Abstract
Background and Objectives: Serum alpha-fetoprotein (AFP) is a recognized affordable oncological marker in patients with hepatocellular carcinoma (HCC). However, AFP’s prognostic role has been assessed mainly after specific treatments, and no unanimously recognized cut-offs have been identified. The aim of this study is to investigate the prognostic role of different basal AFP cut-offs on survival and HCC course. Materials and Methods: In this single-center, retrospective study, all patients newly diagnosed with HCC between January 2009 and December 2021 were prospectively enrolled. Only patients suitable for curative HCC treatments were included in the analyses. Patients were stratified according to AFP cut-offs of 20, 200, 400, and 1000 ng/mL, which were correlated with survival outcomes and clinical parameters. Results: A total of 266 patients were analyzed, with a median follow-up time of 41.5 months. Median overall survival (OS) of all cohort was 43 months. At the multivariate Cox-regression analysis, AFP value ≥ 1000 ng/mL correlated with impaired OS (1-year OS: 67% vs. 88%, 5-year OS: 1% vs. 43%; p = 0.005); other risk factors were tumor dimension ≥ 5 cm (HR 1.73; p = 0.002), Child–Pugh class B–C (HR 1.72; p = 0.002), BCLC stage A (vs. 0) (HR 2.4; p = 0.011), and malignant portal vein thrombosis (HR 2.57; p = 0.007). AFP ≥ 1000 ng/mL was also associated with a reduced recurrence-free survival (HR 2.0; p = 0.038), while starting from AFP ≥ 20 ng/mL, a correlation with development of HCC metastases over time (HR 3.5; p = 0.002) was seen. AFP values ≥ 20 ng/mL significantly correlated with tumor size and higher histological grading; starting from AFP values ≥ 400 ng/mL, a significant correlation with Child–Pugh class B–C and female gender was also observed. Conclusions: Basal AFP correlates with relevant outcomes in patients with HCC. It could help identify patients at a higher risk of worse prognosis who might benefit from personalized surveillance and treatment programs. Prospective studies are needed to confirm these results.
Keywords: hepatocellular carcinoma, alpha-fetoprotein, AFP cut-off, survival, prognosis
1. Introduction
Hepatocellular carcinoma (HCC) accounts for more than 80% of primary liver tumors [1,2], representing the seventh most common cancer and the fourth cause of cancer-related death worldwide [3]. The main risk factors are hepatitis B virus, hepatitis C virus, alcohol abuse determining alcoholic steatohepatitis, and nonalcoholic fatty liver disease/steatohepatitis [4,5]. Most HCC cases, approximately 80%, develop from liver cirrhosis, HCC being the most common cirrhosis complication [6]. Such a high incidence of HCC in cirrhotic patients supports the implementation of surveillance programs, with a 6-month surveillance with abdominal ultrasound being recommended by European and American guidelines [7,8]. The gold standard for HCC diagnosis and follow-up in cirrhotic patients is imaging methods, especially contrast-enhanced ultrasound (CEUS), computed tomography (CT), and contrast-enhanced magnetic resonance (MRI) [9]. HCC that arises in the context of cirrhosis is staged not solely based on the extent of the disease but also by considering the degree of liver function, which represents an indispensable parameter for prognostic purposes and choice of treatment [10]. The Barcelona Clinic for Liver Cancer (BCLC) algorithm is the most widely used HCC staging system, which also provides a first-line therapeutic indication for each stage [11]. The prognosis of HCC depends significantly on the staging of the disease and the suitable treatment options; however, these staging systems are suboptimal, and several studies have underlined how other biological markers are associated with tumor prognosis. Additionally, despite improvements in therapeutic approaches, long-term survival remains poor, with 5-year and 10-year survival rates of about 20% and 10%, respectively [10].
Among HCC-related biomarkers, alpha-fetoprotein (AFP) is an oncofetal glycoprotein which is normally produced by the yolk sac in the first trimester of pregnancy and by the fetal liver starting from the eleventh to twelfth week of gestation [12]. In adult life, the production of AFP is drastically reduced and can be measured in minimal concentrations in healthy adults, between 3 and 20 ng/mL [13,14]. The use of AFP as an oncological marker of HCC was first proposed in the 1960s, though AFP is not specific for HCC, as numerous neoplastic and non-neoplastic conditions involving the liver and other organs can cause an increase in it [15]. Despite the cut-off value of 20 ng/mL being commonly used, some studies have shown an increase in specificity for HCC diagnosis by raising the cut-off to 200 ng/mL, and especially 400 ng/mL [16]; however, most small HCC at diagnoses arise in patients with negative serum AFP [17,18,19].
Besides the limited value of AFP for diagnosis, the value of AFP as a prognostic is gaining ground. Indeed, AFP monitoring is very useful for evaluating the response to a specific treatment [20], and patients with low or negative AFP have a lower probability of post-treatment relapse and increased survival [21,22]. Some recent prognostic scores, such as the CLIP score and the ITA.LI.CA score, also include serum AFP, in addition to other clinical and tumor features [23,24]. However, the prognostic role of AFP in these studies depends on patient characteristics, study design, and the values used as cut-offs [25,26], while several studies, on the other hand, seem not to confirm this role [27]. Moreover, studies assessing the relationship between baseline AFP values and prognostic parameters independently from a specific therapy are few and mainly analyze populations different from the Italian one in terms of ethnicity and the distribution of risk factors [28,29].
Based on these premises, our study aimed to assess the role of different baseline AFP cut-offs towards relevant survival outcomes in a cohort of Italian patients with a new diagnosis of HCC suitable for curative treatment.
2. Materials and Methods
2.1. Study Design and Patients
We performed a single-center, retrospective, observational study. Between January 2009 and December 2021, all patients newly diagnosed with HCC were consecutively enrolled and followed-up over time.
We included only HCC patients deemed suitable for a curative treatment after a multidisciplinary discussion in order to obtain a more homogeneous cohort, avoiding biases related to very different disease prognosis. Therefore, all patients included in this study were classified as BCLC 0 (very early) or BCLC A (early) stage to be suitable for curative treatment. We considered the following as curative approaches: liver resection; radiofrequency thermal ablation (RFTA); and percutaneous ethanol injection (PEI). Some patients also underwent transcatheter arterial chemoembolization (TACE) or transarterial chemoembolization (TAE), but always in combination with RFTA or PEI. No patients received concomitant or adjuvant systemic therapy, given the lack of strong evidence of a benefit from this approach at the enrolment period of our study. No patients in our cohort underwent liver transplantation, because our center is not a referral center for liver transplantation, and therefore, we do not routinely follow-up patients after this kind of treatment.
At enrolment, the following baseline parameters were collected: sex; date of HCC diagnosis; etiology of liver disease; size of the nodule; Child–Pugh score; AFP value; histological grading of HCC; and presence of portal thrombosis (PVT) and if malignant. Regarding the etiology of cirrhosis, the patients were divided into two groups, one with positivity for major hepatotropic viruses (HBV, HCV) and one without positivity for major hepatotropic viruses. For convenience, patients with multifactorial etiology but with evidence of viral infections were included in the first group. A subdivision into two groups was also performed for the size of the tumor lesion, identifying a group with HCC < 5 cm in size and a group with HCC > or equal to 5 cm.
The patients were then stratified according to the following baseline AFP cut-off values: < or ≥20 ng/mL; < or ≥200 ng/mL; < or ≥400 ng/mL; and < or ≥1000 ng/mL.
For the final inclusion in the study analyses, patients with at least one of the following criteria were excluded: previous diagnosis of HCC; HCC without pre-existing liver cirrhosis; unknown etiology of liver cirrhosis; baseline AFP value not available; mixed HCC-cholangiocarcinoma (CCC) histotype; and unknown data and/or cause of death or loss at follow-up.
This study was approved by the local Ethics Committee and was performed according to the Helsinki Declaration. All patients gave their written informed consent for inclusion in this study and clinical data collection.
2.2. HCC Assessment and Follow-Up
HCC diagnosis was made radiologically when typical hallmarks were visible at contrast-enhanced CT and/or MRI, or histologically by means of liver biopsy, according to the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases guidelines in force at the time of diagnosis [7,30]. Tumor dimension was assessed at the cross-sectional imaging technique (CT, MRI, and/or US) performed. The presence of PVT and its possible neoplastic nature were assessed by CEUS.
In patients undergoing liver biopsy, the histological grading of HCC was assigned by a group of onco-pathologists with strong expertise and in accordance with the 2019 World Health Organization (WHO) classification [31].
All patients were followed-up by a multidisciplinary team, including oncologists, gastroenterologists, radiologists, and surgeons, with expertise in the management of liver diseases and HCC, with scheduled outpatient visits and imaging examinations. Specifically, after the treatment a contrast-enhanced CT scan was performed at 1 month, and subsequently every 6 months with CEUS or contrast-enhanced CT scan; if a doubt emerged at CEUS or contrast-enhanced CT, then a contrast-enhanced CT or MRI was performed, respectively.
2.3. Outcome Measures
During the follow-up, the following outcome measures were recorded: death, both considered as overall survival (OS) and disease-specific survival (DSS), and HCC recurrence (recurrence-free survival, RFS), which included local recurrence after treatment, new intrahepatic HCC, and development of extrahepatic HCC metastases over time. For DSS, only death related to the liver disease, namely liver failure or progression of liver cancer, was considered.
OS, DSS, and RFS were considered as time-dependent variables, and the time was calculated from HCC diagnosis to death or last follow-up for OS and DSS, and from HCC treatment to recurrence or last follow-up for RFS. Local recurrence after treatment, new intrahepatic HCC, and HCC metastasis development were instead considered as time-independent variables for the purpose of analysis. Surviving patients were followed-up for at least 6 months after HCC diagnosis.
2.4. Study Objectives
The primary objective of this study was to correlate the different AFP cut-offs with the OS. The secondary objectives were as follows:
To correlate the different AFP cut-offs with the other outcome measures;
To correlate the AFP cut-offs with the baseline clinical variables.
2.5. Statistical Analysis
The categorical variables were described as absolute frequency and percentage. The continuous variables were described as median and range. Kaplan–Meier curves were computed to assess the survival outcomes, namely the overall survival, the disease-related survival, and the recurrence-free survival. Survival data were expressed as the median survival time and as the survival at 1-year and 5-year time points, with the relevant 95% confidence intervals (CI). The predictive role of baseline clinical variables towards survival outcomes was assessed by means of Cox regression analysis and the results are expressed as hazard ratio (HR) with 95%CI. Specifically, survival analyses were performed calculating the hazard ratio of death or recurrence. Variables significant in the univariate analysis were entered in the multivariate model. Correlations between the different AFP cut-offs and local recurrence, new intrahepatic HCC, and HCC metastasis development were assessed by using the bivariate logistic regression analysis and expressed as hazard ratio (HR) with 95%CI. Correlations between the different AFP cut-offs and other baseline variables were assessed by using the Mann–Whitney analysis and Fisher’s exact tests for continuous and categorical variables, respectively. All the analyses were carried out by computer software IBM SPSS Statistics (Version 25; IBM Corporation, Armonk, NY, USA), and significance was established at the 0.05 level (two-sided).
3. Results
3.1. Baseline Features
A total of 266 patients newly diagnosed with HCC and eligible for a treatment with curative intent were consecutively enrolled (32% female, median age at enrollment 73 years). The predominant cirrhosis etiology was viral (80%). RFTA was the most frequently performed treatment (76%), followed by PEI (18%); some patients underwent more than one type of treatment. Most patients were in Child–Pugh A class (81%) and had an HCC of grade 1–2 (85%) at diagnosis, while PVT was present in 42 (16%) patients, 37 (14%) of which were of neoplastic nature. Most patients were AFP-negative (<20 ng/mL) at diagnosis (66%): 12% had AFP ≥ 200 ng/mL, 8% ≥ 400 ng/mL, and 6% ≥ 1000 ng/mL. All the demographic and baseline clinical features, both overall and stratified by AFP cut-offs, are reported in Table 1. The median follow-up time was 41.5 months (1–174).
Table 1.
Demographic and clinical characteristics of the 266 patients analyzed.
| Total | |
|---|---|
| Parameter | n = 266 |
| Sex (F), n (%) | 85 (32) |
| Age at HCC enrollment, years, median (range) | 73 (45–87) |
| Etiology (viral vs. others), n (%) | |
| Viral | 212 (80) |
| Others | 54 (20) |
| Dimension, mm, median (range) | 26.5 (12–150) |
| Diameter, n (%) | |
| ≥5 cm | 224 (84) |
| <5 cm | 42 (16) |
| Child–Pugh score, n (%) | |
| A | 215 (81) |
| B–C | 51 (19) |
| BCLC stage | |
| 0 (Very early) | 60 (23) |
| A (Early) | 206 (77) |
| HCC Grade 1 (1–2 vs. 3), n (%) | |
| 1–2 | 201 (85) |
| 3 | 36 (15) |
| PVT, n (%) | 42 (16) |
| Malignant PVT, n (%) | 37 (14) |
| Type of curative treatment, n (%) | |
| RFTA | 201 (76) |
| PEI | 48 (18) |
| Resection | 29 (11) |
| TACE/TAE 2 | 39 (15) |
| Type of curative treatment, n (%) | |
| AFP cut-offs | 91 (34) |
| ≥20 ng/mL | 31 (12) |
| ≥200 ng/mL | 21 (8) |
| ≥400 ng/mL | 15 (6) |
| ≥1000 ng/ml | |
| HCC recurrence, n (%) 3 | 116 (44) |
| Local recurrence after treatment | 41 (15) |
| New intrahepatic | 77 (29) |
| Extrahepatic metastases | 28 (11) |
| Dead at f-up end, n (%) | 212 (80) |
| Disease-related death, n (%) | 157 (59) |
| Follow-up time, months, median (range) | 41.5 (1–174) |
1 Available for 237 patients; 2 always in combination with RFTA or PEI; 3 some patients experienced more than one type of HCC recurrence; AFP: alpha-fetoprotein; BCLC: Barcellona Clinic Liver Cancer; HCC: hepatocellular carcinoma; PVT: portal vein thrombosis; RFTA: radiofrequency thermal ablation; PEI: percutaneous ethanol injection; TACE: transcatheter arterial chemoembolization; TAE: transarterial chemoembolization.
3.2. Correlations between AFP and Survival
During the follow-up, 212 patients (79.7%) died, of whom 157 (59.0%) died due to liver-related disease.
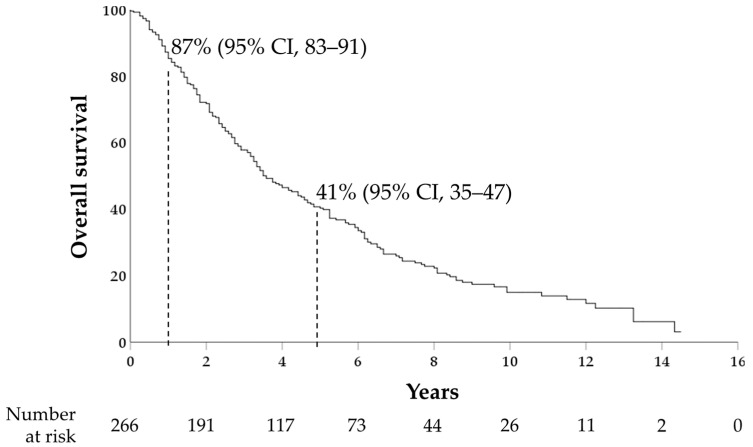
The 1- and 5-year cumulative probabilities of OS were 87% (95%CI, 83–91) and 41% (95%CI, 35–47), respectively, with a median OS of 43 months (95%CI, 35–51) (Figure 1).
Figure 1.
Kaplan–Meier curve for overall survival; 1- and 5-year survival rates are shown.
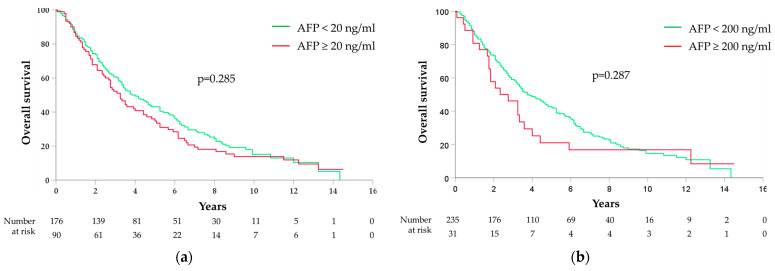
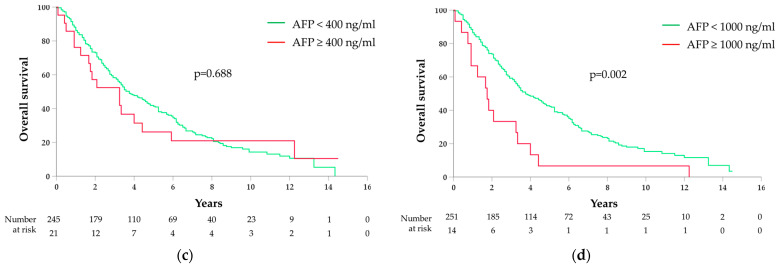
By differentiating between the different AFP cut-offs, no correlation was found between the OS and the AFP cut-offs of 20 ng/mL (median OS 46 months [95%CI 36–56] for AFP < 20 ng/mL and 39 months [95%CI 32–46] for AFP ≥ 20 ng/mL, p = 0.285) (Figure 2a); 200 ng/mL (median OS 46 months [95%CI 37–55] for AFP < 200 ng/mL and 28 months [95%CI 12–44] for AFP ≥ 200 ng/mL, p = 0.287) (Figure 2b); and 400 ng/mL (median OS 45 months [95%CI 36–54] for AFP < 400 ng/mL and 39 months [95%CI 15–63] for AFP ≥ 400 ng/mL, p = 0.688) (Figure 2c).
Figure 2.
Kaplan–Meier curves for overall survival stratified by AFP cut-offs: (a) 20 ng/mL; (b) 200 ng/mL; (c) 400 ng/mL; (d) 1000 ng/mL.
A significant correlation was instead observed between the OS and the AFP cut-off of 1000 ng/mL (HR 2.3; 95%CI 1.3–3.9; p = 0.002), with a median OS of 45 months (95%CI 36–54) for AFP < 1000 ng/mL and 21 months (95%CI 12–30) for AFP ≥ 1000 ng/mL; 1-year and 5-year cumulative probabilities of OS were 88% (95%CI 84–92) vs. 67% (95%CI 43–91) and 43% (95%CI 37–49) vs. 1% (95%CI 0–13), respectively (Figure 2d).
Among other baseline variables, age at enrollment (HR 1.3; 95%CI 0.9–1.9; p = 0.038), tumor dimension ≥ 5 cm (HR 1.8; 95%CI 1.3–2.6; p = 0.001), Child–Pugh class B or C (vs. A) (HR 1.6; 95%CI 1.2–2.3; p = 0.004), BCLC stage A (vs. 0) (HR 2.1; 95%CI 1.5–3.0; p < 0.001) presence of PVT (HR 1.8; 95%CI 1.3–2.6; p = 0.001), and malignant PVT (HR 2.2; 95%CI 1.5–3.2; p < 0.001) were correlated with increased mortality at univariate analysis (Table 2). At the multivariate regression analysis, AFP ≥ 1000 ng/mL (HR 2.0; 95%CI 1.2–3.5; p = 0.009), tumor size ≥ 5 cm (HR 1.5; 95%CI 1.0–2.2; p = 0.034), Child–Pugh class B or C (vs. A) (HR 1.4; 95%CI 1.0–2.0; p = 0.042), BCLC stage A (vs. 0) (HR 1.8; 95%CI 1.2–2.6; p = 0.003), and malignant PVT (HR 2.3; 95%CI 1.2–4.4; p = 0.009) proved to be independent risk factors for mortality (Table 2).
Table 2.
Univariate and multivariate analysis of baseline predictors of overall survival.
| Features | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95%CI) | p Value 1 | HR (95%CI) | p Value 1 | |
| Sex (F vs. M) | 0.96 (0.72–1.28) | 0.764 | ||
| Age at HCC enrollment | 1.29 (0.87–1.92) | 0.038 | 1.02 (1.00–1.04) | 0.120 |
| Etiology (viral vs. others) | 1.19 (0.85–1.69) | 0.314 | ||
| Diameter (≥5 cm vs. <5 cm) | 1.79 (1.26–2.55) | 0.001 | 1.50 (1.03–2.18) | 0.034 |
| Child-Pugh score (B–C vs. A) | 1.63 (1.17–2.27) | 0.004 | 1.41 (0.99–2.01) | 0.042 |
| BCLC stage (A vs. 0) | 2.07 (1.46–2.95) | <0.001 | 1.79 (1.22–2.62) | 0.003 |
| HCC Grade (3 vs. 1–2) | 1.43 (0.98–2.09) | 0.061 | ||
| PVT (yes vs. no) | 1.84 (1.29–2.63) | 0.001 | 1.03 (0.56–1.89) | 0.918 |
| Malignant PVT (yes vs. no) | 2.20 (1.51–3.21) | <0.001 | 2.34 (1.24–4.44) | 0.009 |
| Type of treatment (locoregional vs. surgical) | 1.29 (0.84–2.00) | 0.247 | ||
| AFP ≥ 20 ng/mL (vs. < 20 ng/mL) | 1.17 (0.88–1.55) | 0.285 | ||
| AFP ≥ 200 ng/mL (vs. < 200 ng/mL) | 1.27 (0.82–1.99) | 0.287 | ||
| AFP ≥ 400 ng/mL (vs. < 400 ng/mL) | 1.11 (0.67–1.83) | 0.688 | ||
| AFP ≥ 1000 ng/mL (vs. < 1000 ng/mL) | 2.29 (1.35–3.89) | 0.002 | 2.20 (1.28–3.78) | 0.004 |
1 Cox regression analysis; CI: confidence interval; AFP: alpha-fetoprotein; BCLC: Barcellona Clinic Liver Cancer; HCC: hepatocellular carcinoma; HR: hazard ratio; PVT: portal vein thrombosis.
The 1- and 5-year cumulative probabilities of DSS were 90% (95%CI, 86–94) and 50% (95%CI, 44–56), respectively, with a median of 58 months (95%CI, 47–69). A baseline AFP value ≥ 1000 ng/mL was found to be a risk factor also for DSS, both at the univariate and the multivariate regression analyses (HR 2.1; 95%CI 1.1–4.1; p = 0.019), along with tumor dimension ≥ 5 cm (HR 1.7; 95%CI 1.1–2.6; p = 0.012), Child–Pugh class B or C (HR 1.7; 95%CI 1.2–2.6; p = 0.007), BCLC stage A (vs. 0) (HR 1.9; 95%CI 1.2–2.9; p = 0.008), and malignant PVT (HR 2.2; 95%CI 1.1–4.5; p = 0.023).
3.3. Correlations between AFP and HCC Recurrence
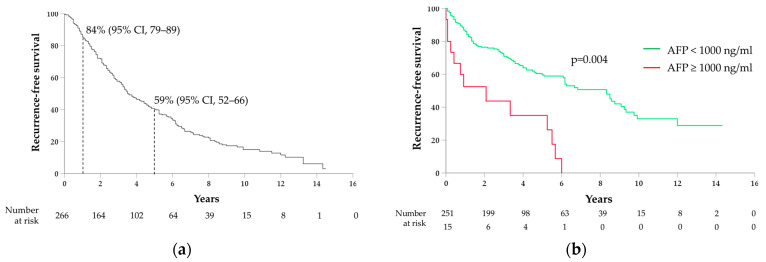
During the follow-up, 116 patients (43.6%) experienced an HCC recurrence. The 1- and 5-year cumulative probabilities of RFS were 86% (95%CI, 82–90) and 80% (95%CI, 74–86), respectively, with a median RFS of 42 months (95%CI 26–58) (Figure 3a). At univariate analysis, tumor dimension ≥ 5 cm (HR 2.6; 95%CI 1.7–4.1; p < 0.001), BCLC stage A (vs. 0) (HR 2.2; 95%CI 1.2–4.0; p = 0.008), HCC grade 3 (vs. 1–2) (HR 1.4; 95%CI 0.8–2.4; p = 0.032), PVT (HR 2.4; 95%CI 1.5–3.7; p < 0.001), malignant PVT (HR 2.4; 95%CI 1.5–3.9; p < 0.001), and AFP ≥ 1000 ng/mL (HR 1.8; 95%CI 1.2–4.1; p = 0.021) were correlated with a reduced RFS. At multivariate regression analysis, tumor dimension ≥ 5 cm (HR 2.3; 95%CI 1.5–3.8; p = 0.001), BCLC stage A (vs. 0) (HR 2.4; 95%CI 1.2–4.8; p = 0.011), HCC grade 3 (vs. 1–2) (HR 1.7; 95%CI 1.0–2.8; p = 0.003), and AFP ≥ 1000 ng/mL (HR 2.0; 95%CI 1.0–3.7; p = 0.038) (Figure 3b) were confirmed to be independent risk factors for recurrence (Table 3).
Figure 3.
Kaplan–Meier curves for recurrence-free survival: (a) overall, with 1- and 5-year survival rates shown; (b) stratified by AFP cut-off of 1000 ng/mL.
Table 3.
Univariate and multivariate analysis of baseline predictors of recurrence-free survival.
| Features | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95%CI) | p Value 1 | HR (95%CI) | p Value 1 | |
| Sex (F vs. M) | 0.69 (0.46–1.05) | 0.091 | ||
| Age at HCC enrollment | 1.01 (0.99–1.04) | 0.207 | ||
| Etiology (viral vs. others) | 0.88 (0.57–1.35) | 0.560 | ||
| Diameter (≥5 cm vs. <5 cm) | 2.60 (1.67–4.07) | <0.001 | 2.36 (1.45–3.83) | 0.001 |
| Child-Pugh score (B–C vs. A) | 1.47 (0.94–2.29) | 0.092 | ||
| BCLC stage (A vs. 0) | 2.21 (1.24–3.96) | 0.008 | 2.41 (1.22–4.75) | 0.011 |
| HCC Grade (3 vs. 1–2) | 1.41 (0.84–2.38) | 0.032 | 1.65 (1.04–4.67) | 0.003 |
| PVT (yes vs. no) | 2.39 (1.54–3.71) | <0.001 | 1.55 (0.60–4.01) | 0.363 |
| Malignant PVT (yes vs. no) | 2.40 (1.48–3.88) | <0.001 | 1.69 (0.62–4.61) | 0.304 |
| Type of treatment (locoregional vs. surgical) | 1.41 (0.81–2.48) | 0.229 | ||
| AFP ≥ 20 ng/mL (vs. < 20 ng/mL) | 1.23 (0.84–1.79) | 0.283 | ||
| AFP ≥ 200 ng/mL (vs. < 200 ng/mL) | 1.04 (0.54–1.99) | 0.412 | ||
| AFP ≥ 400 ng/mL (vs. < 400 ng/mL) | 1.97 (1.03–2.17) | 0.104 | ||
| AFP ≥ 1000 ng/mL (vs. < 1000 ng/mL) | 1.82 (1.20–4.13) | 0.021 | 1.95 (1.04–3.68) | 0.038 |
1 Cox regression analysis; CI: confidence interval; AFP: alpha-fetoprotein; BCLC: Barcellona Clinic Liver Cancer; HCC: hepatocellular carcinoma; HR: hazard ratio; PVT: portal vein thrombosis.
Forty-one patients (15.4%) experienced a local HCC recurrence after treatment, with a median time to recurrence of 12 months (95%CI 2–22). AFP ≥ 1000 ng/mL (HR 2.8; 95%CI 1.4–7.3; p = 0.005) and HCC grade 3 (vs. 1–2) (HR 2.8; 95%CI 1.0–8.1; p = 0.047) were correlated with an increased risk of LR.
Development of a new intrahepatic HCC was observed in 77 (28.9%) patients, without correlations with the AFP cut-offs or other baseline clinical variables.
Twenty-eight patients developed at least one extrahepatic HCC metastasis during the follow-up. Patients with an AFP value ≥ 20 ng/mL showed an increased risk of developing HCC metastases over time compared with patients with AFP < 20 ng/mL (HR 3.5; 95%CI 1.6–7.8; p = 0.002). This correlation was also confirmed for the other higher AFP cut-offs. Other baseline factors associated with extrahepatic HCC recurrence were tumor dimension ≥ 5 cm (HR 4.3; 95%CI 1.9–10.1; p = 0.001), HCC grade 3 (vs. 1–2) (HR 3.5; 95%CI 1.4–9.1; p = 0.009), and malignant PVT (HR 3.6; 95%CI 1.5–8.6; p = 0.005).
3.4. Correlations between AFP and Baseline Variables
The AFP cut-offs were progressively correlated with other baseline variables at HCC diagnosis. A baseline AFP ≥ 20 ng/mL was associated with tumor dimension ≥ 5 cm (p = 0.008) and histological tumor grading G3 (p < 0.001). Starting from a value ≥ 400 ng/mL, AFP showed a correlation with Child–Pugh score B or C (p = 0.038). No further correlations were observed for AFP ≥ 1000 ng/mL (Table 4). The AFP absolute values were also correlated with cirrhosis etiology, without a difference in median AFP between patients with viral etiology vs. others (18 ng/mL vs. 13 ng/mL, respectively; p = 0.096).
Table 4.
Correlation between the increasing AFP cut-offs and the baseline clinical variables.
| AFP Cut-Offs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 ng/mL | 200 ng/mL | 400 ng/mL | 1000 ng/mL | |||||||||
| Parameter | < | ≥ | p 2 | < | ≥ | p 2 | < | ≥ | p 2 | < | ≥ | p 2 |
| Sex (F), % | 30 | 32 | 0.368 | 31 | 39 | 0.102 | 30 | 50 | 0.045 | 31 | 47 | 0.020 |
| Age at HCC enrollment, years, median | 73 | 72 | 0.672 | 73 | 72 | 0.753 | 73 | 72 | 0.903 | 73 | 73 | 0.669 |
| Viral etiology, % | 76 | 87 | 0.073 | 79 | 89 | 0.242 | 79 | 91 | 0.201 | 79 | 87 | 0.743 |
| Diameter ≥ 5 cm, % | 11 | 24 | 0.006 | 14 | 30 | 0.027 | 15 | 29 | 0.032 | 15 | 33 | 0.045 |
| Child-Pugh score A, % | 82 | 79 | 0.566 | 83 | 63 | 0.061 | 82 | 62 | 0.022 | 83 | 53 | 0.012 |
| BCLC A, % | 75 | 81 | 0.354 | 76 | 89 | 0.153 | 77 | 86 | 0.426 | 77 | 87 | 0.532 |
| HCC Grade 1 1–2, % | 84 | 69 | <0.001 | 88 | 52 | <0.001 | 87 | 53 | <0.001 | 88 | 36 | <0.001 |
| PVT, % | 13 | 20 | 0.178 | 16 | 19 | 0.612 | 15 | 19 | 0.670 | 16 | 20 | 0.713 |
| Malignant PVT, % | 12 | 18 | 0.192 | 14 | 11 | 0.713 | 14 | 9 | 0.748 | 14 | 13 | 0.947 |
1 Available for 237 patients; 2 Mann–Whitney analysis or Fisher’s exact test; AFP: alpha-fetoprotein; BCLC: Barcellona Clinic Liver Cancer; HCC: hepatocellular carcinoma; PVT: portal vein thrombosis.
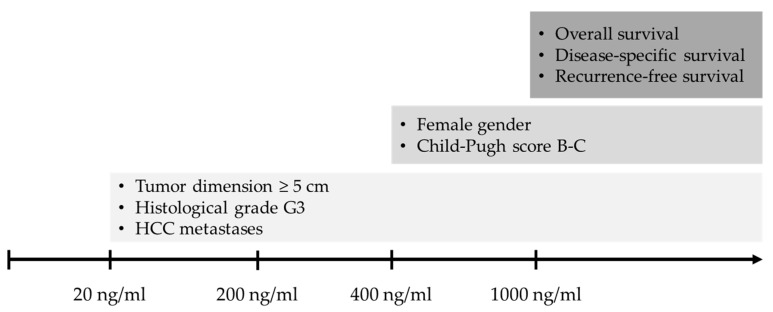
All correlations between AFP cut-offs and outcomes and baseline variables are summarized in Figure 4.
Figure 4.
Summary figure showing the predictive power of the different AFP cut-offs towards outcomes and baseline clinical variables.
4. Discussion
In this study, we investigate the association between AFP, as measured at HCC diagnosis, and survival outcomes and HCC features in a cohort of patients with newly diagnosed HCC suitable for curative treatment. One-year and five-year OS rates in our cohort were 87% and 41%, respectively. This survival rate is higher than the 20% 5-year survival rate reported from the overall Italian nationwide cohort of HCC patients [32], while it is similar to the survival rates reported for the patients with HCC at very early and early stages (BCLC 0-A) [33,34]. This reflects the specific cohort we analyzed, which is composed only of patients suitable for curative treatment and is characterized by a relatively limited (<3 cm) median tumor dimension, which results from the strict adherence of our patients to surveillance programs and allowed most patients to be eligible for local ablation therapies.
Regarding our primary outcome, we found that a very high level of baseline AFP, specifically ≥1000 ng/mL, correlated with impaired survival, both considered overall and liver-related. In our cohort, the median OS in HCC patients with AFP in this range was 21 months, similar to what is reported in patients with unresectable HCC (BCLC B) [7,35]. AFP ≥ 1000 ng/mL also proved to be an independent risk factor for mortality in the multivariate Cox regression analysis. Our results agree with previous evidence from the literature. In a systematic review and meta-analysis, Hakeem et al. [27] analyzed a total of more than 12.000 patients and demonstrated better survival for patients with a preliver transplant AFP level < 1000 ng/mL. They also found that high pretransplant AFP was associated with poor tumor differentiation, in accordance with what we found in our cohort, where positive AFP was associated with histological HCC grade 3. More recently, Silva et al. [36] analyzed a large registry of more than 40.000 HCC patients and found that patients with elevated (200–1999 ng/mL) and highly elevated (≥2000 ng/mL) basal AFP levels had impaired OS, regardless of the HCC treatment plan. In a very recently published study, Yao et al. [37] assessed the predictive role of AFP in patients undergoing liver resection of early-stage HCC and showed that patients with high (400–999 ng/mL) and extremely high (≥1000 ng/mL) preoperative AFP were characterized by worse OS compared with patients with low (<400 ng/mL) AFP. Several other studies showed similar results [38,39,40,41,42].
We failed to find a discriminating role of the 400 ng/mL basal AFP cut-off toward survival outcomes. On the contrary, combined with previous literature evidence, our results seem to confirm the 1000 ng/mL cut-off as a reliable tool to identify patients with significantly different survival among those undergoing an HCC curative treatment, and its application in clinical practice in order to select patients needing a more aggressive follow-up and treatment approach may be considered.
In our cohort, AFP ≥ 1000 ng/mL was also associated with an increased risk of HCC recurrence and reduced recurrence-free survival. This result was expected and directly follows the correlation with survival, since HCC recurrence (intra- or extra-hepatic) is a major determinant of poor prognosis, and most patients in our cohort died of liver-related conditions. By differentiating between different patterns of recurrence, AFP ≥ 1000 ng/mL was correlated with local HCC recurrence after treatment, while AFP values ≥ 20 ng/mL with the development of metastases from HCC. These results also agree with previously published data [15,41,43,44,45]. In particular, numerous studies in the literature demonstrated that AFP is capable of activating the expression of metastasis-related factors through the PI3K/AKT179 pathway, therefore increasing the risk of metastasis development [28,46,47,48,49].
At the multivariate analysis, our results show that in addition to AFP ≥ 1000 ng/mL, tumor size ≥ 5 cm, Child–Pugh functional class B or C, BCLC stage A (early) compared with stage 0 (very early), and malignant PVT were independent risk factors for increased mortality. All these correlations agree with previous evidence. The 5 cm dimension cut-off has proved to predict more aggressive tumor behavior and prognosis in several studies [33,50,51,52], and the prognostic role of the Child–Pugh score in cirrhotic patients has been widely demonstrated [53,54,55]. Malignant PVT is an expression of advanced diseases and significantly affected survival in our cohort, similar to what has been shown in many previous studies [56,57,58,59,60].
In our study, the basal AFP value was significantly correlated with several clinical and HCC-related features at diagnosis. Regarding the size of the nodule, HCC with AFP ≥ 20 ng/mL showed larger dimensions, and statistical significance was maintained moving to groups with higher AFP cut-offs. The correlation between high values of AFP and HCC size ≥ 5 cm is widely reported [28,40,61,62]. Starting from the cut-off of 400 ng/mL, we observed that female patients and patients in more advanced functional classes (i.e., Child–Pugh B and C) were more represented. Also, these correlations are consistent with some studies in the literature [22,41,46,63,64,65], even though they are not confirmed by others [66,67,68]. Many previous studies reported a connection between PVT and neoplastic PVT, and higher values of AFP [46,69,70]. However, we did not find a correlation between these baseline variables and the various AFP cut-off groups, even at the highest 1000 ng/mL cut-off, a result that is in a certain sense unexpected, particularly considering that malignant PVT was a predictor of relevant outcomes, as described above; this could be due to the small number of patients with malignant PVT in our cohort, which yielded insufficient statistical power to find this connection. Finally, in relation to histological grading, our study showed a prevalence of HCC with poorly differentiated histological grade (G3) starting from AFP values ≥ 20 ng/mL, and this difference remained statistically significant progressing towards higher AFP cut-offs. These data are confirmed by numerous studies and could indicate that HCCs with elevated AFP values are more aggressive but also that a loss of cellular differentiation could lead to an increased production of AFP [71,72,73].
Our study has some limitations, of which the sample size and the retrospective nature of this study are the main ones. The sample size, although sufficient to demonstrate numerous correlations, was relatively small, especially for the highest AFP cut-offs, and some correlations could have not emerged. Moreover, the decision not to analyze a cohort of patients undergoing a single specific HCC treatment could be considered a limit of the study; however, this decision was driven by the purpose of assessing the predictive role of different AFP cut-offs in a wider, real-world-setting cohort, irrespective of the curative treatment performed. The retrospective nature of this study makes it necessary to validate our results on prospective cohorts. The strengths of this study were the homogeneity of the study cohort, with particular reference to regularity and adherence to the follow-up after HCC diagnosis and after treatment, the relevance of the outcomes analyzed, and the long-term follow-up that make these outcomes more robust and our results more reliable.
5. Conclusions
AFP is a simple and cost-saving biomarker of HCC. We demonstrated a predictive role of different AFP cut-offs, as assessed at HCC diagnosis, toward relevant outcomes in patients with HCC, mainly overall survival. We also found correlations between AFP and baseline features of the tumor. All these findings allow us to assess that a high basal AFP level correlates with more aggressive tumor behavior. Therefore, baseline AFP could help to immediately identify patients at higher risk of unfavorable disease evolution in order to refer them to a personalized therapeutic and follow-up approach, in accordance with the concept of precision medicine, with the aim of improving their quality of life and survival. Our results require validation in prospective and larger studies.
Acknowledgments
We would like to thank Sandro Rossi, the non-profit CMT foundation, for his important scientific achievements.
Author Contributions
Conceptualization, S.M., C.F., V.R. and A.A.; methodology, S.M., C.F., V.R. and A.A.; formal analysis, S.M.; resources, V.R. and A.A.; data curation, S.M., A.M., F.T.V., C.B., C.S. and L.V.; writing—original draft preparation, S.M., C.F. and D.A.; writing—review and editing, A.M., F.T.V., D.S., M.B., L.R., S.A., E.S., L.P., M.M., V.R. and A.A.; supervision, V.R. and A.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Fondazione IRCCS Policlinico San Matteo (number 0048910/22, approved 27 September 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rumgay H., Ferlay J., de Martel C., Georges D., Ibrahim A.S., Zheng R., Wei W., Lemmens V., Soerjomataram I. Global, regional and national burden of primary liver cancer by subtype. Eur. J. Cancer. 2022;161:108–118. doi: 10.1016/j.ejca.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Rutherford M.J., Arnold M., Bardot A., Ferlay J., De P., Tervonen H., Little A., Bucher O., St Jacques N., Gavin A., et al. Comparison of liver cancer incidence and survival by subtypes across seven high-income countries. Int. J. Cancer. 2021;149:2020–2031. doi: 10.1002/ijc.33767. [DOI] [PubMed] [Google Scholar]
- 3.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Toh M.R., Wong E.Y.T., Wong S.H., Ng A.W.T., Loo L.H., Chow P.K., Ngeow J. Global Epidemiology and Genetics of Hepatocellular Carcinoma. Gastroenterology. 2023;164:766–782. doi: 10.1053/j.gastro.2023.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Huang D.Q., El-Serag H.B., Loomba R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021;18:223–238. doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai A., Sandhu S., Lai J.P., Sandhu D.S. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World J. Hepatol. 2019;11:1. doi: 10.4254/wjh.v11.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Singal A.G., Llovet J.M., Yarchoan M., Mehta N., Heimbach J.K., Dawson L.A., Jou J.H., Kulik L.M., Agopian V.G., Marrero J.A., et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922–1965. doi: 10.1097/HEP.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich C.F., Nolsoe C.P., Barr R.G., Berzigotti A., Burns P.N., Cantisani V., Chammas M.C., Chaubal N., Choi B.I., Clevert D.A., et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020;46:2579–2604. doi: 10.1016/j.ultrasmedbio.2020.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Daniele B., Mauro Borzio A.E.B., Fiore F., Daniele G., Cabibbo G., Casadei Gardini A., Gian Grazi L., Lorenza Rimassa L., Dionisi F., ASSOCIAZIONE ITALIANA ONCOLOGIA MEDICA Linee Guida AIOM Epatocarcinoma. 2020. [(accessed on 1 February 2023)]. Available online: https://www.aiom.it/linee-guida-aiom-2020-epatocarcinoma/
- 11.Llovet J.M., Bru C., Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 12.Gitlin D., Perricelli A., Gitlin G.M. Synthesis of -fetoprotein by liver, yolk sac, and gastrointestinal tract of the human conceptus. Cancer Res. 1972;32:979–982. [PubMed] [Google Scholar]
- 13.Ball D., Rose E., Alpert E. Alpha-fetoprotein levels in normal adults. Am. J. Med. Sci. 1992;303:157–159. doi: 10.1097/00000441-199203000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Masseyeff R., Gilli J., Krebs B., Calluaud A., Bonet C. Evolution of alpha-fetoprotein serum levels throughout life in humans and rats, and during pregnancy in the rat. Ann. N. Y. Acad. Sci. 1975;259:17–28. doi: 10.1111/j.1749-6632.1975.tb25398.x. [DOI] [PubMed] [Google Scholar]
- 15.Omata M., Cheng A.L., Kokudo N., Kudo M., Lee J.M., Jia J., Tateishi R., Han K.H., Chawla Y.K., Shiina S., et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan S.L., Mo F., Johnson P.J., Siu D.Y., Chan M.H., Lau W.Y., Lai P.B., Lam C.W., Yeo W., Yu S.C. Performance of serum alpha-fetoprotein levels in the diagnosis of hepatocellular carcinoma in patients with a hepatic mass. HPB. 2014;16:366–372. doi: 10.1111/hpb.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T., Zhang K.H. New Blood Biomarkers for the Diagnosis of AFP-Negative Hepatocellular Carcinoma. Front. Oncol. 2020;10:1316. doi: 10.3389/fonc.2020.01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carr B.I., Akkiz H., Uskudar O., Yalcin K., Guerra V., Kuran S., Karaogullarindan U., Altintas E., Ozakyol A., Tokmak S., et al. HCC with low- and normal-serum alpha-fetoprotein levels. Clin. Pract. 2018;15:453–464. doi: 10.4172/clinical-practice.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schutte K., Schulz C., Link A., Malfertheiner P. Current biomarkers for hepatocellular carcinoma: Surveillance, diagnosis and prediction of prognosis. World J. Hepatol. 2015;7:139–149. doi: 10.4254/wjh.v7.i2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L., Zhao Y., Jia J., Chen H., Bai W., Yang M., Yin Z., He C., Zhang L., Guo W., et al. The Prognostic Value of Alpha-Fetoprotein Response for Advanced-Stage Hepatocellular Carcinoma Treated with Sorafenib Combined with Transarterial Chemoembolization. Sci. Rep. 2016;6:19851. doi: 10.1038/srep19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agopian V.G., Harlander-Locke M.P., Markovic D., Zarrinpar A., Kaldas F.M., Cheng E.Y., Yersiz H., Farmer D.G., Hiatt J.R., Busuttil R.W. Evaluation of Patients With Hepatocellular Carcinomas That Do Not Produce alpha-Fetoprotein. JAMA Surg. 2017;152:55–64. doi: 10.1001/jamasurg.2016.3310. [DOI] [PubMed] [Google Scholar]
- 22.Nomura F., Ohnishi K., Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patients. Cancer. 1989;64:1700–1707. doi: 10.1002/1097-0142(19891015)64:8<1700::aid-cncr2820640824>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 23.A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients: The Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 24.Borzio M., Dionigi E., Rossini A., Marignani M., Sacco R., De Sio I., Bertolini E., Francica G., Giacomin A., Parisi G., et al. External validation of the ITA.LI.CA prognostic system for patients with hepatocellular carcinoma: A multicenter cohort study. Hepatology. 2018;67:2215–2225. doi: 10.1002/hep.29662. [DOI] [PubMed] [Google Scholar]
- 25.Kanwal F., Singal A.G. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology. 2019;157:54–64. doi: 10.1053/j.gastro.2019.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniele B., Bencivenga A., Megna A.S., Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127:S108–S112. doi: 10.1053/j.gastro.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Hakeem A.R., Young R.S., Marangoni G., Lodge J.P., Prasad K.R. Systematic review: The prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2012;35:987–999. doi: 10.1111/j.1365-2036.2012.05060.x. [DOI] [PubMed] [Google Scholar]
- 28.Jearth V., Patil P.S., Mehta S., Sundaram S., Seth V., Goel M., Patkar S., Bal M., Rao V. Correlation of Clinicopathological Profile, Prognostic Factors, and Survival Outcomes with Baseline Alfa-Fetoprotein Levels in Patients With Hepatocellular Carcinoma: A Biomarker that is Bruised but Not Broken. J. Clin. Exp. Hepatol. 2022;12:841–852. doi: 10.1016/j.jceh.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu C.Y., Liu P.H., Lee Y.H., Hsia C.Y., Huang Y.H., Lin H.C., Chiou Y.Y., Lee F.Y., Huo T.I. Using serum alpha-fetoprotein for prognostic prediction in patients with hepatocellular carcinoma: What is the most optimal cutoff? PLoS ONE. 2015;10:e0118825. doi: 10.1371/journal.pone.0118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marrero J.A., Kulik L.M., Sirlin C.B., Zhu A.X., Finn R.S., Abecassis M.M., Roberts L.R., Heimbach J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 31.Nagtegaal I.D., Odze R.D., Klimstra D., Paradis V., Rugge M., Schirmacher P., Washington K.M., Carneiro F., Cree I.A., Board W.C.o.T.E. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Associazione Italiana Oncologia Medica (AIOM) I NUMERI DEL CANCRO IN ITALIA 2023. [(accessed on 1 February 2023)]. Available online: https://www.aiom.it/i-numeri-del-cancro-in-italia/
- 33.Wang C.Y., Li S. Clinical characteristics and prognosis of 2887 patients with hepatocellular carcinoma: A single center 14 years experience from China. Medicine. 2019;98:e14070. doi: 10.1097/MD.0000000000014070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi J.W., Kang S., Lee J., Choi Y., Kim H.C., Chung J.W. Prognostication and risk factor stratification for survival of patients with hepatocellular carcinoma: A nationwide big data analysis. Sci. Rep. 2023;13:10388. doi: 10.1038/s41598-023-37277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biolato M., Gallusi G., Iavarone M., Cabibbo G., Racco S., De Santis A., Corte C.D., Maida M., Attili A.F., Sangiovanni A., et al. Prognostic ability of BCLC-B Subclassification in Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Ann. Hepatol. 2018;17:110–118. doi: 10.5604/01.3001.0010.7542. [DOI] [PubMed] [Google Scholar]
- 36.Silva J.P., Gorman R.A., Berger N.G., Tsai S., Christians K.K., Clarke C.N., Mogal H., Gamblin T.C. The prognostic utility of baseline alpha-fetoprotein for hepatocellular carcinoma patients. J. Surg. Oncol. 2017;116:831–840. doi: 10.1002/jso.24742. [DOI] [PubMed] [Google Scholar]
- 37.Yao L.Q., Fan Z.Q., Wang M.D., Diao Y.K., Chen T.H., Zeng Y.Y., Chen Z., Wang X.M., Zhou Y.H., Li J., et al. Prognostic Value of Serum α-Fetoprotein Level as an Important Characteristic of Tumor Biology for Patients Undergoing Liver Resection of Early-Stage Hepatocellular Carcinoma (BCLC Stage 0/A): A Large Multicenter Analysis. Ann. Surg. Oncol. 2024;31:1219–1231. doi: 10.1245/s10434-023-14525-w. [DOI] [PubMed] [Google Scholar]
- 38.Bai D.S., Zhang C., Chen P., Jin S.J., Jiang G.Q. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci. Rep. 2017;7:12870. doi: 10.1038/s41598-017-12834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tandon P., Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: A systematic review of 72 studies. Liver Int. 2009;29:502–510. doi: 10.1111/j.1478-3231.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng S.Y., Chen W.J., Lai P.L., Jeng Y.M., Sheu J.C., Hsu H.C. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: Significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int. J. Cancer. 2004;112:44–50. doi: 10.1002/ijc.20279. [DOI] [PubMed] [Google Scholar]
- 41.Tangkijvanich P., Anukulkarnkusol N., Suwangool P., Lertmaharit S., Hanvivatvong O., Kullavanijaya P., Poovorawan Y. Clinical characteristics and prognosis of hepatocellular carcinoma: Analysis based on serum alpha-fetoprotein levels. J. Clin. Gastroenterol. 2000;31:302–308. doi: 10.1097/00004836-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Sternby Eilard M., Holmberg E., Naredi P., Söderdahl G., Rizell M. Addition of alfa fetoprotein to traditional criteria for hepatocellular carcinoma improves selection accuracy in liver transplantation. Scand. J. Gastroenterol. 2018;53:976–983. doi: 10.1080/00365521.2018.1488180. [DOI] [PubMed] [Google Scholar]
- 43.Bruix J., Cheng A.L., Meinhardt G., Nakajima K., De Sanctis Y., Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J. Hepatol. 2017;67:999–1008. doi: 10.1016/j.jhep.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 44.Heimbach J.K., Kulik L.M., Finn R.S., Sirlin C.B., Abecassis M.M., Roberts L.R., Zhu A.X., Murad M.H., Marrero J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 45.Kudo M., Matsui O., Izumi N., Iijima H., Kadoya M., Imai Y., Okusaka T., Miyayama S., Tsuchiya K., Ueshima K., et al. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458–468. doi: 10.1159/000343875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chi X., Jiang L., Yuan Y., Huang X., Yang X., Hochwald S., Liu J., Huang H. A comparison of clinical pathologic characteristics between alpha-fetoprotein negative and positive hepatocellular carcinoma patients from Eastern and Southern China. BMC Gastroenterol. 2022;22:202. doi: 10.1186/s12876-022-02279-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y.Q., Wang A.J., Zhang T.T., Chen S.H. Association of alpha-fetoprotein and metastasis for small hepatocellular carcinoma: A propensity-matched analysis. Sci. Rep. 2022;12:15676. doi: 10.1038/s41598-022-19531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katyal S., Oliver J.H., Peterson M.S., Ferris J.V., Carr B.S., Baron R.L. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 49.Yokoo T., Patel A.D., Lev-Cohain N., Singal A.G., Yopp A.C., Pedrosa I. Extrahepatic metastasis risk of hepatocellular carcinoma based on α-fetoprotein and tumor staging parameters at cross-sectional imaging. Cancer Manag. Res. 2017;9:503–511. doi: 10.2147/CMAR.S147097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shinkawa H., Tanaka S., Kabata D., Takemura S., Amano R., Kimura K., Kinoshita M., Kubo S. The Prognostic Impact of Tumor Differentiation on Recurrence and Survival after Resection of Hepatocellular Carcinoma Is Dependent on Tumor Size. Liver Cancer. 2021;10:461–472. doi: 10.1159/000517992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minagawa M., Ikai I., Matsuyama Y., Yamaoka Y., Makuuchi M. Staging of hepatocellular carcinoma: Assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann. Surg. 2007;245:909–922. doi: 10.1097/01.sla.0000254368.65878.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goh B.K., Teo J.Y., Chan C.Y., Lee S.Y., Jeyaraj P., Cheow P.C., Chow P.K., Ooi L.L., Chung A.Y. Importance of tumor size as a prognostic factor after partial liver resection for solitary hepatocellular carcinoma: Implications on the current AJCC staging system. J. Surg. Oncol. 2016;113:89–93. doi: 10.1002/jso.24099. [DOI] [PubMed] [Google Scholar]
- 53.Peng Y., Qi X., Guo X. Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine. 2016;95:e2877. doi: 10.1097/MD.0000000000002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cholongitas E., Papatheodoridis G.V., Vangeli M., Terreni N., Patch D., Burroughs A.K. Systematic review: The model for end-stage liver disease--should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment. Pharmacol. Ther. 2005;22:1079–1089. doi: 10.1111/j.1365-2036.2005.02691.x. [DOI] [PubMed] [Google Scholar]
- 55.Durand F., Valla D. Assessment of prognosis of cirrhosis. Semin. Liver Dis. 2008;28:110–122. doi: 10.1055/s-2008-1040325. [DOI] [PubMed] [Google Scholar]
- 56.Kogo M., Kano A., Kiuchi Y., Mitamura K., Yoneyama K. Prognostic index for survival in patients after treatment for primary hepatocellular carcinoma. Dig. Dis. Sci. 2007;52:2444–2451. doi: 10.1007/s10620-006-9137-x. [DOI] [PubMed] [Google Scholar]
- 57.Nam J.Y., Lee Y.B., Lee J.H., Yu S.J., Kim H.C., Chung J.W., Yoon J.H., Kim Y.J. A Prognostic Prediction Model of Transarterial Radioembolization in Hepatocellular Carcinoma: SNAP-HCC. Dig. Dis. Sci. 2022;67:329–336. doi: 10.1007/s10620-021-06843-4. [DOI] [PubMed] [Google Scholar]
- 58.Karaoğullarindan Ü., Gümürdülü Y., Üsküdar O., Odabaş E., Güler H.S., Tozluklu N., Bağir E., Kuran S. Prognostic value and morphological findings of overexpression of glypican-3 in hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2023;35:89–93. doi: 10.1097/MEG.0000000000002452. [DOI] [PubMed] [Google Scholar]
- 59.Yu J.J., Li Y.N., Shu C., Yang H.Y., Huang Z., Tao R., Chen Y.Y., Chen X.P., Xiao W. Prognostic value of preoperative circulating tumor cells for hepatocellular carcinoma with portal vein tumor thrombosis: A propensity score analysis. J. Cancer Res. Clin. Oncol. 2023;149:8981–8991. doi: 10.1007/s00432-023-04834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim N., Yu J.I., Park H.C., Hong J.Y., Lim H.Y., Goh M.J., Paik Y.H. Nomogram for predicting overall survival in patients with large (>5 cm) hepatocellular carcinoma based on real-world practice. J. Liver Cancer. 2023;23:350–361. doi: 10.17998/jlc.2023.08.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farinati F., Marino D., De Giorgio M., Baldan A., Cantarini M., Cursaro C., Rapaccini G., Del Poggio P., Di Nolfo M.A., Benvegnù L., et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: Both or neither? Am. J. Gastroenterol. 2006;101:524–532. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 62.Rusie D., Mercan Stanciu A., Toma L., Iliescu E.L. Correlation Between Serum Alpha-Fetoprotein and Tumour Size in Patients With Hepatocellular Carcinoma Treated With Direct-Acting Antivirals. Cureus. 2022;14:e24506. doi: 10.7759/cureus.24506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trevisani F., D’Intino P.E., Caraceni P., Pizzo M., Stefanini G.F., Mazziotti A., Grazi G.L., Gozzetti G., Gasbarrini G., Bernardi M. Etiologic factors and clinical presentation of hepatocellular carcinoma. Differences between cirrhotic and noncirrhotic Italian patients. Cancer. 1995;75:2220–2232. doi: 10.1002/1097-0142(19950501)75:9<2220::aid-cncr2820750906>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 64.Lu L., Shen L., Wu Z., Shi Y., Hou P., Xue Z., Lin C., Chen X., Group F.H.-b.S. Trajectories of serum α-fetoprotein and intermediate-stage hepatocellular carcinoma outcomes after transarterial chemoembolization: A longitudinal, retrospective, multicentre, cohort study. EClinicalMedicine. 2022;47:101391. doi: 10.1016/j.eclinm.2022.101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yen Y.H., Kee K.M., Li W.F., Liu Y.W., Wang C.C., Hu T.H., Tsai M.C., Lin C.Y. Stationary Trend in Elevated Serum Alpha-Fetoprotein Level in Hepatocellular Carcinoma Patients. Cancers. 2023;15:1222. doi: 10.3390/cancers15041222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arrieta O., Cacho B., Morales-Espinosa D., Ruelas-Villavicencio A., Flores-Estrada D., Hernández-Pedro N. The progressive elevation of alpha fetoprotein for the diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. BMC Cancer. 2007;7:28. doi: 10.1186/1471-2407-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bolondi L., Benzi G., Santi V., Gaiani S., Li Bassi S.L., Zironi G., Mazziotti A., Sama C., Grigioni W., Gozzetti G. Relationship between alpha-fetoprotein serum levels, tumour volume and growth rate of hepatocellular carcinoma in a western population. Ital. J. Gastroenterol. 1990;22:190–194. [PubMed] [Google Scholar]
- 68.Lee H.S., Chung Y.H., Kim C.Y. Specificities of serum alpha-fetoprotein in HBsAg+ and HBsAg- patients in the diagnosis of hepatocellular carcinoma. Hepatology. 1991;14:68–72. doi: 10.1002/hep.1840140112. [DOI] [PubMed] [Google Scholar]
- 69.Carr B.I., Guerra V. Low Alpha-Fetoprotein Levels Are Associated with Improved Survival in Hepatocellular Carcinoma Patients with Portal Vein Thrombosis. Dig. Dis. Sci. 2016;61:937–947. doi: 10.1007/s10620-015-3922-3. [DOI] [PubMed] [Google Scholar]
- 70.Siddiqui M.T.U., Fareed G., Khan M.R., Riaz A., Hamid S.S. Portal vein thrombosis in patients with hepatocellular carcinoma and early cirrhosis-prevalence and risk factors. Ecancermedicalscience. 2023;17:1581. doi: 10.3332/ecancer.2023.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blank S., Wang Q., Fiel M.I., Luan W., Kim K.W., Kadri H., Mandeli J., Hiotis S.P. Assessing prognostic significance of preoperative alpha-fetoprotein in hepatitis B-associated hepatocellular carcinoma: Normal is not the new normal. Ann. Surg. Oncol. 2014;21:986–994. doi: 10.1245/s10434-013-3357-z. [DOI] [PubMed] [Google Scholar]
- 72.Sauzay C., Petit A., Bourgeois A.M., Barbare J.C., Chauffert B., Galmiche A., Houessinon A. Alpha-foetoprotein (AFP): A multi-purpose marker in hepatocellular carcinoma. Clin. Chim. Acta. 2016;463:39–44. doi: 10.1016/j.cca.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 73.Wei Z., Zhang Y., Lu H., Ying J., Zhao H., Cai J. Serum alpha-fetoprotein as a predictive biomarker for tissue alpha-fetoprotein status and prognosis in patients with hepatocellular carcinoma. Transl. Cancer Res. 2022;11:669–677. doi: 10.21037/tcr-21-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The research data are stored in an institutional repository and will be shared upon request to the corresponding author.