Abstract
Herpes simplex virus (HSV) is a causative agent of fever blister, genital herpes, and neonatal herpes. Nowadays, edible algae are recognized as health food due to high nutrition content and their many active compounds that are beneficial to health. The purpose of this study is to investigate the inhibitory effects of algal polysaccharide extract from Cladophora spp. against herpes simplex virus type 1 and type 2 on Vero cells. In this study, the structure of polysaccharide extract is presented as S=O and C–O–S of the sulfate group, as identified by the FT-IR technique. The toxicity of algal polysaccharide extract on Vero cells was determined by MTT assay. The algal extract showed low toxicity on the cells, with 50% cytotoxic concentration (CC50) value greater than 5000 µg mL−1. The inhibition of HSV infection by the algal extract was then evaluated on Vero cells using plaque reduction assay. The 50% effective concentration (EC50) values of algal extract exhibited antiviral activity against HSV-1 upon treatment before, during, and after viral adsorption with and without removal of the extract were 70.31, 15.17, > 5000 and 9.78 µg mL−1, respectively. Additionally, the EC50 values of algal extract against HSV-2 upon treatment before, during and after viral adsorption with, and without removal of the extract were 5.85, 2.57, > 5000 and 26.96 µg mL−1, respectively. Moreover, the algal extract demonstrated direct inactivation of HSV-1 and HSV-2 virions as well as inhibitory effect against HSV replication. Accordingly, algal polysaccharide extract containing sulfated polysaccharides showed strong activity against HSV. Therefore, it is proved to be useful to apply Cladophora spp. polysaccharide extract as an anti-HSV agent.
Subject terms: Herpes virus, Pharmaceutics, Outcomes research
Introduction
Herpes simplex is caused by herpes simplex virus (HSV) infection, particularly HSV-1 and HSV-2. The herpes simplex virus is categorized as a group I double-stranded DNA virus and is a member of the Herpesviridae family1,2. HSV-1 and HSV-2 are pervasive human pathogens that cause localized skin infections. An HSV-1 infection can cause herpes labialis, whereas an HSV-2 infection can cause herpes genitalist3,4. The viruses remain a prominent problem in human public health because of their high transmission rate between people. HSV-1 is transmitted primarily through direct human skin contact, whereas HSV-2 is transmitted through sexual contact involving exposure to the mucous membrane within lesions or infections, or from mucosal secretion. Moreover, HSV-2 can be transmitted horizontally and vertically during asymptomatic shedding, and is incurable, causing a latent infection in the ganglia5–8.
The nucleoside analogues, especially acyclovir and related analogues, e.g., valacyclovir, penciclovir, and famciclovir, were tested as standard remedies for HSV infection9. The main action mode of nucleoside analogues is targeting the viral DNA polymerase. However, nucleoside drug analogues continue to have side effects in the case of long-term usage and the virus may develop a drug resistance. Drug-resistant HSV strains are caused by mutations in viral thymidine kinase and viral DNA polymerase genes, and the mutant viruses do not respond to commercial drug treatment10–12. Moreover, the epidemiology of HSV and drug resistant HSV have increased13. Nowadays, there is a lot of interest in natural substances as remedies for many ailments, including herpes simplex virus infection.
Several biologically active compounds exhibit antiviral activity, such as polysaccharides, peptides, proteins, phenolic compounds, and other organic compounds14,15. The efficacy of biologically active compounds against viruses indicates their broad antiviral activities on different pathways of the viral multiplication cycle. Antiviral activity is observed on the viral entry, penetration, replication, assembly, and egression16,17. The previous reports indicated that there has been extensive research on the anti-HSV properties of various natural substances. For instance, terpenes isolated from Melia azedarach showed high antiviral activity against HSV-1 in cell culture experiments18. Additionally, β-orcinol depsidone derived from lichen Usnea fruticose was found to inactivate HSV-1 DNA-polymerase during HSV replication19. Moreover, catechin purified from Limonium sinense exhibited greater antiviral activity than ACV by reducing the expression of ICP0 and ICP4 genes. Polyphenols isolated from tea plants such as Camellia sinensis could interfere the fusion process between the viral and cellular membranes by aggregating HSV glycoproteins B and D on the viral surface20,21. Furthermore, the griffithsin (GRFT) peptide isolated from red algae directly effect on the viral glycoproteins B, D, and heterodimers of gH/gL, which are essential for virus entry and cell-to-cell spread of HSV22,23. Crude aqueous and organic solvent extracts from algae, including green algae (Chlorella vulgaris and Spirogyra neglecta), brown algae (Durvillaea antarc), and red algae (Hypnea musciformis), demonstrated potent anti-HSV activities24,25. Polysaccharides, especially sulfated polysaccharides, have high potential antiviral activity against infections during the viral adsorption to the host cell. Moreover, biological and synthetic sulfated polyanions are able to inhibit the replication of various mammalian viruses26,27. Sulfated polysaccharides are found in some microorganisms, plants, and animals; however, the highest level is found mostly in algae. Sulfated glucan, sulfated galactan, and sulfated arabinogalactan are the main sulfated polysaccharides found in green macroalgae28,29. Marine algae are also sources of various structures of sulfated polysaccharides varying with the algal species. The major sulfated polysaccharides found in marine algae include ulvan of green algae (Chlorophyceae), fucoidan and laminarans of brown algae (Phaeophyceae), and carrageenan of red algae (Rhodophyceae)30. Moreover, sulfated polysaccharides display several physiochemical and biological features of potential interest for food, agricultural, and pharmaceutical applications. Furthermore, sulfated polysaccharides demonstrate additional properties including anticoagulant, antiviral, antioxidant, anticancer, and immunomodulating activity31–33.
Therefore, the purpose of research is to study the characteristics of polysaccharide extract from Cladophora spp. and investigate the inhibitory effects of algal polysaccharide extract against herpes simplex virus infections.
Results
Algal polysaccharide extract
The extraction yield and chemical composition including total carbohydrate, protein, and sulfate content of crude Cladophora spp. algal polysaccharide extract from Cladophora spp. is shown in Table 1, as taken from this study. The extraction yield for crude polysaccharide extract was approximately 30.10% w w−1. The algal polysaccharide extract consisted of 51.37% w w−1 carbohydrate, 13.69% w w−1 protein, and 7.31% w w−1 sulfate.
Table 1.
Yield and chemical composition of algal polysaccharide extract from Cladophora spp.
| Chemical content (% w w−1) | Yield | Total carbohydrate | Protein | Sulfate |
|---|---|---|---|---|
| 30.10 | 51.37 | 13.69 | 7.31 |
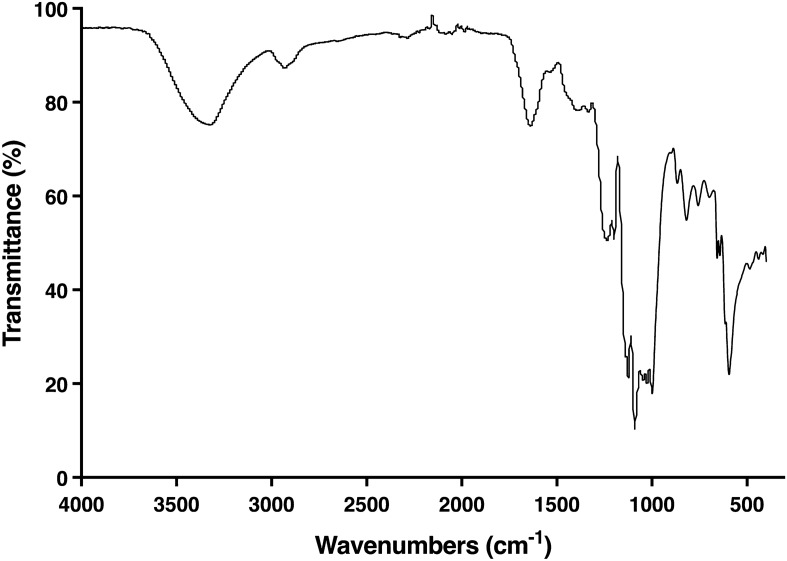
The FT-IR spectrum of polysaccharide extract from Cladophora spp. is presented in Fig. 1. A characteristic band at 3321.1 cm−1 corresponds to the –OH stretching vibrations of the hydroxy groups and the N–H stretching vibrations of the amide group. A small band at 2931.6 cm−1 is attributed to the –CH2 or –CH3 stretching vibrations of the alkyl group, or the aliphatic hydrocarbon chain. Strong transmission at 1639.7 cm−1 indicates the C = O asymmetric stretching vibrations of the amide I group, and also implicates the N–H bending vibrations of the amide II group, indicating the presence of amino acid. The 1124.3 cm−1 peak represents the C–O stretching vibrations of the polysaccharide ether. Moreover, the signal at 1025.9 cm−1 links to the stretching vibrations of the C–O–C bridge of the glucosides and sugar ring. Specifically, four bands at 1333.5, 1235.5, 866.4, and 594.1 cm−1 are consistent with the S=O stretching vibrations of the sulfonamides group, the S=O asymmetric stretching vibrations of the sulfated ester substitutions, the C–O–S bending vibrations of the sulfate group and the C–S stretching vibrations of the sulfide ester substitutions, respectively. These results confirm the presence of sulfate groups (7.31%) in the polysaccharide structure.
Figure 1.
Infrared transmittance spectrum of algal polysaccharide extract from Cladophora spp.
Cytotoxicity of algal polysaccharide extract
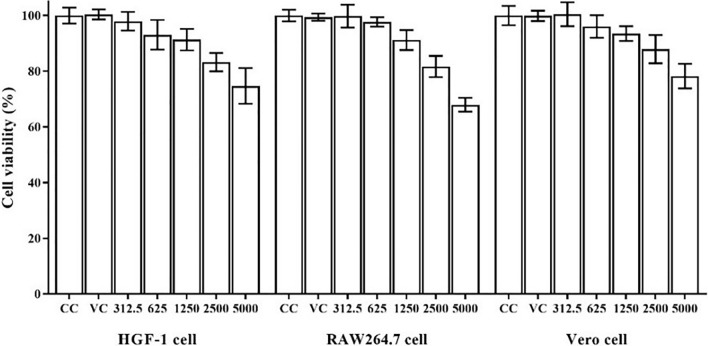
The cytotoxicity test of algal polysaccharide extract from Cladophora spp. was performed in vitro to detect cytotoxic activity on a normal human primary cell line (HGF-1 cells), immune cells (RAW264.7 cells) and a viral susceptible cell line (Vero cells). The stock solution of the algal polysaccharide extract was prepared at a concentration of 50 mg mL−1 by dissolving it with deionized water. The stock extract was diluted by two-fold dilution with DMEM growth medium at extract concentrations of 312.5, 625, 1250, 2500, and 5000 µg mL−1. The DMEM growth medium was also used as the basal media for the cell control (CC) and sterile distilled water was used as the vehicle control (VC). The results obtained are shown in Fig. 2. Algal polysaccharide extract had a low toxicity on HGF-1 cells, RAW264.7 cells and Vero cells. The 50% cytotoxic concentration (CC50) value of algal polysaccharide extract used to treat all cell lines was greater than 5000 µg mL−1, therefore, extract with a maximal concentration of 5000 µg mL−1 was used to determine the anti-HSV activity, viral particle inactivation, and antiviral replication kinetics.
Figure 2.
Cytotoxic effects of algal polysaccharide extract from Cladophora spp. on HGF-1 cells, RAW264.7 cells and Vero cells. Bar graph and error bars are based on mean ± SD of three experiments.
Anti-viral activities of algal polysaccharide extract
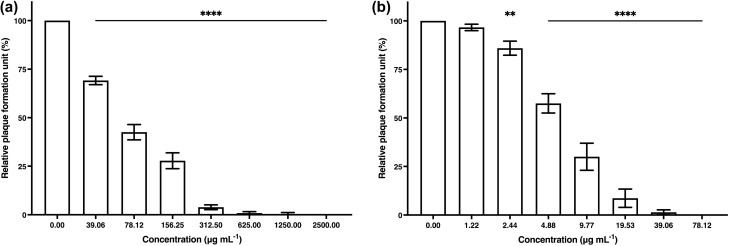
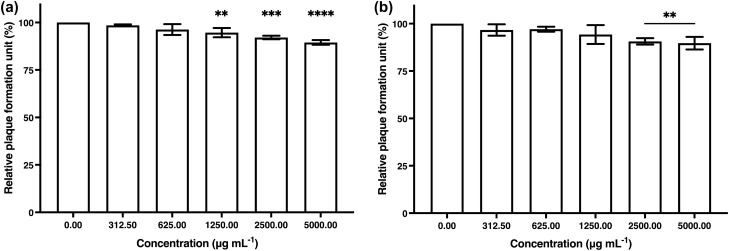
The antiviral activity of algal polysaccharide extract from Cladophora spp. against HSV-1 and HSV-2 was evaluated by plaque reduction assay. The Vero cells and the HSV-infected Vero cells were treated with different concentrations of algal polysaccharide extract at a maximal concentration of 5000 μg mL−1. Algal polysaccharide extract at concentrations of 78.12 and 1250 μg mL−1 showed efficient inhibition of HSV-1 and HSV-2 upon treatment before viral adsorption to the Vero cells, with both percentages of inhibition at 100% (Fig. 3). Heparin at concentration of 20 mg mL−1 was used as a positive control to inhibit HSV-1 and HSV-2 upon treatment before viral adsorption to the Vero cells, with the percentage of inhibition of 13.15 and 10.61%, respectively.
Figure 3.
The plaque reduction of (a) HSV-1 and (b) HSV-2 activity by algal polysaccharide extract from Cladophora spp. upon treatment before viral adsorption on Vero cells. Graphs and error bars are based on mean ± SD of three experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
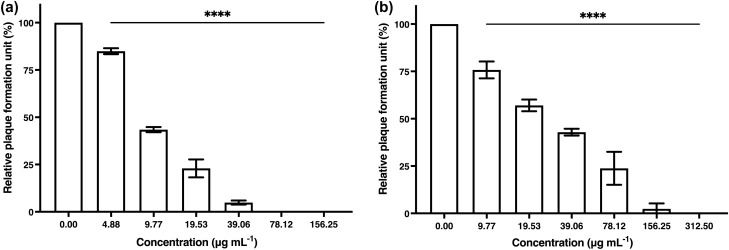
Additionally, algal polysaccharide extract at concentrations of 39.06 and 312.50 μg mL−1 showed potential to eliminate HSV-1 and HSV-2 upon treatment during viral adsorption to the Vero cells, with both percentages of inhibition at 100% (Fig. 4). Heparin at a concentration of 20 mg mL−1 was also used as a positive control to inhibit HSV-1 and HSV-2 upon treatment during viral adsorption to the Vero cells, with the percentage of inhibition at 97.23 and 86.59%, respectively.
Figure 4.
The plaque reduction of (a) HSV-1 and (b) HSV-2 activity by algal polysaccharide extract from Cladophora spp. upon treatment during viral adsorption on Vero cells. Bar graph and error bars are based on mean ± SD of three experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
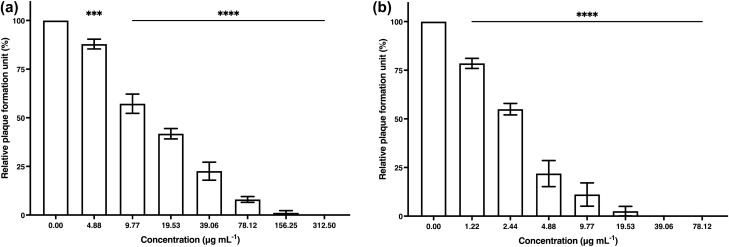
The treated cells were incubated at room temperature for 1 h. After incubation, the algal polysaccharide extract was removed, and the treated cells were washed twice with phosphate-buffered saline solution to determine the single cycle of HSV-2 infection. The resulted showed that algal polysaccharide extract at the highest concentration of 5000 μg mL−1 demonstrated low percentage of inhibition by 20% (Fig. 5). Moreover, ACV at a concentration of 10 μg mL−1 was also used as a positive control to inhibit HSV-1 and HSV-2 viral infection on Vero cell, with the percentage of inhibition at 90.54 and 31.88%, respectively.
Figure 5.
The plaque reduction of (a) HSV-1 and (b) HSV-2 activity by algal polysaccharide extract from Cladophora spp. upon treatment after viral adsorption on Vero cells with removal of the extract after treatment. Bar graph and error bars are based on mean ± SD of three experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Furthermore, algal polysaccharide extract at concentrations of 156.25 and 312.50 μg mL−1 also showed high potential to eradicate HSV-1 and HSV-2 by 100% upon treatment after viral adsorption without removal of the algal polysaccharide extract (Fig. 6). ACV at a concentration of 10 μg mL−1 was used as a positive control to inhibit HSV-1 and HSV-2 upon treatment after viral adsorption to the Vero cell, with the percentage of inhibition at 97.64 and 49.25%, respectively.
Figure 6.
The plaque reduction of (a) HSV-1 and (b) HSV-2 activity by algal polysaccharide extract from Cladophora spp. upon treatment after viral adsorption on Vero cells without removal of the extract after treatment. Bar graph and error bars are based on mean ± SD of three experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
The 50% effective concentration (EC50) values of algal polysaccharide extract from Cladophora spp. against HSV-1 upon treatment before, during and after viral adsorption with, and without removal of the extract were 70.31, 15.17, > 5000 and 9.78 µg mL−1, respectively. In addition, the EC50 values of algal polysaccharide extract from Cladophora spp. against HSV-2 when treated before, during and after viral adsorption with, and without removal of the extract were 5.85, 2.57, > 5000 and 26.96 µg mL−1, respectively (Table 2).
Table 2.
Inhibition of HSV by algal polysaccharide extract from Cladophora spp. upon treatment before, during and after viral adsorption with and without removal the algal extract from the infected Vero cells.
| Inhibition mechanisms | HSV-1 | HSV-2 | |
|---|---|---|---|
| Before viral adsorption | EC50 (µg mL−1) | 70.31 | 5.85 |
| Selective Index (SI)* | > 71.11 | > 854.70 | |
| During viral adsorption | EC50 (µg mL−1) | 15.17 | 2.57 |
| Selective Index (SI) * | > 329.60 | > 1945.52 | |
| After viral adsorption with removal of the algal extract | EC50 (µg mL−1) | > 5000 | > 5000 |
| Selective Index (SI)* | 1 | 1 | |
| After viral adsorption without removal of the algal extract | EC50 (µg mL−1) | 9.78 | 26.96 |
| Selective Index (SI) * | > 511.25 | > 185.46 | |
*Selective Index (SI) = 50% Cytotoxic dose (CD50)/50% Effective dose (ED50).
Furthermore, the selectivity index (SI) values of algal polysaccharide extract from Cladophora spp. were calculated from 50% cytotoxic dose (CD50)/50% effective dose (ED50). As 50% Cytotoxic dose (CD50) of the algal polysaccharide extract from Cladophora spp. was more than 5000 ug mL−1. Thus, SI values of algal polysaccharide extract from Cladophora spp. against HSV-1 upon treatment before, during and after viral adsorption with, and without removal of the extract were more than 71.11, 329.60, 1.00 and 511.25, respectively. Additionally, the SI values of the Cladophora spp. polysaccharide extract against HSV-2 upon treatment before, during and after viral adsorption with, and without removal of the extract were more than 854.70, 1945.52, 1.00 and 185.46, respectively (Table 2).
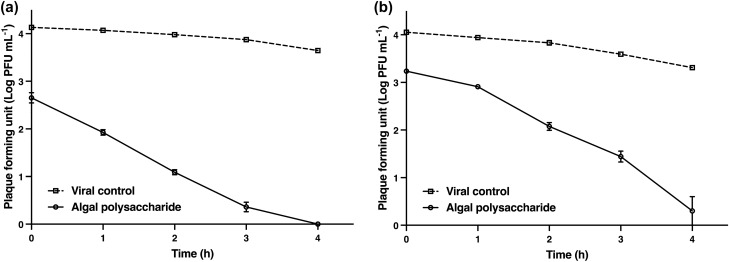
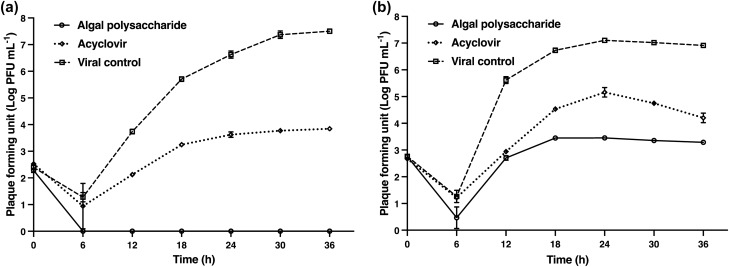
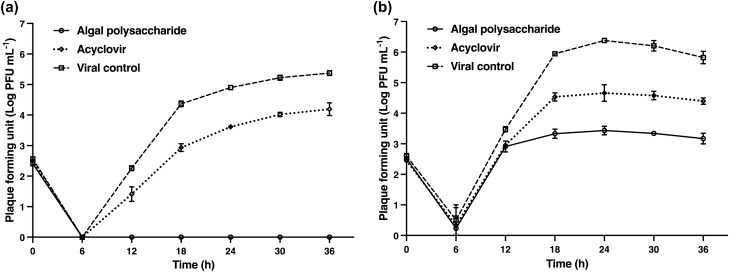
The activity of algal polysaccharide extract on the direct inactivation of HSV particles was determined using plaque titration assay and was compared with the virus control. HSV-1 and HSV-2 particles at titers of 1.0 × 104 PFU mL−1 were treated with algal polysaccharide extract at a concentration of 5000 μg mL−1 for 1, 2, 3, and 4 h. The inhibitory effect of the extract on HSV viral particles was observed to be the highest when the incubation period increased (Fig. 7). The results signify that algal polysaccharide extract at a concentration of 5000 µg mL−1 could reduce the plaque number of HSV-1 and HSV-2 after treatment greater than 2 log PFU mL−1 when incubation for 1 and 3 h, respectively. Algal polysaccharide extract reduced the plaque number of HSV-1 by 1.47, 2.14, 2.89, 3.49, and 3.65 log PFU mL−1 when treated at 0, 1, 2, 3, and 4 h compared to the virus control in each period, respectively. Similarly, algal polysaccharide extract reduced the plaque number of HSV-2 by 0.81, 1.03, 1.75, 2.14, and 3.01 log PFU mL−1 when treated at 0, 1, 2, 3, and 4 h compared to the virus control in each period, respectively. Moreover, algal polysaccharide extract at a concentration of 5000 μg mL−1 was also tested for the repression of HSV replication. The replication of HSV was carried out at 0, 6, 12, 18, 24, 30, and 36 h after treatment with algal polysaccharide extract, and was then compared to the virus control and ACV. ACV had 50% inhibitory concentration (IC50) values of 1.54 and 12.75 μg mL−1 for HSV‐1 and HSV‐2 treatment, respectively. These experimental conditions allow for the synchronization of the HSV replication steps. The results reveal that the extracellular HSV-1 and HSV-2 yields were inhibited 6 h after the infected cells were treated with algal polysaccharide extract. In addition, the extract also proved to repress the intracellular HSV yield. The inhibition trend of the intracellular HSV yield of HSV-1 and HSV-2 replication was greater than the action of ACV (Figs. 8 and 9).
Figure 7.
The virucidal activities of algal polysaccharide extract from Cladophora spp. against (a) HSV-1 and (b) HSV-2 particles in comparison with the virus control. Bar graph and error bars are based on mean ± SD of three experiments.
Figure 8.
The inhibition of (a) extracellular HSV-1 yield and (b) intracellular HSV-1 yield upon treatment with algal polysaccharide extracts from Cladophora spp. for 0, 6, 12, 18, 24, 30, and 36 h, compared to the ACV as a positive control and viral control. Bar graph and error bars are based on mean ± SD of three experiments.
Figure 9.
The inhibition of (a) extracellular HSV-2 yield and (b) intracellular HSV-2 yield upon treatment with algal polysaccharide extracts from Cladophora spp. for 0, 6, 12, 18, 24, 30, and 36 h, compared to the ACV as a positive control and viral control. Bar graph and error bars are based on mean ± SD of three experiments.
Discussion
Extract derived from natural substances provides effective treatment for various diseases. Polysaccharide extract from natural substances has broad potential effects against various viruses, especially enveloped viruses34. In previous studies, polysaccharide extract has proven its antiviral potency against human immunodeficiency viruses, Japanese encephalitis virus, dengue virus, yellow fever virus, influenza virus, avian influenza virus, and measles virus35–38. Studies of investigating the antiherpetic effects of sulfated polysaccharides have been conducted using polysaccharides from various sources. Sulfated polysaccharide extracts from green algae such as Monostroma nitidum, Caulerpa brachypus, C. okamurai, C. scapelliformis, Chaetomorpha crassa, Ch. spiralis, Codium adhaerens, Co. fragille, and Co. latum exhibited potent anti-HSV-1 activities during viral attachment, with 50% inhibitory concentrations (IC50) ranging from 0.38 to 8.5 μg mL−139. The sulfated polysaccharides from these green algae exerted higher anti-HSV-1 activity than polysaccharide extract from Cladophora spp., which had EC50 value of 15.17 μg mL−1 in this study.
Moreover, the sulfated polysaccharide extract obtained from brown algae (Sargassum muticum) using enzyme-assisted and hot water extraction methods demonstrated its effectiveness in protecting Vero cells against HSV-1 infection, with EC50 values ranging from 37.7 to 48.0 μg mL−1. Similarly, the sulfated polysaccharide extract obtained from brown algae exhibited significant anti-HSV-1 activity in a post-HSV infection assay, with EC50 values ranging from 1.2 to 2.4 μg mL−140. However, algal polysaccharide extract from Cladophora spp. in this study inhibited HSV-1 infection with lower activity when treatment before and after viral attachment with EC50 values of 70.31 and 9.78 μg mL−1, respectively.
In addition, the fractionated polysaccharide extract from red algae (Callophyllis variegata) exhibited anti-HSV-1 activities during viral attachment, with IC50 values ranging from 0.16 to 1.55 μg mL−1, and demonstrated anti-HSV-2 activities, with IC50 values ranging from 0.21 to 2.19 μg mL−141. Moreover, the polysaccharide derived from Spirulina platensis (calcium spirulan) displayed potent anti-HSV-1 activity during viral attachment with an EC50 of 0.86 μg mL−142. Thus, the polysaccharide from Callophyllis variegata and calcium spirulan polysaccharide from Spirulina platensis demonstrated higher activity than polysaccharide extract from Cladophora spp. in this study since the polysaccharide extract from Cladophora spp. inhibited HSV-1 and HSV-2 infection during viral attachment with EC50 values of 15.17 and 2.57 μg mL−1, respectively.
The study on polysaccharide extracts from seaweed including Rhodymenia pseudopalmata, Solieria filiformis, Hydropuntia cornea (Rhodophyta) and Sargassum fluitans (Phaeophyceae) was performed against HSV-1 infection. The result showed that all polysaccharide extracts had low toxicity on Vero cell with CC50 more than 200 μg mL−1. The polysaccharide extract from Solieria filiformis exhibited effective antiviral effect when treatment before viral attachment with EC50 of 136.0 μg mL−1 and SI value of 1.47 whereas the polysaccharide extract of Sargassum fluitans demonstrated antiviral activity with EC50 of 42.8 μg mL−1 and SI value of 4.67. The anti-HSV-1 activity of the algal polysaccharide extract from Cladophora spp. in this study was compared and the inhibition of HSV-1 infection before viral attachment (EC50 = 70.31, SI > 71.11) demonstrated higher anti-HSV-1 activity than the polysaccharide extract from Solieria filiformis. However, the polysaccharide extract of Sargassum fluitans showed stronger anti-HSV-1 activity than the polysaccharide extract from Cladophora spp. In contrast, Rhodymenia pseudopalmata and Hydropuntia cornea polysaccharide extracts did not show the anti-HSV-1 activity43.
Other study demonstrated anti-HSV activities of natural sulfated polysaccharides (SPs) from green algae; Enteromorpha compressa and Monostroma nitidum. The polysaccharide extracts showed low toxicity on Vero cell with CC50 values > 1000 and 4100 μg mL−1, respectively. The sulphated polysaccharides also exerted high efficacy of antiviral activity against HSV-1 infection during viral adsorption with the EC50 values of 0.49 and 0.4 μg mL−1 and the SI values > 200 and 1000, respectively. Moreover, the sulfated polysaccharide isolated from Caulerpa brachypus, Caulerpa okamurai, and Caulerpa scapelliformis also demonstrated low toxicity on Vero cell with CC50 values of 4700, 6400, and 6400 μg mL−1, respectively. This sulphated polysaccharide could inhibit HSV-1 during viral attachment with the EC50 of 1.9, 0.65, 0.55 μg mL−1 and SI values of 2500, 9800, and 12,000, respectively39. Therefore, the sulfated polysaccharide isolated from green algae showed the high potency of anti-HSV activity greater than the algal polysaccharide extract from Cladophora spp. that observed from this study (EC50 = 15.17, SI > 329.60).
Furthermore, a recent study revealed that marine polysaccharide extracts, such as sulfated polysaccharide extract from sea cucumber, fucoidan extract from brown algae, and ι-carrageenan extract from red algae, were able to inhibit SARS-CoV-2 infection on Vero E6 cells44. Other marine polysaccharides such as polysaccharide extracts from brown marine algae; Undaria pinnatifida sporophyll, Laminaria japonica, Hizikia fusiforme, and Sargassum horneri and green marine algae (Codium fragile), have demonstrated inhibitory activity against SARS-CoV-2 virus entry using the test model of SARS-CoV-2 pseudo-virus infection in ACE-2 overexpressed HEK293T cells45. In the same manner, sulfated polysaccharide extracts from algae have also demonstrated various effective biological activities, including immunomodulation, antiviral, antioxidant, antihyperlipidemic and anticancer activity46.
Hence, this study focused on algal polysaccharide extract from Cladophora spp. The algal polysaccharide extract from Cladophora spp. contains sulfated polysaccharides with high levels of carbohydrates and a very low protein and sulfate content. However, when the extraction involves a purification step, such as ion exchange chromatography, it could eliminate proteins and other organic compounds. Algal polysaccharide extract from Cladophora glomerata Kützing has been extracted with a purification step using DEAE-Sepharose fast flow ion exchange chromatography column, resulting in a reduced protein level from 17.3 to 13.0% w w−1. This purification step also increased the sulfate content of algal polysaccharide extract from 19.9 to 23.5% w w−147. The FT-IR spectra shows the characteristics of algal polysaccharide extracts related to other algal polysaccharide extracts from an algal sample in the same order of Cladophorales, such as Cladophora glomerata, Cladophora crispate, Cladophora surera, Cladophora rupestris, Chaetomorpha gracilis Kützing, or Rhizoclonium hieroglyphicum (C.Agardh) Kützing48–52. The structural characteristics demonstrate the main functional group of the C–O–C bridge of the glycosidic linkage, and the sulfate group. Moreover, algal polysaccharide extract contains the functional group C=O of carboxylated sugars, which is similar to an analysis of sulfated glucan and sulfated galactan in a previous study. In accordance with this study, sulfated glucan and sulfated galactan are the main composition of the cell wall of the polysaccharide of Cladophora spp.53.
Algal polysaccharide extract from Cladophora spp. has a low toxicity on various cell lines, such as HGF-1 cells, RAW264.7 cells, and Vero cells. The results of this study are concordant with previous studies that evaluated the cytotoxicity of other algal polysaccharide extracts in cell culture, such as Cladophora spp., Codium tomentosum, Ulva armoricana, Ulva intestinalis, Ulva lactuca, and Ulva pertusa54–58. Furthermore, Cladophora spp. is proved to be experimentally safe by acute cytotoxicity tests on rats59.
Algal polysaccharide extract from Cladophora spp. shows high potential against HSV infection in Vero cells in a few different ways. The antiviral activity of algal polysaccharide extract against HSV prior and during viral adsorption on Vero cells is attributed to the protective and interfering properties of extract. Before viral adsorption, treatment of algal polysaccharide extract protects Vero cells by inhibiting HSV and during viral adsorption, the extracts interfere the viral infection step and blocks infection in Vero cells. Effect of algal polysaccharide extract on the single cycle of HSV-2 infection was determined by removal of the algal polysaccharide extract after viral adsorption. The efficacy of the algal polysaccharide extract after viral adsorption was rather low when the extract was removed by washing with phosphate-buffered saline after incubation for 1 h. This might be from the large molecular weight of algal polysaccharide extract that could not pass through the cell via cell membrane to inactivate the viral particles60. However, treatment of algal polysaccharide extract after viral adsorption without removal of the extract resulted in high efficacy of HSV inhibition since new viral particles might have time to expose to algal polysaccharide extract and the new viral particles were inactivated after viral egress from the infected cells.
The effectiveness of algal polysaccharides in inhibiting HSV-1 and HSV-2 infections on Vero cells varies depending on the infectious mechanism of each type of HSV. HSV infections involve viral glycoproteins binding to host cell receptors during viral fusion with the cytoplasmic membrane of host cell. The main receptors for cell entry are nectin-1, nectin-2, herpesvirus entry mediator (HVEM), and 3-O heparan sulfate (3-O HS), with HVEM and nectin-1 being used by both HSV-1 and HSV-2. However, 3-O HS is specific to HSV-1, while nectin-2 has a greater effect on HSV-2 entry than on HSV-161,62. This suggests that algal polysaccharide extracts with polymer structures containing sulfate groups can interfere with the binding of viral glycoproteins to host cell receptors. Consequently, the algal polysaccharide extract has effect on anti-HSV infection during viral adsorption in Vero cells greater than other infection mechanisms. Moreover, similar results were obtained when cells were treated against enveloped viruses with green algal polysaccharides, sulfated polysaccharides and ulvan39,63.
Sulfated polysaccharides demonstrated antiviral activity against a wide range of viruses due to their unique polyanion structure, which carried a strong negative charge64. This characteristic enables them to inhibit viruses by interacting with the positive charges on the surface of host cells, thereby preventing virus penetration65. For example, sulfated polysaccharides can disrupt the viral glycoprotein region on viral particles by binding to the heparan sulfate proteoglycan region of the host cell surface66. These interactions correspond to the results of antiviral activity of algal polysaccharide extract against HSV both before and during viral adsorption on Vero cells. Moreover, the results of antiviral activity assays on algal polysaccharides against herpes simplex viruses indicate that sulfated polysaccharides can also disrupt the process of viral egress from host cells after viral adsorption and replication. Therefore, it would be beneficial to investigate and utilize natural compounds derived from Cladophora spp. algal extract that act in accordance with synthetic antiviral drugs on different targets of the HSV infection process.
Materials and methods
Cell line and viruses
In the cytotoxicity test, the human gingival fibroblasts cell line (HGF-1 cell; ATCC-CRL-2014) was used to represent a normal human primary cell, and the murine macrophage cell line originated from Mus musculus (RAW264.7; ATCC-TIB-71) was used to represent an immune cell. The African green monkey kidney cells (Vero cells) were kindly obtained from the Microbiology Section, Department of Medical Technology, Faculty of Associated Medical Science, Chiang Mai University, Chiang Mai, Thailand and was used as a model to study viral infections. All of the cell lines were cultured as a monolayer in a growth medium, Dulbecco’s modified Eagle medium; DMEM (Gibco, UK) supplemented with 10% v v−1 heat inactivated fetal bovine serum, FBS (Gibco, UK), 100 ug mL−1 streptomycin and 100 U mL−1 penicillin (Gibco, UK), 10 mM of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES). The cell was grown in a humidified 5% v v−1 CO2 atmosphere at 37 °C using a CO2 incubator until 80–90% confluence was observed. The standard strains of herpes simplex virus type 1 strain F (HSV-1F) and herpes simplex virus type 2 strain G (HSV-2G) were propagated on Vero cells cultured in DMEM medium containing 2% v v−1 FBS using a multiplicity of infection (MOI) of 1.0. The viral culture supernatant was collected to obtain the virus, and the titers of virus were quantified by plaque titration assay.
Algal polysaccharide extraction
The specimen of Cladophora spp. was collected from the Mekong River, Wiang Sub-District, Chiang Khong District, Chiang Rai Province, Thailand and kindly verified by the applied algal research laboratory (AARL), Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand. The Cladophora spp. specimen was dried at 60 °C and blended into a powder. The algal polysaccharides were extracted using a hot water extraction method. The dried Cladophora spp. was boiled in distilled water at 98 °C for 1 h with the ratio of 20 g algal powder to 1 L distilled water. Next, the extract solution was filtered and concentrated by a rotary evaporator. The extract was precipitated by 95% ethanol at 4 °C for 24 h. After precipitation, the precipitate was centrifugated and lyophilized to eliminate the ethanol from the extract67.
Algal polysaccharide chemical profile analysis
The Cladophora spp. algal polysaccharide extract was analyzed for carbohydrate, protein, and sulfate content by colorimetric analysis technique. The total carbohydrate content was measured by phenol–sulfuric acid assay using D-glucose as a standard68. The algal polysaccharide extract was mixed with 5% w v−1 phenol solution. Thereafter, 98% w w−1 sulfuric acid was carefully added and the mixture was incubated for 10 min in the dark. After incubation, the reaction absorbance was measured at 490 nm by spectrophotometry (Thermo Scientific, USA). The protein content was determined by Lowry assay using bovine serum albumin as a standard69. The algal polysaccharide extract was complexed with Lowry's protein complex-forming reagent. After complexing, the reaction was mixed with 50% v v−1 Folin-Ciocalteu's phenol reagent, and was incubated for 30 min in the dark. The reaction absorbance was detected at 750 nm by spectrophotometry. Hydrolysis of the polysaccharides was performed with 4 M of hydrochloric acid at 100 °C for 2 h, according to the sulfate turbidity method using potassium sulfate as a standard, followed by an estimation of the sulfate content70. The digested algal polysaccharides were reacted with a sulfate turbidity conditioning reagent. The reaction was then gently mixed with 6% w v−1 barium chloride and the reaction absorbance was immediately measured at 420 nm by spectrophotometry.
Algal polysaccharide structure characterization
The functional groups of sulphated polysaccharides of Cladophora spp. were analyzed by Fourier-transform infrared (FT-IR) spectroscopy using a Nicolet 6700 FT-IR spectrometer (Thermo Scientific, USA). The fully dried algal polysaccharide extract was embedded in potassium bromide (KBr) by the potassium bromide-pellet technique. The pellet was scanned in a wave number range of 4000–400 cm−1 with a resolution of 4 cm−1 using transmittance mode71.
Cytotoxicity test of algal polysaccharides
The cytotoxic effects of algal polysaccharides from Cladophora spp. were determined on HGF-1 cells and RAW264.7 cells by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay72. The cytotoxicity was also tested on Vero cells to determine a concentration of extract that could be used in subsequent antiviral studies. The algal polysaccharide extract at a maximal concentration of 5000 µg mL−1 was prepared in DMEM growth medium. The cultured cells were exposed to different concentrations of the extract, and the treated cells were incubated at 37 °C in a CO2 incubator for 48 h. After incubation, the MTT assay was prepared following the manufacturer’s instructions. A 5 mg mL−1 of MTT reagent (Bio Basic Inc., Canada) was added to each well and incubated at 37 °C for 4 h. Then, MTT-formazan from the treated cells was dissolved by dimethyl sulfoxide and the color of the formazan solution was determined using microplate readers (Biochrom, UK) by measuring the absorbance at 540 nm, with a reference wavelength of 630 nm. The cell viability percentage was calculated from the ratio between the absorbance values of both the treated cells and the untreated cells73.
Antiviral assay of algal polysaccharides against herpes simplex viruses
The antiviral activities of Cladophora spp. algal polysaccharides were observed by plaque reduction assay with antiviral mechanisms, including the antiviral activity upon treatment before, during and after viral adsorption74.
The antiviral activity upon treatment before viral adsorption was evaluated. The Vero cells were seeded in a 24-well plate at a density of 1.0 × 105 cells well−1. After incubation for 24 h, the cultured cell monolayer was treated with a non-toxic concentration of algal polysaccharide extract. The treatments were incubated at room temperature for 1 h. Then, the algal polysaccharide extract was disposed and the treated cells were washed twice with phosphate-buffered saline solution. The cells were then infected with HSV at the titers of 1.0 × 102 PFU mL−1. The overlay medium containing 0.5% w v−1 carboxymethyl cellulose in growth medium was added to the wells for viral plaque forming. The infected cells were then incubated at 37 °C in a CO2 incubator for 72 h.
The antiviral activity upon treatment during viral adsorption was evaluated. The Vero cells were seeded in a 24-well plate at a density of 1.0 × 105 cells well−1. After incubation for 24 h, a non-toxic concentration of algal polysaccharide extract and HSV at the titers of 1.0 × 102 PFU mL−1 were inoculated onto the cell monolayer. The mixture was incubated at room temperature for 1 h. After incubation, the mixture was removed and the infected cells were washed twice with phosphate-buffered saline solution. The overlay medium containing 0.5% w v−1 carboxymethyl cellulose in growth medium was added to the wells for viral plaque forming. The infected cells were then incubated at 37 °C in a CO2 incubator for 72 h.
The antiviral activity upon treatment after viral adsorption was evaluated. The Vero cells were seeded in a 24-well plate at a density of 1.0 × 105 cells well−1. After incubation for 24 h, the cultured cell monolayer was infected with HSV at the titers of 1.0 × 102 PFU mL−1. The infected cells were incubated at room temperature for 1 h. The residual inoculum was then eliminated and the infected cells were washed twice with phosphate-buffered saline solution after the infected cells were treated with a non-toxic concentration of algal polysaccharide extract in the first group. Then, DMEM medium was added and further incubation during the remaining time up to 72 h. Whereas, the infected cells were not washed after treatment with the algal polysaccharide in the second group. The overlay medium containing 0.5% w v−1 carboxymethyl cellulose in growth medium was added to the wells for viral plaque forming. The infected cells were then incubated at 37 °C in a CO2 incubator for 72 h.
After 72 h of incubation in all antiviral assays, the viral plaques were stained with 0.1% crystal violet in 0.5% ethanol. The viral plaque formation was counted and the percentage of relative plaque forming units was calculated from the amount of plaque forming units remaining from the inactive by the algal polysaccharide extract, related to the amount in the virus control. The viral inhibition efficiency was compared to acyclovir and heparin. Accordingly, the acyclovir drug, ACV (Sigma-Aldrich, USA), was used as the positive control for antiviral activity upon treatment after viral adsorption. On the contrary, the heparin sodium salt was used as the positive control of antiviral activity upon treatment before viral adsorption and antiviral activity upon treatment during viral adsorption. The 50% effective concentration (EC50) values of the extract against the virus were calculated. The selective index (SI) values were also analyzed from the ratio between the CC50 value and EC50 value75.
Virucidal assay of algal polysaccharides on herpes simplex viruses
A viral suspension of HSV particles at the titers of 1.0 × 104 PFU mL−1 was mixed with the equivalent volume of a non-toxic concentration of Cladophora spp. algal polysaccharide extract. The mixtures were incubated at room temperature for 1, 2, 3, and 4 h to allow the algal polysaccharide extract to have an effect on the virus particles. After incubation for the respective times, the residual virus was diluted 100 times using DMEM to eliminate the effects of the remaining algal polysaccharide extract on subsequent binding events. In this case, the titer of the virus and the concentration of the extract tested are 1.0 × 102 PFU mL−1 and 50 µg mL−1, respectively. This latter concentration is close to, but not greater than, EC50, so it has no significant influence on virus inhibition. The diluted virus was infected into Vero cells and incubated at room temperature for 1 h. The HSV titer was determined by plaque titration assay76.
Antiviral replication assay of algal polysaccharides against herpes simplex viruses
Vero cells were seeded in a 6-well plate at a density of 1 × 105 cells well−1. After incubation for 24 h, the cultured cell monolayer was infected with HSV at an MOI of 0.1. The mixture was incubated at room temperature for 1 h. After incubation, the residual inoculum was removed and the infected cells were washed twice with phosphate-buffered saline solution. The infected cells were treated with non-toxic concentrations of Cladophora spp. algal polysaccharide extract and were incubated at 37 °C in a CO2 incubator. Next, the supernatants were collected at 0, 6, 12, 18, 24, 30, and 36 h after viral infection to harvest the extracellular virus. In a like manner, the infected cells were also frozen and thawed twice to harvest the intracellular virus. The harvested virus was kept at -80 °C before the determination of virus titers using plaque titration assay77.
Statistical analysis
Statistical analyses were executed by IBM SPSS Statistics 20 software (IBM Corp., USA). A one-way analysis of variance (ANOVA) was performed and Tukey's honestly significant difference (HSD) post hoc test was used to establish the significance among all groups with the significant difference at p-values less than 0.05 (p < 0.05). The EC50 values were estimated using probit analysis by PriProbitNM 1.63 software (Kyoto University, Japan)78.
Conclusions
The characterization of Cladophora spp. by chemical analysis established that the structure of algal polysaccharide extract is sulfated polysaccharides. Algal polysaccharide extract exhibited low toxicity on HGF-1 cells, RAW264.7 cells, and Vero cells, and showed high anti-herpetic activity against both HSV-1 and HSV-2 infections upon treatment before and during viral adsorption on Vero cells. The algal polysaccharide extract also had high antiviral activity on both HSV-1 and HSV-2 when treatment after viral adsorption and leaving for 72 h of viral infection on Vero cells without removing the extract from the infected cells. Moreover, algal polysaccharide extract demonstrated direct inactivation of HSV viral particles in virucidal assay. Furthermore, algal polysaccharide extract showed greater inhibitory effect on HSV replication than the IC50 dose of the ACV drug.
Acknowledgements
This research was partially supported by Chiang Mai University and Center of Excellence on Biodiversity (BDC), Office of Higher Education Commission (BDC-PG3-160018). Research and Researcher for Industry from Thailand Science Research and Innovation (MSD61I0107) and Green Diamond Co., Ltd. are also acknowledged for their financial support. We would like to thank Applied Algal Research Laboratory, Chiang Mai University (AARL CMU) for collecting and identifying the algae sample. Moreover, Natural Extracts and Innovative Products for Alternative Healthcare Research Group, Chiang Mai University, Chiang Mai, Thailand were also grateful acknowledged.
Author contributions
P.S. carried out conceptualization, methodology, formal analysis and investigation, collection of resources, wrote the original draft, and edited the manuscript. S.S. carried out formal analysis, investigation, and reviewed and edited the manuscript. A.P. and J.P. carried out supervision. Y.T. carried out conceptualization, methodology, formal analysis and investigation, reviewed and edited the manuscript, funding acquisition, and supervision. All authors read and approved the manuscript for submission.
Data availability
The data presented in this study are available on request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chayavichitsilp P, Buckwalter JV, Krakowski AC, Friedlander SF. Herpes simplex. Pediatr. Rev. 2009;30:119–129. doi: 10.1542/pir.30.4.119. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn, J. H. Virus Taxonomy. Encyclopedia of Virology 28–37 (2021).
- 3.Trybala E, Liljeqvist JA, Svennerholm B, Bergström T. Herpes simplex virus types 1 and 2 differ in their interaction with heparan sulfate. J. Virol. 2000;74:9106–9114. doi: 10.1128/JVI.74.19.9106-9114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crimi S, et al. Herpes virus, oral clinical signs and QoL: Systematic review of recent data. Viruses. 2019;11:463. doi: 10.3390/v11050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wald A. Genital HSV-1 infections. Sex. Transm. Infect. 2006;82:189–190. doi: 10.1136/sti.2006.019935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koelle DM, Corey L. Herpes simplex: Insights on pathogenesis and possible vaccines. Annu Rev Med. 2008;59:381–395. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- 7.Schiffer JT, Mayer BT, Fong Y, Swan DA, Wald A. Herpes simplex virus-2 transmission probability estimates based on quantity of viral shedding. J. R. Soc. Interface. 2014;11:20140160. doi: 10.1098/rsif.2014.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicoll MP, Proença JT, Efstathiou S. The molecular basis of herpes simplex virus latency. FEMS Microbiol. Rev. 2012;36:684–705. doi: 10.1111/j.1574-6976.2011.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith JP, et al. Pharmacokinetics of acyclovir and its metabolites in cerebrospinal fluid and systemic circulation after administration of high-dose valacyclovir in subjects with normal and impaired renal function. Antimicrob. Agents Chemother. 2010;54:1146–1151. doi: 10.1128/AAC.00729-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frobert E, et al. Genotypic detection of acyclovir-resistant HSV-1: Characterization of 67 ACV-sensitive and 14 ACV-resistant viruses. Antivir. Res. 2008;79:28–36. doi: 10.1016/j.antiviral.2008.01.153. [DOI] [PubMed] [Google Scholar]
- 11.Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: Mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 2011;55:459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang YC, Feng H, Lin YC, Guo XR. New strategies against drug resistance to herpes simplex virus. Int. J. Oral Sci. 2016;8:1–6. doi: 10.1038/ijos.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gershengorn HB, Darby G, Blower SM. Predicting the emergence of drug-resistant HSV-2: New predictions. BMC Infect. Dis. 2003;3:1. doi: 10.1186/1471-2334-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin LT, Hsu WC, Lin CC. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014;4:24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alkhatib A. Antiviral functional foods and exercise lifestyle prevention of coronavirus. Nutrients. 2020;12:2633. doi: 10.3390/nu12092633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammadi Pour P, Fakhri S, Asgary S, Farzaei MH, Echeverría J. The Signaling pathways, and therapeutic targets of antiviral agents: Focusing on the antiviral approaches and clinical perspectives of anthocyanins in the management of viral diseases. Front. Pharmacol. 2019;10:1207. doi: 10.3389/fphar.2019.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xian Y, et al. Bioactive natural compounds against human coronaviruses: A review and perspective. Acta Pharm. Sin. B. 2020;10:1163–1174. doi: 10.1016/j.apsb.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim M, et al. Antiviral effects of 28-deacetylsendanin on herpes simplex virus-1 replication. Antivir. Res. 1999;43:103–112. doi: 10.1016/S0166-3542(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 19.Hassan STS, Šudomová M, Berchová-Bímová K, Šmejkal K, Echeverría J. Psoromic acid, a lichen-derived molecule, inhibits the replication of HSV-1 and HSV-2, and inactivates HSV-1 DNA polymerase: Shedding light on antiherpetic properties. Molecules. 2019;24:2912. doi: 10.3390/molecules24162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin LC, Kuo YC, Chou CJ. Anti-herpes simplex virus type-1 flavonoids and a new flavanone from the root of Limonium sinense. Planta Med. 2000;66:333–336. doi: 10.1055/s-2000-8540. [DOI] [PubMed] [Google Scholar]
- 21.Isaacs CE, et al. Digallate dimers of (-)-epigallocatechin gallate inactivate herpes simplex virus. Antimicrob. Agents Chemother. 2011;55:5646–5653. doi: 10.1128/AAC.05531-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nixon B, et al. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J. Virol. 2013;87:6257–6269. doi: 10.1128/JVI.00012-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Sand L, et al. Antiviral active compounds derived from natural sources against herpes simplex viruses. Viruses. 2021;13:1386. doi: 10.3390/v13071386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendes GS, Bravin IC, Yoneshigue-Valentin Y, Yokoya NS, Romanos MTV. Anti-HSV activity of Hypnea musciformis cultured with different phytohormones. Rev. Bras. Farmacogn. 2012;22:789–794. doi: 10.1590/S0102-695X2012005000054. [DOI] [Google Scholar]
- 25.Castillo E, et al. Anti-herpetic activity of Macrocystis pyrifera and Durvillaea antarctica algae extracts against HSV-1 and HSV-2. Front. Microbiol. 2020;11:2006. doi: 10.3389/fmicb.2020.02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Wang SX, Guan HS. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs. 2012;10:2795–2816. doi: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teng YF, et al. Recent progresses in marine microbial-derived antiviral natural products. Arch. Pharm. Res. 2020;43:1215–1229. doi: 10.1007/s12272-020-01286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa LS, et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010;64:21–28. doi: 10.1016/j.biopha.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Cheng YS, Zheng Y, Labavitch JM, Vander Gheynst JS. The impact of cell wall carbohydrate composition on the chitosan flocculation of Chlorella. Process Biochem. 2011;46:1927–1933. doi: 10.1016/j.procbio.2011.06.021. [DOI] [Google Scholar]
- 30.Wijesekara I, Pangestuti R, Kim SK. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011;84:14–21. doi: 10.1016/j.carbpol.2010.10.062. [DOI] [Google Scholar]
- 31.Jung WK, Je JY, Kim HJ, Kim SK. A novel anticoagulant protein from Scapharca broughtonii. J. Biochem. Mol. Biol. 2002;35:199–205. doi: 10.5483/bmbrep.2002.35.2.199. [DOI] [PubMed] [Google Scholar]
- 32.Damonte EB, Matulewicz MC, Cerezo AS. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2004;11:2399–2419. doi: 10.2174/0929867043364504. [DOI] [PubMed] [Google Scholar]
- 33.Leiro JM, Castro R, Arranz JA, Lamas J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int. Immunopharmacol. 2007;7:879–888. doi: 10.1016/j.intimp.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Laurienzo P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs. 2010;8:2435–2465. doi: 10.3390/md8092435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez MJB, Olmo LMBD, Benito PB. Antiviral activities of polysaccharides from natural sources. Stud. Nat. Prod. Chem. 2005;30:393–418. doi: 10.1016/S1572-5995(05)80038-9. [DOI] [Google Scholar]
- 36.Cunha L, Grenha A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs. 2016;14:42. doi: 10.3390/md14030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L, Huang G. The antiviral activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018;115:77–82. doi: 10.1016/j.ijbiomac.2018.04.056. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y, Ren WZL, Zhang Y, Liu D, Liu Y. A review of the pharmacological action of Astragalus polysaccharide. Front. Pharmacol. 2020;11:349. doi: 10.3389/fphar.2020.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JB, Hayashi K, Maeda M, Hayashi T. Antiherpetic activities of sulfated polysaccharides from green algae. Planta Med. 2004;70:813–817. doi: 10.1055/s-2004-827228. [DOI] [PubMed] [Google Scholar]
- 40.Pliego-Cortés H, et al. Sulfated Polysaccharides from seaweed strandings as renewable source for potential antivirals against herpes simplex virus 1. Mar. Drugs. 2022;20:116. doi: 10.3390/md20020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodríguez MC, et al. Galactans from cystocarpic plants of the red seaweed Callophyllis variegata (Kallymeniaceae, Gigartinales) Carbohydr. Res. 2005;340:2742–2751. doi: 10.1016/j.carres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi T, Hayashi K, Maeda M, Kojima I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J. Nat. Prod. 1996;59:83–87. doi: 10.1021/np960017o. [DOI] [PubMed] [Google Scholar]
- 43.Gilles B, et al. Antiviral and cytotoxic activities of polysaccharides extracted from four tropical seaweed species. Nat. Prod. Commun. 2017;12:807–811. [Google Scholar]
- 44.Song S, et al. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020;11:7415–7420. doi: 10.1039/D0FO02017F. [DOI] [PubMed] [Google Scholar]
- 45.Yim SK, et al. Inhibition of SARS-CoV-2 virus entry by the crude polysaccharides of seaweeds and Abalone Viscera in vitro. Mar. Drugs. 2021;19:219. doi: 10.3390/md19040219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kidgell JT, Magnusson M, de Nys R, Glasson CR. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019;39:101422. doi: 10.1016/j.algal.2019.101422. [DOI] [Google Scholar]
- 47.Surayot U, et al. Structural characterization of sulfated arabinans extracted from Cladophora glomerata Kützing and their macrophage activation. Biosci. Biotechnol. Biochem. 2016;80:972–982. doi: 10.1080/09168451.2015.1132149. [DOI] [PubMed] [Google Scholar]
- 48.Mungmai L, Jiranusornkul S, Peerapornpisal Y, Sirithunyalug B, Leelapornpisid P. Extraction, characterization and biological activities of extracts from freshwater macroalga [Rhizoclonium hieroglyphicum (C. Agardh) Kützing] cultivated in Northern Thailand. Chiang Mai J. Sci. 2014;41:14–26. [Google Scholar]
- 49.Çelekli A, Hamdullah A, Aykaç Ç, Gültekin E, Bozkurt H. Biochemical responses of filamentous algae in different aquatic ecosystems in South East Turkey and associated water quality parameters. Ecotoxicol. Environ. Saf. 2016;133:403–412. doi: 10.1016/j.ecoenv.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Arata PX, et al. Sulfated polysaccharides in the freshwater green macroalga Cladophora surera not linked to salinity adaptation. Front. Plant Sci. 2017;8:1927. doi: 10.3389/fpls.2017.01927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sucaldito MR, Camacho DH. Characteristics of unique HBr-hydrolyzed cellulose nanocrystals from freshwater green algae (Cladophora rupestris) and its reinforcement in starch-based film. Carbohydr. Polym. 2017;169:315–323. doi: 10.1016/j.carbpol.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 52.Freile-Pelegrín Y, et al. Valorization of the filamentous seaweed Chaetomorpha gracilis (Cladophoraceae, Chlorophyta) from an IMTA system. J. Appl. Phycol. 2020;32:2295–2306. doi: 10.1007/s10811-020-02066-8. [DOI] [Google Scholar]
- 53.Lahaye M, Robic A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules. 2007;8:1765–1774. doi: 10.1021/bm061185q. [DOI] [PubMed] [Google Scholar]
- 54.Matloub AA, et al. In vitro antiviral, cytotoxic, antioxidant and hypolipidemic activities of polysaccharide isolated from marine algae. Int. J. Pharmacogn. Phytochem. Res. 2015;7:1099–1111. [Google Scholar]
- 55.Hardouin K, et al. Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae) Algal Res. 2016;16:233–239. doi: 10.1016/j.algal.2016.03.013. [DOI] [Google Scholar]
- 56.Song L, et al. Characterization and comparison of the structural features, immune-modulatory and anti-avian influenza virus activities conferred by three algal sulfated polysaccharides. Mar. Drugs. 2016;14:4. doi: 10.3390/md14010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Navya P, Khora SS. In vitro cytotoxicity analysis of sulfated polysaccharides from green seaweed Codium tomentosum Stackhouse, 1797. J. Appl. Pharm. Sci. 2017;7:033–036. [Google Scholar]
- 58.Ruangrit K, et al. A successful biorefinery approach of macroalgal biomass as a promising sustainable source to produce bioactive nutraceutical and biodiesel. Biomass Convers. Biorefin. 2021;13:1089–1099. doi: 10.1007/s13399-021-01310-6. [DOI] [Google Scholar]
- 59.Munir M, Qureshi R, Bibi M, Khan A. Pharmaceutical aptitude of Cladophora: A comprehensive review. Algal Res. 2019;39:1–10. doi: 10.1016/j.algal.2019.101476. [DOI] [Google Scholar]
- 60.Hao C, Xu Z, Xu C, Yao R. Anti-herpes simplex virus activities and mechanisms of marine derived compounds. Front. Cell. Infect. Microbiol. 2024;13:1302096. doi: 10.3389/fcimb.2023.1302096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spear PG. Herpes simplex virus: Receptors and ligands for cell entry. Cell Microbiol. 2004;6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 62.Akhtar J, Shukla D. Viral entry mechanisms: Cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009;276:7228–7236. doi: 10.1111/j.1742-4658.2009.07402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopes N, et al. Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit herpes simplex virus. Int. J. Biol. Macromol. 2017;102:605–612. doi: 10.1016/j.ijbiomac.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 64.Chen X, Han W, Wang G, Zhao X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int. J. Biol. Macromol. 2020;164:331–343. doi: 10.1016/j.ijbiomac.2020.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huleihel M, Ishanu V, Tal J, Arad SM. Antiviral effect of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. J Appl. Phycol. 2001;13:127–134. doi: 10.1023/A:1011178225912. [DOI] [Google Scholar]
- 66.Ahmadi A, Moghadamtousi SZ, Abubakar S, Zandi K. Antiviral potential of algae polysaccharides isolated from marine sources: A review. Biomed. Res. Int. 2015;2015:825203. doi: 10.1155/2015/825203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paradossi G, Cavalieri F, Pizzoferrato L, Liquori AM. A physico-chemical study on the polysaccharide ulvan from hot water extraction of the macroalga Ulva. Int. J. Biol. Macromol. 1999;25:309–315. doi: 10.1016/S0141-8130(99)00049-5. [DOI] [PubMed] [Google Scholar]
- 68.Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 69.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- 70.Dodgson KS, Price RG. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962;84:106–110. doi: 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fernando IPS, et al. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. Algae. 2017;32:75–86. doi: 10.4490/algae.2017.32.12.1. [DOI] [Google Scholar]
- 72.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 73.Alves A, Sousa RA, Reis RL. In vitro cytotoxicity assessment of ulvan, a polysaccharide extracted from green algae. Phytother Res. 2013;27:1143–1148. doi: 10.1002/ptr.4843. [DOI] [PubMed] [Google Scholar]
- 74.Yucharoen R, Chansakaow S, Tragoolpua Y. Inhibitory effect of aromatic herbs, lavender, sage and chamomile against herpes simplex virus infection. Afr. J. Biotechnol. 2011;10:15394–15401. doi: 10.5897/AJB11.2393. [DOI] [Google Scholar]
- 75.Deethae A, Peerapornpisal Y, Pekkoh J, Sangthong P, Tragoolpua Y. Inhibitory effect of Spirogyra spp. algal extracts against herpes simplex virus type 1 and 2 infection. J. Appl. Microbiol. 2018;124:1441–1453. doi: 10.1111/jam.13729. [DOI] [PubMed] [Google Scholar]
- 76.Nikomtat J, Meepowpan P, Tragoolpua Y. Inhibition of Inula cappa (Ham. ex D. Don) DC. extracts on herpes simplex virus infection in vitro. Afr. J. Microbiol. Res. 2011;5:4049–4058. [Google Scholar]
- 77.Chaliewchalad P, Chansakaow S, Tragoolpua Y. Efficacy of Houttuynia cordata. Thunb extracts against herpes simplex virus infection. Chiang Mai J Sci. 2015;42:317–330. [Google Scholar]
- 78.Srisai, P., Pekkoh, J., & Tragoolpua, Y. Anti-herpes simplex virus effect of algal polysaccharide extract from Ulva reticulata. Proceedings of RSU International Research Conference (2020) 671–680 (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.