Predicting disease on the basis of biological markers, such as serum cholesterol concentration or blood pressure, is not new; the ability to predict disease using DNA is. As the scope for genetic testing extends beyond testing for single gene disorders to testing large sections of the population for genes associated with common disorders it is important to consider what effect this will have on individuals and on society as a whole. Research into the psychological impact of genetic testing in Huntington’s disease, cystic fibrosis, breast cancer, and ovarian cancer has shown that an individual’s decision to undergo testing and his or her response on receiving the results are influenced by many factors. This article discusses the nature of these factors and the implication they have for the introduction of widespread genetic screening.
Summary points
As genetic screening becomes widespread, its psychological impact on individuals, their families, and society as a whole needs to be assessed
The psychological impact of predictive genetic testing for Huntington’s disease, breast cancer, and ovarian cancer, when offered with expert counselling before and after testing, depends more on pretest expectations, mood, and social support than the results of the test itself
Distress associated with screening may be reduced by careful assessment before and after testing, counselling, and support
Research is needed to determine the most effective and practical counselling strategies for the increasingly large number of people who will be offered genetic tests for treatable conditions
The psychological impact of screening for biological markers associated with increased risk of disease has been well researched.1 Extrapolating from these findings to predict the impact of population based genetic screening of asymptomatic individuals is difficult, though—partly because the predictive value of genetic tests for some disorders is high, and partly because the results of genetic testing impact on families as well as individuals.
At present, most of the genetic tests that are carried out are reproductive tests which provide information about the chances of genetic disorders in future children—for example, carrier testing for cystic fibrosis. But predictive tests, which give people information about their own chances of developing a disease, are being carried out with increasing frequency. These include presymptomatic tests for genetic mutations associated with dominantly inherited conditions with complete penetrance (having the mutation is invariably associated with disease, as for Huntington’s disease) and predispositional tests, which test for gene mutations that are risk factors for a disease (having the mutation means an increased risk, but not a certainty, of developing a disease—for example, genetic testing for hereditary breast and ovarian cancer). Predispositional testing is set to become the main type of genetic test offered in the near future, as genes predisposing to common diseases such as cancer, Alzheimer’s disease, heart disease, and diabetes continue to be discovered.2
Factors influencing the decision to undergo genetic testing
Uptake rates for genetic tests are higher when there are effective ways of treating or preventing the condition. If little can be offered, most people do not want information about their risk status. Thus the uptake for DNA predictive testing is about 10% for Huntington’s disease, for which there is no treatment3,4; for breast cancer, for which there is some possibility of prevention and treatment, it is about 50%5; and it is around 80% for familial adenomatous polyposis, for which there is effective treatment.6
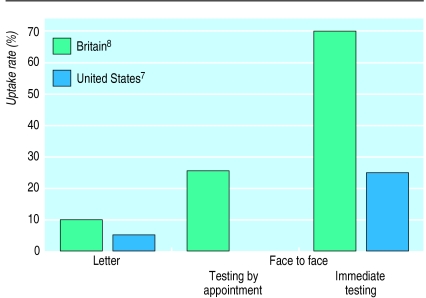
Uptake of genetic tests also depends on how a test is offered. This has been shown most clearly in population screening for cystic fibrosis. When people get invited by letter, fewer than 10% come forward—but uptake is greatly increased when testing is offered in person and if, in addition, it can be carried out immediately (fig).7,8
Which method of invitation is preferable is debatable. For conditions for which there is an effective treatment, high uptake may be the most important goal. If the only intervention being offered is termination of pregnancy, the quality of the decision to undergo a test, or not, may be a more appropriate goal than high uptake. There is some evidence that methods of offering tests that result in high uptake are associated with decisions based on less information and hence of poorer quality.7
Interest in undergoing testing is more strongly related to perceived risk than objective risk.9,10 The extent to which individuals feel uncertain about their risk for a particular condition, their need for certainty, and the extent to which tests will provide that certainty are each important in determining whether they undergo a particular genetic test. Reducing uncertainty is one of the most common reasons for undergoing a predictive DNA test (A Binchy et al, unpublished data).3 Women are more likely than men to undergo carrier tests,8 presymptomatic tests,11 and predispositional tests.6 This may be because of differences in their knowledge about health threats and a difference in the way they cope with adverse information about their health, with men being more likely than women to engage in minimisation.11,12 Societal and cultural factors are also important. Thus while uptake rates in the United States and Britain are similar for Huntington’s disease and breast cancer, rates for cystic fibrosis are lower in the United States.3–5,7,8 This may reflect a more negative attitude to termination of pregnancy as well as greater concern about insurance.
How individuals respond to genetic testing
People who find out that they carry a mutation that predisposes them or a possible child to a disease tend to be more distressed than those whose test results are negative, although the distress is usually within a normal range. The prediction of catastrophic reactions, including suicide, among those at risk for Huntington’s disease who received unfavourable test results have not been fulfilled. Indeed, people receiving positive test results have, overall, experienced some decrease in psychological distress as the uncertainty over their genetic status has decreased.13 Unexpectedly, some people receiving negative test results experience difficulties in adjusting to their revised risk status.13 Among women undergoing predictive DNA testing for breast cancer, the test result seems to have relatively little impact on general levels of anxiety or depression.5,14 Long term follow up data on psychological morbidity are available only on patients who have undergone predictive testing for Huntington’s disease. They suggest that carriers do not become more distressed over time and people with negative results do not experience further decreases in psychological distress.15 This illustrates that factors other than test results are important in predicting and understanding responses to genetic testing.
The importance of individual characteristics
Most people participating in population based screening programmes expect negative test results. Most of those who are known to be, or believe themselves to be, at high risk expect positive results.16 This may explain why people who are aware that they are at risk experience less distress after getting the results of their test than do those who are not aware of being at risk.17,18 There are gender differences too, with women more likely than men to report negative feelings after genetic testing.11,12,19 The amount of social support and the psychological resources that people have also affect their ability to cope.20
In Huntington’s disease, a person’s mood before testing is a better indication of how they react to their test result than is the result of the test itself.11,21 Those who are distressed before they undergo genetic testing are particularly at risk of an adverse psychological outcome after testing, and they need to be identified and given additional support early on.
For many people the term genetic, in relation to an illness, carries negative connotations. They wrongly assume that an illness with a genetic cause is not preventable and not treatable. In a recent study people were asked to imagine that they had been tested by their general practitioner and found to have an increased risk of heart disease.22 Half the participants were told that this increased susceptibility had been determined by a genetic test. For the other half, the type of test was unspecified. When risk was determined by a genetic test, heart disease was seen as less preventable.
The extent to which individuals consider a condition to be preventable is an important predictor of whether they follow advice on how to reduce the risk of developing the condition or ameliorate the condition once it has developed.23 This may mean that if people do not consider genetic tests in the same light as tests for biological risk factors of disease they will not be motivated to change their behaviour. This view is supported by a study in which smokers randomly allocated to be tested for a genetic susceptibility to lung cancer were found to be no more likely to quit smoking than those who were not tested.24 Those who were tested, however, perceived their risks of lung cancer as greater and were more fearful of this than those not given such information.
How testing is conducted
Initial studies of testing for Huntington’s disease and breast cancer have included standardised protocols involving one or more consultations to help people decide whether to proceed with testing. The offer of the test is separated in time from the taking of a biological sample to conduct the DNA test. Such programmes also offer counselling after test results are revealed. The reason such programmes have not had catastrophic effects is likely to be due to such counselling. We do not yet know whether such elaborate counselling is necessary for people undergoing predictive testing for other conditions for which treatment is available, such as breast cancer and ovarian cancer. Research is needed to determine the most effective components of pretest and post-test counselling and the most efficient ways of providing it in anticipation of the increasingly large group of people who will be offered genetic tests.
The way risk information is presented affects how it is perceived and responded to. For example, doctors are more willing to prescribe drugs if evidence of their effectiveness is presented in terms of relative risks rather than absolute risks.25,26 The way genetic risk information is given varies widely—as relative and absolute risks, probabilities and percentages, and numerous verbal descriptors.27–29 One experiment with university students showed that judgments about personal vulnerability to a range of negative outcomes were sensitive to relative, but not absolute, information on risk.30 The impact of presenting the results of genetic tests in different ways has yet to be determined.
Impact of genetic testing on families
Relationships among siblings, parents, and offspring can be complicated by the different test results that individuals receive. For example, some of those found not to carry the gene for Huntington’s disease were rejected by their families when they were found no longer to have one of the key bonds that had previously tied them together: being at risk for Huntington’s disease.31 Partners may be affected more than those who undergo testing—again in Huntington’s disease, a study showed that the partners of those who tested positive experienced more post-test distress and poorer quality of life than did the carrier.16 Partners of non-carriers, however, experienced less hopelessness than their tested partners. These findings suggest the importance of providing support for relatives as well as for the individual undergoing testing.
Impact on society
The conduct of research into the genetic basis for disease and, more recently, of complex behaviours, as well as the clinical provision of genetic testing, all serve to emphasise the inherited component of the human condition. Beliefs about the causes of any problem, in particular how controllable it is seen to be, influence how others respond to people who have the problem.32 For example, health professionals have a more positive attitude towards helping patients with heart disease who do not smoke.33 How might a genetic emphasis on the causes of illness or behaviour affect general attitudes and in particular those of health professionals?
If the effect is to make the outcome seem less controllable, a genetic emphasis may have a positive effect. So, for example, the claim of homosexuality as genetic in origin was greeted by some gay rights activists as heralding the end of blame for such a sexual orientation. If, on the other hand, the effect of a genetic screening programme is to emphasise the importance of taking preventive actions to avoid that risk, people may regard an adverse outcome as controllable. This may result in people with such outcomes receiving less help and more blame.
In line with this, concerns have been expressed that genetic testing in pregnancy together with the offer of termination of affected pregnancies will make us less tolerant as a society towards disability and difference—and might lead to blaming of parents who do not use genetic tests and subsequently give birth to a child with a disability. Though there is indirect evidence to suggest that parents may be blamed for not undergoing tests,34 this remains an important and little researched question.
Conclusion
Research concerning the psychological impact of genetic testing is limited, but it does suggest that adverse psychological reactions are uncommon when testing is provided within a testing programme that separates the offer of testing from the taking of a biological sample for the test, and that provides clear information and emotional support both before and after testing (box). Research is now needed to determine how much and what type of information and support are required for the increasing numbers of people being offered genetic testing, and how these are most efficiently provided, to achieve good understanding of a test and its results, as well as facilitating behaviours to reduce risk, without high levels of emotional distress or false reassurance.
Best practice in genetic testing
In the light of current evidence, best practice for the conduct of genetic testing (presymptomatic, predispositional, and prenatal) includes the following points:
The written protocol for the conduct of the testing programme should include how the laboratory tests are to be conducted and how communication with patients is to be managed
Before they decide whether to undergo a test, clear and simple information should be presented to those eligible for testing. Such information should include the advantages and disadvantages of testing, as well as the meaning of any possible test result
The initial offer of a test should be separated in time (a day or more) from a biological sample being taken
Test results should be explained and support offered to all those tested and their relatives
The effectiveness of a testing programme in achieving good understanding as well as facilitating behaviours that reduce risk, without high levels of emotional distress or false reassurance, needs to be assessed, not assumed
Figure.
Uptake of carrier testing for cystic fibrosis in non-pregnant populations, according to method of offering the test
Figure.
Amount of social support affects people’s ability to cope with results of genetic testing
Footnotes
Funding: TMM is supported by the Wellcome Trust; RTC is supported by grants from the National Cancer Institute.
Conflict of interest: None.
References
- 1.Croyle RT, editor. Psychosocial effects of screening for disease prevention and detection. New York: Oxford University Press; 1995. [Google Scholar]
- 2.Bell J. Genetic advances: implications for clinical medicine. BMJ. 1998;316:618–620. [Google Scholar]
- 3.Craufurd D, Dodge A, Kerzin-Storrar L, Harris R. Uptake of presymptomatic predictive testing for Huntington’s disease. Lancet. 1989;i:603–605. doi: 10.1016/s0140-6736(89)90722-8. [DOI] [PubMed] [Google Scholar]
- 4.Quaid KA, Morris M. Reluctance to undergo predictive testing: the case of Huntington disease. Am J Med Genet. 1993;45:42–45. doi: 10.1002/ajmg.1320450112. [DOI] [PubMed] [Google Scholar]
- 5.Lerman C, Narod S, Schulman K, Hughes C, Gomez-Caminero A, Bonney G. BRCA1 testing in families with hereditary breast-ovarian cancer. A prospective study of patient decision making and outcomes. JAMA. 1996;275:1885–1892. [PubMed] [Google Scholar]
- 6.Evans DGR, Maher ER, Maclead R, Davies DR, Craufurd D. Uptake of genetic testing for cancer predisposition—ethical issues. J Med Genet. 1997;34:746–749. doi: 10.1136/jmg.34.9.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tambor ES, Bernhardt BA, Chase GA, Faden RR, Geller G, Hofman KJ, et al. Offering cystic fibrosis carrier screening to an HMO population: factors associated with utilization. Am J Hum Genet. 1994;55:626–637. [PMC free article] [PubMed] [Google Scholar]
- 8.Bekker H, Modell M, Dennis G, Silver A, Mathew C, Bobrow M, et al. Uptake of cystic fibrosis carrier testing in primary care: supply push or demand pull? BMJ. 1993;306:1584–1586. doi: 10.1136/bmj.306.6892.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Struewing JP, Lerman C, Kase RG, Giambarresi TR, Tucker MA. Anticipated uptake and impact of genetic testing in hereditary breast and ovarian cancer families. Cancer Epidemiol Biomarkers Prev. 1995;4:169–173. [PubMed] [Google Scholar]
- 10.Marteau TM, Kidd J, Cook R, Michie S, Johnston M, Slack J, et al. Perceived risk not actual risk predicts uptake of amniocentesis. Br J Obstet Gynaecol. 1991;98:282–286. doi: 10.1111/j.1471-0528.1991.tb13394.x. [DOI] [PubMed] [Google Scholar]
- 11.Tibben A, Frets PG, van den Kamp JJP, Niermeijer MF, Vegter-van der Vlis M, Roos RAC, et al. On attitudes and appreciation 6 months after predictive DNA test for Huntington disease in the Dutch program. Am J Med Genet 1993;48:103-11. [DOI] [PubMed]
- 12.Marteau TM, Dundas R,, Axworthy D. Long term cognitive and emotional impact of genetic testing for carriers of cystic fibrosis: the effects of gender and test result. Health Psychol 1997;16:51-62. [DOI] [PubMed]
- 13.Wiggins S, Whyte P, Huggins M, Adam S, Theilmann J, Bloch M, et al. The psychological consequences of predictive testing for Huntington’s disease. N Engl J Med 1992;1401-5. [DOI] [PubMed]
- 14.Croyle RT, Smith KR, Botkin JR, Baty B, Nash J. Psychological responses to BRCA1 mutation testing: preliminary findings. Health Psychol. 1997;16:63–72. doi: 10.1037//0278-6133.16.1.63. [DOI] [PubMed] [Google Scholar]
- 15.Tibben A, Timman R, Bannink EC, Duivenvoorden HJ. Three-year follow-up after presymptomatic testing for Huntington’s disease in tested individuals and partners. Health Psychol. 1997;16:48–63. doi: 10.1037//0278-6133.16.1.20. [DOI] [PubMed] [Google Scholar]
- 16.Lynch HT, Watson P, Conway TA, Lynch JF, Slominski-Caster SM, Narod SA, et al. DNA screening for breast/ovarian cancer susceptibility based on linked markers. Arch Intern Med. 1993;153:1979–1987. [PubMed] [Google Scholar]
- 17.Marteau T, Anionwu E. Evaluating carrier testing: objectives and outcomes. In: Marteau TM, Richards M, eds. The troubled helix: social and psychological implications of the new human genetics. Cambridge: Cambridge University Press, 1996:123-39.
- 18.Marteau TM, Kidd J, Cook R, Johnston M, Michie S, Shaw RW, et al. Screening for Down’s syndrome [letter] BMJ. 1988;297:1469. doi: 10.1136/bmj.297.6661.1469-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson EK, Mayall E, Chapple J, Dalziel M, Harrington K, Williams C, et al. Screening for carriers of cystic fibrosis through primary health care services. BMJ. 1991;303:504–507. doi: 10.1136/bmj.303.6801.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazarus RS, Folkman S. Stress, appraisal and coping. New York: Springer; 1984. [Google Scholar]
- 21.Decruyenaere M, Evers-Kiebooms G, Boogaerts A, Cassiman JJ, Cloostermans T, Demyttenaere K. Prediction of psychological functioning one year after the predictive test for Huntington’s disease and impact of the test result on reproductive decision making. J Human Genet. 1996;33:737–743. doi: 10.1136/jmg.33.9.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marteau TM, Senior V. Illness representations after the human genome project: the perceived role of genes in causing illness. In: Petrie K, Weinman J, eds. Perceptions of illness and treatment: current psychological research and implications. Amsterdam: Harwood Academic Press, 1997:241-66.
- 23.Skinner EA. Personality processes and individual differences: A guide to constructs of control. J Pers Soc Psychol. 1996;71:549–570. doi: 10.1037//0022-3514.71.3.549. [DOI] [PubMed] [Google Scholar]
- 24.Lerman C, Gold K, Audrain J, Lin TH, Boyd NR, Orleans CT, et al. Incorporating biomarkers of exposure and genetic susceptibility into smoking cessation treatment: effects on smoking-related cognitions, emotions, and behavior change. Health Psychol. 1997;16:87–99. doi: 10.1037//0278-6133.16.1.87. [DOI] [PubMed] [Google Scholar]
- 25.Bobbio M, Demichelis B, Giustetto G. Completeness of reporting trial results: effects on physicians willingness to prescribe. Lancet. 1994;343:1209–1211. doi: 10.1016/s0140-6736(94)92407-4. [DOI] [PubMed] [Google Scholar]
- 26.Bucher HC, Weinbacher M, Gyr K. Influence of method of reporting study results on decision of physicians to prescribe drugs to lower cholesterol concentration. BMJ. 1994;309:761–764. doi: 10.1136/bmj.309.6957.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allanson A, Michie S, Marteau TM. Presentation of screen negative results on serum screening for Down syndrome: variations across Britain. J Med Screening. 1997;4:21–22. doi: 10.1177/096914139700400108. [DOI] [PubMed] [Google Scholar]
- 28.Marteau TM, Plenicar M, Kidd J. Obstetricians presenting amniocentesis to pregnant women: practice observed. J Reprod Infant Psychol. 1993;11:3. [Google Scholar]
- 29.Hallowell N, Statham H, Murton F, Green J, Richards MPM. “Talking about chance”: the presentation of risk information during genetic counseling for breast and ovarian cancer. J Genet Counseling. 1997;6:269–286. doi: 10.1023/A:1025624221369. [DOI] [PubMed] [Google Scholar]
- 30.Klein WM. Objective standards are not enough: affective, self-evaluative behavioral responses to social comparison information. J Pers Soc Psychol. 1997;72:763–774. doi: 10.1037//0022-3514.72.4.763. [DOI] [PubMed] [Google Scholar]
- 31.Tibben A, Vegter-van der Vlis M, Niermeijer MF, van der Kamp JJP, Roos RAC, Rooijmans HGM, et al. Testing for Huntington’s disease with support for all parties [letter]. Lancet 1990;335:553. [DOI] [PubMed]
- 32.Billings PR, Kohn MA, de Cuevas M, Beckwith J, Alper JS, Natowicz MR. Eugenics: past, present and the future. Am J Hum Genet. 1991;49:1109–1118. [PMC free article] [PubMed] [Google Scholar]
- 33.Holtzman NA, Shapiro D. Genetic testing and public policy. BMJ (in press). [DOI] [PMC free article] [PubMed]
- 34.Marteau TM, Drake H. Attributions for disability: the influence of genetic screening. Soc Sci Med. 1995;40:1127–1132. doi: 10.1016/0277-9536(94)00180-2. [DOI] [PubMed] [Google Scholar]