Abstract
Setting
Insulin resistance (IR) and compensatory hyperinsulinemia are considered contributing factors toward polycystic ovary syndrome (PCOS).
Objectives
This study evaluates the frequency of metabolic abnormalities in PCOS patients and the effects of myo-inositol (MI) and D-chiro-inositol (DCI), in a 40:1 ratio on hormonal and metabolic parameters.
Participants
Thirty-four women with PCOS phenotype A (endocrine-metabolic syndrome [EMS-type 1]) between the ages of 20–40.
Design
Open prospective study with phenotype A (EMS-type I, n = 34) supplemented with 2,255 mg/day of inositol (MI and DCI in a 40:1 ratio) for 3 months.
Methods
The following were measured before and after treatment: serum levels of follicular stimulating hormone, luteinizing hormone (LH), estradiol, total and free testosterone, sex hormone-binding globulin (SHBG), free androgen index (FAI), anti-Müllerian hormone, glucose, insulin, HOMA-IR, and body mass index (BMI).
Results
55.9% of the enrolled patients were overweight or obese, 50% affected by IR, 17.6% with a history of gestational diabetes mellitus, and 61.8% had familial diabetes mellitus. At the conclusion of the study, BMI (p = 0.0029), HOMA-IR (p < 0.001) significantly decreased, along with decreased numbers of patients with elevated insulin levels. The supplementation resulted in decreased total testosterone (p < 0.001), free testosterone (p < 0.001), FAI (p < 0.001), and LH (p < 0.001); increased SHBG (p < 0.001) and estradiol (p < 0.001).
Limitations
The present analysis was limited to a 12-week follow-up, which precluded a long-term evaluation of the effects of MI and DCI combination. Also, this period was insufficient to achieve and analyze clinical changes such as restoration of the menstrual cycle, restoration of reproductive function, and clinical manifestations of hyperandrogenism.
Conclusions
Supplementation improved metabolic and hormonal profile in PCOS phenotype A (EMS-type I) patients. This builds upon previous work that demonstrated that combined inositol treatment may be effective in PCOS. The study presented herein, used a reduced concentration than in prior literature; however, a significant change in hormonal and metabolic parameters was still observed.
Keywords: Polycystic ovarian syndrome, Insulin resistance, Myo-inositol, D-chiro-inositol
Introduction
Polycystic ovary syndrome (PCOS) represents the most common endocrine disorder in women of reproductive age and is associated with a variety of symptoms, including reproductive and metabolic issues. In recent years, the metabolic component of this syndrome has been carefully considered, as such insulin resistance (IR) and the correlated compensatory hyperinsulinemia have been considered hallmarks of PCOS [1]. This is mirrored in patient populations, as 50–80% of women with PCOS are affected by IR [2, 3], 61% are overweight/obese [4], and >50% suffer from type 2 diabetes mellitus and metabolic syndrome before the age of 40 [5]. Moreover, it has been widely discussed that PCOS-associated hyperandrogenism and reproductive problems, may be a consequence of metabolic disorders, and thus have been defined as “metabolic hyperandrogenism” by some members of the field [6]; therefore, PCOS may be more accurately described as “metabolic reproductive syndrome,” as defined previously by the National Institutes of Health (NIH) evidence-based methodology workshop of PCOS 2012 [7]. At the same time, elevated androgen levels intern promotes the accumulation and redistribution of adipose tissue in women, causing damage to insulin signaling pathways, creating a vicious cycle leading to further impaired glucose metabolism and IR [8]. The Experts Group on Inositol in Basic and Clinical Research (EGOI) has recently proposed a new set of criteria that considers the importance of the metabolic nature of PCOS and more clearly distinguishes between the phenotypes that were originally put forward with the Rotterdam criteria [6, 9].
As a result of the often termed “ovarian paradox,” whereby ovaries, unlike other organs and tissues, maintain insulin signaling networks in the event of systemic IR; therefore, its steroidogenesis is carried out under conditions of compensatory hyperinsulinemia [10]. High insulin levels reduce the sensitivity of granulosa cells and oocytes to the action of follicle-stimulating hormone (FSH), simultaneously increasing the pulsatile secretion of gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) through their central receptors, thus inhibiting folliculogenesis and oocyte maturation [11]. Excessive LH in combination with a direct stimulation of theca cell proliferation by insulin increases androgen synthesis in the ovaries, while blocking aromatase activity impairs their conversion to estrogens. In addition, hyperinsulinemia inhibits hepatic synthesis of sex hormone-binding globulin (SHBG), resulting in an increase in both total and free androgen levels, which leads to clinical manifestations of hyperandrogenism and ovulatory disorders [12]. Thus, IR and compensatory hyperinsulinemia can be described as triggers of the PCOS pathogenesis.
Insulin may further indirectly affect steroidogenesis by altering the ovarian homeostasis between stereoisomers of inositol in ovarian tissue – myo-inositol (MI) and D-chiro-inositol (DCI) [6]. MI and DCI are secondary messengers of insulin, where MI is responsible for the intracellular glucose transport and is simultaneously converted to DCI, which facilitates glycogen storage. The conversion of MI to DCI is mediated by the tissue-specific epimerase enzyme under insulin stimulation, which regulates the intracellular ratio of MI and DCI [13, 14]. The physiological ratio of MI to DCI changes depending on tissue type, for example, in serum the MI:DCI ratio is 40:1, whereas in ovaries it is much higher, reaching 100:1, with a greater need for MI due to its role in FSH signaling [13]. Patients with hyperinsulinemia commonly present increased levels MI to DCI epimerization, leading to an MI deficiency in the ovaries, resulting in impaired folliculogenesis, anovulation, and decreased oocyte quality [15]. The increase of DСI concentration promotes insulin-mediated androgen synthesis by ovarian theca cells, in addition to hindering the conversion of androgens to estrogens via inhibiting aromatase activity and thereby leads to increased hyperandrogenism in women with IR [16]. Experimental and clinical studies have shown that MI, alone or combined with DCI in a 40:1 ratio similar to physiological plasma concentration, is a promising treatment for metabolic, hormonal, and reproductive clinical features commonly observed in hyperinsulinemic PCOS patients [17–19]. This combined treatment leverages the insulin-sensitizing properties of both stereoisomers, while restoring physiological ratios which may become perturbed.
The clinical presentation of PCOS is heterogeneous and is represented by four different phenotypes, initial laid out by the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine in Rotterdam in 2003 [20], and later expanded upon by various groups as understanding of the condition has increased. The Rotterdam criteria describe someone as having PCOS if they have 2 of the 3 following clinical features: clinical and/or biochemical hyperandrogenism, ovulatory dysfunction, and polycystic ovaries according as measured by ultrasound analysis. Phenotype A (termed Endocrine-metabolic syndrome (EMS) type I by Unfer V. et al. [6]) is the only phenotype which has all the above criteria and as such is sometimes termed “classic PCOS.” In contrast, phenotypes B, C, and D have only 2 of these criteria and are often under or incorrectly diagnosed. As the underlying pathophysiology of PCOS is not fully understood, current therapeutic approaches are typically directed at symptoms rather than targeting a specific etiologic pathway. Recommendations on diet and lifestyle modification, hormonal therapy, treatments for androgen-dependent dermatological disorders, and fertility care are commonly prescribed without taking into consideration pathogenetic differences [21], and the updated International PCOS recommendation 2023 do not consider any information about phenotypes [22]. Traditionally, therapeutic approaches for the treatment of PCOS patients have rarely focused on need for correction of metabolic disorders and IR treatment, with medicines that address these symptoms being largely limited to the treatment of type 2 diabetes mellitus and cardiovascular diseases prevention, rather than gynecological diseases. In recent years, the use of insulin-sensitizing drugs, such as metformin and inositol in scientific and clinical practice have gained traction, and the use of these molecules in combination with diet and lifestyle modifications, have shown a positive therapeutic effect in a number of women with PCOS [6, 23]. Considering the above, we decided to evaluate the frequency of metabolic disorders in classical PCOS phenotype patients and evaluate the effectiveness of the 40:1 MI and DCI combination in improving hormonal and metabolic parameters.
Methods
We evaluated PCOS patients aged between 20 and 40 years who were classified as phenotype A according to the Rotterdam criteria (ovulatory dysfunction, hyperandrogenism (clinical and/or biochemical), and the presence of polycystic ovaries detected via ultrasound and attended Moscow clinics from January 2022 to February 2023. All women signed a consent form for the evaluation of personal data.
Ovulatory dysfunction was diagnosed by the presence of oligo-amenorrhea (<8 cycles per year) and/or infertility (type IV – PCOS, according to the updated FIGO classification of ovulatory dysfunction [24]). Clinical hyperandrogenism included acne and/or hirsutism by a modified Ferriman Gallwey score (mFG) of 4–6. Biochemical hyperandrogenism was defined by elevated total or free testosterone level on days 2–3 of the menstrual cycle above levels of 1.72 nmol/L and 0.034 nmol/L, respectively. The ultrasound image of polycystic ovaries was characterized by the presence ≥20 follicles measuring 2–9 mm in diameter [22]. The exclusion criteria were defined as follows: administration of estroprogestin or other hormonal treatment in the previous 3 months; the absence of one of three features of Rotterdam criteria, congenital adrenal hyperplasia, and/or other endocrine abnormalities (hyperprolactinemia, thyroid diseases, type 2 diabetes mellitus), tubal-peritoneal and uterine factor of infertility, serious illnesses.
All enrolled patients were supplemented with 2,255 mg of inositol daily for 3 months by taking two soluble tablets of “Actifert-Gyno” containing 1,100 mg MI and 27.5 mg DCI (in a 40:1 ratio) with 400 μg folic acid a day, without any diet and/or lifestyle modifications. At the start and end of the study, on days 2–3 of the menstrual cycle, serum levels of FSH, LH, estradiol, total and free testosterone, free androgen index (FAI), SHBG, anti-Müllerian hormone (AMH), glucose, insulin were measured in addition to HOMA-IR and body mass index (BMI). HOMA-IR was calculated using the formula: (fasting glycemia [mmol/L] × fasting insulin [µED/L])/22.5. FAI was calculated by the formula: (total testosterone [nmol/L]/SHBG [nmol/L] × 100.
Results
Forty PCOS patients were enrolled in the study, six of whom were subsequently excluded because they did not return for laboratory testing after the end of therapy. Demographic, anthropometric, metabolic, hormonal, and historical characteristics of the 34 patients are presented in Table 1.
Table 1.
Demographic, anthropometric, metabolic, hormonal, and historical characteristics of PCOS patients (n = 34)
| Characteristics | n = 34 |
|---|---|
| Age, M (SD), years | 31.1 (6.7) |
| BMI, M (SD), kg/m2 | 33.25 (3.77) |
| BMI ≥25 kg/m2, n (%) | 19 (55.9) |
| Ovulatory dysfunction, n (%) | 34 (100) |
| Oligo-amenorrhea, n (%) | 17 (50.0) |
| Anovulatory infertility, n (%) | 20 (58.8) |
| Hyperandrogenism, n (%) | 34 (100) |
| Clinical (acne and/or hirsutism), n (%) | 28 (82.4) |
| Biochemical (elevated total or free testosterone level), n (%) | 23 (67.6) |
| Polycystic ovaries, n (%) | 34 (100) |
| IR, n (%) | 17 (50.0) |
| Gestational diabetes mellitus, n (%) | 6 (17.6) |
| Familial diabetes mellitus, n (%) | 21 (61.8) |
| Anti-Mullerian hormone, M (SD), ng/mL | 6 (2.8) |
All patients were classified as phenotype A (EMS-type I), had ovulatory dysfunction (50% – oligo-amenorrhea and 58.8% – anovulatory infertility), polycystic ovaries morphology, clinical hyperandrogenism (82.4%), and/or biochemical hyperandrogenism (67.6%) as defined by excess total and/or free testosterone. A strong association with metabolic disorders was observed in the studied PCOS patient group: 55.9% were overweight or obese, 50% were hyperinsulinemic (insulin level >10.4 μU/mL and/or HOMA-IR >2.7), 17.6% described a background history of gestational diabetes mellitus and 61.8% a history of familial diabetes mellitus. High serum AMH level (>7.35 ng/mL) was detected in 41.2% of the examined patients with an average value of 6 ± 2.8 ng/mL.
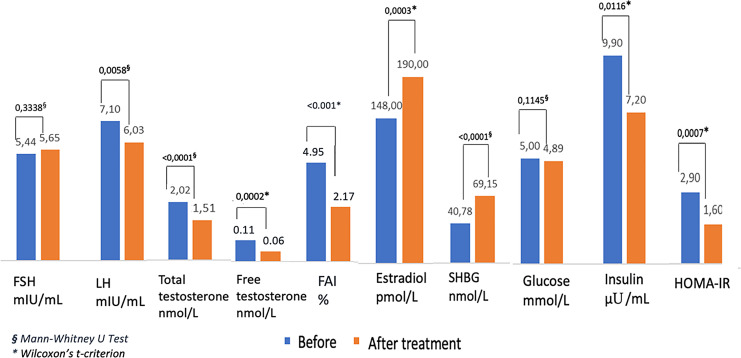
Comparative assessment of hormonal and metabolic characteristics, before and after 3 months of MI and DCI (40:1) supplementation are presented in Table 2 and Figure 1. Levels of total, free testosterone, and FAI demonstrated a significant decrease (p < 0.001); notably, this was coupled with a significant increase in estradiol and SHBG (p < 0.001) levels. 70% of patients had an increased LH:FSH ratio on the 2–3 days of the cycle. After 3 months of treatment, the average LH level had significantly decreased (p < 0.001), while FSH level remained stable. Metabolic profile significantly improved, as evidenced by decreased insulin levels (p = 0.0116), HOMA-IR (p < 0.001), and BMI (p = 0.0029). No adverse events in MI/DCI-treated patients during the therapy period were observed.
Table 2.
Comparative assessment of hormonal and metabolic characteristics of PCOS patients before and after 3 months of MI and DHI (40:1) supplementation (n = 34)
| Characteristics | Before, M (SD) | After, M (SD) | Differences (after-before) | p value |
|---|---|---|---|---|
| *Me (Q1; Q3) | *Me (Q1; Q3) | Me (Q1; Q3) | ||
| FSH, mIU/mL | 5.44 (4.5; 6.5) | 5.65 (4.7; 6.8) | 0.1 (−0.3; 1) | 0.3338§ |
| LH, mIU/mL | 7.10 (4.5; 9.0) | 6.03 (4.8; 7.4) | −1 (−2.3; 0.8) | 0.0058§ |
| Estradiol, pmol/L | 147.5 (111; 286) | 190 (150; 316) | 27 (0; 57.0) | <0.001* |
| Total testosterone, nmol/L | 2.02 (1.7; 2.4) | 1.5 (1.2; 1.72) | −0.41 (−0.95; −0.1) | <0.001* |
| Free testosterone, pg/mL | 0.16 (0.05; 0.16) | 0.07 (0.04; 0.09) | −0.03 (−0.04; −0.02) | <0.001* |
| SHBG, nmol/L | 40.78 (19; 62) | 69.15 (60; 88) | 29 (3.0; 49.0) | <0.001§ |
| FAI, % | 4.95 (3.89; 8.95) | 2.17 (1.95; 2.48) | −2.60 (−3.85; −0.56) | <0.001* |
| Fasting glucose, mmol/L | 5.00 (4.8; 5.3) | 4.89 (4.7; 5.1) | −0.05 (−0.3; 0.1) | 0.1145§ |
| Insulin, μU/mL | 9.9 (5.4; 14.8) | 7.2 (5.6; 9.1) | −0.8 (−6; 0.0) | 0.0116* |
| HOMA-IR | 2.9 (1.0; 5.0) | 1.6 (1.0; 2.8) | −0.25 (−1.4; 0.0) | <0.001* |
| BMI, M (SD), kg/m2 | 33.25 (3.77) | 31.00 (3.83) | −2 (−2.1; 0.0) | 0.0029§ |
§Mann-Whitney U test.
*Wilcoxon’s t-criterion.
Fig. 1.
Changes of hormonal and metabolic characteristics of PCOS patients before and after 3 months of MI and DHI (40:1) supplementation.
According to the presence of IR (HOMA-IR >2.7), the patients were divided into two groups: PCOS with IR (group 1, n = 17) and normoinsulinemic PCOS (group 2, n = 17) (Table 3). Interestingly, almost 60% of PCOS patients reported familial diabetes mellitus, regardless of IR status. A greater number of the IR patients were overweight/obese (82.4%) and had elevated blood testosterone levels (88.2%) compared with 29.4% (p = 0.0019) and 47.1% in the normoinsulinemic PCOS group.
Table 3.
Demographic, anthropometric, metabolic, hormonal, and historical characteristics of PCOS patients with IR (group 1) and normoinsulinemic PCOS (group 2)
| Characteristics | Group 1: PCOS with IR (n = 17) | Group 2: PCOS without IR (n = 17) | p value |
|---|---|---|---|
| Age, M (SD), years | 30.1 (7.3) | 32.2 (5.9) | >0.05 |
| BMI, M (SD), kg/m2 | 34 (6.4) | 28 (4.6) | 0.0032 |
| BMI ≥25 kg/m2, n (%) | 14 (82.4) | 5 (29.4) | 0.0019 |
| Ovulatory dysfunction, n (%) | 17 (100) | 17 (100) | >0.05 |
| Oligo-amenorrhea, n (%) | 8 (47.1) | 9 (52.9) | >0.05 |
| Anovulatory infertility, n (%) | 11 (64.7) | 9 (52.9) | >0.05 |
| Hyperandrogenism, n (%) | 17 (100) | 17 (100) | >0.05 |
| Clinical (acne and/or hirsutism), n (%) | 13 (76.5) | 15 (88.2) | >0.05 |
| Biochemical (elevated total or free testosterone level), n (%) | 15 (88.2) | 8 (47.1) | 0.0076 |
| Polycystic ovaries, n (%) | 17 (100) | 17 (100) | >0.05 |
| Gestational diabetes mellitus, n (%) | 11 (64.7) | 10 (58.8) | >0.05 |
| Familial diabetes mellitus, n (%) | 6,17 (2.87) | 5,74 (2.81) | >0.05 |
p value, Student’s t-criterion.
Comparative assessment of the pre- and post-hormonal and metabolic characteristics demonstrated significantly increased serum levels of estradiol and SHBG and decreased LH, FAI, total, and free testosterone level following a 3-month supplementation of MI and DCI in a 40:1 ratio in both groups (Table 4). Notably, at baseline excess total testosterone had 88.2% patients with IR and only 47.1% normoinsulinemic patients, but the rate of increased level of free testosterone was the same (52.9% and 58.8%, respectively). Moreover, androgen changes after inositol supplementation did not differ between the two groups: the number of patients with normal androgen levels increased 2.5–3 fold. Total testosterone level remained elevated only in 35.3% of patients with IR and 17.7% of normoinsulinemic patients, excess-free testosterone – in 17.7% of both groups. After 3 months of inositol supplementation, IR reduced in the PCOS-IR group as demonstrated by significant decreases in insulin level (p = 0.0056) and HOMA-IR (p = 0.0027).
Table 4.
Comparative assessment of hormonal and metabolic characteristics of PCOS patients before and after 3 months of MI and DHI (40:1) supplementation of PCOS patients with IR (group 1) and normoinsulinemic PCOS (group 2)
| Characteristics | Group 1: PCOS with IR (n = 17) | Group 2: PCOS without IR (n = 17) | p 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| before, M (SD) | after, M (SD) | differences (after-before) | p 1 | before, M (SD) | after, M (SD) | differences (after-before) | p 2 | ||
| *Me (Q1; Q3) | *Me (Q1; Q3) | Me (Q1; Q3) | *Me (Q1; Q3) | *Me (Q1; Q3) | Me (Q1; Q3) | ||||
| FSH, mIU/mL | 5.20 (4.5; 6.3) | 5.65 (5.0; 6.0) | 0.6 (0.0; 1.0) | 0.3255§ | 5.4 (4.5; 7.0) | 5.0 (4.5; 6.8) | 0 (−0.3; 0.4) | 0.9628§ | 0.313/0.993§ |
| LH, mIU/mL | 7.09 (4.3; 8.9) | 6.03 (5.0; 7.8) | −1 (2.6; 0.8) | 0.1739§ | 5.8 (5.0; 9.3) | 6.0 (4.8; 7.0) | −1 (−2.0; 0.0) | 0.2426§ | 0.775/0.652§ |
| Estradiol, pmol/L | 140 (95.0; 316.0) | 170 (128.0; 355.0) | 20 (0.0;57.0) | 0.0186* | 148 (112.0; 249.0) | 190 (160.0; 304.0) | 29 (19.0; 55.0) | 0.0057* | 0.098/0.809* |
| Total testosterone, nmol/L | 2.2 (1.8; 2.7) | 170 (120.0; 355.0) | 20 (0.0; 57.0) | <0.001* | 1.70 (1.5; 2.12) | 1.30 (1.0; 1.63) | −0.3 (−0.56; 0.0) | 0.0069* | 0.972/0.917* |
| Free testosterone, pg/mL | 0.10 (0.05; 0.16) | 0.06 (0.04;0.09) | −0.01(−0.07; -0.00) | <0.001* | 0.10 (0.07; 0.14) | 0.07 (0.04; 0.09) | −0.02 (−0.07; −0.03) | 0.0046* | 0.061/0.138* |
| SHBG, nmol/L | 25 (12; 51) | 63 (54; 65) | 29 (3.0; 51.0) | 0.0010§ | 51 (26; 62) | 80 (65; 90) | 28 (3.0; 45.0) | 0.0001§ | 0.056/0.019§ |
| FAI, % | 5.29 (1.3; 8.8) | 2.52 (2.41; 2.77) | −2.4 (−0.83; 4.46) | <0.001* | 3.42 (3.13; 5.77) | 1.63 (1.54; 1.81) | −1.4 (−2.3; −0.11) | 0.008* | 0.002/0.084* |
| Fasting glucose, mmol/L | 5.1 (4.8; 5.3) | 4.9 (4.7; 5.1) | −0.06 (−0.4; 0.1) | 0.3977§ | 4.9 (4.8; 5.2) | 5 (4.5; 5.1) | −0.05 (−0.2; −0.11) | 0.5059§ | 0.408/0.416§ |
| Insulin, μU/mL | 15 (11.8; 16.2) | 9.1 (7.9; 14.7) | −6 (−8.0; −2.1) | 0.0056* | 5.4 (5.1; 6.0) | 5.6 (5.1; 6.0) | 0 (−0.8; 0.0) | 0.8785* | <0.001/<0.001* |
| HOMA-IR | 3.3 (3.0; 6.0) | 2.1 (1.6; 3.8) | −1 (−1.9; −0.2) | 0.0027* | 1.0 (0.8; 2.1) | 1.0 (1.0; 1.6) | 0 (−0.3; 0.0) | 0.0754* | 0.004/0.004* |
§Mann-Whitney U test.
*Wilcoxon’s t-criterion.
p3, differences between groups 1 (p1) and 2 (p2) before/after.
Discussion
The results of our study indicate a high prevalence of metabolic disorders among patients with classical PCOS (phenotype A or EMS-type I): 50% of the enrolled women had IR, 61.8% familial diabetes mellitus, and 55.9% were overweight or obese. Notably, familial diabetes mellitus is a proven risk factor for the development of PCOS and metabolic disorders [25]. However, the frequency of familial diabetes mellitus did not differ between the normoinsulinemic group and the IR group. It may indicate that serum insulin level and HOMA-IR are not sufficient to detect the initial signs of IR. In this case, the 75 g oral glucose tolerance test (OGTT) with additional insulin detection may provide a more accurate diagnostic tool [26].
The study demonstrated the beneficial effect of daily supplementation with 2,255 mg of inositol (MI and DCI in a 40:1 ratio) on metabolic parameters in patients with PCOS phenotype A (EMS-type I). After 3 months of treatment, BMI and HOMA-IR decreased (p = 0.0029; p = 0.0007) and the number of normoinsulinemic patients increased by 2.4 times, as a consequence of a reduction in hyperandrogenism. The fasting serum glucose level always remained within the normal range and showed a mild decrease. No adverse events during the treatment period were observed.
These results mirror the complex relationship between inositol and glucose, whereby it has been found that MI supplementation can significantly inhibit duodenal glucose absorption reducing blood glucose levels [27]. In contrast, glucose significantly reduces the uptake of inositol with it being postulated that glucose and inositol share transporter systems [28]. In vitro studies further evidenced that inositol uptake is significantly inhibited through the administration of 20 mm of glucose to cultured human retinal pigment epithelial cells [29]. Furthermore, it has been suggested that high levels of glucose may induce MI deficiency, as IR and hyperglycemia are through to affect the cellular isomeric ratio between MI and DCI [30, 31]. Therefore, it is probable that restoration of MI levels would reduce the metabolic morbidities commonly associated with PCOS [32].
An elevated LH:FSH ratio in early follicular phase is characteristic of PCOS [32] and was detected in 70.6% of the patient’s cohort regardless of IR status. Excessive pulsatile LH secretion stimulates the proliferation and production of androgens by thecal cells, while suppressing the aromatase activity, which converts androgens into estrogens. Moreover, LH excess may cause premature arrest of the antral follicle [28]. After 3 months of combined MI:DCI supplementation the LH levels decreased from 7.10 (4.5; 9.0) to 6.03 (4.8; 7.4) (Me (Q1; Q3) (p = 0.0058), but the number of patients with increased LH:FSH ratio did not significantly reduced. It is possible that beneficial effects can be achieved with long-term therapy and/or higher dose.
At baseline, 41.2% of the enrolled women demonstrated elevated AMH level and consequently a large number of preantral and small antral follicles were observed in the ovaries, which are considered as a marker of PCOS [33]. We did not observe any benefit after inositol administration, which may also be due to an insufficiently long course or dose of therapy.
In contrast, androgen level demonstrated a significant decrease following 3 months of therapy. At baseline, total and free testosterone exceeded healthy levels in the majority of the study cohort (67.6% and 55.9%, respectively), following treatment this fell 23.5 (p = 0.013) and 14.7% (p = 0.0002), coupled with a decrease in FAI (−2.60 [−3.85; −0.56], p < 0.001). FAI is decreased (−2.60 [−3.85; −0.56], p < 0.001). In a similar trend, average SHBG level demonstrated a significant increased (p < 0.0001). This reduction of androgens is extremely important in PCOS patients who are seeking pregnancy, as increased androgen levels cause a lack of negative feedback, leading to an abnormal LH response on the hypothalamic-pituitary axis resulting in increased GnRH pulse frequency [34]. The abnormal response occurs during intrauterine development and is associated with increased AMH production in pregnant PCOS women. In addition to a direct stimulating effect on the pulsatile LH release, AMH inhibits aromatase activity in the placenta, thereby increasing the bioavailability of androgens to the fetus [35]. Thus, the hyperandrogenic status in the mother leads to an androgen-associated reprogramming of the neuronal response to GnRH in uteri and the birth of a child with impaired excessive LH synthesis and the increased risk of development of PCOS in female offspring [36].
Importantly, MI and DCI in a 40:1 ratio restored serum estradiol levels, associated with a decrease in testosterone. However, DCI at high doses and prolonged treatments has been demonstrated to block aromatase expression and lead to hyperandrogenism [37]. In mouse studies, Bevilaqua et al. [38] demonstrated that prolonged doses of DCI contributed to the formation of a PCO-like phenotype. Further evidence was later provided by Nordio et al. [39], who observed significant increases in testosterone levels as a result of DCI supplementation at 1,200 mg/day. As a consequence of this, the trial was abandoned due to safety reasons, casting further doubt on the suitability of DCI as a monotherapy for PCOS.
The IR cohort showed a higher incidence of obesity (82.4%) and biochemical hyperandrogenism (88.2%) compared to normoinsulinemic patients (29.4% and 47.1%, respectively), adding more credence to the correlation between obesity and hyperandrogenism. But no significant difference could be identified between the hormonal profiles of both groups after inositol supplementation; this was somewhat unexpected due to the reported mode of action of inositol. In our opinion, this indicates not that the use of inositol is highly efficacious in normoinsulinemic PCOS patients, but rather there was a wider prevalence of metabolic disorders among the patients included in the study and the insulin tests were insufficiently sensitive.
Study Limitations
The present analysis was limited to a 12-week follow-up, which precluded a long-term evaluation of the effects of MI and DCI combination. Also, this period was insufficient to achieve and analyze clinical changes such as restoration of the menstrual cycle, restoration of reproductive function, and clinical manifestations of hyperandrogenism. These limitations demonstrate the need for further studies with a larger sample size and the use of more sensitive tests to detect IR.
Conclusions
Our study demonstrated that the classical PCOS phenotype is correlated with a high frequency of metabolic disorders: about 80% of the enrolled women were affected by IR and/or had a familial diabetes mellitus. This study added further evidence to the use of 40:1 MI:DCI (2,255 mg) in the modulation of metabolic (decreases in BMI, insulin level, and HOMA-IR) and hormonal parameters (decreases in the total and free testosterone level, and FAI, paired with increases in SHBG, and estradiol) in women with PCOS. These positive results were observed in both IR hyperinsulinemic and normoinsulinemic patients; however, it is thought this was largely due to a lack of sensitivity in detecting IR in the normoinsulinemic cohort. This represents a lower dose than has been previously used in literature, demonstrating some positive effects can be obtained even at a reduced dose, despite higher doses or longer treatments being needed to reduce the LH:FSH ratio which remained evaluated after treatment. In total, this study builds upon prior work to further encourage the investigation of inositol as a treatment for PCOS, particularly in IR or otherwise metabolically comprised patients.
Statement of Ethics
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Private Educational Institution of Supplementary Education “Academy of Medical Education named after F.I. Inosemtsev.” Written informed consent was obtained for all participants.
Conflict of Interest Statement
S.H.M. and V.U. are employees of Lo.LI Pharma s.r.l; the other authors declare no conflict of interest.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
Pustotina O.: collection of clinical data and creation of an electronic database for the results, analysis of the results of statistical processing of the clinical material and their interpretation, writing, correcting, and editing of the article before the publication. Myers S.H. and Unfer V.: writing, correcting, and editing of the article before the publication. Rasulova I.: collection of clinical data and creation of an electronic database for the results, approval of the final version of the article before the publication.
Funding Statement
This study was not supported by any sponsor or funder.
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy reasons but are available from the corresponding author upon reasonable request.
References
- 1.Ding H, Zhang J, Zhang F, Zhang S, Chen X, Liang W, et al. Resistance to the insulin and elevated level of androgen: a major cause of polycystic ovary syndrome. Front Endocrinol. 2021;12:741764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tosi F, Bonora E, Moghetti P. Insulin resistance in a large cohort of women with polycystic ovary syndrome: a comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum Reprod. 2017;32(12):2515–21. [DOI] [PubMed] [Google Scholar]
- 3.Cassar S, Misso ML, Hopkins WG, Shaw CS, Teede HJ, Stepto NK. Insulin resistance in polycystic ovary syndrome: a systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum Reprod. 2016;31(11):2619–31. [DOI] [PubMed] [Google Scholar]
- 4.Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(6):618–37. [DOI] [PubMed] [Google Scholar]
- 5.Ovalle F, Azziz R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil Steril. 2002;77(6):1095–105. [DOI] [PubMed] [Google Scholar]
- 6.Unfer V, Dinicola S, Russo MAPCOS P. Does inositol therapy find a rationale in all the different phenotypes? Int J Mol Sci. 2023;24(7):6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Final report of the National Institutes of Health (NIH) evidence-based methodology workshop of PCOS 2012.
- 8.Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27–36. [DOI] [PubMed] [Google Scholar]
- 9.Myers SH, Russo M, Dinicola S, Forte G, Unfer V. Questioning PCOS phenotypes for reclassification and tailored therapy. Trends Endocrinol Metab. 2023;34(11):694–703. [DOI] [PubMed] [Google Scholar]
- 10.Tuorila K, Ollila MM, Järvelin MR, Tapanainen JS, Franks S, Puukka K, et al. Hyperandrogenemia in early adulthood is an independent risk factor for abnormal glucose metabolism in middle age. J Clin Endocrinol Metab. 2021;106(11):e4621–e4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlomagno G, Unfer V, Roseff S. The D-chiro-inositol paradox in the ovary. Fertil Steril. 2011;95(8):2515–6. [DOI] [PubMed] [Google Scholar]
- 12.Joham AE, Norman RJ, Stener-Victorin E, Legro RS, Franks S, Moran LJ, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10(9):668–80. [DOI] [PubMed] [Google Scholar]
- 13.Unluhizarci K, Karaca Z, Kelestimur F. Role of insulin and insulin resistance in androgen excess disorders. World J Diabetes. 2021;12(5):616–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unfer V, Carlomagno G, Papaleo E, Vailati S, Candiani M, Baillargeon JP. Hyperinsulinemia alters myoinositol to d-chiroinositol ratio in the follicular fluid of patients with PCOS. Reprod Sci. 2014;21(7):854–8. [DOI] [PubMed] [Google Scholar]
- 15.Heimark D, McAllister J, Larner J. Decreased myo-inositol to chiro-inositol (M/C) ratios and increased M/C epimerase activity in PCOS theca cells demonstrate increased insulin sensitivity compared to controls. Endocr J. 2014;61(2):111–7. [DOI] [PubMed] [Google Scholar]
- 16.Laganà AS, Garzon S, Casarin J, Franchi M, Ghezzi F. Inositol in polycystic ovary syndrome: restoring fertility through a pathophysiology-based approach. Trends Endocrinol Metab. 2018;29(11):768–80. [DOI] [PubMed] [Google Scholar]
- 17.Monastra G, Vazquez-Levin M, Bezerra Espinola MS, Bilotta G, Laganà AS, Unfer V. D-chiro-inositol, an aromatase down-modulator, increases androgens and reduces estrogens in male volunteers: a pilot study. Basic Clin Androl. 2021;31(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordio M, Basciani S, Camajani E. The 40:1 myo-inositol/D-chiro-inositol plasma ratio is able to restore ovulation in PCOS patients: comparison with other ratios. Eur Rev Med Pharmacol Sci. 2019;23(12):5512–21. [DOI] [PubMed] [Google Scholar]
- 19.Bevilacqua A, Dragotto J, Giuliani A, Bizzarri M. Myo-inositol and D-chiro-inositol (40:1) reverse histological and functional features of polycystic ovary syndrome in a mouse model. J Cel Physiol. 2019;234(6):9387–98. [DOI] [PubMed] [Google Scholar]
- 20.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group . Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7. [DOI] [PubMed] [Google Scholar]
- 21.Pustotina OP. Polycystic ovary syndrome phenotypes. Farmateka. 2023;30(4–5):156–63. [Google Scholar]
- 22.Teede HJ, Tay CT, Laven JJ, Dokras A, Moran LJ, Piltonen TT, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2023;38(9):1655–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pustotina PO, Dikke DG, Ostromensky OV. Inositol and human reproduction. Obstet Gynecol. 2022;2_2022:111–8. [Google Scholar]
- 24.Munro MG, Balen AH, Cho SH, Critchley HOD, Díaz I, Ferriani R, et al. The FIGO ovulatory disorders classification system. Fertil Steril. 2022;118(4):768–86. [DOI] [PubMed] [Google Scholar]
- 25.Genazzani A, Battipaglia C, Petrillo T. HIE (hepatic insulin extraction) index in overweight/obese PCOS patients with or without familial diabetes. Gynecol Reprod Endocr Metabol. 2022;3(1):57–68. [Google Scholar]
- 26.Bock G, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Chandramouli V, et al. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes. 2007;56(6):1703–11. [DOI] [PubMed] [Google Scholar]
- 27.Chukwuma CI, Ibrahim MA, Islam MS. Myo-inositol inhibits intestinal glucose absorption and promotes muscle glucose uptake: a dual approach study. J Physiol Biochem. 2016;72(4):791–801. [DOI] [PubMed] [Google Scholar]
- 28.Bevilacqua A, Bizzarri M. Inositols in insulin signaling and glucose metabolism. Int J Endocrinol. 2018;2018:1968450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas TP, Feldman EL, Nakamura J, Kato K, Lien M, Stevens MJ, et al. Ambient glucose and aldose reductase-induced myo-inositol depletion modulate basal and carbachol-stimulated inositol phospholipid metabolism and diacylglycerol accumulation in human retinal pigment epithelial cells in culture. Proc Natl Acad Sci U S A. 1993;90(20):9712–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamenov Z, Gateva A. Inositols in PCOS. Molecules. 2020;25(23):5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karkera S, Agard E, Sankova L. The clinical manifestations of polycystic ovary syndrome (PCOS) and the treatment options. Eur J Biol Med Scien Res. 2023;11(1):57–91. [Google Scholar]
- 32.De Leo V, Musacchio MC, Cappelli V, Massaro MG, Morgante G, Petraglia F. Genetic, hormonal, and metabolic aspects of PCOS: an update. Reprod Biol Endocrinol. 2016;14(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lie Fong S, Laven JSE, Duhamel A, Dewailly D. Polycystic ovarian morphology and the diagnosis of polycystic ovary syndrome: redefining threshold levels for follicle count and serum anti-Müllerian hormone using cluster analysis. Hum Reprod. 2017;32(8):1723–31. [DOI] [PubMed] [Google Scholar]
- 34.Wawrzkiewicz-Jałowiecka A, Kowalczyk K, Trybek P, Jarosz T, Radosz P, Setlak M, et al. In search of new therapeutics-molecular aspects of the PCOS pathophysiology: genetics, hormones, metabolism and beyond. Int J Mol Sci. 2020;21(19):7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva MSB, Giacobini P. New insights into anti-Müllerian hormone role in the hypothalamic-pituitary-gonadal axis and neuroendocrine development. Cell Mol Life Sci. 2021;78(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–88. [DOI] [PubMed] [Google Scholar]
- 37.Sacchi S, Marinaro F, Tondelli D, Lui J, Xella S, Marsella T, et al. Modulation of gonadotrophin induced steroidogenic enzymes in granulosa cells by d-chiroinositol. Reprod Biol Endocrinol. 2016;14(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bevilacqua A, Dragotto J, Lucarelli M, Di Emidio G, Monastra G, Tatone C. High doses of D-chiro-inositol alone induce a PCO-like syndrome and other alterations in mouse ovaries. Int J Mol Sci. 2021;22(11):5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nordio M, Bezerra Espinola MS, Bilotta G, Capoccia E, Montanino Oliva M. Long-lasting therapies with high doses of D-chiro-inositol: the downside. J Clin Med. 2023;12(1):390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy reasons but are available from the corresponding author upon reasonable request.