Abstract
Background:
Due to variations in perforator vasculature, deep inferior epigastric artery perforator (DIEP) flap preoperative imaging can minimize operative time required to locate the most suitable perforators. Dedicated computed tomography angiography (CTA) has been the gold standard; however, many patients have already undergone a staging computed tomography (CT) per oncologic workup. The benefits from CTA may also be realized with a staging CT or CT with IV contrast.
Methods:
Ten patients who underwent DIEP flap reconstruction with staging CT and CTA within 3 years of one another were included in this study. Reviewers evaluated axial views of both imaging modalities separately to identify each visible perforator in reference to the pubic symphysis from the xiphoid to the pubic symphysis. An intraclass correlation coefficient (ICC) was used to determine agreement in location of perforators between the two imaging studies. Statistical analysis was performed using an ICC and Wilcoxon signed rank-tests.
Results:
The identified perforators within the patient cohort had an excellent correlation between their location on CT and CTA based upon ICC. The mean number of perforators identified in the CT group was 15.3 (SD 4.9) and in the CTA group was 18.8 (SD 6.4), which was not statistically different (P = 0.247).
Conclusions:
CT has similar efficacy in identifying number of perforators and perforator location to dedicated CTA for preoperative planning in DIEP flaps. This has the potential for decreased patient contrast and ionizing radiation exposure as well as improved patient and healthcare resource utilization.
Takeaways
Question: Can preexisting oncologic staging computed tomography (CT) with IV contrast be used instead of dedicated computed tomography angiography (CTA) for preoperative DIEP flap planning with similar efficacy for perforator identification?
Findings: Retrospective comparison of 10 patients who underwent DIEP flap reconstruction with CTA within 3 years of staging CT revealed excellent correlation between perforator location. Mean number of perforators identified in the CT versus CTA groups was 15.3 versus 18.8, respectively (P = 0.247).
Meaning: Preexisting staging CT can be used instead of dedicated CTA without compromising accuracy of preoperative DIEP flap planning, meanwhile minimizing contrast induced nephropathy and radiation exposure.
INTRODUCTION
The deep inferior epigastric artery perforator (DIEP) flap procedure is the gold standard for autologous breast reconstruction options after mastectomy for breast cancer.1 The vascular anatomy of the deep inferior epigastric system has multiple variants, with anatomical differences in almost all patients. These variations can lead to additional dissection in the operating room to locate the most suitable perforators to optimize postoperative outcomes for the patient. Due to the extensive variations of the vasculature, studies have shown the utility of utilizing preoperative computed tomography angiography (CTA) imaging to aid in the visualization of a patient’s deep perforating arteries. CTA has been used as the gold standard to provide high-quality imaging and localization of perforating arteries, more accurately and detailed than ultrasound, the previous standard of care.2 CTA has been shown to be extremely accurate in mapping DIEP artery courses for dissection, benefitting surgeons perioperatively.3 Studies have shown that using CTA (instead of ultrasound) reduces the average operative time by approximately an hour.2,4,5 Other benefits include decreased morbidity and decreased risk of flap failure.2 By using CTA, the perforating arteries can be located and marked superficially on the patient before surgery, thus decreasing time in the operating room and improving patient outcomes.4,6–12 Using CTA imaging instead of ultrasound or magnetic resonance arteriography reduces overall healthcare costs by decreasing operation time.13,14 By using radiographic measurements in the images, the corresponding DIEP can be identified and dissected much more quickly in the operating room. However, the use of CTA exposes the patient to radiation and increases healthcare burden and costs.1 It has been shown clinically that up to 5.8% of patients who receive IV contrast necessary for CTA develop an acute kidney injury.15 Per oncology workup, some DIEP flap candidates have already undergone staging computed tomography (CT) to determine tumor burden.
By evaluating the previously conducted staging CT for the same anatomical landmarks that are typically visualized on the CTA preoperative imaging, it may be possible to achieve the same benefit gained from the CTA by solely using the staging CT. If this is the case, the DIEP flap procedure may be performed with the same confidence without ordering and processing a preoperative CTA. The potential benefits of doing so are decreasing radiation and contrast exposure to the patient, as well as decreasing the total cost of the DIEP flap procedure on the healthcare system. The aim of this study was to assess if using preexisting staging CT offers similar imaging data regarding perforator vessels, eliminating the need for the preoperative CTA.
METHODS
Institutional review board approval was obtained from the Bell Chapter of the Hawkins Foundation in Akron, Ohio before collecting data. Patients who underwent DIEP flap reconstruction with both staging CT and preoperative CTA between January 2017 and February 2021 by the senior author (D.C.) were included in this study. As standard DIEP reconstruction protocol, all patients received preoperative CTA imaging (protocol acquisition parameters outlined in Table 1); however, only selected patients had preexisting staging CT for staging of their breast cancer. Standard radiation dose comparison for the two study protocols of interest is outlined in Table 2. Patients who did not receive both staging CT and preoperative CTA were excluded from this study.
Table 1.
Acquisition Parameters for CTA Abdomen/Pelvis DIEP Protocol
| Parameter | CTA DIEP Protocol |
|---|---|
| Acquisition (mm) | 32 × 0.7 |
| Pitch range and pitch increment | 0.35–1.5 (0.05) |
| Tube voltage (kV) | 80 |
| Tube current (mA) | 12–400 |
| Rotation time (s) | 0.8 |
| Reconstruction section width (mm) | 0.6–10 |
| Reconstruction slice increment (mm) | 0.1–10 |
Table 2.
Radiation Dose Comparison of CT Abdomen/Pelvis with Contrast versus CTA Abdomen Pelvis (DIEP)
| Scan/Recon | kV | Quality Ref. mAs | mA | CTDI Vol. (mGy) | DLP (mGy*cm) |
|---|---|---|---|---|---|
| CT abdomen/pelvis with IV contrast | 130 | 99 | 10.6 | 350 | |
| Topogram | 110 | 15 | 0.03 | 1.60 |
|
| CTA abdomen/pelvis DIEP | 110 | 94 | 6.54 | 287 | |
| Topogram | 110 | 15 | 0.03 | 2.21 | |
| Premonitoring | 110 | 22 | 1.44 | 1.44 | |
| Monitoring | 110 | 22 | 43.1 | 43.1 |
CTDI, CT dose index; mGy, milligray; DLP, dose length product.
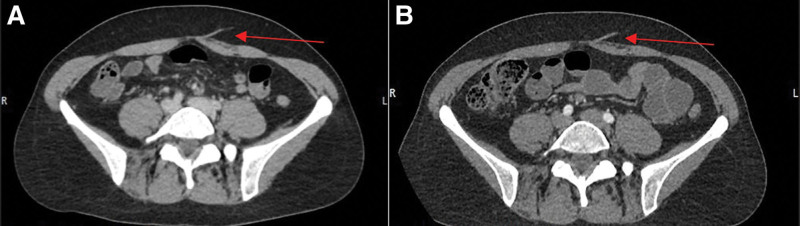
There were over 150 flaps in the study period, of which 10 patients were identified who met the inclusion criteria. These records were divided and reviewed by a plastic surgery fellow and two medical students. Data were subsequently verified by two senior radiologists trained in identifying perforators for preoperative evaluation of free flap reconstruction. For each patient, the staging CT was reviewed first, followed by a review of the preoperative CTA. An example side-by-side comparison of the same perforator in both imaging modalities is depicted in Figure 1. Reviewers first identified the center of the pubic symphysis in the axial view, to serve as a point of reference for additional measurements. Each hemi-abdominal wall was then scanned from pubic symphysis to xiphoid process in axial view. Perforators were identified exiting the deep fascia and measured in reference to the pubic symphysis. After identification of perforators, the renal artery was identified in the coronal view, and Hounsfield units were recorded. These steps were then repeated for the preoperative CTA. Statistical analysis was performed using an intraclass correlation coefficient (ICC) and Wilcoxon signed rank-tests.
Fig. 1.
CT vs CTA for preoperative identification and planning. Comparison of CT (A) to CTA (B) of the same perforator (arrows).
RESULTS
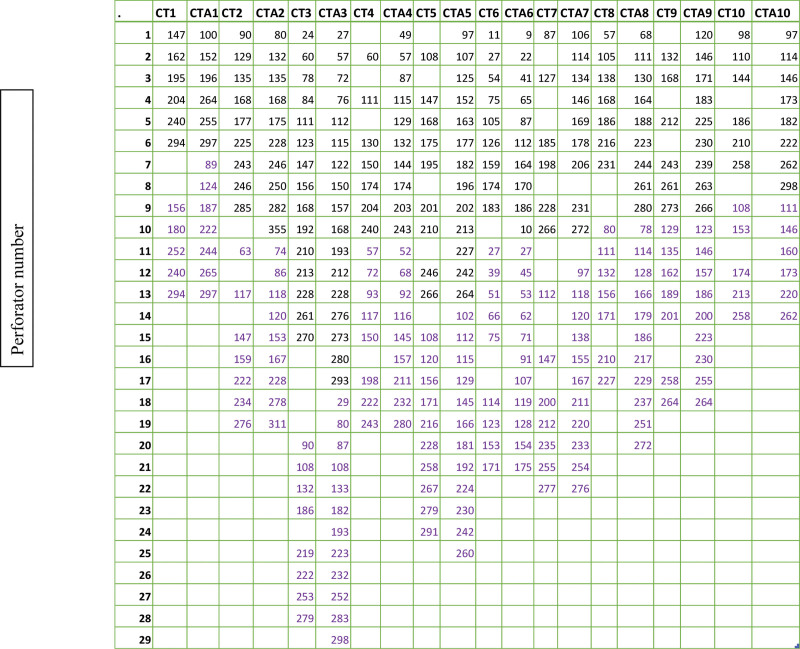
Figure 2 shows the distance from the pubic symphysis of all identified perforators on the 10 identified patients. An ICC was used to determine the meaning in agreement of the values in the location of the perforators. The ICC values can be seen in Table 3. The data show that the identified perforators within the patient cohort had excellent correlation between their location on CT and CTA.
Fig. 2.
Location of perforators on CT vs CTA in millimeters superior to pubic symphysis. The location of the perforators for each scan (CT1 and CTA 1 are patient 1) in millimeters from the pubic symphysis. Black is the right hemiabdomen, purple is the left hemiabdomen.
Table 3.
Intraclass Correlation Coefficient
| ICC | 95% CI | P | |
|---|---|---|---|
| Perforator #1 CT versus CTA | 0.997 | (0.990–0.999) | <0.01 |
| Perforator #2 CT versus CTA | 0.998 | (0.992–0.999) | <0.01 |
| Perforator #3 CT versus CTA | 0.999 | (0.994–1.00) | <0.01 |
| Perforator #4 CT versus CTA | 0.998 | (0.990–0.999) | <0.01 |
| Perforator #5 CT versus CTA | 0.997 | (0.988–0.999) | <0.01 |
| Perforator #6 CT versus CTA | 0.999 | (0.995–1.00) | <0.01 |
| Perforator #7 CT versus CTA | 0.998 | (0.998–1.00) | <0.01 |
| Perforator #8 CT versus CTA | 0.996 | (0.982–0.999) | <0.01 |
| Perforator #9 CT versus CTA | 0.998 | (0.992–1.00) | <0.01 |
| Perforator #10 CT versus CTA | 0.998 | (0.991–1.00) | <0.01 |
| Perforator #11 CT versus CTA | 0.994 | (0.940–0.999) | <0.01 |
| Perforator #12 CT versus CTA | 0.996 | (0.960–1.00) | <0.01 |
| Perforator #13 CT versus CTA | 0.961 | (0.120–0.999) | <0.01 |
| Perforator #14 CT versus CTA | 0.994 | (0.794–1.00) | <0.01 |
| Perforator #15 CT versus CTA | 0.991 | (-0.510 to 1.00) | NS |
| Perforator #16 CT versus CTA | 0.993 | (-0.383 to 1.00) | NS |
Koo and Li16 give the following parameters to provide meaning in the agreement of the values: below 0.50 = poor; between 0.50 and 0.75 = moderate; between 0.75 and 0.90 = good; and above 0.90 = excellent.
P < 0.05 defines statistical significance.
Table 4 lists the number of perforators identified for all the patients. The mean number of perforators identified in the CT group was 15.3 (SD 4.9), and in the CTA group was 18.8 (SD 6.4), which was not statistically significant P = 0.247. Table 4 also lists the Hounsfield units for each of the scans on the patients. The average Hounsfield units of the CT group was 148.4 (SD 44.7), and of the CTA group was 317 (SD 139.4), which was statistically significant (P < 0.01).
Table 4.
No. Perforators and Hounsfield Units Identified in CT versus CTA
| No. Perforators | Hounsfield Units | |||||
|---|---|---|---|---|---|---|
| Imaging Modality | Mean (n) | SD | P | Mean | SD | P |
| CT | 15.3 | 4.9 | 0.247 | 148.4 | 44.7 | <0.01 |
| CTA | 18.8 | 6.4 | 317 | 139.4 | ||
P < 0.05 defines statistical significance.
DISCUSSION
The results of this study indicate an excellent correlation between perforator location on CT and CTA. We feel there are several significant clinical implications of these findings. CTA is currently the preferred imaging modality for preoperative mapping of the DIEP perforators, given the increased sensitivity compared with ultrasound and spatial resolution to that of MRI; however, it is not without certain pitfalls.1 These may be of particular importance in patients who have already undergone staging CT with intravenous contrast as part of their breast cancer workup. Repeat CT imaging exposes the patient to more radiation, of whom nearly 6% will experience contrast-induced nephropathy.15 Additionally, it consumes more patient and healthcare resources that may not be necessary. Our data demonstrate that images obtained from the staging CT correlates very well to the data obtained from a dedicated CTA for DIEP preoperative planning. Avoiding an additional CT scan decreases patient radiation and contrast exposure, and avoids another costly imaging study.
Based on current Center for Medicare and Medicaid Services Current Procedural Terminology (CPT) codes and the 2023 Physician Fee Schedule, the CPT 74174 (CTA abdomen/pelvis with contrast including noncontrast images, if performed, and image postprocessing) total Medicare allowable fee is $373.91. This includes a technical fee of $271.24 and professional fee of $102.67. In comparison, the CPT 74177 (CT abdomen/pelvis with IV contrast) total Medicare allowable fee is $300.25. This includes a technical fee of $214.58 and professional fee of $85.67.17,18 Further, preoperative CT for DIEP flap planning has been shown to reduce operative time by approximately one hour compared with ultrasound.2,4,5 Existing literature concluded that 1 hour of operative time in California costs approximately $36–37 per minute in 2014.19 Accounting for inflation, these data indicate that preoperative CT saves approximately $2707.25 in operative time costs in 2022, given the average 1 hour reduction per case.
The potential advantages of obtaining preoperative imaging are not only financial in nature, as it has been well documented that preoperative CTA reduces flap loss and overall morbidity.9 This study demonstrates the efficacy of preexisting staging CT to identify perforator vessel number and location compared with dedicated CTA, for the purpose of preoperative DIEP mapping.
There are limitations to this study. It should be noted that although there was no significant difference in location or number of perforators identified, the CTA group had significantly higher Hounsfield units. This suggests that perforators may be more easily identified on CTA, specifically through their intramuscular course to aid in dissection planning. Although this study simply concluded a preexisting oncologic CT abdomen/pelvis with intravenous contrast is as efficient as CTA in identifying number and location of perforators, it did not explicitly evaluate accuracy of intramuscular perforator course mapping. A comparison of mapping perforator pathways may be difficult to quantify, but a future study should focus on comparing what the best perforator identified is in terms of location, caliber, and pathway through the rectus muscles.
Further, despite excellent ICC based on over 180 perforators evaluated, the study is limited by sample size. Some element of the small sample size is due to early detection in breast cancer with modern screening mammography, thus precluding the need for a staging CT abdomen/pelvis in many patients. Nonetheless, this surgical population will continue to be studied to gather more data.
CONCLUSIONS
This retrospective study demonstrates that preoperative staging computerized tomography with intravenous contrast has similar efficacy to dedicated CTA for preoperative planning in free deep inferior epigastric perforator flaps. There was no significant difference in number of perforators identified or perforator location in relation to the pubic symphysis. The potential impacts of this study include avoidance of the adverse effects of repeated contrast and radiation exposure, while simultaneously decreasing overall healthcare expenditures.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Published online 20 May 2024.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Phillips TJ, Stella DL, Rozen WM, et al. Abdominal wall CT angiography: a detailed account of a newly established preoperative imaging technique. Radiology. 2008;249:32–44. [DOI] [PubMed] [Google Scholar]
- 2.Rozen WM, Phillips TJ, Ashton MW, et al. Preoperative imaging for DIEA perforator flaps: a comparative study of computed tomographic angiography and Doppler ultrasound. Plast Reconstr Surg. 2008;121:9–16. [DOI] [PubMed] [Google Scholar]
- 3.Rozen WM, Ashton MW, Stella DL, et al. The accuracy of computed tomographic angiography for mapping the perforators of the DIEA: a cadaveric study. Plast Reconstr Surg. 2008;122:363–369. [DOI] [PubMed] [Google Scholar]
- 4.Casey WJ, III, Chew RT, Rebecca AM, et al. Advantages of preoperative computed tomography in deep inferior epigastric artery perforator flap breast reconstruction. Plast Reconstr Surg. 2009;123:1148–1155. [DOI] [PubMed] [Google Scholar]
- 5.Teunis T, van Voss MH, Kon M, et al. CT-angiography prior to DIEP flap breast reconstruction: a systematic review and meta-analysis. Microsurgery. 2013;33:496–502. [DOI] [PubMed] [Google Scholar]
- 6.Ohkuma R, Mohan R, Baltodano PA, et al. Abdominally based free flap planning in breast reconstruction with computed tomographic angiography: systematic review and meta-analysis. Plast Reconstr Surg. 2014;133:483–494. [DOI] [PubMed] [Google Scholar]
- 7.Karunanithy N, Rose V, Lim AK, et al. CT angiography of inferior epigastric and gluteal perforating arteries before free flap breast reconstruction. Radiographics. 2013;31:1307–1319. [DOI] [PubMed] [Google Scholar]
- 8.Lam DL, Mitsumori LM, Neligan PC, et al. Pre-operative CT angiography and three-dimensional image post processing for deep inferior epigastric perforator flap breast reconstructive surgery. Br J Radiol. 2012;85:e1293–e1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connor EF, Rozen WM, Chowdhry M, et al. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surg. 2016;5:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozen WM, Ashton MW, Stella DL, et al. The accuracy of computed tomographic angiography for mapping the perforators of the deep inferior epigastric artery: a blinded, prospective cohort study. Plast Reconstr Surg. 2008;122:1003–1009. [DOI] [PubMed] [Google Scholar]
- 11.Ghattaura A, Henton J, Jallali N, et al. One hundred cases of abdominal-based free flaps in breast reconstruction. The impact of preoperative computed tomographic angiography. J Plast Reconstr Aesthet Surg. 2010;63:1597–1601. [DOI] [PubMed] [Google Scholar]
- 12.Keys KA, Louie O, Said HK, et al. Clinical utility of CT angiography in DIEP breast reconstruction. J Plast Reconstr Aesthet Surg. 2013;66:e61–e65. [DOI] [PubMed] [Google Scholar]
- 13.Rozen WM, Ashtonm MW, Whitaker IS, et al. The financial implications of computed tomographic angiography in DIEP flap surgery: a cost analysis. Microsurg. 2009;29:168–169. [DOI] [PubMed] [Google Scholar]
- 14.Cina A, Barone-Adesi L, Rinaldi P, et al. Planning deep inferior epigastric perforator flaps for breast reconstruction: a comparison between multidetector computed tomography and magnetic resonance angiography. Eur Radiol. 2013;23:2333–2343. [DOI] [PubMed] [Google Scholar]
- 15.Weber R, van Hal R, Stracke P, et al. Incidence of acute kidney injury after computed tomography angiography±computed tomography perfusion followed by thrombectomy in patients with stroke using a postprocedural hydration protocol. J Am Heart Assoc. 2020;9:e014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CMS.gov. License agreement. Available at https://www.cms.gov/license/ama?file=/files/zip/list-codes-effective-january-1-2022-published-november-19-2021.zip. Published 2022. Accessed August 16, 2022. [Google Scholar]
- 18.CMS.gov. Physician fee schedule (no date). Available at https://www.cms.gov/medicare/payment/fee-schedules/physician. Accessed September 8, 2023. [Google Scholar]
- 19.Childers C, Maggard-Gibbons M. Understanding costs of care in the operating room. JAMA Surg. 2018;153:e176233. [DOI] [PMC free article] [PubMed] [Google Scholar]