Abstract
Viral co-infections are frequently observed among children, but whether specific viral interactions enhance or diminish the severity of respiratory disease is still controversial. This study aimed to investigate the type of viral mono- and co-infections by also evaluating viral correlations in 3525 respiratory samples from 3525 pediatric in/outpatients screened by the Allplex Respiratory Panel Assays and with a Severe Acute Respiratory Syndrome-COronaVirus 2 (SARS-CoV-2) test available. Overall, viral co-infections were detected in 37.8% of patients and were more frequently observed in specimens from children with lower respiratory tract infections compared to those with upper respiratory tract infections (47.1% vs. 36.0%, p = 0.003). SARS-CoV-2 and influenza A were more commonly detected in mono-infections, whereas human bocavirus showed the highest co-infection rate (87.8% in co-infection). After analyzing viral pairings using Spearman’s correlation test, it was noted that SARS-CoV-2 was negatively associated with all other respiratory viruses, whereas a markedly significant positive correlation (p < 0.001) was observed for five viral pairings (involving adenovirus/human bocavirus/human enterovirus/metapneumoviruses/rhinovirus). The correlation between co-infection and clinical outcome may be linked to the type of virus(es) involved in the co-infection rather than simple co-presence. Further studies dedicated to this important point are needed, since it has obvious implications from a diagnostic and clinical point of view.
Keywords: respiratory viruses, co-infection, multiplex PCR, children
1. Introduction
Acute respiratory infections (ARIs) are the leading cause of infections in pediatric populations worldwide and are one of the most frequent causes of consultation or admission to healthcare facilities in pediatric services [1]. ARIs are classified as upper and lower respiratory tract infections, based on the respiratory tract involved. Clinically, upper respiratory tract infections are usually characterized by mild symptoms and are generally self-limiting, whereas lower respiratory tract infections may cause severe clinical manifestations (such as bronchitis, bronchiolitis, and/or pneumonia) [2]. A variety of respiratory viruses are involved in the etiology of respiratory tract infections in pediatric populations, including adenovirus (ADV), human bocavirus (HBoV), human enterovirus (HeV), human rhinoviruses (HRV), respiratory syncytial viruses (RSV A and B), metapneumoviruses (MPV), four parainfluenza viruses (PIVs 1–4), influenza viruses (Flu A and B), and human coronaviruses (HCoVs). Some of these viruses, such as RSV or Flu A and B, typically show extensive seasonal variation in prevalence, whereas others are commonly present throughout all seasons [3]. In the last two years, with the introduction of control measures due to the Severe Acute Respiratory Syndrome COronaVirus 2 (SARS-CoV-2) pandemic, the epidemic seasonality of other respiratory viruses changed [4,5,6,7]. However, the mitigation measures after the pandemic allowed these viruses, including SARS-CoV-2, to begin circulating once again [8]. Currently, multiplex polymerase chain reaction (PCR) panel assays, which allow for the simultaneous detection of different pathogens in a specimen with high sensitivity and short turnaround time, are becoming more commonly used in diagnostics [9]. Thanks to the introduction of these molecular assays also in the diagnosis of respiratory tract infections, it is now possible to better study and understand the epidemiology and pathogenesis of respiratory viruses. Since various respiratory viruses may be circulating in the same period, they can infect the same respiratory tract either concurrently or sequentially [10]. As a result, there are three possible major effects of these interactions: viral synergy, viral interference, and viral non-interference [11]. In fact, infection by a first virus can have a synergistic interaction or an antagonistic mechanism, thus promoting or reducing the infection and replication of another virus, by several pathophysiological factors, including synergistic damage to the epithelium or, by contrast, to the production of interferon [10]. If the presence of the first virus has no effect on the replication of another, this is defined a non-interference [11]. Viral co-infections in pediatric populations with ARIs are frequently reported by multiplex PCR, particularly in those who are hospitalized [12,13,14,15]. The impact of viral co-infection and its correlation with the disease severity of respiratory tract infection in the pediatric population remains unclear. In some studies, viral co-infection was associated with disease progression and the worsening of symptoms [12,14]. In contrast, no differences were observed by other authors in the disease outcome compared to viral mono-infection [16,17]. This variability can be driven by various factors, including the number of patients under studies (often limited) and the lack of correlation among specific viruses. Convincing evidence in this setting has obvious diagnostic and therapeutic implications. In general, virus–virus interactions and their occurrence at the population level are still poorly understood and need to be thoroughly investigated, especially after the SARS-CoV-2 pandemic. Indeed, as shown above, whether specific viral interactions enhance or diminish the severity of respiratory disease is still controversial. Therefore, starting from these different observations, the preliminary aim of the study was to describe the presence of viruses in the respiratory specimens collected from a large cohort of children and adolescents (0–18 years old) admitted to a large tertiary pediatric hospital in Rome between 1 January 2022 and 30 June 2023. Then, our main aim was to investigate the distribution and type of viral mono- and co-infections in respiratory samples included in the analysis. In a subgroup of pediatric patients whose ARI (upper or lower) diagnosis was available, we also evaluated the type of viral detections.
2. Materials and Methods
2.1. Sample Characteristics and Inclusion Criteria of the Analysis
Between 1 January 2022 and 30 June 2023, 10,018 respiratory samples from 5171 children and adolescents (0–18 years, both inpatients and outpatients) with clinically relevant respiratory symptoms were tested using the Allplex Respiratory Panel Assays (Seegene, Seoul, Republic of Korea) at the Bambino Gesù Children’s Hospital in Rome, Italy. If a patient had multiple respiratory samples, we selected the first one carried out. In most cases, this was the one performed at the time of hospital admission (inpatient and outpatient). Respiratory samples belonging to the same patient with a temporal distance >30 days were considered as a new case. Data from 3525 pediatric patients with inclusion criteria were integrated with SARS-CoV-2 test results obtained by either molecular or antigenic methods if the test was performed within 72 h before or after the multiplex respiratory panel test (Figure S1). Clinical information regarding the type of ARI (upper or lower respiratory tract infections) diagnosed at the time of hospital admission was collected for a large subgroup of respiratory samples from 1011 pediatric patients (Figure S1).
2.2. Allplex Respiratory Panel
The Allplex Respiratory Panel Assay is a closed multiplex PCR system for the simultaneous detection of virus respiratory pathogens that includes extraction, amplification, detection, and analysis of samples. All steps, from nucleic acid extraction to final pathogen detection, were carried out automatically. Nucleic acid extraction and PCR preparation were performed in a one-step process using Hamilton (Seegene Inc., Seoul, Republic of Korea). A multiplex PCR assay for 16 different respiratory tract viruses was performed in three different PCR multiplex panels. These panels allow for the identification of 16 different viruses: Flu A and B, RSV A and B, ADV, HeV, PIV1-4, MPV, HBoV, HRV, and three human coronaviruses (CoV NL63/229E/OC43). Each reaction mixture contained 8 µL of extracted nucleic acid and 17 µL of one-step RT-PCR master mix (5 µL of RP MOM, 5 µL of RNase-free water, 5 µL of 5× real-time one-step buffer, and 2 µL of real-time one-step enzyme) at a final volume of 25 µL. Multiplex RT-PCR was performed using a CFX96™ Real-time PCR System (Bio-Rad Laboratories, Hercules, CA, USA). The results were analyzed automatically using Seegene Viewer V3.0 (Seegene Inc., Seoul, Republic of Korea) and interpreted according to the manufacturer’s instructions. Following the datasheet indications for interpretation of the results, the sample was considered positive for the presence of the pathogen if the Cycle threshold (Ct) value was ≤42 cycles. A negative specimen was defined by the absence of amplification for any pathogen with the presence of amplification of the internal control.
2.3. SARS-CoV-2 Tests
Data of multiplex respiratory panel tests were integrated with SARS-CoV-2 test results assessed by antigenic or molecular tests. SARS-CoV-2 antigen testing was performed using the electrochemiluminescence immunoassay (ECLIA) Roche Elecsys SARS-CoV-2 Antigen on a Roche Cobas e411 (Roche Diagnostics GmbH, Mannheim, Germany) or the fluorescent immunoassay RADT STANDARD F COVID-19 Ag FIA on an F2400 analyzer (SD BIOSENSOR, Korea). According to the manufacturers’ instructions, a result of cut-off index (COI) ≥ 1.0 was interpreted as reactive for SARS-CoV-2 antigen. Molecular tests were performed for the detection of the SARS-CoV-2 virus through reverse transcription (RT), followed by real-time PCR from RNA extracted from respiratory samples by different assays according to the manufacturers’ instructions. The Cepheid Xpert Xpress CoV-2 plus performed on Cepheid’s GeneXpert®DX system (Cepheid, Sunnyvale, CA, USA) is an assay that amplifies and detects unique sequences in the nucleocapsid (N), envelope (E), and RNA-dependent RNA polymerase (RdRP) genes of the SARS-CoV-2 genome. The SARS-CoV-2 ELITe MGB Kit® (Elitechgroup, Turin, Italy), performed using the ELITe InGenius instrument (Elitechgroup, Turin, Italy), is an assay that amplifies and detects unique sequences in the RdRp and ORF8 genes of the SARS-CoV-2 genome.
2.4. Interpretation of Data and Statistical Analysis
Based on the results obtained, samples were classified as positive or negative for each pathogen. Among the positive results, the percentage of samples with mono-infection and co-infection was determined based on the number of pathogens identified. Co-infection was defined as positive detection of >1 virus in the same sample. Descriptive statistics are expressed as median values and interquartile range (IQR) for continuous data and number (percentage) for categorical data. Statistical comparisons were performed using Fisher’s exact and Mann–Whitney tests for categorical and continuous variables, respectively. A value of p less than 0.05 was considered statistically significant. The Spearman’s rank correlation coefficient was calculated to evaluate correlations between respiratory viruses. Data were analyzed using statistical software package SPSS (v32.0; SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Seasonal Distribution of Non-SARS-CoV-2 Respiratory Viruses in Samples Analyzed
During the study period, a total of 10,018 respiratory samples from 5171 children and adolescents with a median age of 3.0 years (interquartile range [IQR]: 0.5–6.9) were screened for respiratory viruses at the Bambino Gesù Children’s Hospital in Rome, Italy. Of these, 6061 (60.5%) were positive for at least one virus (Figure S1). For some viruses, a different seasonal pattern was observed during 2022 and the first part of 2023. As expected, RSV, Flu A and B were mainly detected in the cold months, but with some differences during the two years (Figure S2). Considering RSV, the detection of this virus was highest from November to February 2022. During the cold months of January–February 2022, RSV A was more prevalent than RSV B (20.8% vs. 7.8%, p < 0.001). In contrast, between November 2022 and January 2023, RSV B circulated predominantly compared to RSV A (57.9% vs. 10.9%, p < 0.001). A different prevalence was also observed for Flu A and Flu B during the cold months of 2022 and 2023. In particular, the prevalence of Flu A was the highest from November 2022 to January 2023. By comparing January–February 2022 and January–February 2023, a significant increase in Flu A prevalence was observed (0.7% vs. 15.7%, p < 0.001). Circulation of Flu B was rarely observed during 2022 (below 1%), whereas the prevalence of this virus increased significantly in 2023, with the highest detection in March 2023 (3.6%, N = 23). Other viruses like ADV, HBoV, HeV, and HRV were detected throughout the 2022 year, with no significant fluctuation in prevalence during the different months, independent of the cold temperatures (Figure S2). A similar trend was also observed during the winter and spring seasons of 2023 (Figure S2).
3.2. General Results of Samples Included in the Analysis
Of the 10,018 respiratory specimens initially considered, 3525 of them, each taken from 3525 patients, met the inclusion criteria, i.e., multiplex respiratory panel test and a SARS-CoV-2 test performed within 72 h available (see Section 2.1 and Figure S1). Of them, 1881 (53.4%) were from males, and 1644 (46.6%) were from from females. The median age was 3.3 years (IQR: 2.7–4.9). Samples were also stratified according to Eunice Kennedy Shriver National Institute of Child Health and Human Development age stage terminology [18]. By stratifying samples based on these categorical ages, most of them were from early childhood (2.1 to 5 years of age; N = 1028, 29.2%) and infants (29 days to 12 months; N = 770, 21.8%), followed by middle childhood (6 to 11 years of age; N = 579, 16.4%), toddlers (13 months to 2 years; N = 405, 11.5%), neonates (birth to 28 days; N = 377, 10.7%), and adolescents (12 to 18 years of age; N = 366, 10.4%). The large majority of respiratory specimens was collected by a nasopharyngeal aspirate (N = 2406, 68.3%) or nasopharyngeal swab (N = 1089, 30.9%; see Table 1).
Table 1.
General characteristics of 3525 patients included in the analysis.
| Total, N | 3525 |
| Demographic characteristics | |
| Male, N (%) | 1881 (53.4) |
| Female, N (%) | 1644 (46.6) |
| Age, years, median (IQR) | 3.3 (2.7–4.9) |
| Age category | |
| Neonates (birth–28 days), N (%) | 377 (10.7) |
| Infant (29 days–12 months), N (%) | 770 (21.8) |
| Toddler (13 months–2 years), N (%) | 405 (11.5) |
| Early childhood (2.1–5 years), N (%) | 1028 (29.2) |
| Middle childhood (6–11 years), N (%) | 579 (16.4) |
| Adolescence (12–18 years), N (%) | 366 (10.4) |
| Sample type | |
| Nasopharyngeal aspirate, N (%) | 2406 (68.3) |
| Nasopharyngeal swab, N (%) | 1089 (30.9) |
| Bronchoalveolar lavage, N (%) | 30 (0.85) |
IQR, interquartile range.
3.3. Detection of Respiratory Viruses
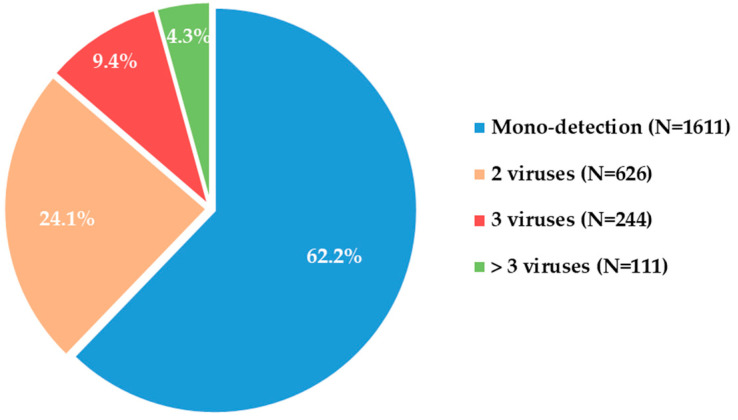
Of the 3525 respiratory samples analyzed from the corresponding 3.525 patients, 73.5% (N = 2592) were positive for at least one virus. Viral mono-infection was found in 62.2% (N = 1611) of positive samples, and co-infection was observed in 37.8% (N = 1981) of the specimens. Often, co-infection involved two viruses (24.1%, N = 626), and the detection of >3 viruses was less frequent but still remarkable (9.4%, N = 244 for 3, and 4.3%, N = 111 for >3 viruses, Figure 1).
Figure 1.
Distribution of viral detections in positive respiratory samples with a SARS-CoV-2 test available (N = 2592).
A different distribution of viral mono- and co-infections was observed among age groups. Indeed, viral co-infection was significantly higher in samples from toddlers and early childhood (55.0%, N = 183 and 46.0%, N = 383, respectively) compared to samples from adolescents and neonates (18.4%, N = 155 and 22.4%, N = 210, respectively) (p < 0.001) (Figure S3).
3.4. Distribution of Detected Viruses and Viral Pair Relations
Overall, HRV was detected in 48.5% of all samples, followed by ADV (16.5%), SARS-CoV-2 (16.0%), HBoV (12.1%), and RSV B (11.9%) (Table 2).
Table 2.
Absolute number and percentage of positive samples for 17 different viruses as mono-detection or co-detection (2 viruses or >2 viruses).
| AdV (N = 428) |
HBoV (N = 314) |
HCoV-229A (N = 36) |
HCoV-NL63 (N = 45) |
HCoV-OC43 (N = 132) |
HeV (N = 278) |
Flu A (N = 174) |
Flu B (N = 36) |
MPV (N = 234) |
PIV1 (N = 54) |
PIV2 (N = 64) |
PIV3 (N = 193) |
PIV4 (N = 40) |
HRV (N = 1256) |
RSV A (N = 79) |
RSV B (N = 308) |
SARS-CoV-2 (N = 414) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Virus (N = 1611) |
122 (28.5) |
38 (12.0) |
14 (38.9) |
13 (28.9) |
37 (28.0) |
54 (19.4) |
96 (55.1) |
19 (52.8) |
81 (34.6) |
14 (25.9) |
30 (46.9) |
80 (41.4) |
14 (35.0) |
566 (45.1) |
36 (45.6) |
135 (43.8) |
262 (63.3) |
| 2 Viruses (N = 626) |
140 (32.7) |
105 (33.4) |
13 (36.1) |
16 (35.5) |
43 (32.6) |
79 (28.4) |
46 (26.4) |
9 (25.0) |
77 (32.9) |
15 (27.8) |
17 (26.6) |
59 (30.6) |
20 (50.0) |
399 (31.8) |
26 (32.9) |
99 (32.1) |
89 (21.5) |
| >2 Viruses (N = 355) |
166 (38.8) |
171 (54.4) |
9 (25.0) |
16 (35.5) |
52 (39.4) |
145 (52.1) |
32 (18.4) |
8 (22.2) |
76 (32.5) |
25 (46.3) |
17 (26.6) |
54 (27.8) |
6 (15.0) |
291 (23.2) |
17 (21.5) |
74 (24.0) |
63 (15.2) |
| p-value * | <0.001 | <0.001 | 0.005 | <0.001 | <0.001 | <0.001 | 0.052 | 0.299 | <0.001 | <0.001 | 0.013 | <0.001 | 0.001 | <0.001 | 0.003 | <0.001 | 0.619 |
* Two-sided p-values were calculated by Fisher’s exact test.
By analyzing the distribution of each virus in mono- or co-infection, substantial differences were observed. The majority of the viruses were more often detected as a part of a co-infection: the highest co-infection rate was observed for HBoV (87.8% co-infection vs. 12.2% mono-infection, p < 0.001) and HeV (80.5% co-infection vs. 19.5% mono-infection, p < 0.001) (Table 2). Considering viral co-infections, viral pairings frequently detected mainly involved HRV with other viruses, thus reflecting the high number of this virus detection in the overall samples analyzed (Table 2 and Table S1). In particular, HRV was observed more often in co-infection (55.0% co-infection vs. 45.0% mono-infection, p < 0.001). In contrast, Flu A was mainly seen in mono-infection (55.1% mono-infection vs. 44.9% co-infection, p = 0.052) (Table 2 and Table S1). SARS-CoV-2 was also mainly seen in mono-infection (63.3%, N = 262 mono-infection vs. 36.7%, N = 152 co-infection, p = 0.619). Moreover, a pairwaise analysis between SARS-CoV-2 and other viruses was performed. This analysis highlighted a statistically significant distribution of this virus mainly in mono-infection (p < 0.05).
3.5. Correlations between Respiratory Viruses
Correlations between respiratory viruses were also evaluated with Spearman’s rank correlation test. The data showed 13 positive correlations and 19 negative correlations that were statistically significant among the viral pairs identified (Table 3). In particular, a markedly significant correlation (p < 0.001) was observed for five positive associations and for four negative associations. Significantly positive correlations were found between ADV and HBoV (r = 0.106, p < 0.001), ADV and HeV (r = 0.094, p < 0.001), HBoV and HeV (r = 0.085, p < 0.001), HBoV and MPV (r = 0.081, p < 0.001), and HeV and HRV (r = 0.110, p < 0.001) (Table 3). In contrast, significantly negative correlations were observed between OC43 and HRV (r = −0.077, p < 0.001), Flu A and HRV (r = −0.0164, p < 0.001), HRV and RSV A (r = −0.091, p < 0.001), and HRV and RSV B (r = −0.127, p < 0.001) (Table 3). In addition, SARS-CoV-2 was negatively associated with all other respiratory viruses tested (p < 0.05), thus confirming the high prevalence of this virus in mono-detection. Thus, some viruses had a significant association (either negative or positive) with other viruses, confirming that their co-presence is not casual, but is regulated by complex factors that can include cellular target(s), replication capacity, pathogenetic characteristics, seasonality, etc.
Table 3.
Correlations among respiratory viruses.
| Virus 1 | Virus 2 | Spearman’s Rho | p-Value |
|---|---|---|---|
| Positive correlations | |||
| ADV | HBoV | 0.106 | <0.001 |
| ADV | HeV | 0.094 | <0.001 |
| HBoV | HeV | 0.085 | <0.001 |
| HBoV | MPV | 0.081 | <0.001 |
| HeV | HRV | 0.110 | <0.001 |
| ADV | NL63 | 0.044 | 0.024 |
| HBoV | HRV | 0.068 | 0.001 |
| HBoV | OC43 | 0.048 | 0.014 |
| HBoV | PIV1 | 0.045 | 0.021 |
| OC43 | RSVA | 0.061 | 0.002 |
| OC43 | RSVB | 0.045 | 0.022 |
| HeV | MPV | 0.056 | 0.004 |
| HeV | PIV1 | 0.045 | 0.021 |
| Negative correlations | |||
| OC43 | HRV | −0.077 | <0.001 |
| Flu A | HRV | −0.164 | <0.001 |
| HRV | RSV A | −0.091 | <0.001 |
| HRV | RSV B | −0.127 | <0.001 |
| ADV | Flu A | −0.061 | 0.002 |
| ADV | RSV B | −0.067 | 0.001 |
| ADV | RSV A | −0.055 | 0.005 |
| HEV | RSV B | −0.050 | 0.011 |
| HEV | Flu A | −0.048 | 0.014 |
| MPV | HRV | −0.066 | 0.001 |
| MPV | RSV A | −0.048 | 0.014 |
| MPV | PIV2 | −0.041 | 0.035 |
| PIV 3 | RSV B | −0.059 | 0.003 |
| PIV 3 | HRV | −0.054 | 0.006 |
| Flu A | PIV3 | −0.047 | 0.017 |
| Flu A | MPV | −0.041 | 0.035 |
| 229E | HEV | −0.041 | 0.036 |
| 229E | HRV | −0.043 | 0.030 |
| PIV 2 | RSV B | −0.043 | 0.026 |
3.6. Analysis of Viral Detection in Samples of Patients with ARI Diagnosis
The next step of the work was to evaluate viral distribution in a subset of 1011 samples, each belonging to pediatric patients with a defined diagnosis of ARI (upper or lower) at the time of hospital admission. Among the 1011 samples, 378 (37.0%) were from an upper ARI, and 633 (63.0%) were from from a lower ARI. We evaluated the correlation of mono-coinfection with the epidemiological and clinical data. Looking at demographic characteristics, no statistically significant differences were observed between groups, except for a higher prevalence of early childhood and longer duration of hospitalization in the group with lower ARI (p: 0.012 and <0.001, respectively) (Table 4).
Table 4.
General characteristics of samples belonging to pediatric patients with a diagnosis of acute respiratory infection (ARI, upper or lower) at the time of hospital admission.
| Total ARI (N = 1011) |
Upper ARI (N = 378) |
Lower ARI (N = 633) |
p-Value * | |
|---|---|---|---|---|
| Male, N (%) | 553 (54.5) | 195 (51.6) | 358 (56.5) | 0.133 |
| Age, years, median (IQR) | 0.9 (0.2–3.6) | 0.8 (0.1–3.1) | 1.0 (3.0–7.9) | 0.451 |
| Neonates, N (%) | 177 (17.5) | 69 (18.2) | 108 (17.0) | 0.669 |
| Infant, N (%) | 351 (34.7) | 135 (35.7) | 216 (34.1) | 0.633 |
| Toddler, N (%) | 112 (11.1) | 49 (13.0) | 63 (9.9) | 0.148 |
| Early childhood, N (%) | 225 (22.2) | 68 (18.0) | 157 (24.8) | 0.012 |
| Middle childhood, N (%) | 89 (8.8) | 39 (10.3) | 50 (7.9) | 0.207 |
| Adolescence, N (%) | 57 (5.6) | 18 (4.8) | 39 (6.3) | 0.399 |
| Length of hospitalization (days, median IQR) | 4.3 (2.9–7.1) | 3.6 (2.7–5.5) | 5.0 (3.0–7.9) | <0.001 |
| Clinical manifestation | ||||
| Bronchitis | 178 (17.6) | - | 178 (28.1) | - |
| Bronchiolitis | 257 (25.4) | - | 257 (40.6) | - |
| Pneumonia | 198 (19.6) | - | 198 (31.3) | - |
IQR, interquartile range; * two-sided p-values were calculated by Fisher’s exact test or Mann–Whitney test, as appropriate.
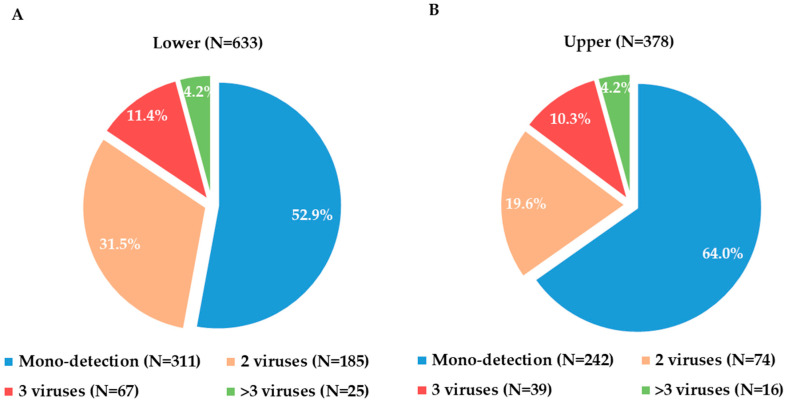
By analyzing the distribution of viral detection in the two groups, a higher prevalence of co-infection was observed in the lower respiratory tract compared to the upper respiratory tract infections (47.1% vs. 36.0%, p = 0.003) (Figure 2). Overall, SARS-CoV-2 was the virus mainly detected in upper ARI, mostly (see above) in mono-infection (66.1% in mono-infection vs. 33.9% in co-infection, p = 0.390). RSV B and (not totally unexpected, see the Discussion section) HRV were the most frequently detected viruses in lower ARI; both were mainly involved in co-infections (HRV in particular: 69.8% in co-infection vs. 30.2% in mono-infection, p < 0.001, whereas RSV B was found 52.6% of the time in co-infection vs. 47.4% of the time in mono-infection, p = 0.07).
Figure 2.
Distribution of viral detections in lower (panel A) and upper (panel B) acute respiratory infections (ARIs).
4. Discussion
Two years after of the coronavirus disease 2019 (COVID-19) pandemic, and as a result of the relaxation of the mitigation measures, there is now a co-circulation of SARS-CoV-2 with other respiratory viruses. In this new epidemiological scenario, the role of viral interactions due to co-infection is yet to be thoroughly investigated. Respiratory viruses may infect various cells within the respiratory tract, including ciliated epithelial cells, alveolar cells, and immune cells. The co-presence of different respiratory viruses in the same respiratory tract can lead to specific viral interactions that depend on several aspects, including host factors, the seasonality of viruses, and their cell tropism. Indeed, if two viruses infect the same cell, there is a competition mechanism in which one virus can inhibit the replication of the other. Instead, if two viruses infect different cell types, they are more likely to be co-present. What is driving these positive and negative interactions during co-infections is unknown, but studying viral correlations at the population level can help to provide insight into these mechanisms and the possible role in the disease severity. This may be particularly important for the pediatric population, as viral co-infections are frequently observed among children compared to adults [19]. For this reason, this study investigated as a primary objective the distribution and type of viral mono- and co-infections by also evaluating the type of viral correlations in a large number of respiratory samples collected from children and adolescents admitted to the Bambino Gesù Children’s Hospital in Rome between 1 January 2022 and 30 June 2023. A preliminary epidemiological analysis was also performed to evaluate the distribution of respiratory viruses other than SARS-CoV-2.
By analyzing the overall dataset of 10,018 respiratory samples (Figure S1), collected in a post-pandemic period, a substantial re-circulation of respiratory viruses other than SARS-CoV-2 was observed. A variation in seasonal pattern was observed particularly for RSV and influenza viruses in the 2022–2023 season, which marked the return of the circulation of these viruses at almost pre-pandemic levels, as also observed in other studies [20,21,22,23]. In this setting, considering the principal aim of this study, we evaluated the number and type of viral detection in a subgroup of 3525 patients screened for respiratory viruses, also considering SARS-CoV-2. Overall, the majority of positive samples (62.2%) were characterized by the presence of a single virus. Co-infection was found in 37.8% of respiratory specimens analyzed, particularly in samples from toddlers (13 months to 2 years) and early childhood (2 to 5 years of age). This result is in line with recent studies, which found that the age group with the highest viral co-infection rate is, in general, that of children aged 0–5 years old [4,24,25]. By analyzing the distribution of each virus in mono- or co-infection, the data show that the highest co-infection rate was observed for HBoV (87.8% in co-infection). This virus was also involved in some significantly positive correlations with ADV, MPV, and HeV. HBoV is usually characterized by prolonged viral shedding, with a persistence of its DNA described for up to 3 months in outpatients and up to 1 year in hospitalized children after acute infection [26]. This can in part explain the high frequency of HBoV co-detection with other respiratory viruses [26,27]. In this study, a single sample per patient was analyzed, and in most cases, this coincided with that performed at hospital admission. For patients with an HBoV-positive respiratory test, we assessed whether longitudinal samples were also present, and indeed, a persistence of HBoV up to 4 months was observed.
The phenomenon described above raises the question as to whether HBoV has a causative pathogenetic role for co-infection with other viruses, or whether it is found only as a co-detected virus, since it can persist in the host for a long time. Recent studies reported that low HBoV viral loads were detected more often in co-infection [26,28]. In contrast, high HBoV loads were observed mainly in the absence of other respiratory viruses, suggesting a causative role of this virus in the etiology of the respiratory disease [26,28]. By analyzing the threshold cycles (Ct) of HBoV-positive samples, we also observed a trend toward lower CT values (that is, high viral load) when HBoV was detected in mono-infection versus when it was detected in co-infection, though the difference was not statistically significant. Therefore, in the study of viral co-infection, viral load may serve also as an important value to understand which virus may have a greater influence on the clinical severity of the respiratory disease. This aspect became particularly relevant during the COVID-19 pandemic. Indeed, Ct values can be used to estimate the viral load of SARS-CoV-2 as a marker of infectivity and to identify patients at higher risk for morbidity or severe outcome [29].
In our study, SARS-CoV-2 and Flu A tend to be found mostly in mono-infection. Interestingly; another recently published study reports that in children, most viral co-infections were found at significantly reduced rates relative to that expected from the incidence of each virus, especially those involving SARS-CoV-2 and influenza [30].
Another aspect analyzed in this study included the interactions between respiratory viruses through analysis of correlation. Significantly negative associations were observed particularly for SARS-CoV-2 with all other respiratory viruses tested. Moreover, a negative correlation was also observed for HRV with RSV A and RSV B and for Flu A with HRV. The biological mechanism related to viral interference may in part help to explain the negative correlations observed in this study. Indeed, recent findings reported that SARS-CoV-2 replication can be impaired by a primary HRV infection [31]. It has also been found that SARS-CoV-2 is particularly susceptible to negative interference by Flu and RSV, i.e., viruses that trigger a strong IFN response [32]. It has also been well demonstrated that a previous infection with rhinovirus inhibits infection with influenza A virus, by activating antiviral defenses in the target tissue against both viruses [33]. A negative association between HRV and RSV was also reported in different epidemiological studies including large pediatric populations [34]. In fact, it has been shown that children with prior exposure to RSV had a lower incidence of HRV than those without a previous RSV infection. Other factors unrelated to viral interference could also contribute to the negative correlations between specific viruses, such as differences in virus seasonality based on environmental factors or differences in virus host range (e.g., viruses that preferentially infect different age groups). Further longitudinal studies in a large cohort of pediatric patients are needed to clarify this point.
Evidence concerning the severity of disease in the presence of a viral co-infections compared to single viral infections is conflicting. Indeed, some studies have reported an increased risk for lower respiratory tract infections in hospitalized children [15,35], whereas others were unable to identify clinical differences [36,37]. In our study, no analysis related to disease severity was performed. However, we investigated the type of viral detections in a subgroup of pediatric patients whose ARI (upper or lower) diagnosis was available. We observed a higher prevalence of viral co-infections particularly in samples from patients with a lower respiratory tract infection compared to patients with an upper respiratory tract infection. The viruses that mainly characterized viral mono- and co-infection in lower respiratory tract infection were HRV and RSV B. These data are concordant with the evidence showing that HRV may also be associated with lower respiratory tract infections alongside RSV in infants/children [38]. Moreover, despite the negative interaction between RSV and HRV, when co-infection is present, an increase in disease severity is reported, as compared to a single RSV infection [34].
One limitation of this study is that it did not also consider the presence of potential bacterial coinfections. Another limitation is that it was not possible to retrieve information on the patients’ vaccination status (i.e., influenza vaccine). Vaccination can impact viral dynamics and kinetics. Vaccinated individuals may have a shorter infectivity duration and a lower viral load. Therefore, this can potentially impact viral interactions in coinfection. This aspect is still poorly investigated and deserves further investigation.
Taken together, all our data suggest that the correlation between co-infection and clinical outcome may be linked to the type of virus(es) involved in the co-infection rather than a simple co-presence. Further studies dedicated to this important point are needed, since it has obvious implications from a diagnostic and clinical point of view (i.e., identification of patients that may have a worse clinical outcome when carrying a specific association of viruses rather than a general mono- vs. multiple infection). Lastly, by considering the upper respiratory tract infection, SARS-CoV-2 was the most frequently detected pathogen. Currently, there is a global dominance of the Omicron variant [39]. Compared with previous variants, it has shown greater tropism for the upper respiratory tract and/or bronchial tissue [40,41,42]. This change in tropism can help to explain the greater concentration of the virus in the upper respiratory tract, which makes its detection in this respiratory tract more likely.
5. Conclusions
In conclusion, in this study, the high number of respiratory samples from pediatric patients analyzed highlighted different patterns of positive and negative correlations between viral pairings. In particular, SARS-CoV-2 was negatively associated with all other respiratory viruses, whereas a markedly significant positive correlation was observed for five viral pairings (involving adenovirus/human bocavirus/human enterovirus/metapneumoviruses/rhinovirus). Viral co-infection was frequently observed in samples from children with a lower respiratory tract infection compared to those with an upper respiratory tract infection, thus suggesting a role for co-infections in the prognosis of these patients. Further studies are required to confirm the significance of the viral pairings observed in this analysis and to elucidate the virologic mechanisms underlying the association that may lead to cooperation or competition between co-detected viruses. Clinical data will help to elucidate the role of multiple viral co-infections on the severity of respiratory disease.
Acknowledgments
The authors also thank Valerio Di Gioacchino and Diana Fortini, as well as the whole staff of the Microbiology and Virology Laboratory of Bambino Gesù Children’s Hospital IRCCS for outstanding technical support in processing samples, performing laboratory analyses, and data management.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/v16050750/s1: Figure S1. Sample characteristics and inclusion criteria of the analysis; Figure S2. Distribution of community respiratory viruses from January 2022 to June 2023; Figure S3. Distribution and prevalence of viral mono- and co-detection within age groups; Table S1. Matrix table showing viral pairings observed in the samples analyzed.
Author Contributions
Conceptualization, C.F.P., V.C.D.M. and R.S.; methodology, V.C.D.M. and R.S.; formal analysis, V.C.D.M., R.S. and L.C. (Luna Colagrossi); resources, E.G. and L.F.; data curation, L.C. (Luana Coltella), S.R., G.L., L.G., A.C.V., M.P. and S.C.; writing—original draft preparation V.C.D.M. and R.S.; writing—review and editing, C.F.P., V.C.D.M. and R.S.; supervision, C.F.P., C.R., P.B., M.R. and A.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Due to the retrospective nature of the study, informed consent of the patients was not deemed necessary, as all the data were collected in a completely anonymous and aggregate form.
Data Availability Statement
Data are contained within the article (and its Supplementary Materials).
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This work was supported by EU funding within the Next-Generation EU-MUR PNRR Extended Partnership Initiative on Emerging Infectious Diseases (Project No. PE00000007, INF-ACT). This work was also supported by the Italian Ministry of Health with “Current Research funds” and by the ANIA foundation.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization (WHO) Severe Acute Respiratory Infections Treatment Centre. Practical Manual to Set Up and Manage a SARI Treatment Centre and a SARI Screening Facility in Health Care Facilities. [(accessed on 25 March 2024)]. Available online: https://www.who.int/publications/i/item/10665-331603.
- 2.Simoes E.A.F., Cherian T., Chow J., Shahid-Salles S.A., Laxminarayan R., John T.J. Disease Control Priorities in Developing Countries. 2nd ed. Oxford University Press; New York, NY, USA: 2006. Chapter 25 Acute Respiratory Infections in Children. [Google Scholar]
- 3.Chadha M., Hirve S., Bancej C., Barr I., Baumeister E., Caetano B., Chittaganpitch M., Darmaa B., Ellis J., Fasce R., et al. Human Respiratory Syncytial Virus and Influenza Seasonality Patterns—Early Findings from the WHO Global Respiratory Syncytial Virus Surveillance. Influenza Other Respir. Viruses. 2020;14:638–646. doi: 10.1111/irv.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maison N., Omony J., Rinderknecht S., Kolberg L., Meyer-Bühn M., von Mutius E., Hübner J., von Both U. Old Foes Following News Ways?—Pandemic-Related Changes in the Epidemiology of Viral Respiratory Tract Infections. Infection. 2024;52:209–218. doi: 10.1007/s15010-023-02085-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattana G., Albitar-Nehme S., Cento V., Colagrossi L., Piccioni L., Raponi M., Raucci U., Vittucci A.C., Reale A., Villani A., et al. Back to the Future (of Common Respiratory Viruses) J. Glob. Antimicrob. Resist. 2022;28:223–225. doi: 10.1016/j.jgar.2022.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciofi degli Atti M., Rizzo C., D’Amore C., Ravà L., Reale A., Barbieri M.A., Bernaschi P., Russo C., Villani A., Perno C.F., et al. Acute Respiratory Infection Emergency Access in a Tertiary Care Children Hospital in Italy, Prior and after the SARS-CoV-2 Emergence. Influenza Other Respir. Viruses. 2023;17:e13102. doi: 10.1111/irv.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida T., Guimarães J.T., Rebelo S. Epidemiological Changes in Respiratory Viral Infections in Children: The Influence of the COVID-19 Pandemic. Viruses. 2023;15:1880. doi: 10.3390/v15091880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow E.J., Uyeki T.M., Chu H.Y. The Effects of the COVID-19 Pandemic on Community Respiratory Virus Activity. Nat. Rev. Microbiol. 2022;21:195–210. doi: 10.1038/s41579-022-00807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dien Bard J., McElvania E. Panels and Syndromic Testing in Clinical Microbiology. Clin. Lab. Med. 2020;40:393–420. doi: 10.1016/j.cll.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piret J., Boivin G. Viral Interference between Respiratory Viruses. Emerg. Infect. Dis. 2022;28:273–281. doi: 10.3201/eid2802.211727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du Y., Wang C., Zhang Y. Viral Coinfections. Viruses. 2022;14:2645. doi: 10.3390/v14122645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malveste Ito C.R., Moreira A.L.E., da Silva P.A.N., Santos M.d.O., dos Santos A.P., Rézio G.S., de Brito P.N., Rezende A.P.C., Fonseca J.G., Peixoto F.A.d.O., et al. Viral Coinfection of Children Hospitalized with Severe Acute Respiratory Infections during COVID-19 Pandemic. Biomedicines. 2023;11:1402. doi: 10.3390/biomedicines11051402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bermúdez-Barrezueta L., López-Casillas P., Rojo-Rello S., Sáez-García L., Marugán-Miguelsanz J.M., Pino-Vázquez M.d.l.A. Outcomes of Viral Coinfections in Infants Hospitalized for Acute Bronchiolitis. Virol. J. 2023;20:235. doi: 10.1186/s12985-023-02197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Althouse B.M., Flasche S., Toizumi M., Nguyen H.-A.T., Vo H.M., Le M.N., Hashizume M., Ariyoshi K., Anh D.D., Rodgers G.L., et al. Differences in Clinical Severity of Respiratory Viral Infections in Hospitalized Children. Sci. Rep. 2021;11:5163. doi: 10.1038/s41598-021-84423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabatabai J., Ihling C.M., Manuel B., Rehbein R.M., Schnee S.V., Hoos J., Pfeil J., Grulich-Henn J., Schnitzler P. Viral Etiology and Clinical Characteristics of Acute Respiratory Tract Infections in Hospitalized Children in Southern Germany (2014–2018) Open Forum Infect. Dis. 2023;10:ofad110. doi: 10.1093/ofid/ofad110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scotta M.C., Chakr V.C.B.G., de Moura A., Becker R.G., de Souza A.P.D., Jones M.H., Pinto L.A., Sarria E.E., Pitrez P.M., Stein R.T., et al. Respiratory Viral Coinfection and Disease Severity in Children: A Systematic Review and Meta-Analysis. J. Clin. Virol. 2016;80:45–56. doi: 10.1016/j.jcv.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asner S.A., Science M.E., Tran D., Smieja M., Merglen A., Mertz D. Clinical Disease Severity of Respiratory Viral Co-Infection versus Single Viral Infection: A Systematic Review and Meta-Analysis. PLoS ONE. 2014;9:e99392. doi: 10.1371/journal.pone.0099392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams K., Thomson D., Seto I., Contopoulos-Ioannidis D.G., Ioannidis J.P.A., Curtis S., Constantin E., Batmanabane G., Hartling L., Klassen T. Standard 6: Age Groups for Pediatric Trials. Pediatrics. 2012;129:S153–S160. doi: 10.1542/peds.2012-0055I. [DOI] [PubMed] [Google Scholar]
- 19.Maltezou H.C., Papanikolopoulou A., Vassiliu S., Theodoridou K., Nikolopoulou G., Sipsas N.V. COVID-19 and Respiratory Virus Co-Infections: A Systematic Review of the Literature. Viruses. 2023;15:865. doi: 10.3390/v15040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierangeli A., Nenna R., Fracella M., Scagnolari C., Oliveto G., Sorrentino L., Frasca F., Conti M.G., Petrarca L., Papoff P., et al. Genetic Diversity and Its Impact on Disease Severity in Respiratory Syncytial Virus Subtype-A and -B Bronchiolitis before and after Pandemic Restrictions in Rome. J. Infect. 2023;87:305–314. doi: 10.1016/j.jinf.2023.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Vittucci A.C., Antilici L., Russo C., Musolino A.M.C., Cristaldi S., Cutrera R., Persia S., Di Maio C.V., Raponi M., Perno C.F., et al. Respiratory Syncytial Virus: Can We Still Believe That after Pandemic Bronchiolitis Is Not a Critical Issue for Public Health? Eur. J. Pediatr. 2023;182:5303–5313. doi: 10.1007/s00431-023-05201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Centre for Disease Prevention and Control (ECDC) Seasonal Influenza 2022–2023. Annual Epidemiological Report for 2023. [(accessed on 25 March 2024)]. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/seasonal-influenza-annual-epidemiological-report-2022-2023.pdf.
- 23.Pierangeli A., Piralla A., Uceda Renteria S., Giacomel G., Lunghi G., Pagani E., Giacobazzi E., Vian E., Biscaro V., Piccirilli G., et al. Multicenter Epidemiological Investigation and Genetic Characterization of Respiratory Syncytial Virus and Metapneumovirus Infections in the Pre-Pandemic 2018–2019 Season in Northern and Central Italy. Clin. Exp. Med. 2022;23:2725–2737. doi: 10.1007/s10238-022-00973-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandelia Y., Procop G.W., Richter S.S., Worley S., Liu W., Esper F. Dynamics and Predisposition of Respiratory Viral Co-Infections in Children and Adults. Clin. Microbiol. Infect. 2021;27:631.e1–631.e6. doi: 10.1016/j.cmi.2020.05.042. [DOI] [PubMed] [Google Scholar]
- 25.Westbrook A., Wang T., Bhakta K., Sullivan J., Gonzalez M.D., Lam W., Rostad C.A. Respiratory Coinfections in Children With SARS-CoV-2. Pediatr. Infect. Dis. J. 2023;42:774–780. doi: 10.1097/INF.0000000000003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verbeke V., Reynders M., Floré K., Vandewal W., Debulpaep S., Sauer K., Cardoen F., Padalko E. Human Bocavirus Infection in Belgian Children with Respiratory Tract Disease. Arch. Virol. 2019;164:2919–2930. doi: 10.1007/s00705-019-04396-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schildgen O., Müller A., Allander T., Mackay I.M., Völz S., Kupfer B., Simon A. Human Bocavirus: Passenger or Pathogen in Acute Respiratory Tract Infections? Clin. Microbiol. Rev. 2008;21:291–304. doi: 10.1128/CMR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang W., Yin F., Zhou W., Yan Y., Ji W. Clinical Significance of Different Virus Load of Human Bocavirus in Patients with Lower Respiratory Tract Infection. Sci. Rep. 2016;6:20246. doi: 10.1038/srep20246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maltezou H.C., Raftopoulos V., Vorou R., Papadima K., Mellou K., Spanakis N., Kossyvakis A., Gioula G., Exindari M., Froukala E., et al. Association Between Upper Respiratory Tract Viral Load, Comorbidities, Disease Severity, and Outcome of Patients With SARS-CoV-2 Infection. J. Infect. Dis. 2021;223:1132–1138. doi: 10.1093/infdis/jiaa804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weidmann M.D., Green D.A., Berry G.J., Wu F. Assessing Respiratory Viral Exclusion and Affinity Interactions through Co-Infection Incidence in a Pediatric Population during the 2022 Resurgence of Influenza and RSV. Front. Cell Infect. Microbiol. 2023;13:1208235. doi: 10.3389/fcimb.2023.1208235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Essaidi-Laziosi M., Alvarez C., Puhach O., Sattonnet-Roche P., Torriani G., Tapparel C., Kaiser L., Eckerle I. Sequential Infections with Rhinovirus and Influenza Modulate the Replicative Capacity of SARS-CoV-2 in the Upper Respiratory Tract. Emerg. Microbes. Infect. 2022;11:413–424. doi: 10.1080/22221751.2021.2021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dee K., Schultz V., Haney J., Bissett L.A., Magill C., Murcia P.R. Influenza A and Respiratory Syncytial Virus Trigger a Cellular Response That Blocks Severe Acute Respiratory Syndrome Virus 2 Infection in the Respiratory Tract. J. Infect. Dis. 2023;227:1396–1406. doi: 10.1093/infdis/jiac494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu A., Mihaylova V.T., Landry M.L., Foxman E.F. Interference between Rhinovirus and Influenza A Virus: A Clinical Data Analysis and Experimental Infection Study. Lancet Microbe. 2020;1:e254–e262. doi: 10.1016/S2666-5247(20)30114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Achten N.B., Wu P., Bont L., Blanken M.O., Gebretsadik T., Chappell J.D., Wang L., Yu C., Larkin E.K., Carroll K.N., et al. Interference Between Respiratory Syncytial Virus and Human Rhinovirus Infection in Infancy. J. Infect. Dis. 2017;215:1102–1106. doi: 10.1093/infdis/jix031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richard N., Komurian-Pradel F., Javouhey E., Perret M., Rajoharison A., Bagnaud A., Billaud G., Vernet G., Lina B., Floret D., et al. The Impact of Dual Viral Infection in Infants Admitted to a Pediatric Intensive Care Unit Associated with Severe Bronchiolitis. Pediatric Infectious Dis. J. 2008;27:213–217. doi: 10.1097/INF.0b013e31815b4935. [DOI] [PubMed] [Google Scholar]
- 36.Peng D., Zhao D., Liu J., Wang X., Yang K., Xicheng H., Li Y., Wang F. Multipathogen Infections in Hospitalized Children with Acute Respiratory Infections. Virol. J. 2009;6:155. doi: 10.1186/1743-422X-6-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wishaupt J.O., van der Ploeg T., de Groot R., Versteegh F.G.A., Hartwig N.G. Single- and Multiple Viral Respiratory Infections in Children: Disease and Management Cannot Be Related to a Specific Pathogen. BMC Infect. Dis. 2017;17:62. doi: 10.1186/s12879-016-2118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ljubin-Sternak S., Meštrović T. Rhinovirus—A True Respiratory Threat or a Common Inconvenience of Childhood? Viruses. 2023;15:825. doi: 10.3390/v15040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization (WHO) Tracking SARS-CoV-2 Variants. [(accessed on 26 March 2024)]. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants.
- 40.Hui K.P.Y., Ng K.-C., Ho J.C.W., Yeung H.-W., Ching R.H.H., Gu H., Chung J.C.K., Chow V.L.Y., Sit K.-Y., Hsin M.K.Y., et al. Replication of SARS-CoV-2 Omicron BA.2 Variant in Ex Vivo Cultures of the Human Upper and Lower Respiratory Tract. EBioMedicine. 2022;83:104232. doi: 10.1016/j.ebiom.2022.104232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan K.S., Ong S.W.X., Koh M.H., Tay D.J.W., Aw D.Z.H., Nah Y.W., Abdullah M.R.B., Coleman K.K., Milton D.K., Chu J.J.H., et al. SARS-CoV-2 Omicron Variant Shedding during Respiratory Activities. Int. J. Infect. Dis. 2023;131:19–25. doi: 10.1016/j.ijid.2023.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hui K.P.Y., Ho J.C.W., Cheung M., Ng K., Ching R.H.H., Lai K., Kam T.T., Gu H., Sit K.-Y., Hsin M.K.Y., et al. SARS-CoV-2 Omicron Variant Replication in Human Bronchus and Lung Ex Vivo. Nature. 2022;603:715–720. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article (and its Supplementary Materials).