Abstract
Based on structural and functional properties, three groups of large staphylococcal multiresistance plasmids have been recognized, viz., the pSK1 family, pSK41-like conjugative plasmids, and β-lactamase–heavy-metal resistance plasmids. Here we describe an analysis of the replication functions of a representative of each of these plasmid groups. The replication initiation genes from the Staphylococcus aureus plasmids pSK1, pSK41, and pI9789::Tn552 were found to be related to each other and to the Staphylococcus xylosus plasmid pSX267 and are also related to rep genes of several plasmids from other gram-positive genera. Nucleotide sequence similarity between pSK1 and pI9789::Tn552 extended beyond their rep genes, encompassing upstream divergently transcribed genes, orf245 and orf256, respectively. Our analyses revealed that genes encoding proteins related to the deduced orf245 product are variously represented, in several types of organization, on plasmids possessing six seemingly evolutionarily distinct types of replication initiation genes and including both theta-mode and rolling-circle replicons. Construction of minireplicons and subsequent functional analysis demonstrated that orf245 is required for the segregational stability of the pSK1 replicon. In contrast, no gene equivalent to orf245 is evident on the conjugative plasmid pSK41, and a minireplicon encoding only the pSK41 rep gene was found to exhibit a segregational stability approaching that of the parent plasmid. Significantly, the results described establish that many of the large multiresistance plasmids that have been identified in clinical staphylococci, which were formerly presumed to be unrelated, actually utilize an evolutionarily related theta-mode replication system.
Clinical Staphylococcus aureus strains often harbor multiple plasmids, ranging from small rolling-circle (RC) replicating plasmids that are cryptic or encode only a single resistance determinant to larger multiresistance and conjugative plasmids (12, 32, 36). Three groups of multiresistance plasmids have been recognized in staphylococci. Isolates from the 1960s and 1970s were commonly found to carry multiresistance plasmids conferring resistance to penicillin and heavy metals or other inorganic ions (48). Such β-lactamase–heavy-metal resistance plasmids characteristically contain the β-lactamase-encoding transposon Tn552 or a derivative and operons mediating resistance to arsenical, cadmium, and/or mercuric ions (36). Some β-lactamase–heavy-metal resistance plasmids also contain Tn551, conferring resistance to macrolide-lincosamide-streptogramin type B antibiotics (33); an IS256-bounded composite aminoglycoside resistance transposon, Tn4001 (30); and/or a qacA or qacB antiseptic and disinfectant multidrug resistance determinant (30).
pSK41-like conjugative multiresistance plasmids were first detected in strains isolated in the mid 1970s. Such plasmids commonly contain a Tn4001 hybrid structure (6) and IS257-flanked cointegrated copies of small plasmids, such as the aminoglycoside resistance plasmid pUB110 (7). Other determinants encoded by cointegrated episomes carried by pSK41 family plasmids include smr, which confers multidrug resistance to antiseptics and disinfectants, and dfrA, which mediates trimethoprim resistance (4). Mupirocin resistance plasmids related to pSK41 have also been identified (31).
Clinical S. aureus strains isolated in Australia and the United Kingdom in the 1980s typically contained a multiresistance plasmid related to pSK1 (28, 30, 50, 55). pSK1 family plasmids commonly carry a qacA gene (40, 53), Tn4001 (39), and an IS257-containing structure termed Tn4003, which encodes trimethoprim resistance (11, 41); some pSK1 family plasmids also possess a Tn552-like β-lactamase resistance transposon (17).
Although the replication of staphylococcal RC plasmids has been studied in considerable detail, comparatively little attention has been paid to the replication systems of larger plasmids from this genus, which are presumed to replicate via the theta mode (21, 49). To date, the replication initiation region of only a single staphylococcal multiresistance plasmid has been characterized, that of the β-lactamase–heavy-metal resistance plasmid pSX267 from Staphylococcus xylosus (16, 19). To broaden our understanding of the replicative mechanisms of staphylococcal multiresistance plasmids and the evolutionary relationships among them, we have characterized the replication regions from the prototypes of the pSK1 and pSK41 plasmid families and a β-lactamase–heavy-metal resistance plasmid, pI9789::Tn552.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The Escherichia coli host strain used was DH5α (F− endA hsdR17 supE44 thi-1 λ− recA1 gyrA96 relA1 φ80dlacZΔM15; Bethesda Research Laboratories), while the S. aureus host strains used were RN4220 (23) and the rifampin- and novobiocin-resistant strain SK982 (29). The S. aureus strain RN1965 (43) was used as a source of a reference set of plasmids for plasmid incompatibility tests (see below). pUC18 (58) and derivatives were used as cloning vectors. The general culture conditions were 37°C on Luria-Bertani (LB) agar or in LB medium (45). Where appropriate, antimicrobial agents were used at the following concentrations: ampicillin, 100 μg/ml; cadmium chloride, 50 μM; erythromycin, 20 μg/ml; gentamicin, 20 μg/ml; neomycin, 10 μg/ml; novobiocin, 2 μg/ml; rifampin, 20 μg/ml; tetracycline, 10 μg/ml; trimethoprim, 100 μg/ml.
DNA manipulations.
Plasmid DNA was isolated from E. coli using a Quantum Prep plasmid miniprep kit (Bio-Rad), while DNA was isolated from S. aureus as described by Lyon et al. (28). Protoplast transformation of S. aureus was performed as described by Götz et al. (18). Electroporation of S. aureus with a Bio-Rad gene pulser was performed as described previously (25). DNA cloning and transformation of E. coli was performed using standard techniques (45). Restriction endonucleases and T4 DNA ligase (Promega) were used in accordance with the manufacturer's instructions. PCRs were carried out with Pfu DNA polymerase (Stratagene) according to the manufacturer's recommendations. Oligonucleotide primers for PCR and nucleotide sequencing were made with a Beckman Oligo 1000 synthesizer.
The pSK1-derived fragment carried by pSK4829 was amplified with the rep upstream primer, 5′-GCGAAGCTTCCCTAGATAATTCTTCTGATAATTTAG-3′, and the downstream primer, 5′-GCGGGATCCTTTTCTGTTGACTTAATTCC-3′, whereas a different upstream primer, 5′-GCGAAGCTTGTTACATTCAATTCATCAGCAAACC-3′, was used to amplify the fragment carried by pSK4833. The HindIII and BamHI sites (underlined) incorporated into the primers allowed ligation of the restricted PCR products into similarly cleaved pWE180 vector DNA. pWE180 was constructed by ligation of a blunted PstI-ClaI fragment of pE194, encoding the ermC erythromycin resistance determinant (22), into a blunted NdeI site of pUC18. The pSK41-derived fragment carried by pSK5413 was amplified with the rep upstream primer, 5′-AATGGATCCATATAGTTTTTGTATACGGTATTC-3′, and the downstream primer, 5′-CTCGGATCCACTAATTTATCATGTCAGTGTTC-3′. The BamHI site (underlined) incorporated into the upstream primer and an internal HindIII site within the pSK41 sequence (at nucleotide 14485 of GenBank entry AF051916) allowed ligation of the restricted PCR product into similarly cleaved pUC18 vector DNA. BamHI and SacI double digestion of this plasmid, and subsequent ligation with a DNA fragment encoding a tetA(K) tetracycline resistance gene (20), amplified from the chromosome of a clinical S. aureus isolate (A. E. Simpson, R. A. Skurray, and N. Firth, unpublished data), generated pSK5413.
Nucleotide sequence determination and data analysis.
Dideoxy sequencing (46) was performed with SequiTherm cycle-sequencing kits (Epicentre Technologies) or Sequenase version 2 sequencing kits (United States Biochemical) according to the manufacturers' recommendations. All restriction sites were crossed, and all sequence was determined on both DNA strands; derivatives of pBR322 (5) or pUC119 (56) containing pSK1 or pI9789::Tn552 restriction fragments were utilized as double-stranded sequencing substrates. Sequences were stored and assembled with the program SEQUENCHER (Gene Codes Corporation) and analyzed with the Genetics Computer Group (9) and PHYLIP (10) packages maintained by the Australian National Genomic Information Service, University of Sydney. Sequence similarities were assessed with pairwise alignments generated with the program GAP (9) using the Dayhoff 250 PAM matrix (8). Statistical significance (Z) was calculated using the formula Z = (a − m)/ς, where a is the alignment score, m is the mean of 100 alignment scores where one of the sequences has been randomly shuffled, and ς is the standard deviation of m (37). Z scores greater than 3, 6, or 10 were taken to be indicative of “possible,” “probable,” or “highly probable” evolutionary relatedness, respectively (26). Phylogenetic trees were drawn by using the program TREEVIEW (34).
Assay of plasmid segregational stability.
An overnight culture of the strain(s) to be assayed, grown in medium selective for the plasmid, was diluted in saline, and a viable count was performed using nonselective LB agar plates. Additionally, 100 μl of the 10−2 dilution was used to inoculate 10 ml of LB medium. After overnight growth without selection, this culture was diluted, counted, and subcultured as before; this process was repeated until approximately 100 generations in the nonselective medium were achieved. One hundred colonies from each day's viable-count plates were patched onto media with and without selection for the plasmid so that the proportion of the population retaining the resistance phenotype conferred by the plasmid could be quantitated. DNA was isolated from selected colonies to confirm the absence or presence of the relevant plasmid.
Plasmid incompatibility tests.
S. aureus strain RN1965 (43) contains RC plasmids representing incompatibility groups (Inc) 3 to 5 and larger plasmids defining Inc1, Inc2, and Inc6. The six plasmids carried by this strain are pRN3025 (Inc1), pRN2003 (Inc2), pT127 (Inc3), pC221 (Inc4), pS177 (Inc5), and pK545 (Inc6), which confer resistance to erythromycin, cadmium, tetracycline, chloramphenicol, streptomycin, and neomycin, respectively. Plasmid incompatibility was determined by using mixed-culture transfer experiments (29) employing RN1965 as the donor and SK982 containing the plasmid under test as the recipient. Transferrants were selected on LB agar containing rifampin, novobiocin, and selection for the incoming plasmid only, and were subsequently patched onto media selective for both incoming and resident plasmids to determine coexistence and hence compatibility. This was confirmed by inoculating the cultures into 10 ml of LB medium selective for only the incoming plasmid, plating them with the same selection for single colonies, and patching them onto LB agar selective for both incoming and resident plasmids; maintenance of the resident plasmid by more than 90% of the cells carrying the incoming plasmid was taken to indicate compatibility. DNA isolations were subsequently performed on representative isolates to confirm the presence of both plasmids.
Nucleotide sequence accession numbers.
The nucleotide sequences of the pSK1 and pI9789::Tn552 replication regions are available under the GenBank accession numbers AF203376 and AF203377, respectively.
RESULTS
Replication regions of the multiresistance plasmids pSK1 and pI9789::Tn552.
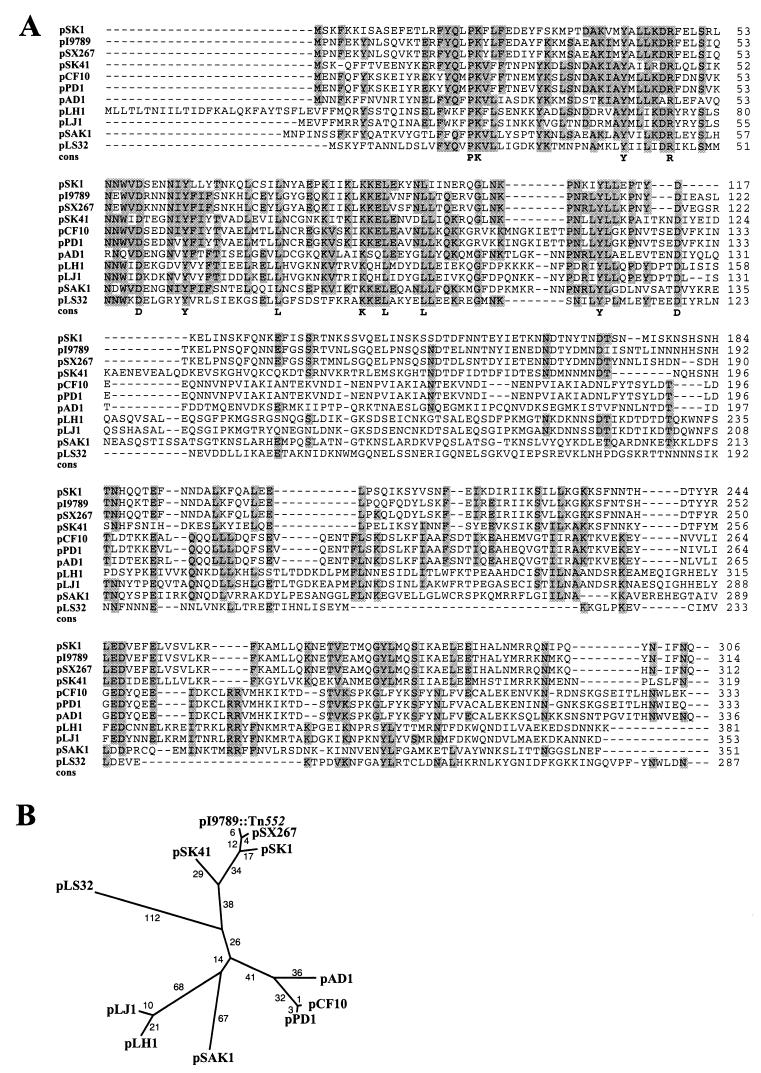
The putative replication regions from the prototype of the pSK1 family of plasmids (12) and the β-lactamase–heavy-metal resistance plasmid pI9789::Tn552 (42, 48) were identified based on similarity to the predicted rep region of the conjugative S. aureus multiresistance plasmid, pSK41 (4), and the demonstrated rep region of the S. xylosus β-lactamase–heavy-metal resistance plasmid, pSX267, which is believed to replicate via the theta mode (16). The functionality of the pSK1 and pI9789::Tn552 rep regions was subsequently demonstrated in S. aureus (see below). The extent of nucleotide sequence similarity among the replication regions of pSK1, pI9789::Tn552, and pSK41, which represent the three groups of large staphylococcal multiresistance plasmids identified to date (12), is illustrated in the multiple sequence alignment shown in Fig. 1; due to the high degree of identity (89%) between the pI9789::Tn552 and pSX267 replication regions, for clarity, only the longer pI9789::Tn552 sequence is shown. The replication initiation genes of these staphylococcal plasmids encode products similar (17 ≤ Z ≤ 43) to those of plasmids from other gram-positive genera, including pLS32 from Bacillus natto (52), pLJ1 (51), pLH1 (54), and pSAK1 (GenBank entry Z50862) from Lactobacillus species, and the enterococcal plasmids pAD1 (57), pCF10 (44), and pPD1 (14) (Fig. 2A), a number of which have been proposed to utilize the theta mode of replication (see also reference 21). Phylogenetic analysis (Fig. 2B) revealed that the Rep proteins fall into generic clusters, providing no evidence of horizontal transmission of the rep genes among these genera.
FIG. 1.
Nucleotide sequence alignment of the replication regions of pSK1, pI9789::Tn552, and pSK41. The pSK41 sequence shown corresponds to nt 12991 to 14216 of GenBank entry AF051917. Nucleotides present in two or more sequences at any one position are shaded, and insertions or deletions are denoted by dashes. The nucleotide sequences are numbered on the right. Translation start and stop codons of the indicated genes (outlined) are boxed. Directly repeated sequences are indicated by arrows, whereas inverted repeats are shown by half arrows.
FIG. 2.
(A) Multiple sequence alignment of the deduced pSK1 and pI9789::Tn552 rep products and related proteins. The amino acid sequences of the replication initiation proteins from the indicated plasmids were obtained from the following GenBank entries: pSX267, X92404; pSK41, AF051916; pCF10, L14285; pPD1, D78016; pAD1, L01794; pLH1, AJ222725; pLJ1, J04240; pSAK1, Z50862; and pLS32, D49467. Residue numbering is shown on the right. Amino acids common to five or more sequences at any position are shaded, globally conserved residues are indicated in the consensus (cons) line, and insertions or deletions are denoted by dashes. (B) Phylogenetic analysis of the Rep proteins shown in panel A. The unrooted tree was constructed by using the programs PROTDIST and NEIGHBOR (10) from the multiple alignment generated by PILEUP (9) shown in panel A. Branch lengths in arbitrary units are indicated. All nodes had bootstrap values of greater than 80%, and an equivalent tree topology was obtained with the program PROTPARS (10).
The phylogenetic analysis also suggested that the pSK1 replicon type is more closely related to that of the β-lactamase–heavy-metal resistance plasmids than it is to that of pSK41-like plasmids. Consistent with this notion, nucleotide sequence similarity between pSK41 and both pSK1 and pI9789::Tn552 is primarily confined to the rep gene coding sequences, whereas similarity between pSK1 and pI9789::Tn552 continues beyond this gene in both directions (Fig. 1). Downstream of rep, similarity extends across a region, corresponding to sequences beyond nucleotide 2280 of pI9789::Tn552 (Fig. 1), that appears to represent a remnant of a cointegrated RC plasmid which has been described previously on pSX267 (16). This region of similarity, which ends adjacent to an inverted repeat in pSK1, bears greater evidence of genetic drift than the neighboring rep coding segment, including two probable deletions within the pSK1 sequence (Fig. 1). Nevertheless, the degree of conservation of this vestige on pSK1 and pI9789::Tn552 argues that the lineages of these plasmids diverged quite recently in evolutionary time.
Upstream of the respective rep genes, recognizable nucleotide sequence similarity between pSK1 and pI9789::Tn552 encompasses the intergenic region and a divergently transcribed open reading frame, orf245 on pSK1 and orf256 on pI9789::Tn552 (Fig. 1). The deduced products of these genes share statistically significant amino acid sequence similarity with proteins encoded by plasmids from other gram-positive genera, including a large number of lactococcal plasmids related to pUCL22 (13, 47), and the Tetragenococcus halophilus plasmid pUCL287 (3). In contrast to the divergent organizations of pSK1 orf245 and pI9789::Tn552 orf256 in relation to their respective rep genes, the homologous genes on these other plasmids are located immediately downstream of, and probably cotranscribed with, their cognate plasmid replication initiation genes, which do not appear to be evolutionarily related to those of the staphylococcal plasmids.
Gering et al. (16) have demonstrated that the origin of pSX267 replication resides within its rep coding sequence and suggested that repeats present within rep may be involved. pSK1, pI9789::Tn552, and pSK41 possess equivalent arrays of direct and inverted repeats within their respective rep genes (Fig. 1). The pSK1 replication region also contains a series of direct repeats located upstream of orf245. Seven copies matching the 12-nucleotide (nt) consensus sequence (C/T)-T-(A/T)-(A/G)-(C/G)-(C/T)-(A/G)-C-C-T-A-A are evident (Fig. 1). The significance of these repeats is unknown at this time, although it is tempting to speculate that they might be involved in the regulation of orf245 and/or replication. However, the 12-bp repeats appear to be less well conserved on pI9789::Tn552 and pSX267 (Fig. 1) and are outside the minimal replicating fragment defined for the latter plasmid (16).
Functional analysis of the pSK1 replication region.
To confirm the functionality of the pSK1 replication region identified above and to investigate the role, if any, of orf245 in the process, two minireplicons, pSK4829 and pSK4833, were constructed in E. coli using the plasmid pWE180. This plasmid is a derivative of pUC18 (59) containing an erythromycin resistance determinant, ermC. Although ermC confers resistance in both S. aureus and E. coli, the ColE1-derived replicon of pWE180 is functional only in the latter. The two pSK1 DNA segments amplified and separately cloned into appropriately restricted pWE180 corresponded to the rep gene and upstream intergenic region in the case of pSK4833 (nt 842 to 2287 [Fig. 1]) and a larger fragment also encompassing orf245 in pSK4829 (nt 3 to 2287 [Fig. 1]). pSK4829 and pSK4833 plasmid DNA isolated from E. coli was able to transform S. aureus RN4220 cells to erythromycin resistance via protoplast transformation. Plasmid isolation and agarose gel electrophoresis demonstrated the presence of pSK4829 and pSK4833 DNA in the RN4220 transformants, confirming that both of these plasmids contain a replication origin that functions in S. aureus. It should be noted that a recombinant plasmid equivalent to pSK4833, but containing the pI9789::Tn552 rep region rather than that from pSK1, was similarly shown to replicate in S. aureus RN4220.
The replication proficiency of pSK4833 in S. aureus established that orf245 is not essential for replication initiating at the pSK1 origin and suggests that rep is the only coding sequence absolutely required, as has been shown to be the case in the related replication region of pSX267 (16). However, the orf245 homolog encoded by pUCL287, repB287, although similarly dispensable for replication, has been shown to influence the segregational stability and copy number of this plasmid (3). To investigate the possibility that orf245 plays an equivalent role in the replication of pSK1, the segregational stabilities of pSK4829 and pSK4833 in the absence of selection were determined and compared to that of pSK1 in its entirety.
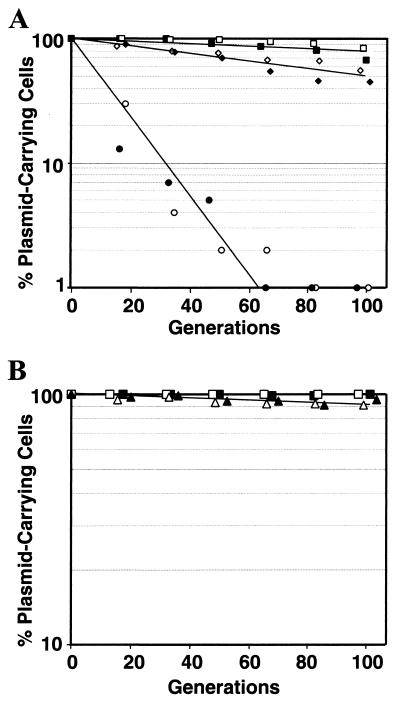
As shown in Fig. 3A, after 100 generations of growth without plasmid selection, pSK1 was maintained by approximately 80% of an S. aureus RN4220 population. pSK4833, encoding only the pSK1 rep gene and upstream intergenic sequence, exhibited significantly lower segregational stability, being entirely lost from the bacterial population by approximately the 80th generation. In comparison, pSK4829, which additionally encodes orf245, was found to be almost as stable as the parent plasmid, pSK1. These data strongly suggest that orf245 contributes to the stable maintenance of pSK1 in S. aureus.
FIG. 3.
Segregational stabilities of pSK1, pSK41, and derivatives in S. aureus strain RN4220. The values indicate the proportion of cells in a population, cultured in the absence of plasmid selection for the indicated number of generations, subsequently able to grow when plated on medium containing selection for the plasmid. Open and solid shapes distinguish data for each of two independent experiments. (A) Values for pSK1, pSK4829, and pSK4833, tested in S. aureus strain RN4220, are denoted by squares, diamonds and circles, respectively. The presence of pSK1 was indicated by growth in the presence of 100 μg of trimethoprim/ml, whereas retention of pSK4829 or pSK4833 was indicated by growth in the presence of 20 μg of erythromycin/ml. (B) Values for pSK41 and pSK5413, tested in S. aureus strains SK982 and RN4220, respectively, are denoted by squares and triangles, respectively. The presence of pSK41 was indicated by growth in the presence of 20 μg of gentamicin/ml, whereas retention of pSK5413 was indicated by growth in the presence of 10 μg of tetracycline/ml.
Functional analysis of the replication region from the conjugative multiresistance plasmid pSK41.
The results discussed above implicating orf245 in the stable maintenance of pSK1 prompted us to investigate the replication system of pSK41, since this plasmid possesses a related rep gene but lacks an orf245 homolog. However, in an equivalent position and orientation, pSK41 possesses a gene, orf86, whose deduced product contains a putative helix-turn-helix DNA-binding domain (4), a feature shared by Orf245 and homologs (3, 47). We therefore intended to construct two pSK41 minireplicons, one containing pSK41 rep and the upstream intervening sequence and the other containing orf86 through rep, to determine if orf86 plays a role equivalent to that of orf245 on pSK1; however, despite numerous attempts we have not been able to obtain a recombinant plasmid containing orf86, either in the presence of rep or in isolation. pSK5413 was constructed by ligating an amplified fragment corresponding to nt 12795 to 14727 of pSK41 (GenBank entry AF051917), encoding the rep gene and the upstream intergenic region, into pUC18 and the subsequent insertion of the staphylococcal tetracycline resistance determinant, tetA(K) (20), as a marker. pSK41 was maintained in a population with almost absolute fidelity after 100 generations (Fig. 3B). After electroporation into S. aureus RN4220, segregational stability assays indicated that pSK5413 was maintained almost as efficiently as pSK41 (Fig. 3B), suggesting that orf86 does not contribute to the segregational stability of pSK41 and that the replication machinery of pSK41 requires no accessory gene equivalent to pSK1 orf245 for maximal efficiency.
Previous studies suggested that pSK1 belongs to staphylococcal plasmid incompatibility group 1 (Inc1) (27), which includes α and γ family β-lactamase–heavy-metal resistance plasmids such as pI9789 (48) and pSX267 (19). To investigate the incompatibility classification of the pSK41 replicon, a mixed-culture transfer experiment using the multiplasmid strain RN1965 (43) as the donor and SK982 harboring pSK5413 as the recipient was performed. These studies demonstrated that plasmids from the non-RC plasmid incompatibility groups, Inc1, Inc2, and Inc6, could each coexist with pSK5413, indicating that pSK41 does not belong to any of these groups. pSK41 may therefore belong to either of the two non-RC Inc groups that we were unable to test, Inc7 or Inc15, or define a new incompatibility group.
DISCUSSION
The nucleotide sequence identity evident among pSK1, pI9789::Tn552, pSX267, and pSK41 is consistent with incompatibility classifications determined here and previously (19, 27, 48). In particular, the sequence repeats present within the rep genes, which are likely to represent the replication origin of each plasmid, are largely conserved on the Inc1 plasmids, pSK1, pI9789::Tn552, and pSX267, whereas the repeats present within the rep gene of pSK41, which does not belong to Inc1, differ considerably (Fig. 1). As is the case for pSX267, database entries for three other staphylococcal plasmids contain incomplete sequences of probable orf245-like genes. It is therefore likely that the multiresistance plasmids, pSK156 (35), pIP630 (1), and pIP1156 (2), all belong to the same plasmid replication complex as pSK1 and pI9789::Tn552.
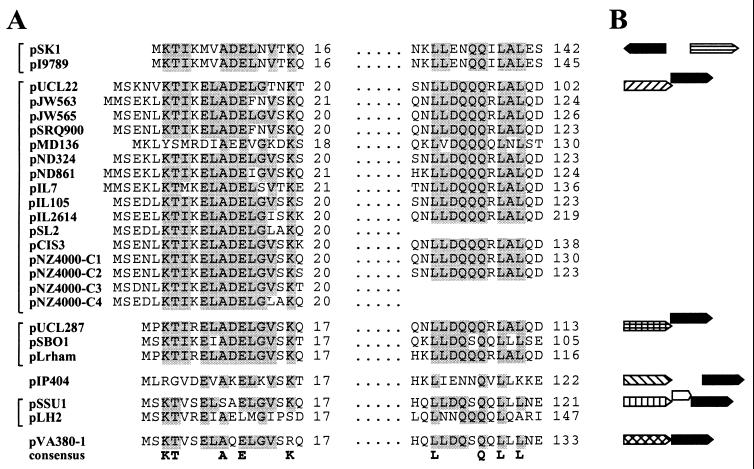
The staphylococcal multiresistance plasmids described here represent the third type of theta-mode replicon to possess an orf245-like gene, with pUCL22- and pUCL287-like plasmids representing the other two. Comparative sequence analyses reveal that genes encoding products sharing statistically significant amino acid sequence similarity to pSK1 Orf245 are represented on plasmids possessing yet more classes of replication initiation genes. Across the entire family of these proteins, sequence conservation is restricted to two linear segments, as shown in Fig. 4; viz., an N-terminal region confined to the first half of a predicted helix-turn-helix DNA-binding domain present in each protein and a segment located within the C-terminal halves of the proteins. Another theta-replicating plasmid, pIP404 from Clostridium perfringens (15), which encodes a replication initiation gene related to those of the broad-host-range plasmids pAMβ1 and pIP501, also possesses a gene encoding an Orf245 homolog (Z = 4.4). As on pUCL22 and pUCL287, the pIP404 orf245-like gene is encoded downstream of the rep gene, but rather than having an overlapping organization, in this case the two genes are separated by 757 nt of noncoding sequence (Fig. 4) which includes the probable origin of replication. Notably, our analysis has also identified orf245-like genes on members of two distinct classes of plasmids that replicate via an RC mechanism. The streptococcal plasmid pSSU1 and the Lactobacillus plasmid pLH2 (38) belong to the pE194/pMV158 family of RC plasmids. On pSSU1, the rep and orf245-like genes are separated by a small open reading frame that slightly overlaps them (Fig. 4), whereas on pLH2 these genes are separated by almost 2 kb. Finally, another streptococcal plasmid, pVA380-1, which belongs to the pC194/pUB110 RC plasmid family, contains an orf245 homolog immediately downstream of its replication initiation gene (Fig. 4); interestingly, this gene has been assigned a role in the mobilization of pVA380-1 (24).
FIG. 4.
(A) Multiple amino acid sequence alignment of segments conserved in Orf245 and related proteins. Residues present in at least 75% of the sequences at any one position are shaded, and amino acids present in at least 90% are indicated in a consensus line at the bottom. The dots denote sequences between the conserved regions that have been omitted for clarity. The amino acid sequences were obtained from the following GenBank entries: pUCL22, X60454; pJW563, X85168; pJW565, Y12736; pSRQ900, AF001314; pMD136, AF069302; pND324, U44843; pND861, AF034786; pIL7, Z25475; pIL105, AF116286; pIL2614, U90222; pSL2, X56550; pCIS3, AF153414; pNZ4000 (C1 to C4), AF036485; pUCL287, X75607; pSBO1, AB021464; pLrham (unnamed plasmid from Lactobacillus rhamnosus), AF037091; pIP404, M32882; pSSU1, AB019522; pLH2, X81981; and pVA380-1, L23803. For each segment, the amino acid number of the rightmost amino acid of a sequence is shown. Proteins from plasmids possessing related replication initiation genes are bracketed. The Orf245-like protein from pSL2, and two of the four homologs carried by pNZ4000, appear to be truncated and therefore do not possess the second conserved region. (B) For the top plasmid of each replicon class shown in panel A, the genetic organization of the gene encoding the Orf245-like protein, with respect to its associated rep gene, is represented diagrammatically. Distinct rep genes are represented by different patterned boxes, whereas orf245 homologs are denoted by solid boxes, and the genes' directions of transcription are indicated by arrowheads on the boxes; note that genes are not drawn to scale.
Despite the prevalence of rep gene-associated orf245 homologs, only the plasmid maintenance assays of the pSK1 minireplicons described here and the findings of Benachour et al. (3) concerning the stabilities and copy numbers of pUCL287 derivatives have implied a role for these genes in plasmid replication. The association of orf245 homologs with a range of seemingly distinct types of plasmid replication initiation genes is both intriguing and perplexing. Such a distribution implies a significant role for this gene, a notion supported experimentally for pUCL287 and now pSK1. However, for each of the six replicon types represented in Fig. 4, there are examples of naturally occurring plasmids which lack an orf245-like gene. Such a “patchy” distribution is consistent with plasmids variously acquiring and losing a gene of this type, possibly indicating that orf245-like genes play a functionally and evolutionarily significant role only under certain conditions. For example, it is conceivable that such a gene is required for plasmid survival in some hosts and/or environments but not in others. The precise role of orf245 and related genes has yet to be elucidated, although the effect on plasmid copy number observed when repB287 was removed from pUCL287 (3) suggests that at least in this case the gene influences replication directly rather than by enhancing plasmid maintenance as a stand-alone segregation mechanism. We are pursuing the working hypothesis that orf245 exerts a regulatory effect on rep transcription. It is hoped that such studies will ultimately provide insights into the interaction among plasmids, bacterial hosts, and their environment.
The sequence similarity detected between the pSK1 replication region and those of the β-lactamase-heavy-metal resistance plasmids and pSK41-like conjugative plasmids establishes that all three recognized groups of large staphylococcal multiresistance plasmids utilize evolutionarily related theta-mode replication systems. It is sobering to consider the impact of this single replicon type on the worldwide development of antimicrobial-resistant staphylococcal strains.
ACKNOWLEDGMENTS
We thank Carol Scaramuzzi for helpful discussions.
This work was supported in part by Project Grant 980075 from the National Health and Medical Research Council (Australia) and in part by the E.P.A. Cephalosporin Fund (Oxford). S.A. was a recipient of an Australian Agency for International Development (AusAID) scholarship. T.B. was the recipient of an Australian Postgraduate Award.
ADDENDUM IN PROOF
The recently published nucleotide sequence of the pIP630 replication region (J. Allignet and N. El Solh, Plasmid 42:134–138, 1999) has confirmed our suggestion that this plasmid belongs to the same plasmid replication complex as pSK1 and pI9789::Tn552.
REFERENCES
- 1.Allignet J, Loncle V, El Solh N. Sequence of a staphylococcal plasmid gene, vga, encoding a putative ATP-binding protein involved in resistance to virginiamycin A-like antibiotics. Gene. 1992;117:45–51. doi: 10.1016/0378-1119(92)90488-b. [DOI] [PubMed] [Google Scholar]
- 2.Allignet J, Loncle V, Mazodier P, El Solh N. Nucleotide sequence of a staphylococcal plasmid gene, vgb, encoding a hydrolase inactivating the B components of virginiamycin-like antibiotics. Plasmid. 1988;20:271–275. doi: 10.1016/0147-619x(88)90034-0. [DOI] [PubMed] [Google Scholar]
- 3.Benachour A, Frere J, Flahaut S, Novel G, Auffray Y. Molecular analysis of the replication region of the theta-replicating plasmid pUCL287 from Tetragenococcus (Pediococcus) halophilus ATCC33315. Mol Gen Genet. 1997;255:504–513. doi: 10.1007/s004380050523. [DOI] [PubMed] [Google Scholar]
- 4.Berg T, Firth N, Apisiridej S, Hettiaratchi A, Leelaporn A, Skurray R A. Complete nucleotide sequence of pSK41: evolution of staphylococcal conjugative plasmids. J Bacteriol. 1998;180:4350–4359. doi: 10.1128/jb.180.17.4350-4359.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 6.Byrne M E, Gillespie M T, Skurray R A. Molecular analysis of a gentamicin resistance transposonlike element on plasmids isolated from North American Staphylococcus aureus strains. Antimicrob Agents Chemother. 1990;34:2106–2113. doi: 10.1128/aac.34.11.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne M E, Gillespie M T, Skurray R A. 4′,4" adenyltransferase activity on conjugative plasmids isolated from Staphylococcus aureus is encoded on an integrated copy of pUB110. Plasmid. 1991;25:70–75. doi: 10.1016/0147-619x(91)90008-k. [DOI] [PubMed] [Google Scholar]
- 8.Dayhoff M O, Schwartz R M, Orcutt B C. A model of evolutionary changes in proteins. In: Dayhoff M O, editor. Atlas of protein sequence and structure. Washington, D.C.: National Biomedical Research Foundation; 1978. pp. 324–352. [Google Scholar]
- 9.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felsenstein J. PHYLIP. Phylogeny Inference Package Cladistics. 1989;5:164–166. [Google Scholar]
- 11.Firth N, Skurray R A. Mobile elements in the evolution and spread of multiple-drug resistance in staphylococci. Drug Resistance Updates. 1998;1:49–58. doi: 10.1016/s1368-7646(98)80214-8. [DOI] [PubMed] [Google Scholar]
- 12.Firth N, Skurray R A. The Staphylococcus—genetics: accessory elements and genetic exchange. In: Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Gram-positive pathogens. Washington, D.C.: American Society for Microbiology; 2000. pp. 326–338. [Google Scholar]
- 13.Frère J, Novel M, Novel G. Molecular analysis of the Lactococcus lactis subspecies lactis CNRZ270 bidirectional theta replicating lactose plasmid pUCL22. Mol Microbiol. 1993;10:1113–1124. doi: 10.1111/j.1365-2958.1993.tb00981.x. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto S, Tomita H, Wakamatsu E, Tanimoto K, Ike Y. Physical mapping of the conjugative bacteriocin plasmid pPD1 of Enterococcus faecalis and identification of the determinant related to the pheromone response. J Bacteriol. 1995;177:5574–5581. doi: 10.1128/jb.177.19.5574-5581.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garnier T, Cole S T. Complete nucleotide sequence and genetic organization of the bacteriocinogenic plasmid, pIP404, from Clostridium perfringens. Plasmid. 1988;19:134–150. doi: 10.1016/0147-619x(88)90052-2. [DOI] [PubMed] [Google Scholar]
- 16.Gering M, Götz F, Bruckner R. Sequence and analysis of the replication region of the Staphylococcus xylosus plasmid pSX267. Gene. 1996;182:117–122. doi: 10.1016/s0378-1119(96)00526-4. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie M T, Lyon B R, Skurray R A. Structural and evolutionary relationships of β-lactamase transposons from Staphylococcus aureus. J Gen Microbiol. 1988;134:2857–2866. doi: 10.1099/00221287-134-11-2857. [DOI] [PubMed] [Google Scholar]
- 18.Götz F, Ahrne S, Lindberg M. Plasmid transfer and genetic recombination by protoplast fusion in staphylococci. J Bacteriol. 1981;145:74–81. doi: 10.1128/jb.145.1.74-81.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Götz F, Zabielski J, Philipson L, Lindberg M. DNA homology between the arsenate resistance plasmid pSX267 from Staphylococcus xylosus and the penicillinase plasmid pI258 from Staphylococcus aureus. Plasmid. 1983;9:126–137. doi: 10.1016/0147-619x(83)90015-x. [DOI] [PubMed] [Google Scholar]
- 20.Guay G G, Khan S A, Rothstein D M. The tet(K) gene of plasmid pT181 of Staphylococcus aureus encodes an efflux protein that contains 14 transmembrane helices. Plasmid. 1993;30:163–166. doi: 10.1006/plas.1993.1045. [DOI] [PubMed] [Google Scholar]
- 21.Helinski D R, Toukdarian A E, Novick R P. Replication control and other stable maintenance mechanisms of plasmids. In: Neidhardt F C, Curtiss R, Ingraham J L, Lin E C C, Low K B Jr, Magasanik B, Reznikoff W, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 2295–2324. [Google Scholar]
- 22.Horinouchi S, Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982;150:804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreiswirth B N, Lofdahl S, Betley M J, O'Reilly M, Schlievert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 24.LeBlanc D J, Chen Y Y, Lee L N. Identification and characterization of a mobilization gene in the streptococcal plasmid, pVA380-1. Plasmid. 1993;30:296–302. doi: 10.1006/plas.1993.1063. [DOI] [PubMed] [Google Scholar]
- 25.Leelaporn A, Paulsen I T, Tennent J M, Littlejohn T G, Skurray R A. Multidrug resistance to antiseptics and disinfectants in coagulase-negative staphylococci. J Med Microbiol. 1994;40:214–220. doi: 10.1099/00222615-40-3-214. [DOI] [PubMed] [Google Scholar]
- 26.Lipman D J, Pearson W R. Rapid and sensitive protein similarity searches. Science. 1985;227:1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- 27.Lyon B R. Ph.D. thesis. Melbourne, Australia: Monash University; 1985. [Google Scholar]
- 28.Lyon B R, May J W, Skurray R A. Analysis of plasmids in nosocomial strains of multiple-antibiotic-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1983;23:817–826. doi: 10.1128/aac.23.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyon B R, May J W, Skurray R A. Tn4001: a gentamicin and kanamycin resistance transposon in Staphylococcus aureus. Mol Gen Genet. 1984;193:554–556. doi: 10.1007/BF00382099. [DOI] [PubMed] [Google Scholar]
- 30.Lyon B R, Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987;51:88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morton T M, Johnston J L, Patterson J, Archer G L. Characterization of a conjugative staphylococcal mupirocin resistance plasmid. Antimicrob Agents Chemother. 1995;39:1272–1280. doi: 10.1128/aac.39.6.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novick R P. Staphylococcal plasmids and their replication. Annu Rev Microbiol. 1989;43:537–565. doi: 10.1146/annurev.mi.43.100189.002541. [DOI] [PubMed] [Google Scholar]
- 33.Novick R P, Edelman I, Schwesinger M D, Gruss A D, Swanson E C, Pattee P A. Genetic translocation in Staphylococcus aureus. Proc Natl Acad Sci USA. 1979;76:400–404. doi: 10.1073/pnas.76.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page R D. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 35.Paulsen I T, Brown M H, Skurray R A. Characterization of the earliest known Staphylococcus aureus plasmids encoding multidrug efflux systems. J Bacteriol. 1998;180:3477–3479. doi: 10.1128/jb.180.13.3477-3479.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paulsen I T, Firth N, Skurray R A. Resistance to antimicrobial agents other than β-lactams. In: Crossley K B, Archer G L, editors. The Staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 175–212. [Google Scholar]
- 37.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pridmore D, Stefanova T, Mollet B. Cryptic plasmids from Lactobacillus helveticus and their evolutionary relationship. FEMS Microbiol Lett. 1994;124:301–305. doi: 10.1111/j.1574-6968.1994.tb07300.x. [DOI] [PubMed] [Google Scholar]
- 39.Rouch D A, Byrne M E, Kong Y C, Skurray R A. The aacA-aphD gentamicin and kanamycin resistance determinant of Tn4001 from Staphylococcus aureus: expression and nucleotide sequence analysis. J Gen Microbiol. 1987;133:3039–3052. doi: 10.1099/00221287-133-11-3039. [DOI] [PubMed] [Google Scholar]
- 40.Rouch D A, Cram D S, DiBerardino D, Littlejohn T G, Skurray R A. Efflux-mediated antiseptic resistance gene qacA from Staphylococcus aureus: common ancestry with tetracycline- and sugar-transport proteins. Mol Microbiol. 1990;4:2051–2062. doi: 10.1111/j.1365-2958.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 41.Rouch D A, Messerotti L J, Loo L S, Jackson C A, Skurray R A. Trimethoprim resistance transposon Tn4003 from Staphylococcus aureus encodes genes for a dihydrofolate reductase and thymidylate synthetase flanked by three copies of IS257. Mol Microbiol. 1989;3:161–175. doi: 10.1111/j.1365-2958.1989.tb01805.x. [DOI] [PubMed] [Google Scholar]
- 42.Rowland S-J, Dyke K G H. Characterization of the staphylococcal β-lactamase transposon Tn552. EMBO J. 1989;8:2761–2773. doi: 10.1002/j.1460-2075.1989.tb08418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruby C, Novick R P. Plasmid interactions in Staphylococcus aureus: nonadditivity of compatible plasmid DNA pools. Proc Natl Acad Sci USA. 1975;72:5031–5035. doi: 10.1073/pnas.72.12.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruhfel R E, Manias D A, Dunny G M. Cloning and characterization of a region of the Enterococcus faecalis conjugative plasmid, pCF10, encoding a sex pheromone-binding function. J Bacteriol. 1993;175:5253–5259. doi: 10.1128/jb.175.16.5253-5259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 46.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seegers J F, Bron S, Franke C M, Venema G, Kiewiet R. The majority of lactococcal plasmids carry a highly related replicon. Microbiology. 1994;140:1291–1300. doi: 10.1099/00221287-140-6-1291. [DOI] [PubMed] [Google Scholar]
- 48.Shalita Z, Murphy E, Novick R P. Penicillinase plasmids of Staphylococcus aureus: structural and evolutionary relationships. Plasmid. 1980;3:291–311. doi: 10.1016/0147-619x(80)90042-6. [DOI] [PubMed] [Google Scholar]
- 49.Sheehy R J, Novick R P. Studies on plasmid replication. V. Replicative intermediates. J Mol Biol. 1975;93:237–253. doi: 10.1016/0022-2836(75)90130-8. [DOI] [PubMed] [Google Scholar]
- 50.Skurray R A, Rouch D A, Lyon B R, Gillespie M T, Tennent J M, Byrne M E, Messerotti L J, May J W. Multiresistant Staphylococcus aureus: genetics and evolution of epidemic Australian strains. J Antimicrob Chemother. 1988;21:19–38. doi: 10.1093/jac/21.suppl_c.19. [DOI] [PubMed] [Google Scholar]
- 51.Takiguchi R, Hashiba H, Aoyama K, Ishii S. Complete nucleotide sequence and characterization of a cryptic plasmid from Lactobacillus helveticus subsp. jugurti. Appl Environ Microbiol. 1989;55:1653–1655. doi: 10.1128/aem.55.6.1653-1655.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka T, Ogura M. A novel Bacillus natto plasmid pLS32 capable of replication in Bacillus subtilis. FEBS Lett. 1998;422:243–246. doi: 10.1016/s0014-5793(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 53.Tennent J M, Lyon B R, Midgley M, Jones I G, Purewal A S, Skurray R A. Physical and biochemical characterization of the qacA gene encoding antiseptic and disinfectant resistance in Staphylococcus aureus. J Gen Microbiol. 1989;135:1–10. doi: 10.1099/00221287-135-1-1. [DOI] [PubMed] [Google Scholar]
- 54.Thompson J K, Foley S, McConville K J, Nicholson C, Collins M A, Pridmore R D. Complete sequence of plasmid pLH1 from Lactobacillus helveticus ATCC15009: analysis reveals the presence of regions homologous to other native plasmids from the host strain. Plasmid. 1999;42:221–235. doi: 10.1006/plas.1999.1428. [DOI] [PubMed] [Google Scholar]
- 55.Townsend D E, Ashdown N, Bolton S, Bradley J, Duckworth G, Moorhouse E C, Grubb W B. The international spread of methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1987;9:60–71. doi: 10.1016/0195-6701(87)90097-1. [DOI] [PubMed] [Google Scholar]
- 56.Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 57.Weaver K E, Clewell D B, An F. Identification, characterization, and nucleotide sequence of a region of Enterococcus faecalis pheromone-responsive plasmid pAD1 capable of autonomous replication. J Bacteriol. 1993;175:1900–1909. doi: 10.1128/jb.175.7.1900-1909.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]