Abstract
Adenoid cystic carcinoma is a rare malignant tumor that primarily occurs in the salivary glands. There are few reports of sublingual gland adenoid cystic carcinoma with lung metastases on which 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) was performed. We report the case of a 57-year-old Japanese woman with an adenoid cystic carcinoma of the sublingual gland with lung metastases in whom the FDG uptake of the lung metastasis was low despite high FDG uptake in the primary lesion. The pathological examination revealed that solid components were more visible and the Ki-67 index was more positive in the primary lesion compared to the metastatic lesion. We speculate that differences in tumor growth ability might have resulted in the differences in FDG uptake. This case demonstrates that significant differences might occur in the FDG uptake between primary and metastatic tumors.
Keywords: Adenoid cystic carcinoma, Lung metastasis, 18F-FDG-PET/CT, Sublingual gland, FDG uptake, Ki-67
Introduction
Adenoid cystic carcinoma (ACC) is a malignant tumor that primarily occurs in the salivary glands [1]. It accounts for 1% of the cancers of the head and neck and 5%-10% of malignant salivary gland tumors [1,2]. Among the observed cases of salivary gland ACCs, the sublingual gland is a rare site [3,4]. ACC is characterized by a long clinical course, slow growth, and a high recurrence rate [5,6]. Distant metastases usually occur in the lung [7,8].
There are few reports of a sublingual gland ACC with lung metastases in which 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) was performed. In general, the FDG uptake of metastatic lesions tends to be correlated to that of primary lesions. We present the case details of a patient with a sublingual gland ACC with lung metastases, in whom the FDG uptake of the lung metastasis was low despite high FDG uptake in the primary lesion.
Case report
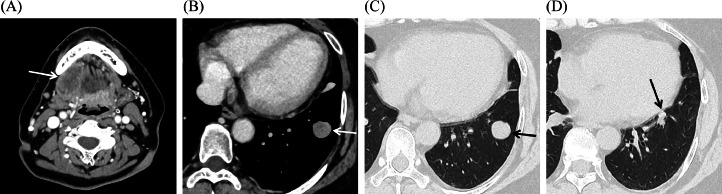
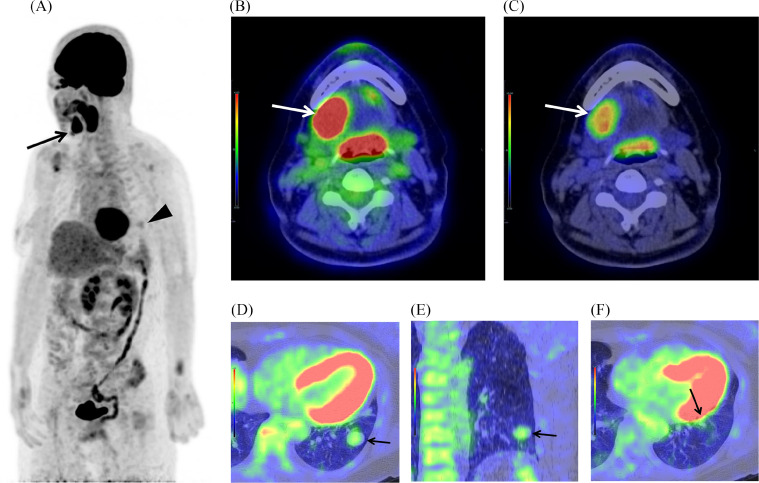
A 57-year-old Japanese woman who noticed swelling under her right jaw was referred to our hospital. She had undergone an epiglottis cystectomy, and she was a carrier of hepatitis B. Computed tomography (CT) revealed a 4 cm mass in the right sublingual gland (Fig. 1A) and 2 well-circumscribed nodules (long diameters 2 cm and 0.8 cm) in the lower lobe of the left lung (Figs. 1B–D). 18F-FDG-PET/CT was scheduled, and 283.3 MBq FDG was injected. The early phase was obtained one hour after injection, and the delayed phase was obtained 2 hours after injection. 18F-FDG-PET/CT showed focal FDG uptake in the right sublingual gland mass, with the maximum standardized uptake value (SUVmax) 10.5 in the early phase and SUVmax 12.5 in the delayed phase (Figs. 2A–C). There was slight FDG uptake in the larger 2 cm nodule, with the SUVmax 2.6 in the early phase and SUVmax 2.6 in the delayed phase (Fig. 2, Fig. 2), and almost no FDG uptake in the smaller 0.8 cm nodule (Fig. 2F). We expected the lung nodules to be unrelated process considering the discordant low uptake of FDG.
Fig. 1.
CT images. (A and B) Mediastinal window: (A) a mass (white arrow) in the right sublingual gland, and (B) a well-circumscribed nodule (white arrow) with relatively homogeneous enhancement in the lower lobe of the left lung. (C and D) Lung window: (C) a well-circumscribed nodule (black arrow) same as (B), and (D) another smaller nodule (black arrow) in the lower lobe of the left lung.
Fig. 2.
18F-FDG-PET/CT images. (A) Maximal intensity projection (MIP) in the early phase: focal FDG uptake (black arrow) in the right sublingual gland mass and slight FDG uptake (black arrowhead) in the lower lobe of the left lung. (B and C) Fused axial CT and FDG-PET image (mediastinal window) in the early phase, showing in the different SUV scales: focal FDG uptake (white arrow) in the right sublingual gland mass, with the SUVmax 10.5. (D and E) Fused CT and FDG-PET image (lung window) of the larger nodule in the early phase: (D) axial image, (E) coronal image: slight FDG uptake (black arrow) with the SUVmax 2.6. (F) Fused axial CT and FDG-PET image (lung window) of the smaller nodule in the early phase: almost no FDG uptake (black arrow).
Two months after the patient's first visit, a right sublingual gland resection for the malignant tumor and a right neck dissection were performed, and the tumor was pathologically diagnosed as an ACC. Cancer cells had infiltrated nerves and the capsule and were observed near the resection margin. No metastasis was detected in lymph nodes. The patient received postoperative radiotherapy (60 Gy). The follow-up CT revealed slight enlargement of the lung nodules, and since lung metastases could not be ruled out, lung surgery was performed. Video-assisted thoracic surgery, i.e., a left basal segmentectomy was performed approx. 6 months after the patient's first visit. The postoperative pathological diagnosis was metastatic lung tumors from the ACC of the right sublingual gland.
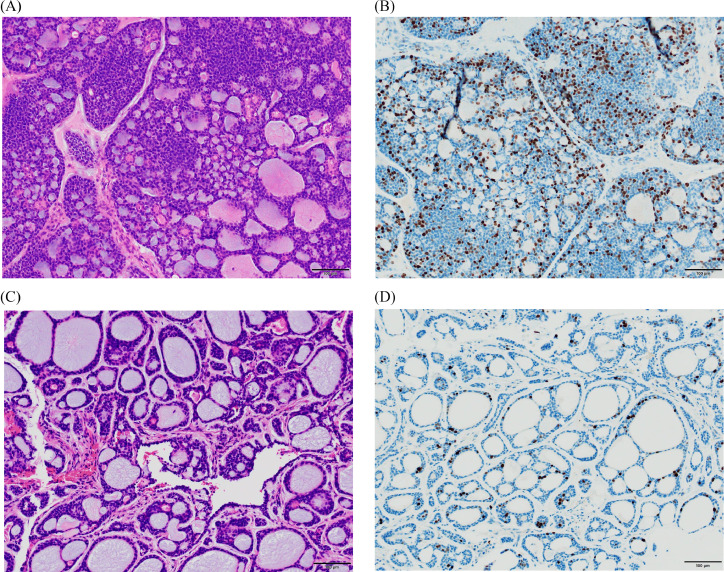
We compared the histological findings of the primary and metastatic lesions. Solid components were more visible and the Ki-67 labeling index was more positive in the primary lesion compared to the metastatic lesion (Figs. 3A–D). The Ki-67 value was 28.5% (793/2,787) in the primary lesion and 6.6% (124/1,884) in the metastatic lesion, thus higher in the primary lesion compared to the metastatic lesion.
Fig. 3.
Histological findings of the primary and metastatic lesions. (A) Hematoxylin and eosin (HE) staining of the primary lesion. (B) The Ki-67 value of the primary lesion. (C) HE staining of the metastatic lesion. (D) The Ki-67 value of the metastatic lesion. Solid components were more visible and the Ki-67 index was more positive in the primary lesion compared to the metastatic lesion.
Discussion
18F-FDG-PET/CT was performed for this patient with a sublingual gland ACC and lung metastases. Cases of sublingual gland ACC are rare [3,4], and there are few reports of ACC having both a primary lesion and pulmonary metastases at the time of the 18F-FDG-PET/CT examination. Distance metastases in ACC are also rare at the initial diagnosis [9].
The SUVmax of the primary lesion in our patient was 10.5 in the early phase and 12.5 in the delayed phase and appears to be relatively high compared to previous cases. In the patient series described by Jeong et al., the SUVmax values for ACC of a salivary gland were 1.5-6.2 [10], and Ruhlmann et al. reported that the mean SUVmax across all recurrent or residual ACCs was 6.8 ± 3.4 (median 5.7, range 2.0–15.0) [9]. In our patient's case, the SUVmax of the primary lesion seemed to be high as that of the ACC.
The standard diagnostic SUV threshold of 2.5 is generally used to designate a scan of a solitary pulmonary nodule as positive [11], and the SUVmax of our patient's lung metastasis was 2.6 in the early phase and thus might be considered barely positive. There are few reports on 18F-FDG-PET/CT findings for metastatic pulmonary ACC. The SUVmax values of secondary ACCs of the lung were 1.0-5.3 in a study by Sun et al. [12]. Ito et al. reported that the SUVmax of a metastatic lung tumor from an ACC resected 16 years earlier in the left upper lung field was 2.5, and that of a metastatic ACC in the left lower lobe of the lungs was 4.5 [13]. In our patient's case, the SUVmax of the lung metastasis seemed to be low considering its size. The low SUVmax made it difficult to consider the lung nodule metastasis.
We observed a significant difference in the SUVmax between our patient's primary lesion and the lung metastasis. Comparing the histological findings of the primary and metastatic lesions, glandular components were more prominent and there was a lower Ki-67 labeling index in the metastatic lesion compared to the primary lesion. Ki-67 is a protein expressed in all phases of the cell cycle except the G0-phase, and its value can be used to estimate the fraction of proliferative cells in tissues [14]. The principle hypothesis explaining why tumor proliferation and glucose metabolism displayed by 18F-FDG-PET/CT are linked to each other is that tumor cell proliferation depends mainly on glycolysis for energy [15]. Meyer et al. described a weak correlation between SUV values and the Ki-67 proliferation index in a large series of patients with head and neck squamous cell carcinoma [16], but the SUV values and Ki-67 index were assumed to be correlated within single patients. We thus speculate that differences in tumor growth ability might have resulted in the differences in FDG uptake in the present patient.
In general, the FDG uptake of metastatic lesions tends to be correlated with that of primary lesions. In their investigation of lung cancer patients, Kosaka et al. observed that the SUVs of most of the metastatic lesions ranged from half to double those of the primary lesions [17]. In our patient's case, the low FDG uptake in the lung metastasis despite the high FDG uptake in the primary lesion made it difficult to diagnose the lung nodules as metastases before surgery. 18F-FDG-PET/CT has been found to be far superior to CT for predicting the TNM stages of high-grade salivary cancer [10]. However, in the present patient, the 18F-FDG-PET/CT examination led to an underestimation of tumor staging. Radiologists should thus be aware of the possibility of underestimation when seeking to achieve a diagnosis based on 18F-FDG-PET/CT.
Conclusion
We treated a patient with a sublingual gland ACC with lung metastases in whom the FDG uptake of the lung metastasis was low despite high FDG uptake in the primary lesion. There was a significant difference in the SUVmax values between the primary lesion and the lung metastasis which might have been caused by differences in tumor growth ability. This case highlights the possibility that significant differences might occur in FDG uptake between primary and metastatic tumors.
Author contributions
All authors provided final approval of the submitted version of this article.
Patient consent
Informed consent was obtained from the patient for the publication of this report and any accompanying images.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments: We would like to thank KN International (www.kninter.co.jp) for English language editing.
References
- 1.Coca-Pelaz A, Rodrigo JP, Bradley PJ, Vander Poorten V, Triantafyllou A, Hunt JL, et al. Adenoid cystic carcinoma of the head and neck: an update. Oral Oncol. 2015;51(7):652–661. doi: 10.1016/j.oraloncology.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Sentani K, Ogawa I, Ozasa K, Sadakane A, Utada M, Tsuya T, et al. Characteristics of 5015 salivary gland neoplasms registered in the Hiroshima Tumor Tissue Registry over a period of 39 years. J Clin Med. 2019;8(5):566. doi: 10.3390/jcm8050566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjørndal K, Krogdahl A, Therkildsen MH, Overgaard J, Johansen J, Kristensen CA, et al. Salivary gland carcinoma in Denmark 1990-2005: a national study of incidence, site and histology. Results of the Danish Head and Neck Cancer Group (DAHANCA) Oral Oncol. 2011;47(7):677–682. doi: 10.1016/j.oraloncology.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 4.Atallah S, Casiraghi O, Fakhry N, Wassef M, Uro-Coste E, Espitalier F, et al. A prospective multicentre REFCOR study of 470 cases of head and neck Adenoid cystic carcinoma: epidemiology and prognostic factors. Eur J Cancer. 2020;130:241–249. doi: 10.1016/j.ejca.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Locati LD, Guzzo M, Bossi P, Massone PP, Conti B, Fumagalli E, et al. Lung metastasectomy in adenoid cystic carcinoma (ACC) of salivary gland. Oral Oncol. 2005;41(9):890–894. doi: 10.1016/j.oraloncology.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Spiro RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg. 1997;174(5):495–498. doi: 10.1016/s0002-9610(97)00153-0. [DOI] [PubMed] [Google Scholar]
- 7.van der Wal JE, Becking AG, Snow GB, van der Waal I. Distant metastases of adenoid cystic carcinoma of the salivary glands and the value of diagnostic examinations during follow-up. Head Neck. 2002;24(8):779–783. doi: 10.1002/hed.10126. [DOI] [PubMed] [Google Scholar]
- 8.Bobbio A, Copelli C, Ampollini L, Bianchi B, Carbognani P, Bettati S, et al. Lung metastasis resection of adenoid cystic carcinoma of salivary glands. Eur J Cardiothorac Surg. 2008;33(5):790–793. doi: 10.1016/j.ejcts.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 9.Ruhlmann V, Poeppel TD, Veit J, Nagarajah J, Umutlu L, Hoffmann TK, et al. Diagnostic accuracy of 18F-FDG PET/CT and MR imaging in patients with adenoid cystic carcinoma. BMC Cancer. 2017;17(1):887. doi: 10.1186/s12885-017-3890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong HS, Chung MK, Son YI, Choi JY, Kim HJ, Ko YH, et al. Role of 18F-FDG PET/CT in management of high-grade salivary gland malignancies. J Nucl Med. 2007;48(8):1237–1244. doi: 10.2967/jnumed.107.041350. [DOI] [PubMed] [Google Scholar]
- 11.Groheux D, Quere G, Blanc E, Lemarignier C, Vercellino L, de Margerie-Mellon C, et al. FDG PET-CT for solitary pulmonary nodule and lung cancer: Literature review. Diagn Interv Imaging. 2016;97(10):1003–1017. doi: 10.1016/j.diii.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Sun X, Gu W, Yuan H, Wang S, Yang Y, Evangelista L, et al. Clinical use of 18F-FDG PET/CT in the differential diagnosis of patients with primary and secondary adenoid cystic carcinoma of the lung: a retrospective cohort study. Transl Lung Cancer Res. 2022;11(8):1643–1656. doi: 10.21037/tlcr-22-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito M, Okita R, Tsutani Y, Mimura T, Kawasaki Y, Miyata Y, et al. Lung metastasis of adenoid cystic carcinoma, which mimicked primary lung cancer. Thorac Cancer. 2013;4(3):327–329. doi: 10.1111/j.1759-7714.2012.00147.x. [DOI] [PubMed] [Google Scholar]
- 14.Denkert C, Budczies J, von Minckwitz G, Wienert S, Loibl S, Klauschen F. Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast. 2015;24(Suppl 2):S67–S72. doi: 10.1016/j.breast.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Deng SM, Zhang W, Zhang B, Chen YY, Li JH, Wu YW. Correlation between the uptake of 18F-fluorodeoxyglucose (18F-FDG) and the expression of proliferation-associated antigen Ki-67 in cancer patients: a meta-analysis. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0129028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer HJ, Gundermann P, Surov A. Associations between FDG-PET and Ki 67-index in head and neck cancer: a meta-analysis. Medicine (Baltimore) 2019;98(40):e17472. doi: 10.1097/MD.0000000000017472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosaka N, Tsuchida T, Tsuji K, Shimizu K, Kimura H. Standardized uptake value differences between primary and metastatic lesions in 18F-FDG PET/CT of patients with lung cancer. Acta Radiol. 2015;56(11):1329–1335. doi: 10.1177/0284185114556705. [DOI] [PubMed] [Google Scholar]