Abstract
Leptospirosis is an uncommon infectious illness – a spirochetal zoonosis – caused by Leptospira species and the primary cause of human leptospirosis is exposure to the urine of infected rodents. Clinical manifestations of human leptospirosis are diverse, ranging from asymptomatic infection to severe life-threatening with multiorgan dysfunction. The severe condition is known as Weil’s disease, which is characterized by feverish illness with jaundice, acute kidney damage, and bleeding. The aim of this case report was to present a Weil’s disease which occurred simultaneously with a community-acquired pneumonia (CAP) resulting in serious complications. A 41-year-old man with Weil’s disease, as well as CAP caused by Streptococcus pneumoniae, and septic shock was presented. The patient was treated accordingly after establishing the diagnosis through history taking, physical examination, and laboratory tests. In this instance, the score for diagnosing leptospirosis based on Modified Faine’s Criteria was calculated resulting possible diagnoses; and therefore, therapeutic management was initiated. Despite presenting with severe symptoms, the patient recovered completely after receiving antibiotics and supportive care. This study highlights that when a patient has Weil’s disease and a CAP infection, which could cause unfavorable consequence, a prompt diagnosis and proper treatment could result satisfied patient recovery.
Keywords: Leptospirosis, Weil’s disease, multiple organ dysfunction, community-acquired pneumonia, septic shock
Introduction
Leptospirosis is an emerging zoonotic disease caused by Leptospira sp. [1] and becoming more common all over the world [2]. Each year, 1.03 million cases of leptospirosis are reported, with 58,900 fatalities [3]. Leptospirosis is anticipated to be the primary zoonotic cause of sickness and mortality. Furthermore, illness and death rates were the highest in the world’s poorest regions and in regions where monitoring is not frequently done [3]. Cuts and abrasions, as well as mucous membranes such as the conjunctiva, and oral, or vaginal surfaces, may serve as entrance points. Exposure may occur via direct contact with infected animals or by indirect contact with soil or water contaminated with infected animals’ urine [4]. Indirect contact with Leptospira- contaminated water or soil is much more prevalent, and it may be linked to recreational or occupational activities [4]. In addition to the dangers associated with outdoor labor, sewage work, military training, and agriculture in tropical locations with considerable rainfall are all recognized, with the last being the most significant in terms of numbers.
The clinical manifestation of leptospirosis ranges from subclinical or asymptomatic, modest indicator, self-limited symptoms to severe lethal presentations. A significant number of patients in endemic locations may have subclinical illness detected only by serology [5]. Leptospirosis may cause life-threatening consequences such as renal failure, meningitis, pulmonary hemorrhage, and multi-organ failure. Weil’s syndrome, a severe form of leptospirosis with high mortality, is characterized by liver dysfunctions accompanied by renal failure and hemorrhage [6]. Patients with severe leptospirosis should receive prompt diagnosis and intensive treatment. Although immediate antibiotic therapy may lessen the severity of leptospirosis, diagnosis is often delayed, resulting in a high fatality rate [7].
Patients infected with L. interrogans – one of the species of Leptospira – typically suffer from multiorgan dysfunction due to the difficulties in early detection of the disease [7]. A diagnosis based only on clinical examination is not always correct, but a specific diagnosis may only be ascertained after clinical suspicion has been established. The diagnosis of leptospirosis must be confirmed by blood or urine cultures, or serological testing, in addition to the presence of clinical symptoms. The World Health Organization (WHO) has proposed “Faine’s criteria” for leptospirosis diagnosis, which now has been modified to improve diagnostic sensitivity [8,9]. Prompt initiation of anti-biotherapy is essential for both disease control and preventing the microorganism’s urinary spread [10]. The aim of this case report was to describe a patient who experienced Weil’s disease with multiple organ dysfunction and a community-acquired pneumonia (CAP) due to Streptococcus pneumoniae. The patient experienced serious complications, including septic shock, but the condition improved with prompt diagnosis and treatments.
Case
A 41-year-old male from Grobogan, Karanganyar, Central Java, Indonesia, presented to the emergency department of Universitas Sebelas Maret Hospital, Surakarta, Central Java, Indonesia, on March 17, 2023 with a fever of 39oC, headache, malaise, nausea, and vomiting, which he had experienced for three days. The patient had developed jaundice, continual fatigue, and severe calf pain five days before hospital admission. The patient also complained of coughing for the last seven days, coughing up yellow mucus that grew increasingly aggravating. Additionally, the patient had also felt chest tightness for the past three days. The feces were a brownish-yellow color with a soft consistency and no blood or mucus; the urine was a dark color, and the volume was less than usual.
The patient works for the city’s sanitation service, scrubbing the drains along municipal streets every day. Approximately three days before complaining the health, an object injured the right leg while was working. The patient confessed that the boots were damaged at the time, but continued to wear them to work and was submerged in the water.
The patient reported having previously smoked. Recent travel, alcohol consumption, recent antibiotic exposure, or contact with sick individuals were denied. The patient also denied taking injectable drugs, engaging in deviant sexual behavior, or having several relationships. The patient experienced an ST-elevation myocardial infarction in 2017 while taking acetylsalicylic acid 1×80mg, atorvastatin 1×20mg, perindopril 1×4mg, and bisoprolol 1×5mg as routine medication. Later, however, the patient discontinued treatment on his own and was no longer routinely monitored by the physician, as no more complaints.
Physical examination at the emergency room revealed somnolence, low blood pressure of 70/30 mmHg with mean arterial pressure (MAP) 43.3 mmHg, bradycardia with a pulse rate of 43 beats per min (bpm), high body temperature (39°C), and tachypnea with a respiratory rate of 50x/min. The initial oxygen saturation was 86% and 10 liters per min non-rebreathing mask (NRM) oxygen was administered resulting the saturation rose to 98%. Conjunctival suffusion and icteric scleral were found in both eyes. Thoracic examination revealed the presence of coarse crackles in both lungs. There was tenderness on the right hypochondriac region of the abdomen, pain in the bilateral gastrocnemius, prolonged capillary refill time (CRT) of the lower extremities, and warm and sweaty skin. The patient was admitted to the intensive care unit (ICU) with the diagnosis of septic shock, Weil’s disease suspicion, and community-acquired pneumonia.
Blood laboratory on the first day of hospitalization showed a hemoglobin (Hb) level of 12 g/dL, thrombocytopenia with platelet counts of 51,000/μL, and leukocytosis (15,510/μL) with lymphocytopenia (4.2%) and neutrophilia (87.4%) (Table 1). Blood biochemical examinations revealed abnormal liver function with an increase of total bilirubin (2.04 mg/dL), direct bilirubin (1.32 mg/dL), aspartate transaminase (AST) (189 U/L), and alanine transaminase (ALT) (92 U/L). A rise in blood urea nitrogen (148 mg/dL) and serum creatinine (5.66 mg/dL) indicated impaired renal function were also found. Moreover, laboratory findings also showed electrolyte imbalance with severe hyponatremia (121.81 mmol/L) and hypocalcemia (1.01 mmol/L). Immuno-serological test for anti-Leptospira IgM was negative on the first day of hospitalization. Arterial blood gas analysis showed partially compensated metabolic acidosis with pH 7.310, PCO2 24 mmHg, PO2 119 mmHg, HCO3 12.3 mmHg, and lactate 4.20 mmol/L. Urinalysis test revealed deep yellow and slightly cloudy urine with hematuria (3+). Cultures on the patient’s sputum and blood were also conducted, in which blood culture did not show any growth of bacteria. Sputum culture results that came out five days after the examination yielded S. pneumoniae as the cause of pneumonia. Various serologic tests were also performed with anti-HAV, anti-HBsAg, anti-HCV, and anti-HIV were all negative. The test of dengue IgM and Salmonella typhi IgM were also negative (Table 1).
Table 1.
Serial results of blood tests
| Indicator | Normal value | Day 1 | Day 2 | Day 3 | Day 5 | Day 7 |
|---|---|---|---|---|---|---|
| Hematology | ||||||
| Leukocytes (103/mm3) | 4.2–9.3 | 15.51 | 15.68 | 8.69 | 10.13 | 9.2 |
| Thrombocytes (103/mm3) | 150–450 | 51 | 13 | 26 | 45 | 98 |
| Erythrocytes (106/mm3) | 4–5 | 3.94 | 3.85 | 4.25 | 4.16 | 4.45 |
| Hemoglobin (g/dL) | 12–15 | 12.0 | 11.6 | 12.8 | 12.4 | 12.8 |
| Hematocrit (%) | 37–43 | 31 | 30 | 33 | 33 | 37 |
| MCV (µm3) | 80–100 | 79.4 | 78.2 | 78.1 | 79.6 | 80.1 |
| MCH (pg) | 26–34 | 30.5 | 30.1 | 30.1 | 29.8 | 30.6 |
| MCHC (g/dL) | 32–36 | 38.3 | 38.5 | 38.6 | 37.5 | 35.6 |
| Diff count | ||||||
| Lymphocytes (%) | 20–40 | 4.2 | 3.8 | 5.5 | 16.3 | 19.1 |
| Monocytes (%) | 4–12 | 4.6 | 6.0 | 5.9 | 14.8 | 12.9 |
| Neutrophil (%) | 50–70 | 87.4 | 89.8 | 88.4 | 68.1 | 65 |
| Eosinophil (%) | 0.5–7 | 3.5 | 0.1 | 0.0 | 0.6 | 0.6 |
| Basophil (%) | 0-2 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 |
| Liver function | ||||||
| ALT (IU/L) | <31 | 189 | 64 | 29 | ||
| AST (IU/L) | 9–36 | 92 | 38 | 35 | ||
| Indirect bilirubin mg/dl | 0.20–0.80 | 0.72 | ||||
| Total bilirubin mg/dl | 0.10–1.00 | 2.04 | ||||
| Direct bilirubin mg/dl | 0.00–0.20 | 1.32 | ||||
| Kidney function | ||||||
| Urea (mg/dL) | 10–45 | 148 | 206 | 139 | 40 | |
| Creatinine (mg/dL) | 0.5–1.2 | 5.66 | 4.83 | 1.67 | 1.1 | |
| Transient blood glucose (mg/dL) | 75–200 | 109 | 125 | 130 | ||
| Electrolyte serum | ||||||
| Sodium mmol/L | 135–145 | 121.81 | 126.08 | 135.90 | ||
| Potassium mmol/L | 3.50–5.50 | 3.89 | 3.51 | 3.55 | ||
| Calcium mmol/L | 1.10–1.35 | 1.11 | 1.10 | 1.12 | ||
| Chloride | 96–106 | 104.06 | 113.80 | 100 |
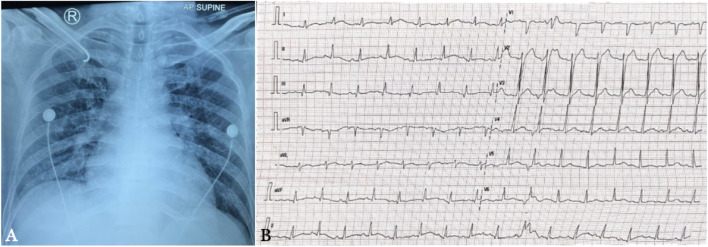
Chest X-ray showed prominent broncho vascular marking, right para cardiac infiltrates, and an increased cardiothoracic ratio, suggesting cardiomegaly and bronchopneumonia (Figure 1A). Electrocardiography (ECG) of day 2 of admission showed borderline 1st degree AV block and lateral ST elevation, thus, acute ST-elevation myocardial infarction (STEMI) was considered (Figure 1B).Report
Figure 1.
Chest radiograph on the first day of hospitalization (A) and electrocardiography (ECG) on day 2 of admission (B). The chest radiograph reveals right paracardial infiltrates, a higher cardiothoracic ratio, and significant broncho vascular marking and the ECG suggests borderline 1st degree AV block and lateral ST elevation.
On the first day of hospital admission, the patient was promptly transported from the emergency department to the ICU for continuous monitoring. The patient was diagnosed with septic shock, suspected leptospirosis, CAP with a pneumonia severity index score of 91 and a risk class of 4, and cardiogenic pulmonary edema. Nutritional therapy in addition to oxygen therapy was initiated. Rapid administration of 30 mL/kg 0.9% NaCl intravenous fluid with attention to the presence of overhydration, infusion of norepinephrine 0.2 mcg/kg/min, and dobutamine 5 mcg/kg/min were administered. A 3% NaCl infusion over 18 h was also given to correct severe hyponatremia. The patient was given 750 mg of levofloxacin on the first day, followed by 500 mg every 48 h, 2 g of ceftriaxone every 24 h, and 300 mg acetylcysteine infusion in 100 mL of normal saline every 12 h. Amino acid infusion 200 mg every 24 h, paracetamol infusion 1 g every 8 h (if necessary), folic acid 800 mcg once daily, and curcuma one tablet every 8 hours were also initiated. This patient was also prescribed oral atorvastatin 20 mg once daily due to the possibility of acute STEMI.
On the second day of admission, blood tests revealed a decrease in platelets to 13,000/L. Due to the patient’s severe thrombocytopenia and hematuria, four units (200 ml) of thrombocyte concentrate were administered. Post-transfusion blood exam showed an increase in platelet counts to 26,000/L. After serum electrolytes were corrected and re-examined, sodium levels increased to 126.08 mmol/L. A 3% NaCl drip over 18 h was administered to achieve normal sodium levels the following day. On the fourth day of admittance, hemodialysis was performed as the patient was diagnosed with stage 3 acute kidney injury, with a 24-h decrease in urination output of only 0.2 mL/kg/hour. The patient’s consciousness improved after hemodialysis, and the urine output increased to 0.5 cc/kg/h. Due to the suspected leptospirosis, the Leptospira IgM test was repeated on the fifth day and yielded positive. Following ICU treatment and monitoring for a duration of 5 days, which involved oxygenation therapy, fluid and nutritional therapy, electrolyte correction, administration of antibiotics based on clinical conditions, administration of vasopressors, transfusion of thrombocyte concentrate, and one session of hemodialysis due to a 24-hour urine output of 0.2 mL/kg/hour, the patient’s condition progressively stabilized and improved. On the sixth day of treatment, the patient was transferred from the ICU to the general ward.
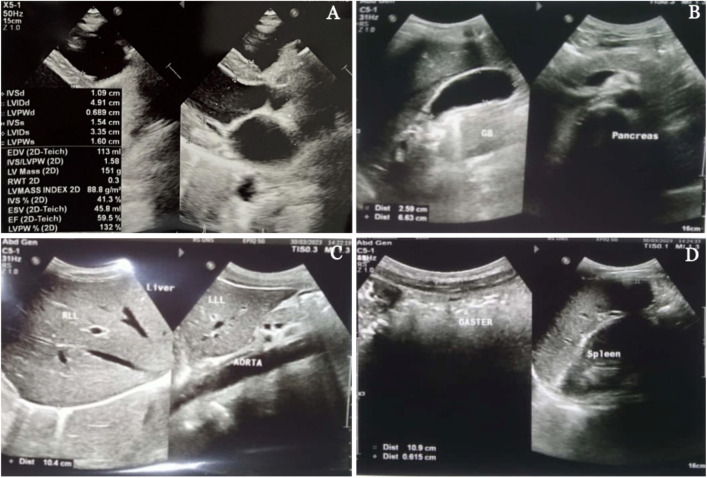
A transthoracic echocardiogram performed on the eighth day of admission revealed grade 1 diastolic dysfunction, mild pulmonic regurgitation, and mild tricuspid regurgitation, with a low likelihood of pulmonary hypertension (Figure 2A). The liver, gallbladder, spleen, pancreas, kidneys, and bladder were all normal, according to the abdominal ultrasound results (Figure 2B-D).
Figure 2.
Transthoracic echocardiography (A) and ultrasound (USG) of the abdomen (B-D) of the patient on the eighth day of hospital admission. The transthoracic echocardiography reveals grade 1 diastolic dysfunction, mild pulmonic regurgitation, and mild tricuspid regurgitation, with a low likelihood of pulmonary hypertension (A) and abdominal USG within normal limits (B-D).
The patient’s condition improved after eight days of hospitalization as his complaints of fever, tightness, jaundice, myalgia, and gastrointestinal issues diminished. His vital signs were stable, and he could communicate and eat normally. The patient was then discharged after eight days of hospitalization.
Discussion
In this report, a case of severe leptospirosis, also known as Weil’s disease, accompanied by CAP and S. pneumonia and a septic shock was described. On admission, the patient had a fever, headache, conjunctival suffusion, dark urine, myalgias, leukocytosis, thrombocytopenia, acute kidney injury, liver failure, hyperbilirubinemia, and shortness of breath. The patient also encountered septic shock and was treated according to the guidelines for septic shock management. The following treatments were administered: oxygenation, nutritional therapy, aggressive hydration, electrolyte imbalance therapy, vasopressors, and antibiotics. Due to severe thrombocytopenia and hematuria, the patient was administered a thrombocyte concentrate transfusion. Moreover, the patient underwent a single hemodialysis treatment due to a 24-hour urine output of 0.2 cc/kg. Strict hemodynamic, respiratory, hematological, hepatic, and kidney monitoring was performed. The patient’s condition improved with the treatment, and recovered after eight days.
Leptospirosis is a zoonosis that is prevalent in tropical countries and poses a considerable public health risk, with an estimated annual morbidity of 39.2 cases per 100.000 people in Indonesia [3,11]. Mud fever, slime fever, swamp fever, autumnal fever, infectious jaundice, field fever, and cane fever are all names for Leptospirosis. Leptospirosis is caused by spirochetes bacteria of the genus Leptospira, family Treponema. Leptospira infection may occur in humans either through direct contact (blood, urine, or other bodily fluids exchange) or indirect contact with the urine of infected animals that usually live in stagnant water, rivers, lakes, ditches, or feces. Leptospira can enter the human circulation via intact mucosa, and systemic vasculitis may be seen in both major and small blood vessels in addition to other organs [6]. According to the patient’s account, he was working hard to clear ditches when he sustained an injury on his right limb and accidentally immersed it in the water that was in the ditch.
The progression of the disease is comprised of two distinct clinical phases, septicemic and immune [12,13]. In the initial phase, humans exposed to the bacteria may exhibit flu-like symptoms 7 to 14 days after exposure [12]. During this time frame, patients may appear with conjunctival suffusion, a symptom that normally does not occur with other nonspecific tropical febrile infections such as dengue, typhoid, or malaria. Patients may also come with a fever [12,13]. After one month of contact with the bacteria second phase of leptospirosis may continue [12,14]. During this phase, the immune system responds to bacterial infection by unleashing and regulating cytokines 3 and 7. The body effectively balances these responses in moderate cases, resulting in asymptomatic resolution [12,15]. In extreme circumstances, however, the immune system would react more forcefully, resulting in a cytokine storm that damages many organs, including the liver, kidney, and heart [15]. Cytokine storms are caused when the immune system releases a large number of cytokines all at once. As a consequence of this phenomenon, abnormal laboratory findings such as higher levels of bilirubin, liver enzymes, renal function, and thrombocytopenia could be observed [12,16].
People who are infected with leptospirosis may present with asymptomatic illness, a self-limiting systemic infection, or severe and possibly deadly clinical manifestations. The clinical symptoms of leptospirosis span a wide spectrum and include all of these possibilities [14]. Headache, fever, malaise, myalgia, conjunctival suffusion, and a fleeting rash are among the symptoms that suddenly appear after the commencement of the condition [6,17]. Research has shown that only a small percentage of exposed people acquire severe types of diseases like Weil’s illness, whereas the vast majority merely have mild symptoms [6,17].
Weil’s disease is a severe type of leptospirosis that has a high death rate, characterized by hepatic dysfunctions associated with renal failure and hemorrhages. Weil’s disease accounts for 10% of all cases of leptospirosis [6]. Patients suffering from Weil’s disease should get an early diagnosis and comprehensive medical therapy as soon as possible. At this time, neither the pathophysiology of leptospirosis nor the variables that lead to severe leptospirosis are completely understood [18]. Like this patient, leptospirosis was characterized by severe manifestations, including jaundice, AKI, and hemorrhage [19,20].
The polymerase chain reaction (PCR) test is now considered to be the most accurate method for detecting leptospirosis [21,22]. A quick serology test using microscopic agglutination test (MAT) may be used as an alternative in secondary healthcare institutions that are unable to execute the test [13,21]. Therefore, early diagnosis and prompt treatment are pivotal to avoid illness development, which might lead to multiple organ failures and ultimate fatality [13,23].
Clinical history (Part A), epidemiological history (Part B), and laboratory parameters (Part C) make up the three parts of the Faine’s criteria that the WHO created for the diagnosis of leptospirosis [2]. Since then, Faine’s criteria have seen an improvement in their diagnostic sensitivity [8]. The patient in this case, had a fever of 39oC (2), headache (2), conjunctival suffusion (4), myalgia (4), jaundice (1), and dyspnea (2), according to the Modified Faine’s Criteria (Table 2). Epidemiological factors revealed contact with a contaminated environment (4), and bacteriological and laboratory findings revealed the presence of IgM antibodies to Leptospira (15). The total Modified Faine’s Criteria score for this patient was 34, which indicated leptospirosis as a possible diagnosis.
Table 2.
Scoring system for the diagnosis of leptospirosis based on the Modified Faine’s Criteria
| Criteria | Score | Patient core |
|---|---|---|
| Part A: Clinical data | ||
| Headache | 2 | 2 |
| Fever | 2 | |
| Fever >39°C | 2 | 2 |
| Conjunctival suffusion | 4 | 4 |
| Meningism | 4 | |
| Myalgia | 4 | 4 |
| Conjunctival suffusion with meningism and myalgia | 10 | |
| Jaundice | 1 | 1 |
| Albuminuria/nitrogen retention | 2 | |
| Hemoptysis/dyspnea | 2 | 2 |
| Part B: Epidemiological factors | ||
| Rainfall | 5 | |
| Contact with contaminated environment | 4 | 4 |
| Animal contact | 1 | |
| Part C: Bacteriological and laboratory findings | ||
| Isolation of Leptospira in culture-Diagnosis certain | ||
| Polymerase chain reaction | 25 | |
| Positive serology | ||
| Enzyme-linked Immunosorbent assay Ig M positive | 15 | 15 |
| Slide Agglutination test positive | 15 | |
| Other rapid test-latex agglutination tests | 15 | |
| Microscopic agglutination test-single positive in high titer | 15 | |
| Microscopic agglutination test-rising titer/seroconversion (paired sera) | 25 | |
| Presumptive diagnosis of leptospirosis is made of: | ||
| Part A or Part A and Part B score: 26 or more | ||
| Part A, B, C (total): 25 or more | 34 | |
| A score between 20 and 25 suggest leptospirosis as a possible diagnosis |
The vast majority of leptospirosis is considered to be minor and self-limiting. The development of illness might be avoided in some individuals by starting antibiotic treatment early. As soon as there is a reason to think that a patient has leptospirosis, empirical therapy should be initiated. Patients diagnosed with severe leptospirosis are often given intravenous penicillin (1.5 million units IV every six hours), ampicillin (0.5–1 g IV qid), ceftriaxone (1 g IV daily), or cefotaxime (1 g IV qid) as part of their treatment. It has been established that ceftriaxone is just as effective as penicillin in treating severe leptospirosis [6,17].
Additionally, the patient, in this instance, has experienced chest tightness for the past three days. The patient also complained of coughing with increasingly yellow mucous for seven days. Upon examination of the chest, the pulmonary fields of both lungs revealed coarse crackles. The chest x-ray showed prominent broncho vascular signs and right pericardiac infiltration, suggesting CAP. S. pneumonia is a common cause of CAP, as indicated by the sputum culture results in this patient. The antibiotic sensitivity test revealed that the organism was susceptible to ceftriaxone and levofloxacin, administered a few days prior.
Based on the 2007 IDSA/ATS CAP guidelines one significant or three minor criteria are needed to define severe pneumonia requiring ICU admission [24]. One primary criterion found in this patient was septic shock with the need for vasopressors. Besides, three minor criteria were also found, including the respiratory rate of more than 30 breaths/min, uremia (148 mg/dL), and thrombocytopenia (51,000/μl). Thus, the patient needed to be admitted to the ICU. CAP patients with ICU admission are given beta-lactam antibiotics (ampicillin-sulbactam, cefotaxime, or ceftriaxone) plus either a macrolide or a fluoroquinolone including levofloxacin.
In 2017, the patient had an ST-elevation myocardial infarction. Additionally, the chest X-ray revealed a heightened cardiothoracic ratio. During this hospitalization, the patient also encountered shortness of breath. The ECG showed an anomalous ECG with probable first-degree AV block and lateral ST elevation. Therefore, acute STEMI should be considered. Later conducted serial ECGs demonstrated that acute STEMI was ruled out. A transthoracic echocardiogram on the eighth day revealed grade 1 diastolic dysfunction, mild pulmonic regurgitation, and mild tricuspid regurgitation, with a low likelihood of pulmonary hypertension.
The patient was also treated for septic shock with rapid administration of NaCl intravenous fluid, norepinephrine, and dobutamine. Sepsis and septic shock are medical emergencies requiring early fluid resuscitation as the first-line therapy. The resuscitation requires at least 30 mL/kg of crystalloid fluid (including NaCl) within the first three hours [25,26]. Fluid resuscitation in septic shock is given to correct hypotension associated with hypovolemia. The mechanism of action of vasopressors, including norepinephrine, is to increase peripheral vascular resistance to increase blood pressure in patients with hypotension despite adequate fluid resuscitation or cardiogenic pulmonary edema [26,27]. Inotropes, such as dobutamine, can be administered in cases of myocardial dysfunction to increase cardiac output by increasing cardiac contractility. Patients with persistent hypoperfusion and low cardiac output despite adequate fluid resuscitation and the use of vasopressor agents may be given dobutamine.
Conclusion
This is a case report of a 41-year-old man with severe manifestations of leptospirosis (Weil’s disease), along with CAP S. pneumonia, and septic shock. Leptospirosis was diagnosed using the Modified Faine’s Criteria assessment, which confirmed the possibility of infection. The disease was diagnosed based on clinical data, epidemiological factors, bacteriological and laboratory findings, and then antibiotics and other symptomatic treatments were administered. Despite presenting with severe symptoms, the patient recovered completely after eight days, highlighting the importance of early diagnosis and prompt treatment.
Acknowledgments
This case report study is an outcome of Hibah Research Group (HGR) Internal Medicine of UNS (IMUNS).
Ethics approval
The patient provided written informed consent to be published as case report.
Competing interests
The authors declare that there is no conflict of interest.
Funding
This study received no external funding.
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
How to cite
Hermawati BD, Hapsari BDA, Wulandari EL, et al. Weil’s disease with multiple organ dysfunction, community-acquired pneumonia and septic shock: The role of rapid diagnosis and management. Narra J 2024; 4 (1): e587 - http://doi.org/10.52225/narra.v4i1.587.
References
- 1.Jarocka-Karpowicz I, Biernacki M, Wroński A, et al. Cannabidiol effects on phospholipid metabolism in keratinocytes from patients with Psoriasis Vulgaris. Biomolecules 2020;10:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blake T, Gullick NJ, Hutchinson CE, et al. Psoriatic disease and body composition: A systematic review and narrative synthesis. PLoS One 2020;15:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azuaga AB, Ramírez J, Cañete JD. Psoriatic arthritis: Pathogenesis and targeted therapies. Int J Mol Sci 2023;24:4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelfand JM, Wang S.. Expanding the global perspective on psoriasis. JAMA 2023;369:1–3. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet 2021;397:1301–1315. [DOI] [PubMed] [Google Scholar]
- 6.Nair PA, Badri T.. Psoriasis. StatPearls Publ. 2023. [PubMed] [Google Scholar]
- 7.Gisondi P, Bellinato F, Targher G, et al. Biological disease-modifying antirheumatic drugs may mitigate the risk of psoriatic arthritis in patients with chronic plaque psoriasis. Ann Rheum Dis 2022;81:68–73. [DOI] [PubMed] [Google Scholar]
- 8.Liu P, Kuang Y, Ye L, et al. Predicting the risk of psoriatic arthritis in plaque psoriasis patients: development and assessment of a new predictive nomogram. Front Immunol 2022;12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolbinger F, Di Padova F, Deodhar A, et al. Secukinumab for the treatment of psoriasis, psoriatic arthritis, and axial spondyloarthritis: Physical and pharmacological properties underlie the observed clinical efficacy and safety. Pharmacol Ther 2022;229:107925. [DOI] [PubMed] [Google Scholar]
- 10.Aboobacker S, Kurn H, Al Aboud AM. Secukinumab. StatPearls Publ. 2023. [PubMed] [Google Scholar]
- 11.Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: A systematic literature review and experts recommendations. PLoS One 2021;16:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ocampo VD, Gladman D.. Psoriatic arthritis. F1000Research 2019;8:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol 2019;80:251–265. [DOI] [PubMed] [Google Scholar]
- 14.Gialouri CG, Evangelatos G, Iliopoulos A, et al. Late-onset psoriatic arthritis: are there any distinct characteristics? A retrospective cohort data analysis. Life 2023;13:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farci F, Mahabal GD. Hyperkeratosis. StatPearls Publ. 2023. [PubMed] [Google Scholar]
- 16.Listiawan MY, Fadila A, Wiratama PA, et al. Evaluation of histopathology findings of clinically confirmed psoriasis vulgaris. Berk Ilmu Kesehat Kulit Kelamin 2023;35:21–26. [Google Scholar]
- 17.Coates LC, Merola JF, Grieb SM, et al. Methotrexate in psoriasis and psoriatic arthritis. J Rheumatol 2020;96:31–35. [DOI] [PubMed] [Google Scholar]
- 18.Felten R, Cursay GL, Lespessailles E.. Is there still a place for methotrexate in severe psoriatic arthritis? Ther Adv Musculoskelet Dis 2022;14:259–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frieder J, Kivelevitch D, Menter A.. Secukinumab: A review of the anti-IL-17A biologic for treatment of psoriasis. Ther Adv Vaccines 2018;9:259–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yunita I, Anggraeni S.. Secukinumab therapy in psoriasis management. Berkala Ilmu Kesehat Kulit Kelamin 2022;34:59–65. [Google Scholar]
- 21.Blauvelt A. Safety of secukinumab in the treatment of psoriasis. Expert Opin Drug Saf 2016;15:1413–20. [DOI] [PubMed] [Google Scholar]
- 22.Abrouk M, Gandy J, Nakamura M, et al. Secukinumab in the treatment of psoriasis and psoriatic arthritis: A review of the literature. Skin Therapy Lett 2017;22:1–6. [PubMed] [Google Scholar]
- 23.Blair HA. Secukinumab: A review in psoriatic arthritis. Drugs 2021;81:483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aboobacker S, Kurn H, Aboud AM. Secukinumab. StatPearls Publ. 2023. [PubMed] [Google Scholar]
- 25.Zhao Y, Cai L, Liu XY, et al. Efficacy and safety of secukinumab in Chinese patients with moderate-to-severe plaque psoriasis: A real-life cohort study. Chin Med J (Engl) 2021;134:1324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the results are available as part of the article and no additional source data are required.