Abstract
To evaluate radiological and clinical features in metastatic anaplastic lymphoma kinase+ non-small cell lung cancer patients and crizotinib efficacy in different lines. This national, non-interventional, multicenter, retrospective archive screening study evaluated demographic, clinical, and radiological imaging features, and treatment approaches in patients treated between 2013-2017. Totally 367 patients (54.8% males, median age at diagnosis 54 years) were included. Of them, 45.4% were smokers, and 8.7% had a family history of lung cancer. On radiological findings, 55.9% of the tumors were located peripherally, 7.7% of the patients had cavitary lesions, and 42.9% presented with pleural effusion. Pleural effusion was higher in nonsmokers than in smokers (37.3% vs. 25.3%, P = .018). About 47.4% of cases developed distant metastases during treatment, most frequently to the brain (26.2%). Chemotherapy was the first line treatment in 55.0%. Objective response rate was 61.9% (complete response: 7.6%; partial response: 54.2%). The highest complete and partial response rates were observed in patients who received crizotinib as the 2nd line treatment. The median progression-free survival was 14 months (standard error: 1.4, 95% confidence interval: 11.2–16.8 months). Crizotinib treatment lines yielded similar progression-free survival (P = .078). The most frequent treatment-related adverse event was fatigue (14.7%). Adrenal gland metastasis was significantly higher in males and smokers, and pleural involvement and effusion were significantly higher in nonsmokers—a novel finding that has not been reported previously. The radiological and histological characteristics were consistent with the literature data, but several differences in clinical characteristics might be related to population characteristics.
1. Introduction
The Global Burden of Disease study reported that more than 2.2 million new lung cancer cases were diagnosed, and 2 million lung cancer patients lost their lives in 2019.[1] This disease burden makes lung cancer the leading cause of cancer deaths globally, despite advances in diagnosis and treatment.[2] About 85% of the lung cancer cases are histologically classified as non-small cell lung cancer (NSCLC), including adenocarcinoma, squamous cell carcinoma, and large-cell carcinoma.[3] Advances in identifying driver mutations in NSCLC have led to the development of novel treatments like tyrosine kinase inhibitors (TKI), immune checkpoint inhibitors, or antiangiogenic molecules.[4]
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase and is found to be rearranged in about 3% to 7% of the NSCLC cases, particularly among adenocarcinoma subgroups of never/light young smokers.[5] Current evidence suggests that inhibition of ALK activity results in antitumoral efficiency in advanced NSCLC.[6,7] However, gene rearrangement must first be documented to make the ALK inhibitor therapy available for these patients. Several previous studies have evaluated ALK-positive (ALK+) NSCLC cases to predict gene rearrangement based on clinical and radiological features of the disease and reported several features of these tumors regarding tumor size, appearance, and localization.[8] However, the topic still needs further research to obtain more robust characteristics. Based on this background, this study aimed to evaluate the radiological and clinical features, and crizotinib efficacy in different lines in ALK+ NSCLC patients.
2. Patients and methods
This study was designed as a national, non-interventional, multicenter, retrospective archive study and included ALK+ NSCLC patients treated with ALK inhibitors between January 1, 2013 and December 31, 2017, in the participating centers in Türkiye. The inclusion criteria were being ≥ 18 years of age, having a diagnosis of advanced ALK+ NSCLC (confirmed by fluorescence in situ hybridization), having been treated with chemotherapy and/or ALK inhibitor at any stage, and having a computed tomography and positron emission tomography at diagnosis. Drop-outs were excluded. The protocol of the study was approved by the Non-interventional Clinical Research Ethics Committee of Hacettepe University (approval number: GO 18/610; date: January 8, 2019). This study was performed in line with the principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
The present study evaluated demographic (age, gender), clinical (date of diagnosis, stage of cancer, pathological/histological sub-type of cancer, metastatic sites, progression), radiological imaging characteristics at diagnosis, and treatment outcomes in patients with ALK+ NSCLC. ALK gene rearrangement in NSCLCs constitutes a distinct molecular phenotype that significantly benefits from the ALK-directed TKI treatment.[9] Although the currently available methods like next generation sequencing, fluorescence in situ hybridization, immunohistochemistry, and polymerase chain reaction (RT-PCR) can successfully detect ALK gene rearrangement, factors such as availability, cost, and accessibility can limit their use in clinical practice. Thus, the low rates of ALK positivity in high-incidence NSCLC underlies the importance of feasible and cost-effective methods that can be utilized, at least to make a distinction towards possible ALK positivity in NSCLC. With this regard, the radiographic and clinical features of patients emerge as possible signal-creating features.
Radiological imaging reports (such as X-ray, computed tomography, positron emission tomography) were obtained from hospital records of patients diagnosed with ALK+ NSCLC, and retrospectively evaluated to determine the radiological and clinical features at diagnosis. The radiological findings were based on the reports of the imaging studies and not reevaluated from raw images. Treatment outcomes were reported in terms of complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), objective response rate (ORR), disease control rate (DCR), and progression-free survival (PFS). Progression was defined by response evaluation criteria in solid tumors (RECIST Version 1.1).[10] Any use of ALK inhibitors after radiological progression was defined as Continuum beyond Progression Disease, which is the time interval until the switch to the subsequent systemic therapy, i.e. a next-generation TKI via a clinical trial or an early access program or chemotherapy or best supportive care.
2.1. Statistical analyses
Descriptive statistics were presented using median and interquartile range (25th–75th percentiles) for continuous data and frequency and percentage for categorical data. Continuous variables were compared between independent groups using the Mann–Whitney U test, and categorical variables were compared using the Chi-square test (or Fischer’s exact test when assumptions are not met). Survival analyses were done using the Kaplan–Meier method and compared between groups using the log-rank test. The median follow-up time was analyzed using reverse Kaplan–Meier method. Statistical significance was considered as a P-value < .05. All statistical analyses were performed using PASW Statistics for Windows, Version 18.0 (SPSS Inc., Chicago, IL).
3. Results
3.1. Demographic characteristics
A total of 367 patients with ALK+ NSCLC (54.8% males; median age at diagnosis 54 years) were included in the analyses. Smokers consisted 45.4% of the population, and 8.7% of the population had a family history of lung cancer. Histological type was adenocarcinoma in 97.3%. The epidermal growth factor receptor and ROS-1 were positive in 0.5% and 0.8% of the cases. The most frequent symptom was cough (53.1%) and majority of the patients had an Eastern Cooperative Oncology Group Performance Score of 1 (46.0%) at baseline. At follow-up, progressive disease was observed in 272 patients (74.0%). Comparison of baseline demographic and clinical characteristics between genders showed that smoking rate was significantly higher among males (P < .001); however, the presence of asthma/chronic obstructive pulmonary disease (P = .01) and acinar-cell carcinoma (P = .048) were significantly higher in females. Moreover, history of lung cancer in the family (P = .002) and hemoptysis (P = .03) was significantly higher among current smokers, however, cough symptoms (P = .007) were less frequent. Baseline demographic and clinical characteristics of the patients are presented in Table 1.
Table 1.
Baseline demographic and clinical characteristics of patients with ALK-positive non-small cell lung cancer.
| All patients | Gender | Smoking | |||||
|---|---|---|---|---|---|---|---|
| n = 367 | Male n = 201 |
Female n = 166 |
P | Absent (n = 185) |
Present (n = 154) |
P | |
| Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | |||
| Age at diagnosis, years | 54 [46–62] | 57 [48–62] | 52 [43–62] | .099 | 52 [43–62] | 56 [48–62] | .07 |
| n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Gender | <.001 | ||||||
| Female | 166 (42.2) | 130 (70.3) | 24 (15.6) | ||||
| Male | 201 (54.8) | 55 (29.7) | 130 (84.4) | ||||
| Smoking | 154 (45.4)* | 130 (64.7) | 24 (14.5) | <.001 | |||
| Asthma/COPD | 19 (5.2) | 5 (2.5) | 14 (8.4) | .01 | 13 (7) | 6 (3.9) | .21 |
| Family history of lung cancer | 32 (8.7) | 20 (10) | 12 (7.2) | .36 | 9 (4.9) | 23 (14.9) | .002 |
| Histology | .19 | .07 | |||||
| Adenocarcinoma | 357 (97.3) | 193 (96) | 164 (98.8) | 181 (97.8) | 149 (96.8) | ||
| NOS | 4 (1.1) | 2 (1) | 2 (1.2) | 3 (1.6) | – | ||
| Neuroendocrine | 3 (0.8) | 3 (1.5) | – | – | 3 (1.9) | ||
| Mixed | 3 (0.8) | 3 (1.5) | – | 1 (0.5) | 2 (1.3) | ||
| EGFR + | 2 (0.5) | 1 (0.5) | 1 (0.6) | 1 | 1 (0.5) | 1 (0.6) | 1 |
| ROS-1 + | 3 (0.8) | 3 (1.5) | – | .26 | – | 3 (1.9) | .09 |
| Symptoms | |||||||
| Cough | 195 (53.1) | 100 (49.8) | 95 (57.2) | .15 | 116 (62.7) | 74 (48.1) | .007 |
| Dyspnea | 177 (48.2) | 92 (45.8) | 85 (51.2) | .3 | 100 (54.1) | 73 (47.4) | .22 |
| Pain | 130 (35.4) | 70 (34.8) | 60 (36.1) | .79 | 71 (38.4) | 55 (35.7) | .61 |
| Weight loss | 103 (28.1) | 59 (29.4) | 44 (26.5) | .55 | 48 (25.9) | 53 (34.4) | .09 |
| Hemoptysis | 33 (9) | 21 (10.4) | 12 (7.2) | .28 | 11 (5.9) | 20 (13) | .03 |
| ECOG score at baseline | .34 | .13 | |||||
| 0 | 106 (28.9) | 60 (29.9) | 46 (27.7) | 49 (26.5) | 49 (31.8) | ||
| 1 | 169 (46) | 87 (43.3) | 82 (49.4) | 93 (50.3) | 68 (44.2) | ||
| ≥2 | 78 (21.3) | 44 (21.9) | 34 (20.5) | 39 (21.1) | 36 (23.3) | ||
Bold values indicate statistical significance at P < .05.
COPD = chronic obstructive pulmonary disease, ECOG = Eastern Cooperative Oncology Group, EGFR = epidermal growth factor receptor, IQR = inter-quartile range, NOS = not otherwise specified.
Because of missing data, percentages were reported over 339 patients who had complete data for smoking.
3.2. Radiological characteristics
At baseline, a total of 261 patients had radiological data. Analyses of radiological features detected that 44.1% of the cases had centrally and 55.9% had peripherally localized lesions. In addition, cavitary lesions were present in 7.7% and pleural effusion in 42.9% of patients. At baseline, the most common sites of metastatic disease were lungs (47.1%), bones (41.1%), and pleura (34.9%). Brain metastasis was present in 25.1% of the patients. There was no significant difference regarding radiological features and metastatic disease between males and females at baseline. However, comparison between patients according to smoking status showed that nonsmokers had a higher frequency of pleural effusion (37.3% vs 25.3%, P = .018), and pleural involvement (41.6% vs 28.6%, P = .013) than that of smokers, but adrenal gland metastasis was more prevalent among smokers (22.1% vs 12.5%, P = .018) (Table 2).
Table 2.
Radiological features and presence of metastatic disease at baseline.
| All patients | Gender | Smoking | |||||
|---|---|---|---|---|---|---|---|
| n = 367 | Male n = 201 |
Female n = 166 |
P | Absent n = 185 |
Present n = 154 |
P | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Radiological findings | |||||||
| Localization of primary | .48 | .82 | |||||
| Central | 115 (44.1)* | 64 (31.8) | 51 (30.7) | 59 (31.9) | 48 (31.2) | ||
| Peripheral | 146 (55.9)* | 84 (41.8) | 62 (37.3) | 71 (38.4) | 64 (41.6) | ||
| Cavitary lesion | 20 (7.7)* | 10 (5) | 10 (6) | .66 | 11 (5.9) | 7 (4.5) | .57 |
| Effusion | 112 (42.9)* | 54 (26.9) | 58 (34.9) | .10 | 69 (37.3) | 39 (25.3) | .018 |
| Metastatic disease | |||||||
| Lung | 173 (47.1) | 94 (46.8) | 79 (47.6) | .88 | 85 (45.9) | 79 (51.3) | .33 |
| Bone | 151 (41.1) | 88 (43.8) | 63 (38) | .26 | 71 (38.4) | 62 (40.3) | .72 |
| Pleural involvement | 128 (34.9) | 64 (31.8) | 64 (38.6) | .18 | 77 (41.6) | 44 (28.6) | .013 |
| Distant LAP | 114 (31.1) | 58 (28.9) | 56 (33.7) | .32 | 60 (32.4) | 46 (29.9) | .61 |
| Brain | 92 (25.1) | 55 (27.4) | 37 (22.3) | .26 | 44 (23.8) | 44 (28.6) | .32 |
| Liver | 67 (18.3) | 38 (18.9) | 29 (17.5) | .72 | 35 (18.9) | 27 (17.5) | .74 |
| Adrenal gland | 62 (16.9) | 41 (20.4) | 21 (12.7) | .05 | 23 (12.4) | 34 (22.1) | .018 |
LAP = lymphadenopathy.
Percentages were reported over 261 patients who had complete data for radiological characteristics.
3.3. Outcomes and side effects
The median follow-up was 32.2 months. The outcomes are presented in Table 3. The number of patients received crizotinib were 136 in the 1st line, 148 in the 2nd line, 36 in the 3rd line, and 20 in other lines. As per the reimbursement policies at the time, platinum-based chemotherapy was the first line treatment in 55.0% of the patients, and crizotinib was the ALK inhibitor given to 30.8%.
Table 3.
Crizotinib treatment outcomes.
| Overall | Line of treatment | ||||
|---|---|---|---|---|---|
| 1st line | 2nd line | 3rd line | Other | ||
| (n = 136) | (n = 148) | (n = 36) | (n = 20) | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Complete response | 28 (7.6) | 8 (6.2) | 16 (11.2) | 3 (9.4) | 1 (5.6) |
| Partial response | 199 (54.2) | 77 (59.2) | 93 (65.0) | 17 (53.1) | 9 (50.0) |
| Stable disease | 40 (10.9) | 14 (10.8) | 18 (12.6) | 4 (12.5) | 3 (16.7) |
| Progressive disease | 62 (16.9) | 31 (23.8) | 16 (11.2) | 8 (25) | 5 (27.8) |
| Objective response rate | 227 (61.9) | 85 (65.5) | 109 (76.2) | 20 (62.5) | 10 (55.6) |
| Disease control rate | 267 (72.8) | 99 (76.3) | 127 (88.8) | 24 (75.0) | 13 (72.3) |
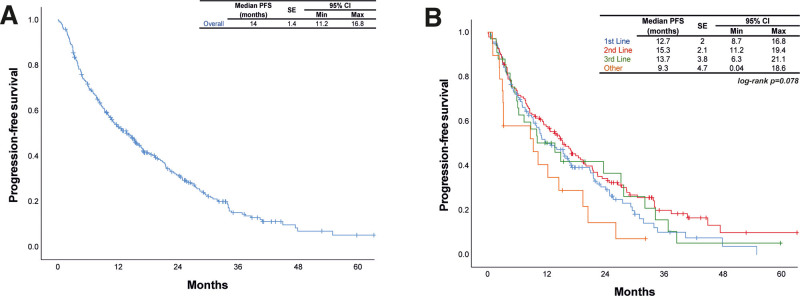
On ALK inhibitors, CR was achieved in 7.6% of patients, PR in 54.2%, SD in 10.9%, and progressive disease in 16.9%. The highest CR and PR rates were observed among patients who received crizotinib as 2nd line treatment. The median PFS was 14 months (standard error [SE]: 1.4, 95% confidence interval: 11.2–16.8 months) among all patients. Comparison of PFS between crizotinib treatment groups showed that the outcomes were similar (P = .078) (Fig. 1). The median overall survival (OS) was 25.2 months (SE: 3.2, 95% confidence interval: 19–31.4 months) after crizotinib treatment.
Figure 1.
Progression-free survival (A) in all patients and (B) crizotinib treatment groups. CI = confidence interval; PFS = progression-free survival; Max = maximum; Min = minimum.
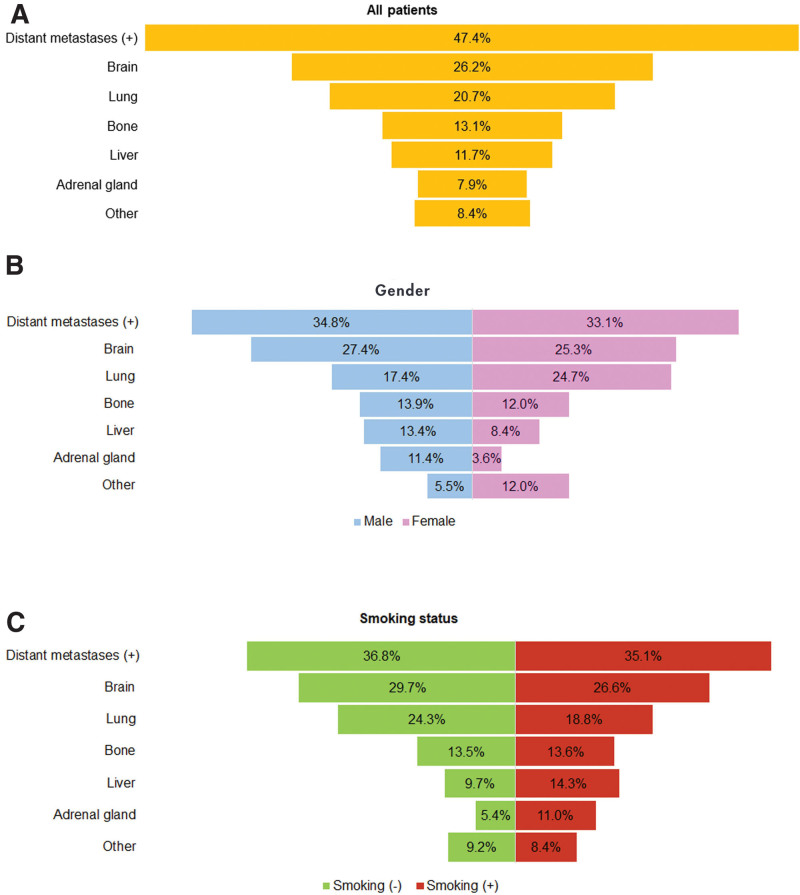
Most frequent treatment-related adverse event was fatigue in 14.7% of patients, followed by dyspnea and anemia (5.2%, each) (Table 4). Distant metastasis under crizotinib treatment was observed in 47.4% of the cases and the most common site of progression was the brain (26.2%). Comparison of metastatic sites between genders and according to smoking status detected that the only significant difference was for the adrenal gland, in which males (P = .006) and smokers (P = .046) had significantly higher metastasis rates (Fig. 2).
Table 4.
Treatment-related adverse events due to crizotinib.
| All-grade adverse events | n (%) |
|---|---|
| Fatigue | 54 (14.7) |
| Dyspnea | 19 (5.2) |
| Anemia | 19 (5.2) |
| Peripheral edema | 18 (4.9) |
| Visual impairment | 16 (4.4) |
| Newly developed effusion | 13 (3.5) |
| Abdominal pain | 12 (3.3) |
| Muscle cramps | 10 (2.7) |
| Stomatitis | 10 (2.7) |
| Venous thrombosis | 9 (2.5) |
| Neutropenia | 6 (1.6) |
| Hypothyroidism | 4 (1.1) |
| Decrease in heart rate | 3 (0.8) |
| Hyperlipidemia | 3 (0.8) |
| Prolongation of QT interval | 1 (0.3) |
| Thrombocytopenia | 1 (0.3) |
Figure 2.
Progression of metastatic disease during crizotinib treatment (A) in all patients, (B) according to gender, and (C) according to smoking status.
4. Discussion
This present study evaluated the general characteristics of patients with ALK+ NSCLC treated between 2013 to 2017 to identify the radiological features that might affect the diagnosis and the clinical features. To the best of our knowledge, it is the largest retrospective analysis, including the highest number of ALK+ NSCLC patients.[11–13] To summarize the role of radiological imaging methods in diagnosis and differential diagnosis of patients with ALK+ NSCLC, about 44.1% of patients had centrally, and 55.9% had peripherally localized lesions. In addition, cavitation was reported in only 7.7%, while effusion was present in about one-third of cases; 25.1% of the patients had brain metastasis and the median PFS was 14 months.
Radiologic features of ALK+ lung cancer were evaluated in several previous studies, which generally reported that these tumors were more centrally located, absent of pleural tail, had associated large pleural effusions, were relatively small in size, had lower tumor disappearance rate after treatment, and may appear as more solid masses with lobulated margins.[8,14,15] Some of those findings were also reported in different studies that underlined that these lesions had a more solid pattern, pleural and soft tissue metastasis, metastatic sclerotic bone lesions, and when compared to epidermal growth factor receptor mutant patients, had positive bronchoscopic findings suggesting a central localization.[8,14,16–19] Our results partly complied with these results that the solid pattern, high-frequency of bone metastasis, and pleural involvement were also observed in our cases, about 44% of patients had centrally located lesions, but more than half of the patients had a peripherally located tumor. However, tumor localization may vary between studies, i.e. Halpenny and colleagues found no significant difference in location.[20] The sites of distant metastasis were also similar in studies that bone, pleura, brain, and lymph-node metastases were the most common metastatic sites, like in our study.[21] Additionally, it is worth mentioning that although the radiological features are still being researched in ALK+ lung cancer, they are far from replacing immunohistochemistry, which is the cheapest and prevalently available method to detect ALK positivity.
Our study also evaluated the radiological findings and presence of metastatic disease according to gender and smoking status, and found that adrenal gland metastasis was significantly higher in males and smokers, and pleural involvement and effusion were significantly higher in nonsmokers. This is a novel finding that has not been reported previously but should be interpreted cautiously. Previous studies showed that smoking compounds downregulates cell cycle and inhibits apoptosis, while inducing inflammation and altering cell adhesion.[22] Moreover, animal models also showed that smoking-induced inflammation might play a possible role in benign and/or malignant pleural effusion in naïve mice.[23] Based on these previous reports, our results about the increased pleural involvement and effusion among nonsmokers seems discrepant, but these comparisons are only primary comparisons between demographic subgroups and lacks to suggest a causal relationship between these conditions. However, these results might imply that gender and smoking may be significant factors to be considered in the clinical management of ALK+ NSCLC. Previous studies reported that patients with ALK+ disease were younger (49–54 years old) and never or light smokers.[8,14,16,24] Nevertheless, there were conflicting reports regarding gender predominance, in which some studies reported a male predominance,[19,21,25,26] whereas some studies reported higher proportions of females.[8,16,27] Our results also related that the median age at diagnosis was 54 years, similar to the literature data. For the gender predominance, we found that more than half of the patients were males. However, for smoking history, nearly half (45.4%) of the cases were smokers.
The role of crizotinib for ALK+ NSCLC was first evaluated in an international, multicenter Phase I study (PROFILE 1001),[28,29] where ORR was 57%, with 33% achieving SD. In 2009, a single-arm, global Phase II study of crizotinib (PROFILE 1005) recruited 136 patients with ALK+ NSCLC who progressed after initial chemotherapy. The ORR was 51%, and DCR at 12 weeks was 74%, which was impressive in a group of pretreated patients.[30] The subsequent Phase III trial PROFILE 1007 demonstrated that when compared with second-line chemotherapy, crizotinib prolonged PFS, increased response rates, and improved the quality of life in patients with advanced, previously treated ALK+ NSCLC.[31] PFS was significantly improved in the crizotinib arm (median PFS: 7.7 months vs 3 months), but there was no OS benefit. The most recent phase III PROFILE 1014 trial compared crizotinib with chemotherapy as first-line treatment in patients with ALK+ advanced NSCLC, and reported that OS was not reached with crizotinib and 47.5 months with chemotherapy, 4-year survival probability was 56.6% with crizotinib and 49.1% with chemotherapy, and highlighted the benefit of crizotinib for prolonging survival in this patient population.[32] In another retrospective study in a single Chinese Cancer Center, crizotinib was used as first-line treatment in 60.6%, second-line treatment in 28.8%, and third-line or later in 10.6% of 104 patients with ALK+ NSCLC, which reported that ORR and DCR were 82.7% and 98.1%, PFS and OS were 13.0 months and 36.0 months.[21] The outcomes in our study in terms of treatment responses: ORR was 61.9%, while DCR was 72.8%. Median PFS was 14 months (SE: 1.4 months) in the entire study group, but 12.7 months (SE: 2 months), 15.3 months (SE: 2.1 months), 13.7 months (SE: 3.8 months), and 9.3 months (SE: 4.7 months) in patients using crizotinib as first, second, third, and other lines of treatment, respectively. Although PFS after 2nd-line treatment was higher, comparisons showed that PFS was similar between all lines of treatment. One reason for this higher PFS in the 2nd-line was that at the time of data collection the local regulations in the country allowed crizotinib over chemotherapy, thus crizotinib was used for patients following 1 or 2 cycles of chemotherapy, which might also explain the difference with PROFILE trial. Another reason for the PFS after 2nd-line treatment being higher might be due to the effect of survival bias.
Crizotinib was shown to be tolerable with a good safety profile, and the main adverse effects included visual disturbances, gastrointestinal side effects, and pneumonitis. In our study, the most common treatment-related adverse event was fatigue, followed by dyspnea and anemia. The treatment outcomes and side effects had a partial concordance with the available evidence in the literature.
Besides the strengths of this study, there were also several limitations. First, this is a retrospective study, which is a limited design regarding data collection. Second, the limited availability of the radiological and adverse event data restricted further assessments with the related parameters. Third, radiological assessments were done locally in each center. Fourth, since the primary focus was on crizotinib treatment in this study, other ALK inhibitors were not included, and comparative analyses were not performed, which might have been of interest to some readers. Moreover, all patients did not have detailed radiological reports, and the data regarding adverse events were also limited, such as a strikingly low number of QT prolongations, which was expected to be higher from a clinical perspective.
5. Conclusion
The present study is the first and largest study to evaluate the radiological and clinical characteristics in ALK+ NSCLC cases in the Turkish population. Accordingly, the PFS data were in accordance with the previous studies in the literature. Additionally, the results suggested that the pleural effusion and involvement, particularly in nonsmokers, and the brain metastases in radiological assessments, should alert the physicians in terms of ALK positivity in this patient group. Adrenal gland metastasis was significantly more frequent in males and smokers, and pleural involvement and effusion were significantly more frequent in nonsmokers. This is a novel finding that has not been reported previously.
Acknowledgments
The medical writing support and editorial support were provided at Omega CRO, Ankara, Türkiye and were funded by Pfizer. The statistical analyses of the study were also provided at Omega CRO, Ankara, Türkiye and were also funded by Pfizer.
Author contributions
Conceptualization: Saadettin Kilickap, Perran Fulden Yumuk, Mehmet Ali Nahit Sendur, Mustafa Erman.
Investigation: Saadettin Kilickap, Akin Ozturk, Nuri Karadurmus, Taner Korkmaz, Perran Fulden Yumuk, Irfan Cicin, Semra Paydas, Ebru Cilbir, Teoman Sakalar, Mukremin Uysal, Havva Yesil Cinkir, Necdet Uskent, Necla Demir, Abdullah Sakin, Oldac Uras Dursun, Birkan Aver, Nazim Serdar Turhal, Serkan Keskin, Deniz Tural, Yesim Eralp, Fatma Bugdayci Basal, Hatime Arzu Yasar, Mehmet Ali Nahit Sendur, Umut Demirci, Erdem Cubukcu, Mustafa Karaagac, Burcu Cakar, Ali Murat Tatli, Tarkan Yetisyigit, Semiha Urvay, Pinar Gursoy, Basak Oyan, Zeynep Hande Turna, Abdurrahman Isikdogan, Omer Fatih Olmez, Ozan Yazici, Devrim Cabuk, Mehmet Metin Seker, Olcun Umit Unal, Nezih Meydan, Sadi Kerem Okutur, Didem Tunali, Mustafa Erman.
Methodology: Saadettin Kilickap.
Project administration: Saadettin Kilickap.
Supervision: Saadettin Kilickap.
Validation: Saadettin Kilickap.
Visualization: Saadettin Kilickap.
Writing – original draft: Saadettin Kilickap, Akin Ozturk, Nuri Karadurmus, Taner Korkmaz, Perran Fulden Yumuk, Irfan Cicin, Semra Paydas, Ebru Cilbir, Teoman Sakalar, Mukremin Uysal, Havva Yesil Cinkir, Necdet Uskent, Necla Demir, Abdullah Sakin, Oldac Uras Dursun, Birkan Aver, Nazim Serdar Turhal, Serkan Keskin, Deniz Tural, Yesim Eralp, Fatma Bugdayci Basal, Hatime Arzu Yasar, Mehmet Ali Nahit Sendur, Umut Demirci, Erdem Cubukcu, Mustafa Karaagac, Burcu Cakar, Ali Murat Tatli, Tarkan Yetisyigit, Semiha Urvay, Pinar Gursoy, Basak Oyan, Zeynep Hande Turna, Abdurrahman Isikdogan, Omer Fatih Olmez, Ozan Yazici, Devrim Cabuk, Mehmet Metin Seker, Olcun Umit Unal, Nezih Meydan, Sadi Kerem Okutur, Didem Tunali, Mustafa Erman.
Writing – review & editing: Saadettin Kilickap, Akin Ozturk, Nuri Karadurmus, Taner Korkmaz, Perran Fulden Yumuk, Irfan Cicin, Semra Paydas, Ebru Cilbir, Teoman Sakalar, Mukremin Uysal, Havva Yesil Cinkir, Necdet Uskent, Necla Demir, Abdullah Sakin, Oldac Uras Dursun, Birkan Aver, Nazim Serdar Turhal, Serkan Keskin, Deniz Tural, Yesim Eralp, Fatma Bugdayci Basal, Hatime Arzu Yasar, Mehmet Ali Nahit Sendur, Umut Demirci, Erdem Cubukcu, Mustafa Karaagac, Burcu Cakar, Ali Murat Tatli, Tarkan Yetisyigit, Semiha Urvay, Pinar Gursoy, Basak Oyan, Zeynep Hande Turna, Abdurrahman Isikdogan, Omer Fatih Olmez, Ozan Yazici, Devrim Cabuk, Mehmet Metin Seker, Olcun Umit Unal, Nezih Meydan, Sadi Kerem Okutur, Didem Tunali, Mustafa Erman.
Abbreviations:
- ALK
- Anaplastic lymphoma kinase
- ALK+
- ALK-positive
- CR
- complete response
- DCR
- disease control rate
- NOS
- not otherwise specified
- NSCLC
- non-small cell lung cancer
- ORR
- objective response rate
- OS
- overall survival
- PD
- progressive disease
- PR
- partial response
- SD
- stable disease
- SE
- standard error
- TKI
- tyrosine kinase inhibitors
The medical writing support and editorial support as well as statistical analyses of the study were funded by Pfizer.
OUD and BA are the employees of Pfizer Biopharmaceuticals Group, Istanbul, Türkiye. BOU reports research support for clinical trials through institution from Novartis, GSK, Astra Zeneca; honoraria from BMS, Amgen, Novartis, Pfizer, Astra Zeneca, Roche, MSD; support for attending meetings from Roche, Pfizer, Novartis and is on the advisory boards of Takeda, Roche, Astra Zeneca, MSD, Novartis, Amgen, Gilead. ME has support funding for medical writing from Pfizer, provides lectures for Pfizer, Novartis, Roche, Astellas, Janssen, MSD, Gen, Nobel, Deva, Eczacibasi, BMS, Takeda, Astra Zeneca, has support for attending meetings from Roche, has participation on advisory board of Novartis, Pfizer, Roche, Astellas, MSD, Deva, Astra Zeneca. The remaining authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Kilickap S, Ozturk A, Karadurmus N, Korkmaz T, Yumuk PF, Cicin I, Paydas S, Cilbir E, Sakalar T, Uysal M, Yesil Cinkir H, Uskent N, Demir N, Sakin A, Dursun OU, Aver B, Turhal NS, Keskin S, Tural D, Eralp Y, Bugdayci Basal F, Yasar HA, Sendur MAN, Demirci U, Cubukcu E, Karaagac M, Cakar B, Tatli AM, Yetisyigit T, Urvay S, Gursoy P, Oyan B, Turna ZH, Isikdogan A, Olmez OF, Yazici O, Cabuk D, Seker MM, Unal OU, Meydan N, Okutur SK, Tunali D, Erman M. A multicenter, retrospective archive study of radiological and clinical features of ALK-positive non-small cell lung cancer patients and crizotinib efficacy. Medicine 2024;103:21(e37972).
All authors certify that the manuscript is a unique submission and is not being considered for publication by any other source in any medium. The manuscript has not been published, in part or in full, in any form.
Contributor Information
Akin Ozturk, Email: onkoakin@gmail.com.
Nuri Karadurmus, Email: drnkaradurmus@yahoo.com.
Taner Korkmaz, Email: taner.korkmaz@gmail.com.
Perran Fulden Yumuk, Email: fuldenyumuk@yahoo.com; fyumuk@ku.edu.tr.
Irfan Cicin, Email: irfancicin@hotmail.com.
Semra Paydas, Email: sepay@cu.edu.tr.
Ebru Cilbir, Email: ebrucilbir@hotmail.com.
Teoman Sakalar, Email: drteomansakalar@gmail.com.
Mukremin Uysal, Email: mukreminuysal@yahoo.com.
Havva Yesil Cinkir, Email: drhavva1982@gmail.com.
Necdet Uskent, Email: nuskent@yahoo.com.
Necla Demir, Email: dralcen@hotmail.com.
Abdullah Sakin, Email: drsakin@hotmail.com.
Oldac Uras Dursun, Email: OldacUras.Dursun@pfizer.com.
Birkan Aver, Email: Birkan.Aver@pfizer.com.
Nazim Serdar Turhal, Email: turhal@superonline.com.
Serkan Keskin, Email: serkan.keskin@memorial.com.tr.
Deniz Tural, Email: deniztural@gmail.com.
Yesim Eralp, Email: yeralp@yahoo.com.
Fatma Bugdayci Basal, Email: dr.fatmabb@gmail.com.
Hatime Arzu Yasar, Email: arzuyasar@gmail.com.
Mehmet Ali Nahit Sendur, Email: masendur@yahoo.com.tr.
Umut Demirci, Email: drumutdemirci@gmail.com.
Erdem Cubukcu, Email: erdemcubukcu@uludag.edu.tr.
Mustafa Karaagac, Email: ermanm1968@gmail.com.
Burcu Cakar, Email: burcu.cakar@gmail.com.
Ali Murat Tatli, Email: alimurattat@hotmail.com.
Tarkan Yetisyigit, Email: drtarkan@yahoo.com.
Semiha Urvay, Email: s.elmaci@yahoo.com.tr.
Pinar Gursoy, Email: pinargursoy77@gmail.com.
Basak Oyan, Email: basakou@yahoo.com.
Zeynep Hande Turna, Email: hande.turna@gmail.com.
Abdurrahman Isikdogan, Email: drisikdogan@hotmail.com.
Omer Fatih Olmez, Email: olmezof@gmail.com.
Ozan Yazici, Email: drozanyazici@gmail.com.
Devrim Cabuk, Email: devrimcabuk@yahoo.com.
Mehmet Metin Seker, Email: mmetinseker@yahoo.com.tr.
Olcun Umit Unal, Email: drolcun@hotmail.com.
Nezih Meydan, Email: nezihmeydan@yahoo.com.
Sadi Kerem Okutur, Email: keremokutur@gmail.com.
Didem Tunali, Email: didemtun@hotmail.com.
Mustafa Erman, Email: ermanm1968@gmail.com.
References
- [1].GBD. 2019 Respiratory Tract Cancers Collaborators. Global, regional, and national burden of respiratory tract cancers and associated risk factors from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Respir Med. 2021;9:1030–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen YL, Chen WL, Cheng YC, et al. Development of a novel ALK rearrangement screening test for non-small cell lung cancers. PLoS One. 2021;16:e0257152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31:992–1001. [DOI] [PubMed] [Google Scholar]
- [4].Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–54. [DOI] [PubMed] [Google Scholar]
- [5].Shaw AT, Engelman JA. ALK in lung cancer: past, present, and future. J Clin Oncol. 2013;31:1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ardini E, Magnaghi P, Orsini P, Galvani A, Menichincheri M. Anaplastic lymphoma kinase: role in specific tumours, and development of small molecule inhibitors for cancer therapy. Cancer Lett. 2010;299:81–94. [DOI] [PubMed] [Google Scholar]
- [7].Camidge DR, Dziadziuszko R, Peters S, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the Global Phase III ALEX study. J Thorac Oncol. 2019;14:1233–43. [DOI] [PubMed] [Google Scholar]
- [8].Nakada T, Okumura S, Kuroda H, et al. Imaging characteristics in ALK fusion-positive lung adenocarcinomas by using HRCT. Ann Thorac Cardiovasc Surg. 2015;21:102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].The official site of the RECIST Working Group. RECIST (Response Evaluation Criteria in Solid Tumours). Available at: http://recist.eortc.org. Access date March 02, 2022. [Google Scholar]
- [11].Zhang B, Zhang Y, Xu J, et al. Characteristics and response to crizotinib in ALK-rearranged, advanced non-adenocarcinoma, non-small cell lung cancer (NA-NSCLC) patients: a retrospective study and literature review. Target Oncol. 2018;13:631–9. [DOI] [PubMed] [Google Scholar]
- [12].Auliac JB, Monnet I, Dubos-Arvis C, et al. Non-small-cell lung cancer (NSCLC) harboring ALK Translocations: clinical characteristics and management in a real-life setting: a French retrospective analysis (GFPC 02-14 study). Target Oncol. 2017;12:833–8. [DOI] [PubMed] [Google Scholar]
- [13].Cameron LB, Hitchen N, Chandran E, et al. Targeted therapy for advanced anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer. Cochrane Database Syst Rev. 2022;1:CD013453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Choi CM, Kim MY, Hwang HJ, Lee JB, Kim WS. Advanced adenocarcinoma of the lung: comparison of CT characteristics of patients with anaplastic lymphoma kinase gene rearrangement and those with epidermal growth factor receptor mutation. Radiology. 2015;275:272–9. [DOI] [PubMed] [Google Scholar]
- [15].Yamamoto S, Korn RL, Oklu R, et al. ALK molecular phenotype in non-small cell lung cancer: CT radiogenomic characterization. Radiology. 2014;272:568–76. [DOI] [PubMed] [Google Scholar]
- [16].Mendoza DP, Lin JJ, Rooney MM, et al. Imaging features and metastatic patterns of advanced ALK-rearranged non-small cell lung cancer. AJR Am J Roentgenol. 2020;214:766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim TH, Woo S, Yoon SH, Halpenny DF, Han S, Suh CH. CT characteristics of non-small cell lung cancer with anaplastic lymphoma kinase rearrangement: a systematic review and meta-analysis. AJR Am J Roentgenol. 2019;213:1059–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang EW, Digumarthy SR. Non small cell lung cancer with targetable driver alterations: imaging perspective. Integr. Cancer Sci. Ther. 2019;6:1–6. [Google Scholar]
- [19].Kang HJ, Lim HJ, Park JS, et al. Comparison of clinical characteristics between patients with ALK-positive and EGFR-positive lung adenocarcinoma. Respir Med. 2014;108:388–94. [DOI] [PubMed] [Google Scholar]
- [20].Halpenny DF, Riely GJ, Hayes S, et al. Are there imaging characteristics associated with lung adenocarcinomas harboring ALK rearrangements? Lung Cancer. 2014;86:190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu C, Yu H, Long Q, et al. Real world experience of crizotinib in 104 patients with ALK rearrangement non-small-cell lung cancer in a single chinese cancer center. Front Oncol. 2019;9:1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pezzuto A, Citarella F, Croghan I, Tonini G. The effects of cigarette smoking extracts on cell cycle and tumor spread: novel evidence. Future Sci OA. 2019;5:FSO394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Magkouta S, Glynos K, Pappas A, Papapetropoulos A, Kalomenidis I. Effect of smoking on experimental malignant pleural effusion. Eur Respir J. 2015;46:4343. [Google Scholar]
- [24].Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorc Oncol. 2008;3:13–7. [DOI] [PubMed] [Google Scholar]
- [25].Britschgi C, Addeo A, Rechsteiner M, et al. Real-world treatment patterns and survival outcome in advanced anaplastic lymphoma kinase (ALK) rearranged non-small-cell lung cancer patients. Front Oncol. 2020;10:1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chia PL, Mitchell P, Dobrovic A, John T. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clin. Epidemiol. 2014;6:423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ock CY, Yoo SH, Keam B, et al. Clinical factors affecting progression-free survival with crizotinib in ALK-positive non-small cell lung cancer. Korean J Intern Med. 2019;34:1116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Crinò L, Kim D, Riely GJ, et al. Initial phase II results with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC): PROFILE 1005. J Clin Oncol. 2011;29(15_Suppl):Abstract 7514. [Google Scholar]
- [31].Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. [DOI] [PubMed] [Google Scholar]
- [32].Solomon BJ, Kim DW, Wu YL, et al. Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non-small-cell lung cancer. J Clin Oncol. 2018;36:2251–8. [DOI] [PubMed] [Google Scholar]