Abstract
Objectives
To determine the role of diffusion-weighted imaging (DWI) for predicting response to neoadjuvant therapy (NAT) in pancreatic cancer.
Materials and methods
MEDLINE, EMBASE, and Cochrane Library databases were searched for studies evaluating the performance of apparent diffusion coefficient (ADC) to assess response to NAT. Data extracted included ADC pre- and post-NAT, for predicting response as defined by imaging, histopathology, or clinical reference standards. ADC values were compared with standardized mean differences. Risk of bias was assessed using the Quality Assessment of Diagnostic Studies (QUADAS-2).
Results
Of 337 studies, 7 were included in the analysis (161 patients). ADC values reported for the pre- and post-NAT assessments overlapped between responders and non-responders. One study reported inability of ADC increase after NAT for distinguishing responders and non-responders. A correlation with histopathological response was reported for pre- and post-NAT ADC in 4 studies. DWI’s diagnostic performance was reported to be high in three studies, with a 91.6–100% sensitivity and 62.5–94.7% specificity. Finally, heterogeneity and high risk of bias were identified across studies, affecting the domains of patient selection, index test, reference standard, and flow and timing.
Conclusion
DWI might be useful for determining response to NAT in pancreatic cancer. However, there are still too few studies on this matter, which are also heterogeneous and at high risk for bias. Further studies with standardized procedures for data acquisition and accurate reference standards are needed.
Clinical relevance statement
Diffusion-weighted MRI might be useful for assessing response to neoadjuvant therapy in pancreatic cancer. However, further studies with robust data are needed to provide specific recommendations for clinical practice.
Key Points
•The role of DWI with ADC measurements for assessing response to neoadjuvant therapy in pancreatic cancer is still unclear.
•Pre- and post-neoadjuvant therapy ADC values overlap between responders and non-responders.
•DWI has a reported high diagnostic performance for determining response when using histopathological or clinical reference standards; however, studies are still few and at high risk for bias.
Keywords: Diffusion magnetic resonance imaging, Neoadjuvant therapy, Pancreatic carcinoma, Meta-analysis
Introduction
Pancreatic cancer is one of the most lethal cancers worldwide, with surgical resection as the only potentially curative therapeutic option [1, 2]. Nevertheless, most patients are not surgical candidates, many presenting with locally advanced disease. These patients can undergo neoadjuvant chemotherapy or chemoradiotherapy, attempting to downstage the disease and allowing a successful surgical resection [3]. Evaluating these patients, however, is a difficult task, as imaging studies do not accurately reflect response to neoadjuvant therapy (NAT) [4–7]. Traditional size criteria in cross-sectional imaging are known to be unreliable, as the primary pancreatic lesion may undergo minimal or no size reduction, due to remaining tumoral fibrotic stroma even when a response in the cancer cells has already occurred [8].
Diffusion-weighted MRI (DWI), quantified by apparent diffusion coefficient (ADC), is a cornerstone in the MRI evaluation of pancreatic cancer, especially for diagnostic purposes [9]. This technique probes microstructural characteristics of biological tissues, namely cell density and extracellular compartment’s composition, therefore being regarded as a useful biomarker for characterizing neoplastic lesions [10, 11]. However, its value in the assessment of response to NAT in pancreatic cancer is still undetermined, as studies have reported inconclusive and diverse results, both regarding the classification of patients as responders and non-responders and the correlation with histopathological response [12–16].
This study aims to determine the role of DWI in the evaluation of response to NAT in pancreatic cancer, by systematically reviewing the published literature and performing a meta-analysis of reported data.
Materials and methods
This study is presented according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [17]. The study protocol was registered in an international database prior to data search and collection — PROSPERO: CRD42022309467 [18]. Eligibility criteria were defined according to the following PICOs: (P) patients with pancreatic cancer undergoing neoadjuvant chemotherapy, radiotherapy, or chemoradiotherapy; (I) diffusion-weighted MRI (DWI) is the index test; (C) no comparison is to be performed; (O) outcome is assessed with an acceptable reference standard for determining response status to NAT — post-operative histopathology, clinical follow-up, imaging follow-up.
Search strategy
MEDLINE, EMBASE, and Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Clinical Answers) databases were used for the literature search, and the final search was undertaken on 17/01/2023. The search strategy was first defined by an abdominal radiologist with 4 years of experience (C.B.), and reviewed by two radiologists (L.A. and C.M., with 7 and 35 years of experience, respectively). The search query including title and abstract was the following: “pancreatic OR pancreas AND neoadjuvant OR chemotherapy OR radiotherapy OR chemoradiotherapy AND diffusion OR apparent diffusion coefficient OR ADC OR diffusion-weighted MRI OR DWI.” Only studies in the English literature were included; study publication dates were not restricted to a certain period.
The search results were first filtered for relevance by title and abstract review by two reviewers (C.B. and L.A.); the same reviewers then independently performed the full-text reviews of the resulting studies, determining if these fulfilled the criteria for inclusion and solving discrepancies in a consensus meeting. Finally, reference lists of the included studies were additionally searched for more relevant studies.
Inclusion criteria
Patients were included according to the following: diagnosis of pancreatic cancer; NAT was performed with chemo-, radio-, or chemoradiotherapy; DWI with retrievable ADC measurements was reported for tumor imaging before, after, or before and after NAT; a measure of response to NAT was provided, including histopathological response scores, clinical follow-up evaluation, correlation with RECIST criteria.
Exclusion criteria
If studies were found using overlapping groups of patients, one of the studies would be excluded to avoid duplication of included patients. Studies where a reference standard was not used to determine response to NAT were excluded. Studies including metastatic (stage IV) patients were excluded, in order to exclude patients undergoing palliative and not neoadjuvant therapy. As only human data was collected, studies performed in animals were excluded. Conference abstracts, letters, and comments were excluded from the analysis.
Risk of bias assessment
A QUADAS-2 assessment tool was used, based on the domains of patient selection, index test, reference standard, and flow and timing [19]. The tool was adapted to the current review by C.B. and L.A. in consensus, defining quality standards for each of the domains assessed, and then applied by both authors independently to the included studies. Discrepancies in evaluation between both readers were solved in a consensus meeting.
Data extraction
Two authors (C.B. and L.A.) independently extracted the following data: first author name, year of publication, journal name, type of study (retrospective, prospective), institution(s) and study dates, number of patients, patients’ age, resectability status, chemotherapy and/or radiotherapy details (protocols and timings), MR system field strength, DWI acquisition b values, DWI timing (before and/or after NAT), MRI readers’ experience, tumor location in the pancreas, tumor size pre- and post-NAT, ADC values pre- and post-NAT for responders and non-responders, histopathological analysis for NAT response grading, clinical follow-up data when used to determine response to NAT.
Whenever possible, ADC data was retrieved as a 2 × 2 table, as defined: true positives were cases where a positive index text was confirmed with the reference standard; true negatives were cases where a negative index test was confirmed with the reference standard; false positives were cases where a positive index test was not confirmed with the reference standard; false negatives were cases where a negative index test was not confirmed with the reference standard.
When available, Pearson’s correlation coefficient values between ADC and histological response grades were retrieved, including ADC measurement timing (pre- or post-NAT) and histological grading system (based either on Evans or College of American Pathologists classification systems) [20, 21].
When the published data for any specific study was considered insufficient or incomplete, an attempt to contact the study’s corresponding author by e-mail was conducted, and the new data added to the analysis, if then provided.
Data analysis
The collected data regarding pre-NAT ADC values in responders and non-responders were summarized in forest plots.
Standardized mean differences with random effects and Z test for overall effects were used for comparing ADC values in the pre-NAT period, between responders and non-responders.
Sources of heterogeneity between studies were recorded and qualitatively discussed [22].
Data extraction and analysis were performed using Microsoft Excel® and Review Manager (RevMan) 5.4. (The Cochrane Collaboration, 2020).
Results
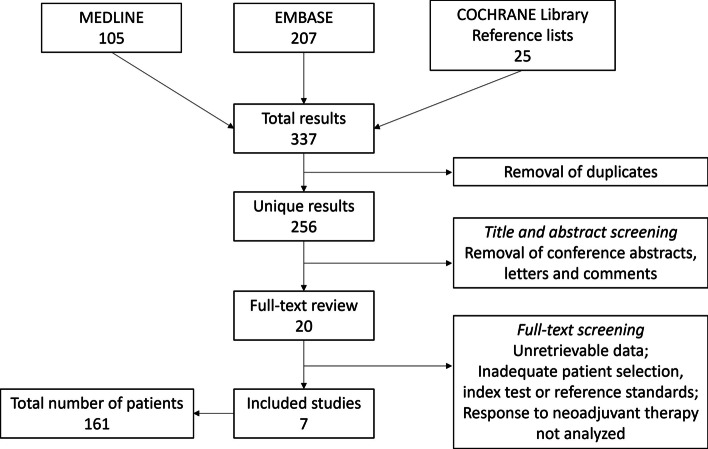
The initial search yielded 337 studies; after removal of duplicates, title and abstract screening, and full-text screening, 7 studies with 161 patients (6–41 patients per study) were finally included (Fig. 1) [12–14, 23–26]. One study’s corresponding author was contacted with a request for further information: however, a reply was not obtained.
Fig. 1.
Study flowchart
Studies’ and patients’ characteristics
The included studies’ characteristics are described in Table 1. Most studies were recent (6/7 from 2017 onwards; date range of publication: 2014–2022), and prospective (5/7 prospective). Five out of seven studies were performed on 3-T MRI systems, and most (6/7) used high b values for DWI (800 s/mm2 and higher). All studies used mono-exponential models for ADC calculations. The number and experience of readers were inconsistently reported. Most studies (5/7) performed DWI before and after NAT. For determining response to NAT, 4 studies used histopathological criteria (3 using a modified Evans classification, 1 using the College of American Pathologists classification), 2 studies used RECIST 1.1 criteria, and 1 study used clinical criteria (multidisciplinary assessment of surgical indication).
Table 1.
Studies characteristics
| Study | Journal | Year | Study design | Magnetic field | B values (s/mm2) | Number of readers | Readers experience | ROI drawing process | DWI timing | Reference standard |
|---|---|---|---|---|---|---|---|---|---|---|
| Cuneo KC, et al [23] | Transl Oncol | 2014 | Prospective | ND | 0, 100, 500, 800 | 2 | Radiologist + researcher (ND) | Manual delineation of tumor volume | Before NT | Pathological (Evans) |
| Dalah E, et al [24] | Transl Oncol | 2018 | Retrospective | 3 T | 0, 500, 1000 | ND | ND | Manual delineation of tumor volume | After NT | Pathological (CAP) |
| Hussien N, et al [12] | Front Oncol | 2022 | Prospective | 3 T | 0, 400, 800 | ND | ND | ND | Before and after NT | Clinical (multidisciplinary — surgical indication) |
| Kang JH, et al [14] | Eur Radiol | 2021 | Prospective | 3 T | 0, 30, 60, 120, 200, 400, 800 | 2 | Radiologists (8 + 10 years) | Average of two largest manual cross-sectional ROIs; intra- and inter-observer variability assessed | Before and after NT | RECIST 1.1 + tumor-vessel contact |
| Kinh Do R, et al [25] | Tomography | 2020 | Prospective | 3 T | 0, 500 | ND | Radiologists + radiation oncologists (ND) | Manual delineation of tumor ROIs | Before and after NT | RECIST 1.1 |
| Okada KI, et al [26] | J Hepatobiliary Pancreat Sci | 2017 | Retrospective | 1.5 T | 0, 50, 800 | 2 | Radiologists (2) | Manual ROI: largest cross-sectional area based on CT | Before and after NT | Pathological (Evans) |
| Okada KI, et al [13] | Langenbecks Arch Surg | 2020 | Prospective | 3 T | 0, 50, 1000 | 1 | Radiologist (1) | Manual ROIs: 3–4 largest cross-sectional areas based on CT | Before and after NT | Pathological (Evans and CAP) |
ND, non-disclosed; NT, neoadjuvant therapy; NM, nuclear medicine consultant; CAP, College of American Pathologists; CT, computerized tomography; ROI, region of interest
Included patients’ characteristics are summarized in Table 2. The number of patients included in each study ranged from 6 to 41, and the patients’ mean age ranged from 52.5 to 69 years. Most studies (5/7) included borderline resectable patients; 2/7 included locally advanced patients. Six studies described tumor locations, most often in the head of the pancreas (5/6). Mean tumor size pre-NAT ranged from 27.8 to 51.5 mm, and post-NAT ranged from 22.3 to 55.5 mm. Chemotherapy regimens varied, gemcitabine being included in most studies (6/7); 4 studies included radiotherapy.
Table 2.
Patient, disease, and treatment data collected
| Study | Number of patients | Age (years) | Initial staging | Resectability criteria | Tumor size (pre-NT) | Tumor size (post-NT) | Tumor location | Resection surgery | Chemotherapy | Radiotherapy |
|---|---|---|---|---|---|---|---|---|---|---|
| Cuneo KC, et al [23] | 10 (7*) | 60.7 | Resectable | ND | ND | ND | ND | All patients | Gemcitabine + oxaliplatin | 30 Gy in 2 fractions (method ND) |
| Dalah E, et al [24] | 25 | ND | Resectable and borderline | ND | 37.79 ± 12.1 cc | 9.75 ± 5.93 cc | 100% head | All patients | Gemcitabine or Capecitabine + FOLFIRINOX or gemcitabine-Abraxane | 50.4 Gy in 28 fractions, IMRT |
| Hussien N, et al [12] | 30 | 52.5 | Borderline | NCCN (v2.2012) | ND | ND | 23.3% head 76.7% body/tail | 11 patients | 63.3% FOLFIRINOX 36.7% gemcitabine/cisplatin | No |
| Kang JH, et al [14] | 41 | 60.3 | Borderline and locally advanced | NCCN (v2.2017) | 36 ± 8 | ND | 65.9% head 34.1% body/tail | 25 patients | FOLFIRINOX | No |
| Kinh Do R, et al [25] | 10 (6*) | 64 | Locally advanced | ND | 51.5 ± 24.3 | R: 42 ± 2.8 NR: 55.5 ± 26 | 50% head 50% body/tail | None | 80% FOLFIRINOX 10% GEM/NAB 10% FOLFIRINOX + GEM/NAB | 27–33 Gy in 3 fractions, SBRT |
| Okada KI, et al (2017) [26] | 24 | 67 | Borderline | NCCN (v2.2015) | 28.9 ± 12.5 | 26.9 ± 12.2 | 54.2% head 45.8% body/tail | All patients | S-1 + gemcitabine or FIRINOX or GEM/NAB or S-1 + radiation | 50 Gy, EBRT (4 patients) |
| Okada KI, et al (2020) [13] | 28 | 69 | Borderline | NCCN (v2.2015) | 27.8 ± 9.36 | 22.3 ± 9.8 | 68% head 32% body/tail | All patients | GEM/NAB | No |
*Number of patients eligible for response assessment. If not otherwise mentioned, tumor size is expressed in mm. ND, non-disclosed; R, responders; NR, non-responders; pre-NT, pre-neoadjuvant therapy; post-NT, post-neoadjuvant therapy; NCCN, National Comprehensive Cancer Network
DWI for response assessment
Table 3 summarizes ADC measurements and their performance in assessing response to NAT. ADC values overlapped for responders and non-responders between studies: in the pre-NAT period, these ranged from 1.0 to 1.61 × 10−3 mm2/s for responders, and from 1.25 to 1.5 × 10−3 mm2/s for non-responders. In the post-NAT period, only one included study reported ADC values: 1.4 × 10−3 mm2/s for responders, and 1.3 × 10−3 mm2/s for non-responders. One study reported ADC increase from the pre- to the post-NAT period: 14.9% for responders and 10.3% for non-responders.
Table 3.
ADC measurements and response assessment
| Study | R: pre-ADC | NR: pre-ADC | R: post-ADC | NR: post-ADC | R: ADC increase | NR: ADC increase | Response assessment | Diagnostic performance | |
|---|---|---|---|---|---|---|---|---|---|
| Criteria | Sensitivity/specificity | ||||||||
| Cuneo KC, et al [23] | 1.61 ± 0.05 | 1.25 ± 0.16 | ND | ND | ND | ND | Pre-ADC correlates with pathological response grade | NA | NA |
| Dalah E, et al [24] | ND | ND | ND | ND | ND | ND | Post-ADC correlates with pathological response | NA | NA |
| Hussien N, et al [12] | 1.0 (1.0–1.3) | 1.4 (1.0–1.4) | 1.4 (1.3–1.7) | 1.3 (1.0–1.7) | ND | ND | ADC increase in responders | Stationary vs. regressive ADC | 100%/94.7% |
| Kang JH, et al [14] | 1.46 ± 0.23 | 1.40 ± 0.19 | ND | ND | 14.9 ± 13.8% | 10.3 ± 27.6% | No significant differences in ADC | NA | NA |
| Kinh Do R, et al [25] | 1.60 ± 0.17 | 1.50 ± 0.05 | ND | ND | ND | ND | No significant differences in ADC | NA | NA |
| Okada KI, et al (2017) [26] | ND | ND | ND | ND | ND | ND | Pre- and post-ADC correlate with pathological response grade | Pre-ADC (≥ 1.2) | 100%/75% |
| Okada KI, et al (2020) [13] | ND | ND | ND | ND | ND | ND | Pre-ADC, post-ADC, and ADC increase correlate with pathological response grade | Pre-ADC (≥ 1.2) | 91.6%/62.5% |
| Post-ADC (≥ 1.4) | 100%/81% | ||||||||
R, responders; NR, non-responders; pre-ADC, ADC value pre-neoadjuvant therapy; post-ADC, ADC value post-neoadjuvant therapy; ND, non-disclosed; NA, not assessed. ADC values (× 10−3 mm2/s) are expressed as means ± standard deviations or medians and ranges (in parentheses)
Two studies, both using RECIST 1.1 criteria for assessing response status, reported no significant differences in ADC values for differentiating responders from non-responders [14, 25]. All other studies (5/7) reported ADC being able to determine response status, using histopathological or clinical criteria as reference standard. The 4 studies evaluating histopathological response (Table 4) reported a correlation between both pre- and post-NAT ADC values and response grade, which was positive when using the modified Evan’s classification and negative when using the College of American Pathologists’ classification [13, 23, 24, 26].
Table 4.
Correlation coefficients of studies using histopathological criteria to determine response to neoadjuvant therapy
| Study | Classification | DWI timing | Correlation coefficient |
|---|---|---|---|
| Cuneo KC, et al [23] | Evans* | Pre | 0.94* (p = 0.001) |
| Dalah E, et al [24] | College of American Pathologists | Post | − 0.52 (p < 0.05) |
| Okada KI, et al (2017) [26] | Evans* | Pre | 0.63 (p = 0.001) |
| Post | 0.41 (p = 0.144) | ||
| Okada KI, et al (2020) [13] | Evans* | Pre | 0.63 (p < 0.001) |
| Post | 0.71 (p < 0.001) | ||
| Pre/Post Difference | 0.29 (p = 0.138) |
*Modified version of the Evans classification [20]. Pre, pre-neoadjuvant therapy; Post, post-neoadjuvant therapy
Three studies reported DWI’s diagnostic performance, with sensitivity values ranging from 91.6 to 100% and specificity values from 62.5 to 94.7% [12, 13, 26]. Noteworthy, these studies used different criteria for determining response status: stationary vs. regressive ADC; pre-NAT ADC ≥ 1.2 × 10−3 mm2/s; post-NAT ADC ≥ 1.4 × 10−3 mm2/s.
Synthesis of collected data
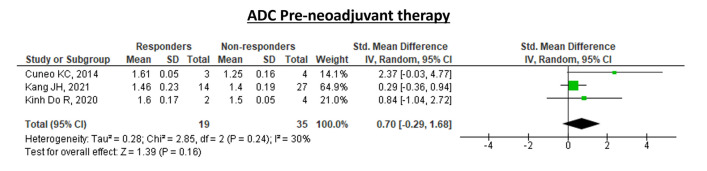
Figure 2 summarizes the pooled analysis for pre-NAT ADC values reported in responders and non-responders. When testing for overall effects, ADC values were not significantly different between groups of patients (Z = 1.39, p = 0.16, I2 = 30%).
Fig. 2.
Pooled analysis results of ADC values pre-neoadjuvant therapy. Test for overall effects was not statistically significant, with Z = 1.39 (p = 0.16)
Studies were considered too few and heterogeneous to perform a pooled analysis regarding post-NAT ADC values (1 study), ADC variance from pre- to post-NAT (1 study), and diagnostic performance with summary ROC statistics (3 studies with high heterogeneity).
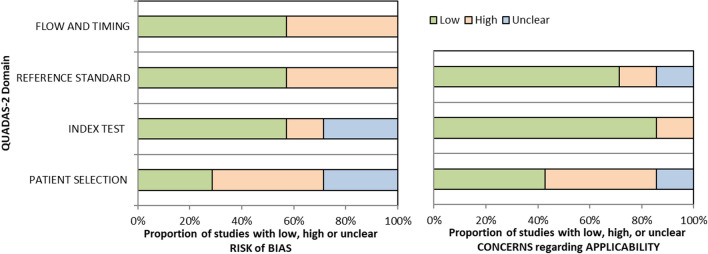
Risk of bias and heterogeneity
Figure 3 illustrates the QUADAS-2 evaluation results, regarding risk of bias and applicability concerns. High risk of bias and applicability concerns were identified across studies, especially for patient selection, but also for the other domains. The main causes for concern regarding patient selection were the exclusion of borderline or locally advanced stages, retrospective studies, non-disclosure of resectability criteria, and patients excluded due to missing or inadequate index test. For the reference standard, the dependance on RECIST criteria, interpretation of the reference standard with knowledge of the index test (DWI), and the use of clinical multidisciplinary evaluation were considered the major causes of concern. Regarding the index test, the major sources of concern stemmed from reader’s number and experience not being disclosed, region of interest (ROI) drawing process not being disclosed or vaguely described, the use of poorly defined diagnostic criteria, and the use of highest b values lower than the usual standards (500 s/mm2). Finally, in the flow and timing domain, concerns were expressed due to widely variable MRI timings both before and after NAT.
Fig. 3.
QUADAS-2 chart, representing the estimated risk for bias and applicability concerns for all studies included
Heterogeneity was also analyzed in a qualitative manner across studies. Regarding the reference standard, the use of RECIST 1.1 criteria was considered a probable source of heterogeneity, as the two studies using it did not report ADC to be of diagnostic value while all others did, when using either clinical or histopathological criteria. For the studies using histopathological response assessment, the correlation coefficients with ADC values were positive when using the modified Evans classification and negative when using the College of American Pathologists classification [20, 21]. Since these classifications’ gradings are the inverse of each other — higher tumor cell destruction translates into higher degrees in the Evans but lower degrees in the College of American Pathologists classifications — the results between studies can be considered concordant, as higher pre- and post-NAT ADC values correlate with higher tumor cell destruction. Another identified source of heterogeneity directly influencing ADC values obtained in each study was the variability in b values used for ADC calculation. Although 1/7 studies used low b values for ADC calculation, also used for IVIM (intravascular incoherent motion) assessment, this study also used a mono-exponential calculation method for ADC. Also, 1/7 studies used highest b values lower than the usual standards (500 s/mm2), while all other studies used b values of 800–1000 s/mm2.
Discussion
This systematic review determines that DWI with ADC values for the assessment of response to NAT in pancreatic cancer may be useful, with reported high diagnostic performance. However, the included number of studies was small and high risk for bias was identified across studies, prompting a cautious interpretation of these results.
As ADC values observed across studies overlapped for responders and non-responders and no overall effects were seen in the pre-NAT period, an evaluation of absolute ADC values for this purpose appears to not be useful. The reproducibility of ADC values in the pancreas has been shown to be imperfect, and some variability should be expected across individuals, MRI scanners, and even anatomical regions of the pancreas [27–31]. Despite this, a correlation between ADC values and histopathological response was reported in 4 studies, both for the pre- and post-NAT assessments; therefore, high ADC values may still have a role in predicting response to NAT, but more studies providing further characterization in this regard are needed.
Technical issues affecting the reproducibility of ADC values were an important concern in this study, with values varying widely between studies, for both responders and non-responders. For future studies, the use of standardized protocols for image acquisition with motion-robust techniques, post-processing and corrective methods should be effective for diminishing these differences and improve ADC values’ reproducibility [32–37]. Also, the process of ROI placement for ADC measurement, which was inconsistently reported in studies in this review, is known to have inherent variability [38, 39]. This is another aspect that could be improved in further studies, exploring the variability of ADC measurements derived from the measurement process itself, and its effect on the magnitude of ADC differences between groups of responders and non-responders.
The diagnostic performance of DWI for determining response to NAT was high in all three included studies, which is encouraging for implementing its use in clinical practice. Nevertheless, studies used both different DWI-based metrics (1/3 used an ADC increase measure; 2/3 used cutoff ADC values) and different reference standards for this purpose (1/3 clinical; 2/3 histopathological). Therefore, the most adequate methodology when using DWI to differentiate responders from non-responders remains undetermined.
Response assessment after NAT in pancreatic cancer remains a challenge in clinical practice, with most centers relying on both analytical (serum CA19-9 concentration) and imaging data to assess response, the latter based on traditional size criteria: increase in tumor size being suggestive of progressive disease, stability or reduction in tumor size being considered a response and warranting surgical exploration [4, 40, 41]. This approach, however, may lead to unnecessary surgery being performed in cases of persistent advanced disease, or potentially resectable patients being excluded from surgery. Novel biomarkers have therefore been sought, with DWI emerging as a potential imaging candidate, but with inconclusive/insufficient results thus far [42–44]. Our analysis helps establishing DWI with ADC measurement as useful biomarker for assessing response, but current data is still not enough to recommend its implementation in clinical practice.
This systematic review and meta-analysis is limited by the small number of studies, which precluded subgroup analysis and statistical exploration of heterogeneity factors. Studies were also considered heterogeneous and at high risk for bias. We identified similar important sources for both heterogeneity and risk for bias, the most obvious being the variability in resectability status of patients included in each study, NAT regimens, DWI b values, readers’ experience, and reference standards (RECIST, clinical and histopathological). In an ideal world, we would expect studies to replicate the best clinical practice and control for interfering variables — include all patients undergoing NAT, use only standardized NAT regimens and DWI acquisition parameters with experienced readers, and use the most accurate reference standards. However, these studies provide real-world data, reflecting the difficulties in managing patients with pancreatic cancer, with imperfect staging systems after NAT, and the evolution of NAT regimens and treatment recommendations in recent years [45, 46].
In conclusion, this systematic review and meta-analysis helps establish DWI as a useful biomarker for determining response to NAT in pancreatic cancer. However, few studies were included and were considered heterogeneous and at high risk for bias. Further data, best provided by studies with standardized procedures for data acquisition and accurate reference standards, are needed.
Acknowledgements
The authors would like to thank the collaboration of Prof. Tiago Bilhim (Interventional Radiology Unit, Centro Hospitalar Universitário de Lisboa Central, Portugal) in the initial elaboration of the study’s protocol, and the collaboration of Prof. Thierry Metens (Université Libre de Bruxelles, Brussels) in the final manuscript review with insightful discussions.
Abbreviations
- ADC
Apparent diffusion coefficient
- DWI
Diffusion-weighted imaging
- MRI
Magnetic resonance imaging
- NAT
Neoadjuvant therapy
- NCCN
National Comprehensive Cancer Network
- ROC
Receiver operating characteristics
- ROI
Region of interest
Funding
Open access funding provided by FCT|FCCN (b-on). The authors state that this work has not received any funding.
Declarations
Guarantor
The scientific guarantor of this publication is Dr. Celso Matos.
Conflict of interest
Carlos Bilreiro is a member of European Radiology’s Editorial Board – Oncology section. They have not taken part in the review or selection process.
The other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was not required for this study because it is a systematic review and meta-analysis.
Ethical approval
Institutional Review Board approval was not required because this study is a systematic review and meta-analysis.
Study subjects or cohorts overlap
Since this study is a systematic review and meta-analysis, all data was collected from previously published studies.
Methodology
•diagnostic or prognostic study
•multicentre study
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/CAAC.21708. [DOI] [PubMed] [Google Scholar]
- 2.Ducreux M, Cuhna AS, Caramella C et al (2015) Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(Suppl 5):v56–v68. 10.1093/ANNONC/MDV295 [DOI] [PubMed]
- 3.Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J (2010) Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 7(4):e1000267. 10.1371/JOURNAL.PMED.1000267 [DOI] [PMC free article] [PubMed]
- 4.Zins M, Matos C, Cassinotto C. Pancreatic adenocarcinoma staging in the era of preoperative chemotherapy and radiation therapy. Radiology. 2018;287:374–390. doi: 10.1148/RADIOL.2018171670. [DOI] [PubMed] [Google Scholar]
- 5.Cassinotto C, Cortade J, Belleannée G, et al. An evaluation of the accuracy of CT when determining resectability of pancreatic head adenocarcinoma after neoadjuvant treatment. Eur J Radiol. 2013;82:589–593. doi: 10.1016/J.EJRAD.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Katz MHG, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749–5756. doi: 10.1002/CNCR.27636. [DOI] [PubMed] [Google Scholar]
- 7.Wagner M, Antunes C, Pietrasz D, et al. CT evaluation after neoadjuvant FOLFIRINOX chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur Radiol. 2017;27:3104–3116. doi: 10.1007/S00330-016-4632-8. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda Y, Inoue Y, Hiratsuka M et al (2019) Encapsulating fibrosis following neoadjuvant chemotherapy is correlated with outcomes in patients with pancreatic cancer. PLoS One 14(9):e0222155. 10.1371/JOURNAL.PONE.0222155 [DOI] [PMC free article] [PubMed]
- 9.Barral M, Taouli B, Guiu B, et al. Diffusion-weighted MR imaging of the pancreas: current status and recommendations. Radiology. 2015;274:45–63. doi: 10.1148/radiol.14130778. [DOI] [PubMed] [Google Scholar]
- 10.Qayyum A (2009) Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics 29:1797–1810. 10.1148/RG.296095521 [DOI] [PMC free article] [PubMed]
- 11.Morone M, Bali MA, Tunariu N et al (2017) Whole-body MRI: current applications in oncology. AJR Am J Roentgenol 209:W336–W349. 10.2214/AJR.17.17984 [DOI] [PubMed]
- 12.Hussien N, Hussien RS, Saad DHA, El Kassas M, Elkhatib WF, Ezz El Din M (2022) The role of MRI pancreatic protocol in assessing response to neoadjuvant therapy for patients with borderline resectable pancreatic cancer. Front Oncol 11:796317. 10.3389/FONC.2021.796317 [DOI] [PMC free article] [PubMed]
- 13.Okada Kichi, Kawai M, Hirono S et al (2020) Diffusion-weighted MRI predicts the histologic response for neoadjuvant therapy in patients with pancreatic cancer: a prospective study (DIFFERENT trial). Langenbecks Arch Surg 405:23–33. 10.1007/S00423-020-01857-4 [DOI] [PubMed]
- 14.Kang JH, Lee SS, Kim JH, et al. Multiparametric MRI for prediction of treatment response to neoadjuvant FOLFIRINOX therapy in borderline resectable or locally advanced pancreatic cancer. Eur Radiol. 2021;31:864–874. doi: 10.1007/S00330-020-07134-8. [DOI] [PubMed] [Google Scholar]
- 15.Bali MA, Pullini S, Metens T, et al. Assessment of response to chemotherapy in pancreatic ductal adenocarcinoma: comparison between diffusion-weighted MR quantitative parameters and RECIST. Eur J Radiol. 2018;104:49–57. doi: 10.1016/J.EJRAD.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Harder FN, Jungmann F, Kaissis GA et al (2021) [18F]FDG PET/MRI enables early chemotherapy response prediction in pancreatic ductal adenocarcinoma. EJNMMI Res 11(1):70. 10.1186/S13550-021-00808-4 [DOI] [PMC free article] [PubMed]
- 17.Page MJ, Moher D, Bossuyt PM et al (2021) PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372:n160. 10.1136/BMJ.N160 [DOI] [PMC free article] [PubMed]
- 18.Bilreiro C, Andrade L, Bilhim T, Matos C (2022) Diffusion-weighted MRI for assessing response to neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis. PROSPERO CRD42022309467. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022309467 [DOI] [PMC free article] [PubMed]
- 19.Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 20.Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–1339. doi: 10.1001/ARCHSURG.1992.01420110083017. [DOI] [PubMed] [Google Scholar]
- 21.Kakar S, Shi C, Adsay V et al (2017) Protocol for the examination of specimens from patients with carcinoma of the pancreas, version pancreas exocrine 4.0.0.1. College of American Pathologists. Available at: https://documents.cap.org/protocols/cp-pancreas-exocrine-17protocol-4001.pdf
- 22.Deeks JJ, Bossuyt PM, Leeflang MM, Takwoingi Y (eds) (2023) Cochrane handbook for systematic reviews of diagnostic test accuracy. Version 2.0 (updated July 2023). Cochrane. Available from https://training.cochrane.org/handbook-diagnostic-test-accuracy/current [DOI] [PMC free article] [PubMed]
- 23.Cuneo KC, Chenevert TL, Ben-Josef E, et al. A pilot study of diffusion-weighted MRI in patients undergoing neoadjuvant chemoradiation for pancreatic cancer. Transl Oncol. 2014;7:644–649. doi: 10.1016/J.TRANON.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalah E, Erickson B, Oshima K, et al. Correlation of ADC with pathological treatment response for radiation therapy of pancreatic cancer. Transl Oncol. 2018;11:391–398. doi: 10.1016/J.TRANON.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Do RK, Reyngold M, Paudyal R et al (2020) Diffusion-weighted and dynamic contrast-enhanced MRI derived imaging metrics for stereotactic body radiotherapy of pancreatic ductal adenocarcinoma: preliminary findings. Tomography 6:261–271. 10.18383/J.TOM.2020.00015 [DOI] [PMC free article] [PubMed]
- 26.Okada KI, Hirono S, Kawai M, et al. Value of apparent diffusion coefficient prior to neoadjuvant therapy is a predictor of histologic response in patients with borderline resectable pancreatic carcinoma. J Hepatobiliary Pancreat Sci. 2017;24:161–168. doi: 10.1002/JHBP.430. [DOI] [PubMed] [Google Scholar]
- 27.Malyarenko D, Galbán CJ, Londy FJ, et al. Multi-system repeatability and reproducibility of apparent diffusion coefficient measurement using an ice-water phantom. J Magn Reson Imaging. 2013;37:1238–1246. doi: 10.1002/JMRI.23825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donati OF, Chong D, Nanz D, et al. Diffusion-weighted MR imaging of upper abdominal organs: field strength and intervendor variability of apparent diffusion coefficients. Radiology. 2014;270:454–463. doi: 10.1148/RADIOL.13130819. [DOI] [PubMed] [Google Scholar]
- 29.Ye XH, Gao JY, Yang ZH, Liu Y. Apparent diffusion coefficient reproducibility of the pancreas measured at different MR scanners using diffusion-weighted imaging. J Magn Reson Imaging. 2014;40:1375–1381. doi: 10.1002/JMRI.24492. [DOI] [PubMed] [Google Scholar]
- 30.Rosenkrantz AB, Oei M, Babb JS, et al. Diffusion-weighted imaging of the abdomen at 3.0 tesla: image quality and apparent diffusion coefficient reproducibility compared with 1.5 tesla. J Magn Reson Imaging. 2011;33:128–135. doi: 10.1002/jmri.22395. [DOI] [PubMed] [Google Scholar]
- 31.Schoennagel BP, Habermann CR, Roesch M, et al. Diffusion-weighted imaging of the healthy pancreas: apparent diffusion coefficient values of the normal head, body, and tail calculated from different sets of b-values. J Magn Reson Imaging. 2011;34:861–865. doi: 10.1002/JMRI.22743. [DOI] [PubMed] [Google Scholar]
- 32.McTavish S, Van AT, Peeters JM et al (2022) Gradient nonlinearity correction in liver DWI using motion-compensated diffusion encoding waveforms. MAGMA 35:827–841. 10.1007/S10334-021-00981-6/FIGURES/8 [DOI] [PMC free article] [PubMed]
- 33.Hu L, Zhou DW, Fu CX, et al. Calculation of apparent diffusion coefficients in prostate cancer using deep learning algorithms: a pilot study. Front Oncol. 2021;11:697721. doi: 10.3389/FONC.2021.697721/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sedlaczek OL, Kleesiek J, Gallagher FA, et al. Quantification and reduction of cross-vendor variation in multicenter DWI MR imaging: results of the Cancer Core Europe imaging task force. Eur Radiol. 2022;32:8617–8628. doi: 10.1007/S00330-022-08880-7/FIGURES/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barral M, Taouli B, Guiu B et al (2014) Diffusion-weighted MR imaging of the pancreas: current status and recommendations. Radiology 274:45–63. 10.1148/RADIOL.14130778 [DOI] [PubMed]
- 36.Virostko J, Craddock RC, Williams JM et al (2021) Development of a standardized MRI protocol for pancreas assessment in humans. PLoS One 16(8):e0256029. 10.1371/JOURNAL.PONE.0256029 [DOI] [PMC free article] [PubMed]
- 37.Geng R, Zhang Y, Starekova J, et al. Characterization and correction of cardiovascular motion artifacts in diffusion-weighted imaging of the pancreas. Magn Reson Med. 2021;86:1956–1969. doi: 10.1002/MRM.28846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma C, Li J, Boukar MB, et al. Optimized ROI size on ADC measurements of normal pancreas, pancreatic cancer and mass-forming chronic pancreatitis. Oncotarget. 2017;8:99085–99092. doi: 10.18632/ONCOTARGET.18457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma C, Guo X, Liu L, et al. Effect of region of interest size on ADC measurements in pancreatic adenocarcinoma. Cancer Imaging. 2017;17:1–7. doi: 10.1186/S40644-017-0116-6/TABLES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cassinotto C, Mouries A, Lafourcade JP, et al. Locally advanced pancreatic adenocarcinoma: reassessment of response with CT after neoadjuvant chemotherapy and radiation therapy. Radiology. 2014;273:108–116. doi: 10.1148/RADIOL.14132914. [DOI] [PubMed] [Google Scholar]
- 41.Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16:11–26. doi: 10.1038/S41571-018-0112-1. [DOI] [PubMed] [Google Scholar]
- 42.Zaharia C, Søreide K. Call for better response evaluation after neoadjuvant therapy in pancreatic cancer. Br J Surg. 2023;110:294–296. doi: 10.1093/BJS/ZNAC452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J, Zhan H, Li F, Hu S, Wang L (2021) Neoadjuvant therapy for pancreatic cancer: limitations and advances of response assessment (Review). Oncol Rep 45(4):26. 10.3892/OR.2021.7977 [DOI] [PubMed]
- 44.Soloff EV, Al-Hawary MM, Desser TS, et al. Imaging assessment of pancreatic cancer resectability after neoadjuvant therapy: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:570–581. doi: 10.2214/AJR.21.26931. [DOI] [PubMed] [Google Scholar]
- 45.Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18:2–11. doi: 10.1016/J.PAN.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:439–457. doi: 10.6004/JNCCN.2021.0017. [DOI] [PubMed] [Google Scholar]