Abstract
The presence of N-nitroso compounds, particularly N-nitrosamines, in pharmaceutical products has raised global safety concerns due to their significant genotoxic and mutagenic effects. This systematic review investigates their toxicity in active pharmaceutical ingredients (APIs), drug products, and pharmaceutical excipients, along with novel analytical strategies for detection, root cause analysis, reformulation strategies, and regulatory guidelines for nitrosamines. This review emphasizes the molecular toxicity of N-nitroso compounds, focusing on genotoxic, mutagenic, carcinogenic, and other physiological effects. Additionally, it addresses the ongoing nitrosamine crisis, the development of nitrosamine-free products, and the importance of sensitive detection methods and precise risk evaluation. This comprehensive overview will aid molecular biologists, analytical scientists, formulation scientists in research and development sector, and researchers involved in management of nitrosamine-induced toxicity and promoting safer pharmaceutical products.
Keywords: Carcinogenicity, Genotoxicity, Mutagenicity, Molecular toxicity, Nitrosamine impurities, Nitrosamine drug substance-related impurities
Graphical abstract
Highlights
-
•
The nitrosamines crisis in pharmaceuticals is discussed.
-
•
We delve into the toxicological implications and toxicity profiles of N-nitroso compounds.
-
•
We discussed root cause analysis, risk assessment, and mitigation strategies for nitrosamine impurities.
-
•
We highlighted GC and LC methods for detection and quantification of N-nitroso compounds in pharmaceuticals.
-
•
The emphasis is on developing nitrosamine-free pharmaceutical products to ensure regulatory compliance.
1. Introduction
Nitrosamine impurities, even in trace amounts, are highly toxic and mutagenic, capable of damaging DNA, and subsequently increase the risk of cancer incidence [1]. Regulatory authorities have identified nitrosamine impurities in various active pharmaceutical ingredients (APIs) and other approved medications leading to the abrupt recall of drugs like sartans (valsartan), ranitidine (zantac), nizatidine, metformin, and varenicline due to unacceptable levels of nitrosamine impurities [2].
In recent times, regulatory bodies have shifted their focus to a newer class of nitrosamine impurities known as nitrosamine drug substance-related impurities (NDSRIs), which share structural similarity with the API. Recent incidents involving NDSRIs include quinapril hydrochloride where the presence of N-nitroso-quinapril was detected [3]. Orphenadrine citrate enteric release tablets which exhibited N-nitroso orphenadrine as an impurity [3]. N-nitroso-varenicline (NNV) in varenicline is formed through a reacting between drug substance and nitrites present in the excipients [4].
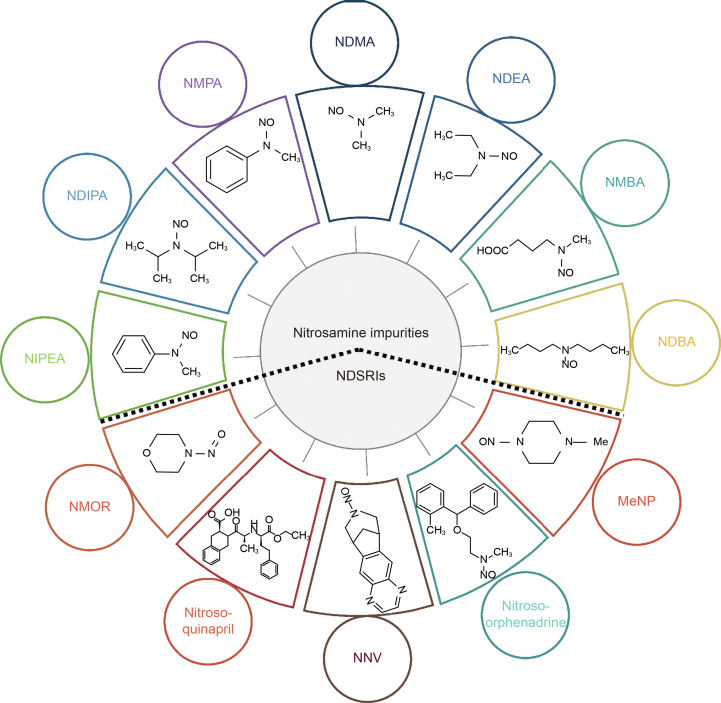
Nitrosamine impurities are classified as Class 1 known mutagenic impurities according to The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) M7 guidelines [5]. The International Agency for Research on Cancer (IARC) categorized these impurities into Groups 2A and 2B based on epidemiological data [6] with N-nitrosodimethylamine (NDMA) and NDEA falling under Group 2A, while other nitrosamines are classified as Group 2B human carcinogens [7]. The chemical structures of selected nitrosamines and NDSRIs are represented in Fig. 1.
Fig. 1.
Chemical structures of various nitrosamine impurities and nitrosamine drug substance-related impurities (NDSRIs). NDMA: N-nitrosodimethylamine; NDEA: N-nitrosodiethylamine; NMBA: N-nitroso-N-methyl-4-aminobutanoic acid; NDBA: N-nitrosodibutylamine; MeNP: 1-methyl-4-nitrosopiperazine; NNV: N-nitroso-varenicline; NMOR: N-nitrosomorpholine; NIPEA: N-nitrosoisopropylethyl amine; NDIPA: N-nitrosodiisopropylamine; NMPA: N-nitrosomethylphenylamine.
To address the nitrosamine crisis, the United States Pharmacopeia (USP) is hosting a virtual platform called the “nitrosamines exchange”. This platform brings together pharmaceutical companies, contract research organizations (CROs), API manufacturers, and excipients suppliers to facilitate real-time communication, updates, and risk assessments related to the nitrosamines issue. Additionally, USP has established a “nitrosamine analytical hub” to provide updates on novel analytical methods for testing nitrosamines.
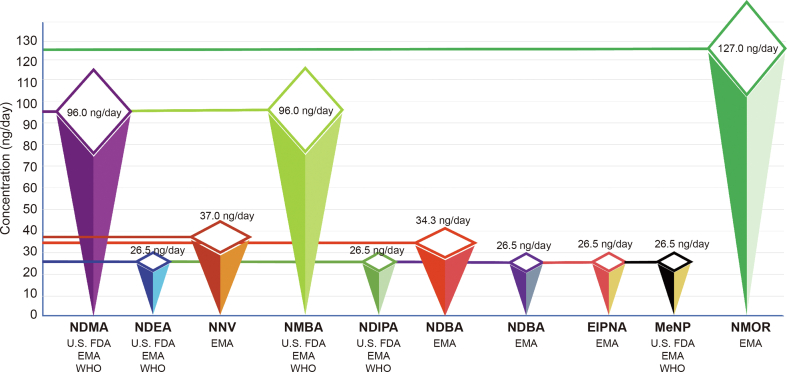
Regulatory authorities for drug control have specified acceptable daily intake limits for various nitrosamine impurities and NDSRIs in APIs and medicinal products. For instance these limits include 96 ng/day for N-nitrosodimethylamine (NMDA) and N-nitroso-N-methyl-4-aminobutanoic acid (NMBA), 26.5 ng/day for N-nitrosomethylphenylamine (NMPA), N-nitrosodiisopropylamine (NDIPA), N-nitrosoisopropylethyl amine (NIPEA), N-nitrosodiethylamine (NDEA), and 1-methyl-4-nitrosopiperazine (MeNP), 34.3 ng/day for NMPA, 37 ng/day for NNV, and 127 ng/day for N-nitrosomorpholine (NMOR) [5,8,9]. These limits are represented in Fig. 2.
Fig. 2.
Acceptable daily intake limits for selected nitrosamines impurities and nitrosamine drug substance-related impurities (NDSRI) in medicinal products as per regulatory authorities. NDMA: N-nitrosodimethylamine; U.S. FDA: United States food and drug administration; EMA: European medicines agency; WHO: World Health Organization; NDEA: N-nitrosodiethylamine; NNV: N-nitroso-varenicline; NMBA: N-nitroso-N-methyl-4-aminobutanoic acid; NDIPA: N-nitrosodiisopropylamine; NMPA: N-nitrosomethylphenylamine; NDBA: N-nitrosodibutylamine; EIPNA: N-nitrosoethylisopropylamine; MeNP: 1-methyl-4-nitrosopiperazine; NMOR: N-nitrosomorpholine.
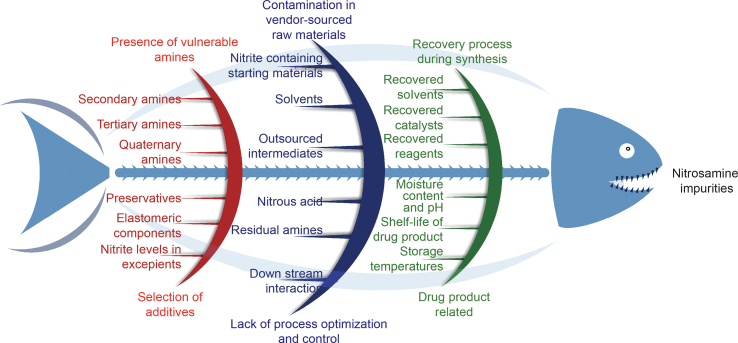
Conditions that fosters nitrosamine formation include the presence of vulnerable amines, such as secondary and tertiary amines [10]. These amines react with nitrous acid in acidic conditions, leading to the formation of nitrosamines. Nitrous acid itself is unstable and is formed from nitrites (NO2−) under acidic conditions. Nitrosamine impurities may also originate from contamination in vendor-sourced raw materials, including nitrite-containing starting materials, intermediates, and the reuse of solvents, reactants, catalysts and reagents during synthesis. During the manufacturing of drug products, several factors, such as the selection of additives, preservatives, excipients, and elastomeric components, should be carefully assessed, as they can contribute to the generation of nitrosamines in pharmaceutical products. Additionally, post-production factors such as moisture content, pH, and storage temperatures of the product might also lead to generation of nitrosamines [11]. Formulators of APIs or drug products should consider these factors and take necessary steps to mitigate nitrosamine impurities in pharmaceutical products. Moreover, nitrosamines may also form in the gastrointestinal tract if vulnerable amines, along with nitrites, are consumed [12]. The root causes and sources of nitrosamine impurities in pharmaceutical products are represented in the form of a fishbone diagram in Fig. 3.
Fig. 3.
Root causes and sources of nitrosamine impurities in active pharmaceutical ingredients (APIs) and medicinal products.
The recall of drugs due to the nitrosamine crisis has resulted in disruptions in supply chains and shortage of several drugs in markets, raising concerns among patients globally. It has also impacted the clinical drug development process. Drug interaction studies were affected by the recall of key drugs such as metformin, rifampin, ranitidine, nizatidine, and propranolol. Experimental studies and in silico approaches could be handy to theoretically predict the formation of nitrosamines in numerous drugs containing secondary and tertiary amines.
This systematic review significantly examines and discusses the toxicity of N-nitroso compounds, primarily focusing on N-nitrosamines, in drug products, pharmaceutical excipients, and APIs. Additionally, it encompasses recent updates on the molecular toxicity profiles of these impurities within physiological systems. Furthermore, the review investigates novel, updated analytical strategies for the sensitive detection and quantification of these hazardous impurities. It delves into the root causes for the presence of nitrosamine impurities and offers insightful reformulation strategies for pharmaceutical products. Additionally, it provides regulatory updates aimed at mitigating the occurrence of these nitrosamine impurities.
2. Literature search
We conducted a literature search by accessing public databases, including PubMed, Medline, eMedicine, the National Library of Medicine (NLM), and ReleMed. We focused on published articles, including original research, review articles, case reports, and other systematic reviews, both with and without comprehensive meta-analysis, related to N-nitroso compounds in pharmaceutical excipients, APIs, and drug products. Subsequently, we collected information relevant to the updated molecular toxicity implications associated with nitrosamine impurities and the implications of multilevel approaches for sensitive detection. Our primary screening of 180 articles yielded the relevant data for this study. All data gathered for this study adhered to the guidelines outlined in the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement. We conducted an automatic search with manual sorting of the selected articles.
2.1. Study selection
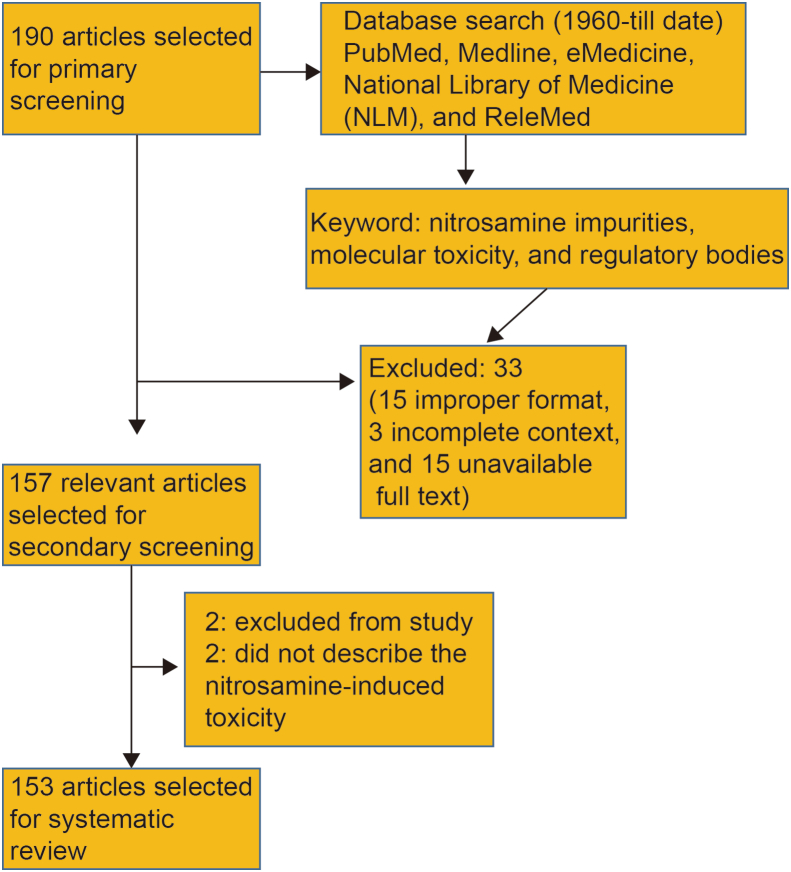
For this systematic review, all published or accepted articles pertaining to nitrosamine-related topics, specifically focusing on toxicity and analytical implications in pharmaceuticals, were included. A total of 180 articles spanning from 1960 to the present day, including original research, reviews, case reports and studies reporting nitrosamine impurities above the no-observed-adverse-effect levels (NOAEL) established by regulatory agencies, were initially screened. During the primary screening, we considered factors such as relevance, publication date, access to the full article text, and content. Subsequently, in the secondary screening phase, we excluded the 33 reports including 15 with improper formats (such as case reports, letters, and short communications), 3 with incomplete content, and 15 with unavailable full texts. This process resulted in the selection of 147 articles for further evaluation. Of these, 2 articles were excluded from the study and 2 articles did not describe nitrosamine-induced toxicity or its pharmaceutical or analytical implications. In the end, 143 articles were chosen for systematic review. The schematic depiction of the exclusion and inclusion criteria for articles included in the study through primary and secondary screening is represented as Scheme 1.
Scheme 1.
The schematic depiction of the exclusion and inclusion criteria for articles included in the study through primary and secondary screening.
Our initial focus was to gather information on nitrosamine-induced toxicity, side effects, or physiological toxicity triggered by nitrosamine impurities. Additionally, we aimed to explore analytical techniques for sensitive detection of nitrosamine impurities and to put forth regulatory updates on nitrosamine impurities.
2.2. Data extraction
In accordance with international standards outlined in the “Systematic review of observational studies in epidemiology”, data selection and categorization followed specific criteria. These criteria included the number of articles addressing nitrosamine-induced toxicity, other neurological or systemic complications, year and type of publication, and the preclinical studies describing signaling pathways implicated in nitrosamine-induced toxicity for toxicity management. We organized the data into three main groups to facilitate categorization: articles that describe “side effects or physiological toxicity triggered by nitrosamine impurities”, those focusing on “analytical techniques for sensitive detection of nitrosamine impurities”, and those aiming to “put forth regulatory updates on nitrosamine impurities”. The results of these three categories were reviewed and analyzed separately. This systematic approach allowed us to address the crucial inferences, which may prove valuable to molecular biologists, analytical scientists, formulation scientists in research and development sector, and researchers involved in management of nitrosamine-induced toxicity.
3. Molecular toxicity of nitrosamine impurities
NDMA, NDEA, N-nitrosoethylisopropylamine (NEIPA), NDIPA, N-nitrosodibutylamine (NDBA), and NMBA are the predominant nitrosamines that can foster the carcinogenic effects via genotoxicity or mutagenicity in the physiological system. In recent years, it has become evident that impurities in several pharmaceutical products pose significant human health risks, as observed in rodent models. For example, NMBA and NDEA have metabolic similarities to the NMDA and 12 carcinogenic N-nitrosomethyl-N-alkylamines (NMAs). Both genotoxicity and tumorigenicity are assessed through the examination of chromosomal aberrations, sister chromatid exchange, and micronuclei formation during studies on nitrosamines-induced toxicity studies.
3.1. Genotoxicity
Nitrosamine impurities such as NDMA, NDEA, and others can induce mutagenic effects and subsequently thereby leading to carcinogenicity. NDMA has been shown to induce mutations due to the genotoxicity in vivo models [[13], [14], [15], [16], [17], [18]]. Cytochrome P450 (CYP450) enzymes such as CYP2E1 and CYP2A6 are involved in the metabolism of N-nitrosamines and form N-nitrosodialkylamines [[19], [20], [21]]. For instance, in rodent models, CYP2E1 is implicated in the metabolism of several substrates including organic solvents, nitrosamines, and other drug molecules [22]. Additionally, CYP3A4 plays a role in catalyzing the activation of larger N-nitrosamines [23]. The differential expression of CYP2E1 modulates the nitrosamines metabolism in different in vitro or in vivo models [21,24,25].
Genotoxicity of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-mediated DNA damage includes the formation of methyl DNA base adducts, such as O6-Me-dGuo and N7-Me-dGuo. Other genotoxic products resulting from the activity of N-nitrosamines in the healthy cells include methyl DNA base adducts and pyridyloxobutyl (POB) DNA base adducts including O6-POB-dGuo, and O2-POB-Thd. Aldehyde DNA adducts are also significant genotoxic products formed due to the presence of nitrosamine impurities. However, pyridylhydroxybutyl DNA adducts require further extensive studies to explore the exact nature of nitrosamine impurities [1,26,27]. Table 1 [7,[28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55]] outlines the carcinogenic potential of N-nitroso compounds across various routes of administration in several in vivo models.
Table 1.
The carcinogenic potential of N-nitroso compounds across various routes of administration in several in vivo models.
| N-nitroso compounds | No. | Route of administration | Animal models | Tumors | Refs. |
|---|---|---|---|---|---|
| N-nitrosodimethylamine (NMDA) | 1 | Oral | Mice, rats, hamsters, rabbits, guinea pigs, and fish | Benign and malignant tumors of the liver | [7,28] |
| 2 | Inhalation | Mice, prenatal exposure in mice | Benign and malignant tumors of the liver | [7,28] | |
| 3 | Subcutaneous | Hamsters, mastomys, and newborn and suckling mice and rats | Benign and malignant tumors of the liver | [7,28] | |
| 4 | Intraperitoneal | Adult and newborn mice and in newts | Benign and malignant tumors of the liver | [7,28] | |
| 5 | Intramuscular | Rats | Benign and malignant tumors of the liver | [7,28] | |
| 6 | Oral | Frogs and fish | Benign and malignant tumors of the liver | [7,28] | |
| 7 | Oral | Lung tumors in mice | Tumors of the respiratory tract | [7,28] | |
| 8 | Inhalation | Mice and rats | Lung tumors | [7,28] | |
| 9 | Inhalation | Rats | Nasal-cavity tumors | [7,28] | |
| 10 | Subcutaneous | Adult, newborn, and suckling mice and nasal cavity tumors in adult hamsters | Lung tumors | [7,28] | |
| 11 | Intraperitoneal | Adult and newborn mice | Lung tumors | [7,28] | |
| 12 | Inhalation | Rats | Nasal-cavity tumors | [7,28] | |
| 13 | Inhalation | Mice | Lung tumors | [7,28] | |
| 14 | Oral exposure | Mice, rats, and hamsters | Blood-vessel tumors | [7,28] | |
| 15 | Subcutaneous | Hamsters and adult, newborn, and suckling mice | Blood-vessel tumors | [7,28] | |
| 16 | Intraperitoneal | Mice | Blood-vessel tumors | [7,28] | |
| 17 | Oral | Frogs | Tumors of the hematopoietic system | [7,28] | |
| 18 | Oral | Mollusks | Tumors of the digestive gland and hematopoietic system | [29] | |
| 19 | Subcutaneous | Female hamsters | Ovarian tumors | [30] | |
| 20 | Oral | Foxes | Liver tumors | [31] | |
| 21 | Intraperitoneal | Rats | Lung and liver tumors | [32] | |
| 22 | Oral | Humans | Oropharyngeal cancer | [33] | |
| 23 | Oral | Stomach | [34,35] | ||
| 24 | Oral | Esophageal | [[36], [37], [38], [39], [40]] | ||
| 25 | Oral | Colorectal | [41] | ||
| N-nitrosodiethylamine (NDEA) | 1 | Inhalation | Rats | Liver tumors | [7,28] |
| 2 | Rectal | Rats | Liver tumors | [7,28] | |
| 3 | Intramuscular | Chickens and cats | Liver tumors | [42] | |
| 4 | Intraperitoneal | Newborn mice | Liver tumors | [43,44] | |
| 5 | Intratracheal | Hamsters of both sexes | Tumors of the lung or trachea | [[45], [46], [47]] | |
| 6 | Subcutaneous | Rabbits | Tumors of the lung or trachea | [48] | |
| 7 | Intraperitoneal | Newborn mice | Tumors of the lung or trachea | [44] | |
| 8 | Oral | Larval or juvenile fish | Pancreatic tumors | [49] | |
| 9 | Oral | Mollusks | Tumors of the digestive gland and hematopoietic system | [29] | |
| 10 | Intraperitoneal | Pregnant hamsters, prenatally exposed offspring, and second generation of offspring | Benign laryngotracheal tumors | [50] | |
| N-nitrosomorpholine (NMOR) | 1 | Oral | Male hamsters, female rats, and male hamsters | Respiratory or digestive-tract tumors | [51] |
| 2 | Intravesicular | Rats | Tumors of internal organs | [52] | |
| 3 | Oral | Female rats | Tumors of the esophagus | [7] | |
| 4 | Oral | Male mice | Hepatocellular adenoma | [7] | |
| 5 | Oral | Rats | Hepatocellular carcinoma and cholangiofibroma | [7] | |
| 6 | Intravenous | Rats | Liver cancer | [7] | |
| 7 | Oral | Syrian golden hamsters | Malignant liver tumors | [[53], [54], [55]] | |
| 8 | Oral | Hamsters | Respiratory or digestive-tract tumors | [55] | |
| 9 | Oral | Hamsters | Tumors of the respiratory tract and upper digestive tract | [55] |
Furthermore, DNA lesions are formed due to the activity of these nitrosamines in pharmaceutical ingredients or drugs, leading to the formation of O6-methylguanine or 3-methyladenine (3MeA) [1,[56], [57], [58], [59]]. DNA methylation is primarily induced by NDMA, and the unrepaired 3MeA can promote mutagenicity, subsequently leading to cancer. Typically, the DNA damage induced by 3MeA is repaired by the alkyladenine glycosylase, which eliminates the methylated bases, triggering base excision repair [1]. Therefore, the activity of alkyladenine glycosylase is crucial in minimizing the mutagenicity induced by nitrosamine impurities such as NDMA [[60], [61], [62]].
3.2. Carcinogenicity
Several epidemiological reports have described the influence of nitrosamines on the risk of developing esophageal cancers, colon cancers, hepatocellular cancers, and other devastating forms of cancers [[63], [64], [65]].
3.2.1. Lung cancer
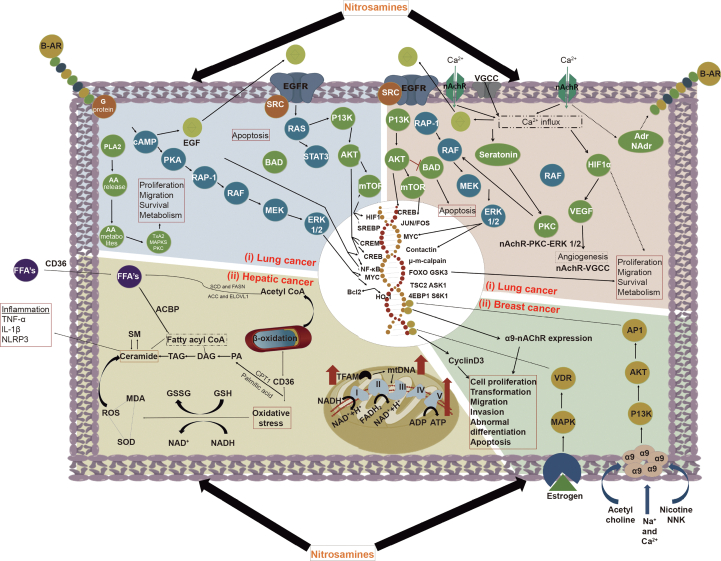
Lung cancer can be induced by the nitrosamine impurities, as they have the ability to stimulate proliferation, migration, and cancer cell survival by modulating G-protein signaling, nicotinic acetylcholine receptor (nAchR) signaling, and epidermal growth factor receptor (EGFR) signaling. For instance, nitrosamines' influence on EGFR signaling can induce changes in the cyclic adenosine monophosphate-protein kinase A-rapidly accelerated fibrosarcoma-mitogen-activated protein kinase kinase-extracellular signal-regulated kinase 1/2 (cAMP-PKA-RAF-MEK-ERK1/2) axis, subsequently promoting cancer cell proliferation. Similarly, the EGFR modulation could cause alterations in the phosphoinositide-3-kinase-Akt-mammalian target of rapamycin (PI3K-Akt-Mtor) signaling pathway to fostering cancer cell survival. Nitrosamines also induce changes in nAChR signaling, voltage gated calcium channel (VGCC), calcium signaling, and protein kinase C (PKC) modulation, which contribute to cancer progression through migration and survival. HIF1-alpha signaling is also modulated by the beta-adrenergic signaling when nitrosamines interact with these receptors which subsequently cause angiogenesis for nutrient supply to the proliferating cancer cells.
3.2.2. Esophageal cancer
Esophageal cancer has higher incidence rates and wide geographical prevalence, indicating the involvement of environmental factors in its development. Past reports in toxicogenomics have described the role of nitrosamines in esophageal carcinogenesis. A study by Zhao et al. [64] elucidated the potential of nitrosamines to induce carcinogenicity in the esophageal epithelium. In this study, the authors examined the urinary levels of nitrosamines in patients associated with esophageal squamous cell carcinoma (ESCC), basal cell hyperplasia, and precancerous lesions, comparing them to healthy individuals. The risk was found to be higher in ESCC patients with elevated levels of NMEA, NDBA, nitrosopyrrolidine (NPYR), and NMOR compared to the control group. As per this report, there is a higher risk of ESCC in patients with hazardous exposure to N-nitrosomethylethylamine (NMEA), NDBA, and NPYR, suggesting a specific dose-response relationship between nitrosamine exposure and the incidence of esophageal cancer [64].
3.2.3. Hepatocellular and pancreatic cancer
Hepatocellular cancer is one of the lethal cancers that can result from the metabolism of nitrosamines with the involvement of P450 enzymes. Lifestyle factors, such as dietary exposure to higher levels of nitrosamines or N-nitroso compounds, have been linked to the development of cancer [66,67]. These compounds specifically form DNA adducts in the nuclei of cells located in digestive organs and the liver contributing to the carcinogenicity of nitroso compounds [68,69]. Furthermore, food processing additives containing these compounds serve as a significant source of nitrosamine exposure in humans [70,71]. A study by Zheng et al. [65] explored the relationship between the dietary intake of specific N-nitroso compounds, total N-nitroso compounds, and the development of hepatocellular carcinoma. The authors concluded that higher consumption of N-nitroso compounds could increase the risk of hepatocellular carcinoma incidence, in conjunction with other risk factors such as hepatitis B virus (HBV) or hepatitis C virus (HCV) infection or alcohol intake.
Mainly, NDEA and NDMA are significant nitroso compounds found in pharmaceuticals and these are classified as Group 2A carcinogens for humans. Metabolic activation by CYP450 enzymes can induce electrophilic alkylation, leading to the formation of mutation-induced DNA adducts [68]. Hepatic enzymes play a substantial role in the metabolism of nitrosamines, with greater activity observed in the liver when compared to the other extrahepatic tissues [72]. Dose-response relationships concerning nitrosamines have shown that NDMA and NDEA induce hepatic tumors in in vivo models. DNA adduct formation has been observed in both in vivo models and human in liver tumors with significant nitrosamines exposure [[72], [73], [74], [75], [76], [77]]. N-nitrosamines induce the hydroxylation of an alpha-carbon atom within the alkyl group, generating hydroxyl nitrosamines in liver cells and subsequently fostering DNA alkylation. These sequential events further cause gene damage, mutations, and confers malignant transformation of liver cells into cancer cells by triggering oncogenic signaling [[78], [79], [80], [81]] as represented in Fig. 4. Furthermore, exposure to N-nitrosamines affects hepatic lipid metabolism through alterations in mitochondrial DNA, oxidative phosphorylation (OXPHOS) signaling, and other mitochondrial metabolic pathways. Another mechanism of nitrosamines involves the induction of inflammation in liver tissue through oxidative stress, adenosine triphosphate (ATP) depletion, and a decline in enzymes related to fatty acid oxidation [82].
Fig. 4.
Molecular signaling pathway for nitrosamines in various types of cancers. (i) Lung cancer: nitrosamines can stimulate proliferation, migration and cancer cell survival by modulating G-protein signaling, nAchR signaling, and EGFR signaling. (ii) Breast cancer: nitrosamines modulates adenylyl cyclase/cAMP/PKA/CREB pathway, which mediates EGFR signaling pathway transactivation. (iii) Hepatic cancer: N-nitrosamines exposure affects hepatic lipid metabolism through the alteration of mitochondrial DNA, OXPHOS signaling, and other mitochondrial metabolic pathways. I: nicotinamide adenine dinucleotide (NADH)-ubiquinone oxidoreductase complex; II: succinic acid-ubiquinone oxidoreductase complex; III: cytochrome Bc1 complex; IV: cytochrome C oxidase complex; and V: adenosine triphosphate synthase complex; B-AR: β-adrenergic receptor; Adr: adrenaline; NAdr: noradrenaline; HIF1α: hypoxia inducible factor 1α; VEGF: protein kinase B; RAF: rapidly accelerated fibrosarcoma; PKC: protein kinase C; ERK: extracellular signal-regulated kinase; MEK: mitogen‑activated protein kinase kinase; RAP-1: Ras-related protein 1; BAD: BCL2 associated agonist of cell death protein mTor: mammalian target of rapamycin; PI3K: phosphoinositide 3-kinase; VGCC: voltage gated calcium channel; nAchR: nicotinic acetylcholine receptors; CREB: cAMP response element-binding protein; FOS, JUN and Myc: transcription factors; μ-m calpain: isozymes of calpain; FOXO: forkhead transcription factor; GSK3: glycogen synthase kinase 3; TSC2: tuberous sclerosis 2; ASK1: apoptosis signal-regulating kinase 1; 4EBP1: eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1; S6K1: S6 kinase 1; PLA2: phospholipase-A2; PKA: protein kinase A; TxA2: thromboxane A2; SREBP-1: sterol regulatory element-binding protein 1; CREM: cAMP Response Element Modulating protein; NF-kB: nuclear factor kappa B; Bcl2: B cell lymphoma 2; HO-1: heme oxygenase-1; AA: arachidonic acid; RAS: rat sarcoma proteins; STAT: signal transducer and activator of transcription; AP1: activator protein 1; PI3K: phosphoinositide 3-kinase; MAPK: mitogen-activated protein kinase; VDR: vitamin D receptor; FASN: fatty acid synthase; ROS: reactive oxygen species; CD36: fatty acid translocase; SOD: superoxide dismutase; ACC: acetyl-CoA carboxylase; TNF-α: tumor necrosis factor alpha; IL-1β: interleukin-1β; SCD: stearoyl-CoA desaturase; ELOVL1: ELOVL fatty acid elongase 1; ACBP: acyl-CoA binding protein, TAG: triacylglycerols; DAG: diacylglycerols; PA: phosphatidic acids; CPT1: carnitine palmitoyltransferase 1; GSH: glutathione; GSSG: glutathione disulfide; MDA: malondialdehyde; NAD: nicotinamide adenine dinucleotide; TFAM: mitochondrial transcription factor-A.
NDEA and NDMA are considered as the pancreatic carcinogens as indicated by the positive correlation for the intake of certain foods that can induce generation of N-nitroso compounds [71]. This study also investigated the dietary intake of factors such as cigar smoking, vitamin C, vitamin E, or red meat in relation to the incidence of pancreatic cancer. Notably, the intake of vitamins C and E could be considered as nitrosation inhibitors while the consumption of heme iron in red meat may contribute to the endogenous production of N-nitroso compounds [70,[83], [84], [85], [86]].
Pentoxifylline has demonstrated the potential to ameliorate hepatotoxicity and oxidative stress induced by N-nitrosamines in experimental rodent models [87]. The N-nitrosamines role in damaging mitochondrial lipid metabolism yet requires substantial studies to investigate the exact toxicology of these impurities in pharmaceuticals [82,[88], [89], [90]].
3.2.4. Breast cancer
Breast cancer remains a major cause of mortality and morbidity worldwide, often attributed to unhealthy lifestyle patterns and dietary habits. Tobacco contains a wide range of nitrosamines, with NNK being a significant carcinogen known to promote the malignant cancer cells [91]. These tobacco derived carcinogens are particularly relevant to the development of various cancers including breast cancer. NNK plays a significant role in the development and progression of breast cancer by interacting with the nAChR present in cancer cells. Notably, the α9nAChR receptor is implicated in the development and progression of breast cancer cells [92,93]. Additionally, nicotine and NNK can interact with this receptor system, subsequently influencing cancer cell proliferation by modulating crucial cell signaling pathways like mitogen-activated protein kinase (MAPK) and PI3K-Akt pathways. These signaling pathways are stimulated by the activity of nicotine and estrogen hormone stimulation. The activation of α9nAChR by NNK and nicotine forms a significant feedback loop through PI3K-Akt and MAPK signaling, which could further initiate activator protein 1 (AP1) and vitamin D receptor (VDR) transcription factors stimulation to confer to the cancer development [[94], [95], [96], [97]]. This phenomenon has been confirmed through gene knockdown studies of α9nAChR using small interfering RNA (siRNAs) [[98], [99], [100]]. However, the current nitrosamine impurities found in pharmaceutical formulations and APIs are yet to be investigated for their potential to modulate the activity of α9nAChR in fostering breast cancer through in vitro and in vivo studies.
Moreover, the beta-adrenergic receptor system, part of the G-protein coupled receptor system, and its downstream signaling play a significant role in promoting cancer cell proliferation upon NNK activity [101,102]. This signaling pathway subsequently fosters the modulation of the adenylyl cyclase/cAMP/PKA/cAMP response element-binding protein (CREB) pathway, which, in turn, mediates the transactivation of EGFR signaling pathway [103,104]. However, the specific impact of nitrosamine impurities on the modulation of these G-protein coupled signaling pathways and EGFR pathways is yet to be investigated vividly through in vitro and in vivo studies for their potential role in incidence of cancers.
3.3. Physiological alterations: nitrosamine impurities in angiotensin receptor blockers
NDMA, NDEA, NEIPA, NDIPA, NDBA, and NMBA are major nitrosamines known to induce carcinogenicity in the physiological system [105]. Additionally, these impurities found in pharmaceutical drugs could cause alterations in the physiological system. For instance, angiotensin receptor blockers (ARBs) such as losartan, valsartan, olmesartan, irbesartan, and telmisartan have been recalled from US markets due to elevated levels of nitrosamine impurities in their products, which can disrupt physiological homeostasis [105]. The presence of these nitrosamines in ARBs is primarily attributed to improper manufacturing process [106].
NMDA is metabolized by the P450 2E1 in hepatic microsomes in humans [20]. Methyl group oxidation could induce the formation of to α-hydroxy-NDMA and this is mutagenic which can simultaneously decompose and generate two reactive species including formaldehyde and methyl diazohydroxide [20,107]. These two metabolites may have significant effect on the physiological systems [108]. In case of NDEA, the metabolic activation that cause oncogenesis is profoundly mediated by the P450 2E1 and P450 2A6 [20,109,110]. Ethyl diazohydroxide is formed from the P-450 mediated hydroxylation of NDEA, which further forms ethyldiazonium that can interact with DNA and induce formation of DNA adducts. The formation of DNA could cause the significant alteration in the physiology of healthy cells in human tissues and transform into neoplastic cell by the oncogenicity. Rodent studies pertinent to the effects of NDEA described the incidence of several kinds of liver cancers, esophageal cancers, and nasopharyngeal cancers, because toxic metabolite induced physiological alterations and oncogenicity [16,111].
DNA adducts such as O6-methylguanine (O6-Me-Gua) and N7-Me-Gua induced by the NDMA when incubated with esophagus cell cultures obtained from human tissues [112]. Methylated DNA adducts observed across the human tissues could typically damage the healthy cells [[113], [114], [115]]. In case of liver cell DNA, the NDMA-poisoned victims, O6-Me-Gua and N7-Me-Gua formation was observed. In case of NMEA, N7-Me-Gua is methylated form of DNA adducts formed substantially in hepatic region and renal region followed by the esophagus and lungs. These toxic DNA adducts may further harm the cell protein formation at transcriptional and translational levels which required substantial future studies to unravel the underlying mechanisms behind these N-nitroso compounds-induced physiological alterations [116].
4. Analytical techniques to enhance sensitive detection of nitrosamine impurities
For many years, nitrosamine impurities have been reported in pharmaceutical products. However, there are still numerous products containing these impurities that have not been thoroughly explored yet. The recent surge in the withdrawals of pharmaceutical products from markets has raised significant concerns among regulatory bodies worldwide. As a response, regulatory authorities like United States Food and Drug Administration (U.S. FDA), European Medicines Agency (EMA), World Health Organization (WHO) are urging all the concerned stakeholders to perform stringent identification, reporting of these impurities, and are recommending APIs and drug product manufacturers to develop highly sensitive analytical methods, especially gas and liquid chromatographic methods capable of quantifying nitrosamines with lower limit of quantitation (LOQ) levels. This is essential for the effective detection and quantification of trace levels of these impurities in raw materials, intermediates in drug synthesis, and finished drug products, ultimately ensuring the safety of pharmaceuticals for consumers. The development of such novel and sensitive methods is a challenging task that demands substantial expertise and research experience. Major challenges in development of analytical methods can arise from issues such as inadequate ionization of analytes and interference of matrices. When it comes to NDSRIs, lower molecular weights, complexity in the structure, and lack of readily available standards can pose difficulties in achieving sensitive detection. It also necessitates access to high-cost instrumentation, including liquid chromatography tandem mass spectrometry (LC-MS/MS) and gas chromatography-tandem mass spectrometry (GC-MS/MS).
LC-MS/MS and GC-MS techniques are widely employed in nitrosamine detection as they offer good sensitivity with nanograms level detection of nitrosamines. Orbitrap and quadrupole time of flight (Q-TOF) analyzers are preferable for trace-level detection of these impurities. USP's initiative “Nitrosamines Exchange” provides access to industry experts, researchers, and drug manufacturers for the latest advancements in chromatographic techniques for nitrosamine detection. A sensitive analytical method should be capable of quantifying specific nitrosamine impurities in certain matrices (APIs or drug products) to 10% of its acceptable intake limit for that particular nitrosamine impurity [117].
GC-MS/MS is a preferable analytical tool for the identification and quantification of volatile and semi-volatile analytes. Chemical ionization offers better specificity and sensitivity for the analysis of nitrosamine impurities than electron impact ionization [118]. The stationary phases used in GC-MS/MS for nitrosamine analysis include polyethylene glycol (PEG)-coated capillary columns [[119], [120], [121], [122], [123]], cyanopropylphenyl dimethylpolysiloxane [124], and phenyl methylpolysiloxane [125]. Several detectors employed for nitrosamine analysis include the flame ionization detector [125], nitrogen phosphorous detector (NPD) [[126], [127], [128], [129], [130]], and chemiluminescence detector [[127], [128], [129]]. GC-MS/MS analysis can have potential interference with residual solvents, which could affect the robustness and sensitivity of the analytical method [118], for instance, dimethylformamide (DMF) interference in the analysis of NDMA in metformin [131,132]. Interferences from complex matrices can be overcome by additional clean-up and pre-treatment steps like solid-phase extraction (SPE) [124] or liquid-liquid extraction (LLE) [126,133], and the usage of internal standards [121]. Controlling and mitigating the coelution of matrices is essential as it can lead to false positive results, shorten the life span of GC columns, and contaminate the mass spectrometer [134]. This issue can be overcome by using head space (HS) injection mode [[120], [121], [122]]. However, the HS injection mode is not suitable for the analysis of thermolabile analytes like metformin and ranitidine [120,122,125].
Other alternative approaches employed in GC-MS/MS analysis include solvent-free-HS-GC/MS [122] and full-evaporation headspace-GC/MS, which offers higher sensitivity than HS-GC/MS [132]. The burden of procuring costly isotopic internal standards can be overcome by using approaches such as HS-GC/MS [120], HS-solid-phase microextraction (SPME)-GC/MS [121], dispersive liquid-liquid microextraction (DLLME)-GC/MS [125], and full evaporation static headspace gas chromatography method with nitrogen phosphorous detection [132] which do not require the usage of internal standards.
LC-MS/MS is a preferable analytical tool for the identification and quantification of polar, nonpolar, and non-volatile analytes [135]. Lee et al. [136] stated that atmospheric pressure chemical ionization (APCI) has higher sensitivity for nitrosamine analysis compared to electro spray ionization (ESI). ESI is the preferred choice for the analysis of N-nitrosodipropylamine (NDPA) [137,138]. Interference from the matrix can be minimized by using atmospheric pressure photo ionization (APPI) [136]. The positive mode of ionization is suitable for most nitrosamines, whereas the negative mode is preferred in the case of NMBA [[139], [140], [141]]. The U.S. FDA suggests the usage of LC-MS/MS for the analysis of metformin and ranitidine due to their thermally unstable nature [[120], [121], [122],125]. Deuterated isotopic standards are commonly used in LC-MS/MS analysis. Sample preparation steps are also involved in LC-MS/MS analysis. LLE is more cost effective than SPE [134]. Dichloromethane is the preferred extraction solvent chosen for NDMA analysis [[142], [143], [144]]. Matrix interference in LC-MS/MS can be overcome by an online diverting valve, which bypasses matrices present in the sample [134].
In this systematic review, we have diligently summarized the latest developments in analytical methods, specifically gas and liquid chromatographic methods, for testing nitrosamines in APIs and drug products. This comprehensive summary aims to provide valuable insights to researchers interested in developing more sensitive methods for nitrosamine detection in various drugs. Table S1 [121,124,[139], [140], [141],[145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175]] presents LC-MS/MS, GC-MS/MS, and HPLC methods that have been recommended by regulatory bodies, independent researchers, and industry experts for nitrosamine analysis.
5. Regulatory updates on nitrosamine impurities
In mid-2018, the initial detection of nitrosamines began with NDMA in sartan APIs, which prompted the recall of batches from the markets. International regulatory authorities, such as U.S. FDA, EMA, European Directorate for the Quality of Medicines & Healthcare (EDQM), Health Sciences Authority (HAS, Singapore), Pharmaceuticals and Medical Devices Agency (PMDA, Japan), Therapeutic Goods Administration (TGA, Australia), and Health Canada (HC, Canada), collaborated to alert API manufacturers. They put forth several regulations, recommendations, and guidances to review their manufacturing process and to initiate risk assessments regarding nitrosamines formation [[176], [177], [178]]. The primary focus was on evaluating raw materials, intermediates, solvents, and reagents used in API manufacturing for the presence of vulnerable amines [176]. In 2018, valsartan products were recalled from the market. In 2019, ranitidine, nizatidine, and metformin extended-release products were found to contain nitrosamines, leading to their recall from the market in 2020. The root cause of elevated nitrosamines levels in ranitidine products was linked to storage conditions [179]. Subsequently, attention shifted onto examining key factors that might cause the formation of nitrosamines in drug products.
Regulatory bodies have intended the manufacturers to perform root cause analysis to determine how nitrosamines are incorporated into drugs and drug products. The U.S. FDA put forth a three-step mitigation strategy to control and prevent nitrosamine formation in APIs and drug products. Step 1, manufacturers should prioritize risk assessments for their API and drug product portfolios, and they must document and report their findings to regulatory bodies by March 31, 2021 (U.S. FDA). EMA gave a timeline of March 31, 2021 (chemical medicines), and till July 1, 2021 (biological medicines). Step 2, confirmatory testing using sensitive analytical techniques to quantify nitrosamines is necessary until October 1, 2023 (U.S. FDA), September 26, 2022 for chemical medicines, and July 1, 2023 for biological medicines by EMA. Step 3, manufacturers must report the identified root causes of nitrosamine formation and implement of changes to their processes or approaches to eliminate nitrosamine formation [176,180].
In 2020, Lhasa Limited (UK) initiated the creation of a database on nitrite levels in excipients used in drug product manufacturing. This initiative involved representation from industry experts and researchers, as nitrite levels in excipients make drug products more prone to nitrosamine impurities [181]. Additionally, in 2020, the Committee for Medicinal Products for Human Use (CHMP), constituted by EMA, conducted scientific review of the entire situation and submitted its report in this regard. In 2021, the European medicines regulatory network established an intellectual group known as “Nitrosamine Implementation Oversight Group” to discuss developments and the current scenario regarding the emergence of nitrosamine impurities. EMA asked marketing authorization holders of sartans, rifampicin, ranitidine, metformin-containing, and varenicline medicines to release their products into the markets only after stringent testing [180].
The U.S. FDA primarily recommended following steps 2 and 3 of the three-step mitigation strategies in response to the emergence of NDSRI issues. They also called upon all concerned stakeholders to review, discuss, provide information, and update their suggestions and approaches for regulatory bodies to effectively address this problem. The recent findings on nitrosamines in several products and the subsequent recalls represent just the tip of the iceberg. There is a looming possibility of a higher incidence and risk associated with numerous products already in the market in the coming days. To address this potential crisis, a united task force should be formed in collaboration with all global regulatory bodies with an aim of devising a strategy to tackle the upcoming crisis.
6. Nitrosamine risk assessment and reformulation strategies to mitigate incorporation of nitrosamine impurities into pharmaceutical products
Root causes analysis on sources of nitrosamines incorporation must be conducted if nitrosamines are reported in the initial analysis. Subsequently, reformulation strategies must be designed to minimize the risk of nitrosamine impurities formation in pharmaceutical products. Research on reformulation strategies to mitigate nitrosamines in drug products shall be highly beneficial to tackle the crisis caused by nitrosamines.
U.S. FDA recommends the inclusion of antioxidants like ascorbic acid and α-tocopherol, as they have shown the potential to inhibit the generation of nitrosamines within the human body [182,183]. Additionally, the usage of excipients like sodium carbonate, which create a basic pH environment in vivo, could be an effective mitigation strategy because the generation of nitrosamines occurs mostly in an acidic medium [182]. Such reformulation strategies can be designed and suggested to the formulators across various pharmaceutical industries and also communicated to regulatory authorities through supplements or amendments submitted by the formulators.
Nitrosamines can be incorporated into drug products in multiple routes, during the API multi-step synthesis process, as well as drug product storage and transport. In context of API synthesis, drugs containing vulnerable amines are particularly prone to nitrosamine contamination [184]. In such cases, API manufacturers should adopt a meticulous approach when selecting catalysts, reagents, and solvents for the multi-step synthesis process [184].
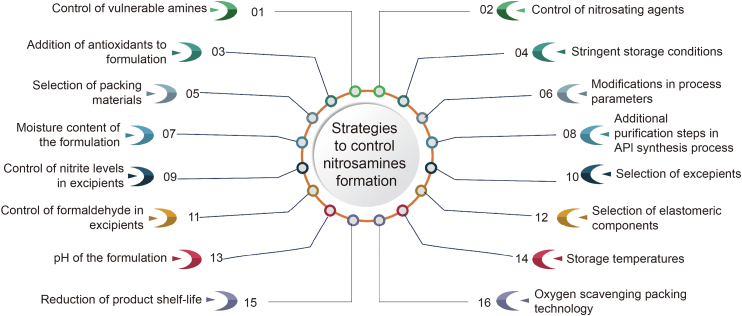
During multi-step API synthesis, incorporating additional purification steps can be effective in eliminating nitrosamine formation. It is suggested to avoid reagents containing primary or secondary amine and nitrosating agents and solvents during synthesis process. In assessing the risk of nitrosamine contamination in drug products, various factors, like pH, moisture content, and particle size distribution [185] of the formulation, are to be considered. Material characteristics of drug primary packing materials and excipients used in formulations should also be taken into account. For instance, the choice of blistering material or primer in lidding foil, such as nitrocellulose, can be critical. Nitrocellulose can act as a nitrosating agent when exposed to secondary amines like dimethylamine and diethylamine, which might be present in printing ink. During the blistering process, which is usually done at elevated temperatures, any formed nitrosamine can evaporate and potentially enter into the drug product [186]. Storage conditions may also impact the formation of nitrosamines. For example, the levels of NDMA in ranitidine API and finished products can increase with rising storage temperatures [187]. To mitigate this, Harmon et al. [188] proposed oxygen scavenging packing technology to counter autoxidation, a possible mechanism for the formation of NDMA in ranitidine hydrochloride. Harmon et al. [188] also proposed that formaldehyde, a constituent of various excipients, catalyzes nitrosating reactions and leads to nitrosamine formation. Manufacturers should exercise caution when selecting excipients for their formulations, particularly considering nitrite levels in excipients. This is especially important when drug substance and the drug product contain vulnerable amines in them [189]. Additionally in drug product risk assessment, elastomeric components should also be considered [190]. In context of drug product storage, storage temperatures should be carefully evaluated [117]. Fig. 5 delineates the reformulation strategies to mitigate the incorporation of nitrosamine impurities into pharmaceutical products.
Fig. 5.
Reformulation strategies to mitigate incorporation of nitrosamine impurities into pharmaceutical products. API: active pharmaceutical ingredient.
7. Conclusion
The systematic review provides a vivid precise risk evaluation of the molecular toxicity pertinent to the nitrosamine impurities, enabling effective root cause analysis and reformulation strategies to mitigate their presence in drug products. The development of highly sensitive analytical methods simplifies the detection process of these impurities. Furthermore, proactive measures by regulatory bodies and research communities are essential in addressing potential nitrosamine impurity-related issues in pharmaceutical products. This approach ensures patient safety and fosters confidence in the recurrent use of medications for various ailments. The presence of N-nitroso compounds, particularly N-nitrosamines, in drug products, excipients, and APIs poses significant risks to human health due to their genotoxic and mutagenic effects. Emphasis on toxicological data, devising effective methods to calculate acceptable intake limits and exploring structural activity relationships, could significantly help to tackling the crisis of nitrosamines. Sharing knowledge of in-silico predictions, in vivo studies and analytical testing on nitrosamines by concerned stakeholders and experts globally can also significantly help in avoid repetitive testing and cost reduction. The review demonstrates the detrimental impact of nitrosamine impurities on physiological systems, emphasizing the importance of novel analytical strategies for sensitive detection and quantification. Additionally, the review highlights the hypothesis that understanding root causes, implementing reformulation strategies, and incorporating regulatory updates can effectively mitigate nitrosamine impurities in pharmaceutical formulations, ensuring the development of safer products.
CRediT author statement
Hemanth P.R. Vikram: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Investigation, Writing - Original draft preparation; Tegginamath Pramod Kumar: Conceptualization, Data curation, Formal analysis, Supervision, Funding acquisition, Visualization; Gunjan Kumar: Conceptualization, Supervision, Funding acquisition; Narasimha M. Beeraka and Rajashree Deka: Writing - Reviewing and Editing; Sheik Mohammed Suhail, Sandeep Jat, Namitha Bannimath, and Gayatiri Padmanabhan: Visualization; Ravandur S. Chandan: Supervision; Pramod Kumar: Conceptualization, Supervision; Bannimath Gurupadayya: Supervision, Writing - Reviewing and Editing, Funding acquisition.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors would like to express their heartfelt gratitude to Shri Gunjan Kumar, Director of Xenone Healthcare Pvt. Ltd, New Delhi, and JSS Academy of Higher Education and Research (JSSAHER), India for unwavering support during the entire work and SERB-CII Prime Minister Fellowship, Government of India for the fellowship awarded to Mr. Hemanth P.R. Vikram.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2023.12.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Kay J.E., Corrigan J.J., Armijo A.L., et al. Excision of mutagenic replication-blocking lesions suppresses cancer but promotes cytotoxicity and lethality in nitrosamine-exposed mice. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharate S.S. Critical analysis of drug product recalls due to nitrosamine impurities. J. Med. Chem. 2021;64:2923–2936. doi: 10.1021/acs.jmedchem.0c02120. [DOI] [PubMed] [Google Scholar]
- 3.Pfizer Pfizer voluntary nationwide recall of lots of Accupril® (Quinapril HCl) due to N-nitroso-quinapril content. https://www.pfizer.com/news/announcements/pfizer-voluntary-nationwide-recall-lots-accuprilr-quinapril-hcl-due-n-nitroso
- 4.Agency E.M. Champix (varenicline) - lots to be recalled due to presence of impurity N-nitroso-varenicline above the Pfizer acceptable daily intake limit. https://www.ema.europa.eu/en/medicines/dhpc/champix-varenicline-lots-be-recalled-due-presence-impurity-n-nitroso-varenicline-above-pfizer
- 5.Singh J. International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. J. Pharmacol. Pharmacother. 2015;6:185–187. doi: 10.4103/0976-500X.162004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loprieno N. Letter: International Agency for Research on Cancer (IARC) monographs on the evaluation of carcinogenic risk of chemicals to man: “Relevance of data on mutagenicity”. Mutat. Res. 1975;31 [PubMed] [Google Scholar]
- 7.World Health Organization . Vol. 30. International Agency for Research on Cancer; 1983. (IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans). [Google Scholar]
- 8.World Health Organization Evaluation of Certain Food Additives: Eighty-sixth Report of the Joint FAO/WHO Expert Committee on food Additives. https://www.who.int/publications/i/item/9789241210232
- 9.Rose U. European pharmacopoeia activities on control of nitrosamines and other DNA-reactive impurities. J. Pharm. Sci. 2023;112:1163–1165. doi: 10.1016/j.xphs.2022.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Ashworth I.W., Dirat O., Teasdale A., et al. Potential for the formation of N-nitrosamines during the manufacture of active pharmaceutical ingredients: An assessment of the risk posed by trace nitrite in water. Org. Process Res. Dev. 2020;24:1629–1646. [Google Scholar]
- 11.Shaikh T., Gosar A., Sayyed H. Nitrosamine impurities in drug substances and drug products. J. Adv. Pharm. Pract. 2020;2:48–57. [Google Scholar]
- 12.Sen N.P., Smith D.C., Schwinghamer L. Formation of N-nitrosamines from secondary amines and nitrite in human and animal gastric juice. Food Cosmet. Toxicol. 1969;7:301–307. doi: 10.1016/s0015-6264(69)80366-4. [DOI] [PubMed] [Google Scholar]
- 13.Dass S.B., Hammons G.J., Bucci T.J., et al. Susceptibility of C57BL/6 mice to tumorigenicity induced by dimethylnitrosamine and 2-amino-1-methyl-6-phenylimidazo[4, 5-b]pyridine in the neonatal bioassay. Cancer Lett. 1998;124:105–110. doi: 10.1016/s0304-3835(97)00462-x. [DOI] [PubMed] [Google Scholar]
- 14.Dass S.B., Bucci T.J., Heflich R.H., et al. Evaluation of the transgenic p53+/- mouse for detecting genotoxic liver carcinogens in a short-term bioassay. Cancer Lett. 1999;143:81–85. doi: 10.1016/s0304-3835(99)00196-2. [DOI] [PubMed] [Google Scholar]
- 15.Nishikawa A., Furukawa F., Kasahara K., et al. Comparative study on organ-specificity of tumorigenicity, mutagenicity and cell proliferative activity induced by dimethylnitrosamine in Big Blue mice. Cancer Lett. 1997;117:143–147. doi: 10.1016/s0304-3835(97)00225-5. [DOI] [PubMed] [Google Scholar]
- 16.Peto R., Gray R., Brantom P., et al. Dose and time relationships for tumor induction in the liver and esophagus of 4080 inbred rats by chronic ingestion of N-nitrosodiethylamine or N-nitrosodimethylamine. Cancer Res. 1991;51:6452–6469. [PubMed] [Google Scholar]
- 17.Vesselinovitch S.D. The sex-dependent difference in the development of liver tumors in mice administered dimethylnitrosamine. Cancer Res. 1969;29:1024–1027. [PubMed] [Google Scholar]
- 18.Weghorst C.M., Pereira M.A., Klaunig J.E. Strain differences in hepatic tumor promotion by phenobarbital in diethylnitrosamine- and dimethylnitrosamine-initiated infant male mice. Carcinogenesis. 1989;10:1409–1412. doi: 10.1093/carcin/10.8.1409. [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki H., Oda Y., Funae Y., et al. Participation of rat liver cytochrome P450 2E1 in the activation of N-nitrosodimethylamine and N-nitrosodiethylainine to products genotoxic in an acetyltransferase-overexpressing Salmonella typhimurium strain (NM2009) Carcinogenesis. 1992;13:979–985. doi: 10.1093/carcin/13.6.979. [DOI] [PubMed] [Google Scholar]
- 20.Yamazaki H., Inui Y., Yun C.-H., et al. Cytochrome P450 2E1 and 2A6 enzymes as major catalysts for metabolic activation of N-nitrosodialkylamines and tobacco-related nitrosamines in human liver microsomes. Carcinogenesis. 1992;13:1789–1794. doi: 10.1093/carcin/13.10.1789. [DOI] [PubMed] [Google Scholar]
- 21.Nedelcheva V., Gut I. P450 in the rat and man: Methods of investigation, substrate specificities and relevance to cancer. Xenobiotica. 1994;24:1151–1175. doi: 10.3109/00498259409038673. [DOI] [PubMed] [Google Scholar]
- 22.Martignoni M., Groothuis G.M., de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2006;2:875–894. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- 23.Cross K.P., Ponting D.J. Developing structure-activity relationships for N-nitrosamine activity. Comput. Toxicol. 2021;20 doi: 10.1016/j.comtox.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillipson C.E., Ioannides C. A comparative study of the bioactivation of nitrosamines to mutagens by various animal species including man. Carcinogenesis. 1984;5:1091–1094. doi: 10.1093/carcin/5.8.1091. [DOI] [PubMed] [Google Scholar]
- 25.Synder S., Hsu I.C., Trump B.F. Comparison of metabolic activation of carcinogens in human, rat, and hamster hepatocytes. Mutat. Res. 1987;182:31–39. doi: 10.1016/0165-1161(87)90005-7. [DOI] [PubMed] [Google Scholar]
- 26.Glowienke S., Onken U., Elhajouji A., et al. Genotoxicity evaluation of a valsartan-related complex N-nitroso-impurity. Regul. Toxicol. Pharmacol. 2022;134 doi: 10.1016/j.yrtph.2022.105245. [DOI] [PubMed] [Google Scholar]
- 27.Li X., He X., Le Y., et al. Genotoxicity evaluation of nitrosamine impurities using human TK6 cells transduced with cytochrome P450s. Arch. Toxicol. 2022;96:3077–3089. doi: 10.1007/s00204-022-03347-6. [DOI] [PubMed] [Google Scholar]
- 28.Cumulative Index to IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans, IARC Monogr. Eval. Carcinog. Risk. Chem. Hum. 1986;39:379–403. [PubMed] [Google Scholar]
- 29.Khudoley V.V., Syrenko O.A. Tumor induction by N-nitroso compounds in bivalve mollusks Unio pictorum. Cancer Lett. 1978;4:349–354. doi: 10.1016/s0304-3835(78)95722-1. [DOI] [PubMed] [Google Scholar]
- 30.Richter-Reichhelm H.B., Green U., Ketkar M.B., et al. The carcinogenic effect of dimethylnitrosamine in laboratory bred European hamsters (Cricetus cricetus) Cancer Lett. 1978;4:1–4. doi: 10.1016/s0304-3835(78)92972-5. [DOI] [PubMed] [Google Scholar]
- 31.Koppang N., Helgebostad A., Armstrong D., et al. Toxic and carcinogenic effects of dimethylnitrosamine (DMNA) in the blue fox (Alopex lagopus) Acta Vet. Scand. 1981;22:501–516. doi: 10.1186/BF03548675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noronha R.F., Goodall C.M. Enhancement by testosterone of dimethylnitrosamine carcinogenesis in lung, liver and kidney of inbred NZR/Gd female rats. Carcinogenesis. 1983;4:613–616. doi: 10.1093/carcin/4.5.613. [DOI] [PubMed] [Google Scholar]
- 33.De Stefani E., Oreggia F., Ronco A., et al. Salted meat consumption as a risk factor for cancer of the oral cavity and pharynx: A case-control study from Uruguay. Cancer Epidemiol. Biomarkers Prev. 1994;3:381–385. [PubMed] [Google Scholar]
- 34.La Vecchia C., D’Avanzo B., Airoldi L., et al. Nitrosamine intake and gastric cancer risk. Eur. J. Cancer Prev. 1995;4:469–474. doi: 10.1097/00008469-199512000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Pobel D., Riboli E., Cornée J., et al. Nitrosamine, nitrate and nitrite in relation to gastric cancer: A case-control study in Marseille, France. Eur. J. Epidemiol. 1995;11:67–73. doi: 10.1007/BF01719947. [DOI] [PubMed] [Google Scholar]
- 36.Lu S.H., Yang W.X., Guo L.P., et al. IARC Sci. Publ.; 1987. Determination of N-nitrosamines in gastric juice and urine and a comparison of endogenous formation of N-nitrosoproline and its inhibition in subjects from high- and low-risk areas for oesophageal cancer; pp. 538–543. [PubMed] [Google Scholar]
- 37.Rogers M., Vaughan T.L., Davis S., et al. Consumption of nitrate, nitrite, and nitrosodimethylamine and the risk of upper aerodigestive tract cancer. Cancer Epidemiol. Biomarkers Prev. 1995;4:29–36. [PubMed] [Google Scholar]
- 38.Siddiqi M., Tricker A.R., Preussmann R. Formation of N-nitroso compounds under simulated gastric conditions from Kashmir foodstuffs. Cancer Lett. 1988;39:259–265. doi: 10.1016/0304-3835(88)90068-7. [DOI] [PubMed] [Google Scholar]
- 39.Lin K., Shen W., Shen Z., et al. Dietary exposure and urinary excretion of total N-nitroso compounds, nitrosamino acids and volatile nitrosamine in inhabitants of high- and low-risk areas for esophageal cancer in Southern China. Int. J. Cancer. 2002;102:207–211. doi: 10.1002/ijc.10698. [DOI] [PubMed] [Google Scholar]
- 40.Lin K., Shen Z., Lu S.H., et al. Intake of volatile N-nitrosamines and their ability to exogenously synthesize in the diet of inhabitants from high-risk area of esophageal cancer in Southern China, Biomed. Environ. Sci. 2002;15:277–282. [PubMed] [Google Scholar]
- 41.Knekt P., Järvinen R., Dich J., et al. Risk of colorectal and other gastro-intestinal cancers after exposure to nitrate, nitrite and N-nitroso compounds: A follow-up study. Int. J. Cancer. 1999;80:852–856. doi: 10.1002/(sici)1097-0215(19990315)80:6<852::aid-ijc9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 42.Schmähl D., Habs M., Ivankovic S. Carcinogenesis of N-nitrosodiethylamine (DENA) in chickens and domestic cats. Int. J. Cancer. 1978;22:552–557. doi: 10.1002/ijc.2910220508. [DOI] [PubMed] [Google Scholar]
- 43.Lai D.Y., Arcos J.C. Minireview: Dialkylnitrosamine bioactivation and carcinogenesis. Life Sci. 1980;27:2149–2165. doi: 10.1016/0024-3205(80)90379-3. [DOI] [PubMed] [Google Scholar]
- 44.Vesselinovitch S.D., Koka M., Mihailovich N., et al. Carcinogenicity of diethylnitrosamine in newborn, infant, and adult mice. J. Cancer Res. Clin. Oncol. 1984;108:60–65. doi: 10.1007/BF00390974. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto A., Hisanaga A., Ishinishi N. Comparative study on the carcinogenicity of N-nitrosodiethylamine and benzo[a]pyrene to the lung of Syrian golden hamsters induced by intermittent instillations to the trachea. Cancer Lett. 1985;25:271–276. doi: 10.1016/s0304-3835(15)30006-9. [DOI] [PubMed] [Google Scholar]
- 46.Ishinishi N., Tanaka A., Hisanaga A., et al. Comparative study on the carcinogenicity of N-nitrosodiethylamine, N-nitrosodimethylamine, N-nitrosomorpholine, N-nitrosopyrrolidine and N-nitrosodi-n-propylamine to the lung of Syrian golden hamsters following intermittent instillations to the trachea. Carcinogenesis. 1988;9:947–950. doi: 10.1093/carcin/9.6.947. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka A., Hisanaga A., Inamasu T., et al. A comparison of the carcinogenicity of N-nitrosodiethylamine and N-nitrosodimethylamine after intratracheal instillation into Syrian golden hamsters. Food Chem. Toxicol. 1988;26:847–850. doi: 10.1016/0278-6915(88)90025-7. [DOI] [PubMed] [Google Scholar]
- 48.Huntrakoon M., Menon C.D., Hung K.S. Diethylnitrosamine-induced pulmonary endocrine cell hyperplasia and its association with adenomatosis and adenocarcinoma in rabbits. Am. J. Pathol. 1989;135:1119–1128. [PMC free article] [PubMed] [Google Scholar]
- 49.Thiyagarajah A., Grizzle J.M. Diethylnitrosamine-induced pancreatic neoplasms in the fish Rivulus ocellatus marmoratus. J. Natl. Cancer Inst. 1986;77:141–147. [PubMed] [Google Scholar]
- 50.Mohr U., Emura M., Kamino K., et al. Increased risk of cancer in the descendants of Syrian hamsters exposed prenatally to diethylnitrosamine (DEN) Int. J. Cancer. 1995;63:86–91. doi: 10.1002/ijc.2910630116. [DOI] [PubMed] [Google Scholar]
- 51.Klein R.G., Spiegelhalder B., Preussmann R. Inhalation carcinogenesis of N-nitrosomorpholine (NMOR) in rats and hamsters. Exp. Pathol. 1990;40:189–195. doi: 10.1016/s0232-1513(11)80293-8. [DOI] [PubMed] [Google Scholar]
- 52.Lijinsky W., Thomas B.J., Kovatch R.M. Local and systemic carcinogenic effects of alkylating carcinogens in rats treated by intravesicular administration. Jpn. J. Cancer Res. 1991;82:980–986. doi: 10.1111/j.1349-7006.1991.tb01931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ketkar M.B., Holste J., Preussmann R., et al. Carcinogenic effect of nitrosomorpholine administered in the drinking water to Syrian golden hamsters. Cancer Lett. 1983;17:333–338. doi: 10.1016/0304-3835(83)90172-6. [DOI] [PubMed] [Google Scholar]
- 54.Lijinsky W., Kovatch R.M., Knutsen G.L. Carcinogenesis by nitrosomorpholines, nitrosooxazolidines and nitrosoazetidine given by gavage to Syrian golden hamsters. Carcinogenesis. 1984;5:875–878. doi: 10.1093/carcin/5.7.875. [DOI] [PubMed] [Google Scholar]
- 55.Cardesa A., García-Bragado F., Ramírez J., et al. Histological types of laryngotracheal tumors induced in Syrian golden hamsters by nitrosomorpholine and nitrosopiperidine. Exp. Pathol. 1990;40:267–281. doi: 10.1016/s0232-1513(11)80311-7. [DOI] [PubMed] [Google Scholar]
- 56.Swenberg J.A., Hoel D.G., Magee P.N. Mechanistic and statistical insight into the large carcinogenesis bioassays on N-nitrosodiethylamine and N-nitrosodimethylamine. Cancer Res. 1991;51:6409–6414. [PubMed] [Google Scholar]
- 57.Pegg A.E., Hui G. Formation and subsequent removal of O6-methylguanine from deoxyribonucleic acid in rat liver and kidney after small doses of dimethylnitrosamine. Biochem. J. 1978;173:739–748. doi: 10.1042/bj1730739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Souliotis V.L., van Delft J.H., Steenwinkel M.J., et al. DNA adducts, mutant frequencies and mutation spectra in lambda lacZ transgenic mice treated with N-nitrosodimethylamine. Carcinogenesis. 1998;19:731–739. doi: 10.1093/carcin/19.5.731. [DOI] [PubMed] [Google Scholar]
- 59.Beranek D.T. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat. Res. 1990;231:11–30. doi: 10.1016/0027-5107(90)90173-2. [DOI] [PubMed] [Google Scholar]
- 60.Calvo J.A., Moroski-Erkul C.A., Lake A., et al. Aag DNA glycosylase promotes alkylation-induced tissue damage mediated by Parp1. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crosbie P.A., Watson A.J., Agius R., et al. Elevated N3-methylpurine-DNA glycosylase DNA repair activity is associated with lung cancer. Mutat. Res. 2012;732:43–46. doi: 10.1016/j.mrfmmm.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Hall J., Brésil H., Donato F., et al. Alkylation and oxidative-DNA damage repair activity in blood leukocytes of smokers and non-smokers. Int. J. Cancer. 1993;54:728–733. doi: 10.1002/ijc.2910540504. [DOI] [PubMed] [Google Scholar]
- 63.Ghaffari H.R., Nasseri S., Yunesian M., et al. Monitoring and exposure assessment of nitrate intake via fruits and vegetables in high and low risk areas for gastric cancer. J. Environ. Health Sci. Eng. 2019;17:445–456. doi: 10.1007/s40201-019-00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao C., Zhou J., Gu Y., et al. Urinary exposure of N-nitrosamines and associated risk of esophageal cancer in a high incidence area in China. Sci. Total Environ. 2020;738 doi: 10.1016/j.scitotenv.2020.139713. [DOI] [PubMed] [Google Scholar]
- 65.Zheng J., Daniel C.R., Hatia R.I., et al. Dietary N-nitroso compounds and risk of hepatocellular carcinoma: A USA-based study. Hepatology. 2021;74:3161–3173. doi: 10.1002/hep.32046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.World Cancer Research Fund and American Institute for Cancer Research Food, nutrition, physical activity, and the prevention of cancer: A global perspective. https://www3.paho.org/hq/dmdocuments/2011/nutrition-AICR-WCR-food-physical-activ.pdf
- 67.Shephard S.E., Schlatter C., Lutz W.K. Assessment of the risk of formation of carcinogenic N-nitroso compounds from dietary precursors in the stomach. Food Chem. Toxicol. 1987;25:91–108. doi: 10.1016/0278-6915(87)90311-5. [DOI] [PubMed] [Google Scholar]
- 68.Tricker A.R., Preussmann R. Carcinogenic N-nitrosamines in the diet: Occurrence, formation, mechanisms and carcinogenic potential. Mutat. Res. 1991;259:277–289. doi: 10.1016/0165-1218(91)90123-4. [DOI] [PubMed] [Google Scholar]
- 69.Freedman N.D., Cross A.J., McGlynn K.A., et al. Association of meat and fat intake with liver disease and hepatocellular carcinoma in the NIH-AARP cohort. J. Natl. Cancer Inst. 2010;102:1354–1365. doi: 10.1093/jnci/djq301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lijinsky W. N-nitroso compounds in the diet. Mutat. Res. 1999;443:129–138. doi: 10.1016/s1383-5742(99)00015-0. [DOI] [PubMed] [Google Scholar]
- 71.Zheng J., Stuff J., Tang H., et al. Dietary N-nitroso compounds and risk of pancreatic cancer: Results from a large case-control study. Carcinogenesis. 2019;40:254–262. doi: 10.1093/carcin/bgy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bartsch H., Montesano R. Relevance of nitrosamines to human cancer. Carcinogenesis. 1984;5:1381–1393. doi: 10.1093/carcin/5.11.1381. [DOI] [PubMed] [Google Scholar]
- 73.Peto R., Gray R., Brantom P., et al. IARC Sci. Publ.; 1984. Nitrosamine carcinogenesis in 5120 rodents: Chronic administration of sixteen different concentrations of NDEA, NDMA, NPYR and NPIP in the water of 4440 inbred rats, with parallel studies on NDEA alone of the effect of age of starting (3, 6 or 20 weeks) and of species (rats, mice or hamsters) pp. 627–665. [PubMed] [Google Scholar]
- 74.Kimbrough R.D. Pathological changes in human beings acutely poisoned by dimethylnitrosamine. Banbury Rep. 1982;12:25–35. [Google Scholar]
- 75.Lijinsky W. Life-span and cancer: The induction time of tumors in diverse animal species treated with nitrosodiethylamine. Carcinogenesis. 1993;14:2373–2375. doi: 10.1093/carcin/14.11.2373. [DOI] [PubMed] [Google Scholar]
- 76.Flaks B., Challis B.C. Fine structure of rat liver during chronic intoxication with two heterocyclic N-nitrosamines: N-nitrosopiperidine and the non-carcinogen, 2,2',6,6'-tetramethyl-N-nitrosopiperidine. Carcinogenesis. 1980;1:961–974. doi: 10.1093/carcin/1.12.961. [DOI] [PubMed] [Google Scholar]
- 77.Wong H.L., Murphy S.E., Wang M., et al. Comparative metabolism of N-nitrosopiperidine and N-nitrosopyrrolidine by rat liver and esophageal microsomes and cytochrome P450 2A3. Carcinogenesis. 2003;24:291–300. doi: 10.1093/carcin/24.2.291. [DOI] [PubMed] [Google Scholar]
- 78.Zhang H., Zhao C., Liu Q., et al. Dysregulation of fatty acid metabolism associated with esophageal inflammation of ICR mice induced by nitrosamines exposure. Environ. Pollut. 2022;297 doi: 10.1016/j.envpol.2021.118680. [DOI] [PubMed] [Google Scholar]
- 79.Neves Cruz J., Santana de Oliveira M., Gomes Silva S., et al. Insight into the interaction mechanism of nicotine, NNK, and NNN with cytochrome P450 2A13 based on molecular dynamics simulation. J. Chem. Inf. Model. 2020;60:766–776. doi: 10.1021/acs.jcim.9b00741. [DOI] [PubMed] [Google Scholar]
- 80.Guo J., Zhu X., Badawy S., et al. Metabolism and mechanism of human cytochrome P450 enzyme 1A2. Curr. Drug Metab. 2021;22:40–49. doi: 10.2174/1389200221999210101233135. [DOI] [PubMed] [Google Scholar]
- 81.Juvonen R.O., Jokinen E.M., Huuskonen J., et al. Molecular docking and oxidation kinetics of 3-phenyl coumarin derivatives by human CYP2A13. Xenobiotica. 2021;51:1207–1216. doi: 10.1080/00498254.2021.1898700. [DOI] [PubMed] [Google Scholar]
- 82.Zhang H., Lu L., Zhao C., et al. Lipid metabolism disorders contribute to hepatotoxicity of ICR mice induced by nitrosamines exposure. Environ. Int. 2022;167 doi: 10.1016/j.envint.2022.107423. [DOI] [PubMed] [Google Scholar]
- 83.Stuff J.E., Goh E.T., Barrera S.L., et al. Construction of an N-nitroso database for assessing dietary intake. J. Food Compost Anal. 2009;22:S42–S47. doi: 10.1016/j.jfca.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bartsch H., Ohshima H., Pignatelli B. Inhibitors of endogenous nitrosation. Mechanisms and implications in human cancer prevention. Mutat. Res. 1988;202:307–324. doi: 10.1016/0027-5107(88)90194-7. [DOI] [PubMed] [Google Scholar]
- 85.Lunn J.C., Kuhnle G., Mai V., et al. The effect of haem in red and processed meat on the endogenous formation of N-nitroso compounds in the upper gastrointestinal tract. Carcinogenesis. 2007;28:685–690. doi: 10.1093/carcin/bgl192. [DOI] [PubMed] [Google Scholar]
- 86.Aschebrook-Kilfoy B., Cross A.J., Stolzenberg-Solomon R.Z., et al. Pancreatic cancer and exposure to dietary nitrate and nitrite in the NIH-AARP Diet and Health Study. Am. J. Epidemiol. 2011;174:305–315. doi: 10.1093/aje/kwr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roshankhah S., Salahshoor M., Jalili C., et al. Pentoxifylline modulation hepatotoxicity and apoptosis induced by nitrosamine in rats. Biomed. Biotechnol. Res. J. BBRJ. 2020;4 [Google Scholar]
- 88.Li K., Ricker K., Tsai F.C., et al. Estimated cancer risks associated with nitrosamine contamination in commonly used medications. Int. J. Environ. Res. Public Health. 2021;18 doi: 10.3390/ijerph18189465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Druckrey H., Preussmann R., Ivankovic S., et al. Organotropic carcinogenic effects of 65 various N-nitroso- compounds on BD rats. Z. Krebsforsch. 1967;69:103–201. [PubMed] [Google Scholar]
- 90.M Barnes J., Magee P.N. Some toxic properties of dimethylnitrosamine. Br. J. Ind. Med. 1954:167–174. doi: 10.1136/oem.11.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gankhuyag N., Lee K.H., Cho J.Y. The role of nitrosamine (NNK) in breast cancer carcinogenesis. J. Mammary Gland Biol. Neoplasia. 2017;22:159–170. doi: 10.1007/s10911-017-9381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee C.H., Chang Y.C., Chen C.S., et al. Crosstalk between nicotine and estrogen-induced estrogen receptor activation induces α9-nicotinic acetylcholine receptor expression in human breast cancer cells. Breast Cancer Res. Treat. 2011;129:331–345. doi: 10.1007/s10549-010-1209-0. [DOI] [PubMed] [Google Scholar]
- 93.Lee C.-H., Huang C.-S., Chen C.-S., et al. Overexpression and activation of the α9-nicotinic receptor during tumorigenesis in human breast epithelial cells. J. Natl. Cancer Inst. 2010;102:1322–1335. doi: 10.1093/jnci/djq300. [DOI] [PubMed] [Google Scholar]
- 94.Improgo M.R., Soll L.G., Tapper A.R., et al. Nicotinic acetylcholine receptors mediate lung cancer growth. Front. Physiol. 2013;4 doi: 10.3389/fphys.2013.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang F., Gerzanich V., Wells G.B., et al. Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits. J. Biol. Chem. 1996;271:17656–17665. doi: 10.1074/jbc.271.30.17656. [DOI] [PubMed] [Google Scholar]
- 96.Groot-Kormelink P.J., Boorman J.P., Sivilotti L.G. Formation of functional α3β4α5 human neuronal nicotinic receptors in Xenopus oocytes: A reporter mutation approach. Br. J. Pharmacol. 2001;134:789–796. doi: 10.1038/sj.bjp.0704313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramirez-Latorre J., Yu C.R., Qu X., et al. Functional contributions of α5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- 98.Callaghan B., Adams D.J. Analgesic α-conotoxins Vc1.1 and RgIA inhibit N-type calcium channels in sensory neurons of α9 nicotinic receptor knockout mice. Channels (Austin) 2010;4:51–54. doi: 10.4161/chan.4.1.10281. [DOI] [PubMed] [Google Scholar]
- 99.Ho Y.S., Lee C.H., Wu C.H. The alpha 9-nicotinic acetylcholine receptor serves as a molecular target for breast cancer therapy. J. Exp. Clin. Med. 2011;3:246–251. [Google Scholar]
- 100.Katz E., Verbitsky M., Rothlin C.V., et al. High calcium permeability and calcium block of the α9 nicotinic acetylcholine receptor. Hear. Res. 2000;141:117–128. doi: 10.1016/s0378-5955(99)00214-2. [DOI] [PubMed] [Google Scholar]
- 101.Weddle D.L., Tithoff P., Williams M., et al. β-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22:473–479. doi: 10.1093/carcin/22.3.473. [DOI] [PubMed] [Google Scholar]
- 102.Schuller H.M., Cole B. Regulation of cell proliferation by β- adrenergic receptors in a human lung adenocarcinoma cell line. Carcinogenesis. 1989;10:1753–1755. doi: 10.1093/carcin/10.9.1753. [DOI] [PubMed] [Google Scholar]
- 103.Slomiany B.L., Slomiany A. Src-kinase-dependent epidermal growth factor receptor transactivation in salivary mucin secretion in response to β-adrenergic G-protein-coupled receptor activation. Inflammopharmacology. 2004;12:233–245. doi: 10.1163/1568560042342329. [DOI] [PubMed] [Google Scholar]
- 104.Dorsam R.T., Gutkind J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 105.Zhang J., Selaya S.D., Shakleya D., et al. Rapid quantitation of four nitrosamine impurities in angiotensin receptor blocker drug substances. J. Pharm. Sci. 2023;112:1246–1254. doi: 10.1016/j.xphs.2022.12.005. [DOI] [PubMed] [Google Scholar]
- 106.Shephard E.A., Nawarskas J.J. Nitrosamine impurities in angiotensin receptor blockers. Cardiol. Rev. 2020;28:262–265. doi: 10.1097/CRD.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 107.Mochizuki M., Osabe M., Anjo T., et al. Mutagenicity of α-hydroxy N-nitrosamines in V79 Chinese hamster cells. J. Cancer Res. Clin. Oncol. 1984;108:290–295. doi: 10.1007/BF00390460. [DOI] [PubMed] [Google Scholar]
- 108.Li Y., Hecht S.S. Metabolic activation and DNA interactions of carcinogenic N-nitrosamines to which humans are commonly exposed. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23094559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang C.S., Yoo J.S., Ishizaki H., et al. Cytochrome P450IIE1: Roles in nitrosamine metabolism and mechanisms of regulation. Drug Metab. Rev. 1990;22:147–159. doi: 10.3109/03602539009041082. [DOI] [PubMed] [Google Scholar]
- 110.Verna L., Whysner J., Williams G.M. N-nitrosodiethylamine mechanistic data and risk assessment: Bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol. Ther. 1996;71:57–81. doi: 10.1016/0163-7258(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 111.Peto R., Gray R., Brantom P., et al. Effects on 4080 rats of chronic ingestion of N-nitrosodiethylamine or N-nitrosodimethylamine: A detailed dose-response study. Cancer Res. 1991;51:6415–6451. [PubMed] [Google Scholar]
- 112.Harris C.C., Autrup H., Stoner G.D., et al. Metabolism of benzo(a)pyrene, N-nitrosodimethylamine, and N-nitrosopyrrolidine and identification of the major carcinogen-DNA adducts formed in cultured human esophagus. Cancer Res. 1979;39:4401–4406. [PubMed] [Google Scholar]
- 113.Kyrtopoulos S.A. DNA adducts in humans after exposure to methylating agents. Mutat. Res. 1998;405:135–143. doi: 10.1016/s0027-5107(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 114.Phillips D.H. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis. 2002;23:1979–2004. doi: 10.1093/carcin/23.12.1979. [DOI] [PubMed] [Google Scholar]
- 115.Ma B., Stepanov I., Hecht S.S. Recent studies on DNA adducts resulting from human exposure to tobacco smoke. Toxics. 2019;7 doi: 10.3390/toxics7010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Herron D.C., Shank R.C. Methylated purines in human liver DNA after probable dimethylnitrosamine poisoning. Cancer Res. 1980;40:3116–3117. [PubMed] [Google Scholar]
- 117.Schlingemann J., Burns M.J., Ponting D.J., et al. The landscape of potential small and drug substance related nitrosamines in pharmaceuticals. J. Pharm. Sci. 2023;112:1287–1304. doi: 10.1016/j.xphs.2022.11.013. [DOI] [PubMed] [Google Scholar]
- 118.Parr M.K., Joseph J.F. NDMA impurity in valsartan and other pharmaceutical products: Analytical methods for the determination of N-nitrosamines. J. Pharm. Biomed. Anal. 2019;164:536–549. doi: 10.1016/j.jpba.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 119.U.S. Food and Drug Administration Combined N-Nitrosodimethylamine (NDMA) and N-Nitrosodiethylamine (NDEA) Impurity Assay by GC/MS-Headspace. https://www.fda.gov/media/117843/download
- 120.Wichitnithad W., Sudtanon O., Srisunak P., et al. Development of a sensitive headspace gas chromatography-mass spectrometry method for the simultaneous determination of nitrosamines in losartan active pharmaceutical ingredients. ACS Omega. 2021;6:11048–11058. doi: 10.1021/acsomega.1c00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alshehri Y.M., Alghamdi T.S., Aldawsari F.S. HS-SPME-GC-MS as an alternative method for NDMA analysis in ranitidine products. J. Pharm. Biomed. Anal. 2020;191 doi: 10.1016/j.jpba.2020.113582. [DOI] [PubMed] [Google Scholar]
- 122.Lee D.H., Hwang S.H., Park S., et al. A solvent-free headspace GC/MS method for sensitive screening of N-nitrosodimethylamine in drug products. Anal. Methods. 2021;13:3402–3409. doi: 10.1039/d1ay01036k. [DOI] [PubMed] [Google Scholar]
- 123.Chang S.-H., Ho H.-Y., Zang C.-Z., et al. Screening of nitrosamine impurities in sartan pharmaceuticals by GC-MS/MS. Mass Spectrom. Lett. 2021;12:31–40. [Google Scholar]
- 124.Lim H.H., Oh Y.S., Shin H.S. Determination of N-nitrosodimethylamine and N-nitrosomethylethylamine in drug substances and products of sartans, metformin and ranitidine by precipitation and solid phase extraction and gas chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2020;189 doi: 10.1016/j.jpba.2020.113460. [DOI] [PubMed] [Google Scholar]
- 125.Giménez-Campillo C., Pastor-Belda M., Campillo N., et al. Development of a new methodology for the determination of N-nitrosamines impurities in ranitidine pharmaceuticals using microextraction and gas chromatography-mass spectrometry. Talanta. 2021;223 doi: 10.1016/j.talanta.2020.121659. [DOI] [PubMed] [Google Scholar]
- 126.Takatsuki K., Kikuchi T. Determination of N-nitrosodimethylamine in fish products using gas chromatography with nitrogen-phosphorus detection. J. Chromatogr. 1990;508:357–362. doi: 10.1016/s0021-9673(00)91278-0. [DOI] [PubMed] [Google Scholar]
- 127.Grebel J.E., Mel Suffet I.H. Nitrogen-phosphorus detection and nitrogen chemiluminescence detection of volatile nitrosamines in water matrices: Optimization and performance comparison. J. Chromatogr. A. 2007;1175:141–144. doi: 10.1016/j.chroma.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 128.Grebel J.E., Young C.C., Suffet I.H. Solid-phase microextraction of N-nitrosamines. J. Chromatogr. A. 2006;1117:11–18. doi: 10.1016/j.chroma.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 129.Jurado-Sánchez B., Ballesteros E., Gallego M. Comparison of the sensitivities of seven N-nitrosamines in pre-screened waters using an automated preconcentration system and gas chromatography with different detectors. J. Chromatogr. A. 2007;1154:66–73. doi: 10.1016/j.chroma.2007.03.117. [DOI] [PubMed] [Google Scholar]
- 130.Zheng J., Kirkpatrick C.L., Lee D., et al. A full evaporation static headspace gas chromatography method with nitrogen phosphorous detection for ultrasensitive analysis of semi-volatile nitrosamines in pharmaceutical products. AAPS J. 2022;24 doi: 10.1208/s12248-021-00669-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zmysłowski A., Książek I., Szterk A. N-nitrosodimethylamine contamination in the metformin finished products. Molecules. 2020;25 doi: 10.3390/molecules25225304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang J., Marzan T.A., Ye W., et al. A cautionary tale: Quantitative LC-HRMS analytical procedures for the analysis of N-nitrosodimethylamine in metformin. AAPS J. 2020;22 doi: 10.1208/s12248-020-00473-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Al-Kaseem M., Al-Assaf Z., Karabeet F. Development and validation of GC-FID method for the determination of volatile N-nitrosamines in meat. Int. J. Pharm. Sci. Rev. Res. 2014;25:59–64. [Google Scholar]
- 134.Wichitnithad W., Nantaphol S., Noppakhunsomboon K., et al. Current status and prospects of development of analytical methods for determining nitrosamine and N-nitroso impurities in pharmaceuticals. Talanta. 2023;254 doi: 10.1016/j.talanta.2022.124102. [DOI] [PubMed] [Google Scholar]
- 135.Banerjee S., Mazumdar S. Electrospray ionization mass spectrometry: A technique to access the information beyond the molecular weight of the analyte. Int. J. Anal. Chem. 2012;2012 doi: 10.1155/2012/282574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee J.H., Lee S.U., Oh J.E. Analysis of nine nitrosamines in water by combining automated solid-phase extraction with high-performance liquid chromatography-atmospheric pressure chemical ionisation tandem mass spectrometry. Int. J. Environ. Anal. Chem. 2013;93:1261–1273. [Google Scholar]
- 137.Chang S., Chang C.C., Wang L., et al. A multi-analyte LC-MS/MS method for screening and quantification of nitrosamines in sartans. J. Food Drug Anal. 2020;28:292–301. doi: 10.38212/2224-6614.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim H., Sung D., Yu H., et al. Comparison of EI-GC-MS/MS, APCI-LC-MS/MS, and ESI-LC-MS/MS for the simultaneous analysis of nine nitrosamines eluted from synthetic resins into artificial saliva and health risk assessment. Toxics. 2021;9 doi: 10.3390/toxics9100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.European Directorate for the Quality of Medicines & HealthCare, LCMS/MS method “190321 NMBA APCI POS waste” (Shimadzu HPLC + Sciex QTrap 5500) https://www.edqm.eu/en/ad-hoc-projects-of-the-omcl-network
- 140.European Directorate for the Quality of Medicines & HealthCare, OMCLs release three methods for determination of NDMA in sartans. https://www.edqm.eu/en/-/omcls-release-three-methods-for-determination-of-ndma-in-sartans-1
- 141.U.S. Food and Drug Administration Liquid chromatography-high resolution mass spectrometry (LC-HRMS) method for the determination of six nitrosamine impurities in ARB drugs. https://www.fda.gov/media/125478/download
- 142.Cárdenes L., Ayala J.H., González V., et al. Fast microwave-assisted dansylation of N-nitrosamines. Analysis by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. A. 2002;946:133–140. doi: 10.1016/s0021-9673(01)01547-3. [DOI] [PubMed] [Google Scholar]
- 143.Kokotou M.G., Mantzourani C., Bourboula A., et al. A liquid chromatography-high resolution mass spectrometry (LC-HRMS) method for the determination of free hydroxy fatty acids in cow and goat milk. Molecules. 2020;25 doi: 10.3390/molecules25173947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mutsuga M., Yamaguchi M., Kawamura Y. Analysis of N-nitrosamine migration from rubber teats and soothers. Am. J. Anal. Chem. 2013;4:277–285. [Google Scholar]
- 145.U.S. Food and Drug Administration Liquid chromatography-high resolution mass spectrometry (LC-HRMS) method for the determination of NDMA in ranitidine drug substance and drug product. https://www.fda.gov/media/130801/download
- 146.U.S. Food and Drug Administration Liquid chromatography-high resolution mass spectrometry (LC-ESI-HRMS) method for the determination of MNP in rifampin and CPNP in rifapentine drug substance and drug product. https://www.fda.gov/media/142092/download
- 147.U.S. Food and Drug Administration Liquid chromatography-electrospray ionization-high resolution mass spectrometry (LC-ESI-HRMS) method for the determination of nitrosamine impurities in metformin drug substance and drug product. https://www.fda.gov/media/138617/download
- 148.European Directorate for the Quality of Medicines & HealthCare Determination of NDMA and NDEA in SARTAN drug substances by HPLC/UV. https://www.edqm.eu/documents/52006/71923/Ad-hoc-projects-OMCL-Network-ansm.pdf/40f8b0b4-a829-39c4-9f9b-1b95ac6e200f?t=1628667749603
- 149.Mohammed M., Gandhimathi R. New validated LC-MS method development and estimation of nitrosamine impurities in canaglifozin. Int. J. Pharm. Qual. Assur. 2023;14:1094–1099. [Google Scholar]
- 150.Health Sciences Authority Determination of NDMA in ranitidine products by LC-MS/MS. https://www.hsa.gov.sg/docs/default-source/announcements/safety-alerts/determination-of-ndma-in-ranitidine-products-by-lcmsms.pdf
- 151.European Directorate for the Quality of Medicines & HealthCare Test method for the determination of NDMA by LC-MS/MS in ranitidine drug substance and film coat tablets. https://www.edqm.eu/documents/52006/290719/CVUA+Karlsruhe+method+based+on+UHPLC-APCI-MS_MS.pdf/e198028d-734c-0b92-10e9-083ee1094d8a?t=1638888048231
- 152.European Directorate for the Quality of Medicines & HealthCare, AZBT impurity in valsartan, irbesartan, losartan, candesartan LC-MS/MS method (Shimadzu HPLC + AB Sciex QTrap 5500) https://www.edqm.eu/documents/52006/71923/general-methodparameters-azbt-lcms.pdf/e37991e8-2f0f-4d2d-6e79-dff141005157?t=1628668153524
- 153.European Directorate for the Quality of Medicines & HealthCare, CVUA Karlsruhe method by UHPLC-APCI-MS/MS and allows determination of NDMA and NDEA in sartan drug substances and drug products. https://www.edqm.eu/en/ad-hoc-projects-of-the-omcl-network
- 154.Shaik K.M., Sarmah B., Wadekar G.S., et al. Regulatory updates and analytical methodologies for nitrosamine impurities detection in sartans, ranitidine, nizatidine, and metformin along with sample preparation techniques. Crit. Rev. Anal. Chem. 2022;52:53–71. doi: 10.1080/10408347.2020.1788375. [DOI] [PubMed] [Google Scholar]
- 155.Yamamoto E., Kan-No H., Tomita N., et al. Isolation of N-nitrosodimethylamine from drug substances using solid-phase extraction-liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2022;210 doi: 10.1016/j.jpba.2021.114561. [DOI] [PubMed] [Google Scholar]
- 156.Chidella K.S., Dasari V.B., Anireddy J. Ultra-sensitive LC-MS/MS method for the trace level quantification of six potential genotoxic nitrosamine impurities in telmisartan. Am. J. Anal. Chem. 2021;12:227–240. [Google Scholar]
- 157.Patel R., Purohit S., Solanki R., et al. Development and validation of an analytical method for trace-level quantification of genotoxic nitrosamine impurities in losartan and hydrochlorothiazide fixed-dose combination tablets using ultra performance liquid chromatography triple quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. 2023;37 doi: 10.1002/rcm.9488. [DOI] [PubMed] [Google Scholar]
- 158.Solanki R., Wadhwana P., Patel R., et al. Analytical method capable of quantifying eight nitrosamine impurities from five different commercially available metformin formulations with glipizide, glibenclamide, gliclazide, evogliptin, and glimepiride by ultra high performance liquid chromatography triple quadrupole mass spectrometry. J. Pharm. Sci. 2023;112:1268–1276. doi: 10.1016/j.xphs.2023.02.016. [DOI] [PubMed] [Google Scholar]
- 159.European Directorate for the Quality of Medicines & HealthCare, Nitrosamines by GC-MS/MS. https://www.edqm.eu/documents/52006/71923/Ad-hoc-projects-OMCL-Network-Swissmedic.pdf/76853d6b-4c19-c054-5bb7-ee4e77232cdf?t=1628667616159
- 160.Kosuri E.R., Bhanti M., Jaywant M.A., et al. A GC-MS/MS method for trace level quantification of six nitrosamine impurities (NDMA, NDEA, NEIPA, NDIPA, NDPA, and NDBA) in commercially used organic solvents: Dichloromethane, ethyl acetate, toluene, and o-xylene. J. Pharm. Sci. 2023;112:1225–1230. doi: 10.1016/j.xphs.2022.11.024. [DOI] [PubMed] [Google Scholar]
- 161.European Directorate for the Quality of Medicines & HealthCare, Determination of NDMA (HS-GC-MS) https://www.edqm.eu/documents/52006/286293/PALG+method+based+on+Headspace+GC-MS+(single+quad).pdf/0ce8ad50-e57a-7186-6f81-e319ec8120e2?t=1638887392387
- 162.Health Canada Nitrosamine impurities in medications: Test methods and test results. https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/information-health-product/drugs/nitrosamine-impurities/test-results.html
- 163.U.S. Food and Drug Administration GC/MS headspace method for NDMA in valsartan drug substance and drug products. https://www.fda.gov/media/115965/download
- 164.U.S. Food and Drug Administration Combined direct injection N-Nitrosodimethylamine (NDMA), N-nitrosodiethylamine (NDEA), N-nitrosoethylisopropylamine (NEIPA), N-nitrosodiisopropylamine (NDIPA) and N-nitrosodibutylamine (NDBA) impurity assay by GC-MS/MS. https://www.fda.gov/media/123409/download
- 165.U.S. Food and Drug Administration Combined headspace N-nitrosodimethylamine (NDMA), N-nitrosodiethylamine (NDEA), N-nitrosoethylisopropylamine (NEIPA), N-nitrosodiisopropylamine (NDIPA) impurity assay by GC-MS/MS. https://www.fda.gov/media/124025/download
- 166.Health Sciences Authority Determination of N-nitrosodimethylamine (NDMA) in metformin products by HRAM-GCMS. https://www.hsa.gov.sg/docs/default-source/announcements/safety-alerts/determination-of-ndma-in-metformin-products-by-hram-gcms.pdf
- 167.Witkowska A.B., Giebułtowicz J., Dąbrowska M., et al. Development of a sensitive screening method for simultaneous determination of nine genotoxic nitrosamines in active pharmaceutical ingredients by GC-MS. Int. J. Mol. Sci. 2020;23 doi: 10.3390/ijms232012125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Al-Kaseem M., Al-Assaf Z., Karabeet F., rapid A. validated RP-HPLC method for the determination of seven volatile N-nitrosamines in meat. Pharmacol. Pharm. 2014;5:298–308. [Google Scholar]
- 169.Li W., Chen N., Zhao Y., et al. Online coupling of tandem liquid-phase extraction with HPLC-UV for the determination of trace N-nitrosamines in food products. Anal. Methods. 2018;10:1733–1739. [Google Scholar]
- 170.Cha W., Fox P., Nalinakumari B. High-performance liquid chromatography with fluorescence detection for aqueous analysis of nanogram-level N-nitrosodimethylamine. Anal. Chim. Acta. 2006;566:109–116. [Google Scholar]
- 171.Lu S., Wu D., Li G., et al. Facile and sensitive determination of N-nitrosamines in food samples by high-performance liquid chromatography via combining fluorescent labeling with dispersive liquid-liquid microextraction. Food Chem. 2017;234:408–415. doi: 10.1016/j.foodchem.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 172.Boczar D., Wyszomirska E., Zabrzewska B., et al. Development and validation of a method for the semi-quantitative determination of N-nitrosamines in active pharmaceutical ingredient enalapril maleate by means of derivatisation and detection by HPLC with fluorimetric detector. Appl. Sci. 2021;11 [Google Scholar]
- 173.Masada S., Tsuji G., Arai R., et al. Rapid and efficient high-performance liquid chromatography analysis of N-nitrosodimethylamine impurity in valsartan drug substance and its products. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-48344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pérez-Ruiz T., Martínez-Lozano C., Tomás V., et al. Automated solid-phase extraction and high-performance liquid chromatographic determination of nitrosamines using post-column photolysis and tris(2,2'-bipyridyl) ruthenium(III) chemiluminescence. J. Chromatogr. A. 2005;1077:49–56. doi: 10.1016/j.chroma.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 175.Bodiwala K.B., Panchal B.G., Savale S.S., et al. Simultaneous estimation of six nitrosamine impurities in valsartan using liquid chromatographic method. J. AOAC Int. 2022;105:1–10. doi: 10.1093/jaoacint/qsab100. [DOI] [PubMed] [Google Scholar]
- 176.U.S. Food and Drug Administration Control of Nitrosamine Impurities in Human DrugsGuidance for Industry. https://www.fda.gov/media/141720/download
- 177.Sedlo I., Kolonić T., Tomić S. Presence of nitrosamine impurities in medicinal products. Arch. Ind. Hyg. Toxicol. 2021;72:1–5. doi: 10.2478/aiht-2021-72-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Elder D.P., Johnson G.E., Snodin D.J. Tolerability of risk: A commentary on the nitrosamine contamination issue. J. Pharm. Sci. 2021;110:2311–2328. doi: 10.1016/j.xphs.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 179.King F.J., Searle A.D., Urquhart M.W. Ranitidine—Investigations into the root cause for the presence of N-nitroso-N,N-dimethylamine in ranitidine hydrochloride drug substances and associated drug products. Org. Process Res. Dev. 2020;24:2915–2926. [Google Scholar]
- 180.Ruepp R., Frötschl R., Bream R., et al. The EU response to the presence of nitrosamine impurities in medicines. Front. Med. 2021;8 doi: 10.3389/fmed.2021.782536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Torres S., Boetzel R., Gatimu E., et al. ICH Q3D drug product elemental risk assessment: The use of an elemental impurities excipients database. J. Pharm. Sci. 2022;111:1421–1428. doi: 10.1016/j.xphs.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 182.Ziebarth D., Scheunig G. IARC Sci. Publ.; 1976. Effects of some inhibitors on the nitrosation of drugs in human gastric juice; pp. 279–290. [PubMed] [Google Scholar]
- 183.Mergens W.J. Efficacy of vitamin E to prevent nitrosamine formation. Ann. N. Y. Acad. Sci. 1982;393:61–69. doi: 10.1111/j.1749-6632.1982.tb31232.x. [DOI] [PubMed] [Google Scholar]
- 184.Schlingemann J., Boucley C., Hickert S., et al. Avoiding N-nitrosodimethylamine formation in metformin pharmaceuticals by limiting dimethylamine and nitrite. Int. J. Pharm. 2022;620 doi: 10.1016/j.ijpharm.2022.121740. [DOI] [PubMed] [Google Scholar]
- 185.Hao G., Hu R., Wang X., et al. N-Nitrosodimethylamine formation in metformin hydrochloride sustained-release tablets: Effects of metformin and hypromellose used in drug product formulation. J. Pharm. Biomed. Anal. 2023;222 doi: 10.1016/j.jpba.2022.115066. [DOI] [PubMed] [Google Scholar]
- 186.Golob N., Grahek R., Ross M., et al. Nitrocellulose blister material as a source of N-nitrosamine contamination of pharmaceutical drug products. Int. J. Pharm. 2022;618 doi: 10.1016/j.ijpharm.2022.121687. [DOI] [PubMed] [Google Scholar]
- 187.Wagner J.A., Dinh J.C., Lightdale J.R., et al. Is this the end for ranitidine? NDMA presence continues to confound. Clin. Transl. Sci. 2021;14:1197–1200. doi: 10.1111/cts.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Harmon P. Trace aldehydes in solid oral dosage forms as catalysts for nitrosating secondary amines. J. Pharm. Sci. 2023;112:1216–1219. doi: 10.1016/j.xphs.2022.10.033. [DOI] [PubMed] [Google Scholar]
- 189.Boetzel R., Schlingemann J., Hickert S., et al. A nitrite excipient database: A useful tool to support N-nitrosamine risk assessments for drug products. J. Pharm. Sci. 2023;112:1615–1624. doi: 10.1016/j.xphs.2022.04.016. [DOI] [PubMed] [Google Scholar]
- 190.Boltres B. Evaluating nitrosamines from elastomers in pharmaceutical primary packaging. PDA J. Pharm. Sci. Technol. 2022;76:136–150. doi: 10.5731/pdajpst.2021.012645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.