Abstract
This study investigated the effects of supplemental nucleotides, autolyzed yeast (Saccharomyces cerevisiae), and sodium butyrate in diets for nursery pigs on growth performance, diarrhea incidence, blood profile, intestinal morphology, mRNA expression of nutrient transporters, inflammatory markers, antioxidant profile, and tight junction proteins in the small intestine. One hundred eighty 21-day-old pigs (5.17 ± 0.57 kg) were assigned in a randomized block design to 1 of 4 dietary treatments: (1) CON: control, basal diet, (2) NUC: CON + nucleotides, (3) YSC: CON + lysed yeast S. cerevisiae, (4) ASB: CON + acidifier sodium butyrate. Pigs were fed for 24 days, phase 1 (21–32 days) and 2 (32–45 days). During phase 1, YSC and ASB improved average daily gain (ADG) and feed conversion (FC) compared with CON. At the overall period, ASB improved ADG and YSC improved FC compared with CON. The NUC diet did not affect growth performance. The ASB increased ileal villus height compared to CON. The YSC and ASB reduced the number of Peyer’s patches in the ileum compared with CON. The YSC increased mRNA expression of nutrient transporters (SMCT2, MCT1, and PepT1), tight junction proteins (OCL and ZO-1), antioxidants (GPX), and IL1-β in the jejunum compared with CON. The ASB increased mRNA expression of nutrient transporters (SGLT1 and MCT1), tight junction proteins (OCL and ZO-1), and antioxidants (GPX and SOD) compared with CON. In conclusion, autolyzed yeast and sodium butyrate promoted growth performance by improving the integrity of the intestinal barrier, the mRNA expression of nutrient transporters, and antioxidant enzymes in the jejunum of nursery pigs whereas supplementation of nucleotides did not show such effects.
Keywords: Autolyzed yeast, Gut health, Performance, Piglets, Nucleotides, Sodium butyrate
Subject terms: Animal physiology, Immunology, Molecular biology, Biomarkers, DNA
Introduction
Weaning is considered the “critical window” in the piglet’s life because it is associated with several stress factors, such as loss of contact with the mother and original litter, solid diet, environmental and structural changes, and the establishment of a new hierarchy1,2. Abrupt separation from the sow and dietary transition are critical events causing intestinal challenges and compromising immune functions affecting growth of pigs3,4. Dietary interventions with various functional additives have been introduced and implemented to cope with challenges to the intestine and immune functions upon weaning. Nucleotides5,6, yeast7,8 and organic acids9,10 have appeared on the market as feeding strategies to improve intestinal health and, consequently, promote better growth performance of weaned piglets. Globally, their use as in-feed additives in pig diets has become more frequent, especially during the weaning period11,12. However, factors such as the form of supplementation, the type of additive, and the site of action make each application unique and complex in animal responses and should be better understood in studies involving pigs fed different feed additives13–15.
Nucleotides are monomers that serve as building blocks of nucleic acids (DNA and RNA) and are synthesized by animals through the de novo pathway or the salvage pathway. These monomers function as physiological mediators, coenzyme components, and contributors to cell growth and division and are crucial to the rapid proliferation of intestinal mucosa cells and immune cells16,17. Thus, the supplementation of nucleotides to the diet can bring benefits in periods of rapid growth and development (e.g. digestive organs, immune systems, cell renewal) helping in circumstances associated with intestinal injuries and stress, such as post-weaning period5,6.
The lysed yeast (S. cerevisiae) contains in the cell wall a complex polymer and is composed of β-glucans, α-mannans, mannoproteins, and a minor component of chitin18, in addition, the cytoplasm contains B complex vitamins, highly digestible proteins and free amino acids15. However, monogastric animals do not have enzymes to digest polysaccharides such as those present in the yeast cell wall, therefore, the breakdown of yeast cell wall by lysing would improve the bioavailability of yeast components for animal nutrition7. The major polysaccharide constituents of the yeast cell wall, β-glucans and α-mannans, have been recognized to be capable of modulation of the mucosa immune system, promoting beneficial effects on pig health (e.g. inhibition of pathogen adhesion to gastrointestinal epithelial tissue and stimulation of immunocompetent cells in Peyer's patches)19,20. Thus, yeast products, mainly based on S. cerevisiae, are widely used as feed additives.
Organic acids (e.g. sodium butyrate) act by reducing the pH of digesta in the gastrointestinal tract, stimulating the activity of digestive enzymes, inhibiting the proliferation of pathogenic bacteria, and improving the digestibility of nutrients9,21. Sodium butyrate is a short-chain fatty acid that acts by improving villus height, decreasing crypt, and improving the expression of tight junction protein in the gut14. As a result, it beneficially affects the reabsorption of fluids and electrolytes which is associated with reduced diarrhea22, moreover modulating intestinal permeability will reduce translocation of intraluminal toxins and antigens from the lumen into subepithelial tissues and systemic blood circulation23. Together with improving gut health, the ability of sodium butyrate to alleviate oxidative stress and improve immunological profile will result in improved pig weight gain14,22,24. However, free organic acids dissociate and lose most of their antibacterial capacity before reaching the distal part of the digestive system25. Therefore, sodium butyrate in encapsulated form allows progressive reach to the distal parts of the gastrointestinal tract without being fully dissociated, because the product is gradually released compared to an uncoated product10.
In view of the above, the hypothesis of the study was that the dietary supplementation of purified nucleotide, lysed yeast (S. cerevisiae), or encapsulated sodium butyrate would improve the growth performance of weaned piglets by beneficially stimulating the immune response and supporting intestinal physiological and health functions. Therefore, the objective of this study was to evaluate the effects of purified nucleotide, lysed yeast, and sodium butyrate in diets for nursery pigs on growth performance, diarrhea incidence, blood profile, intestinal morphology, mRNA expression of nutrients transporter, inflammatory markers, antioxidant profile, and tight junction proteins.
Results
Growth performance
During phase 1 (21–32 days), pigs fed YSC or ASB diets showed (P < 0.05) improvements in ADG, FC, and BW compared with pigs fed CON diet; however, ADFI was not affected by dietary treatments (Table 1). In phase 2 (32–45 days), pigs fed ASB diet had higher (P < 0.05) BW compared to pigs fed CON diet. In addition, there was a trend to increase the BW (P = 0.095) in pigs fed YSC diet compared to those fed CON diet. Pigs fed NUC diet tended to present lower ADG (P = 0.080). However, FC was not affected by dietary treatments. During the overall period, ADG was higher (P < 0.05) in pigs fed ASB diet than those with CON diet. In addition, FC was better (P < 0.05), and a trend was observed to improve ADG (P = 0.095) in pigs fed YSC diet compared with pigs on CON diet. Moreover, ADFI was not affected by dietary treatments.
Table 1.
Growth performance of piglets fed diets supplemented or not with feed additives (n = 9 pens replicates per dietary treatment and 5 piglets per pen as an experimental unit).
| Itema | Dietary treatmentb | SEMc | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | NUC | YSC | ASB | CON × NUC | CON × YSC | CON × ASB | ||
| Body weight, kg | ||||||||
| Initial | 5.22 | 5.22 | 5.22 | 5.22 | – | – | – | – |
| 32 days of age | 6.83 | 6.89 | 7.18 | 7.18 | 0.11 | 0.693 | 0.034 | 0.031 |
| 45 days of age | 12.64 | 12.36 | 13.12 | 13.32 | 0.19 | 0.321 | 0.095 | 0.019 |
| Phase 1, 21–32 days | ||||||||
| ADFI, g/day | 296 | 286 | 281 | 302 | 7.02 | 0.319 | 0.133 | 0.546 |
| ADG, g/day | 146 | 152 | 178 | 178 | 10.08 | 0.687 | 0.035 | 0.030 |
| FC, g/g | 2.05 | 1.97 | 1.61 | 1.74 | 0.09 | 0.549 | < 0.010 | 0.034 |
| Phase 2, 32–45 days | ||||||||
| ADFI, g/day | 627 | 600 | 638 | 668 | 17.35 | 0.263 | 0.678 | 0.105 |
| ADG, g/day | 486 | 454 | 494 | 510 | 12.57 | 0.080 | 0.674 | 0.187 |
| FC, g/g | 1.29 | 1.32 | 1.29 | 1.31 | 0.02 | 0.336 | 0.940 | 0.602 |
| Overall period | ||||||||
| ADFI, g/day | 449 | 431 | 448 | 473 | 10.88 | 0.237 | 0.902 | 0.141 |
| ADG, g/day | 309 | 297 | 329 | 337 | 8.20 | 0.326 | 0.095 | 0.019 |
| FC, g/g | 1.46 | 1.45 | 1.36 | 1.40 | 0.02 | 0.848 | < 0.010 | 0.103 |
aAverage daily feed intake (ADFI, g/day), average daily weight gain (ADG, g/day), feed conversion ratio (FC, g:g).
bDietary treatment: phase 1 (21–32 days) and phase 2 (32–45 days), respectively: (1) CON: control, basal diet, (2) NUC: CON + 1 g/kg and 0.5 g/kg of nucleotides, (3) YSC: CON + 20 g/kg and 10 g/kg of lysed yeast S. cerevisiae, (4) ASB: CON + 1.5 g/kg and 1 g/kg of acidifier sodium butyrate.
cPooled standard error of the mean.
Diarrhea incidence
There was a trend to reduce the diarrhea incidence (P = 0.098) in pigs fed NUC diet and improved fecal score (P = 0.090) in pigs fed YSC diet than pigs fed CON diet in the phase 1 (Table 2). In phase 2, there was a trend to reduce the diarrhea incidence (P = 0.089) and improved fecal score (P = 0.091) in pigs fed YSC diet than in those fed CON diet.
Table 2.
Diarrhea incidence and fecal score of piglets fed diets supplemented or not with feed additives (n = 9 pens replicate per dietary treatment and 5 piglets per pen as an experimental unit).
| Item | Dietary treatmenta | SEMb | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | NUC | YSC | ASB | CON × NUC | CON × YSC | CON × ASB | ||
| Phase 1 | ||||||||
| Diarrhea incidence, % | 20.01 | 12.10 | 14.33 | 15.56 | 3.28 | 0.098 | 0.230 | 0.345 |
| Fecal score | 0.80 | 0.64 | 0.61 | 0.69 | 0.07 | 0.161 | 0.090 | 0.341 |
| Phase 2 | ||||||||
| Diarrhea incidence, % | 15.50 | 16.00 | 6.70 | 7.16 | 3.55 | 0.927 | 0.089 | 0.106 |
| Fecal score | 0.53 | 0.60 | 0.32 | 0.37 | 0.11 | 0.516 | 0.091 | 0.193 |
aDietary treatment: phase 1 (24–32 days) and phase 2 (32–45 days), respectively: (1) CON: control, basal diet, (2) NUC: CON + 1 g/kg and 0.5 g/kg of nucleotides, (3) YSC: CON + 20 g/kg and 10 g/kg of lysed yeast S. cerevisiae, (4) ASB: CON + 1.5 g/kg and 1 g/kg of acidifier sodium butyrate.
bPooled standard error of the mean.
Blood profile
There was no effect of dietary treatments on IgG, creatinine, and urea concentrations (Table 3).
Table 3.
Blood profile of piglets fed diets supplemented or not with feed additives on d 32 (n = 9 pigs replicates per dietary treatment).
| Item | Dietary treatmenta | SEMb | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | NUC | YSC | ASB | CON × NUC | CON × YSC | CON × ASB | ||
| Urea, mg/dL | 16.55 | 16.89 | 15.55 | 13.11 | 1.58 | 0.882 | 0.658 | 0.133 |
| Creatinine, mg/dL | 0.64 | 0.70 | 0.68 | 0.66 | 0.06 | 0.416 | 0.330 | 0.458 |
| IgG, mg/dL | 272.74 | 306.67 | 266.07 | 273.69 | 23.42 | 0.157 | 0.777 | 0.968 |
aDietary treatment: CON control, basal diet, NUC CON + 1 g/kg of nucleotides, YSC CON + 20 g/kg of lysed yeast S. cerevisia, ASB CON + 1.5 g/kg of acidifier sodium butyrate.
bPooled standard error of the mean.
Intestinal morphology
Dietary treatments had no effects on villus height, crypt depth, villus:crypt ratio, and proportion of goblet cells in the duodenum and jejunum (Table 4). In the ileum, the dietary treatments had no effects on crypt depth and proportion of goblet cells. However, villus height was increased (P < 0.05) and villus:crypt ratio trended to increase (P = 0.066) in pigs fed ASB diet than pigs fed CON diet. In addition, the number of Peyer’s patches in pigs fed YSC or ASB diets was lower (P < 0.05) compared to pigs that received CON diet. There was a trend towards an increase (P = 0.071) villus height and reduce (P = 0.078) the number of Peyer’s patches in pigs fed NUC diet than those fed the CON diet.
Table 4.
Intestinal morphology of piglets fed diets supplemented or not with feed additives on d 32 (n = 9 pigs replicates per dietary treatment).
| Item | Dietary treatmenta | SEMb | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | NUC | YSC | ASB | CON × NUC | CON × YSC | CON × ASB | ||
| Duodenum | ||||||||
| Villus height, μm | 307 | 297 | 298 | 300 | 19.09 | 0.662 | 0.689 | 0.753 |
| Crypt depth, μm | 178 | 176 | 182 | 177 | 9.23 | 0.840 | 0.761 | 0.926 |
| Villus:crypt ratio | 1.72 | 1.70 | 1.63 | 1.71 | 0.08 | 0.812 | 0.387 | 0.857 |
| Goblet cells, % | 32.50 | 34.25 | 33.51 | 36.79 | 3.00 | 0.656 | 0.790 | 0.227 |
| Jejunum | ||||||||
| Villus height, μm | 264 | 255 | 261 | 268 | 24.09 | 0.771 | 0.922 | 0.880 |
| Crypt depth, μm | 150 | 139 | 143 | 157 | 11.12 | 0.474 | 0.640 | 0.590 |
| Villus:crypt ratio | 1.80 | 1.83 | 1.82 | 1.71 | 0.10 | 0.804 | 0.851 | 0.486 |
| Goblet cells, % | 23.15 | 24.62 | 25.16 | 22.79 | 2.76 | 0.683 | 0.562 | 0.912 |
| Ileum | ||||||||
| Villus height, μm | 210 | 241 | 234 | 255 | 12.99 | 0.071 | 0.150 | < 0.010 |
| Crypt depth, μm | 132 | 139 | 138 | 138 | 9.17 | 0.534 | 0.573 | 0.576 |
| Villus:crypt ratio | 1.64 | 1.66 | 1.69 | 1.81 | 0.07 | 0.896 | 0.603 | 0.066 |
| Goblet cells, % | 50.43 | 53.07 | 46.65 | 45.94 | 4.59 | 0.661 | 0.514 | 0.405 |
| Peyer’s patches, n | 48 | 40 | 39 | 38 | 3.33 | 0.078 | 0.042 | 0.017 |
aDietary treatment: CON control, basal diet, NUC CON + 1 g/kg of nucleotides, YSC CON + 20 g/kg of lysed yeast S. cerevisiae, ASB CON + 1.5 g/kg of acidifier sodium butyrate.
bPooled standard error of the mean.
mRNA relative abundance of nutrient transporters
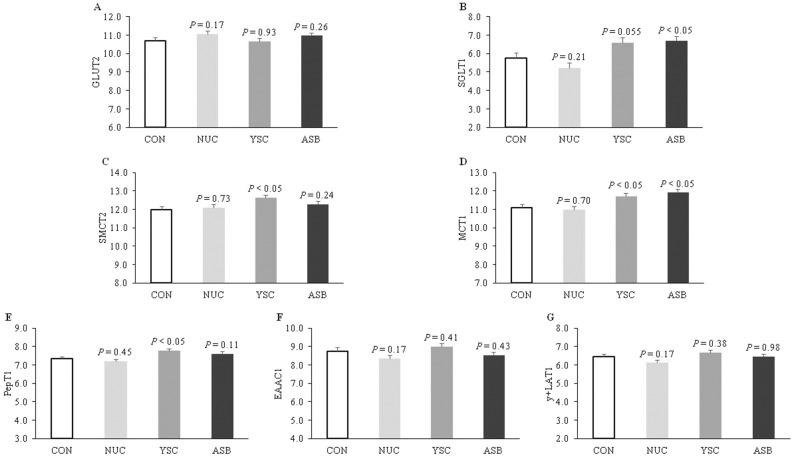
In the jejunum, there was no effect of dietary treatments on the mRNA expression of GLUT2, EAAC1, and y+LAT1 (Fig. 1). However, mRNA expression of SMCT2 and PepT1 was higher (P < 0.05) in pigs fed YSC diet compared to pigs fed CON diet. The mRNA expression of MCT1 in pigs fed ASB and YSC diets was higher (P < 0.05) than in those fed CON diet. In addition, SGLT1 mRNA expression in pigs fed ASB diet was higher (P < 0.05), and YSC diet (P = 0.055) trended upwards compared to pigs fed CON diet.
Figure 1.
mRNA relative abundance of nutrient transporters in the jejunum of piglets fed diets supplemented or not with feed additives on d 32. Dietary treatment: CON, control, basal diet; NUC, CON + 1 g/kg of nucleotides; YSC, CON + 20 g/kg of lysed yeast S. cerevisiae; ASB: CON + 1.5 g/kg of acidifier sodium butyrate. Data are means of 9 pigs replicates per dietary treatment. P-value, preplanned contrasts of each of the treatments (NUC, YSC, and ASB) versus the control treatment (CON). (A) GLUT2, glucose transporter type 2; (B) SGLT1, Na+/glucose co-transporter 1; (C) SMCT2, sodium-coupled monocarboxylate transporter; (D) MCT1, monocarboxylate transporter 1; (E) PepT1, peptide transporter 1; (F) EAAC1, excitatory amino acid carrier 1; (G) y+LAT1, Na+-independent cationic and Na+-dependent neutral amino acid transporter 1.
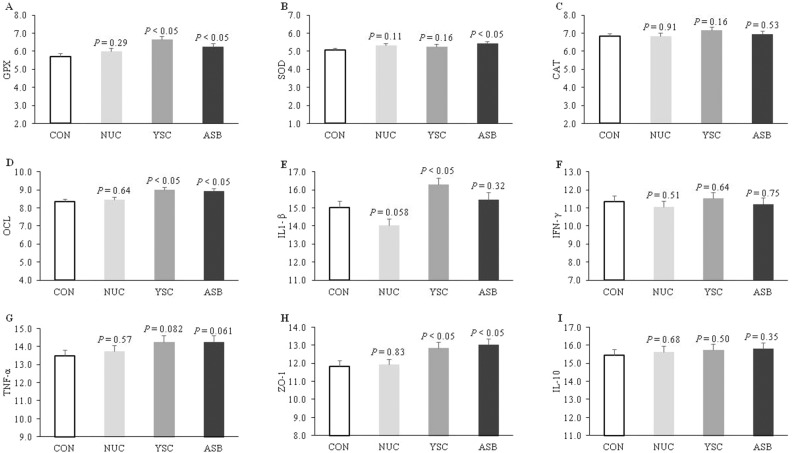
mRNA relative abundance of inflammatory and antioxidants markers, and tight junction proteins
There was no effect of dietary treatments on the mRNA expression of CAT, IFN-γ, and IL-10 (Fig. 2). However, mRNA expression of GPX, OCL, and ZO-1 was higher (P < 0.05) in pigs fed YSC or ASB diets compared to pigs fed CON diet. The mRNA expression of SOD in pigs fed ASB diet, and IL1-β in pigs fed YSC diet was higher (P < 0.05) than in those fed CON diet. In addition, TNF-α mRNA expression in pigs fed YSC diet (P = 0.082) and ASB diet (P = 0.061) trended upwards compared to pigs fed CON diet. There was a downward trend (P = 0.058) in IL1-β mRNA expression of pigs fed NUC diet than pigs that received CON diet.
Figure 2.
mRNA relative abundance of inflammatory and antioxidants markers, and tight junction proteins in the jejunum of pigls fed diets supplemented or not with feed additives on d 32. Dietary treatment: CON, control, basal diet; NUC, CON + 1 g/kg of nucleotides; YSC, CON + 20 g/kg of lysed yeast S. cerevisiae; ASB: CON + 1.5 g/kg of acidifier sodium butyrate. Data are means of 9 pigs replicates per dietary treatment. P-value, preplanned contrasts of each of the treatments (NUC, YSC, and ASB) versus the control treatment (CON). (A) GPX, glutathione peroxidase; (B) SOD, superoxide dismutase; (C) CAT, catalase; (D) OCL, occludin; (E) ZO-1, zonula occludens-1; (F) IFN-γ, interferon gamma; (G) TNF-α, tumor necrosis factor alpha; (H) IL1-β, interleukin 1 beta; (I) IL-10, interleukin 10.
Discussion
The hypothesis of the current experiment was that the dietary supplementation of purified nucleotide, S. cerevisiae yeast, or encapsulated acidifier sodium butyrate would improve the growth performance of weaned piglets and beneficially affect gut parameters. The present results showed that feeding nursery pigs with YSC or ASB diets increased growth performance and improved gut health, whereas no improvements were observed feeding the NUC diet.
Although nucleotides can be synthesized from other precursors in pigs’ diets, in the post-weaning a stressful and limited nutrient intake period, nucleotides could be considered an essential nutrient6. However, in the present study, performance was not influenced by NUC diet, while others have reported positive effects of nucleotide supplementation on piglet growth performance26,27 and feed intake5. The NUC diets correspond to 100 mg nucleotides/kg diet in phase 1 and 75 mg nucleotides/kg in phase 2 in a 24-day trial and were fed purified. On the other side, Superchi et al.26 used a nucleotide yeast-derived source that also contains viable cells, cell wall components, inositol, and functional amino acids. Weaver and Kim27 demonstrated improved growth performance with up to 1000 mg nucleotides/kg during a 28-day trial. Working with different levels of nucleotides (0, 50, 150, 250, and 500 mg/kg) in a 20-day trial with one pig per pen, Jang and Kim5 only found significant improvements in the feed intake, and when feeding the lower supplementation levels (50 and 150 mg/kg). Thus, the discrepancies in growth performance results between studies are justified due to the different sources, the different dosages used, and administration time.
Regarding the YSC diet, the improved growth performance results are attributed to the improvements in intestinal health promoted by the supplementation of S. cerevisiae yeast. This additive contains high amounts of highly digestible protein, essential amino acids, nucleotides, mannanoligosaccharides, and β-glucans15. These components present in yeast may have anti-inflammatory properties to reduce intestinal inflammation and, consequently, minimize diarrheal disorders (as observed in the current study) and nutrient malabsorption1. According to Kogan and Kocher19, β-glucans are capable of blocking fimbriae of pathogenic bacteria and preventing their adhesion to the epithelium of the intestinal mucosa, acting to prevent or eliminate infection. Yeast-based additives support the immune system of piglets by modulating the intestinal microbiota28, which may contribute to improve growth performance29.
An increase in nutrient digestibility as reported by Barbosa et al.30 and Boontiam et al.8 is another mechanism by which yeast supplementation in nursery pigs’ diets can improve the growth performance. Nutrient transporters are proteins expressed in the apical membrane of intestinal cells that absorb nutrients and, therefore, it is possible to improve digestibility by increasing the expression of these transporters31. This is supported by the results of our study, which showed increased mRNA expression of nutrient transporters (SGLT1, SMCT2, MCT1, and PepT1) in the jejunum of pigs. The SGLT1 is responsible for glucose absorption, while PepT1 acts on peptide absorption. The increase in the expression of these transporters suggested that there was greater availability of glucose and amino acids at the cellular level, in agreement with the findings by Clarke et al.31. Similarly, the increased expression of SMCT2 and MCT1 indicated a greater availability of monocarboxylates (e.g. lactate, short-chain fatty acids, and ketone bodies)32, which represent substrates for maintaining the energetic state in cells like the enterocytes.
Regarding the ASB diet, the improvement in growth performance is supported by the increase in SGLT1 and MCT1 mRNA expression in the jejunum of nursery pigs. The MCT1 is expressed in both the small and large intestines, and its function is to transport butyrate into the cell33. Although, in the small intestine, microbial butyrate formation is low or absent34, the addition of butyrate to diets exerts trophic effects35 and stimulates the secretion of digestive enzymes21, and this can result in more efficient digestion and absorption of dietary nutrients, leading to improved performance. Furthermore, butyrate is a source of energy for enterocytes and additionally has an antiapoptotic effect33. Therefore, greater MCT1 and SGLT1 expression may enhance the absorption of nutrients and energy to cope with post-weaning stress36. In the present study, sodium butyrate was added in encapsulated form (composed of a vegetable fat-based coating material) to be enzymatically broken down by lipase secreted in the duodenum, as mentioned by Maito et al.10. According to Tugnoli et al.25, the encapsulation process provides protection that allows a gradual release of the acidifier along the length of the gastrointestinal tract, reducing the dissociation in the stomach and maintaining their efficacy in jejunum and ileum.
The weaning transition promotes physiological changes in the structural and functional aspects of the intestine, causing villous atrophy and increased crypt depth, which in turn reduces the small intestine capacity to absorb nutrients3. In the present study, villus height and villus:crypt ratio in the ileum were higher in pigs fed the ASB diet, indicating improvement in intestinal morphology. Moreover, a greater abundance of jejunal mucosal barrier function-related genes (OCL and ZO-1) was observed in pigs fed the ASB diet. These results indicated that ASB diet was efficient in the structural maintenance of the small intestine, as suggested by others35. The gradual release of sodium butyrate throughout the intestinal tract seems to be critical in achieving the expected target9, allowing the additive to affect different portions of the intestine such as jejunum and ileum. According to Guilloteau et al.33, supplementation with sodium butyrate stimulates the proliferation of epithelial cells, resulting in a greater absorption surface and preservation of villi length, reflecting the improvements observed in growth performance of pigs.
A greater abundance of jejunal OCL and ZO-1 mRNA expression was also observed in pigs fed the YSC. According to Rose et al.37, OCL and ZO-1 are classified as intestinal junction proteins with a role in regulating epithelial permeability. Decreased intestinal permeability can be correlated with reduced oxidative stress, because this results in attenuation of damage to the intestinal mucosal barrier14. Oxidative stress is a physiological stage in which antioxidant defense is inadequate to detoxify the reactive oxygen species, this oxidative process damages essential biomolecules, leading to reduced growth performance29. The GPX and SOD as the key enzymes of the antioxidant system play a crucial role in eliminating free radicals, reducing oxidative damage, and maintaining cell structure is well known38. In our study, in addition to the increase in the expression of tight junction proteins, pigs fed the ASB and YSC diet had greater expression of GPX and SOD mRNA. These results suggested that nursery pigs fed with additives had greater antioxidant capacity than those fed the CON diet, demonstrating that there was an improvement in the intestinal redox state.
The impacts of weaning stress are not only limited to intestinal barrier function and oxidative stress, but an increase in the activation of the immune system in weaned piglets is also observed3. An unregulated enhanced immune response may trigger a negative effect on other metabolic processes and as a result impair growth performance39. Peyer's patches are the major organized lymphoid structures involved in the induction of mucosal immune responses in the intestine40. In the present study, a reduction in Peyer's patch counts was observed in the ileum of pigs fed YSC or ASB diet. This suggested that there was less induction of the mucosal immune system in pigs that received YSC and ASB diets and, consequently, less stimulus to the immune system because Peyer's patches can be considered as immunological sensors of the intestine41. These results suggested that both yeast and sodium butyrate could help enhance the small intestine epithelial barrier, antioxidant capacity, and immune system. Thus, the enhancement of overall gut health helps explain the improvement in the growth performance of pigs fed YSC and ASB diets.
Regarding the production of pro-inflammatory cytokines, it was found that pigs fed the YSC diet showed an increase in IL1-β mRNA expression and a tendency to increase TNF-α, while piglets fed the ASB diet showed a tendency to increase TNF-α mRNA expression. On the other hand, pigs fed the NUC diet had a tendency to reduce IL1-β mRNA expression. Nucleotides, yeast, and acidifier-based additives can improve pig immune responses in the post-weaning period16. These additives can activate immune cells, including macrophages, which produce IL-1β as part of the immune response42,43. The results of the present study indicated a sustained condition of immune response, because in some cases, a controlled and transient increase in IL-1β may be part of a healthy immune response to support the ability of pigs to fight infections or maintain intestinal health18. According to Grimble39, it is considered beneficial the presence of cytokines (e.g. IL-1β and TNF-α) in adequate concentrations during an inflammatory response to infection. It is important to avoid overstimulation of the immune system, as greater expression of pro-inflammatory cytokines can trigger pathological responses in inflammatory conditions44. However, in a previous study, it was observed that TNF-α increases the expression of specific anti-apoptotic proteins, as well as triggers the expression of the survival gene BCL2A1 (not evaluated in the current study) in the intestine of weaned piglets45. Also, the β-glucans present in yeast are recognized by specific receptors (pattern recognition receptors) on immune cells, such as macrophages and neutrophils. In particular, they are recognized by the dectin-1 receptor46. Once β-glucans bind to these receptors, they can trigger an immune response. Upon recognition of β-glucans, immune cells can produce pro-inflammatory cytokines, such as TNF-α, and IL-1β47. Collectively, although these cytokines play a central role in initiating and magnifying the inflammatory response, they did not negatively affect the biological response of nursery pigs fed YSC and ASB diets.
Conclusions
Based on the evaluated criteria, dietary supplementation of autolyzed yeast S. cerevisiae or sodium butyrate promotes better growth performance by improving the integrity of the intestinal barrier, the mRNA expression of nutrient transporters and antioxidant enzymes in the jejunum of nursery pigs, but without major differences in intestinal morphology in those fed with S. cerevisiae yeast. Furthermore, none of the dietary treatments promoted changes in the observed blood metabolites, but a diet containing S. cerevisiae yeast or sodium butyrate provided a sustained immune response in the jejunum of nursery pigs. On the other hand, dietary nucleotide supplementation did not improve growth performance and gut health.
Material and methods
The experimental protocol follows the ethical principles in animal research (CONCEA, 2016) and was approved by the Ethical Committee on Animal Use of Universidade Federal de Viçosa, under protocol no. 0110/2022. All methods were carried out in accordance with relevant guidelines and regulations. All methods were reported in accordance with ARRIVE guidelines (https://arriveguidelines.org/arrive-guidelines).
Animals, experimental design, housing, and diets
The experiment was conducted on a commercial farm in the municipality of Santo Antônio do Grama, MG, Brazil. A total of 180 piglets [PIC 337 (Large White × Landrace × Duroc × Pietrain) × DB 90 (Large White × Landrace)] castrated males and females, weaned at 21 day-old and with 5.17 ± 0.57 kg BW were used in a 24-day trial. Piglets were assigned to a randomized block design based on their initial BW (light 4.7 ± 0.25 kg and heavy 5.6 ± 0.34 kg) into 4 dietary treatments and 9 pens replicates (5 pigs/pen).
The experiment was conducted only in one series, without an adaptation period. The pigs were housed in suspended pens (1.75 × 1.00 m, 0.35 m2/pig), with a plastic floor, semi-automatic feeders and nipple drinkers, with free access to diet and water. The minimum and maximum temperatures in the nursery room were 22.9 ± 1.50 °C and 31.8 ± 2.54 °C, respectively.
Pigs were fed in a two-phase feeding regimen (phase 1: 21–32 days, and phase 2: 32–45 days). All diets were corn and soybean meal-based with industrial amino acids and formulated according to the nutritional recommendations of the Brazilian Tables for Poultry and Swine48 (Table 5), and provided in mash form. During phases 1 and 2, the dietary treatments consisted, respectively, of: (1) CON: control, basal diet, (2) NUC: CON + 1 g/kg and 0.5 g/kg of nucleotides, (3) YSC: CON + 20 g/kg and 10 g/kg of lysed yeast S. cerevisiae, (4) ASB: CON + 1.5 g/kg and 1 g/kg of acidifier sodium butyrate. Feed additives were added in replacement with inert in the CON based on the manufacturer's recommendations.
Table 5.
Ingredients and chemical composition of control diets fed to nursery piglets (g/kg, as-fed basis).
| Itema | Phase 1 | Phase 2 |
|---|---|---|
| 21–32 days | 32–45 days | |
| Ground corn, 7.8% CP | 388.1 | 470.9 |
| Soybean meal, 46.0% CP | 219.7 | 242.2 |
| Dried whey, 12.5% CP | 150.0 | 100.0 |
| Soybean micronized, 36.0% CP | 100.0 | 70.0 |
| Extrude corn, 7.6% CP | 20.0 | 20.0 |
| Plasma protein, 78.0% CP | 20.0 | 10.0 |
| Sugar | 30.0 | 30.0 |
| Inert (kaolin) | 20.0 | 10.0 |
| Dicalcium phosphate | 9.35 | 10.6 |
| Limestone calcitic | 7.69 | 7.34 |
| Soybean oil | 15.93 | 11.1 |
| Zinc oxide | 2.50 | 2.20 |
| Choline chloride | 1.95 | 1.50 |
| L-lys, 78.0% | 4.69 | 4,45 |
| DL-met, 99.0% | 2.32 | 1.96 |
| L-thr, 98.5% | 2.30 | 2.14 |
| L-trp, 99.0% | 0.32 | 0.28 |
| L-val, 96.5% | 1.03 | 0.76 |
| Salt | 1.90 | 2.44 |
| Copper sulfate | 0.60 | 0.60 |
| Vitamin-mineral premixb | 1.40 | 1.40 |
| Phytasec | 0.05 | 0.05 |
| BHT | 0.10 | 0.10 |
| Calculated composition | ||
| ME, kcal/kg | 3400 | 3375 |
| Crude protein, g/kg | 213.00 | 213.00 |
| SIDd lys, g/kg | 14.50 | 13.46 |
| SID met, g/kg | 4.90 | 4.53 |
| SID met + cys, g/kg | 8.13 | 7.54 |
| SID thr, g/kg | 9.72 | 9.02 |
| SID trp, g/kg | 2.76 | 2.56 |
| SID val, g/kg | 10.01 | 9.29 |
| SID ile, g/kg | 8.01 | 7.70 |
| Total Ca, g/kg | 8.50 | 8.25 |
| Available P, g/kg | 5.19 | 5.00 |
| Total Na, g/kg | 2.80 | 2.30 |
| Lactose, g/kg | 112.50 | 75.00 |
aFeed additives were included as a replacement for the inert in the diet. Phase 1 (21 to 32 d) and phase 2(32 to 45 d), respectively: (1) CON: control, basal diet, (2) NUC: CON + 1 g/kg and 0.5 g/kg of nucleotides (Nucleobase 1.5, Aleris Animal Nutrition, Jundiaí, SP, Brazil), (3) YSC: CON + 20 g/kg and 10 g/kg of lysed yeast S. cerevisiae (Sinergis, Aleris Animal Nutrition, Brazil), (4) ASB: CON + 1.5 g/kg and 1 g/kg of acidifier sodium butyrate (Nuttritiva B90CNa, Additiva Nutrition, Monte Alegre do Sul, SP, Brazil).
bComposition per kg of diet: vitamin A, 12,000 IU; vitamin D3, 2,250 IU; vitamin E, 65 IU; vitamin K, 3 mg; thiamine, 2.25 mg; riboflavin, 6 mg; pyridoxine, 2.25 mg; vitamin B12, 27 mcg; folic acid, 400 mcg; biotin, 150 mcg; pantothenic acid, 22.5 mg; niacin, 45 mg; copper sulfate, 10 mg; iodine, 1.5 mg; iron sulfate, 100 mg; manganese sulfate, 40 mg; sodium selenite, 0.3 mg; zinc oxide, 100 mg.
cNatuphos®, Basf enzyme.
dStandardized ileal digestible.
Composition of feed additives
The source of nucleotides (Nucleobase 1.5, Aleris Animal Nutrition, Jundiaí, SP, Brazil) obtained from yeast extract purified with autolyzed yeast, contained 15% free nucleotides and 85% vehicle, corresponding to 100 mg free nucleotides/kg diet (phase 1) and 75 mg free nucleotides/kg (phase 2). The yeast source (Sinergis, Aleris Animal Nutrition, Brazil) contained 100% autolyzed yeast S. cerevisiae. The acidifier source (Nuttritiva B90CNa, Additiva Nutrition, Monte Alegre do Sul, SP, Brazil) contained 90% sodium butyrate in the form of a protected and encapsulated salt with palm oil, microgranulated, corresponding to 1.35 g sodium butyrate/kg diet (phase 1) and 0.90 g sodium butyrate/kg diet (phase 2).
Growth performance and diarrhea incidence
Throughout the trial, the offered diet and leftovers were weighed to calculate average daily feed intake (ADFI). Pigs were individually weighed on d 21, 32, and 45 to determine BW, average daily weight gain (ADG), and feed conversion (FC). The fecal consistency of each pig was visually assessed during phase 1 (24–32 days) and phase 2 (32–45 days), using the method described by Liu et al.49. Fresh feces were ranked on a 4-point scale as follows: 0 = solid, 1 = semi-solid, 2 = semi-liquid, and 3 = liquid. The diarrhea incidence was defined as the consistency of feces at scale 2 or 3 for 2 continuous days. The diarrhea incidence for the pigs was calculated as follows: diarrhea incidence (%) = [(the number of pigs with diarrhea in each pen × number of days of diarrhea) ÷ (total number of pigs in each pen × number of days)] × 100%. Observations were made in the morning, every day throughout the experimental period by a trained evaluator.
Sample collection
At 32 day-old, blood was collected from 1 piglet with BW closest to the average weight of the pigs within its respective pen. The animals were not fasted. Blood was collected (09h00) by orbital sinus puncture with a hypodermic needle (40 × 1.6 mm) into 10 mL tubes without anticoagulants. Samples were immediately sent at room temperature to the Viçosa Clinical Laboratory (Viçosa, MG—Brazil) for determination of serum urea nitrogen (SUN; Ureal Cobas C311, Linklab, software PNCQ), creatinine (Creatinine, WS-Kovalent, kinetic method, ASB-380, Mindray), and immunoglobulin G concentrations (IgG Atellica CH IgG_2, CH Analyzer, Siemens Healthineers).
The same blood donor piglet was electrically stunned (240 V for 3 s) followed by exsanguination to collect samples on d 32. Fragments measuring 2 cm were sampled (8 pigs/treatment) from the duodenum (10 cm from the pylorus junction), jejunum (mid-section), and ileum (5 cm to ileocecal junction) for histological evaluation50. The histological sections were then washed in a physiological solution (0.9% sodium chloride) and fixed in 4% paraformaldehyde solution (100 mL 40% paraformaldehyde, 900 mL distilled water, 2.28 g monobasic sodium phosphate, and 21.74 g dibasic sodium phosphate) for 24 h at room temperature6. Another 2 cm of jejunum was collected and immediately frozen in liquid nitrogen, stored at − 80 °C for RNA extraction and gene expression analysis.
Intestinal morphology, Peyer’s patches, and goblet cells
After 24 h of fixation, the fragments of the duodenum, jejunum, and ileum were transferred to an ethanol solution 70% (v/v). Then, the samples were cut into cross-sections and dried in increasing gradients of ethyl, diaphanized in HistoChoice®, and embedded in liquid Paraplast® at 65 °C. Five cross-sections (5 μm thickness each) were placed per slide and stained with hematoxylin and eosin. The sections were semi-serial using 1 in 10 cuts51. For morphological readings of villus height and crypt depth in the duodenum, jejunum, and ileum, an EVOS™ M5000 Cell Imaging System optical microscope (Invitrogen, Thermo Fisher Scientific) with a 10-objective lens was used. The images were then analyzed using ImageJ 1.50i (Java1.6.0_20; National Institutes of Health, USA). Heights of 20 villus and their 20 crypts were selected and measured. Villus to crypt ratios using the length data were then calculated. All measurements were made by a single trained individual. In the ileum fragment, the total count of the Peyer’s patches was performed at 4 × magnification52.
To assess the goblet cells in the duodenum, jejunum, and ileum, 10 fields per slide were photographed at 20 × magnification. Subsequently, the ImageJ program was used, and perpendicular lines were inserted with markings in uniformly sized quadrants under each image. Then, the total number of intersections in the image and the cells that touched the intersections were counted. The calculation was made according to the methodology proposed by Mandarim de Lacerda53:
Relative mRNA abundance
Total RNA was extracted using a commercial kit (SV Total RNA isolation kit—Promega, Z3100), following the manufacturer's instructions. The RNA concentration was estimated using NanoDrop™ Lite (Thermo Fisher Scientific), and RNA integrity was assessed using 1% agarose gel electrophoresis. Complementary DNA was synthesized according to the GoScript™ Reverse Transcription System protocol (Promega Corporation). GenBank numbers used to access the gene primers are shown in Table 6. Primers were used for reverse transcription quantitative PCR with GoTaq® qPCR Master Mix (Promega) in QuantStudio® 3 (Applied Biosystems, Thermo Fisher Scientific). Geometric mean of Ct value of β-actina was used to normalize the expression of the target genes for the jejunum samples. The relative expression of the gene of interest was calculated by ΔCt54 for glutathione peroxidase (GPX), superoxide dismutase (SOD), catalase (CAT), occludin (OCL), zonula occludens-1 (ZO-1), interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL1-β), interleukin 10 (IL-10), glucose transporter type 2 (GLUT2), Na+/glucose co-transporter 1 (SGLT1), sodium-coupled monocarboxylate transporter (SMCT2), monocarboxylate transporter 1 (MCT1), peptide transporter 1 (PepT1), excitatory amino acid carrier 1 (EAAC1), Na+-independent cationic and Na+-dependent neutral amino acid transporter 1 (y+LAT1).
Table 6.
List of primers used in reverse transcription quantitative-PCR gene expression analysis in weaned piglets.
| Genes | GenBank number | Sequence2 |
|---|---|---|
| GPX | NM_214201.1 | F: 5′GCCCAACTTCATGCTCTTC3′ |
| R: 5′CAGGATCTCCCCATTCTTGGC3′ | ||
| SOD | NM_001190422.1 | F: 5′ATCAAGAGAGGCACGTTGGA3′ |
| R: 5′TCTGCCCAAGTCATCTGGTT3′ | ||
| CAT | NM_214301.2 | F: 5′GCTTTAGTGCTCCCGAACAG3′ |
| R: 5′AGATGACCCGCAATGTTCTC3′ | ||
| OCL | NM_001163647.1 | F: 5′TCCTGGGTGTGATGGTGTTC3′ |
| R: 5′CGTAGAGTCCAGTCACCGCA3′ | ||
| ZO-1 | XM_003353439.2 | F: 5´AAGCCCTAAGTTCAATCACAATCT3′ |
| R: 5´ATCAAACTCAGGAGGCGGC3′ | ||
| IFN- γ | NM_213948 | F: 5′TGGTAGCTCTGGGAAACTGAATG3′ |
| R: 5′GGCTTTGCGCTGGATCTG3′ | ||
| TNF-α | NM_214022.1 | F: 5′CATCGCCGTCTCCTACCA3′ |
| R: 5′CCCAGATTCAGCAAAGTCCA3′ | ||
| IL1- β | NM_214055.1 | F: 5′TCTGCCCTGTACCCCAACTG3′ |
| R: 5′CCCAGGAAGACGGGCTTT3′ | ||
| IL-10 | NM_214041.1 | F: 5′GAAGGACCAGATGGGCGACTT3′ |
| R: 5′CACCTCCTCCACGGCCCTTG3′ | ||
| GLUT2 | FJ209732.1 | F: 5′TTGCCTTGGATGAGTTATGTGA3′ |
| R: 5′GCGTGGTCCTTGACTGAAAA3′ | ||
| SGLT1 | MW_280290.1 | F: 5′GGTTGGAGCTTCTCTGTTTG3′ |
| R: 5′CCAGAACAACCACCCAAATC3′ | ||
| SMCT2 | XM_003122908.1 | F: 5′AGGTCTACCGCTTTGGAGCAT3′ |
| R:5′GAGCTCTGATGTGAAGATGATGACA3′ | ||
| MCT1 | AM_286425.1 | F: 5′GGTGGAGGTCCTATCAGCAG3′ |
| R: 5′AAGCAGCCGCCAATAATCAT3′ | ||
| PepT1 | AY_180903.1 | F: 5′CATGGATGCTGTGGTGTATC3′ |
| R: 5′CATGGAAGCCAGGAACATC3′ | ||
| EAAC1 | NM_001164649.1 | F: 5′CAGTGGATGCCATGTTAGAC3′ |
| R: 5′CGTCTCTGGCTCACTAGAA3′ | ||
| y+LAT1 | EU_047705.1 | F: 5′TGCGTGGAGGACATCTT3′ |
| R: 5′CTCCTTCCAGCGCAAATAG3′ | ||
| β-actin | U07786.1 | F: 5′CTCTTCCATCGTGTCCTTCTAC3′ |
| R: 5′CCTCAGACTTGTCGATCTTCTG3′ |
GPX glutathione peroxidase, SOD superoxide dismutase, CAT catalase, OCL occluding, ZO-1 zonula occludens-1, IFN-γ interferon gamma, TNF-α tumor necrosis factor alpha, IL1-β interleukin 1 beta, IL-10 interleukin 10, GLUT2 glucose transporter type 2, SGLT1 Na + /glucose co-transporter 1, SMCT2 sodium-coupled monocarboxylate transporter, MCT1 monocarboxylate transporter 1, PepT1 peptide transporter 1, EAAC1 excitatory amino acid carrier 1, y + LAT1 Na + -independent cationic and Na + -dependent neutral amino acid transporter 1. 2F and R indicate Forward and Reverse primers, respectively.
Statistical procedures
The pen was considered the experimental unit for growth performance and diarrhea incidence analysis. One pig from each pen was considered the experimental unit for intestinal morphology, gene expression, and blood profile. The statistical model included the fixed effect of dietary treatment, and block and residual error as random factors. The normality of experimental errors was evaluated using Shapiro–Wilk. The data were analyzed using the mixed procedure of SAS 9.4 (SAS Inst., Inc., Cary, NC, USA) via one-way analysis of variance (ANOVA). When an effect was detected in the ANOVA (P < 0.05), differences between means were determined by the preplanned contrasts, in which each of the treatments (NUC, YSC, and ASB) were compared versus the control treatment (CON). The statistical significance and tendency were declared at P < 0.05 and 0.05 ≤ P < 0.10, respectively.
Acknowledgements
The authors thank CNPq—Conselho Nacional de Desenvolvimento Científico e Tecnológico, CAPES – Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, INCT-CA—Instituto Nacional de Ciência e Tecnologia de Ciência Animal, and FAPEMIG—Fundação de Amparo à Pesquisa do Estado de Minas Gerais. Special thanks to Ajinomoto do Brazil for providing the crystalline amino acids used in the experiment.
Author contributions
GCR and AMC: conceptualization, data curation, and project management. GCR, AMC, and FFA: methodology. GCR: software. GCR, AMC, JLG and SWK: statistical analysis, formal analysis, and writing—original draft preparation. GCR and SWK: validation. AMC and FFA: investigation. AMC, JLG, SWK and GCR: writing—review and editing. GCR: supervision. All authors contributed to the article and approved the submitted version.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Genova, J. et al. A summary of feed additives, intestinal health and intestinal alkaline phosphatase in piglet nutrition. Czech J. Anim. Sci. 65, 281–294. 10.17221/70/2020-CJAS (2020).
- 2.Moore, K. L., Mullan, B. P., Pluske, J. R., Kim, J. C. & D'Souza, D. N. The use of nucleotides, vitamins and functional amino acids to enhance the structure of the small intestine and circulating measures of immune function in the post-weaned piglet. Anim. Feed Sci. Technol.165, 184–190. 10.1016/j.anifeedsci.2010.09.013 (2011)
- 3.Kim SW, Duarte ME. Understanding intestinal health in nursery pigs and the relevant nutritional strategies. Anim. Biosci. 2021;34:338–344. doi: 10.5713/ab.21.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moeser AJ, Pohl CS, Rajput M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim. Nutr. 2017;3:313–321. doi: 10.1016/j.aninu.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang KB, Kim SW. Supplemental effects of dietary nucleotides on intestinal health and growth performance of newly weaned pigs. J. Anim. Sci. 2019;97:4875–4882. doi: 10.1093/jas/skz334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valini GAC, et al. Dietary nucleotide supplementation as an alternative to in-feed antibiotics in weaned piglets. Anim. 2021;15:100021. doi: 10.1016/j.animal.2020.100021. [DOI] [PubMed] [Google Scholar]
- 7.Berto, P. N. et al. Dietary supplementation with hydrolyzed yeast and its effect on the performance, intestinal microbiota, and immune response of weaned piglets. Anais Acad. Bras. de Ciên. 92. 10.1590/0001-3765202020180969 (2020). [DOI] [PubMed]
- 8.Boontiam W, Bunchasak C, Kim YY, Kitipongpysan S, Hong J. Hydrolyzed yeast supplementation to newly weaned piglets: Growth performance, gut health, and microbial fermentation. Anim. 2022;12:350. doi: 10.3390/ani12030350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu JD, Bayir HO, Cosby DE, Cox NA, Williams SM, Fowler J. Evaluation of encapsulated sodium butyrate on growth performance, energy digestibility, gut development, and Salmonella colonization in broilers. Poult. Sci. 2017;96:3638–3644. doi: 10.3382/ps/pex174. [DOI] [PubMed] [Google Scholar]
- 10.Maito CD, et al. Simultaneous feeding of calcium butyrate and tannin extract decreased the incidence of diarrhea and proinflammatory markers in weaned piglets. Anim. Biosci. 2022;35:87. doi: 10.5713/ab.21.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pluske JR. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J. Anim. Sci. Biotechnol. 2013;4:1–7. doi: 10.1186/2049-1891-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng L, Duarte ME, Loftus AS, Kim SW. Intestinal health of pigs upon weaning: challenges and nutritional intervention. Front. Vet. Sci. 2021;8:628258. doi: 10.3389/fvets.2021.628258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes MDS, et al. Effect of antibiotics and low-crude protein diets on growth performance, health, immune response, and fecal microbiota of growing pigs. J. Anim. Sci. 2023;101:1. doi: 10.1093/jas/skad357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C, et al. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J. Nutr. 2015;145:2774–2780. doi: 10.3945/jn.115.217406. [DOI] [PubMed] [Google Scholar]
- 15.Xiong X, et al. Dietary supplementation with yeast product improves intestinal function, and serum and ileal amino acid contents in weaned piglets. Livest. Sci. 2015;171:20–27. doi: 10.1016/j.livsci.2014.10.012. [DOI] [Google Scholar]
- 16.Liu Y, et al. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018;4:113–125. doi: 10.1016/j.aninu.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sauer N, Mosenthin R, Bauer E. The role of dietary nucleotides in single stomached animals. Nutr. Res. Rev. 2011;24:46–59. doi: 10.1017/S0954422410000326. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Z, et al. Effects of different forms of yeast Saccharomyces cerevisiae on growth performance, intestinal development, and systemic immunity in early-weaned piglets. J. Anim. Sci. Biotechnol. 2015;6:1–8. doi: 10.1186/s40104-015-0046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kogan G, Kocher A. Role of yeast cell wall polysaccharides in pig nutrition and health protection. Livest. Sci. 2007;109:161–165. doi: 10.1016/j.livsci.2007.01.134. [DOI] [Google Scholar]
- 20.Liu, G. et al. Dietary Saccharomyces cerevisiae cell wall extract supplementation alleviates oxidative stress and modulates serum amino acids profiles in weaned piglets. Oxid. Med. Cell. Longev.2017. 10.1155/2017/3967439 (2017). [DOI] [PMC free article] [PubMed]
- 21.Papatsiros VG, Christodoulopoulos G, Filippopoulos LC. The use of organic acids in monogastric animals (swine and rabbits) J. Cell Anim. Biol. 2012;6:154–159. doi: 10.5897/JCAB11.081. [DOI] [Google Scholar]
- 22.Fang CL, Sun H, Wu J, Niu HH, Feng J. Effects of sodium butyrate on growth performance, haematological and immunological characteristics of weanling piglets. J. Anim. Physiol. Anim. Nutr. 2014;98:680–685. doi: 10.1111/jpn.12122. [DOI] [PubMed] [Google Scholar]
- 23.Lewis K, et al. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm. Bowel Dis. 2010;16:1138–1148. doi: 10.1002/ibd.21177. [DOI] [PubMed] [Google Scholar]
- 24.Li X, et al. Sodium butyrate ameliorates oxidative stress-induced intestinal epithelium barrier injury and mitochondrial damage through AMPK-mitophagy pathway. Oxid. Med. Cell. Longev. 2022;1:1. doi: 10.1155/2022/3745135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tugnoli B, Giovagnoni G, Piva A, Grilli E. From acidifiers to intestinal health enhancers: How organic acids can improve growth efficiency of pigs. Anim. 2020;10:134. doi: 10.3390/ani10010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Superchi P, et al. Effects of dietary nucleotide supplementation on growth performance and hormonal and immune responses of piglets. Anim. 2012;6:902–908. doi: 10.1017/S1751731111002473. [DOI] [PubMed] [Google Scholar]
- 27.Weaver AC, Kim SW. Supplemental nucleotides high in inosine 5′-monophosphate to improve the growth and health of nursery pigs. J. Anim. Sci. 2014;92:645–651. doi: 10.2527/jas.2013-6564. [DOI] [PubMed] [Google Scholar]
- 28.Vries H, et al. Impact of yeast-derived β-glucans on the porcine gut microbiota and immune system in early life. Microorganisms. 2020;8:1573. doi: 10.3390/microorganisms8101573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomes MDS, et al. Effect of amino acid blend as alternative to antibiotics for growing pigs. J. Anim. Sci. 2022;100:1. doi: 10.1093/jas/skac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbosa KA, et al. Effects of combined feed additives in diets to support growth performance and intestinal health profile in nursery piglets. Livest. Sci. 2022;266:105121. doi: 10.1016/j.livsci.2022.105121. [DOI] [Google Scholar]
- 31.Clarke LC, et al. Effect of β-glucanase and β-xylanase enzyme supplemented barley diets on nutrient digestibility, growth performance and expression of intestinal nutrient transporter genes in finisher pigs. Anim. Feed Sci. Technol. 2018;238:98–110. doi: 10.1016/j.anifeedsci.2018.02.006. [DOI] [Google Scholar]
- 32.Metzler-Zebeli BU, Ertl R, Grüll D, Molnar T, Zebeli Q. Enzymatically modified starch up-regulates expression of incretins and sodium-coupled monocarboxylate transporter in jejunum of growing pigs. Anim. 2017;11:1180–1188. doi: 10.1017/S1751731116002615. [DOI] [PubMed] [Google Scholar]
- 33.Guilloteau P, et al. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr. Res. Rev. 2010;23:366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- 34.Bartholome AL, Albin DM, Baker DH, Holst JJ, Tappenden KA. Supplementation of total parenteral nutrition with butyrate acutely increases structural aspects of intestinal adaptation after an 80% jejunoileal resection in neonatal piglets. J. Parenter Enteral Nutr. 2004;28:210–222. doi: 10.1177/0148607104028004210. [DOI] [PubMed] [Google Scholar]
- 35.Liu H, Zhao J, Zhang W, Nie C. Impacts of sodium butyrate on intestinal mucosal barrier and intestinal microbial community in a weaned piglet model. Front. Microbiol. 2023;13:1041885. doi: 10.3389/fmicb.2022.1041885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng Q, et al. Changes in cecal morphology, cell proliferation, antioxidant enzyme, volatile fatty acids, lipopolysaccharide, and cytokines in piglets during the postweaning period. J. Anim Sci. 2020;98:1. doi: 10.1093/jas/skaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose EC, Odle J, Blikslager AT, Ziegler AL. Probiotics, prebiotics and epithelial tight junctions: A promising approach to modulate intestinal barrier function. Int. J. Mol. Sci. 2021;22:6729. doi: 10.3390/ijms22136729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He J, et al. Effects of l-glutamine on growth performance, antioxidant ability, immunity and expression of genes related to intestinal health in weanling pigs. Livest. Sci. 2016;189:102–109. doi: 10.1016/j.livsci.2016.05.009. [DOI] [Google Scholar]
- 39.Grimble RF. Dietary lipids and the inflammatory response. Proc. Nutr. Soc. 1998;57:535–542. doi: 10.1079/PNS19980078. [DOI] [PubMed] [Google Scholar]
- 40.Makala LH, et al. Ontogeny of pig discrete Peyer’s patches: distribution and morphometric analysis. Pathobiol. 2001;68:275–282. doi: 10.1159/000055938. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi N, Takahashi D, Takano S, Kimura S, Hase K. The roles of Peyer's patches and microfold cells in the gut immune system: Relevance to autoimmune diseases. Front. Immunol. 2019;10:2345. doi: 10.3389/fimmu.2019.02345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Y, Zhang W, Gao F, Zhou G. Effect of sodium butyrate on intestinal inflammatory response to lipopolysaccharide in broiler chickens. Can. J. Anim. Sci. 2015;95:389–395. doi: 10.4141/cjas-2014-183. [DOI] [Google Scholar]
- 43.Sanchez NCB, Broadway PR, Carroll JA. Influence of yeast products on modulating metabolism and immunity in cattle and swine. Anim. 2021;11:371. doi: 10.3390/ani11020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett JM, Reeves G, Billman GE, Sturmberg JP. Inflammation–nature’s way to efficiently respond to all types of challenges: implications for understanding and managing “the epidemic” of chronic diseases. Front. med. 2018;5:316. doi: 10.3389/fmed.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novais AK, et al. Weaning differentially affects mitochondrial function, oxidative stress, inflammation and apoptosis in normal and low birth weight piglets. PLoS One. 2021;6:1. doi: 10.1371/journal.pone.0247188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodridge HS, Wolf AJ, Underhill DM. β-glucan recognition by the innate immune system. Immunol. Rev. 2009;230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu R, et al. Yeast hydrolysate attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage in weaned piglets. J. Anim. Sci. Biotechnol. 2023;14:1–15. doi: 10.1186/s40104-023-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rostagno, H. S. et al. Brazilian Tables for Poultry and Swine: composition of feedstuffs and nutritional requirements. Animal Science Department UFV (2017), Viçosa, MG, Brazil, 488.
- 49.Liu PPXS, et al. Chito-oligosaccharide reduces diarrhea incidence and attenuates the immune response of weaned pigs challenged with Escherichia coli K88. J. Anim. Sci. 2010;88:3871–3879. doi: 10.2527/jas.2009-2771. [DOI] [PubMed] [Google Scholar]
- 50.Yang KM, Jiang ZY, Zheng CT, Wang L, Yang XF. Effect of Lactobacillus plantarum on diarreia and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 2014;92:1496–1503. doi: 10.2527/jas.2013-6619. [DOI] [PubMed] [Google Scholar]
- 51.Júnior DTV, et al. Supplementation of Bacillus subtilis DSM 32540 improves performance and intestinal health of weaned pigs fed diets containing different fiber sources. Livest. Sci. 2023;270:105202. doi: 10.1016/j.livsci.2023.105202. [DOI] [Google Scholar]
- 52.Correia AM, Genova JL, Saraiva A, Rocha GC. Effects of crude protein and non-essential amino acids on growth performance, blood profile, and intestinal health of weaned piglets. Front. Vet. Sci. 2023;10:1. doi: 10.3389/fvets.2023.1243357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mandarim De Lacerda, C. A. Métodos quantitativos em morfologia. Rio de Janeiro(1995). Eduerj, 131.
- 54.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.