 95% CI, as shown in Figure 7. Asymmetry suggests the possibility of either publication bias or a systematic difference between studies of higher and lower precision.
Figure 6.
Forest plot of the functional outcome in patients with aSAH analyzed by sex, assessed by the mRS, GOS, and GOSE. The risk ratio for functional outcomes. Comparison between male and female patients with aneurysmal subarachnoid hemorrhage [12,13,44,45,46,47,49,57,59,103,105,106,107,108,109,110,111,112]. CI, confidence interval; RR, risk ratio.
Figure 7.
Contour-enhanced funnel plot. Functional outcome studies with contour levels of 0.9, 0.95, and 0.99, respectively.
4. Discussion
We conducted a systematic review and meta-analysis to update the available evidence on sex-related differences in mortality, risk of DCI, and functional outcomes in patients with aSAH. Overall, 74 studies were included in the analysis, including a sample of 18,534 patients for the outcome mortality, 23,864 patients for DCI, and 7739 for functional outcomes. According to our results, we found that the mortality risk after aSAH is similar in females and males. In terms of functional outcomes, males showed a tendency towards better outcomes than females; however, they did not reach statistical significance. Interestingly, males had a significantly lower risk of developing DCI than females.
4.1. Results in Context
To our knowledge, our systematic review is the first one that evaluates at the same time the sex differences in risk of DCI, functional outcomes, and mortality in patients with aSAH. This was performed to improve the characterization of these outcomes and assess their relevance since patients with DCI have a higher risk of mortality [113] and worse outcomes [114]. Interestingly, contrary to expectations, despite women having a higher risk of DCI, no higher mortality or significantly worse functional outcomes were found in women.
Recently, Rehman et al. performed a systematic review and meta-analysis to investigate if sex is a predictor for DCI in patients with aSAH [54]. In contrast to this previous work, we did not consider asymptomatic vasospasm as DCI; instead, we included only those studies that defined DCI as a clinical deterioration deemed secondary to vasospasm and/or a new cerebral infarct after excluding other possible causes [34,35]. In our opinion, this choice makes the comparisons among studies more homogenous. Additionally, it is important to note that asymptomatic vasospasm does not have the same negative impact on outcomes as symptomatic vasospasm [115], so its relevance to clinical practice may not be as significant. Nevertheless, consistent with the previous findings, we found a higher risk for DCI among women than men. This higher risk for DCI development in women could be a factor to consider for more efficient utilization of resources, which could imply the need for differentiated management where women may require more intensive neuromonitoring and more frequent radiological controls to prevent and detect DCI.
The reason why women are more likely to develop DCI than men is not completely understood. Contrary to the results of experimental studies [116,117], a possible effect of the sexual hormones on the Doppler blood flow velocities in cerebral vessels in patients with aSAH could not be demonstrated [118]. Furthermore, sex-related differences in the management and delivery of care of patients with aSAH to the disadvantage of women—as already shown in other medical conditions—could play a role in the development of DCI, and these should be further investigated [119,120,121].
Considering mortality and functional outcomes, we did not find significant differences between women and men. Some considerations are needed to interpret these findings. Women are generally older when they suffer from aSAH [122,123], and older age is well-known as a determinant for poor clinical outcomes in patients with aSAH [124,125]. Only based on this, one might expect a higher mortality and a worse functional outcome for women. On the other hand, however, we do not know whether women and men in the study population had the same severity of aSAH and the same intensity of treatment. In addition, patients with aSAH often die after a redirection of care to palliation. This could be another factor since sex-related differences in frequency and the kind of limitations of life-sustaining therapies have already been reported [126]. In non-neuro-intensive care settings, the female sex has been found to be associated with a higher likelihood of limitation of life-sustaining therapies [127]. Furthermore, men are more likely to receive intensive care at the end of life, while women are more likely to state a preference for the limitation of life-sustaining therapies [128,129,130,131]. Given that the causes of death were not present in most of the included publications, we are unable to give a solid explanation for the relation between mortality and sex in patients with aSAH.
Since most of the identified studies evaluated the functional outcome in a dichotomized way (favorable vs. unfavorable), we also decided to maintain the same dichotomization in our analysis. However, analyses of trials using such dichotomous approaches could result in a loss of information and a risk of ignoring bi-directional effects, and they often require larger samples than ordinal approaches [132,133,134]. Despite these pitfalls of dichotomization, ordinal analyses continue to be poorly adopted [135,136], and dichotomous approaches continue to be favored as the primary outcome by many high-profile trials [137,138]. One reason for this may be the poor clinical interpretability of conventional ordinal approaches, which provide outputs, like p-values or standard odds ratios, without intuitive effect sizes [139].
We quantified statistical heterogeneity using prediction intervals, in addition to the chi-squared test, and calculated I². Prediction intervals also offer an estimate of where the true effects can be expected for future studies with similar characteristics [40]. Our analysis found that for mortality, DCI, and functional outcomes, the prediction intervals contained the null effect value, indicating that sex may not be a significant factor in some situations [140,141].
4.2. Implications for Practice and for Research
Our research highlights a higher risk of DCI in women, which could imply a need for stricter monitoring at hospitals in those patients, including serial daily transcranial Doppler measurements and the insertion of multimodal neuromonitoring for the early detection of DCI. On the other hand, men with lower risk might require less frequent monitoring of DCI in terms of imaging or be transferred to a regular ward bed or discharged more quickly than women. Therefore, understanding the differences between male and female patients with aSAH can help optimize intensive care resources, as demand often exceeds supply. Furthermore, the early detection of DCI in women can have a positive impact on patients and the community, as DCI patients are often discharged to rehabilitation due to their worse functional status [114], which could lead to increased costs for the health system.
Although our study did not evaluate sex differences between cardiovascular risk factors, it is important to note that studies have shown that women are more severely impacted by cardiovascular risk factors [7]. For instance, smoking has a three-fold higher impact on women than men in the development of aSAH [8]. Therefore, it may be necessary to intensify care in the preventive and educational management of risk factors in women.
In addition, previous studies have suggested that there might be an unconscious gender bias in critical care units, which could lead to less aggressive treatment for women [126]. This bias has been also observed in critically ill patients with cardio- and neurovascular diseases in a large nationwide cohort in Switzerland, where women were less likely to receive ICU treatment, regardless of the severity of their condition [120]. Therefore, it is crucial for intensivists and emergency physicians to carefully reassess whether critically ill women are at risk of not receiving adequate care. To ensure equal application of intensive therapy, we need to address gender biases in algorithms of triage and local protocols. Thus, including a standardized protocol for patients with aSAH and an interdisciplinary approach in neurocritical care units could help minimize the impact of any potential gender bias on medical decisions [142].
In terms of research, as new information is continuously discovered regarding the relationship between sex and aneurysm/SAH outcomes, it is important for studies to include sex as a predictor variable in their analyses and examine sex-specific effects of interventions. This is especially important in randomized trials of medical therapies and interventions, where biologically significant relationships might exist between sex and interventions [9].
Regarding DCI, further research should focus on identifying the factors responsible for its development in women, such as vascular structure, hormones, or genetics. To establish sex-specific management guidelines for aneurysmal subarachnoid hemorrhage (aSAH), there is a need for prospective studies and clinical trials to examine sex differences in aSAH management and outcomes, including complications.
4.3. Limitations/Strengths
This review has some limitations that should be considered while interpreting the findings. First, this review mainly included retrospective studies. Therefore, there could be some missing data or unadjusted data that might have affected the analysis. Second, the outcomes of interest in the included studies were evaluated at different follow-up times, ranging from ICU/hospital discharge to 18 months. Third, there was low to moderate heterogeneity in some outcomes, which could be due to differences in baseline characteristics between males and females along with variations in sample sizes. Fourth, sex-related differences in risk factors, such as smoking and hypertension, for aSAH could not be assessed. Fifth, we limited the assessment of sex differences to mortality, DCI, and functional outcome. Therefore, other outcomes, such as acute kidney injury, intensive care outcomes (i.e., need for tracheostomy, vasoactive drugs, renal replacement therapy, etc.), and subjective quality of life (QoL) measures, should also be considered. Regarding the number of limitations, our findings should be interpreted cautiously.
However, this review has also several strengths. First, according to our knowledge, this is the first systematic review that focuses at the same time on the association between sex and the outcomes of interest (mortality, DCI, and functional outcome) in patients with aSAH, providing the most up-to-date evidence that is based on a large number of patients included in our analysis. Second, we adopted strict inclusion criteria, particularly for the definition of DCI, despite the use of several terms/definitions across various studies, which posed a challenge. According to this, we excluded studies that did not provide a clear definition of DCI or those that involved asymptomatic vasospasm. In our opinion, this choice leads to results that are more homogeneous. Third, our analysis of functional outcome in terms of mRS only considered studies that defined a Modified Rankin Scale score of 0–2 as favorable, as recommended by the European Stroke Organization. As a result, our findings are more comparable and based on more homogeneous studies.
4.4. Conclusions
In conclusion, this study updates the available data on sex-related differences in patients with aSAH considering clinical outcomes. The results indicate that female patients are more likely to experience DCI after aSAH than males. However, there were no significant differences between the two sexes in terms of mortality and functional outcomes. Hence, as women represent a higher risk group for DCI, we suggest that they may require more intensive neuromonitoring during hospitalization.
We found that only a limited number of studies had sufficient gender data to include in our meta-analysis. Therefore, we suggest that more prospective studies with a focus on gender analysis are needed to obtain more robust results regarding sex differences in the clinical outcomes of aSAH patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13102781/s1, Table S1. Characteristics of the included studies for the mortality analysis. Table S2 Characteristics of the included studies for the delayed cerebral ischemia analysis. Table S3. Characteristics of the included studies for the functional outcome analysis.
Author Contributions
Conceptualization, S.B. and G.B.; methodology, S.B. and G.B.; validation, S.B., M.B., A.P. and G.B.; formal analysis, M.B. and A.P.; investigation, S.B. and G.B.; resources, S.B.; data curation, S.B. and G.B; writing—original draft preparation, S.B. and G.B.; writing—review and editing, S.B., M.B., E.K, G.E., A.P. and G.B.; visualization, S.B. and M.B.; supervision, E.K. and G.B.; project administration, S.B. and G.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All studies in this review have been approved by the appropriate ethics committee and have, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data from previously published studies, in which informed consent was obtained, were retrieved and analyzed.
Conflicts of Interest
The authors declare no conflicts of interest. The authors have no competing interests to declare that are relevant to the content of this article.
Consent for Publication
All of the authors of this manuscript have been included, and we warrant that nobody who qualifies for authorship has been excluded. We agree to its submission to the Journal of Clinical Medicine and, if accepted, to its publication in this journal.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.van Gijn J., Kerr R.S., Rinkel G.J. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- 2.Suarez J.I., Tarr R.W., Selman W.R. Aneurysmal subarachnoid hemorrhage. N. Engl. J. Med. 2006;354:387–396. doi: 10.1056/NEJMra052732. [DOI] [PubMed] [Google Scholar]
- 3.Seule M., Oswald D., Muroi C., Brandi G., Keller E. Outcome, Return to Work and Health-Related Costs after Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care. 2020;33:49–57. doi: 10.1007/s12028-019-00905-2. [DOI] [PubMed] [Google Scholar]
- 4.Turan N., Heider R.A., Zaharieva D., Ahmad F.U., Barrow D.L., Pradilla G. Sex Differences in the Formation of Intracranial Aneurysms and Incidence and Outcome of Subarachnoid Hemorrhage: Review of Experimental and Human Studies. Transl. Stroke Res. 2016;7:12–19. doi: 10.1007/s12975-015-0434-6. [DOI] [PubMed] [Google Scholar]
- 5.Handa H., Hashimoto N., Nagata I., Hazama F. Saccular cerebral aneurysms in rats: A newly developed animal model of the disease. Stroke. 1983;14:857–866. doi: 10.1161/01.str.14.6.857. [DOI] [PubMed] [Google Scholar]
- 6.Vajda J. Multiple intracranial aneurysms: A high risk condition. Acta Neurochir. 1992;118:59–75. doi: 10.1007/bf01400727. [DOI] [PubMed] [Google Scholar]
- 7.Feigin V.L., Rinkel G.J., Lawes C.M., Algra A., Bennett D.A., van Gijn J., Anderson C.S. Risk factors for subarachnoid hemorrhage: An updated systematic review of epidemiological studies. Stroke. 2005;36:2773–2780. doi: 10.1161/01.STR.0000190838.02954.e8. [DOI] [PubMed] [Google Scholar]
- 8.Lindekleiv H., Sandvei M., Njølstad I., Løchen M.-L., Romundstad P., Vatten L., Ingebrigtsen T., Vik A., Mathiesen E. Sex differences in risk factors for aneurysmal subarachnoid hemorrhage: A cohort study. Neurology. 2011;76:637–643. doi: 10.1212/WNL.0b013e31820c30d3. [DOI] [PubMed] [Google Scholar]
- 9.Fuentes A.M., Stone McGuire L., Amin-Hanjani S. Sex Differences in Cerebral Aneurysms and Subarachnoid Hemorrhage. Stroke. 2022;53:624–633. doi: 10.1161/STROKEAHA.121.037147. [DOI] [PubMed] [Google Scholar]
- 10.Aarhus M., Helland C.A., Wester K. Differences in anatomical distribution, gender, and sidedness between ruptured and unruptured intracranial aneurysms in a defined patient population. Acta Neurochir. 2009;151:1569–1574. doi: 10.1007/s00701-009-0316-3. [DOI] [PubMed] [Google Scholar]
- 11.Ghods A.J., Lopes D., Chen M. Gender differences in cerebral aneurysm location. Front. Neurol. 2012;3:78. doi: 10.3389/fneur.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamdan A., Barnes J., Mitchell P. Subarachnoid hemorrhage and the female sex: Analysis of risk factors, aneurysm characteristics, and outcomes. J. Neurosurg. 2014;121:1367–1373. doi: 10.3171/2014.7.JNS132318. [DOI] [PubMed] [Google Scholar]
- 13.Oppong M.D., Iannaccone A., Gembruch O., Pierscianek D., Chihi M., Dammann P., Köninger A., Müller O., Forsting M., Sure U., et al. Vasospasm-related complications after subarachnoid hemorrhage: The role of patients’ age and sex. Acta Neurochir. 2018;160:1393–1400. doi: 10.1007/s00701-018-3549-1. [DOI] [PubMed] [Google Scholar]
- 14.Germans M.R., Jaja B.N.R., de Oliviera Manoel A.L., Cohen A.H., Macdonald R.L. Sex differences in delayed cerebral ischemia after subarachnoid hemorrhage. J. Neurosurg. 2018;129:458–464. doi: 10.3171/2017.3.JNS162808. [DOI] [PubMed] [Google Scholar]
- 15.Nieuwkamp D.J., Vaartjes I., Algra A., Bots M.L., Rinkel G.J. Age- and gender-specific time trend in risk of death of patients admitted with aneurysmal subarachnoid hemorrhage in the Netherlands. Int. J. Stroke. 2013;8((Suppl. A100)):90–94. doi: 10.1111/ijs.12006. [DOI] [PubMed] [Google Scholar]
- 16.Chotai S., Ahn S.-Y., Moon H.-J., Kim J.-H., Chung H.-S., Chung Y.-G., Kwon T.-H. Prediction of outcomes in young adults with aneurysmal subarachnoid hemorrhage. Neurol. Med. Chir. 2013;53:157–162. doi: 10.2176/nmc.53.157. [DOI] [PubMed] [Google Scholar]
- 17.Rivero Rodríguez D., Scherle Matamoros C., Fernández Cúe L., Miranda Hernández J.L., Pernas Sánchez Y., Pérez Nellar J. Factors associated with poor outcome for aneurysmal subarachnoid haemorrhage in a series of 334 patients. Neurologia. 2017;32:15–21. doi: 10.1016/j.nrl.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Vergouwen M.D., Ilodigwe D., Macdonald R.L. Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke. 2011;42:924–929. doi: 10.1161/strokeaha.110.597914. [DOI] [PubMed] [Google Scholar]
- 19.Duijghuisen J.J., Greebe P., Nieuwkamp D.J., Algra A., Rinkel G.J. Sex-Related Differences in Outcome in Patients with Aneurysmal Subarachnoid Hemorrhage. J. Stroke Cerebrovasc. Dis. 2016;25:2067–2070. doi: 10.1016/j.jstrokecerebrovasdis.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Rosengart A.J., Schultheiss K.E., Tolentino J., Macdonald R.L. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2315–2321. doi: 10.1161/strokeaha.107.484360. [DOI] [PubMed] [Google Scholar]
- 21.Rowland M.J., Hadjipavlou G., Kelly M., Westbrook J., Pattinson K.T. Delayed cerebral ischaemia after subarachnoid haemorrhage: Looking beyond vasospasm. Br. J. Anaesth. 2012;109:315–329. doi: 10.1093/bja/aes264. [DOI] [PubMed] [Google Scholar]
- 22.van der Harst J.J., Luijckx G.R., Elting J.W.J., Lammers T., Bokkers R.P.H., Bergh W.M.v.D., Eshghi O.S., Metzemaekers J.D.M., Groen R.J.M., Mazuri A., et al. The predictive value of the CTA Vasospasm Score on delayed cerebral ischaemia and functional outcome after aneurysmal subarachnoid hemorrhage. Eur. J. Neurol. 2022;29:620–625. doi: 10.1111/ene.15139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dodd W.S., Laurent D., Dumont A.S., Hasan D.M., Jabbour P.M., Starke R.M., Hosaka K., Polifka A.J., Hoh B.L., Chalouhi N. Pathophysiology of Delayed Cerebral Ischemia after Subarachnoid Hemorrhage: A Review. J. Am. Heart Assoc. 2021;10:e021845. doi: 10.1161/jaha.121.021845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan W., Pan Y., Wang C., Wang Y., Zhao X., Liu L. Risk Factors and Clinical Impact of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage: Analysis from the China National Stroke Registry. Neuroepidemiology. 2018;50:128–136. doi: 10.1159/000487325. [DOI] [PubMed] [Google Scholar]
- 25.de Rooij N.K., Rinkel G.J., Dankbaar J.W., Frijns C.J. Delayed cerebral ischemia after subarachnoid hemorrhage: A systematic review of clinical, laboratory, and radiological predictors. Stroke. 2013;44:43–54. doi: 10.1161/strokeaha.112.674291. [DOI] [PubMed] [Google Scholar]
- 26.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pace A., Mitchell S., Casselden E., Zolnourian A., Glazier J., Foulkes L., Bulters D., Galea I. A subarachnoid haemorrhage-specific outcome tool. Brain. 2018;141:1111–1121. doi: 10.1093/brain/awy003. [DOI] [PubMed] [Google Scholar]
- 28.Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott. Med. J. 1957;2:200–215. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 29.Farrell B., Godwin J., Richards S., Warlow C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: Final results. J. Neurol. Neurosurg. Psychiatry. 1991;54:1044–1054. doi: 10.1136/jnnp.54.12.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jennett B., Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 31.Jennett B., Snoek J., Bond M.R., Brooks N. Disability after severe head injury: Observations on the use of the Glasgow Outcome Scale. J. Neurol. Neurosurg. Psychiatry. 1981;44:285–293. doi: 10.1136/jnnp.44.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson J.T., Pettigrew L.E., Teasdale G.M. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. J. Neurotrauma. 1998;15:573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 33.Bath P.M., Lees K.R., Schellinger P.D., Altman H., Bland M., Hogg C., Howard G., Saver J.L. Statistical analysis of the primary outcome in acute stroke trials. Stroke. 2012;43:1171–1178. doi: 10.1161/strokeaha.111.641456. [DOI] [PubMed] [Google Scholar]
- 34.Frontera J.A., Fernandez A., Schmidt J.M., Claassen J., Wartenberg K.E., Badjatia N., Connolly E.S., Mayer S.A. Defining vasospasm after subarachnoid hemorrhage: What is the most clinically relevant definition? Stroke. 2009;40:1963–1968. doi: 10.1161/strokeaha.108.544700. [DOI] [PubMed] [Google Scholar]
- 35.Vergouwen M.D., Vermeulen M., van Gijn J., Rinkel G.J., Wijdicks E.F., Muizelaar J.P., Mendelow A.D., Juvela S., Yonas H., Terbrugge K.G., et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke. 2010;41:2391–2395. doi: 10.1161/strokeaha.110.589275. [DOI] [PubMed] [Google Scholar]
- 36.Simon S.D. Understanding the odds ratio and the relative risk. J. Androl. 2001;22:533–536. doi: 10.1002/j.1939-4640.2001.tb02212.x. [DOI] [PubMed] [Google Scholar]
- 37.Knol M.J., Algra A., Groenwold R.H. How to deal with measures of association: A short guide for the clinician. Cerebrovasc. Dis. 2012;33:98–103. doi: 10.1159/000334180. [DOI] [PubMed] [Google Scholar]
- 38.Alavi M., Hunt G.E., Visentin D.C., Watson R., Thapa D.K., Cleary M. Using risk and odds ratios to assess effect size for meta-analysis outcome measures. J. Adv. Nurs. 2020;76:3231–3234. doi: 10.1111/jan.14528. [DOI] [PubMed] [Google Scholar]
- 39.Cumpston M., Li T., Page M., Chandler J., Welch V., Higgins J.P., Thomas J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borenstein M. Avoiding common mistakes in meta-analysis: Understanding the distinct roles of Q, I-squared, tau-squared, and the prediction interval in reporting heterogeneity. Res. Synth. Methods. 2023;15:354–368. doi: 10.1002/jrsm.1678. [DOI] [PubMed] [Google Scholar]
- 41.Mantel N., Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 42.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badjatia N., Ryan A., Choi H.A., Parikh G.Y., Jiang X., Day A.G., Heyland D.K. Relationship between Nutrition Intake and Outcome after Subarachnoid Hemorrhage: Results From the International Nutritional Survey. J. Intensive Care Med. 2021;36:1141–1148. doi: 10.1177/0885066620966957. [DOI] [PubMed] [Google Scholar]
- 44.Bögli S.Y., Beham S., Hirsbrunner L., Nellessen F., Casagrande F., Keller E., Brandi G. Sex-specific extracerebral complications in patients with aneurysmal subarachnoid hemorrhage. Front. Neurol. 2023;14:1098300. doi: 10.3389/fneur.2023.1098300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crago E.A., Sherwood P.R., Bender C., Balzer J., Ren D., Poloyac S.M. Plasma Estrogen Levels Are Associated with Severity of Injury and Outcomes after Aneurysmal Subarachnoid Hemorrhage. Biol. Res. Nurs. 2015;17:558–566. doi: 10.1177/1099800414561632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Marchis G.M., Schaad C., Fung C., Beck J., Gralla J., Takala J., Jakob S.M. Gender-related differences in aneurysmal subarachnoid hemorrhage: A hospital based study. Clin. Neurol. Neurosurg. 2017;157:82–87. doi: 10.1016/j.clineuro.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Ding C.Y., Cai H.P., Ge H.L., Yu L.H., Lin Y.X., Kang D.Z. Is Admission Lipoprotein-Associated Phospholipase A2 a Novel Predictor of Vasospasm and Outcome in Patients with Aneurysmal Subarachnoid Hemorrhage? Neurosurgery. 2020;86:122–131. doi: 10.1093/neuros/nyz041. [DOI] [PubMed] [Google Scholar]
- 48.Festic E., Rabinstein A.A., Freeman W.D., Mauricio E.A., Robinson M.T., Mandrekar J., Zubair A.C., Lee A.S., Gajic O. Blood transfusion is an important predictor of hospital mortality among patients with aneurysmal subarachnoid hemorrhage. Neurocrit. Care. 2013;18:209–215. doi: 10.1007/s12028-012-9777-y. [DOI] [PubMed] [Google Scholar]
- 49.Fontana V., Bond O., Spadaro S., Annoni F., Nobile L., Badenes R., Volta C.A., Vincent J.-L., Creteur J., Taccone F.S. Red Cell Distribution Width after Subarachnoid Hemorrhage. J. Neurosurg. Anesth. 2018;30:319–327. doi: 10.1097/ana.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 50.Kinter K.J., Alfaro R., Kinter C., Suder L., Davis Z., Rodriguez P., Ruiz J.G., Zevallos J.C., Elkbuli A. The Effects of Body Mass Index on In-hospital mortality following first ischemic or hemorrhagic stroke events: Does the “obesity paradox” apply? Ann. Med. Surg. 2021;70:102839. doi: 10.1016/j.amsu.2021.102839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S.H., Song K.J., Shin S.D., Ro Y.S., Kim M.J., Holmes J.F. The Relationship between Clinical Outcome in Subarachnoidal Hemorrhage Patients with Emergency Medical Service Usage and Interhospital Transfer. J. Korean Med. Sci. 2015;30:1889–1895. doi: 10.3346/jkms.2015.30.12.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macleod M.R., Andrews P.J. Effect of deprivation and gender on the incidence and management of acute brain disorders. Intensive Care Med. 2002;28:1729–1734. doi: 10.1007/s00134-002-1519-8. [DOI] [PubMed] [Google Scholar]
- 53.Ramakrishna R., Stiefel M., Udoteuk J., Spiotta A., Levine J.M., Kofke W.A., Zager E., Yang W., LeRoux P. Brain oxygen tension and outcome in patients with aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2008;109:1075–1082. doi: 10.3171/jns.2008.109.12.1075. [DOI] [PubMed] [Google Scholar]
- 54.Rehman S., Chandra R.V., Zhou K., Tan D., Lai L., Asadi H., Froelich J., Thani N., Nichols L., Blizzard L., et al. Sex differences in aneurysmal subarachnoid haemorrhage (aSAH): Aneurysm characteristics, neurological complications, and outcome. Acta Neurochir. 2020;162:2271–2282. doi: 10.1007/s00701-020-04469-5. [DOI] [PubMed] [Google Scholar]
- 55.Schertz M., Mehdaoui H., Hamlat A., Piotin M., Banydeen R., Mejdoubi M. Incidence and Mortality of Spontaneous Subarachnoid Hemorrhage in Martinique. PLoS ONE. 2016;11:e0155945. doi: 10.1371/journal.pone.0155945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stegmayr B., Eriksson M., Asplund K. Declining mortality from subarachnoid hemorrhage: Changes in incidence and case fatality from 1985 through 2000. Stroke. 2004;35:2059–2063. doi: 10.1161/01.STR.0000138451.07853.b6. [DOI] [PubMed] [Google Scholar]
- 57.Tujjar O., Belloni I., Hougardy J.-M., Scolletta S., Vincent J.-L., Creteur J., Taccone F.S. Acute Kidney Injury after Subarachnoid Hemorrhage. J. Neurosurg. Anesth. 2017;29:140–149. doi: 10.1097/ana.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 58.Virta J.J., Skrifvars M., Reinikainen M., Bendel S., Laitio R., Hoppu S., Ala-Kokko T., Siironen J., Raj R. Trends in Mortality after Intensive Care of Patients with Aneurysmal Subarachnoid Hemorrhage in Finland in 2003–2019: A Finnish Intensive Care Consortium study. Neurocrit. Care. 2022;37:447–454. doi: 10.1007/s12028-021-01420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lai P.M.R., Gormley W.B., Patel N., Frerichs K.U., Aziz-Sultan M.A., Du R. Age-Dependent Radiographic Vasospasm and Delayed Cerebral Ischemia in Women after Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2019;130:e230–e235. doi: 10.1016/j.wneu.2019.06.040. [DOI] [PubMed] [Google Scholar]
- 60.Abulhasan Y.B., Ortiz Jimenez J., Teitelbaum J., Simoneau G., Angle M.R. Milrinone for refractory cerebral vasospasm with delayed cerebral ischemia. J. Neurosurg. 2020;134:971–982. doi: 10.3171/2020.1.jns193107. [DOI] [PubMed] [Google Scholar]
- 61.Badjatia N., Seres D., Carpenter A., Schmidt J.M., Lee K., Mayer S.A., Claassen J., Connolly E.S., Elkind M.S. Free Fatty acids and delayed cerebral ischemia after subarachnoid hemorrhage. Stroke. 2012;43:691–696. doi: 10.1161/strokeaha.111.636035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bakker A.M., Dorhout Mees S.M., Algra A., Rinkel G.J. Extent of acute hydrocephalus after aneurysmal subarachnoid hemorrhage as a risk factor for delayed cerebral infarction. Stroke. 2007;38:2496–2499. doi: 10.1161/strokeaha.107.484220. [DOI] [PubMed] [Google Scholar]
- 63.Barletta J.F., Figueroa B.E., DeShane R., Blau S.A., McAllen K.J. High glucose variability increases cerebral infarction in patients with spontaneous subarachnoid hemorrhage. J. Crit. Care. 2013;28:798–803. doi: 10.1016/j.jcrc.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 64.Wenneberg S.B., Hendén P.M.L., Oras J., Naredi S., Block L., Ljungqvist J., Hergès H.O. Heart rate variability monitoring for the detection of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Acta Anaesthesiol. Scand. 2020;64:945–952. doi: 10.1111/aas.13582. [DOI] [PubMed] [Google Scholar]
- 65.Carrera E., Schmidt J.M., Oddo M., Fernandez L., Claassen J., Seder D., Lee K., Badjatia N., Connolly E.S., Mayer S.A. Transcranial Doppler for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Neurosurgery. 2009;65:316–323. doi: 10.1227/01.neu.0000349209.69973.88. [DOI] [PubMed] [Google Scholar]
- 66.Chen L., Pandey S., Shen R., Xu Y., Zhang Q. Increased Systemic Immune-Inflammation Index Is Associated with Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage Patients. Front. Neurol. 2021;12:745175. doi: 10.3389/fneur.2021.745175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crobeddu E., Mittal M.K., Dupont S., Wijdicks E.F., Lanzino G., Rabinstein A.A. Predicting the lack of development of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke. 2012;43:697–701. doi: 10.1161/strokeaha.111.638403. [DOI] [PubMed] [Google Scholar]
- 68.Da Silva I.R.F., Gomes J.A., Wachsman A., de Freitas G.R., Provencio J.J. Hematologic counts as predictors of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J. Crit. Care. 2017;37:126–129. doi: 10.1016/j.jcrc.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan B.B., Sun X.C., Huang Z.J., Yang X.M., Guo Z.D., He Z.H. Hypoperfusion assessed by pressure reactivity index is associated with delayed cerebral ischemia after subarachnoid hemorrhage: An observational study. Chin. Neurosurg. J. 2021;7:16. doi: 10.1186/s41016-021-00231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fang Y., Mei S., Lu J., Chen Y., Chai Z., Dong X., Araujo C., Reis C., Zhang J., Chen S. New risk score of the early period after spontaneous subarachnoid hemorrhage: For the prediction of delayed cerebral ischemia. CNS Neurosci. Ther. 2019;25:1173–1181. doi: 10.1111/cns.13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fischer C., Goldberg J., Vulcu S., Wagner F., Schöni D., Söll N., Hänggi M., Schefold J., Fung C., Beck J., et al. Nimodipine-Induced Blood Pressure Changes Can Predict Delayed Cerebral Ischemia. Front. Neurol. 2019;10:1161. doi: 10.3389/fneur.2019.01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fukuda S., Koga Y., Fujita M., Suehiro E., Kaneda K., Oda Y., Ishihara H., Suzuki M., Tsuruta R. Hyperoxemia during the hyperacute phase of aneurysmal subarachnoid hemorrhage is associated with delayed cerebral ischemia and poor outcome: A retrospective observational study. J. Neurosurg. 2019;134:25–32. doi: 10.3171/2019.9.jns19781. [DOI] [PubMed] [Google Scholar]
- 73.Guan J., Karsy M., Brock A., Couldwell W.T. The Utility of Ankle-Brachial Index as a Predictor of Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2016;89:139–146. doi: 10.1016/j.wneu.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 74.Heit J.J., Wintermark M., Martin B.W., Zhu G., Marks M.P., Zaharchuk G., Dodd R.L., Do H.M., Steinberg G.K., Lansberg M.G., et al. Reduced Intravoxel Incoherent Motion Microvascular Perfusion Predicts Delayed Cerebral Ischemia and Vasospasm after Aneurysm Rupture. Stroke. 2018;49:741–745. doi: 10.1161/strokeaha.117.020395. [DOI] [PubMed] [Google Scholar]
- 75.Hirashima Y., Kurimoto M., Hori E., Origasa H., Endo S. Lower incidence of symptomatic vasospasm after subarachnoid hemorrhage owing to ruptured vertebrobasilar aneurysms. Neurosurgery. 2005;57:1110–1116. doi: 10.1227/01.neu.0000185632.69374.c9. [DOI] [PubMed] [Google Scholar]
- 76.Hu P., Yang X., Li Y., Deng G., Xu Y., Ye L., Qi Y., Zong Z., Chen Q. Predictive effects of admission white blood cell counts and hounsfield unit values on delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Clin. Neurol. Neurosurg. 2022;212:107087. doi: 10.1016/j.clineuro.2021.107087. [DOI] [PubMed] [Google Scholar]
- 77.Kasius K.M., Frijns C.J., Algra A., Rinkel G.J. Association of platelet and leukocyte counts with delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. Cerebrovasc. Dis. 2010;29:576–583. doi: 10.1159/000306645. [DOI] [PubMed] [Google Scholar]
- 78.Kaur G., Damodara N., Feldstein E., Dominguez J., Huang K.T., Ogulnick J.V., Nuoman R., Khandelwal P., El-Ghanem M., Gupta G., et al. Relation between brain natriuretic peptide and delayed cerebral ischemia in patients with aneurysmalsubarachnoid hemorrhage. Clin. Neurol. Neurosurg. 2021;211:107031. doi: 10.1016/j.clineuro.2021.107031. [DOI] [PubMed] [Google Scholar]
- 79.Kawabata Y., Horikawa F., Ueno Y., Sawada M., Isaka F., Miyake H. Clinical predictors of delayed cerebral ischemia after subarachnoid hemorrhage: First experience with coil embolization in the management of ruptured cerebral aneurysms. J. Neurointerv. Surg. 2011;3:344–347. doi: 10.1136/jnis.2010.004077. [DOI] [PubMed] [Google Scholar]
- 80.Ko S.-B., Choi H.A., Carpenter A.M., Helbok R., Schmidt J.M., Badjatia N., Claassen J., Connolly E.S., Mayer S.A., Lee K. Quantitative analysis of hemorrhage volume for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Stroke. 2011;42:669–674. doi: 10.1161/strokeaha.110.600775. [DOI] [PubMed] [Google Scholar]
- 81.Kozak N., Bereczki D., Szabo S. Predictors of Symptomatic Vasospasm after Subarachnoid Hemorrhage: A Single Center Study of 457 Consecutive Cases. Turk. Neurosurg. 2016;26:545–549. doi: 10.5137/1019-5149.jtn.14408-15.1. [DOI] [PubMed] [Google Scholar]
- 82.Lee H., Perry J.J., English S.W., Alkherayf F., Joseph J., Nobile S., Zhou L.L., Lesiuk H., Moulton R., Agbi C., et al. Clinical prediction of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2018;130:1914–1921. doi: 10.3171/2018.1.jns172715. [DOI] [PubMed] [Google Scholar]
- 83.Megjhani M., Terilli K., Weiss M., Savarraj J., Chen L.H., Alkhachroum A., Roh D.J., Agarwal S., Connolly E.S., Velazquez A., et al. Dynamic Detection of Delayed Cerebral Ischemia: A Study in 3 Centers. Stroke. 2021;52:1370–1379. doi: 10.1161/strokeaha.120.032546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Naraoka M., Matsuda N., Shimamura N., Ohkuma H. Role of microcirculatory impairment in delayed cerebral ischemia and outcome after aneurysmal subarachnoid hemorrhage. J. Cereb. Blood Flow. Metab. 2022;42:186–196. doi: 10.1177/0271678x211045446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neidert M.C., Maldaner N., Stienen M.N., Roethlisberger M., Zumofen D.W., Marbacher S., Maduri R., Hostettler I.C., Schatlo B., Schneider M.M., et al. The Barrow Neurological Institute Grading Scale as a Predictor for Delayed Cerebral Ischemia and Outcome after Aneurysmal Subarachnoid Hemorrhage: Data From a Nationwide Patient Registry (Swiss SOS) Neurosurgery. 2018;83:1286–1293. doi: 10.1093/neuros/nyx609. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen A.M., Williamson C.A., Pandey A.S., Sheehan K.M., Rajajee V. Screening Computed Tomography Angiography to Identify Patients at Low Risk for Delayed Cerebral Ischemia Following Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 2021;12:740241. doi: 10.3389/fneur.2021.740241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Souza N.V.d.O., Rouanet C., Solla D.J.F., de Lima C.V.B., de Souza C.A., Rezende F., Alves M.M., Manuel A.L.d.O., Neto F.C., Frudit M., et al. The Role of VASOGRADE as a Simple Grading Scale to Predict Delayed Cerebral Ischemia and Functional Outcome after Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care. 2023;38:96–104. doi: 10.1007/s12028-022-01577-1. [DOI] [PubMed] [Google Scholar]
- 88.Park S., Megjhani M., Frey H.-P., Grave E., Wiggins C., Terilli K.L., Roh D.J., Velazquez A., Agarwal S., Connolly E.S., et al. Predicting delayed cerebral ischemia after subarachnoid hemorrhage using physiological time series data. J. Clin. Monit. Comput. 2019;33:95–105. doi: 10.1007/s10877-018-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Platz J., Güresir E., Wagner M., Seifert V., Konczalla J. Increased risk of delayed cerebral ischemia in subarachnoid hemorrhage patients with additional intracerebral hematoma. J. Neurosurg. 2017;126:504–510. doi: 10.3171/2015.12.jns151563. [DOI] [PubMed] [Google Scholar]
- 90.Qureshi A.I., Sung G.Y., Razumovsky A.Y., Lane K., Straw R.N., Ulatowski J.A. Early identification of patients at risk for symptomatic vasospasm after aneurysmal subarachnoid hemorrhage. Crit. Care Med. 2000;28:984–990. doi: 10.1097/00003246-200004000-00012. [DOI] [PubMed] [Google Scholar]
- 91.Raatikainen E., Vahtera A., Kuitunen A., Junttila E., Huhtala H., Ronkainen A., Pyysalo L., Kiiski H. Prognostic value of the 2010 consensus definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J. Neurol. Sci. 2021;420:117261. doi: 10.1016/j.jns.2020.117261. [DOI] [PubMed] [Google Scholar]
- 92.Rautalin I., Juvela S., Martini M.L., Macdonald R.L., Korja M. Risk Factors for Delayed Cerebral Ischemia in Good-Grade Patients with Aneurysmal Subarachnoid Hemorrhage. J. Am. Heart Assoc. 2022;11:e027453. doi: 10.1161/jaha.122.027453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ritzenthaler T., Gobert F., Bouchier B., Dailler F. Amount of blood during the subacute phase and clot clearance rate as prognostic factors for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J. Clin. Neurosci. 2021;87:74–79. doi: 10.1016/j.jocn.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 94.Rowland M.J., Garry P., Westbrook J., Corkill R., Antoniades C.A., Pattinson K.T.S. Acute impairment of saccadic eye movements is associated with delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2017;127:754–760. doi: 10.3171/2016.8.jns16408. [DOI] [PubMed] [Google Scholar]
- 95.Sanelli P., Anumula N., Johnson C., Comunale J., Tsiouris A., Riina H., Segal A., Stieg P., Zimmerman R., Mushlin A. Evaluating CT perfusion using outcome measures of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. AJNR Am. J. Neuroradiol. 2013;34:292–298. doi: 10.3174/ajnr.A3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saripalli M., Tan D., Chandra R.V., Lai L.T. Predictive Relevance of Early Temperature Elevation on the Risk of Delayed Cerebral Ischemia Development Following Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2021;150:e474–e481. doi: 10.1016/j.wneu.2021.03.031. [DOI] [PubMed] [Google Scholar]
- 97.Schembri M., Verbaan D., Emmer B.J., Coert B.A., Majoie C.B.L.M., Vandertop W.P., Berg R.v.D. Cerebral circulation time on DSA during endovascular treatment in WFNS grade I aneurysmal SAH patients-a predictor of DCI? Neuroradiology. 2021;63:2131–2138. doi: 10.1007/s00234-021-02749-0. [DOI] [PubMed] [Google Scholar]
- 98.Sorrentino Z.A., Desai A., Eisinger R.S., Maciel C.B., Busl K.M., Lucke-Wold B. Evaluating analgesic medications utilized in the treatment of aneurysmal subarachnoid hemorrhage and association with delayed cerebral ischemia. J. Clin. Neurosci. 2023;115:157–162. doi: 10.1016/j.jocn.2023.07.023. [DOI] [PubMed] [Google Scholar]
- 99.van der Steen W.E., Marquering H.A., Boers A.M., Ramos L.A., Berg R.v.D., Vergouwen M.D., Majoie C.B., Coert B.A., Vandertop W.P., Verbaan D., et al. Predicting Delayed Cerebral Ischemia with Quantified Aneurysmal Subarachnoid Blood Volume. World Neurosurg. 2019;130:e613–e619. doi: 10.1016/j.wneu.2019.06.170. [DOI] [PubMed] [Google Scholar]
- 100.van Os H.J., Ruigrok Y.M., Verbaan D., Dennesen P., Müller M.C., Coert B.A., Algra A., Vergouwen M.D., Wermer M.J. Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage in Patients with a History of Migraine. Stroke. 2020;51:3039–3044. doi: 10.1161/strokeaha.120.030118. [DOI] [PubMed] [Google Scholar]
- 101.Wu Y., He Q., Wei Y., Zhu J., He Z., Zhang X., Guo Z., Xu R., Cheng C., Huang Z., et al. The association of neutrophil-to-lymphocyte ratio and delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage: Possible involvement of cerebral blood perfusion. Neuropsychiatr. Dis. Treat. 2019;15:1001–1007. doi: 10.2147/ndt.s190477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yao P.S., Chen G.R., Zheng S.F., Kang D.Z. Predictors of Postoperative Cerebral Ischemia in Patients with Ruptured Anterior Communicating Artery Aneurysms. World Neurosurg. 2017;103:241–247. doi: 10.1016/j.wneu.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 103.Young J.B., Singh T.D., Rabinstein A.A., Fugate J.E. SSRI/SNRI Use is Not Associated with Increased Risk of Delayed Cerebral Ischemia after aSAH. Neurocrit. Care. 2016;24:197–201. doi: 10.1007/s12028-015-0190-1. [DOI] [PubMed] [Google Scholar]
- 104.Zhang X., Liu Y., Zhang S., Wang C., Zou C., Li A. Neutrophil-to-Albumin Ratio as a Biomarker of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2021;147:e453–e458. doi: 10.1016/j.wneu.2020.12.084. [DOI] [PubMed] [Google Scholar]
- 105.Bretz J.S., Von Dincklage F., Woitzik J., Winkler M.K.L., Major S., Dreier J.P., Bohner G., Scheel M. The Hijdra scale has significant prognostic value for the functional outcome of Fisher grade 3 patients with subarachnoid hemorrhage. Clin. Neuroradiol. 2017;27:361–369. doi: 10.1007/s00062-016-0509-0. [DOI] [PubMed] [Google Scholar]
- 106.Güresir E., Coch C., Fimmers R., Ilic I., Hadjiathanasiou A., Kern T., Brandecker S., Güresir Á., Velten M., Vatter H., et al. Initial inflammatory response is an independent predictor of unfavorable outcome in patients with good-grade aneurysmal subarachnoid hemorrhage. J. Crit. Care. 2020;60:45–49. doi: 10.1016/j.jcrc.2020.07.018. [DOI] [PubMed] [Google Scholar]
- 107.Ois A., Vivas E., Figueras-Aguirre G., Guimaraens L., Cuadrado-Godia E., Avellaneda C., Bertran-Recasens B., Rodríguez-Campello A., Gracia M.-P., Villalba G., et al. Misdiagnosis Worsens Prognosis in Subarachnoid Hemorrhage with Good Hunt and Hess Score. Stroke. 2019;50:3072–3076. doi: 10.1161/strokeaha.119.025520. [DOI] [PubMed] [Google Scholar]
- 108.Unda S.R., Labagnara K., Birnbaum J., Wong M., de Silva N., Terala H., Ramos R.d.l.G., Haranhalli N., Altschul D.J. Impact of hospital-acquired complications in long-term clinical outcomes after subarachnoid hemorrhage. Clin. Neurol. Neurosurg. 2020;194:105945. doi: 10.1016/j.clineuro.2020.105945. [DOI] [PubMed] [Google Scholar]
- 109.Achrén A., Raj R., Siironen J., Laakso A., Marjamaa J. Spontaneous angiogram-negative subarachnoid hemorrhage: A retrospective single center cohort study. Acta Neurochir. 2022;164:129–140. doi: 10.1007/s00701-021-05069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Galea J.P., Dulhanty L., Patel H.C. Predictors of Outcome in Aneurysmal Subarachnoid Hemorrhage Patients: Observations From a Multicenter Data Set. Stroke. 2017;48:2958–2963. doi: 10.1161/strokeaha.117.017777. [DOI] [PubMed] [Google Scholar]
- 111.Pereira A.R., Sanchez-Peña P., Biondi A., Sourour N., Boch A.L., Colonne C., Lejean L., Abdennour L., Puybasset L. Predictors of 1-year outcome after coiling for poor-grade subarachnoid aneurysmal hemorrhage. Neurocrit Care. 2007;7:18–26. doi: 10.1007/s12028-007-0053-5. [DOI] [PubMed] [Google Scholar]
- 112.Qi M., Jiang L., Xu Y., Qu X., Wang N., Chen W., Cheng W., Wang N. Risk Factors for Prognosis in Elderly Patients with Severe Aneurysmal Subarachnoid Hemorrhage: A Retrospective Study. Adv. Ther. 2021;38:249–257. doi: 10.1007/s12325-020-01531-7. [DOI] [PubMed] [Google Scholar]
- 113.Olsen M.H., Orre M., Leisner A.C.W., Rasmussen R., Bache S., Welling K., Eskesen V., Møller K. Delayed cerebral ischaemia in patients with aneurysmal subarachnoid haemorrhage: Functional outcome and long-term mortality. Acta Anaesthesiol. Scand. 2019;63:1191–1199. doi: 10.1111/aas.13412. [DOI] [PubMed] [Google Scholar]
- 114.Sanelli P.C., Anumula N., Gold R., Elias E., Johnson C., Comunale J., Tsiouris A.J., Segal A.Z. Outcomes-based assessment of a new reference standard for delayed cerebral ischemia related to vasospasm in aneurysmal subarachnoid hemorrhage. Acad. Radiol. 2012;19:1066–1074. doi: 10.1016/j.acra.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shamshad A., Persad-Paisley E.M., Wendell L.C., Thompson B.B., Reznik M.E., Furie K.L., Mahta A. Association of asymptomatic cerebral vasospasm with outcomes in survivors of aneurysmal subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 2022;31:106821. doi: 10.1016/j.jstrokecerebrovasdis.2022.106821. [DOI] [PubMed] [Google Scholar]
- 116.Chang C.-M., Su Y.-F., Chang C.-Z., Chung C.-L., Tsai Y.-J., Loh J.-K., Lin C.-L. Progesterone attenuates experimental subarachnoid hemorrhage-induced vasospasm by upregulation of endothelial nitric oxide synthase via Akt signaling pathway. Biomed. Res. Int. 2014;2014:207616. doi: 10.1155/2014/207616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin C.-L., Shih H.-C., Dumont A.S., Kassell N.F., Lieu A.-S., Su Y.-F., Hwong S.-L., Hsu C. The effect of 17beta-estradiol in attenuating experimental subarachnoid hemorrhage-induced cerebral vasospasm. J. Neurosurg. 2006;104:298–304. doi: 10.3171/jns.2006.104.2.298. [DOI] [PubMed] [Google Scholar]
- 118.Martin J., Plank E., Ulm B., Gempt J., Wostrack M., Jungwirth B., Kagerbauer S.M. Concentrations of estradiol, progesterone and testosterone in sefrum and cerebrospinal fluid of patients with aneurysmal subarachnoid hemorrhage correlate weakly with transcranial Doppler flow velocities. BMC Neurosci. 2021;22:29. doi: 10.1186/s12868-021-00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Modra L.J., Higgins A.M., Pilcher D.V., Bailey M., Bellomo R. Sex Differences in Vital Organ Support Provided to ICU Patients. Crit. Care Med. 2024;52:1–10. doi: 10.1097/ccm.0000000000006058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Todorov A., Kaufmann F., Arslani K., Haider A., Bengs S., Goliasch G., Zellweger N., Tontsch J., Sutter R., Buddeberg B., et al. Gender differences in the provision of intensive care: A Bayesian approach. Intensive Care Med. 2021;47:577–587. doi: 10.1007/s00134-021-06393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang S.S., Bögli S.Y., Nierobisch N., Wildbolz S., Keller E., Brandi G. Sex-Related Differences in Patients’ Characteristics, Provided Care, and Outcomes Following Spontaneous Intracerebral Hemorrhage. Neurocrit Care. 2022;37:111–120. doi: 10.1007/s12028-022-01453-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ziemba-Davis M., Bohnstedt B.N., Payner T.D., Leipzig T.J., Palmer E., Cohen-Gadol A.A. Incidence, epidemiology, and treatment of aneurysmal subarachnoid hemorrhage in 12 midwest communities. J. Stroke Cerebrovasc. Dis. 2014;23:1073–1082. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 123.Bögli S.Y., Utebay D., Smits N., Westphal L.P., Hirsbrunner L., Unseld S., Keller E., Brandi G. Sex-related differences of invasive therapy in patients with aneurysmal subarachnoid hemorrhage. Acta Neurochir. 2022;164:2899–2908. doi: 10.1007/s00701-022-05345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Connolly E.S., Rabinstein A.A., Carhuapoma J.R., Derdeyn C.P., Dion J.E., Higashida R.T., Hoh B.L., Kirkness C.J., Naidech A.M., Ogilvy C.S., et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711–1737. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 125.Nieuwkamp D.J., Setz L.E., Algra A., Linn F.H., de Rooij N.K., Rinkel G.J. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: A meta-analysis. Lancet Neurol. 2009;8:635–642. doi: 10.1016/s1474-4422(09)70126-7. [DOI] [PubMed] [Google Scholar]
- 126.Bögli S.Y., Stretti F., Utebay D., Hitz L., Hertler C., Brandi G. Limitation of life sustaining measures in neurocritical care: Sex, timing, and advance directive. J. Intensive Care. 2024;12:3. doi: 10.1186/s40560-023-00714-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McPherson K., Carlos W.G., 3rd Emmett T.W., Slaven J.E., Torke A.M. Limitation of Life-Sustaining Care in the Critically Ill: A Systematic Review of the Literature. J. Hosp. Med. 2019;14:303–310. doi: 10.12788/jhm.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gott M., Morgan T., Williams L. Gender and palliative care: A call to arms. Palliat. Care Soc. Pract. 2020;14:2632352420957997. doi: 10.1177/2632352420957997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sharma R.K., Prigerson H.G., Penedo F.J., Maciejewski P.K. Male-female patient differences in the association between end-of-life discussions and receipt of intensive care near death. Cancer. 2015;121:2814–2820. doi: 10.1002/cncr.29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Garrett J.M., Harris R.P., Norburn J.K., Patrick D.L., Danis M. Life-sustaining treatments during terminal illness: Who wants what? J. Gen. Intern. Med. 1993;8:361–368. doi: 10.1007/bf02600073. [DOI] [PubMed] [Google Scholar]
- 131.Bookwala J., Coppola K.M., Fagerlin A., Ditto P.H., Danks J.H., Smucker W.D. Gender differences in older adults’ preferences for life-sustaining medical treatments and end-of-life values. Death Stud. 2001;25:127–149. doi: 10.1080/07481180126202. [DOI] [PubMed] [Google Scholar]
- 132.Wilson L., Boase K., Nelson L.D., Temkin N.R., Giacino J.T., Markowitz A.J., Maas A., Menon D.K., Teasdale G., Manley G.T. A Manual for the Glasgow Outcome Scale-Extended Interview. J. Neurotrauma. 2021;38:2435–2446. doi: 10.1089/neu.2020.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ganesh A., Luengo-Fernandez R., Pendlebury S.T., Rothwell P.M. Weights for ordinal analyses of the modified Rankin Scale in stroke trials: A population-based cohort study. EClinicalMedicine. 2020;23:100415. doi: 10.1016/j.eclinm.2020.100415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ganesh A., Luengo-Fernandez R., Wharton R.M., Rothwell P.M. Ordinal vs dichotomous analyses of modified Rankin Scale, 5-year outcome, and cost of stroke. Neurology. 2018;91:e1951–e1960. doi: 10.1212/wnl.0000000000006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nunn A., Bath P.M., Gray L.J. Analysis of the Modified Rankin Scale in Randomised Controlled Trials of Acute Ischaemic Stroke: A Systematic Review. Stroke Res. Treat. 2016;2016:9482876. doi: 10.1155/2016/9482876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maguire J., Attia J. Which version of the modified Rankin Scale should we use for stroke trials? Lump or split? Neurology. 2018;91:947–948. doi: 10.1212/WNL.0000000000006533. [DOI] [PubMed] [Google Scholar]
- 137.Hill M.D., Goyal M., Menon B.K., Nogueira R.G., McTaggart R.A., Demchuk A.M., Poppe A.Y., Buck B.H., Field T.S., Dowlatshahi D., et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): A multicentre, double-blind, randomised controlled trial. Lancet. 2020;395:878–887. doi: 10.1016/s0140-6736(20)30258-0. [DOI] [PubMed] [Google Scholar]
- 138.Ma H., Campbell B.C., Parsons M.W., Churilov L., Levi C.R., Hsu C., Kleinig T.J., Wijeratne T., Curtze S., Dewey H.M., et al. Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke. N. Engl. J. Med. 2019;380:1795–1803. doi: 10.1056/NEJMoa1813046. [DOI] [PubMed] [Google Scholar]
- 139.Howard G., Waller J.L., Voeks J.H., Howard V.J., Jauch E.C., Lees K.R., Nichols F.T., Rahlfs V.W., Hess D.C. A simple, assumption-free, and clinically interpretable approach for analysis of modified Rankin outcomes. Stroke. 2012;43:664–669. doi: 10.1161/strokeaha.111.632935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Riley R.D., Elia E.G., Malin G., Hemming K., Price M.P. Multivariate meta-analysis of prognostic factor studies with multiple cut-points and/or methods of measurement. Stat. Med. 2015;34:2481–2496. doi: 10.1002/sim.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.IntHout J., Ioannidis J.P., Rovers M.M., Goeman J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Raine R., Goldfrad C., Rowan K., Black N. Influence of patient gender on admission to intensive care. J. Epidemiol. Community Health. 2002;56:418–423. doi: 10.1136/jech.56.6.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from previously published studies, in which informed consent was obtained, were retrieved and analyzed.