Abstract
Background and Objective
Gene therapies for sickle cell disease (SCD) may offer meaningful benefits for patients and society. This study evaluated the cost-effectiveness of lovotibeglogene autotemcel (lovo-cel), a one-time gene therapy administered via autologous hematopoietic stem cell transplantation, compared with common care for patients in the United States (US) with SCD aged ≥ 12 years with ≥ 4 vaso-occlusive events (VOEs) in the past 24 months.
Methods
We developed a patient-level simulation model accounting for lovo-cel and SCD-related events, complications, and mortality over a lifetime time horizon. The pivotal phase 1/2 HGB-206 clinical trial (NCT02140554) served as the basis for lovo-cel efficacy and safety. Cost, quality-of-life, and other clinical data were sourced from HGB-206 data and the literature. Analyses were conducted from US societal and third-party payer perspectives. Uncertainty was assessed through probabilistic sensitivity analysis and extensive scenario analyses.
Results
Patients treated with lovo-cel were predicted to survive 23.84 years longer on average (standard deviation [SD], 12.80) versus common care (life expectancy, 62.24 versus 38.40 years), with associated discounted patient quality-adjusted life-year (QALY) gains of 10.20 (SD, 4.10) and direct costs avoided of $1,329,201 (SD, $1,346,446) per patient. Predicted societal benefits included discounted caregiver QALY losses avoided of 1.19 (SD, 1.38) and indirect costs avoided of $540,416 (SD, $262,353) per patient. Including lovo-cel costs ($3,282,009 [SD, $29,690] per patient) resulted in incremental cost-effectiveness ratios of $191,519 and $124,051 per QALY gained from third-party payer and societal perspectives, respectively. In scenario analyses, the predicted cost-effectiveness of lovo-cel also was sensitive to baseline age and VOE frequency and to the proportion of patients achieving and maintaining complete resolution of VOEs.
Conclusions
Our analysis of lovo-cel gene therapy compared with common care for patients in the US with SCD with recurrent VOEs estimated meaningful improvements in survival, quality of life, and other clinical outcomes accompanied by increased overall costs for the health care system and for broader society. The predicted economic value of lovo-cel gene therapy was influenced by uncertainty in long-term clinical effects and by positive spillover effects on patient productivity and caregiver burden.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-024-01385-9.
Key Points for Decision Makers
| One-time gene therapies for sickle cell disease have the potential to deliver meaningful clinical and economic benefits for patients and society. We developed a lifetime economic model to predict the cost-effectiveness, or value for money, of lovo-cel gene therapy for patients with sickle cell disease and recurrent vaso-occlusive events in the United States using evidence from the pivotal HGB-206 clinical trial (NCT02140554) and the published literature. |
| Our analysis predicted meaningful improvements in survival, quality of life, and other clinical outcomes for lovo-cel gene therapy compared with common care. The costs associated with lovo-cel were partially offset by predicted reductions in other health system and societal costs. |
| Our findings highlight the contribution of positive spillover effects, such as patient productivity gains and reduced caregiver burden, to the potential economic value of lovo-cel gene therapy in the United States. The cost-effectiveness of lovo-cel was also found to be sensitive to patients’ baseline age and severity levels and to uncertainty in long-term effects. |
Introduction
Sickle cell disease (SCD) refers to a heterogeneous group of genetic abnormalities affecting hemoglobin, the oxygen-carrying protein in red blood cells (RBCs) [1]. The sickled RBCs characteristic of SCD are more rigid and adherent and have a shorter lifespan than normal RBCs, which impairs blood flow and oxygen delivery to the body and results in vessel obstruction and anemia [1, 2]. Sickle cell disease is characterized by frequent and debilitating vaso-occlusive events (VOEs) (e.g., pain crises), stroke, and multisystem organ damage and organ infarction [2], leading to a lifespan cut short by several decades [3, 4]. These events and complications result in broad impacts to quality of life, social functioning, and attainment of personal, educational, and financial goals [5, 6]. Life expectancy for the approximately 100,000 individuals living with SCD in the United States (US) today is estimated at 52.6 years [7, 8], although for those with hemoglobin SS (HbSS) or Sβ0-thalassemia (HbSβ0) genotypes, disease severity is much greater and life expectancy estimates are closer to 40 years [8, 9].
Although the adoption of clinical practice guidelines for SCD newborn screening, management, and treatment in recent decades has shifted the burden of disease and risk of early death from childhood into adulthood [10, 11], significant unmet need remains. For most individuals with SCD today, disease management involves lifelong use of acute and chronic therapies, including opioid and nonopioid pain management, hydration therapy, RBC transfusion, hydroxyurea (HU), and other newer disease-modifying therapies (l-glutamine, crizanlizumab, and voxelotor) [9, 12]. For the limited group of individuals with a suitable human leukocyte antigen (HLA)–matched sibling donor [13], allogeneic hematopoietic stem cell transplantation (HSCT) offers a potential cure [14].
Lovotibeglogene autotemcel (Lyfgenia™, henceforth “lovo-cel”) is a one-time gene therapy approved by the US Food and Drug Administration (FDA) for the treatment of patients 12 years of age or older with SCD and a history of VOEs [15]. Lovo-cel uses autologous transplantation of hematopoietic stem cells (HSC) transduced with a BB305 lentiviral vector encoding a modified β-globin gene, which produces an anti-sickling hemoglobin (Hb), HbAT87Q. Patients’ HSCs are mobilized and collected via apheresis, transduced in the laboratory with the gene to produce HbAT87Q, and, after myeloablative conditioning, infused back to the patient. Lovo-cel has demonstrated sustained efficacy—as well as a safety profile consistent with autologous HSCT requiring myeloablative conditioning—across a clinical trial program that includes the completed phase 1/2 HGB-205 (NCT02151526) and phase 1/2 HGB-206 (NCT02140554) studies and the ongoing LTF-307 (NCT04628585) and phase 3 HGB-210 (NCT04293185) studies [16]. In the pivotal phase 1/2 HGB-206 primary efficacy analysis, which has follow-up data up to 61 months after transplantation, 87.5% of individuals achieved complete resolution (i.e., 100% reduction) of VOEs (VOE-CR) during months 6 through 18 after transplantation [15]. On secondary endpoints, sustained total Hb levels and HbAT87Q anti-sickling fractions were observed [17]. Because lovo-cel uses autologous HSCT and does not require an HLA-matched donor, its approval in the US could result in expanded access to potentially curative treatments for SCD.
Cell and gene therapies, such as lovo-cel, constitute both an increasing percentage of the total drug development pipeline as well as an increasing share of new drug approvals [18, 19]. These technologies hold great promise for patients, their care networks, and broader society, but they are also prompting important discussions on value, access, and affordability [20, 21]. Economic evaluation is a tool for priority setting of healthcare expenditures and is increasingly employed in the US setting to understand the value of novel therapeutics and to inform coverage and reimbursement decisions. This context has shaped a number of recent economic evaluations of the gene therapies for SCD in the US [22–29]. The aim of this study was to leverage the latest available HGB-206 data from February 2023 in a cost-effectiveness analysis of lovo-cel in comparison with common care for patients with SCD with recurrent VOEs.
Methods
We designed, conducted, and validated our cost-effectiveness analysis in alignment with good modeling practice guidelines and recommendations [30–32]. The scope and design of the model were informed by a structured review of the clinical and economic literature in SCD and refined through a collaborative process with patient and expert clinical perspectives, including an individual with SCD and hematologists with experience treating and managing SCD in pediatric and adult populations. We also convened a panel of independent clinical and economic advisors to solicit feedback on the validity of our approach.
Modeling Approach
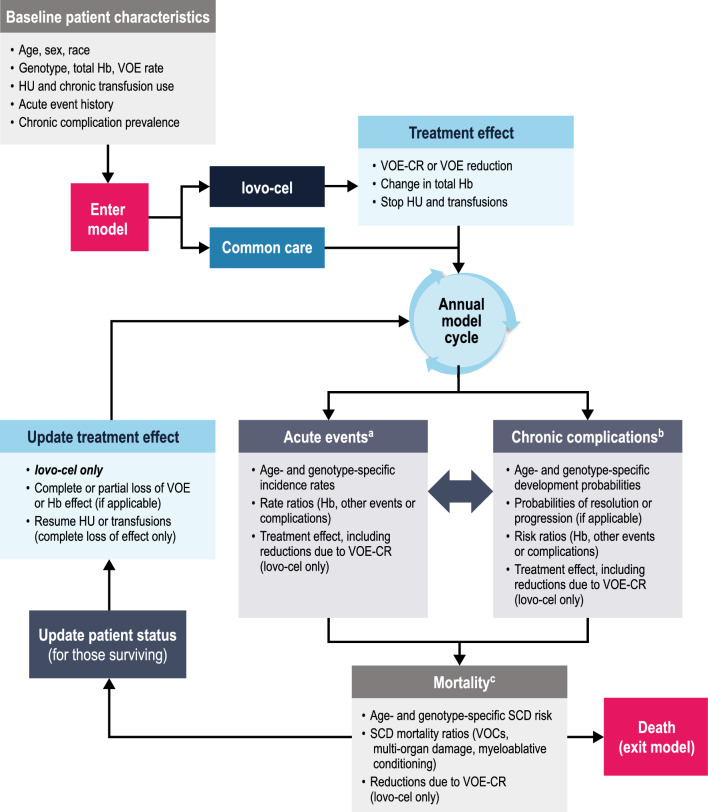
We developed a patient-level simulation modeling approach to predict the lifetime health and economic outcomes of patients with SCD treated with lovo-cel in comparison with common care (Fig. 1). Among economic modeling methodologies [30], this approach is best suited to reflect the heterogeneity of the SCD population, the comprehensive set of SCD-related events and complications potentially impacted by a gene therapy, and the time-dependent relationships between events, complications, and mortality in SCD [30, 33]. This rationale is similarly reflected in other recent cost-effectiveness modeling frameworks for gene therapies in SCD [22, 23, 29].
Fig. 1.
Model structure. Hb hemoglobin, HU hydroxyurea, SCD sickle cell disease, VOC vaso-occlusive crisis, VOE vaso-occlusive event, VOE-CR complete resolution of vaso-occlusive events. a Number of each event per year sampled from annual incidence rates that vary over time. b Timing of incidence for each complication sampled from cumulative complication-free survival curves that are updated each year based on annual development probabilities. c Timing of death sampled from cumulative survival curves that are updated each year based on annual mortality risks
The model specification details are presented in Table 1. The target population was aligned with the transplant population for VOE (TPVOE) baseline criteria in the HGB-206 clinical trial (see Table S1 in the Supplementary Information for subject disposition details) [15, 34, 35]. We modeled a population reflecting the HGB-206 TPVOE criteria (age of ≥ 12 years with ≥ 4 VOEs in the prior 24 months), which is a narrower and potentially more severe subset of the approved lovo-cel indication (age of ≥ 12 years with any history of VOEs) [15]. The target intervention was one-time treatment with lovo-cel gene therapy administered according to the HGB-206 Group C treatment protocol as authorized by the FDA [15, 35]. The comparator was common care for SCD, which includes proportions of patients treated with HU or chronic exchange RBC transfusions. Emerging disease-modifying therapies for SCD (l-glutamine, crizanlizumab, and voxelotor) were not considered as comparators, as their use remains limited in the US [36]. Similarly, allogeneic HSCT was not considered as a comparator due to lovo-cel protocol specifications (exclusion of subjects with a willing matched sibling donor) [35] as well as the low proportion of patients with SCD for whom this treatment is an option [13].
Table 1.
Model characteristics and key parameters
| Base case | Sources and notes | ||
|---|---|---|---|
| Model characteristic | Settings | ||
| Population | US patients with SCD aged ≥ 12 years with genotype HbSS and a history of ≥ 4 VOEs in the prior 24 months | HGB-206 TPVOE population [34, 35] | |
| Target intervention | Lovo-cel, one-time IV infusion after myeloablative conditioning | HGB-206 Group C [15] | |
| Comparator intervention | Common care, including proportions receiving HU or chronic RBC transfusions | Kavanagh et al. [12] | |
| Time horizon | Lifetime a | Sanders et al. [37]; Institute for Clinical and Economic Review [38] | |
| Perspective | Third-party payer and societal (co-base cases) | ||
| Discounting | 3% per year for cost and health outcomes | ||
| WTP threshold (range) | $150,000 ($50,000–$200,000) per QALY gained | ||
| Cost-year | 2022 US $ | ||
| Baseline characteristic | Values | ||
| Model sample size | 2500 simulated patients | See Fig. S7 in the Supplementary Information for convergence plots | |
| Age, years b | 24.2 (7.1) | HGB-206 TPVOE (all treatment groups) (Table S2 in the Supplementary Information) | |
| Sex, % male | 67.5% | ||
| Race, % Black | 95.0% | ||
| SCD genotype, % distribution |
HbSS: HbSβ0: HbSβ+: HbSC/other: |
100% 0% 0% 0% |
HGB-206 TPVOE (all treatment groups) (Table S2 in the Supplementary Information) |
| Total Hb level, g/dL c | 8.38 (1.33) | ||
| Prior VOEs, rate/year b |
All VOEs: Severe VOEs: |
5.70 (4.15) 4.20 (3.11) |
|
| Common care treatment, % distribution | Neither HU nor chronic transfusions: | 57.7% | Derived from Shah et al. [40] |
| HU only: | 18.0% | ||
| Chronic transfusions only: | 24.3% | ||
| Both HU and chronic transfusions: | 0.0% | ||
| Events and complications | Included in the model | ||
| VOEsd | VOC (including % hospitalized), ACS, priapism, splenic sequestration | See Table S8 in the Supplementary Information | |
| Other acute events | VTE (including % pulmonary embolism versus % deep vein thrombosis), stroke (including history of SCI), sepsis/bacteremia | See Table S8 in the Supplementary Information | |
| Chronic complications | PH (including resolution), CKD (including progression to ESRD), retinopathy, AVN, gallstones (including % managed surgically), HF, chronic lung disease, leg ulcers, and neurocognitive impairment (excluding due to overt stroke) | See Table S9 in the Supplementary Information | |
| Mortality | Death | See Table S10 in the Supplementary Information | |
| Lovo-cel efficacy and safety | Values and assumptions | ||
| VOE effect |
VOE-CR achieved: 87.5% of patients Reduction for those not achieving VOE-CR: 68.2% |
HGB-206 TPVOE Group C; assessed during months 6-18 (Table S3 in the Supplementary Information) | |
| Severe VOE effect |
sVOE-CR achieved: 93.8% of patients Reduction for those not achieving sVOE-CR: 64.5% |
||
| Total Hb effect, g/dL b | Change from baseline: 3.50 (1.51) | HGB-206 TP Group C; assessed at 12 months (Table S4 in the Supplementary Information) | |
| Additional effects | Indirect and assumed reductions in other events, complications, and mortality | See Fig. 2 | |
| Safety | SMR from myeloablative conditioning: 1.25 | Kansal et al. [57] | |
| Key assumptions |
VOE-CR and partial VOE reductions start 6 months after transplantation Total Hb effect observed after 1 year Common care treatments discontinued VOE and total Hb effects persist for the remainder of the model horizon AEs (other than conditioning-related mortality) not modeled explicitly |
||
| Lovo-cel costs | Values and assumptions | ||
| Drug product cost | $3,100,000 (one time) | Red Book Online [59] | |
| Mobilization and apheresis |
Ages < 18 years: Ages ≥ 18 years: |
$25,017 (one time) $36,103 (one time) |
See Tables S6, S12, and S13 in the Supplementary Information for microcosting details |
| Conditioning and transplantation |
Ages < 18 years: Ages ≥ 18 years: |
$187,596 (one time) $100,525 (one time) |
|
| Monitoring |
Years 1–2: Years 3–15: |
$2418 (per year) $1748 (per year) |
|
| Fertility preservation |
% opting for preservation: 60% Cost of preservation: $20,000 (assumed to be one time) |
Kansal et al. [57]; assumption | |
| Lovo-cel utilities | Values and assumptions | ||
| Utility gain, PRO improvement | 0.088 (while VOE effect persists) | HBG-206 TP Group C EQ-5D-3L utility index change from baseline; assessed at 12 months (Table S5 in the Supplementary Information) | |
| QALYs lost, transplantation | 0.110 (one time) | Matza et al. [58] | |
| QALYs lost, conditioning | 0.02 (per year starting in year 2) | Kansal et al. [57] | |
ACS acute chest syndrome, AE adverse event, AVN avascular necrosis, CKD chronic kidney disease, ESRD end-stage renal disease, HbSC hemoglobin SC, HbSS hemoglobin SS, HbSβ hemoglobin Sβ-thalassemia, HF heart failure, HU hydroxyurea, IV intravenous, PH pulmonary hypertension, PRO patient-reported outcome, QALY quality-adjusted life-year, RBC red blood cell, SCD sickle cell disease, SCI silent cerebral infarct, SD standard deviation, SMR standardized mortality ratio, sVOE-CR complete resolution of severe vaso-occlusive events, TP transplant population, TPVOE transplant population for vaso-occlusive event, US United States, VOC vaso-occlusive crisis, VOE vaso-occlusive event, VOE-CR complete resolution of vaso-occlusive events, VTE venous thromboembolism, WTP willingness-to-pay
aLifetime time horizon implemented in the model as up to 80 years after model entry
bValues presented as mean (SD) unless otherwise described
cThe TP population in the HGB-206 clinical trial had 52.4% HbS at baseline, which may indicate that some subjects were on disease-modifying therapies at baseline
dThe VOE definition in the HGB-206 clinical trial protocol also included acute hepatic sequestration. This event was not included in the analysis due to a lack of supporting evidence in the SCD clinical literature (see Supplementary Information for additional details)
Other model settings were defined in accordance with US cost-effectiveness modeling guidelines and recommendations [30, 37, 38]. We used a lifetime time horizon to reflect the progressive, lifelong nature of SCD [37, 38]. The analysis perspective determines the scope of outcomes included; we considered co-base-case analyses from both a US third-party payer perspective (including patient health outcomes and direct medical costs only) and a US societal perspective (including caregiver quality-of-life impacts and indirect costs related to productivity loss and unpaid caregiving) [37–39]. Discount rates were used to calculate the present value of future costs and health outcomes [37]. The model was programmed in Microsoft Excel (RRID:SCR_016137) for Windows with Visual Basic for Applications (Microsoft Corporation).
Model Structure
According to the model framework (Fig. 1), a simulated cohort of individual patients meeting the target population criteria was created. Each individual patient was simulated twice, once assuming treatment with common care and once with lovo-cel. Accounting for baseline demographics and SCD status, the model used an annual cycle length to predict patient-level trajectories of acute events, chronic complications, and mortality over time.
Selection of the seven acute events and nine chronic complications included in the model (Table 1 and Fig. S1 in the Supplementary Information) was informed by the HGB-206 protocol-defined VOE endpoint, the SCD clinical and economic literature, and recommendations from clinician and patient advisors. Events in the HGB-206 VOE endpoint (vaso-occlusive crisis [VOC], acute chest syndrome [ACS], priapism, and splenic sequestration) were included in the model as separate events. The primary factors for selecting other events and complications for inclusion in the model were established precedent from prior SCD economic models and the strength of supporting evidence from the literature (see the Supplementary Information for additional details on our literature review). Additional patient-relevant outcomes, such as pain, fatigue, depression, and anxiety, were assumed to be reflected in the health-related quality-of-life (HRQOL) estimates used in the model. The fertility impacts of SCD and of the myeloablative conditioning required for autologous HSCT were not modeled, although fertility preservation costs were included for patients receiving lovo-cel.
The lovo-cel treatment effect was modeled over patients’ remaining lifetimes according to the achievement and maintenance of VOE-CR, reduction in VOEs relative to baseline for those without VOE-CR, and change from baseline in total Hb levels for all patients [15, 17]. The VOE treatment effect also included reduced frequency of severe VOEs as defined in the HGB-206 protocol [15, 35]. We assumed no reduction in VOEs during the first 6 months to account for the lovo-cel engraftment period. All VOE efficacy outcomes reflect HGB-206 TPVOE Group C data after central adjudication of clinician-identified VOEs.
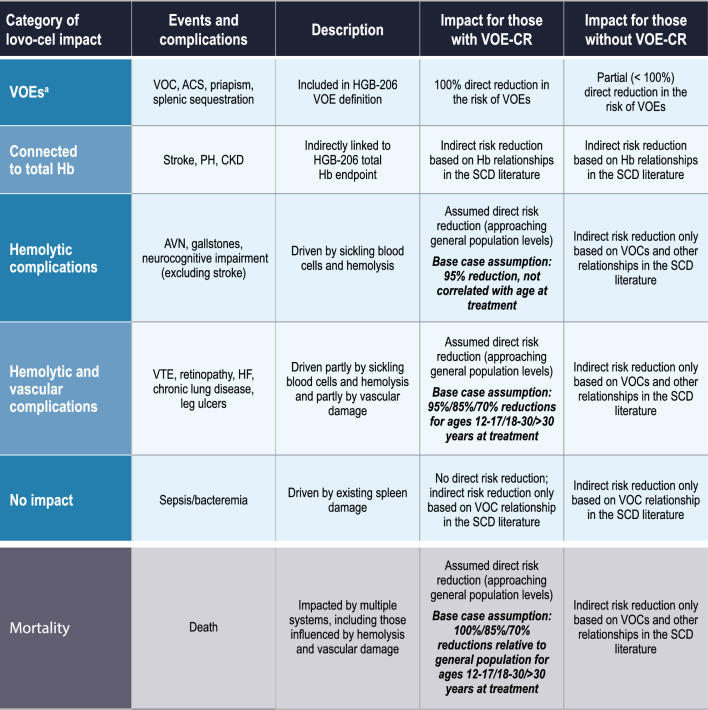
Based on the assumption that VOE-CR represents a curative level of impact for patients receiving lovo-cel, our clinician advisors developed a categorization for the impact of lovo-cel on SCD events, complications, and mortality in which the age of treatment serves as proxy for preexisting disease-related morbidity (Fig. 2). Specifically, this framework categorizes events and complications into those covered by the VOE endpoint (VOC, ACS, priapism, splenic sequestration), those with evidence-based links to total Hb levels (stroke, pulmonary hypertension, and chronic kidney disease), those driven primarily by sickling blood cells and hemolysis (avascular necrosis, gallstones, and neurocognitive impairment [excluding due to overt stroke]), and those driven partly by sickling blood cells and hemolysis and partly by vascular damage (venous thromboembolism, retinopathy, heart failure, chronic lung disease, and leg ulcers). In the context of the framework presented in Fig. 2, direct risk reductions refer to treatment effects applied directly to event and complication risks (e.g., reduction in the rate of VOCs by virtue of the VOE-CR endpoint), and indirect risk reductions refer to those stemming from evidence-based relationships with other events or complications (e.g., reduction in the risk of avascular necrosis by virtue of not having a recent VOC hospitalization).
Fig. 2.
Framework for lovo-cel’s impact on events, complications, and mortality. ACS acute chest syndrome, AVN avascular necrosis, CKD chronic kidney disease, Hb hemoglobin, HF heart failure, PH pulmonary hypertension, SCD sickle cell disease, VOE vaso-occlusive event, VOE-CR complete resolution of vaso-occlusive events, VTE venous thromboembolism. aThe VOE definition in HGB-206 clinical trial protocol also included acute hepatic sequestration. This event was not included in the analysis due to a lack of supporting evidence in the SCD clinical literature (see Supplementary Information for additional details)
The resulting framework for reductions in the risk of events, complications, and mortality for patients with VOE-CR reflects our clinically informed understanding of the relationship between the lovo-cel mechanism of action (erythroid precursor production of anti-sickling HbAT87Q) and disease pathogenesis through hemolysis, vaso-occlusion, and vasculopathy. For patients not achieving VOE-CR, partial reductions in VOE risk and increases in total Hb levels were linked to indirect reductions in the risk of other events, complications, and mortality based on evidence-based relationships identified in the SCD clinical literature. Regardless of VOE-CR status, the model does not assume that lovo-cel reverses any chronic complications present at the time of treatment. This clinician-informed framework is novel to our analysis and is intended to provide a balanced view of the potential long-term clinical benefits following lovo-cel therapy.
Model Parameters
Clinical Parameters
The individual patients included in the modeled cohort were simulated from aggregated demographic and SCD status data from the HGB-206 TPVOE population (Table 1 and Table S2 in the Supplementary Information). Common care treatment use (HU, chronic RBC transfusions, or neither) at baseline was derived from published literature [40] and clinical opinion. For the common care arm, treatment use and associated clinical benefits were assumed to remain constant for the duration of the model.
Data on patients’ acute event histories and existing chronic complications at model entry were obtained from a combination of aggregated HGB-206 TPVOE baseline data (Table S2 in the Supplementary Information) and studies identified in the SCD clinical literature [41–45]. We relied on the SCD clinical literature to identify acute event incidence rates and chronic complication development probabilities, including differences by age and SCD genotype where available. We also identified studies presenting evidence on risk factors for and relationships among SCD events and complications (Table S7 in the Supplementary Information) [46]. We prioritized data from recently published studies to reflect the impact of contemporary HU and chronic transfusion use on SCD events and complications. However, for some events and complications, robust evidence on incidence rates and risk factors were available only from older, landmark SCD cohort studies (e.g., the Cooperative Study of Sickle Cell Disease [47]). A comprehensive presentation of all parameters for events and complications used in the model is provided in Tables S8 and S9 in the Supplementary Information.
Underlying SCD mortality was estimated by applying sex-specific SCD standardized mortality ratios (SMRs) for genotypes HbSS/Sβ0 derived from a landmark SCD cohort [3, 47–49] to age-, sex-, and race-specific general population mortality risks in the US [50]. Similar sex- and genotype-specific SMRs relative to the general US population were not identified for more contemporary SCD cohorts. To facilitate a more contemporary interpretation of this landmark SCD mortality data, we adjusted the SMRs to reflect mortality risks in the absence of VOCs [47, 49]. Acknowledging the likely presence of other SCD events and complications in the selected landmark SCD cohort, and to avoid potential double-counting across the range of risk factors for SCD mortality [44, 49, 51–55], we modeled additional increases in mortality risks associated only with a hospitalized VOC [52] and with multiple end-organ involvement [55]. Additional details and the specific SMRs used in the model are presented in Table S10 in the Supplementary Information.
Lovo-Cel Attributes
Table 1 presents the lovo-cel treatment effect parameters from the HGB-206 trial used in the model. Additional details on the HGB-206 trial data and statistical methodologies are presented in the Supplementary Information. Consistent with the lovo-cel mechanism of action and the available follow-up data through February 2023, our base-case analysis assumed that the lovo-cel treatment effect on VOEs (Table S3 in the Supplementary Information) and changes from baseline in total Hb levels (Table S4 in the Supplementary Information) persist for the remainder of patients’ lifetimes. The magnitude and durability of the VOE and total Hb treatment effects were varied in scenario analyses.
Patients achieving VOE-CR (i.e., 100% reduction) experienced reductions in the risks of other events, complications, and mortality according to the framework in Fig. 2. For primarily hemolytic events and complications, risks were assumed to be reduced by 95% (to a level approximating general population risks) for patients with VOE-CR regardless of the age of treatment with lovo-cel on the basis of the sustained hemolysis improvements observed in the HGB-206 clinical trial [17]. For partly hemolytic and partly vascular events and complications, the magnitude of risk reductions associated with VOE-CR was assumed to decline with increasing age of treatment (95% for patients aged 12–17 years, 85% for patients aged 18–30 years, and 70% for patients aged > 30 years), acknowledging that lovo-cel will not reverse existing vascular damage. Similar treatment age-dependent reductions in the underlying SCD SMRs were assumed for the base case. For patients achieving a partial resolution of VOEs (i.e., < 100% reduction), a more limited indirect treatment effect was modeled, informed by literature-based relationships (Tables S7–S10 in the Supplementary Information). Scenario analyses were conducted on the magnitude of the risk reductions reflected in this framework for the lovo-cel treatment effect on other events, complications, and mortality.
The HGB-206 trial also studied the impact of lovo-cel on patient-reported HRQOL outcomes [17, 56]. The improvement in patient-reported HRQOL observed in the trial was incorporated into the model as a lovo-cel-specific utility gain estimated from the change from baseline in EQ-5D-3L utility values observed in the HGB-206 trial (Table 1 and Table S5 in the Supplementary Information). Utility values are estimates valuing specific health states on a scale where 0 represents death and 1 represents perfect health. The utility gain for lovo-cel was assumed to apply for the remainder of patients’ lives. In scenarios with partial loss of the lovo-cel treatment effect, the lovo-cel utility gain decreased proportionally with the VOE effect.
Parameters describing the safety, costs, and other HRQOL impacts of lovo-cel are presented in Table 1. Safety was considered through the increased risk of mortality (e.g., from the risk of myelodysplastic syndrome or other hematologic malignancies [15, 17]) and the long-term HRQOL impact associated with myeloablative conditioning [57], which were assumed to last for the remainder of patients’ lives. The potential fertility impacts of myeloablative conditioning were captured via a proportion of patients opting for fertility preservation [57]. Other serious adverse events observed in the HGB-206 trial were largely concentrated during the transplantation hospitalization and thus assumed to be captured in the one-time costs and HRQOL impact [58] of transplantation. As an autologous HSCT, lovo-cel does not carry the risk of graft rejection or graft versus host disease. The one-time lovo-cel drug product acquisition price was set to the publicly available list price ($3.1 million) for the base-case analysis [59]. To illustrate the impact of potential rebates on the lovo-cel list price, we included a scenario analysis using the known federal Medicaid Prescription Drug Rebate Program’s statutory minimum rebate [60]. Lovo-cel administration costs (reflecting mobilization, apheresis, conditioning, and transplantation) were estimated from resource utilization observed in the HGB-206 trial (Table S6 in the Supplementary Information) and publicly available sources using a microcosting approach (Table S12 in the Supplementary Information). Annual monitoring costs also were estimated using a microcosting approach [57] (Table S13 in the Supplementary Information) and were assumed for a total of 15 years following lovo-cel treatment [15].
Costs and Health-Related Quality of Life
The direct SCD-related costs captured in the model included costs associated with common care treatments, costs per event for all acute events, and annual costs for all chronic complications. Costs for multiple events and complications present within the same annual model cycle were applied additively. In alignment with literature showing that annual direct costs for patients with SCD are highest between ages 18 and 30 years [61, 62], we applied age-specific multipliers to the costs for all acute events and chronic complications. Although lower annual costs observed in older age groups in the literature may reflect a severity bias (i.e., survivors are those with less severe disease), we chose to use these age-specific differences to balance concerns about double-counting with our additive approach for multiple events or complications. Data sources and parameter values for direct costs used in the base-case analysis are presented in Table S11 in the Supplementary Information. We did not include non-SCD-related direct medical costs during periods of extended survival in the base-case analysis. However, we did consider these costs in a scenario analysis.
Additional indirect costs reflected in the societal perspective included the value of unpaid caregiving and patient productivity impacts. Consistent with the impact of VOE-CR on event and complication risks, the model assumed reductions in unpaid caregiving commensurate with VOE reductions and complication status (100% reduction for patients with VOE-CR and no complications; 75% reduction for patients with VOE-CR and ≥ 1 complication). The model leveraged educational pathways research [63] to project that achieving VOE-CR would support educational and work productivity gains, resulting in higher annual earnings depending on the age at treatment. Specifically, patients achieving VOE-CR with lovo-cel prior to age 15 years (i.e., early enough to affect secondary educational outcomes) would have annual earnings equal to 92% (versus 56% for SCD overall) of race-adjusted general population earnings [63]. Those achieving VOE-CR with lovo-cel after age 15 years would have annual earnings equal to 78% of race-adjusted general population earnings [63]. Data sources and parameter values for indirect costs used in the co-base-case societal perspective are presented in Table S11 in the Supplementary Information. In a scenario analysis, we also included in the societal perspective the additional consumption costs associated with consumer expenditures during periods of extended survival for patients treated with lovo-cel.
The model captured the HRQOL impacts of SCD on both patients (in both co-base-case perspectives) and their caregivers (in the co-base-case societal perspective only) in the form of utility values [64, 65]. Health-related quality-of-life impacts for multiple events and complications present within the same annual model cycle were applied additively. We conservatively assumed one caregiver per patient in our base-case analysis. The impact of lovo-cel on caregiver quality of life was modeled using the same approach as for unpaid caregiving. Data sources and parameter values for patient and caregiver utilities are presented in Table S14 in the Supplementary Information.
Model Outcomes and Analysis
Base-Case Analysis
We used the model to predict lifetime absolute and incremental health and economic outcomes for lovo-cel in comparison with common care for a simulated cohort of 2500 patients, a sample size that was found to be sufficient for convergence in predicted model outcomes (Fig. S7 in the Supplementary Information). Results are presented as the mean per patient across the cohort, with SDs (reflecting variability among patients) and 95% confidence intervals (CIs, reflecting confidence in the mean estimates) for selected outcomes. Survival was estimated in terms of (undiscounted) life-years and age at death. Total (discounted) QALYs, a composite metric accounting for quantity and quality of life, were disaggregated into those attributable to SCD overall, gains associated with treatment, losses due to events and complications, and losses by caregivers. Total (discounted) costs were similarly disaggregated. Additional health outcomes included cumulative lifetime VOCs (the most frequent of the events in the HGB-206 VOE protocol definition) and development of chronic complications. We also estimated the equal value life-year (evLY) and health years in total (HYT) outcomes as alternatives to the QALY outcome [66, 67].
The primary outcome used to estimate the cost-effectiveness of lovo-cel compared with common care was the incremental cost per QALY gained. Incremental costs per evLY gained and per HYT gained were also reported. Due to the limitations of ratio-based outcomes, we estimated the net monetary benefit (NMB) outcome, which monetizes the benefits of a new intervention by applying a willingness-to-pay (WTP) threshold to QALY gains net of associated costs [68]. We considered WTP thresholds ranging from $50,000 to $200,000 per QALY gained [38], with the upper end of this range reflecting society’s higher WTP for more severe conditions [24, 69], to estimate the NMB (mean, SD, and 95% CI) for the base-case cost-effectiveness results. To better understand lovo-cel’s commercial list price relative to its predicted value to society, we also estimated the threshold value-based price for lovo-cel across this same range of WTP thresholds.
Heterogeneity and Uncertainty
Uncertainty analyses are an integral part of economic evaluations [30, 37]. They are particularly relevant for gene therapies, given the one-time, irreversible nature of these therapies and the limited long-term data on their effectiveness [20, 70]. Heterogeneity represents a form of decision uncertainty related to the selection of patients for treatment [71]. Acknowledging the heterogeneity of the SCD patient population, we assessed patient-level variability in predicted outcomes via scatterplots of patient-level incremental outcomes and via the distribution of patient-level NMB estimates.
To assess the impact of parameter uncertainty, we conducted a probabilistic sensitivity analysis (PSA) and extensive scenario analyses. Probability distributions for parameters that were varied in the PSA were selected in accordance with best practices [71, 72] using evidence-based uncertainty estimates where available and reasonable assumptions otherwise (see Table S15 in the Supplementary Information for detailed uncertainty estimates and distributions). The PSA varied all included parameters jointly over their respective probability distributions over 1000 iterations using the same sample of patients for all iterations.
We also evaluated the impact of parameter uncertainty on model predictions through targeted scenario analyses and one-way sensitivity analyses constructed around key model parameters and assumptions (see Table S16 in the Supplementary Information for detailed scenario descriptions). We focused scenarios on key themes of relevance to patient, caregiver, provider, and payer stakeholders: Who should receive lovo-cel? How well does lovo-cel work? How long is the lovo-cel effect expected to last? Additional scenarios were constructed around a known rebate on the lovo-cel list price (as a proxy for other potential payer-specific rebates), parameter uncertainties (e.g., direct costs and disutilities for events and complications), data gaps (e.g., caregiver impacts), and alternative model settings (discounting, inclusion of non-SCD-related medical costs, inclusion of consumer consumption costs). In keeping with good modeling practices for patient-level simulations, we used common random numbers for all sensitivity and scenario analyses [73].
Model Validation
Good practice guidelines for cost-effectiveness model validation emphasize face validity (of the modeling approach and data source), internal validity (of the model programming and predicted outcomes), and external validity (compared with other published studies) [32, 74]. The face validity of our modeling approach is supported by the involvement of clinical and economic advisors and a patient representative in model development and by similar comprehensive economic evaluations for SCD in the literature [24, 29]. We verified the internal validity of the model through a third-person, comprehensive review of all model programming and through extreme value testing. Finally, we assessed the external validity of our model by comparing outcomes with similarly comprehensive cost-effectiveness analyses for SCD gene therapy in the literature (presented in the Discussion section).
Results
Base-Case Analysis
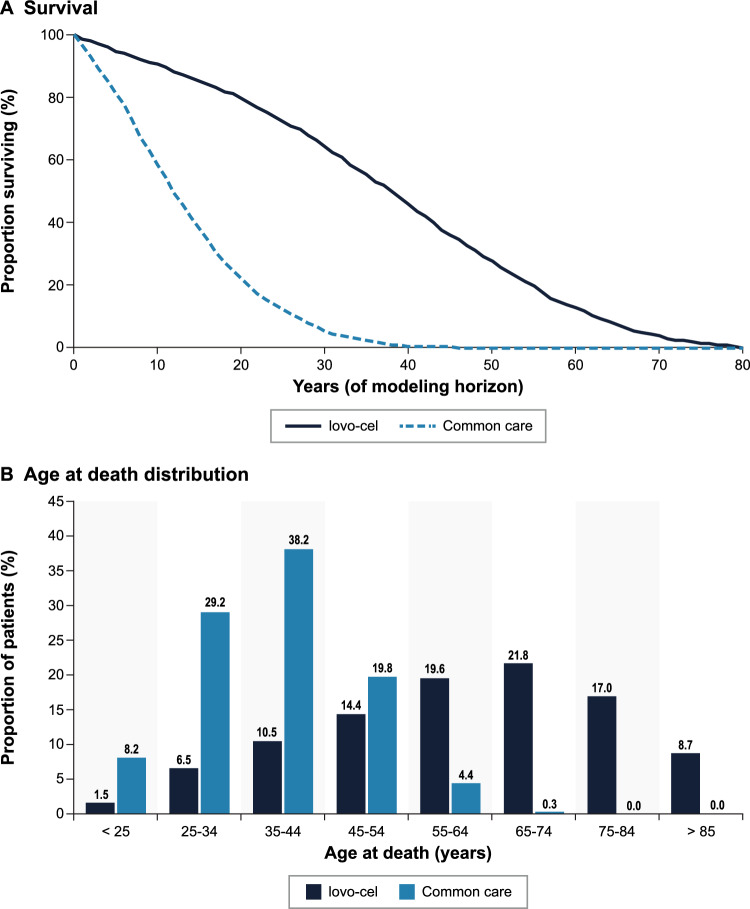
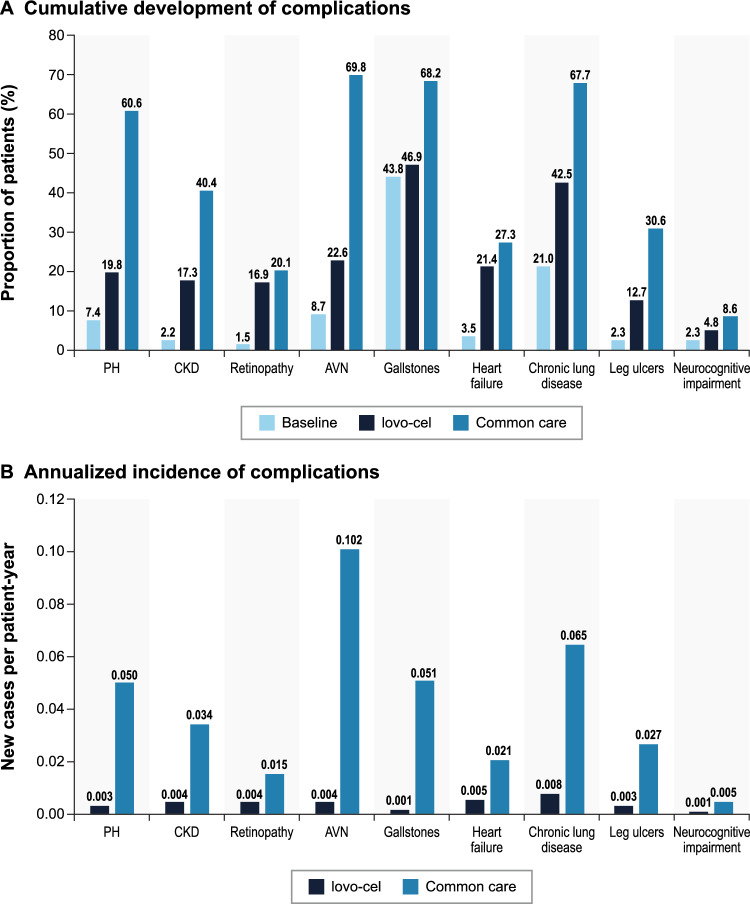
The predicted lifetime outcomes for patients treated with lovo-cel in comparison with common care are presented in Table 2. Patients entered the model with an average baseline age of 24.9 years and an average of 0.93 existing SCD complications. Patients remaining on common care were predicted to survive 13.47 years (SD 9.08; 95% CI 13.12–13.83), with an estimated 67.81 lifetime VOCs and 3.93 cumulative chronic complications per patient. In contrast, patients treated with lovo-cel were predicted to survive 37.32 years (SD 18.97; 95% CI 36.57–38.06), with an estimated 6.75 lifetime VOCs and 2.05 cumulative chronic complications per patient. The predicted increase in survival of 23.84 years (SD 12.80; 95% CI 23.34–24.34) for lovo-cel versus common care translated to the mean age at death increasing from 38.40 to 62.24 years and the proportion of patients surviving past age 65 years increasing from < 1% to nearly 50% (Fig. 3). Of the 6.75 average lifetime VOCs for patients treated with lovo-cel, 2.48 VOCs occurred during the first 6 months (spanning the engraftment period); the remaining VOCs occurred only in those without VOE-CR after the initial 6 months. The cumulative proportions of patients developing specific chronic complications and the associated annualized incidence predicted for lovo-cel compared with common care are presented in Fig. 4. Predicted lifetime and annualized incidence estimates for acute events are presented in Fig. S2 in the Supplementary Information.
Table 2.
Base-case health and economic outcomes
| Model outcome a | Lovo-cel | Common care | Incremental |
|---|---|---|---|
| Survival (undiscounted) | |||
| Life-years, total | 37.32 | 13.47 | 23.84 |
| SD b | 18.97 | 9.08 | 12.80 |
| 95% CI b | 36.57 to 38.06 | 13.12 to 13.83 | 23.34 to 24.34 |
| Age at death, years | 62.24 | 38.40 | 23.84 |
| Other health outcomes (undiscounted) | |||
| Lifetime number of VOCs | 6.75 | 67.81 | −61.06 |
| Prior to 6 months | 2.48 | 2.48 | 0.00 |
| After 6 months | 4.27 | 65.33 | −61.06 |
| Lifetime number of chronic complications | 2.05 | 3.93 | −1.89 |
| QALYs, total (discounted) | 15.25 | 3.86 | 11.39 |
| SD b | 6.09 | 2.05 | 4.91 |
| 95% CI b | 15.01 to 15.49 | 3.78 to 3.94 | 11.19 to 11.58 |
| Patients, total | 16.44 | 6.25 | 10.20 |
| SCD general, including common care | 15.77 | 7.87 | 7.90 |
| Gain associated with lovo-cel | 1.30 | N/A | 1.30 |
| Lost due to acute events | −0.30 | −0.93 | 0.63 |
| Lost due to chronic complications | −0.33 | −0.70 | 0.37 |
| Lost by caregivers | −1.19 | −2.38 | 1.19 |
| evLYs, total (discounted) | 16.50 | 3.86 | 12.63 |
| Patients | 17.15 | 6.25 | 10.91 |
| Lost by caregivers | −0.65 | −2.38 | 1.73 |
| HYT, total (discounted) | 36.28 | 14.57 | 21.71 |
| Patients | 37.47 | 16.95 | 20.52 |
| Lost by caregivers | −1.19 | −2.38 | 1.19 |
| Costs, total (discounted) | $3,773,704 | $2,361,311 | $1,412,393 |
| SD b | $945,131 | $1,556,160 | $1,452,720 |
| 95% CI b | $3,736,655 to $3,810,753 | $2,300,310 to $2,422,313 | $1,355,446 to $1,469,339 |
| Lovo-cel direct costs, total | $3,282,009 | N/A | $3,282,009 |
| Drug product acquisition | $3,100,000 | N/A | $3,100,000 |
| Preparation and administration | $148,634 | N/A | $148,634 |
| Monitoring | $21,319 | N/A | $21,319 |
| Fertility preservation | $12,056 | N/A | $12,056 |
| Other direct costs, total | $860,020 | $2,189,221 | −$1,329,201 |
| Common care | $0 | $168,772 | −$168,772 |
| Acute events | $77,976 | $916,216 | −$838,240 |
| Chronic complications | $782,045 | $1,104,234 | −$322,189 |
| Other societal costs, total | −$368,325 | $172,090 | −$540,416 |
| Value of unpaid caregiving | $86,157 | $172,090 | −$85,933 |
| Patient productivity gain c | −$454,483 | N/A | −$454,483 |
Bold indicates sections of outcomes and overall total outcomes
CI confidence interval, evLY equal value life-year, HYT health years in total, N/A not applicable, QALY quality-adjusted life-year, SCD sickle cell disease, SD standard deviation, VOC vaso-occlusive crisis
aModel outcomes are presented as the predicted means across a sample of 2500 simulated patients
bSDs (reflecting variability across individual patients) and 95% CIs (reflecting confidence in the mean for the simulated sample) are presented for selected outcomes. Convergence plots for incremental costs and QALYs are presented in Fig. S7 in the Supplementary Information
cPatient productivity gain estimated as the difference in predicted lifetime earnings for lovo-cel versus common care ($738,604 versus $284,122)
Fig. 3.
Predicted survival and age at death distributions
Fig 4.
Predicted chronic complication development. AVN avascular necrosis, CKD chronic kidney disease, PH pulmonary hypertension
The improvements in survival, quality of life, and other clinical outcomes predicted for lovo-cel versus common care translated to predicted increases in patient QALYs (16.44 vs. 6.25) and reductions in lifetime direct medical costs associated with common care, events, and complications ($860,020 versus $2,189,221). Reductions in caregiver burden also were predicted in the form of fewer caregiver QALYs lost (1.19 versus 2.38) and unpaid caregiving avoided ($86,157 versus $172,090) per patient. The predicted increase in annual earnings for patients achieving VOE-CR after lovo-cel resulted in a predicted increase in lifetime earnings of $454,483 per patient. The total lovo-cel-related costs (acquisition, preparation and administration, monitoring, and fertility preservation) were estimated to be $3,282,009 per patient.
The cost-effectiveness results for the co-base-case third-party payer and societal perspectives are presented in Table 3. From a third-party payer perspective, the predicted per-patient increases in total direct medical costs of $1,952,808 (SD $1,339,849; 95% CI $1,900,286–$2,005,330) and in patient QALYs of 10.20 (SD 4.10; 95% CI 10.04–10.36) resulted in an incremental cost-effectiveness ratio of $191,519 per QALY gained. From a broader societal perspective, the predicted per-patient increases in total direct and indirect costs of $1,412,393 (SD $1,452,720; 95% CI $1,355,446–$1,469,339) and in total patient and caregiver QALYs of 11.39 (SD 4.91; 95% CI 11.19–11.58) resulted in an incremental cost-effectiveness ratio of $124,051 per QALY gained.
Table 3.
Base-case cost-effectiveness results
| CE outcome | Third-party payer perspective a | Societal perspective a |
|---|---|---|
| Incremental CE ratios | ||
| $/QALY gained | $191,519 | $124,051 |
| $/evLY gained | $179,073 | $111,799 |
| $/HYT gained | $95,163 | $65,058 |
| NMB (by WTP threshold) (95% CI) | ||
| $50,000/QALY gained | −$1,442,988 (−$1,499,008 to −$1,386,968) | −$843,114 (−$906,605 to −$779,622) |
| $100,000/QALY gained | −$933,167 (−$993,558 to −$872,777) | −$273,835 (−$344,578 to −$203,091) |
| $150,000/QALY gained | −$423,347 (−$488,805 to −$357,889) | $295,445 ($216,939 to $373,950) |
| $200,000/QALY gained | $86,473 ($15,399 to $157,548) | $864,724 ($778,082 to $951,365) |
| Threshold VBP (by WTP threshold) | ||
| $50,000/QALY gained | $1,657,012 | $2,256,886 |
| $100,000/QALY gained | $2,166,833 | $2,826,165 |
| $150,000/QALY gained | $2,676,653 | $3,395,445 |
| $200,000/QALY gained | $3,186,473 | $3,964,724 |
CE cost-effectiveness, CI confidence interval, evLY equal value life-year, HYT health years in total, NMB net monetary benefit, QALY quality-adjusted life-year, VBP value-based price, WTP willingness to pay
aThird-party payer perspective results only include direct medical costs (lovo-cel-related and other direct costs) and patient QALYs. Societal perspective results also include other societal costs and caregiver QALYs lost
At a WTP threshold of $150,000 per QALY gained, the NMB for lovo-cel compared with common care was predicted to be −$423,347 (95% CI −$488,805 to −$357,889) from a third-party payer perspective and $295,445 (95% CI $216,939–$373,950) from a societal perspective. At a higher WTP threshold of $200,000 per QALY gained, the NMB was predicted to be positive for both co-base-case perspectives. If incremental QALYs are valued at a WTP threshold of $150,000 per QALY gained (range $50,000–$200,000 per QALY gained) [37, 38], these results indicate that lovo-cel would be cost-effective at a one-time acquisition price of up to $2,676,653 (range $1,657,012–$3,186,473) from a third-party payer perspective and up to $3,395,445 (range $2,256,886–$3,964,724) from a societal perspective.
Heterogeneity and Uncertainty
The results for the base-case analysis indicated significant heterogeneity across patients in incremental costs and outcomes and in the resulting NMB estimates (Figs. S3 and S4 in the Supplementary Information for scatterplots across all patients and by age-specific subgroups). In particular, at a WTP threshold of $150,000 per QALY gained, 38.8% and 54.7% of patients were predicted to have a positive NMB from third-party payer and societal perspectives, respectively (Fig. S5 in the Supplementary Information).
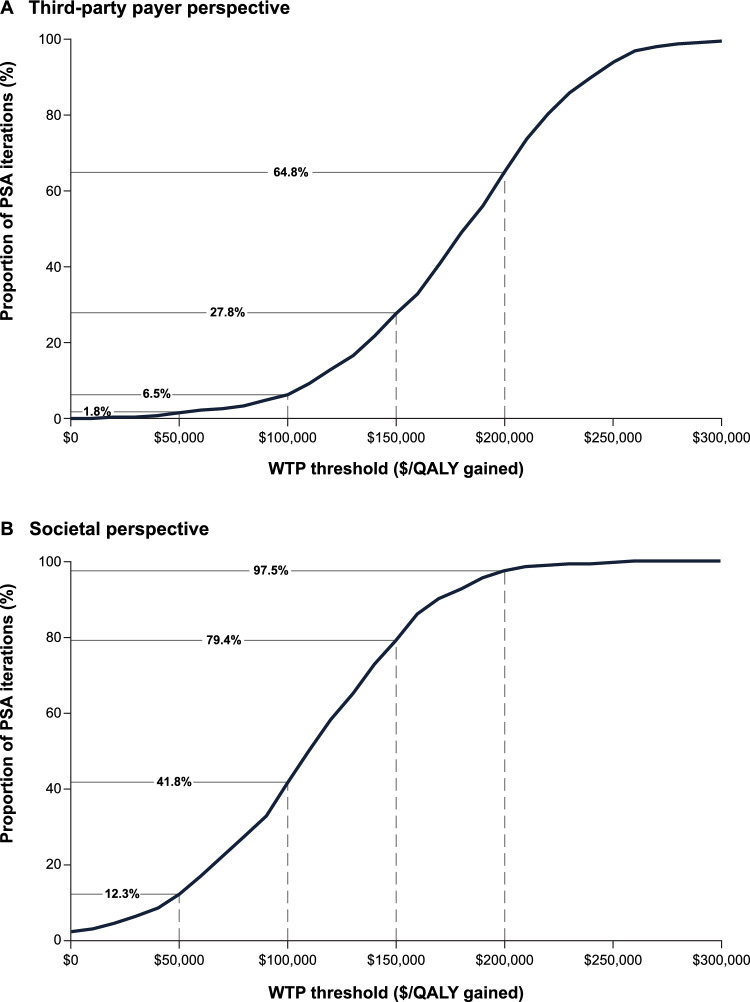
In the PSA assessing the impact of joint parameter uncertainty, the proportions of iterations with a positive NMB at a WTP threshold of $150,000 per QALY gained were 27.8% and 79.4% for the co-base-case third-party payer and societal perspectives, respectively (Figs. 5 and S6 in the Supplementary Information). For the third-party payer perspective, the proportion of iterations with a positive NMB ranged from 1.8% to 64.8% over a WTP threshold range of $50,000 to $200,000 per QALY gained. Over this same WTP threshold range, the proportion of iterations with a positive NMB from a societal perspective ranged from 12.3% to 97.5%.
Fig. 5.
Probabilistic sensitivity analysis results. PSA probabilistic sensitivity analysis, QALY quality-adjusted life-year, WTP willingness-to-pay
Table 4 presents the results from our targeted scenario and one-way sensitivity analyses. Among scenarios evaluating uncertainty regarding who receives lovo-cel, how well it works, and how long the effect lasts, the predicted cost-effectiveness of lovo-cel was most impacted by the age at treatment. Notably, the incremental cost-effectiveness ratios for lovo-cel compared with common care from both third-party payer and societal perspectives were over 40% lower than the base case for patients treated at ages 12–17 years and over 65% higher than the base case for patients treated at ages > 30 years. This variability reflects differences in baseline complication prevalence by age and our assumptions about the age-specific impacts of VOE-CR (Fig. 2). Similarly, relaxing the criteria for lovo-cel eligibility to include patients aged ≥ 12 years with any history of VOEs resulted in incremental cost-effectiveness ratios that were roughly 50% higher than the base case, primarily attributable to the lower VOC rates expected with common care (and thus avoided with lovo-cel).
Table 4.
Scenario results
| Scenario category | Scenario parameters a | Third-party payer perspective | Societal perspective | ||||
|---|---|---|---|---|---|---|---|
| Incremental QALYs | Incremental costs | $/QALY gained (diff. versus base case) | Incremental QALYs | Incremental costs | $/QALY gained (diff. versus base case) | ||
| Base case | 10.20 | $1,952,808 | $191,519 | 11.39 | $1,412,393 | $124,051 | |
| Lovo-cel target population | Age: 12–17 years only b | 12.73 | $1,403,433 | $110,244 (−42.4%) | 14.55 | $705,202 | $48,460 (−60.9%) |
| Age: 18–30 years only b | 10.26 | $1,861,801 | $181,466 (−5.2%) | 11.44 | $1,320,751 | $115,437 (−6.9%) | |
| Age: > 30 years only b | 8.20 | $2,604,663 | $317,739 (+65.9%) | 8.95 | $2,179,382 | $243,405 (+96.2%) | |
| Prior VOE criteria: any history of VOEs | 8.60 | $2,468,155 | $286,896 (+49.8%) | 10.16 | $1,943,252 | $191,320 (+54.2%) | |
| Lovo-cel VOE and total Hb efficacy | VOE-CR: 96.5% of patients; sVOE-CR: 99.2% of patients | 10.92 | $1,921,783 | $176,064 (−8.1%) | 12.29 | $1,326,813 | $107,938 (−13.0%) |
| VOE-CR: 71.0% of patients; sVOE-CR: 79.2% of patients | 8.70 | $2,011,648 | $231,144 (+20.7%) | 9.60 | $1,572,267 | $163,826 (+32.1%) | |
| Partial VOE reduction: excluded | 10.08 | $2,014,516 | $199,907 (+4.4%) | 11.28 | $1,474,645 | $130,762 (+5.4%) | |
| Total Hb change from baseline: 4.10 g/dL (SD, 1.28) | 10.32 | $1,886,663 | $182,787 (−4.6%) | 11.51 | $1,344,109 | $116,778 (−5.9%) | |
| Total Hb change from baseline: 2.86 g/dL (SD, 1.70) | 10.00 | $2,045,616 | $204,517 (+6.8%) | 11.19 | $1,508,562 | $134,774 (+8.6%) | |
| Lovo-cel HRQOL impacts | Utility gain: 0.146 | 11.39 | $1,952,808 | $171,485 (−10.5%) | 12.58 | $1,412,393 | $112,301 (−9.5%) |
| Utility gain: 0.056 | 9.54 | $1,952,808 | $204,715 (+6.9%) | 10.73 | $1,412,393 | $131,651 (+6.1%) | |
| Partial loss of lovo-cel effect | 10% of patients lose 50% of VOE and Hb effects after 5 years | 9.45 | $1,978,202 | $209,276 (+9.3%) | 10.55 | $1,471,792 | $139,481 (+12.4%) |
| VOE-CR impact assumptions for lovo-cel | VOE-CR impact: 90%/80% reduction for ages 18–30/> 30 years | 10.72 | $1,961,672 | $182,992 (−4.5%) | 11.90 | $1,408,479 | $118,404 (−4.6%) |
| VOE-CR impact: 80%/60% reduction for ages 18–30/> 30 years | 9.73 | $1,944,138 | $199,708 (+4.3%) | 10.94 | $1,416,011 | $129,461 (+4.4%) | |
| Lovo-cel drug product price | 23.1% rebate on lovo-cel acquisition price | 10.20 | $1,236,708 | $121,289 (−36.7%) | 11.39 | $696,293 | $61,156 (−50.7%) |
| Event and complication costs and utilities | 25% increase in direct costs | 10.20 | $1,662,701 | $163,067 (−14.9%) | 11.39 | $1,122,285 | $98,571 (−20.5%) |
| 25% decrease in direct costs | 10.20 | $2,242,916 | $219,971 (+14.9%) | 11.39 | $1,702,500 | $149,531 (+20.5%) | |
| 25% increase in disutilities | 10.45 | $1,952,808 | $186,959 (−2.4%) | 11.63 | $1,412,393 | $121,399 (−2.1%) | |
| 25% decrease in disutilities | 9.95 | $1,952,808 | $196,308 (+2.5%) | 11.14 | $1,412,393 | $126,821 (+2.2%) | |
| Other scenarios of interest | VOC rate: increased by 50% for at-home management | 10.44 | $1,927,427 | $191,519 (−3.6%) | 11.62 | $1,386,810 | $119,326 (−3.8%) |
| Unpaid caregiving: $32,808 per year | 10.20 | $1,952,808 | $191,519 (0.0%) | 11.39 | $1,326,460 | $116,503 (−6.1%) | |
| Caregiver disutility: applied to two caregivers per patient | 10.20 | $1,952,808 | $191,519 (0.0%) | 12.57 | $1,412,393 | $112,320 (−9.5%) | |
| Model settings | Discounting: undiscounted | 21.25 | $1,874,012 | $88,184 (−54.0%) | 22.27 | $966,727 | $43,406 (−65.0%) |
| Discounting: 1.5% per year | 14.34 | $1,882,536 | $131,283 (−31.5%) | 15.50 | $1,193,315 | $76,989 (−37.9%) | |
| Unrelated direct medical costs: $3761 per year | 10.20 | $1,992,453 | $195,407 (+2.0%) | 11.39 | $1,452,038 | $127,533 (+2.8%) | |
| Consumption costs: 80.7% of annual earnings | 10.20 | $1,952,808 | $191,519 (0.0%) | 11.39 | $1,826,232 | $160,399 (+29.3%) | |
diff. difference, Hb hemoglobin, HRQOL health-related quality of life, QALY quality-adjusted life-year, SD standard deviation, sVOE-CR complete resolution of severe vaso-occlusive events, VOC vaso-occlusive crisis, VOE vaso-occlusive event, VOE-CR complete resolution of vaso-occlusive events
aAdditional implementation details and relevant sources for all scenarios are presented in Table S15 in the Supplementary Information
bBaseline age scenarios considered as post hoc subgroups (12–17 years: n = 395 [15.8%]; 18–30 years: n = 1,555 [62.2%]; > 30 years: n = 550 [22.0%])
Of the scenarios related to the lovo-cel treatment effect, uncertainty in the proportion of patients achieving VOE-CR had a greater impact on cost-effectiveness results than uncertainty in changes from baseline in total Hb or EQ-5D-3L utility values (Table 4). In general, variations in the proportion of patients achieving VOE-CR and the partial loss of treatment effect had a larger impact on the incremental cost-effectiveness ratios than variations in our assumptions around the age-specific impacts of VOE-CR. Excluding the partial reduction in VOEs for those without VOE-CR had a relatively limited impact on cost-effectiveness results. As anticipated, modeling the known Medicaid Prescription Drug Program rebate [60] (as a proxy for other potential payer-specific rebates) on the lovo-cel commercial list price had a significant impact on cost-effectiveness, lowering the incremental cost-effectiveness ratios by roughly 40–50% across third-party payer and societal perspectives. Variations in the direct costs associated with acute events and chronic complications had a larger impact on cost-effectiveness results than variations in disutilities associated with events and complications. Additional scenarios accounting for the potential impact of VOCs managed at home and related to limitations in the SCD caregiving literature had relatively limited impacts on the incremental cost-effectiveness ratios. Among scenarios considering alternative model settings, reductions in discount rates resulted in meaningfully lower incremental cost-effectiveness ratios, as expected. Although the inclusion of unrelated direct medical costs in years of additional survival for lovo-cel had minimal impact on cost-effectiveness results, the inclusion of consumption costs in years of additional survival offset some of the predicted productivity gains for patients treated with lovo-cel and thus increased the societal incremental cost-effectiveness ratio by nearly 30%.
Discussion
The emergence of one-time gene therapies heralds a new treatment paradigm for SCD with potentially meaningful benefits for patients, their families, the healthcare system, and broader society. This analysis of the cost-effectiveness of lovo-cel gene therapy compared with common care for patients in the US with SCD aged ≥ 12 years with recurrent VOEs is the first economic evaluation to leverage gene therapy clinical trial data with follow-up of up to 61 months after transplantation. Our base-case results estimate the potential economic value of lovo-cel from co-base-case third-party payer and societal perspectives, with predicted incremental cost-effectiveness ratios of $191,519 and $124,051 per QALY gained, respectively. This meaningful difference in the incremental cost-effectiveness ratio between the third-party payer and societal perspectives (falling on either side of the base-case WTP threshold of $150,000 per QALY gained) illustrates the contribution of positive spillover impacts, such as patient productivity gains and reduced caregiver burden, to the potential economic value of lovo-cel. Beyond the choice of perspective and WTP threshold, which can vary across stakeholders within the US, scenario analyses indicated that the predicted cost-effectiveness of lovo-cel was most sensitive to baseline age and VOE frequency. In particular, our predicted incremental cost-effectiveness ratios varied by 40% or greater for the youngest and oldest age subgroups and increased by roughly 50% for an alternative population with a lower historical burden of VOEs. Scenario analyses also illustrated the sensitivity of our cost-effectiveness results to the proportion of patients achieving and maintaining VOE-CR. Our findings also represent a comprehensive estimation of the broad potential clinical impact of lovo-cel that can facilitate open dialogue and shared decision-making among patients, clinicians, and families considering lovo-cel as a treatment option for SCD in the US.
The approval of gene and other advanced therapies with high one-time acquisition costs and uncertain long-term benefits has increased interest in alternative payment models and performance-based managed entry agreements [75–78]. Whether focused at the individual patient or population-based level [76], these agreements are particularly relevant for SCD because the heterogeneity of the population eligible for treatment and the complexity of the disease make defining a simple, uniform, patient-level measure of treatment success impractical. As suggested by the impact on cost-effectiveness results of a rebate on the lovo-cel list price, population-level performance-based agreements negotiated between the lovo-cel manufacturer and US payers have the potential to further mitigate uncertainty about the long-term cost-effectiveness of lovo-cel.
This study contributes to the growing literature on the cost-effectiveness of advanced treatments for SCD, offering synergies and advantages relative to other recent work [79]. The breadth of SCD-related events and complications included in our analysis is comparable to other comprehensive analyses of gene therapies [22–25, 29], and there is general alignment on the importance of considering a societal perspective at least as a co-base case [23–25, 29]. The two recent economic evaluations of gene therapy for SCD most relevant for comparison with our analysis are the evaluation by the Institute for Clinical and Economic Review (ICER) [24] and the Model for Economic Analysis of Sickle Cell Cure (MEASURE), developed as part of the Cure Sickle Cell Initiative [23, 29]. The Hutchinson Institute Sickle Cell Disease Outcomes Research and Economics (HISCORE) model also developed as part of the Cure Sickle Cell Initiative considered a less severe SCD population and a narrower set of events and complications [22, 29].
Our analysis, the MEASURE analysis [29], and the ICER evaluation [24] all compared one-time gene therapy with common care (or the standard of care) in patients with SCD with recurrent VOEs, and all considered a comparably broad set of SCD-related events and complications. The MEASURE analysis used a patient-level simulation approach similar to ours, whereas the ICER evaluation used a Markov-based approach. However, the MEASURE analysis used event and complication risks from interconnected prediction models estimated simultaneously from a US claims database, while our analysis and the ICER evaluation relied primarily on risks from the published literature. All three analyses used VOE reduction as the primary lovo-cel efficacy endpoint, but each differed in how the VOE effect was extended to other events, complications, and mortality. Of note, our analysis and the ICER evaluation used a combination of literature-informed relationships and assumptions to reduce the risks of non-VOE events, complications, and mortality for those with VOE-CR to levels intended to approach general population risks, although the magnitude of the reductions differed between the two approaches. The ICER analysis did not consider partial reductions in VOEs for those without VOE-CR. In contrast, the MEASURE analysis assumed the risk of chronic complications was eliminated beyond 5 years after treatment, did not consider any reduction in non-VOE events other than those estimated indirectly from their statistical prediction models, and applied a curative mortality hazard ratio from patients with SCD treated with allogeneic HSCT. Although each took a societal perspective as at least a co-base case, there were differences in the specific outcomes considered (unpaid caregiving and productivity impacts in all three analyses, caregiver quality of life in our analysis and the MEASURE analysis only, and consumption costs in the MEASURE analysis only) and in the data sources used to inform the approaches.
Despite the similarities in the objectives of these three analyses, the structural differences in how common care outcomes were estimated and valued, how the lovo-cel treatment effect was implemented, and the specific outcomes included resulted in expected differences in model-predicted outcomes (see Table S17 in the Supplementary Information for a comparison). The survival predictions for common care from our analysis and the MEASURE analysis were quite similar (13.5 versus 13.4 years), but both were meaningfully shorter than the common care survival predictions for the ICER evaluation. The survival gains for gene therapy were larger in our analysis (23.8 years) than in the MEASURE analysis (17.4 years) or ICER evaluation (undiscounted outcomes not reported), likely stemming from our use of general population-anchored mortality risk reductions. The gains in patient QALYs for gene therapy versus common care mirrored the differences in survival predictions, although the fundamentally different approaches to quantifying caregiver QALYs (QALYs lost versus QALYs accrued) resulted in overall comparable total QALY gains in our analysis (11.39) and the MEASURE analysis (11.9). Our analysis predicted higher direct medical cost offsets, in part because our lifetime cost prediction for common care (~$2.1 million) was higher than the predictions from the MEASURE (~$1.2 million) and ICER (~$1.5 million) models. The higher prevalence of baseline chronic complications in the MEASURE model also may have limited opportunities for direct medical cost offsets, as gene therapy was not predicted in any model to reverse existing complications. Despite the MEASURE model including additional consumption costs during extended periods of survival associated with lovo-cel, they estimated much higher productivity gains than our model or the ICER evaluation, resulting in net productivity (productivity minus consumption) estimates that were larger than the productivity gains estimated in our model and the ICER evaluation (which both relied on the same educational pathways research to estimate productivity gains). Ultimately, the predictions from each of these models should be further validated against real-world patient outcomes as additional long-term data emerge on patients treated with lovo-cel and other gene therapies for SCD.
The results of this study are subject to the limitations typical of all economic evaluations. Our target population was aligned with the efficacy population in the HGB-206 clinical trial; however, the population treated with lovo-cel in real-world settings may differ from the trial population in terms of baseline age, SCD severity, and comorbidity profile. Additionally, while HGB-206 data up to 61 months after transplantation show a sustained efficacy response [17], longer-term data from ongoing clinical research (including the LTF-307 study) and real-world data are needed to confirm the lifetime impact of lovo-cel. As noted previously, our scenario analysis results illustrate the sensitivity of our cost-effectiveness estimates to uncertainty in both achieving and maintaining VOE-CR. Our analysis relied on simplifying assumptions related to treatment status over time in the common care arm, including the assumption that HU or chronic transfusion use remains constant and the exclusion of common care-specific adverse events (e.g., iron overload). These assumptions likely have counteractive impacts on the predicted cost-effectiveness of lovo-cel compared with common care, and thus we find them unlikely to meaningfully impact our findings.
This study also has several notable strengths. The HGB-206 trial data represent the longest available follow-up of a gene therapy for SCD. Our patient-level simulation methodology, which accounts for the heterogeneity of the SCD population and the interconnectedness of SCD events and complications, was developed collaboratively over several years through a deliberative process relying on the SCD literature, clinician expertise, and patient perspectives. Although assumptions remained necessary to extrapolate the lovo-cel treatment effect to long-term outcomes, our assumptions were tempered to reasonably reflect heterogeneity in patients’ age and comorbid status at the time of treatment. Additionally, we varied key lovo-cel treatment effect assumptions in scenario analyses to illustrate the impact of uncertainty on the predicted clinical and economic value of lovo-cel. Our scenario analyses also explored the impact of uncertainty stemming from gaps in the literature related to SCD caregiver burden and the broader societal costs of SCD. Given the difference between the predicted third-party payer and societal perspective results in our analysis, addressing these data gaps also will be important in estimating the true long-term societal impact of lovo-cel and other gene therapies for SCD.
Although cost-effectiveness analysis is an important tool for value-based decision-making, the traditional framework employed by health technology assessment bodies in the US and globally often fails to account for novel value elements (e.g., value of hope, equity) [39] that may be particularly relevant for one-time therapies with curative intent [20, 21]. Accurately assessing the societal value of new therapies is particularly important for SCD, where the potentially transformative clinical benefit of advanced therapies such as lovo-cel may result in far-reaching effects for a patient population and community that remain marginalized [26, 80]. Stakeholders and decision-makers are encouraged to consider the broad potential societal benefits of these novel therapies when making value-based decisions related to access and reimbursement.
In summary, our findings suggest that lovo-cel gene therapy has the potential to meaningfully improve the lifetime health and economic trajectories of patients with SCD with recurrent VOEs. While data from the ongoing lovo-cel clinical trial program and from real-world studies are required to verify long-term outcomes, our results provide insights for decision-makers and other stakeholders in the US regarding the potential patient, healthcare system, and societal value of lovo-cel as a gene therapy for SCD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
We extend our deepest gratitude to the investigators, patients, and families who participated in or otherwise contributed to the HGB-206 study, thereby making this analysis possible. This study was conducted by RTI Health Solutions in collaboration with bluebird bio, clinical advisors, and a patient representative. Editorial and graphical design assistance was provided by John Forbes, Jonathan Pattishall, Jason Mathes, and Denise Lingenfelser of RTI Health Solutions.
Declarations
Funding
This study was funded by bluebird bio.
Conflict of interest
William L. Herring, Deirdre Mladsi, and Olivia M. Dong are full-time employees of RTI Health Solutions, a nonprofit research organization that received funding for the conduct of this study pursuant to a contract with bluebird bio. Their compensation is unconnected to the studies on which they work. Meghan E. Gallagher, Anjulika Chawla, Jennifer W. Leiding, and Clark Paramore are employees and shareholders of bluebird bio. Nirmish Shah reports research funding from Pfizer and AkiraBio and honorarium or consultancy fees from Novo Nordisk, Pfizer, Vertex, Emmaus, and Alexion. KC Morse reports consultancy fees from bluebird bio. Lixin Zhang is a statistician consultant at bluebird bio, business owner of Solution-Stat Inc., and contractor at Cytel and GlobalTeq Consulting Inc. Biree Andemariam reports research funding from American Society of Hematology, Connecticut Department of Public Health, Hemanext, Health Resources and Services Administration, Imara, Novartis, Novo Nordisk, Patient-Centered Outcomes Research Institute, and Pfizer and honorarium or consultancy fees from Afimmune, Agios, bluebird bio, CVS/Accordant, Emmaus, Forma Therapeutics, GlaxoSmithKline, Global Blood Therapeutics, Hemanext, Novartis, Novo Nordisk, Protagonist Therapeutics, and Vertex.
Availability of data and material
Appropriately deidentified patient-level data sets and supporting documents from the HGB-206 clinical trial (NCT02140554) may be shared following attainment of applicable marketing approvals and consistent with criteria established by bluebird bio and/or industry best practices to maintain the privacy of study participants. For more information, please contact datasharing@bluebirdbio.com. All other data relevant to the study were obtained from the published literature or other publicly available sources and have been presented in the article and the Supplementary Information.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Authors’ contributions
William L. Herring, Deirdre Mladsi, Meghan E. Gallagher, Anjulika Chawla, Biree Andemariam, Clark Paramore, Nirmish Shah, and KC Morse contributed to the conception and design of the study. William L. Herring contributed to programming the model. Olivia M. Dong, William L. Herring, and Meghan E. Gallagher contributed to drafting the manuscript. All authors contributed to the acquisition of data or analysis and interpretation of data, revising the manuscript, and final approval of the manuscript.
References
- 1.National Heart, Lung, and Blood Institute. Sickle cell disease. 2022. https://www.nhlbi.nih.gov/health/sickle-cell-disease. Accessed 26 Aug 2022.
- 2.Kanter J, Kruse-Jarres R. Management of sickle cell disease from childhood through adulthood. Blood Rev. 2013;27(6):279–287. doi: 10.1016/j.blre.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease—life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 4.Lubeck D, Agodoa I, Bhakta N, Danese M, Pappu K, Howard R, et al. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw Open. 2019;2(11):e1915374. doi: 10.1001/jamanetworkopen.2019.15374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dampier C, Lieff S, LeBeau P, Rhee S, McMurray M, Rogers Z, et al. Health-related quality of life in children with sickle cell disease: a report from the Comprehensive Sickle Cell Centers Clinical Trial Consortium. Pediatr Blood Cancer. 2010;55(3):485–494. doi: 10.1002/pbc.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dampier C, LeBeau P, Rhee S, Lieff S, Kesler K, Ballas S, et al. Health-related quality of life in adults with sickle cell disease (SCD): a report from the comprehensive sickle cell centers clinical trial consortium. Am J Hematol. 2011;86(2):203–205. doi: 10.1002/ajh.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiao B, Johnson KM, Ramsey SD, Bender MA, Devine B, Basu A. Long-term survival with sickle cell disease: a nationwide cohort study of Medicare and Medicaid beneficiaries. Blood Adv. 2023;7(13):3276–3283. doi: 10.1182/bloodadvances.2022009202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassell KL. Population estimates of sickle cell disease in the US. Am J Prev Med. 2010;38(4 Suppl):S512–S521. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Pecker LH, Lanzkron S. Sickle cell disease. Ann Intern Med. 2021;174(1):ITC1–ITC16. doi: 10.7326/AITC202101190. [DOI] [PubMed] [Google Scholar]
- 10.National Heart, Lung, and Blood Institute. A century of progress: milestones in sickle cell disease research and care. 2010. https://www.nhlbi.nih.gov/files/docs/public/blood/Tagged2NHLBISickleCellTimeline.pdf. Accessed 16 Dec 2021.
- 11.Centers for Disease Control and Prevention. Sickle cell disease clinical guidelines. 2021. https://www.cdc.gov/ncbddd/sicklecell/recommendations.html. Accessed 16 Dec 2021.
- 12.Kavanagh PL, Fasipe TA, Wun T. Sickle cell disease: a review. JAMA. 2022;328(1):57–68. doi: 10.1001/jama.2022.10233. [DOI] [PubMed] [Google Scholar]
- 13.Sheth S, Licursi M, Bhatia M. Sickle cell disease: time for a closer look at treatment options? Br J Haematol. 2013;162(4):455–464. doi: 10.1111/bjh.12413. [DOI] [PubMed] [Google Scholar]
- 14.Kanter J, Liem RI, Bernaudin F, Bolanos-Meade J, Fitzhugh CD, Hankins JS, et al. American Society of Hematology 2021 guidelines for sickle cell disease: stem cell transplantation. Blood Adv. 2021;5(18):3668–3689. doi: 10.1182/bloodadvances.2021004394C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.bluebird bio. Lyfgenia (lovotibeglogene autotemcel) [package insert]. US Food and Drug Administration website. 2023. https://www.fda.gov/media/174610/download?attachment. Accessed 7 Feb 2024.
- 16.bluebird bio. bluebird bio Announces FDA Priority Review of the Biologics License Application for lovotibeglogene autotemcel (lovo-cel) for patients with sickle cell disease (SCD) 12 years and older with a history of vaso-occlusive events. 2023. https://www.businesswire.com/news/home/20230621695509/en/. Accessed 30 Aug 2023.
- 17.Kanter J, Thompson AA, Kwiatkowski JL, Parikh S, Mapara M, Rifkin-Zenenberg S, et al. 1051 Efficacy, safety, and health-related quality of life (HRQOL) in patients with sickle cell disease (SCD) who have received lovotibeglogene autotemcel (lovo-cel) gene therapy: up to 60 months of follow-up. Presented at the 65th American Society of Hematology (ASH) Annual Meeting and Exposition, San Diego, California. 2023. https://ash.confex.com/ash/2023/webprogram/Paper174229.html. Accessed 7 Feb 2024.
- 18.Young CM, Quinn C, Trusheim MR. Durable cell and gene therapy potential patient and financial impact: US projections of product approvals, patients treated, and product revenues. Drug Discov Today. 2022;27(1):17–30. doi: 10.1016/j.drudis.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Senior M. Fresh from the biotech pipeline: fewer approvals, but biologics gain share. Nat Biotechnol. 2023;41(2):174–182. doi: 10.1038/s41587-022-01630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrison LP, Jr, Jiao B, Dabbous O. Gene therapy may not be as expensive as people think: challenges in assessing the value of single and short-term therapies. J Manag Care Spec Pharm. 2021;27(5):674–681. doi: 10.18553/jmcp.2021.27.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu T, Pochopien M, Liang S, Saal G, Paterak E, Janik J, Toumi M. Gene therapy evidence generation and economic analysis: pragmatic considerations to facilitate fit-for-purpose health technology assessment. Front Public Health. 2022;10:773629. doi: 10.3389/fpubh.2022.773629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winn A, Basu A, Ramsey SD. A framework for a health economic evaluation model for patients with sickle cell disease to estimate the value of new treatments in the United States of America. Pharmacoecon Open. 2023;7(2):313–320. doi: 10.1007/s41669-023-00390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson KM, Jiao B, Bender MA, Ramsey SD, Devine B, Basu A. Development of a conceptual model for evaluating new non-curative and curative therapies for sickle cell disease. PLoS One. 2022;17(4):e0267448. doi: 10.1371/journal.pone.0267448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaudoin F, Thokala P, Nikitin D, Campbell J, Spackman E, McKenna A, et al. Gene therapies for sickle cell disease: effectiveness and value; draft evidence report. Institute for Clinical and Economic Review. 2023. https://icer.org/wp-content/uploads/2023/04/SCD_FOR-PUBLICATION.pdf. Accessed 2 Aug 2023.
- 25.Udeze C, Xie Y, Ogunsile FJ, Mujumdar U, Yang H, Chen X, et al. EE173 Economic evaluation of exagamglogene autotemcel (EXA-CEL) gene-edited therapy in patients with sickle cell disease with recurrent vaso-occlusive crises. Value Health. 2023;26(6):S91. doi: 10.1016/j.jval.2023.03.474. [DOI] [Google Scholar]
- 26.Goshua G, Calhoun C, Ito S, James LP, Luviano A, Krishnamurti L, Pandya A. Distributional cost-effectiveness of equity-enhancing gene therapy in sickle cell disease in the United States. Ann Intern Med. 2023;176(6):779–787. doi: 10.7326/M22-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salcedo J, Bulovic J, Young CM. Cost-effectiveness of a hypothetical cell or gene therapy cure for sickle cell disease. Sci Rep. 2021;11(1):10838. doi: 10.1038/s41598-021-90405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeMartino P, Haag MB, Hersh AR, Caughey AB, Roth JA. A budget impact analysis of gene therapy for sickle cell disease: the Medicaid perspective. JAMA Pediatr. 2021;175(6):617–623. doi: 10.1001/jamapediatrics.2020.7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basu A, Winn AN, Johnson KM, Jiao B, Devine B, Hankins JS, et al. Gene therapy versus common care for eligible individuals with sickle cell disease in the United States: a cost-effectiveness analysis. Ann Intern Med. 2024;177:155–164. doi: 10.7326/M23-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices—overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–1. Med Decis Mak. 2012;32(5):667–677. doi: 10.1177/0272989X12454577. [DOI] [PubMed] [Google Scholar]
- 31.Roberts M, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M, Force I-SMGRPT Conceptualizing a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—2. Value Health. 2012;15(6):804–811. doi: 10.1016/j.jval.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB, Force I-SMGRPT Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—7. Value Health. 2012;15(6):843–850. doi: 10.1016/j.jval.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Purser M, Gallagher M, Mladsi D, Weber JM, Andemariam B, Kaye JA, Chawla A. PBI20 evaluation of published models in sickle cell disease against key criteria for an economic model for a potentially curative one-time treatment. Value Health. 2020;23(2):S414. doi: 10.1016/j.jval.2020.08.098. [DOI] [Google Scholar]
- 34.ClinicalTrials.gov. NCT02140554. 20 April 2020. https://clinicaltrials.gov/ct2/show/NCT02140554. Accessed 1 June 2021.
- 35.Kanter J, Walters MC, Krishnamurti L, Mapara MY, Kwiatkowski JL, Rifkin-Zenenberg S, et al. Biologic and clinical efficacy of LentiGlobin for sickle cell disease. N Engl J Med. 2022;386(7):617–628. doi: 10.1056/NEJMoa2117175. [DOI] [PubMed] [Google Scholar]
- 36.Migotsky M, Beestrum M, Badawy SM. Recent advances in sickle-cell disease therapies: a review of voxelotor, crizanlizumab, and l-glutamine. Pharmacy (Basel). 2022;10(5):123. doi: 10.3390/pharmacy10050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 38.Institute for Clinical and Economic Review. 2020–2023 value assessment framework. 2020. https://icer-review.org/wp-content/uploads/2020/10/ICER_2020_2023_VAF_102220.pdf. Accessed 29 Oct 2020.
- 39.Lakdawalla DN, Doshi JA, Garrison LP, Jr, Phelps CE, Basu A, Danzon PM. Defining elements of value in health care-a health economics approach: an ISPOR Special Task Force Report [3] Value Health. 2018;21(2):131–139. doi: 10.1016/j.jval.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Shah NR, Bhor M, Latremouille-Viau D, Kumar Sharma V, Puckrein GA, Gagnon-Sanschagrin P, et al. Vaso-occlusive crises and costs of sickle cell disease in patients with commercial, Medicaid, and Medicare insurance—the perspective of private and public payers. J Med Econ. 2020;23(11):1345–1355. doi: 10.1080/13696998.2020.1813144. [DOI] [PubMed] [Google Scholar]
- 41.McClish DK, Smith WR, Levenson JL, Aisiku IP, Roberts JD, Roseff SD, Bovbjerg VE. Comorbidity, pain, utilization, and psychosocial outcomes in older versus younger sickle cell adults: the PiSCES project. Biomed Res Int. 2017;2017:1–10. doi: 10.1155/2017/4070547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paramore C, Kong A, Minegishi S, Shi W. Characterizing a population with severe manifestations of sickle cell disease using US real-world evidence. Blood. 2018;132(suppl 1):4811. doi: 10.1182/blood-2018-99-118874. [DOI] [Google Scholar]
- 43.Wilson-Frederick SM, Hulihan M, Blaz J, Young BM, Center for Medicare and Medicare Services. Prevalence of sickle cell disease among Medicare fee-for-service beneficiaries, age 18–75 years, in 2016. 2019. https://www.cms.gov/About-CMS/Agency-Information/OMH/Downloads/Data-Highlight-15-Sickle-Cell-Disease.pdf. Accessed 16 Aug 2021.
- 44.Naik RP, Streiff MB, Haywood C, Jr, Nelson JA, Lanzkron S. Venous thromboembolism in adults with sickle cell disease: a serious and under-recognized complication. Am J Med. 2013;126(5):443–449. doi: 10.1016/j.amjmed.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manwani D, Burnett AL, Paulose J, Yen GP, Burton T, Anderson A, et al. Treatment patterns and burden of complications associated with sickle cell disease: a US retrospective claims analysis. EJHaem. 2022;3(4):1135–1144. doi: 10.1002/jha2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong O, Purser M, Herring W, Mladsi D, Gallagher M, Chawla A, et al. Relationships among sickle cell disease complications and their implications for cost-effectiveness modeling for therapies with curative intent. Poster presented at the ISPOR 2023 Conference; May 7, 2023. Boston, MA. [abstract] Value Health. 2023;26(6):S115–S116. doi: 10.1016/j.jval.2023.03.611. [DOI] [Google Scholar]
- 47.Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease: rates and risk factors. N Engl J Med. 1991;325(1):11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 48.National Institute for Health and Care Excellence. Clinical guideline 143, sickle cell acute painful episode (appendix F). 2012. https://www.nice.org.uk/guidance/cg143/evidence/appendix-f-full-health-economic-report-pdf-186634334. Accessed 26 Aug 2022. [PubMed]
- 49.Maitra P, Caughey M, Robinson L, Desai PC, Jones S, Nouraie M, et al. Risk factors for mortality in adult patients with sickle cell disease: a meta-analysis of studies in North America and Europe. Haematologica. 2017;102(4):626–636. doi: 10.3324/haematol.2016.153791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arias E, Xu J. United States life tables, 2019. Natl Vital Stat Rep. 2022;70(19):1–59. [PubMed] [Google Scholar]
- 51.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine. 2005;84(6):363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 52.Darbari DS, Wang Z, Kwak M, Hildesheim M, Nichols J, Allen D, et al. Severe painful vaso-occlusive crises and mortality in a contemporary adult sickle cell anemia cohort study. PLoS One. 2013;8(11):e79923. doi: 10.1371/journal.pone.0079923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elmariah H, Garrett ME, De Castro LM, Jonassaint JC, Ataga KI, Eckman JR, et al. Factors associated with survival in a contemporary adult sickle cell disease cohort. Am J Hematol. 2014;89(5):530–535. doi: 10.1002/ajh.23683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brunson A, Lei A, Rosenberg AS, White RH, Keegan T, Wun T. Increased incidence of VTE in sickle cell disease patients: risk factors, recurrence and impact on mortality. Br J Haematol. 2017;178(2):319–326. doi: 10.1111/bjh.14655. [DOI] [PubMed] [Google Scholar]
- 55.Chaturvedi S, Ghafuri DL, Jordan N, Kassim A, Rodeghier M, DeBaun MR. Clustering of end-organ disease and earlier mortality in adults with sickle cell disease: a retrospective-prospective cohort study. Am J Hematol. 2018;93(9):1153–1160. doi: 10.1002/ajh.25202. [DOI] [PubMed] [Google Scholar]
- 56.Walters MC, Tisdale JF, Mapara MY, Krishnamurti L, Kwiatkowski JL, Aygun B, et al. Sustained improvements in patient-reported quality of life up to 24 months post-treatment with LentiGlobin for sickle cell disease (bb1111) gene therapy. Blood. 2021;138(1):7. doi: 10.1182/blood-2021-146905. [DOI] [Google Scholar]
- 57.Kansal AR, Reifsnider OS, Brand SB, Hawkins N, Coughlan A, Li S, et al. Economic evaluation of betibeglogene autotemcel (Beti-cel) gene addition therapy in transfusion-dependent beta-thalassemia. J Mark Access Health Policy. 2021;9(1):1922028. doi: 10.1080/20016689.2021.1922028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matza LS, Paramore LC, Stewart KD, Karn H, Jobanputra M, Dietz AC. Health state utilities associated with treatment for transfusion-dependent beta-thalassemia. Eur J Health Econ. 2020;21(3):397–407. doi: 10.1007/s10198-019-01136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Red Book Online. IBM Micromedex [database online]. Truven Health Analytics/IBM Watson Health 2024. https://www.micromedexsolutions.com/home/dispatch/CS/63420F/PFActionId/pf.HomePage/ssl/true. Accessed 9 Feb 2024.
- 60.Dolan R. Understanding the Medicaid prescription drug rebate program. Kaiser Family Foundation (KFF); 2019. https://www.kff.org/medicaid/issue-brief/understanding-the-medicaid-prescription-drug-rebate-program/. Accessed 7 Feb 2024.
- 61.Campbell A, Cong Z, Agodoa I, Song X, Martinez DJ, Black D, et al. The economic burden of end-organ damage among Medicaid patients with sickle cell disease in the United States: a population-based longitudinal claims study. J Manag Care Spec Pharm. 2020;26(9):1121–1129. doi: 10.18553/jmcp.2020.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gallagher ME, Chawla A, Brady BL, Badawy SM. Heterogeneity of the long-term economic burden of severe sickle cell disease: a 5-year longitudinal analysis. J Med Econ. 2022;25(1):1140–1148. doi: 10.1080/13696998.2022.2133824. [DOI] [PubMed] [Google Scholar]
- 63.Graf M, Tuly R, Gallagher M, Sullivan J, Jena AB. Value of a cure for sickle cell disease in reducing economic disparities. Am J Hematol. 2022;97(8):E289–E291. doi: 10.1002/ajh.26617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiao B, Basu A, Ramsey S, Roth J, Bender MA, Quach D, Devine B. Health state utilities for sickle cell disease: a catalog prepared from a systematic review. Value Health. 2022;25(2):276–287. doi: 10.1016/j.jval.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barcelos G, Besser M, O’Flaherty ED, O'Sullivan SB, Bourke S, Oluboyede Y, et al. PCR116 quality of life of and economic burden on caregivers of individuals with sickle cell disease in the UK and France: a cross-sectional survey. Value Health. 2022;25(12):S413. doi: 10.1016/j.jval.2022.09.2051. [DOI] [Google Scholar]
- 66.Basu A, Carlson J, Veenstra D. Health years in total: a new health objective function for cost-effectiveness analysis. Value Health. 2020;23(1):96–103. doi: 10.1016/j.jval.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 67.Campbell JD, Whittington MD, Pearson SD. An alternative measure of health for value assessment: the equal value life-year. Pharmacoeconomics. 2023;41(10):1175–1182. doi: 10.1007/s40273-023-01302-6. [DOI] [PubMed] [Google Scholar]
- 68.York Health Economics Consortium. Net Monetary Benefit. 2024. https://yhec.co.uk/glossary/net-monetary-benefit/. Accessed 16 Feb 2024.
- 69.Lakdawalla DN, Phelps CE. Health technology assessment with diminishing returns to health: the generalized risk-adjusted cost-effectiveness (GRACE) approach. Value Health. 2021;24(2):244–249. doi: 10.1016/j.jval.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 70.Towse A, Fenwick E. Uncertainty and cures: discontinuation, irreversibility, and outcomes-based payments: what is different about a one-off treatment? Value Health. 2019;22(6):677–683. doi: 10.1016/j.jval.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 71.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD, Force I-SMGRPT Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—6. Value Health. 2012;15(6):835–842. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 72.Briggs AH, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006. [Google Scholar]
- 73.Karnon J, Stahl J, Brennan A, Caro JJ, Mar J, Moller J, Force I-SMGRPT Modeling using discrete event simulation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—4. Value Health. 2012;15(6):821–827. doi: 10.1016/j.jval.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 74.Vemer P, Corro Ramos I, van Voorn GA, Al MJ, Feenstra TL. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34(4):349–361. doi: 10.1007/s40273-015-0327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garrison LP, Pezalla E, Towse A, Yang H, Faust E, Wu EQ, et al. Hemophilia gene therapy value assessment: methodological challenges and recommendations. Value Health. 2021;24(11):1628–1633. doi: 10.1016/j.jval.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 76.Wenzl M, Chapman S. Performance-based managed entry agreements for new medicines in OECD countries and EU member states. 2019. https://www.oecd-ilibrary.org/content/paper/6e5e4c0f-en. Accessed 8 Feb 2024.
- 77.Wong CH, Li D, Wang N, Gruber J, Conti RM, Lo AW. Estimating the financial impact of gene therapy in the US. 2021. http://www.nber.org/papers/w28628. Accessed Apr 2021.
- 78.Besley S, Henderson N, Towse A, Cole A. Health technology assessment of gene therapies: are our methods fit for purpose? OHE Consulting Report, London: Office of Health Economics. 2022. www.ohe.org/publications/health-technology-assessment-gene-therapies-are-our-methods-fit-purpose. Accessed 9 Feb 2024.
- 79.Jiao B, Basu A, Roth J, Bender M, Rovira I, Clemons T, et al. The use of cost-effectiveness analysis in sickle cell disease: a critical review of the literature. Pharmacoeconomics. 2021;39(11):1225–1241. doi: 10.1007/s40273-021-01072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Linthicum M, Jalowsky M, Valentine A, Ahmed R, Bright J, Chapman R. Finding equity in value racial and health equity implications of U.S. HTA processes 2 November 2022. https://sickcells.org/wp-content/uploads/2022/10/IVI_Sick-Cells_Equity-in-Value_2022.pdf. Accessed 31 Aug 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.