Abstract
Due to low susceptibility of coronavirus disease of 2019 (COVID-19) in children, limited studies are available regarding COVID-19 in the pediatric population in Tunisia. The current study evaluated the incidence, clinical characteristics, and outcomes of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection among children hospitalized at Béchir Hamza Children’s Hospital. A retrospective cohort analysis was conducted using the hospital database between March 2020 and February 2022 with children aged ≤15 years with SARS-CoV-2 infection (confirmed by RT-PCR). A total of 327 COVID-19 hospitalized patients with a mean age of 3.3 years were included; the majority were male. Neurological disease (20%) was the most common comorbidity, while fever (95.3%) followed by cough (43.7%) and dyspnea (39.6%) were the most frequent symptoms reported. Severe disease with oxygen requirement occurred in 30% of the patients; 13% were admitted in the Intensive Care Unit. The overall incidence rate of COVID-19 hospitalization (in Tunis governorates) was 77.02 per 100,000 while the inpatient case fatality rate was 5% in the study population. The most prevalent circulating variant during our study period was Delta (48.8%), followed by Omicron (26%). More than 45% of the study population were <6 months and one-fourth (n = 25, 26.5%) had at least one comorbidity. Thus, the study findings highlight the high disease burden of COVID-19 in infants.
Keywords: observational study, COVID-19, pediatric population, clinical characteristics, disease severity, comorbidities, study outcomes
1. Introduction
Coronavirus disease 2019 (COVID-19), an infectious disease, has a substantial disease burden globally. The causative agent is the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2). After the confirmation of the first case in December 2019, the disease witnessed exponential growth, with more than 768 million reported cases worldwide, culminating in a pandemic. Mortality data confirm 7 million deaths as of 2 August 2023 [1]. In early March 2020, Tunisia reported its first cases of COVID-19 [2]. Until 26 October 2023, Tunisia recorded 1,156,613 confirmed cases of SARS-CoV-2 infection with 29,494 deaths [3,4].
In the initial phase of the pandemic, COVID-19 infection was comparatively lower in children and adolescents than in the adult population in terms of both severity and frequency [5,6]. The reported cases of infections in children increased dramatically during the Omicron variant stage in early 2022, when there was relaxation in public health and social measures [7]. As the total global data on the incidence and mortality of pediatric COVID-19 cases are limited, it is challenging to assess the course and the effect of the disease in children [8].
Children play a pivotal role in COVID-19 transmission. Being asymptomatic, children may be responsible for the continuous spread of infection. However, a meta-analysis conducted by Chen et al. revealed that children are not actively involved in household transmission. The emergence of novel variants over time is the primary cause of increased transmissibility [9]. According to the study by Dong et al., about 51% of children developed relatively mild disease, while 4.4% were asymptomatic, and 5.3% had severe disease, with 0.6% having critical illness [10].
The introduction of vaccinations to stop the spread of COVID-19 proved successful in reducing disease outbreaks. Even though mRNA and inactivated virus vaccinations have been approved for pediatric patients, only mRNA vaccines are recommended for children in the majority of the African countries. According to the various published reports, the vaccination coverage for children was negligible in Africa [11]. Furthermore, the WHO Strategic Advisory Group of Experts (SAGE) reached a consensus regarding the primary series for COVID-19 vaccinations. They recommended it for healthy children, depending on the disease burden and economic impact within the specified age range as well as the opportunity cost for a particular country. The SAGE group has recommended integration of COVID-19 vaccination into routine immunization schedules (annual uptake) for eligible populations [12,13]. Children with underlying risk conditions such as chronic pulmonary disease, cardiovascular disease, and immunosuppression, and who are less than 1 year of age are at increased risk of severe disease and hospitalization. Therefore, it is important for family members of such children to get a COVID-19 vaccination to prevent occurrence of disease as well as mitigate transmission of disease to this most vulnerable group of children [14].
Given the seemingly low susceptibility of children to COVID-19, studies on the disease incidence, clinical characteristics, and outcomes in the pediatric population are limited in African countries, including Tunisia. Therefore, there is a dearth of data on the impact of COVID-19 in children and infants and the subset of patients hospitalized due to infection. Earlier, Borgi et al. conducted a retrospective case series study in Tunisia to evaluate the clinical characteristics and outcomes of COVID-19 in the pediatric population. The patients admitted to the intensive care unit (ICU) during the period when the Delta variant was predominant were analyzed. However, this study had the limitation of a smaller sample size [15]. Furthermore, for the 17 months following the first case detection in March 2020, high variability in the SARS-CoV-2 lineage was reported in Tunisia [16].
Thus, the current study was conducted to assess the incidence rate of hospitalization per 100,000 pediatric patients with SARS-CoV-2 infection in the age range of 0–15 years hospitalized at Béchir Hamza Children’s Hospital. The secondary objectives of the study were to describe the cases and proportion of underlying medical conditions, clinical presentations and outcomes, and to determine the inpatient case fatality rate of SARS-CoV-2 infection in the study population. The study also determined the viral load based on cycle threshold (CT) values (only for a subset of COVID-19 cases) and their relation to clinical outcomes.
2. Materials and Methods
2.1. Study Design and Data Source
This was a retrospective, observational database study of hospitalized COVID-19 positive children aged 0–15 years in Tunis between March 2020 and February 2022. The hospital’s electronic medical records system was utilized to identify the study population. Each study sample was assigned a unique laboratory code, and all personal and clinical information used in the study was handled with the utmost confidentiality.
The variables collected on the data entry form include the demographic data (age, sex, residence), medical history (underlying medical conditions), clinical presentation (signs and symptoms, duration of hospitalization, oxygen requirement, ICU admission, and disease outcome) and laboratory findings (PCR result with CT value viral load).
2.2. Study Population
The study database contained a total of 2807 hospitalized patients with both negative and positive samples. However, only positive samples with complete data were analyzed. The study included all children aged 0–15 years who were hospitalized at Béchir Hamza Children’s Hospital in the governorate of Tunis between March 2020 and February 2022 and had tested positive for SARS-CoV-2 infection using RT-PCR at the microbiology laboratory, with the results being recorded in the database. The study excluded patients aged > 15 years and patients without a laboratory confirmed SARS-CoV2 infection. The study also analyzed patients admitted to ICU by age group and comorbidity.
Béchir Hamza Children’s Hospital, a first-line center, primarily caters to the medical needs of an urban settlement of more than 2 million habitants. It is also a referral center for all the governorates in Tunisia, offering wider population coverage. Therefore, almost 100% of children’s COVID-19 hospitalizations in Tunis occurred at Béchir Hamza Children’s Hospital.
2.3. Baseline Variables and Study Outcomes
The baseline variables analyzed during the study included demographic data and underlying medical conditions. The study outcomes included descriptions of clinical presentation; assessment of severity of COVID-19 in hospitalized children (oxygen requirement, ICU admission, need for assisted ventilation, and disease outcome); CT value viral load; descriptions of deceased population with respect to age and underlying comorbidities and assessing the incidence rate of hospitalization and inpatient case fatality rate of COVID-19 among the hospitalized children aged < 15 years.
2.4. Complete Genome Sequencing and Genome Analysis
The full genome sequencing, sequences analysis and variant assignment were achieved, as previously described, at Pasteur Institute of Tunis and collaborative centers [17].
2.5. Statistical Analysis
A non-probabilistic sampling strategy was adopted, and all eligible cases were included in the analysis. The sample size was not calculated mathematically, as the entire population was exhaustively included. Data analysis was conducted using R v4.3.1 software. The data were summarized using descriptive statistics. Categorical variables were provided in terms of frequencies and percentages, while continuous variables were shown as means with standard deviations or as medians with interquartile ranges, as appropriate.
The incidence rate of hospitalization per 100,000 affected patients was calculated using the number of positive cases during the study period divided by the overall pediatric population aged 0–15 years in Tunis.
The mortality rate of SARS-CoV-2 infection among the same population was calculated using the number of deaths over the study divided by the total number of infected children.
2.6. Ethical Considerations
The Ethics Committee of Béchir Hamza Children’s Hospital, Tunis approved the study. The study database did not contain any personal identifiers. The study adhered to the outlined protocols and abided by legal and regulatory mandates. The principles laid down by the International Ethical Guidelines for Biomedical Research Involving Human Subjects (Council for International Organizations of Medical Sciences 2002), the ICH Guideline for Good Clinical Practice, and the Declaration of Helsinki were followed.
3. Results
3.1. Baseline Characteristics
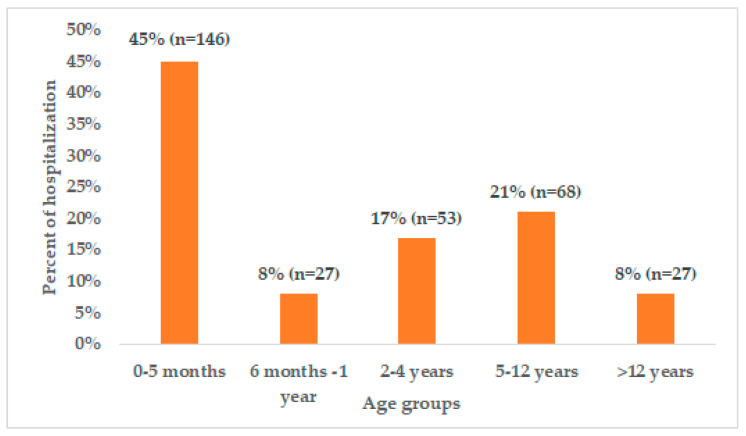
Out of 2790 hospitalized patients, 327 pediatric patients with a COVID-19 diagnosis were included in the study with a mean age of 3.3 years; over 45% of the study patients were <6 months old (Figure 1). Most of the patients (n = 183, 57%) included in the study were male (Table 1). The distribution of confirmed cases among the different provinces revealed that the majority of the included patients (n = 207, 64.5%) were from Tunis Governorate.
Figure 1.
Proportion of children hospitalized due to COVID-19 infections in different age groups.
Table 1.
Baseline characteristics of children with COVID-19 admitted to Béchir Hamza Children’s hospital.
| Characteristics | All Cases (N = 327) |
|---|---|
| Age (years) | |
| Mean (SD) | 3.3 (4.53) |
| Median [25, 75] | 0.8 [0.14; 5.5] |
| Gender, n (%) | |
| Female | 140 (43) |
| Male | 187 (57) |
| Residence Governorate, n (%) | |
| Tunis | 209 (64) |
| Ariana | 33 (10) |
| Ben Arous | 26 (7.9) |
| Mannouba | 20 (6.1) |
| Nabeul | 10 (3) |
| Beja | 7 (2.1) |
| Bizerte | 5 (1.5) |
| Siliana | 4 (1.2) |
| Zaghouan | 4 (1.2) |
| Jendouba | 4 (1.2) |
| Kasserine | 3 (0.9) |
| Elkef | 1 (0.3) |
| Kairouan | 1 (0.3) |
| Comorbidities *, n (%) | |
| Neurological disease | 17 (20) |
| Asthma | 14 (16.47) |
| Congenital heart disease | 14 (16.47) |
| Obesity | 9 (10.59) |
| Diabetes | 7 (8.24) |
| Others ** | 47 (55.29) |
| Reasons for hospitalization, n (%) | |
| Fever | 157 (49) |
| Respiratory problems | 103 (32) |
| Seizures | 17 (5) |
| Gastric problems | 15 (5) |
| Cardiovascular problems | 5 (2) |
| Glycemic problems | 4 (1) |
| Others | 29 (9) |
* Patients can have more than one condition; ** Other comorbidities included genetic disease, cancer, congenital disease, autoimmune disease, and hepatic disease.
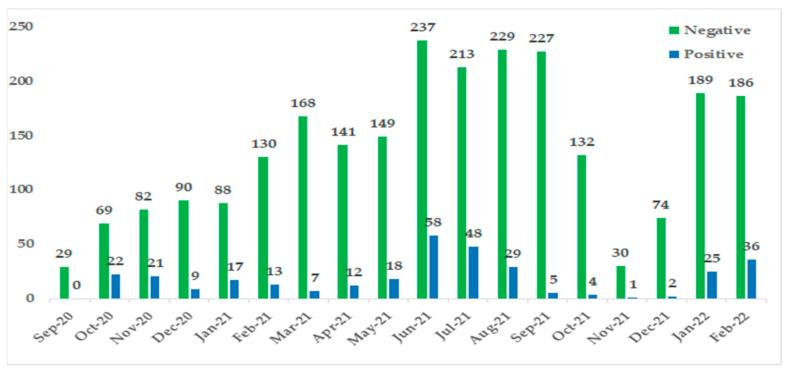
Further, the temporal distribution of confirmed COVID-19 cases years showed a rise in the number of pediatric cases in June 2021 (Figure 2), reflecting the time of surge in the overall number of cases within Tunisia [4].
Figure 2.
Distribution of SARS-CoV-2 test results by sample collection date.
A little more than a quarter (26.5%; n = 85) of the hospitalized COVID-19 children had at least one comorbidity; neurological disease was the most common comorbidity (20%: 17/85) reported among the study patients, followed by asthma and congenital disease (both 16.5%: 14/85). Fever was the most common primary reason for hospitalization (49%), followed by respiratory problems (32%). Table 1 presents the baseline characteristics, underlying comorbidities, and reasons for hospitalization.
Circulating variants:
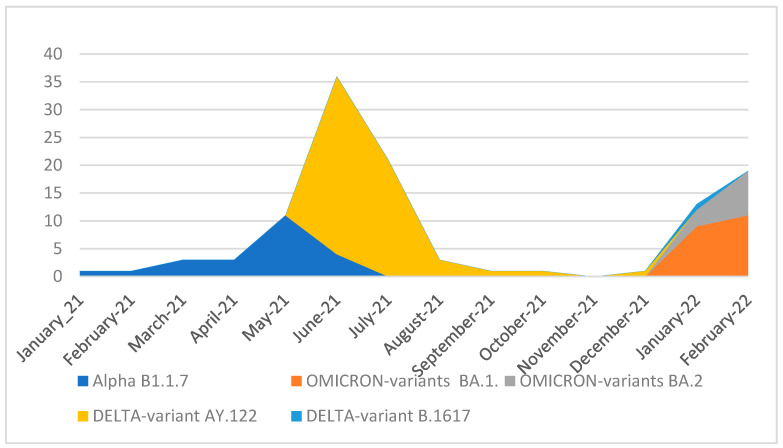
Among the investigated sequences, the detected variants and lineages involved three Variants of Concern (VOCs), Alpha (n = 23), Delta (n = 60), and Omicron (n = 31), as well as three other lineages: B.1.160 (n = 7), B.1.177 (n = 1), and C.36.3 (n = 1). The delta variant was the predominant VOC among the studied population (48.8%), followed by the Omicron and Alpha variants (26% and 19.5%, respectively). The most frequent sub-variant into the Delta variant was AY.122 (98%). For the Omicron variant, the most frequent sub-variant was BA.1 (65%) followed by BA.2 (35%).
Two peaks of case numbers appeared, a Delta peak between June and August 2021 and an Omicron peak in January and February 2022 (Figure 3).
Figure 3.
Evolution of SARS-CoV-2 circulating variants between January 2021 and February 2022.
3.2. Study Outcomes
3.2.1. Clinical Presentation
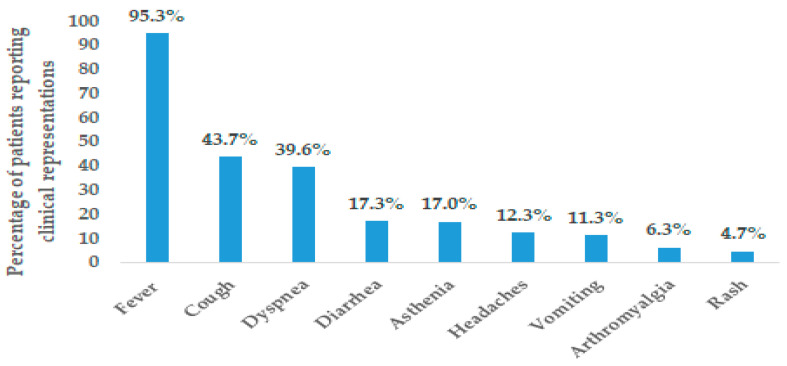
A total of 99% of the study population had symptoms of COVID-19 infection. The majority of hospitalized children presented with fever (95.28%) followed by cough (43.71%) and dyspnea (39.62). The other common clinical presentation noted among the hospitalized children is provided in Figure 4. The viral load of SARS-CoV-2 was moderate; the mean CT value was 26.24 (range: 13–38) for all RT-PCR samples in the study population (SARS-CoV-2 viral load is considered moderate for CT values ranging between 24 to 35) [18].
Figure 4.
Distribution of clinical presentation of COVID-19 in children hospitalized at Béchir Hamza Children’s Hospital.
3.2.2. Disease Severity
About 30% of the study population required oxygen support while about 13% required ICU admission and 12.2% required assisted ventilation. Of the 43 children admitted in the ICU, 33 children were aged below 1 year with seven out of eight deaths occurring in this age group (Table 2). Furthermore, only 3.12% of the patients (n = 10) were hospitalized for more than one month, while 70% of the study population (n = 225) was discharged by the 5th day of hospitalization. A total of 17 deaths (5.3%) due to COVID-19 infection were reported in the study population. Eight deaths occurred in the Pediatric ICU (PICU); the others were not transferred because of the lack of ICU beds and were managed in the pediatric departments.
Table 2.
Total number of children hospitalized in ICU due to COVID-19 infections.
| 0–5 Months | 6 Months–1 Year | 2–4 Years | 5–12 Years | >12 Years | |
|---|---|---|---|---|---|
| Total | 23 | 10 | 3 | 6 | 1 |
| Deaths | 5 | 2 | 0 | 1 | 0 |
| Comorbidities * | |||||
| Obesity | 0 | 0 | 0 | 5 | 1 |
| Neurological disease | 1 | 0 | 0 | 1 | 0 |
| Heart disease | 2 | 0 | 0 | 0 | 0 |
| Asthma | 0 | 0 | 0 | 1 | 0 |
| Diabetes | 0 | 0 | 0 | 0 | 1 |
| Liver disease | 0 | 1 | 0 | 0 | 0 |
| Inherited metabolic disease | 0 | 1 | 0 | 0 | 0 |
* Patients can have more than one condition.
3.2.3. Description of the Deceased Population
In this study, a total of 17 children died due to COVID-19, comprising ten boys and seven girls, with a mean age of 1.9 years. Twelve of them were aged under one year. Most of the deceased patients were hospitalized for fever, dyspnea, and cough, and the severity of infection in the deceased population was determined by the need for oxygenation (n = 14), ICU admission (n = 8), and assisted ventilation (n = 10). Eight of the deceased patients had an underlying comorbidity. Neurological disease and congenital heart disease were the common comorbidities reported in this population. The mean CT of viral load for the deceased was 27.6, ranging from a minimum of 16 to a maximum of 37.
3.2.4. Incidence Rate of Hospitalization and Inpatient Case Fatality Rate
The incidence rate of hospitalization of COVID-19 was found to be 77.02 per 100,000. The total population of children aged ≤ 15 years in Tunis is 268,754. The inpatient case fatality rate of COVID-19 in the study population was 5%.
4. Discussion
The current study reports the demographic characteristics and disease outcomes of the pediatric population with COVID-19 hospitalized at Béchir Hamza Children’s Hospital throughout the various stages of the pandemic in Tunisia. This study had the largest research sample of children with COVID-19 in Tunisia. The current study reported that the incidence rate of hospitalization due to COVID-19 was 77.02 per 100,000 of the child and adolescent population (≤15 years) in Tunis during March 2020 and February 2022. So far, only one previous study assessed the hospitalization rate among Tunisian children and adolescents (≤15 years) and reported that 8.5% of all hospitalizations occurred due to COVID-19 during a similar time period [19]. In the US, the weekly hospitalization rates during 1 March 2020–19 February 2022 in children aged 0–4 years was 14.5, with highest hospitalization rates among infants aged <6 months [20]. In a separate analysis, out of 8121 patients affected with COVID-19 in the age range of 0–9 years, 5.7% required hospitalization, with in-hospital death in 0.2% patients [21]. However, the outcomes of these studies cannot be directly compared to the present study because of possible variations in statistical methods used to calculate the incidence, different demography and age group of patients, and different time periods covered under the analysis. Further, the inpatient case fatality rate in our study was 5% and this was higher than that reported in other similar studies [22,23]. The inpatient fatality rate as per the previous study conducted in Tunisia was 0.46% [19]. Similarly, the inpatient fatality rate was found to be in the range of 1.4–1.8% in the US in pediatric patients hospitalized with COVID-19 [24,25]. This high rate may be attributed to the lack of ICU beds and the young age of patients included in the study (45% of the study patients were below 6 months of age). About half of the deceased patients had underlying comorbidities, thereby emphasizing the importance of monitoring and taking control measures in this high-risk population.
Among the 2790 hospitalized children with acute respiratory infections, the study included 327 SARS-CoV-2-positive children, with a positivity rate of 11.4%. Our study exhibited a higher positivity rate than that reported in the initial phases of the pandemic, where the positivity rate was around 2% in children [26,27]. Nevertheless, a marked increase in the number of children contracting COVID-19 infection was witnessed over time, largely attributable to changing COVID-19 associated symptoms, laboratory testing capacity, and the emergence of different variants [28,29].
The demographic characteristics in the current study demonstrated that COVID-19 infection was more frequent in children below 6 months of age and in boys compared to female children. In the present study, more than half of the patients (53%) were <1 year old, with 45% being less than 6 months old. Similar results have been reported in the meta-analysis study by Bhuiyan et al., wherein 53% of the study population was reported to be less than one year of age [30]. Furthermore, the registry study by Sobolewska-Pilarczyk et al. reported that 23% of children with COVID-19 were aged < 1 year [31]. Although a lower frequency of COVID-19 infection is evident in children, they have a higher susceptibility to contracting it during the first few months of their lives due to their inadequately developed immune systems [30]. Moreover, the majority of the deaths (12 out of a total of 17 deaths) reported, in our study, occurred to infants or children less than 1 year of age. This observation is consistent with a previous study that reported high mortality in children less than 1 year of age [32].
Studies have reported that among infants, although COVID-19 is symptomatically mild, even the low risk of severe infection would require prevention. The presence of comorbid conditions can heighten the risk of infections and their complications in infants [33]. Therefore, it is imperative to focus on protecting the infants from COVID-19 infections. As infants under 6 months of age are ineligible for any existing COVID-19 vaccines, maternal vaccination is the only available option in preventing disease in early infancy. It is noted that infants acquire immunity via the placenta from their mothers [33,34]. A recent study conducted in the US reported that the incidence of hospitalization for COVID-19 was considerably lower for infants aged < 6 months if their mothers were vaccinated compared with infants of unvaccinated mothers (21/100,000 person-years vs. 100/100,000 person-years) [34]. Additionally, it has been noted that infants and children are often infected by coming into contact with any COVID-19 positive patient (whether a parent or any family member). Studies have found that 70–90% of COVID-19 infected children are infected by a household contact/ family member [14,35,36].
Multiple studies have reported higher risk of COVID-19 in males over the period of time [10,27,37]; similarly, a male predominance was observed in our study. The modulation of transmembrane serine protease 2 expression by sex steroids has been put forward as a possible reason for male predominance noted among the COVID-19 population. The existence of TMPRSS2:ERG in prostate cancer, along with the significant modulation of TMPRSS2 by androgens, has led to the speculation that the male predominance in the COVID-19 pandemic may be partly attributed to TMPRSS2 [38].
The temporal distribution of confirmed COVID-19 cases showed a rise in the number of pediatric cases in June 2021. Tunisia experienced the fourth wave of COVID-19 with the Delta variant, which was prevalent during May 2021 [16]. Globally rapid transmission of the Omicron variant caused an unprecedented surge in COVID-19 cases and hospitalizations among children [39].
In the current study, 26% of the children had comorbidities, with neurological disease being the most common comorbidity, followed by asthma, congenital disease, and obesity. Similar to adult patients, children with comorbidities are at increased risk of severe and critical illness and mortality during COVID-19 infection. Evidence from the literature suggests the association of ≥1 comorbidities with severe COVID-19 infection among both adults and the pediatric population [40,41]. The Netherland-based retrospective study by Biharie et al. has reported similar findings, wherein 65.5% of the patients had pre-existing comorbidities and the common comorbidities in their study included obesity (21.7%), respiratory disorders (19.6%), and neurological disorders (17.4%) [42]. Similarly, a population-based surveillance study in the US reported that 55.0% of the study population had ≥1 underlying medical conditions, with obesity, chronic lung disease, neurologic disorders, cardiovascular disease, and blood disorders being the most commonly reported ones [22]. The meta-analysis study by Tsankov et al. reported that children with comorbidities are more susceptible to severe COVID-19 manifestations and associated mortality than healthy children [43]. Obesity has also contributed for severe disease manifestations in children [42,43]. In our study, obesity was reported in 10.59% of the patients. Obesity increases the risk and severity of COVID-19 and leads to nutritional, cardiac, respiratory, and immune response changes, which could worsen the complications from SARS-CoV-2 infection [44]. Additionally, obesity has been identified to increase the risk of hospitalization in the pediatric population. It often leads to immune dysregulation and increased susceptibility to infections [45]. A study conducted in New York using data from a tertiary care unit showed obesity as the most prevalent comorbidity in pediatric patients with COVID-19 [46].
From our study, it was also noticed that 20% of the patients had underlying neurological conditions. Neurological diseases often worsen the symptoms of respiratory diseases, leading to severe illness by reducing muscle tonality and impairing mobility and structural conditions, in turn reducing pulmonary function [47,48]. The COVID-19 pandemic has impacted individuals with neurological conditions, complicating their ability to obtain diagnostic tests, treatments, and therapies [49].
These results substantiate the need to identify and monitor the comorbidities to aid in public health strategies, such as prioritizing vaccinations for at-risk children and implementing targeted prevention measures.
The most common reasons for hospitalization in the current study included fever, followed by cough. The results obtained corroborated previous epidemiological studies, where fever, cough, rhinorrhea, respiratory distress, sore throat, vomiting, and diarrhea were the most commonly reported symptoms [27,28,29,50]. The other symptoms included headaches, anorexia, myalgia, nausea, and fatigue.
In our study, nearly 30% of the patients required oxygen support while approximately 13% required ICU admission. The study by Woodruff et al. reported similar findings, wherein nearly one-third of the study population needed ICU admission or the use of invasive mechanical ventilation. They were also at a greater risk of contracting severe COVID-19 infection [22].
This study has some limitations. Firstly, our study being a retrospective design relied mainly on routinely collected data which might have led to unintentional bias during data extraction and reporting. Furthermore, our study does not have follow-up data on hospitalized children with COVID-19; therefore, the study results are only limited to acute disease burden and do not provide long-term burden of disease. Secondly, the patients were selected from a single hospital and hence the results might be difficult to generalize in a larger number of patients. Furthermore, bias in the results of hospitalization rates needs to be considered. The relationship between extrinsic factors leading to variability of hospitalization among different age groups has not been captured. The geographic restriction to only Tunis does not provide the true representation of COVID-19 in all of Tunisia. Moreover, hospitalization data for different COVID-19 variables was not available in the defined period. Information bias might exist in the study due to inconsistent recording of data, underreporting, and missing data. Additionally, the possibility of missing data of patients who are less likely to be registered for primary care could not be ruled out.
Overall, the number of studies reporting COVID-19-related disease characteristics and outcomes in children is limited in Tunisia. Therefore, the present study contributes to the evidence indicating that children can be affected by COVID-19 and may experience severe illness requiring hospitalization. The study by Borgi et al. has previously described the clinical scenario of COVID-19 infection and hospitalization in the PICU from June 2021 to August 2021 in Tunisia [15]. The study had included only 20 patients with no comorbidities. As the number of patients was limited, the findings from our study also have important implications for public health strategies focused on preventing the spread of COVID-19 infection and mitigating its impact on vulnerable populations, including children. Additionally, comprehending the factors pertaining to the transmission of severe COVID-19 infection in children could reduce infection risk through various mitigation strategies, including the prioritization of vaccinations in children. Furthermore, larger cohort studies across different regions and countries are warranted to identify the various predictors of severe disease in children.
5. Conclusions
COVID-19 infection is more frequent in infants less than 6 months of age compared with older children. The study highlights that children with comorbidities were more susceptible to severe COVID-19-related clinical outcomes. Indeed, more than one-third of ICU admitted patients had at least one comorbidity. Moreover, about 1 in 10 COVID-19 hospitalized children required ICU admission.
Acknowledgments
We thank Allen Kristen, Julia Spinardi, and Hammam Haridy at Pfizer for their support and assistance in this study. The authors would also like to thank Hanae Zarrik, IQVIA Real-World Services, Morocco for her support in statistical analysis.
Author Contributions
Conceptualization, A. B. (Aida Borgi), M.H.K., H.Z. and H. S.; methodology, A.B. (Aida Borgi), K.M. (Khaoula Meftah), I.T. and H.S.; software, A.B. (Aida Borgi), K.M. (Khaoula Meftah), I.T., and H.S.; validation, K.B., M.K., S.B.B., S.B., H.T., K.M (Khaled Menif), and H.S.; formal analysis, A.B. (Aida Borgi), K.M. (Khaoula Meftah); investigation, A.B. (Aida Borgi), K.M. (Khaoula Meftah), I.T., A.B. (Aida Bouafsoun), F.M., N.M., A.A.A., L.E., H.K., S.H.-B., and H.S.; resources, K.M. (Khaoula Meftah), A.B; (Aida Bouafsoun), and H.S; data curation, A.B. (Aida Borgi), K.M. (Khaoula Meftah), I.T, A.B, (Aida Bouafsoun), F.M., N.M., A.A.A., L.E., H.K., S.H.-B., K.B., M.K., S.B.B., S.B., K.M. (Khaled Menif), H.T. and H.S.; writing—original draft preparation, A.B. (Aida Borgi), M.H.K., H.Z. and H.S.; writing—review and editing, A.B. (Aida Borgi), K.M. (Khaoula Meftah), I.T., M.H.K., H.Z., A.B. (Aida Bouafsoun), F.M., N.M., A.A.A., L.E., H.K., S.H.-B., K.B., M.K., S.B.B., S.B., K.M. (Khaled Menif), H.T. and H.S.; visualization, A. B. (Aida Borgi), K.M. (Khaoula Meftah), I.T., M.H.K., H.Z., H.K., S.H.-B. and H.S.; supervision, A.B. (Aida Borgi), M.H.K., H.Z. and H.S.; project administration, M.H.K., H.Z. and H.S.; funding acquisition, M.H.K. and H.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Ethics Committee of Béchir Hamza Children’s Hospital, Tunis approved the study. The study adhered to the outlined protocols and abided by legal and regulatory mandates. The principles laid down by the International Ethical Guidelines for Biomedical Research Involving Human Subjects (Council for International Organizations of Medical Sciences 2002), the ICH Guideline for Good Clinical Practice, and the Declaration of Helsinki were followed.
Informed Consent Statement
Patient consent was waived since the study database did not contain any personal identifiers.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
Moe H. Kyaw and Hela Zaghden are employees of Pfizer and may hold stock options. There are no conflicts of interest for the remaining authors.
Funding Statement
This study was conducted as a collaboration between the Béchir Hamza Children’s Hospital of Tunis and Pfizer. The Béchir Hamza Children’s Hospital is the study sponsor. This study was funded by Pfizer under the number RC01_70095021.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization COVID-19 Weekly Epidemiological Update. [(accessed on 26 October 2023)]. Available online: https://covid19.who.int/
- 2.Talmoudi K., Safer M., Letaief H., Hchaichi A., Harizi C., Dhaouadi S., Derouiche S., Bouaziz I., Gharbi D., Najar N., et al. Estimating transmission dynamics and serial interval of the first wave of COVID-19 infections under different control measures: A statistical analysis in Tunisia from 29 February to 5 May 2020. BMC Infect. Dis. 2020;20:914. doi: 10.1186/s12879-020-05577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ONMNE Tunisia|Ministry of Health, COVID-19 in Numbers. [(accessed on 26 October 2023)]. Available online: https://onmne.tn/
- 4.WHO Tunisia: WHO Coronavirus Disease (COVID-19) Dashboard with Vaccination Data. [(accessed on 26 October 2023)]. Available online: https://covid19.who.int/region/emro/country/tn.
- 5.Baggio S., L’Huillier A.G., Yerly S., Bellon M., Wagner N., Rohr M., Huttner A., Blanchard-Rohner G., Loevy N., Kaiser L., et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Viral Load in the Upper Respiratory Tract of Children and Adults With Early Acute Coronavirus Disease 2019 (COVID-19) Clin. Infect. Dis. 2021;73:148–150. doi: 10.1093/cid/ciaa1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milani G.P., Bottino I., Rocchi A., Marchisio P., Elli S., Agostoni C., Costantino G. Frequency of Children vs Adults Carrying Severe Acute Respiratory Syndrome Coronavirus 2 Asymptomatically. JAMA Pediatr. 2021;175:193–194. doi: 10.1001/jamapediatrics.2020.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Interim Statement on COVID-19 Vaccination for Children. [(accessed on 16 May 2023)]. Available online: https://www.who.int/news/item/11-08-2022-interim-statement-on-covid-19-vaccination-for-children.
- 8.COVID-19 Confrmed Cases and Deaths. United Nations Children’s Fund. [(accessed on 28 July 2023)]. Available online: https://data.unicef.org/resources/covid-19-confrmed-cases-and-deaths-dashboard/
- 9.Chen F., Tian Y., Zhang L., Shi Y. The role of children in household transmission of COVID-19: A systematic review and meta-analysis. Int. J. Infect. Dis. 2022;122:266–275. doi: 10.1016/j.ijid.2022.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., Tong S. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020;145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 11.Spinardi J., Dantas A.C., Carballo C., Thakkar K., Akoury N.A., Kyaw M.H., Del Carmen Morales Castillo G., Srivastava A., Sáfadi M.A.P. Narrative Review of the Evolution of COVID-19 Vaccination Recommendations in Countries in Latin America, Africa and the Middle East, and Asia. Infect. Dis. Ther. 2023;12:1237–1264. doi: 10.1007/s40121-023-00804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO SAGE Roadmap on Uses of COVID-19 Vaccines in the Context of OMICRON and Substantial Population Immunity: An Approach to Optimize the Global Impact of COVID-19 Vaccines at a Time When Omicron and Its Sub-Lineages are the Dominant Circulating Variants of Concern, Based on Public Health Goals, Evolving Epidemiology, and Increasing Population-Level Immunity, First Issued 20 October 2020, Updated: 13 November 2020, Updated: 16 July 2021, Update: 21 January 2022, Latest Update: 30 March 2023. [(accessed on 3 August 2023)]. Available online: https://apps.who.int/iris/handle/10665/366671.
- 13.WHO Highlights from the Meeting of the Strategic Advisory Group of Experts (SAGE) on Immunization. [(accessed on 3 August 2023)]. Available online: https://cdn.who.int/media/docs/default-source/immunization/sage/2023/march-2023/sage_march_2023_meeting_highlights.pdf?sfvrsn=a8e5be9_4.
- 14.Tezer H., Bedir Demirdag T. Novel coronavirus disease (COVID-19) in children. Turk. J. Med. Sci. 2020;50:592–603. doi: 10.3906/sag-2004-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borgi A., Louati A., Miraoui A., Lahmar L., Ayari A., Hajji A., Bouziri A., Menif K., Smaoui H., Jaballah N.B. Critically ill infants with SARS-CoV-2 delta variant infection. Pediatr. Neonatol. 2023;64:335–340. doi: 10.1016/j.pedneo.2022.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chouikha A., Fares W., Laamari A., Haddad-Boubaker S., Belaiba Z., Ghedira K., Kammoun Rebai W., Ayouni K., Khedhiri M., Ben Halima S., et al. Molecular Epidemiology of SARS-CoV-2 in Tunisia (North Africa) through Several Successive Waves of COVID-19. Viruses. 2022;14:624. doi: 10.3390/v14030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khemiri H., Mangone I., Gdoura M., Mefteh K., Chouikha A., Fares W., Lorusso A., Ancora M., Pasquale A.D., Cammà C., et al. Dynamic of SARS-CoV-2 variants circulation in Tunisian pediatric population, during successive waves, from March 2020 to September 2022. Virus Res. 2024;344:199353. doi: 10.1016/j.virusres.2024.199353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabaan A.A., Tirupathi R., Sule A.A., Aldali J., Mutair A.A., Alhumaid S., Muzaheed, Gupta N., Koritala T., Adhikari R., et al. Viral Dynamics and Real-Time RT-PCR Ct Values Correlation with Disease Severity in COVID-19. Diagnostics. 2021;11:1091. doi: 10.3390/diagnostics11061091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben Youssef F., Hechaichi A., Safer M., Letaief H., Dhaouadi S., Mziou E., Bougatef S., Bouabid L., Derouiche S., ép Ben Alaya N.B. Epidemiological profile of children and adolescents with COVID-19 in Tunisia, Mars 2020–August 2022. Popul. Med. 2023;5:A508. doi: 10.18332/popmed/165347. [DOI] [Google Scholar]
- 20.Marks K.J., Whitaker M., Agathis N.T., Anglin O., Milucky J., Patel K., Pham H., Kirley P.D., Kawasaki B., Meek J., et al. Hospitalization of Infants and Children Aged 0–4 Years with Laboratory-Confirmed COVID-19-COVID-NET, 14 States, March 2020–February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:429–436. doi: 10.15585/mmwr.mm7111e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreira A., Chorath K., Rajasekaran K., Burmeister F., Ahmed M., Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur. J. Pediatr. 2021;180:1659–1663. doi: 10.1007/s00431-021-03955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodruff R.C., Campbell A.P., Taylor C.A., Chai S.J., Kawasaki B., Meek J., Anderson E.J., Weigel A., Monroe M.L., Reeg L., et al. Risk Factors for Severe COVID-19 in Children. Pediatrics. 2022;149:e2021053418. doi: 10.1542/peds.2021-053418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zachariah P., Johnson C.L., Halabi K.C., Ahn D., Sen A.I., Fischer A., Banker S.L., Giordano M., Manice C.S., Diamond R., et al. Epidemiology, Clinical Features, and Disease Severity in Patients With Coronavirus Disease 2019 (COVID-19) in a Children’s Hospital in New York City, New York. JAMA Pediatr. 2020;174:e202430. doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldstein L.R., Tenforde M.W., Friedman K.G., Newhams M., Rose E.B., Dapul H., Soma V.L., Maddux A.B., Mourani P.M., Bowens C., et al. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA. 2021;325:1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhalala U.S., Gist K.M., Tripathi S., Boman K., Kumar V.K., Retford L., Chiotos K., Blatz A.M., Dapul H., Verma S., et al. Characterization and Outcomes of Hospitalized Children With Coronavirus Disease 2019: A Report From a Multicenter, Viral Infection and Respiratory Illness Universal Study (Coronavirus Disease 2019) Registry. Crit. Care Med. 2022;50:e40–e51. doi: 10.1097/CCM.0000000000005232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladhani S.N., Amin-Chowdhury Z., Davies H.G., Aiano F., Hayden I., Lacy J., Sinnathamby M., de Lusignan S., Demirjian A., Whittaker H., et al. COVID-19 in children: Analysis of the first pandemic peak in England. Arch. Dis. Child. 2020;105:1180–1185. doi: 10.1136/archdischild-2020-320042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parri N., Magistà A.M., Marchetti F., Cantoni B., Arrighini A., Romanengo M., Felici E., Urbino A., Da Dalt L., Verdoni L., et al. Characteristic of COVID-19 infection in pediatric patients: Early findings from two Italian Pediatric Research Networks. Eur. J. Pediatr. 2020;179:1315–1323. doi: 10.1007/s00431-020-03683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikolopoulou G.B., Maltezou H.C. COVID-19 in Children: Where do we Stand? Arch. Med. Res. 2022;53:1–8. doi: 10.1016/j.arcmed.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lota-Salvado R., Padua J.R., Agrupis K.A., Malijan G.M., Sayo A.R., Suzuki S., Go G.D., Smith C. Epidemiological and clinical characteristics of children with confirmed COVID-19 infection in a tertiary referral hospital in Manila, Philippines. Trop. Med. Health. 2023;51:9. doi: 10.1186/s41182-023-00507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhuiyan M.U., Stiboy E., Hassan M.Z., Chan M., Islam M.S., Haider N., Jaffe A., Homaira N. Epidemiology of COVID-19 infection in young children under five years: A systematic review and meta-analysis. Vaccine. 2021;39:667–677. doi: 10.1016/j.vaccine.2020.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sobolewska-Pilarczyk M., Pokorska-Śpiewak M., Stachowiak A., Marczyńska M., Talarek E., Ołdakowska A., Kucharek I., Sybilski A., Mania A., Figlerowicz M., et al. COVID-19 infections in infants. Sci. Rep. 2022;12:7765. doi: 10.1038/s41598-022-11068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khera N., Santesmasses D., Kerepesi C., Gladyshev V.N. COVID-19 mortality rate in children is U-shaped. Aging. 2021;13:19954–19962. doi: 10.18632/aging.203442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cimolai N. COVID-19 among infants: Key clinical features and remaining controversies. Clin. Exp. Pediatr. 2024;67:1–16. doi: 10.3345/cep.2023.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zerbo O., Ray G.T., Fireman B., Layefsky E., Goddard K., Lewis E., Ross P., Omer S., Greenberg M., Klein N. Maternal SARS-CoV-2 Vaccination and Infant Protection Against SARS-CoV-2 During the First 6 Months of Life. Res. Sq. 2022 doi: 10.21203/rs.3.rs-2143552/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi S.H., Kim H.W., Kang J.M., Kim D.H., Cho E.Y. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin. Exp. Pediatr. 2020;63:125–132. doi: 10.3345/cep.2020.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu X., Zhang L., Du H., Zhang J., Li Y.Y., Qu J., Zhang W., Wang Y., Bao S., Li Y., et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Götzinger F., Santiago-García B., Noguera-Julián A., Lanaspa M., Lancella L., Calò Carducci F.I., Gabrovska N., Velizarova S., Prunk P., Osterman V., et al. COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child. Adolesc. Health. 2020;4:653–661. doi: 10.1016/s2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stopsack K.H., Mucci L.A., Antonarakis E.S., Nelson P.S., Kantoff P.W. TMPRSS2 and COVID-19: Serendipity or Opportunity for Intervention? Cancer Discov. 2020;10:779–782. doi: 10.1158/2159-8290.Cd-20-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naeimi R., Sepidarkish M., Mollalo A., Parsa H., Mahjour S., Safarpour F., Almukhtar M., Mechaal A., Chemaitelly H., Sartip B., et al. SARS-CoV-2 seroprevalence in children worldwide: A systematic review and meta-analysis. eClinicalMedicine. 2023;56:101786. doi: 10.1016/j.eclinm.2022.101786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., Hosein Z., Padda I., Mangat J., Altaf M. Comorbidity and its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020;2:1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preston L.E., Chevinsky J.R., Kompaniyets L., Lavery A.M., Kimball A., Boehmer T.K., Goodman A.B. Characteristics and Disease Severity of US Children and Adolescents Diagnosed With COVID-19. JAMA Netw. Open. 2021;4:e215298. doi: 10.1001/jamanetworkopen.2021.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biharie A., Keuning M.W., Wolthers K.C., Pajkrt D. Comorbidities, clinical characteristics and outcomes of COVID-19 in pediatric patients in a tertiary medical center in the Netherlands. World J. Pediatr. 2022;18:558–563. doi: 10.1007/s12519-022-00564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsankov B.K., Allaire J.M., Irvine M.A., Lopez A.A., Sauvé L.J., Vallance B.A., Jacobson K. Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021;103:246–256. doi: 10.1016/j.ijid.2020.11.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brambilla I., Delle Cave F., Guarracino C., De Filippo M., Votto M., Licari A., Pistone C., Tondina E. Obesity and COVID-19 in children and adolescents: A double pandemic. Acta Biomed. 2022;93:e2022195. doi: 10.23750/abm.v93iS3.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agarwal A., Karim F., Fernandez Bowman A., Antonetti C.R. Obesity as a Risk Factor for Severe Illness From COVID-19 in the Pediatric Population. Cureus. 2021;13:e14825. doi: 10.7759/cureus.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chao J.Y., Derespina K.R., Herold B.C., Goldman D.L., Aldrich M., Weingarten J., Ushay H.M., Cabana M.D., Medar S.S. Clinical Characteristics and Outcomes of Hospitalized and Critically Ill Children and Adolescents with Coronavirus Disease 2019 at a Tertiary Care Medical Center in New York City. J. Pediatr. 2020;223:14–19. doi: 10.1016/j.jpeds.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Havers F., Fry A.M., Chen J., Christensen D., Moore C., Peacock G., Finelli L., Reed C. Hospitalizations Attributable to Respiratory Infections among Children with Neurologic Disorders. J. Pediatr. 2016;170:135–141.e5. doi: 10.1016/j.jpeds.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 48.Keren R., Zaoutis T.E., Bridges C.B., Herrera G., Watson B.M., Wheeler A.B., Licht D.J., Luan X.Q., Coffin S.E. Neurological and neuromuscular disease as a risk factor for respiratory failure in children hospitalized with influenza infection. JAMA. 2005;294:2188–2194. doi: 10.1001/jama.294.17.2188. [DOI] [PubMed] [Google Scholar]
- 49.Valderas C., Mendez G., Echeverria A., Suarez N., Julio K., Sandoval F. COVID-19 and neurologic manifestations: A synthesis from the child neurologist’s corner. World J. Pediatr. 2022;18:373–382. doi: 10.1007/s12519-022-00550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chekhlabi N., El Kettani C., Haoudar A., Bahlaoui A., Mahi M., Ettair S., Dini N. The epidemiological and clinical profile of COVID-19 in children: Moroccan experience of the Cheikh Khalifa University Center. Pan Afr. Med. J. 2020;35((Suppl. S2)):57. doi: 10.11604/pamj.supp.2020.35.2.23571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.