Abstract
Background
The early period following the onset of acute coronary syndrome (ACS) represents a critical stage of coronary heart disease, with a high risk of recurrent events and deaths. The short‐term effects of early treatment with statins on patient‐relevant outcomes in patients suffering from ACS are unclear. This is an update of a review previously published in 2011.
Objectives
To assess the effects, both harms and benefits, of early administered statins in patients with ACS, in terms of mortality and cardiovascular events.
Search methods
We updated the searches of CENTRAL (2013, Issue 3), MEDLINE (Ovid) (1946 to April Week 1 2013), EMBASE (Ovid) (1947 to 2013 Week 14), and CINAHL (EBSCO) (1938 to 2013) on 12 April 2013. We applied no language restrictions. We supplemented the search by contacting experts in the field, by reviewing the reference lists of reviews and editorials on the topic, and by searching trial registries.
Selection criteria
Randomized controlled trials (RCTs) comparing statins with placebo or usual care, with initiation of statin therapy within 14 days following the onset of ACS, follow‐up of at least 30 days, and reporting at least one clinical outcome.
Data collection and analysis
Two authors independently assessed risk of bias and extracted data. We calculated risk ratios (RRs) for all outcomes in the treatment and control groups and pooled data using random‐effects models.
Main results
Eighteen studies (14,303 patients) compared early statin treatment versus placebo or no treatment in patients with ACS. The new search did not identify any new studies for inclusion. There were some concerns about risk of bias and imprecision of summary estimates. Based on moderate quality evidence, early statin therapy did not decrease the combined primary outcome of death, non‐fatal myocardial infarction, and stroke at one month (risk ratio (RR) 0.93, 95% confidence interval (CI) 0.80 to 1.08) or four months (RR 0.93, 95% CI 0.81 to 1.06) of follow‐up when compared to placebo or no treatment. There were no statistically significant risk reductions from statins for total death, total myocardial infarction, total stroke, cardiovascular death, revascularization procedures, and acute heart failure at one month or at four months, although there were favorable trends related to statin use for each of these endpoints. Moderate quality evidence suggests that the incidence of unstable angina was significantly reduced at four months following ACS (RR 0.76, 95% CI 0.59 to 0.96). There were nine individuals with myopathy (elevated creatinine kinase levels more than 10 times the upper limit of normal) in statin‐treated patients (0.13%) versus one (0.015%) in the control groups. Serious muscle toxicity was mostly limited to patients treated with simvastatin 80 mg.
Authors' conclusions
Based on moderate quality evidence, due to concerns about risk of bias and imprecision, initiation of statin therapy within 14 days following ACS does not reduce death, myocardial infarction, or stroke up to four months, but reduces the occurrence of unstable angina at four months following ACS. Serious side effects were rare.
Keywords: Humans; Acute Coronary Syndrome; Acute Coronary Syndrome/drug therapy; Acute Coronary Syndrome/mortality; Angina, Unstable; Angina, Unstable/prevention & control; Cardiovascular Diseases; Cardiovascular Diseases/mortality; Cause of Death; Drug Administration Schedule; Heart Failure; Heart Failure/prevention & control; Hydroxymethylglutaryl‐CoA Reductase Inhibitors; Hydroxymethylglutaryl‐CoA Reductase Inhibitors/adverse effects; Hydroxymethylglutaryl‐CoA Reductase Inhibitors/therapeutic use; Myocardial Infarction; Myocardial Infarction/prevention & control; Myocardial Revascularization; Myocardial Revascularization/statistics & numerical data; Randomized Controlled Trials as Topic; Stroke; Stroke/prevention & control
Plain language summary
Statins for acute coronary syndrome
Long‐term therapy with statins (for at least one year) has been shown to reduce the risk of heart attack, stroke, and all‐cause mortality in patients with and without established coronary heart disease. The early period following an acute coronary syndrome is a critical stage of coronary heart disease, with a high risk of recurrent events and death. We aimed to determine if early initiation of statins improves patient‐relevant outcomes within the first four months following an acute coronary syndrome. This review is an update of a review previously published in 2011 that included 18 studies, enrolling 14,303 patients. The update of this review did not identify any new studies for inclusion. We did not find a significant risk reduction for all‐cause mortality, heart attack, or stroke within the first four months. We had some concerns about risk of bias and imprecision of the results. The risk of unstable angina was reduced by about 25% at four months following acute coronary syndrome. Serious side effects from early treatment with statins were rare (0.1%), and serious muscle toxicity was mostly observed with simvastatin 80 mg.
Summary of findings
Summary of findings for the main comparison. Statins compared to control at 4 months (3 to 6 months) for acute coronary syndrome.
| Statins compared to control at 4 months (3 to 6 months) for acute coronary syndrome | ||||||
| Patient or population: patients with acute coronary syndrome Settings: inpatients, developed countries Intervention: statins Comparison: control at 4 months (3 to 6 months) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control at 4 months (3 to 6 months) | Statins | |||||
| Combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths Follow‐up: 3 to 6 months | 80 per 10001 | 75 per 1000 (65 to 85) | RR 0.93 (0.81 to 1.06) | 9625 (11 studies) | ⊕⊕⊕⊝ moderate2,3 | |
| Death from all causes Follow‐up: 3 to 6 months | 28 per 10001 | 25 per 1000 (20 to 32) | RR 0.9 (0.7 to 1.14) | 9733 (12 studies) | ⊕⊕⊕⊝ moderate2,3 | |
| Fatal and non‐fatal myocardial infarction or reinfarction Follow‐up: 3 to 6 months | 65 per 10001 | 59 per 1000 (50 to 69) | RR 0.91 (0.77 to 1.06) | 9537 (10 studies) | ⊕⊕⊕⊝ moderate2,3 | |
| Fatal and non‐fatal stroke Follow‐up: 3 to 6 months | 10 per 10001 | 7 per 1000 (4 to 11) | RR 0.72 (0.45 to 1.16) | 8536 (7 studies) | ⊕⊕⊕⊝ moderate2,3 | |
| Unstable angina Follow‐up: 3 to 6 months | 63 per 10001 | 48 per 1000 (37 to 60) | RR 0.76 (0.59 to 0.96) | 8770 (9 studies) | ⊕⊕⊕⊝ moderate2,4 | |
| Acute heart failure Follow‐up: mean 4 months | 26 per 10001 | 22 per 1000 (17 to 30) | RR 0.86 (0.65 to 1.15) | 7583 (2 studies) | ⊕⊕⊕⊝ moderate3 | |
| Rhabdomyolysis Follow‐up: mean 4 months | 0 per 10001 | 0 per 1000 (0 to 0) | RR 6.9 (0.36 to 133.47) | 4497 (1 study) | ⊕⊕⊝⊝ low5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The basis of the assumed risk is the average risk of control group patients. 2The largest studies had allocation concealment and used blinding for patients, caregivers, and outcome assessors; however for many studies allocation concealment remained unclear and almost half were open‐label. Sensitivity analyses with high quality studies did not change the point estimate. 3The CI includes effects suggesting benefit as well as no benefit, therefore we decided to downgrade by one level when considering this imprecision together with some concerns about risk of bias. 4There is moderate heterogeneity among studies included in the analysis of unstable angina at four months (I² = 33%). The subgroup analysis for trials with blinded outcome assessment (because of possible subjective component for diagnosis of unstable angina) was still statistically significant, but the estimated risk reduction was smaller. Overall we decided to downgrade by one level when considering the heterogeneity and remaining concerns about the risk of bias. 5The CI includes the possibility of both harms or benefits and there are only three events of rhabdomyolysis in total; therefore we decided to downgrade by two levels for imprecision.
Background
Coronary heart disease accounts for 20% of overall mortality in the United States (AHA 2007). Large trials and meta‐analyses have shown that HMG CoA reductase inhibitors (statins) effectively reduce low‐density lipoprotein (LDL) cholesterol and clinical endpoints such as cardiovascular events and overall mortality in a large spectrum of patients at varying risk of cardiovascular disease (4S 1994; HPS 2002; Shepherd 1995; Studer 2005). A limitation of most of the trials in secondary prophylaxis after acute myocardial infarction or unstable angina is, however, that statins have been started three or more months after an acute event. Acute coronary syndrome (ACS) is defined as a broad spectrum of manifestations that are due to insufficient coronary blood supply. These include ST‐segment elevation myocardial infarction, non‐ST‐segment elevation ACS with or without myocardial cell necrosis (unstable angina and/or non‐ST‐segment elevation myocardial infarction), and ST‐segment depression (non‐Q‐wave) myocardial infarction. The early period following an ACS represents a critical stage of coronary heart disease with a high risk of recurrent events and death due to vessel occlusions from vulnerable coronary plaques (Wood 1998). Therefore, strategies to stabilize vulnerable coronary plaques during this high‐risk period are paramount.
Experimental data indicate that statins may have early beneficial effects by improving the endothelial function of arteries (RECIFE 1999), decreasing platelet aggregability and thrombus formation (Rosenson 1998), and reducing vascular inflammation (Ridker 1998). Each of these mechanisms plays an important role in ACS and they are targets for existing or new drugs in the management of the ACS (Kumar 2009). Statins may exert these additional effects beyond their cholesterol‐lowering effect, which makes them amenable to supplementary therapy of ACS (Sposito 2002).
There is controversial evidence from observational studies that statin therapy prior to or at hospital discharge is associated with reducing short‐term mortality among patients after an ACS (Aronow 2001; Fonarow 2005; Newby 2002; Spencer 2004; Stenestrand 2001). Evidence from randomized controlled trials (RCTs) focusing on patients with an ACS indicates that statins may reduce combined endpoints that include recurrent angina, re‐angioplasty, and re‐hospitalization (Cannon 2004b; L‐CAD 2000; MIRACL 2001; Serruys 2002). These endpoints, however, may be less reliable because they depend to a greater extent on clinical judgement and local practices. Therefore information on 'harder' clinical endpoints, such as definite myocardial infarction, stroke, and coronary heart disease‐specific mortality, is important.
Why it is important to do this review
This is an update of a Cochrane review previously published in 2011. Morrissey et al have questioned the Level of Evidence: 1A recommendation of the current American College of Cardiology/American Heart Association (ACC/AHA) guidelines that statin therapy should be initiated in patients before hospital discharge after an episode of ACS regardless of the baseline LDL level because of a mismatch with the underlying evidence (Morrissey 2009). Two previous meta‐analyses on the topic suggested that early treatment with statins does not reduce death, myocardial infarction, or stroke up to four months following an ACS (Briel 2006a; Hulten 2006). Hulten et al used slightly different eligibility criteria (e.g. they allowed for head to head comparisons of statins to be included), did not contact investigators of primary trials for unpublished data, and pooled hazard ratios instead of risk ratios (Hulten 2006). They concluded that early statin therapy reduces the combined endpoint of death, recurrent ischemia, and recurrent myocardial infarction at six months of treatment and thereafter.
The purpose of the present study is to comprehensively update previous systematic reviews and meta‐analyses of RCTs evaluating the effects of early use of statins on relevant clinical endpoints of cardiovascular morbidity and overall mortality during the early stages at one and four months following the onset of ACS (Briel 2006a; Vale 2011).
Objectives
To assess the effects, both harms and benefits, of early administered statins in patients with ACS, in terms of mortality and cardiovascular events.
Methods
Criteria for considering studies for this review
Types of studies
We include randomized controlled trials (RCTs) comparing statin to placebo or no treatment in patients with an ACS (myocardial infarction or unstable angina). We excluded trials comparing two different statins without a placebo or no treatment control. We only considered trials with at least 30 days of follow‐up of participants after an ACS, reporting at least one clinical outcome.
Types of participants
Adults with recent ACS, regardless of prior lipid levels and prior lipid‐modifying treatment or diet. ACS is defined as a broad spectrum of manifestations that are due to insufficient coronary blood supply. These include ST‐segment elevation myocardial infarction, non‐ST‐segment elevation ACS with or without myocardial cell necrosis (unstable angina and/or non‐ST‐segment elevation myocardial infarction), and ST‐segment depression (non‐Q‐wave) myocardial infarction. We included patients regardless of previous ACS, percutaneous coronary interventions including stents, or co‐morbidities such as atrial fibrillation with or without antithrombotic treatment.
Types of interventions
Initiation of statin therapy (HMG‐CoA reductase inhibitors such as pravastatin, simvastatin, atorvastatin, fluvastatin, lovastatin, rosuvastatin) administered orally at any dosage within 14 days following the onset of an acute coronary syndrome. We only considered trials using cerivastatin for sensitivity analysis since this compound was withdrawn from the market in 2001 (Staffa 2002).
Types of outcome measures
We assessed the following clinical outcomes at one month, four months (range three to six months), and 12 months of follow‐up.
Primary outcome
Combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and death from all causes
Secondary outcomes
Death from all causes
Death from cardiovascular causes
Fatal and non‐fatal myocardial infarction or reinfarction
Fatal and non‐fatal stroke
Revascularization procedures (bypass grafts, angioplasty with or without stenting)
Unstable angina (recurrent myocardial ischemia requiring emergency hospitalization)
Acute (new or worsening) heart failure
Adverse events (rhabdomyolysis, creatinine kinase levels more than 10 times the upper limit of normal values, and liver aminotransferase levels more than three times the upper limit of normal values)
Patient‐perceived quality of life
We considered outcomes and adverse events irrespective of their putative relation to the treatment. To maximize the statistical power of our primary analysis and to recognize the event hierarchy of fatal and non‐fatal events (occurrence of death precludes any other clinical events), we chose a combined primary endpoint to test the most patient‐relevant 'hard' outcomes: death, myocardial infarction, and stroke. Each of the components is highly patient‐relevant and about equally frequent (Montori 2005).
Search methods for identification of studies
To identify relevant trials we updated the searches from February 2010 by re‐running the searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 3), MEDLINE (Ovid, 1946 to April Week 1 2013), EMBASE Classic and EMBASE (Ovid, 1947 to 2013 Week 14), and CINAHL (EBSCO, 1938 to 12 April 2013) on 12 April 2013.
We did not impose any language restrictions. In addition, we searched previous systematic reviews (Briel 2006a; Hulten 2006), reference lists of identified articles, recently published editorials and narrative reviews on the topic, and trial registries (ISRCTN trials registry: isrctn.org/; US National Institute of Health Clinical Trials Registry: www.clinicaltrials.gov/) for further eligible trials. We contacted specialists in the field for any unpublished studies.
The detailed electronic search strategies developed by NB and MB for MEDLINE, EMBASE, CENTRAL, and CINAHL, which include filters for finding RCTs (modified from Dickersin 1994), are listed in Appendix 1. We updated the electronic searches with the help of the Cochrane Heart Group (Appendix 2). We have added some search terms and updated the RCT filter for MEDLINE and EMBASE (Lefebvre 2011).
Data collection and analysis
Selection of studies
Two authors (NV and AJN) independently assessed trial eligibility using a predefined form. We resolved disagreement by discussion and consensus. We excluded double reports. We applied no language restrictions.
Assessment of risk of bias in included studies
Two authors (NV and AJN) independently assessed the risk of bias in the selected trials according to the Cochrane 'Risk of bias' tool (six domains).
We ranked the risk of selection bias with respect to allocation concealment as:
low risk (i.e. central randomization; numbered or coded bottles or containers; drugs prepared by the pharmacy; serially numbered, opaque, sealed envelopes; or other description regarding the methods used that warrants a judgement of adequate allocation concealment)
unclear risk (i.e. unreported)
high risk (for instance, alternation or reference to case record numbers or to dates of birth)
We resolved disagreements by discussion and consensus. We used our 'Risk of bias' assessment of included trials to explore heterogeneity among trials and to perform subgroup analysis.
Data extraction and management
Two authors (NV and AJN) independently extracted trial data in duplicate using predefined forms. We extracted data on patients' characteristics (age, gender, diabetes, hypertension, current smoker, prior myocardial infarction, lipid values at baseline, myocardial infarction as the index event, concomitant treatment for the index event (fibrinolysis, percutaneous coronary intervention), statin regimen (type of statin, daily dosage, starting time, duration), control group therapy (placebo or conventional treatment), follow‐up duration, and measured outcomes for both study groups (proportion of patients with the outcome) at one, four, and 12 months following the onset of the ACS. We resolved any disagreement between authors by discussion and consensus.
Data synthesis
We calculated risk ratios with 95% confidence intervals for all outcomes in the treatment and control groups and pooled them by conducting a random‐effects model meta‐analysis (DerSimonian 1986). We also calculated Peto odds ratios (fixed‐effect model), which are suggested for rare events (Deeks 1998). We investigated the presence of publication bias by means of funnel plots (Sterne 2001). We tested for heterogeneity with the Cochran Q test and measured inconsistency (I2 statistic: the percentage of total variance across studies that is due to heterogeneity rather than chance) of treatment effects across the primary and secondary outcomes (Higgins 2002; Higgins 2003). We did not include studies without events in either group in the analyses.
Sensitivity analysis
We examined treatment effects according to 'Risk of bias' components (concealed treatment allocation, blinding of patients and caregivers, blinded outcome assessment) for the combined primary outcome death, myocardial infarction, and stroke, for death from all causes, and for unstable angina. We also assessed the effect of time to initiation of statins on the combined primary outcome, and the type of statin on the combined primary outcome. We also conducted a sensitivity analysis by including unpublished data from a trial using cerivastatin on death from all causes, total myocardial infarction, total stroke, and unstable angina (PRINCESS 2004).
Results
Description of studies
The combined search of CENTRAL, MEDLINE, EMBASE, and CINAHL identified 2324 potentially relevant articles, of which we excluded all but 63 because it was clear from the abstract that they were not eligible (Figure 1). Full‐text assessment of the 63 potentially relevant articles resulted in the further exclusion of 39 studies (39 articles) because they were not randomized trials, had a follow‐up of less than one month, treatment was given for less than one month, they were head‐to‐head comparisons of statins, statin therapy was initiated beyond 14 days following the onset of ACS, or no or unclear clinical outcome data were reported. In an update of the electronic search on 12 April 2013 we identified another 2268 potentially relevant articles (Appendix 2). After title and abstract screening we checked 24 full texts but found no new eligible studies for inclusion.
1.
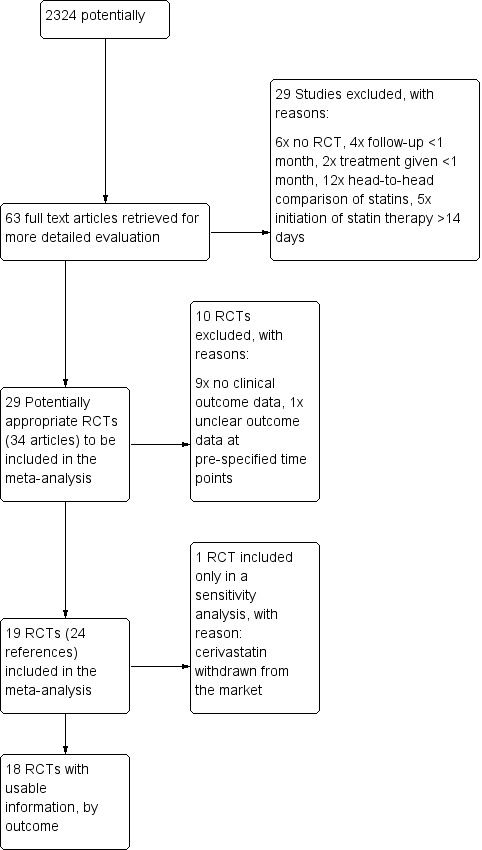
Study flow chart.
For information on the excluded studies please see Characteristics of excluded studies.
Included in the meta‐analysis were 19 RCTs (24 references). One trial using cerivastatin was prematurely stopped because the drug was withdrawn from the market (PRINCESS 2004). We excluded data from the 4.5‐month follow‐up of this trial from the primary analysis, but included the data in a sensitivity analysis. The remaining 18 RCTs enrolled a total of 14,303 patients (7172 treatment, 7131 control). We found no evidence of ongoing eligible trials. Authors of included primary trials contributed additional data relevant for the purpose of this analysis. We were unable to contact the investigators from three trials (LAMIL 1997; Sakamoto 2005; Shal'nev 2007).
Seventeen of the 19 included trials investigated four different statins: pravastatin (seven trials; LAMIL 1997; L‐CAD 2000; OACIS‐LIPID 2008; PACT 2004; PAIS 2001; PTT 2002; RECIFE 1999), atorvastatin (four trials; Colivicchi 2002; ESTABLISH 2004; Macin 2005; MIRACL 2001), fluvastatin (three trials; FACS 2010; FLORIDA 2002; LIPS 2002), and simvastatin (three trials; de Lemos 2004; Ren 2009; Shal'nev 2007) (Table 2). One trial allowed any statin to be used in the intervention group (Sakamoto 2005).
1. Baseline characteristics of included patients.
| Trial (reference) | Randomized individuals, n | Mean age, years (SD) | Men, n (%) | Diabetes, n (%) | Hypertension, n (%) | Current smoker, n (%) | Prior MI, n (%) | MI as index event, n (%) | Fibrinolysis for index event, n (%) | PCI for index event, n (%) | ||||||||||
| Statin | Control | Statin | Control | Statin | Control | Statin | Control | Statin | Control | Statin | Control | Statin | Control | Statin | Control | Statin | Control | Statin | Control | |
| LAMIL 1997 | 36 | 33 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 36 (100) | 33 (100) | NA | NA | NA | NA |
| RECIFE 1999 | 30 | 30 | 55 (2) | 56 (2) | 26 (93) | 22 (81) | 1 (4) | 0 (0) | 5 (18) | 8 (29) | 14 (50) | 17 (63) | 1 (4) | 2 (7) | 11 (39) | 12 (44) | 0 (0) | 0 (0) | 16 (57) | 17 (63) |
| L‐CAD 2000 | 70 | 56 | 55 (10) | 59 (11) | 57 (81) | 44 (79) | 0 (0) | 0 (0) | 22 (31) | 18 (32) | 49 (70) | 36 (64) | 45 (64) | 39 (70) | 32 (46) | 23 (41) | NA | NA | 58 (83) | 50 (89) |
| PAIS 2001 | 50 | 49 | 64 (1) | 63 (2) | 35 (70) | 37 (76) | 8 (16) | 5 (10) | 12 (24) | 16 (33) | 17 (34) | 17 (35) | 14 (28) | 12 (25) | 35 (70) | 31 (63) | 17 (34) | 14 (29) | 0 (0) | 0 (0) |
| PTT 2002 | 79 | 85 | 53 (11) | 52 (10) | 65 (82) | 69 (81) | 14 (18) | 13 (15) | 16 (20) | 21 (25) | 63 (80) | 66 (78) | 0 (0) | 0 (0) | 79 (100) | 85 (100) | 79 (100) | 85 (100) | 0 (0) | 0 (0) |
| PACT 2004 | 1710 | 1698 | 62 (12) | 61 (12) | 1308 (76) | 1285 (76) | 244 (14) | 234 (14) | 700 (41) | 714 (42) | 608 (36) | 575 (34) | 236 (14) | 197 (12) | 1109 (65) | 1111 (65) | 651 (38) | 671 (40) | 414 (24) | 406 (24) |
| LIPS 2002 | 417* | 407* | 61 (10) | 60 (10) | 344 (83) | 336 (83) | 65 (16) | 34 (8) | NA | NA | NA | NA | 184 (44) | 172 (42) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 417 (100) | 407 (100) |
| FLORIDA 2002 | 265 | 275 | 61 (12) | 60 (11) | 214 (81) | 234 (85) | 29 (11) | 31 (11) | 67 (25) | 65 (24) | 140 (53) | 139 (51) | 31 (12) | 31 (11) | 265 (100) | 275 (100) | 137 (52) | 133 (48) | 8 (3) | 10 (4) |
| MIRACL 2001 | 1538 | 1548 | 65 (12) | 65 (12) | 992 (64) | 1020 (66) | 342 (22) | 373 (24) | 843 (55) | 846 (55) | 429 (28) | 430 (28) | 382 (25) | 392 (25) | 812 (53) | 843 (55) | 109 (7) | 137 (9) | 0 (0) | 0 (0) |
| Colivicchi 2002 | 40 | 41 | 69 (14) | 68 (14) | 23 (58) | 24 (59) | 22 (55) | 24 (59) | 35 (88) | 37 (90) | NA | NA | 34 (85) | 35 (85) | NA | NA | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ESTABLISH 2004 | 35 | 35 | 61 (10) | 63 (11) | 30 (86) | 30 (86) | 12 (34) | 11 (31) | 19 (54) | 19 (54) | 24 (69) | 19 (54) | 5 (14) | 5 (14) | 22 (63) | 26 (74) | 7 (20) | 3 (9) | 35 (100) | 35 (100) |
| A‐to‐Z 2004 | 2265 | 2232 | 60 (11) | 61 (11) | 1716 (76) | 1680 (75) | 529 (23) | 530 (24) | 1131 (50) | 1105 (50) | 926 (41) | 915 (41) | 409 (18) | 355 (16) | 1956 (86) | 1919 (86) | 483 (21) | 472 (21) | 979 (43) | 979 (44) |
| Sakamoto 2005 | 237 | 244 | 63 (11) | 65 (12) | 190 (80) | 193 (79) | 83 (35) | 61 (25) | 149 (63) | 142 (58) | 131 (55) | 130 (53) | 10 (4) | 15 (6) | 208 (88) | 219 (90) | 45 (19) | 50 (20) | 215 (91) | 220 (90) |
| Macin 2005 | 44 | 46 | 59 (13) | 61 (12) | 34 (77) | 33 (72) | 10 (23) | 11 (24) | 29 (65.9) | 31 (67.4) | 18 (41) | 19 (41) | 5 (11) | 7 (15) | 23 (52) | 31 (67) | 7 (15.9) | 8 (17.4) | 0 (0) | 0 (0) |
| Sato 2008 | 176 | 177 | 64 (10) | 63 (11) | 129 (73) | 142 (80) | 52 (30) | 59 (34) | 81 (46) | 87 (49) | 98 (56) | 105 (59) | 16 (9) | 19 (10) | NA | NA | NA | NA | 161 (91) | 161 (91) |
| Shal'nev 2007 | 55 | 55 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| FACS 2005 | 78 | 78 | 61 (12) | 63 (11) | 55 (71) | 51 (65) | 14 (18) | 16 (21) | 40 (51) | 40 (51) | 33 (42) | 39 (50) | 4 (5) | 8 (10) | > 47 (60) | > 54 (69) | 0 (0) | 0 (0) | 68 (87) | 71 (91) |
| Ren 2009 | 43 | 43 | 58 (11) | 59 (10) | 27 (63) | 30 (70) | 12 (28) | 10 (23) | 21 (49) | 18 (42) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
* These individuals make up the subgroup of patients with unstable angina (n=824).
MI: myocardial infarction NA: not applicable PCI: percutaneous coronary intervention SD: standard deviation
In accordance with our eligibility criteria, we only included the subgroup of patients with unstable angina from the Lescol Intervention Prevention Study (LIPS 2002). In the A‐to‐Z trial we only used data from the placebo comparison during the first four months of follow‐up (de Lemos 2004).
Of the 19 included trials, four were international, multicenter trials (de Lemos 2004; LIPS 2002; MIRACL 2001; PRINCESS 2004), three were conducted in Japan (ESTABLISH 2004; OACIS‐LIPID 2008; Sakamoto 2005), two in the Netherlands (FLORIDA 2002; PAIS 2001), and one each were conducted in Germany (L‐CAD 2000), Belgium (LAMIL 1997), Argentina (Macin 2005), Australia (PACT 2004), Turkey (PTT 2002), Canada (RECIFE 1999), China (Ren 2009), the Czech Republic and Slovakia (FACS 2010), and Russia (Shal'nev 2007). The earliest included trial started recruitment in April 1996 (LIPS 2002), with the latest concluding in July 2006 (Ren 2009). However, six trials did not specify the recruitment dates (L‐CAD 2000; LAMIL 1997; PACT 2004; PRINCESS 2004; RECIFE 1999; Shal'nev 2007). Eleven trials were reported to be industry‐sponsored (de Lemos 2004; FLORIDA 2002; L‐CAD 2000; LAMIL 1997; LIPS 2002; MIRACL 2001; PACT 2004; PAIS 2001; PRINCESS 2004; RECIFE 1999; FACS 2010). See Characteristics of included studies.
Study population
The reported mean age of participants in the trials ranged from 53 to 69 years (Table 3). All trials enrolled mostly men (range among trials, 59% to 88%). There was considerable variation in the proportion of cardiovascular risk factors and participants with a myocardial infarction prior to the index event (range, 0% to 85%). Due to different trial protocols the proportion of participants with co‐interventions for the index event such as fibrinolytic therapy or percutaneous coronary interventions (PCI) varied widely among trials (range, 0% to 100%).
2. General characteristics of included trials.
| Source | Daily intervention | Control | No. of individuals randomized | Mean initiation of statin after onset of ACS, days | Duration of follow‐up available, months | No. (%) of individuals followed up | Reported concealed allocation/masked patients/caregiver/ assessor |
| LAMIL 1997 | Pravastatin 10 to 20 mg |
Placebo | 69 | 2 | 1 and 3 | 55 (80) | No/yes/yes/no |
| RECIFE 1999 | Pravastatin 40 mg |
Placebo | 60 | 10 | 1.5 | 55 (92) | No/yes/yes/no |
| L‐CAD 2000 | Pravastatin 20 to 40 mg* |
Usual care† | 126 | 6 | 1, 4, and 6 | 126 (100) | No/no/no/no |
| PAIS 2001 | Pravastatin 40 mg |
Placebo | 99 | 2 | 1 and 3 | 97 (98) | No/yes/yes/no |
| PTT 2002 | Pravastatin 40 mg |
Usual care† | 164‡ | 1 | 1 and 6‡ | 164 (100) | No/no/no/no |
| PACT 2004 | Pravastatin 20 to 40 mg |
Placebo | 3408 | 1 | 1 | 3323 (98) | No/yes/yes/no |
| LIPS 2002 | Fluvastatin 80 mg |
Placebo | 824§ | 2 | 1, 4, and 6 | 824 (100) | No/yes/yes/yes |
| FLORIDA 2002 | Fluvastatin 80 mg |
Placebo | 540 | 8 | 1, 4, and 6 | 540 (100) | No/yes/yes/yes |
| MIRACL 2001 | Atorvastatin 80 mg |
Placebo | 3086 | 3 | 1 and 4 | 3075 (99.6) | Yes/yes/yes/yes |
| Colivicchi 2002 | Atorvastatin 80 mg |
Usual care† | 81 | 12 | 1, 3, and 6 | 81 (100) | No/no/no/yes |
| ESTABLISH 2004 | Atorvastatin 20 mg |
Usual care† | 70 | 1 | 1, 4, and 6 | 69 (99) | No/no/no/no |
| A‐to‐Z 2004 | Simvastatin 40 to 80 mg |
Placebo | 4497 | 4 | 1 and 4|| | 4453 (99) | Yes/yes/yes/yes |
| Sakamoto 2005 | Any statin** | Usual care | 486 | 4 | 12 | 407 (84) | No/No/No/Yes |
| Macin 2005 | Atorvastatin 40 mg | Placebo | 90 | 1 | 1 | 89 (99) | No/Yes/Yes/No |
| Sato 2008 | Pravastatin 10 mg | Usual care† | 353 | 7 | 1 and 12 | 348 (99) | No/No/No/Yes |
| Shal'nev 2007 | Simvastatin 40 mg | Usual care | 110 | 1 | 6 | 108 (98) | No/No/No/No |
| FACS 2005 | Fluvastatin 80 mg | Placebo | 156 | 1 | 1, 3, and 12 | 156 (100) | No/Yes/Yes/No |
| Ren 2009 | Simvastatin 40 mg | Placebo | 86 | 3 | 1 | 86 (100) | No/Yes/Yes/Yes |
* 8 of 70 individuals received additionally cholestyramine or nicotinic acid.
† Individuals in the control group were allowed conventional medical treatment including lipid‐lowering therapy.
‡ All 164 individuals were followed‐up for 1 month, a subgroup of 77 (40/37) individuals with additional coronary angioplasty were followed‐up for 6 months.
§ These 824 individuals represent just the subgroup with unstable angina; the LIPS [Lescol Intervention Prevention Study]‐trial originally included another 853 individuals with stable angina.
|| After 4 months individuals in the control group received simvastatin 20mg.
ACS: acute coronary syndrome
Lipid‐lowering effects
The average weighted mean baseline LDL cholesterol level of included participants was 120 mg/dL (3.1 mmol/L) (range, 78 to 178 mg/dL (2.0 to 4.6 mmol/L)) (Table 4). Mean reduction of LDL cholesterol ranged from ‐15% to ‐53% and of total cholesterol from ‐9% to ‐37%, with higher reductions in trials using higher drug doses and/or more potent drugs. The effects on HDL cholesterol and triglycerides were less pronounced and inconsistent among trials (range of average change for HDL cholesterol, ‐9.5% to +13%; and for triglycerides, ‐28% to +10%).
3. Lipid values at baseline and changes during follow‐up.
| Trial (reference) | Intervention | Follow‐up* | Total cholesterol | LDL cholesterol | HDL cholesterol | Triglycerides |
|
Baseline mean, mg/dL (% mean change in difference between treatment and control groups)† |
||||||
| LAMIL 1997 | Pravastatin 10 to 20 mg | 3 months | 228 (‐13) | 158 (‐23) | 36 (+5.3) | NA |
| RECIFE 1999 | Pravastatin 40 mg | 1.5 months | 247 (‐21) | 164 (‐27) | 42 (+13) | 194 (‐21) |
| L‐CAD 2000‡ | Pravastatin 20 to 40 mg | 1 month | 237 (‐24) | 178 (‐25) | 32 (‐6.0) | 142 (±0) |
| PAIS 2001 | Pravastatin 40 mg | 3 months | 255 (‐23) | 176 (‐24) | 43 (+9.1) | 199 (‐13) |
| PTT 2002‡ | Pravastatin 40 mg | 1 month | 230 (‐12) | 133 (‐25) | 39 (+3.0) | 214 (‐5.8) |
| PACT 2004 | Pravastatin 20 to 40 mg | NA | 219 (NA) | NA | NA | NA |
| LIPS 2002 | Fluvastatin 80 mg | 1.5 months | 201 (‐28) | 131 (‐39) | 39 (‐2.0) | 155 (‐21) |
| FLORIDA 2002 | Fluvastatin 80 mg | 12 months | 207 (‐22) | 137 (‐31) | 46 (+3.3) | 146 (‐22) |
| MIRACL 2001 | Atorvastatin 80 mg | 1.5 months | 206 (‐37) | 124 (‐53) | 47 (±0) | 183 (‐28) |
| Colivicchi 2002‡ | Atorvastatin 80 mg | 2 months | 220 (‐9) | 131 (‐15) | 39 (+1.0) | 167 (‐13) |
| ESTABLISH 2004‡ | Atorvastatin 20 mg | 6 months | 191 (‐28) | 124 (‐41) | 44 (‐8.7) | 109 (+4.9) |
| A‐to‐Z 2004 | Simvastatin 40 to 80 mg | 1 month | 184 (‐33) | 112 (‐49) | 39 (+2.0) | 149 (‐22) |
| Sakamoto 2005 | Any statin | 3 months | 207 (‐12) | 134 (‐23) | 47 (+2.2) | 135 (‐5.2) |
| Macin 2005 | Atorvastatin 40 mg | 1 month | 194 (‐19) | 124 (‐30) | 37 (+11) | 189 (+0.8) |
| Sato 2008‡ | Pravastatin 10 mg | 9 months | 220 (NA) | 49 (NA) | 117 (NA) | 148 (NA) |
| Shal'nev 2007 | Simvastatin 40 mg | 0.5 months | 212 (‐29) | 131 (‐46) | 49 (‐9.5) | 146 (‐15) |
| FACS 2005 | Fluvastatin 80 mg | 1 month | 212 (‐26) | 135 (‐31) | 47 (‐4.1) | 162 (+10) |
| Ren 2009 | Simvastatin 40 mg | 1 month | 228 (‐23%) | 139 (‐31) | 40 (+9.6) | NA |
* Lipid values in individual trials were measured at different time points during follow‐up; we report those closest to the 4 months follow‐up date.
† Baseline lipid levels were defined as the average (mean) before treatment in intervention and control groups. The percentage of change for each trial was calculated as the difference in the mean change in lipid levels from baseline to follow‐up in the intervention and the control groups. To convert from mg/dL to mmol/L, multiply by 0.02586 for cholesterol and by 0.01129 for triglycerides.
‡ Individuals in the control group were allowed conventional medical treatment including lipid‐lowering therapy.
HDL: high‐density lipoprotein LDL: low‐density lipoprotein NA: not applicable
Risk of bias in included studies
Risk of bias varied among studies (see 'Risk of bias' summary, Figure 2). Concealed allocation of participants was reported only for three trials (de Lemos 2004; LIPS 2002; MIRACL 2001). Eight trials were reported to have assessed clinical outcomes in a blinded fashion (Colivicchi 2002; de Lemos 2004; FLORIDA 2002; LIPS 2002; MIRACL 2001; OACIS‐LIPID 2008; Sakamoto 2005; Ren 2009), and 11 trials reported blinding of caregivers and patients (de Lemos 2004; FACS 2010; FLORIDA 2002; LAMIL 1997; LIPS 2002; Macin 2005; MIRACL 2001; PACT 2004; PAIS 2001; RECIFE 1999; Ren 2009). Loss to follow‐up was under 2% in all but three studies (RECIFE 1999: 8%, Sakamoto 2005: 16%, LAMIL 1997: 19%). One trial was stopped early for benefit (Colivicchi 2002), with potential overestimation of treatment effects (Bassler 2010).
2.
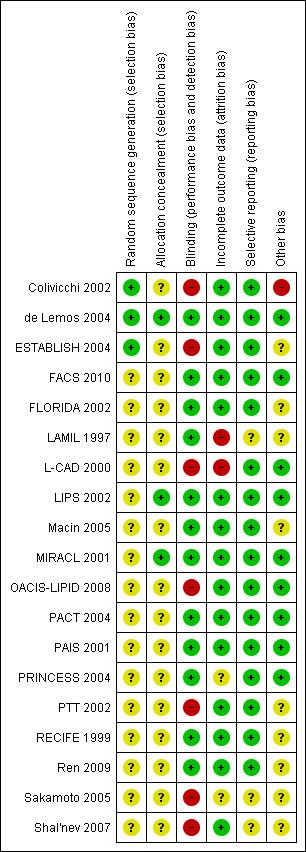
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
Statins versus placebo or no treatment
Analyses for publication bias indicated no evidence for such bias (Figure 3; Figure 4; Figure 5). Since there were only minimal differences in the estimates when calculating risk ratios or Peto odds ratios (fixed‐effect model), we reported outcomes as risk ratios (RR) only.
3.
Funnel plot of comparison: 1 Statins versus control at 1 month, outcome: 1.1 Combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths.
4.
Funnel plot of comparison: 2 Statins versus control at 4 months (3 to 6 months), outcome: 2.1 Combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths.
5.

Funnel plot of comparison: 3 Statins versus control at 12 months, outcome: 3.1 Combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths.
Combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and death from all causes
There was no significant difference in the primary combined outcome with early statin treatment in comparison to placebo or no treatment at one month (RR 0.93, 95% confidence interval (CI) 0.80 to 1.08; 13 studies, 13,484 patients) (Analysis 1.1), four months (RR 0.93, 95% CI 0.81 to 1.06; 11 studies, 9625 participants) (Analysis 2.1), or 12 months (RR 0.80, 95% CI 0.58 to 1.11; six studies, 2080 participants) (Analysis 3.1). We found no evidence of relevant heterogeneity among trials at any follow‐up time points (I² = 0%).
1.1. Analysis.

Comparison 1 Statins versus control at 1 month, Outcome 1 Combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths.
2.1. Analysis.
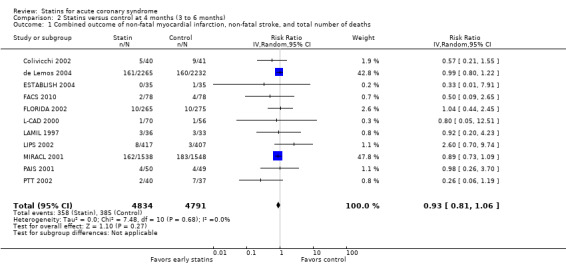
Comparison 2 Statins versus control at 4 months (3 to 6 months), Outcome 1 Combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths.
3.1. Analysis.

Comparison 3 Statins versus control at 12 months, Outcome 1 Combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths.
In sensitivity analyses, summary estimates of the primary endpoint at four months suggested smaller risk reductions for trials with higher methodological quality compared to trials that lacked a respective quality component. In trials with concealed allocation the summary RR for statins compared to control was 0.96 (95% CI 0.79 to 1.16; three studies, 8407 participants) (Analysis 5.1). For trials without concealed allocation the RR was 0.70 (95% CI 0.44 to 1.14; eight studies, 1218 participants) (Analysis 5.1). For trials with blinded outcome assessment the summary RR was 0.94 (95% CI 0.82 to 1.08; five studies, 9028 participants) (Analysis 5.3). For trials without blinded outcome assessment the RR was 0.60 (95% CI 0.30 to 1.22; six studies, 597 participants) (Analysis 5.3). For trials with blinding of patients and caregivers the summary RR was 0.95 (95% CI 0.82 to 1.09; seven studies, 9271 participants; Analysis 5.2). For trials without blinding of patients and caregivers the RR was 0.46 (95% CI 0.21 to 1.00; four studies, 354 participants) (Analysis 5.2).
5.1. Analysis.

Comparison 5 Statins versus control at 4 months: sensitivity analyses, Outcome 1 Allocation concealment ‐ combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths.
5.3. Analysis.
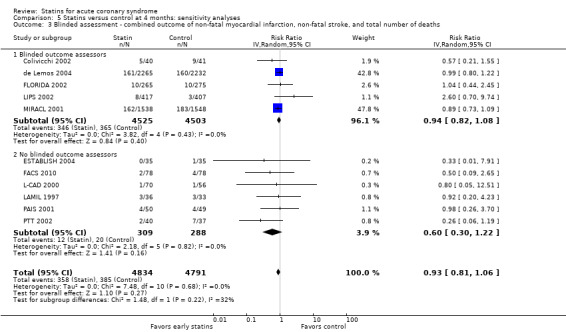
Comparison 5 Statins versus control at 4 months: sensitivity analyses, Outcome 3 Blinded assessment ‐ combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths.
5.2. Analysis.
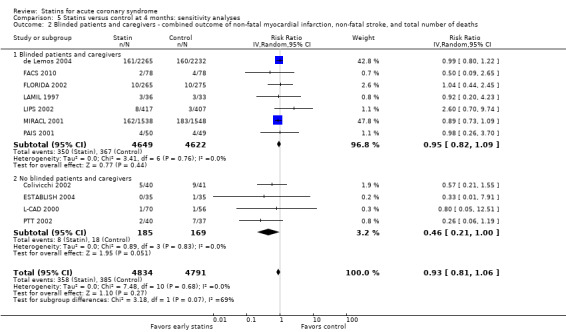
Comparison 5 Statins versus control at 4 months: sensitivity analyses, Outcome 2 Blinded patients and caregivers ‐ combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths.
We did not find different treatment effects for trials with initiation of statin therapy within three days versus up to 14 days (Analysis 5.14), or for trials using different types of statins (Analysis 5.15). Overall, we found moderate quality evidence that early statins provide no relevant risk reduction for the combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total deaths within the first four months following acute coronary syndrome (ACS) (see Table 1).
5.14. Analysis.

Comparison 5 Statins versus control at 4 months: sensitivity analyses, Outcome 14 Initiation of statins.
5.15. Analysis.

Comparison 5 Statins versus control at 4 months: sensitivity analyses, Outcome 15 Types of statins.
Death from all causes
We found no statistically significant difference in death from all causes with early statin therapy compared to placebo or usual care at one month (RR 0.78, 95% CI 0.59 to 1.03; 13 studies, 13,155 patients) (Analysis 1.2), four months (RR 0.90, 95% CI 0.70 to 1.14; 12 studies, 9733 participants) (Analysis 2.2), or 12 months (RR 0.68, 95% CI 0.39 to 1.20; six studies, 2080 participants) (Analysis 3.2). We found no evidence of relevant heterogeneity among trials at any follow‐up time points (I² = 0%).
1.2. Analysis.
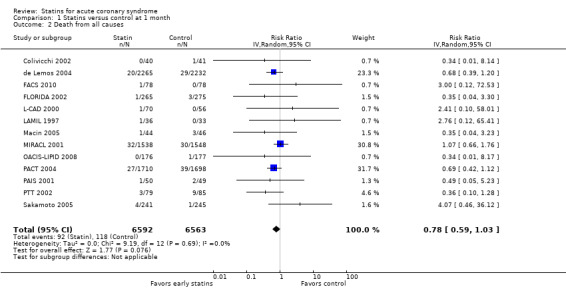
Comparison 1 Statins versus control at 1 month, Outcome 2 Death from all causes.
2.2. Analysis.
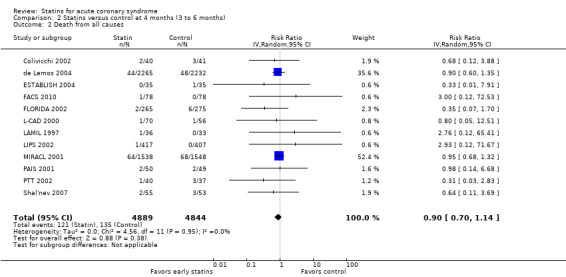
Comparison 2 Statins versus control at 4 months (3 to 6 months), Outcome 2 Death from all causes.
3.2. Analysis.
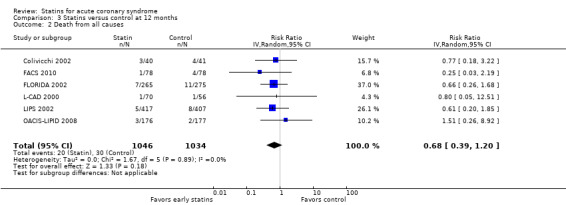
Comparison 3 Statins versus control at 12 months, Outcome 2 Death from all causes.
In sensitivity analyses, summary estimates of the primary endpoint at four months suggested smaller risk reductions for trials with higher methodological quality compared to trials that lacked a respective quality component. For trials with concealed allocation the RR for statins compared to control was 0.94 (95% CI 0.72 to 1.21; three studies, 8407 participants) (Analysis 5.4). For trials without concealed allocation the RR was 0.64 (95% CI 0.31 to 1.31; nine studies, 1326 participants) (Analysis 5.4). For trials with blinded outcome assessment the summary RR was 0.91 (95% CI 0.71 to 1.17; five studies, 9028 participants) (Analysis 5.6). For trials without blinded outcome assessment the RR was 0.77 (95% CI 0.31 to 1.90; seven studies, 705 participants) (Analysis 5.6). For trials with blinding of patients and caregivers the summary RR was 0.93 (95% CI 0.72 to 1.19; seven studies, 9271 participants) (Analysis 5.5). For trials without blinding of patients and caregivers the RR was 0.55 (95% CI 0.21 to 1.44; five studies, 462 participants) (Analysis 5.5). When we additionally included 4.5‐month data from 3605 patients with ACS from the PRINCESS study in a sensitivity analysis (Prevention of Ischemic Events by Early Treatment of Cerivastatin Study, PRINCESS 2004), the summary RR for all‐cause mortality was 0.95 (95% CI 0.78 to 1.17) (Analysis 5.10).
5.4. Analysis.

Comparison 5 Statins versus control at 4 months: sensitivity analyses, Outcome 4 Allocation concealment ‐ death from all causes.
5.6. Analysis.

Comparison 5 Statins versus control at 4 months: sensitivity analyses, Outcome 6 Blinded assessment ‐ death from all causes.
5.5. Analysis.
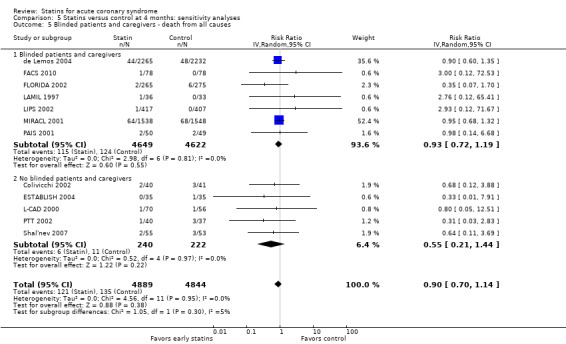
Comparison 5 Statins versus control at 4 months: sensitivity analyses, Outcome 5 Blinded patients and caregivers ‐ death from all causes.
5.10. Analysis.
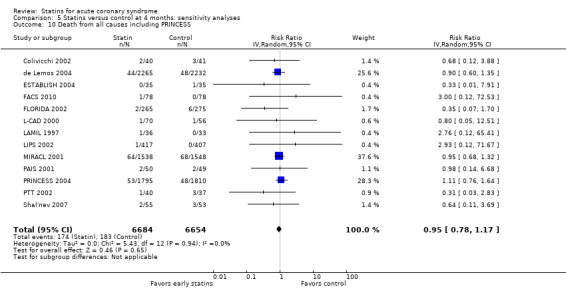
Comparison 5 Statins versus control at 4 months: sensitivity analyses, Outcome 10 Death from all causes including PRINCESS.
Overall, we found moderate quality evidence that early statins provide no relevant risk reduction for the combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total deaths within the first four months following ACS (see Table 1).
Death from cardiovascular causes
There was no statistically significant difference in deaths from cardiovascular causes with early statin treatment in comparison to placebo or no treatment at one month (RR 0.80, 95% CI 0.60 to 1.07; 10 studies, 12,387 participants) (Analysis 1.3), four months (RR 0.84, 95% CI 0.64 to 1.09; eight studies, 9273 participants) (Analysis 2.3), or 12 months (RR 0.55, 95% CI 0.28 to 1.09; five studies, 1954 participants) (Analysis 3.3). We found no evidence of relevant heterogeneity among trials at any follow‐up time points (I² = 0%).
1.3. Analysis.

Comparison 1 Statins versus control at 1 month, Outcome 3 Death from cardiovascular causes.
2.3. Analysis.

Comparison 2 Statins versus control at 4 months (3 to 6 months), Outcome 3 Death from cardiovascular causes.
3.3. Analysis.

Comparison 3 Statins versus control at 12 months, Outcome 3 Death from cardiovascular causes.
Fatal and non‐fatal myocardial infarction or reinfarction
We found no statistically significant difference in fatal and non‐fatal myocardial infarctions with early statin treatment in comparison to placebo or usual care at one month (RR 0.98, 95% CI 0.82 to 1.16; 12 studies, 13,074 participants) (Analysis 1.4), four months (RR 0.91, 95% CI 0.77 to 1.06; 10 studies, 9537 participants) (Analysis 2.4), or 12 months (RR 0.94, 95% CI 0.61 to 1.45; five studies, 1954 participants) (Analysis 3.4). We found no evidence of relevant heterogeneity among trials at any follow‐up time points (I² = 0%).
1.4. Analysis.
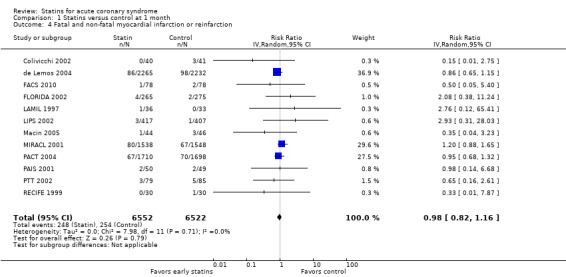
Comparison 1 Statins versus control at 1 month, Outcome 4 Fatal and non‐fatal myocardial infarction or reinfarction.
2.4. Analysis.

Comparison 2 Statins versus control at 4 months (3 to 6 months), Outcome 4 Fatal and non‐fatal myocardial infarction or reinfarction.
3.4. Analysis.
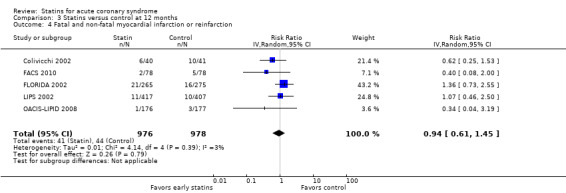
Comparison 3 Statins versus control at 12 months, Outcome 4 Fatal and non‐fatal myocardial infarction or reinfarction.
In a sensitivity analysis we included 4.5‐month data from 3605 patients with ACS from the PRINCESS study and found a summary RR for fatal and non‐fatal myocardial infarctions of 0.90 (95% CI 0.78 to 1.03) (Analysis 5.11).
5.11. Analysis.

Comparison 5 Statins versus control at 4 months: sensitivity analyses, Outcome 11 Fatal and non‐fatal myocardial infarction or reinfarction including PRINCESS.
Due to some concerns about risk of bias and imprecision of results we rated the quality of the available evidence that early statins provide no relevant risk reduction for fatal and non‐fatal myocardial infarction within the first four months following ACS as moderate (see Table 1).
Fatal and non‐fatal stroke
There was no statistically significant difference in fatal and non‐fatal strokes with early statin treatment in comparison to placebo or usual care at one month (RR 0.78, 95% CI 0.47 to 1.29; seven studies, 12,147 participants) (Analysis 1.5), four months (RR 0.72, 95% CI 0.45 to 1.16; seven studies, 8536 participants) (Analysis 2.5), or 12 months (RR 0.38, 95% CI 0.13 to 1.10; four studies, 1130 participants) (Analysis 3.5). We found no evidence of relevant heterogeneity among trials at all follow‐up time points (I² = 0%).
1.5. Analysis.

Comparison 1 Statins versus control at 1 month, Outcome 5 Fatal and non‐fatal stroke.
2.5. Analysis.

Comparison 2 Statins versus control at 4 months (3 to 6 months), Outcome 5 Fatal and non‐fatal stroke.
3.5. Analysis.

Comparison 3 Statins versus control at 12 months, Outcome 5 Fatal and non‐fatal stroke.
In a sensitivity analysis we included 4.5‐month data from 3605 patients with ACS from the PRINCESS study and found a summary RR for fatal and non‐fatal strokes of 0.79 (95% CI 0.52 to 1.18) (Analysis 5.12).
5.12. Analysis.

Comparison 5 Statins versus control at 4 months: sensitivity analyses, Outcome 12 Fatal and non‐fatal stroke including PRINCESS.
Revascularization procedures
There was no statistically significant difference for revascularization procedures (bypass grafts, angioplasty) with early statin treatment compared to placebo or usual care at one month (RR 1.00, 95% CI 0.86 to 1.16; 10 studies, 9668 participants) (Analysis 1.6), or four months (RR 0.92, 95% CI 0.78 to 1.08; nine studies, 9474 participants) (Analysis 2.6). However, at 12 months we found a reduced risk of revascularization procedures with early statins (RR 0.70, 95% CI 0.52 to 0.93; five studies, 1999 participants) (Analysis 3.6). The heterogeneity among treatment effects was low at one month (I² = 0%) and four months (I² = 21%), but moderate at 12 months (I² = 50%). This may be due to differences in settings and definitions of the endpoint of revascularization procedures among trials (Table 5).
1.6. Analysis.
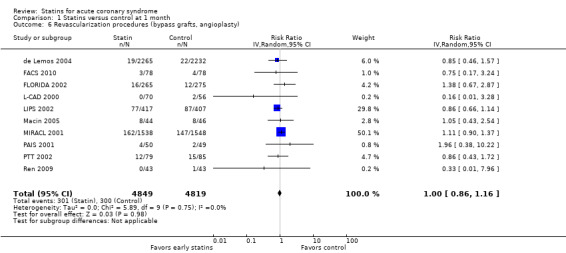
Comparison 1 Statins versus control at 1 month, Outcome 6 Revascularization procedures (bypass grafts, angioplasty).
2.6. Analysis.
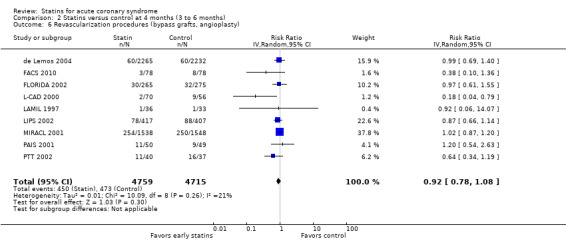
Comparison 2 Statins versus control at 4 months (3 to 6 months), Outcome 6 Revascularization procedures (bypass grafts, angioplasty).
3.6. Analysis.
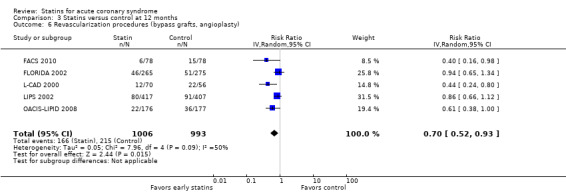
Comparison 3 Statins versus control at 12 months, Outcome 6 Revascularization procedures (bypass grafts, angioplasty).
4. Clinical endpoints in trials of early statin therapy versus control in acute coronary syndromes.
| Trial (reference) | Total death, MI, stroke, n (%) * | Total death, n (%) | Cardiovascular death, n (%) | Total MI, n (%) | Total stroke, n (%) | Revascularization (CABG/PCI), n (%) | Unstable angina, n (%) | Adverse events, n (%) | Acute heart failure | QOL | ||||||||||||||
| Rhabdomyolysis | CK > 10x ULN | ALT > 3x ULN | ||||||||||||||||||||||
| Statin | Control | Statin | Control | Statin | Control | Statin | Control | Statin | Control | Statin | Control | Statin | Control | Statin | Control | Statin | Control | Statin | Control | Statin | Control | Statin | Control | |
| LAMIL 1997 | NA | NA | 1 (2.8) | 0 (0) | 1 (2.8) | 0 (0) | 1 (2.8) | 0 (0.0) | NA | NA | NA | NA | NA | NA | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA |
| 3 (8.3) | 3 (9.1) | 1 (2.8) | 0 (0) | 1 (2.8) | 0 (0) | 3 (8.3) | 3 (9.1) | 0 (0) | 0 (0) | 1 (2.8) | 1 (3.0) | NA | NA | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA | |
| RECIFE 1999 | 0 (0) | 1 (3.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0.0) | 1 (3.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.3) | NA | NA |
| L‐CAD 2000 | 1 (1.4) | 0 (0) | 1 (1.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0) | 0 (0) | 2 (3.6) | NA | NA | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA |
| 1 (1.4) | 1 (1.9) | 1 (1.4) | 1 (1.9) | 0 (0) | 0 (0) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0) | 2 (2.9) | 9 (16.1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 1 (1.4) | 1 (1.9) | 1 (1.4) | 1 (1.9) | 0 (0) | 0 (0) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0) | 6 (8.6) | 12 (21.4) | 6 (8.6) | 10 (17.9) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| PAIS 2001 | 2 (4.0) | 3 (6.1) | 1 (2.0) | 2 (4.1) | 1 (2.0) | 2 (4.1) | 2 (4.0) | 2 (4.1) | 0 (0) | 1 (2.0) | 4 (8.0) | 2 (4.1) | 16 (32.0) † | 11 (22.4) † | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA |
| 4 (8.0) | 4 (8.2) | 2 (4.0) | 2 (4.1) | 2 (4.0) | 2 (4.1) | 4 (8.0) | 2 (4.1) | 0 (0) | 2 (4.1) | 11 (22.0) | 9 (18.4) | 24 (48.0) † | 21 (42.9) † | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA | |
| PTT 2002 | 4 (5.1) | 14 (16.5) | 3 (3.8) | 9 (10.6) | 3 (3.8) | 7 (8.2) | 3 (3.8) | 5 (5.9) | 2 (2.5) | 7 (8.2) | 12 (15.2) | 15 (17.6) | 11 (13.9) † | 25 (29.4) † | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA |
| 2 (5.0) | 7 (18.9) | 1 (2.5) | 3 (8.1) | 0 (0) | 3 (8.1) | 1 (2.5) | 6 (16.2) | 0 (0) | 1 (2.7) | 11 (27.5) | 16 (43.2) | 12 (30.0) † | 22 (59.4) † | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA | |
| PACT 2004 | 86 (5.0) | 96 (5.7) | 27 (1.6) | 39 (2.3) | 26 (1.5) | 34 (2.0) | 67 (3.9) | 70 (4.1) | 8 (0.5) | 10 (0.6) | NA | NA | 123 (7.2) | 126 (7.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (0.4) | 5 (0.3) | 28 (1.6) | 31 (1.8) | NA | NA |
| LIPS 2002 | 3 (0.7) | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (0.7) | 1 (0.2) | 0 (0) || | 0 (0) || | 77 (18.5) | 87 (21.4) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 8 (1.9) | 3 (0.7) | 1 (0.2) | 0 (0) | 1 (0.2) | 0 (0) | 7 (1.7) | 3 (0.7) | 0 (0) || | 0 (0) || | 78 (18.7) | 88 (21.6) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 11 (2.6) | 10 (2.5) | 3 (0.7) | 4 (1.0) | 2 (0.5) | 3 (0.7) | 8 (1.9) | 6 (1.5) | 0 (0) || | 0 (0) || | 79 (18.9) | 88 (21.6) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| FLORIDA 2002 | 5 (1.9) | 5 (1.8) | 1 (0.4) | 3 (1.1) | 1 (0.4) | 3 (1.1) | 4 (1.5) | 2 (0.7) | 0 (0) | 1 (0.4) | 16 (6.0) | 12 (4.4) | 6 (2.3) ¶ | 5 (1.8) ¶ | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 10 (3.8) | 10 (3.6) | 2 (0.8) | 6 (2.2) | 2 (0.8) | 6 (2.2) | 8 (3.0) | 6 (2.2) | 0 (0) | 1 (0.4) | 30 (11.3) | 32 (11.6) | 11 (4.2) ¶ | 9 (3.3) ¶ | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 14 (5.3) | 14 (5.1) | 3 (1.1) | 6 (2.2) | 3 (1.1) | 6 (2.2) | 11 (4.2) | 10 (3.6) | 0 (0) | 1 (0.4) | 36 (13.6) | 41 (14.9) | 14 (5.3) ¶ | 14 (5.1) ¶ | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 28 (10.6) | 28 (10.2) | 7 (2.6) | 11 (4.0) | 6 (2.3) | 11 (4.0) | 21 (7.9) | 16 (5.8) | 2 (0.8) | 5 (1.8) | 46 (17.4) | 51 (18.5) | 16 (6.0)¶ | 18 (6.5)¶ | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| MIRACL 2001 | 101 (6.6) | 96 (6.2) | 32 (2.1) | 30 (1.9) | 27 (1.8) | 21 (1.4) | 80 (5.2) | 67 (4.3) | 7 (0.5) | 10 (0.6) | 162 (10.5) | 147 (9.5) | 72 (4.7) # | 87 (5.6) # | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA | NA | NA |
| 162 (10.5) | 183 (11.8) | 64 (4.2) | 68 (4.4) | 51 (3.3) | 60 (3.9) | 118 (7.7) | 131 (8.5) | 12 (0.8) | 24 (1.6) | 254 (16.5) | 250 (16.1) | 95 (6.2) # | 130 (8.4) # | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 38 (2.5) | 9 (0.6) | 43 (2.8) | 40 (2.6) | NA | NA | |
| Colivicchi 2002 | 0 (0) | 3 (7.3) | 0 (0) | 1 (2.4) | 0 (0) | 1 (2.4) | 0 (0.0) | 3 (7.3) | 0 (0) | 0 (0) | 0 (0) ** | 0 (0) ** | 1 (2.5) # | 1 (2.4) # | 0 (0) | 0 (0) | 1 (2.5)## | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA |
| 5 (12.5) | 9 (22.0) | 2 (5.0) | 3 (7.3) | 1 (2.5) | 2 (4.9) | 3 (7.5) | 7 (17.1) | 1 (2.5) | 1 (2.4) | 0 (0) ** | 0 (0) ** | 2 (5.0) # | 3 (7.3) # | 0 (0) | 0 (0) | 1 (2.5)## | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA | |
| 7 (17.5) | 13 (31.7) | 3 (7.5) | 4 (9.8) | 2 (5.0) | 3 (9.8) | 5 (12.5) | 10 (24.4) | 1 (2.5) | 2 (4.9) | 0 (0) ** | 0 (0) ** | 2 (5.0) # | 6 (14.6) # | 0 (0) | 0 (0) | 1 (2.5)## | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA | |
| ESTABLISH 2004 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA |
| 0 (0) | 1 (2.9) | 0 (0) | 1 (2.9) | 0 (0) | 0 (0) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA | |
| 0 (0) | 1 (2.9) | 0 (0) | 1 (2.9) | 0 (0) | 0 (0) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0) | 8 (22.9) | 8 (22.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA | |
| A‐to‐Z 2004 | 99 (4.4) | 105 (4.7) | 20 (0.9) | 29 (1.3) | 20 (0.9) | 29 (1.3) | 86 (3.8) | 98 (4.4) | 10 (0.4) | 6 (0.3) | 19 (0.8) †† | 22 (1.0) †† | 27 (1.2) ‡‡ | 30 (1.3) ‡‡ | 3 (0.1) | 0 (0) | 0 (0) | 1 (0.04) | 1 (0.04) | 27 (1.2)§§ | 31 (1.4)§§ | NA | NA | NA |
| 161 (7.1) | 160 (7.2) | 44 (1.9) | 48 (2.2) | 42 (1.9) | 48 (2.2) | 130 (5.7) | 140 (6.3) | 16 (0.7) | 12 (0.5) | 60 (2.6) †† | 60 (2.7) †† | 56 (2.5) ‡‡ | 61 (2.7) ‡‡ | 3 (0.1) | 0 (0) | 7 (0.3) | 1 (0.04) | 13 (5.7) | 45 (2.0)§§ | 55 (2.5)§§ | 98 (5.0) | NA | NA | |
| Sakamoto 2005 | 4 (1.7) | 1 (0.4) | 4 (1.7) | 1 (0.4) | 4 (1.7) | 1 (0.4) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 12 (5.0) | 4 (1.6) | 6 (2.5) | 2 (0.8) | 6 (2.5) | 2 (0.8) | NA | NA | 3 (1.2) | 2 (0.8) | 18 (7.5) | 24 (9.8) | 6 (2.5) | 17 (6.9) | NA | NA | NA | NA | NA | NA | 1 (0.4) | 9 (3.7) | NA | NA | |
| Macin 2005 | 3 (6.8) | 5 (10.9) | 1 (2.3) | 3 (6.5) | 1 (2.3) | 3 (6.5) | 1 (2.3) | 3 (6.5) | 0 (0) | 0 (0) | 8 (18.2)¶ | 8 (17.4)¶ | 7 (1.6) | 8 (1.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (9.3) | 10 (21.7) | NA | NA |
| Sato 2008 | 0 (0) | 1 (0.6) | 0 | 1 (0.6) | 0 (0) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0) | 1 (0.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA |
| 4 (2.3) | 7 (4.0) | 3 (1.7) | 2 (1.1) | 1 (0.6) | 2 (1.1) | NA | NA | 0 (0) | 2 (1.1) | 22 (12.7) | 36 (20.6) | 5 (2.9) | 5 (2.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (2.8) | NA | NA | |
| Shal'nev 2007 | NA | NA | 2 (3.6) | 3 (5.5) | NA | NA | NA | NA | NA | NA | NA | NA | 4 (7.3) | 7 (12.7) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| FACS 2005 | 2 (2.6) | 2 (2.6) | 1 (1.3) | 0 (0) | 0 (0) | 0 (0) | 1 (1.3) | 2 (2.6) | 0 (0) | 0 (0) | 3 (3.8) | 4 (5.1) | 2 (2.6) | 6 (7.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA |
| 2 (2.6) | 4 (5.1) | 1 (1.3) | 0 (0) | 0 (0) | 0 (0) | 1 (1.3) | 3 (3.8) | 0 (0) | 1 (1.3) | 3 (3.8) | 8 (10.3) | 3 (3.8) | 12 (15.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA | |
| 4 (5.1) | 10 (12.8) | 1 (1.3) | 4 (5.1) | 0 (0) | 2 (2.6) | 2 (2.6) | 5 (6.4) | 1 (1.3) | 3 (3.8) | 6 (7.7) | 15 (19.2) | 6 (7.7) | 16 (20.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA | NA | NA | |
| Ren 2009 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0.0) | 0 (0.0) | NA | NA | 0 (0) | 1 (2.3) | NA | NA | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | NA |
* Combined primary endpoint; unique patients.
† Patients with “recurrent angina pectoris”.
‡ All 164 individuals were followed‐up for 1 month, a subgroup of 77 individuals with additional coronary angioplasty were followed‐up for 6 months.
§ These 824 individuals represent just the subgroup with unstable angina; the LIPS [Lescol Intervention Prevention Study]‐trial originally included another 853 individuals with stable angina.
|| Fatal strokes only; non‐fatal strokes were not recorded.
¶ Patients with “recurrent ischemia necessitating hospitalization”.
# Patients with “recurrent symptomatic myocardial ischemia with objective evidence and emergency hospitalization”.
** Individuals enrolled into the trial were not amenable for direct revascularization by coronary artery bypass grafting or percutaneous coronary intervention.
†† Revascularization procedures had to be urgent, occur more than 14 days after randomization, and were not allowed to be planned prior to enrollment.
‡‡ Patients with “readmission for acute coronary syndrome”.
§§ Patients with “new onset congestive heart failure requiring admission or initiation of heart failure medications”.
ALT: aminotransferase CABG: coronary artery bypass grafting
CK: creatin kinase MI: myocardial infarction NA: not applicable PCI: percutaneous coronary intervention QOL: quality of life
ULN: upper limit of normal values
Unstable angina
The RR for unstable angina with early statin therapy compared to placebo or no treatment at one month was 0.89 (95% CI 0.76 to 1.05; 10 studies, 12,181 participants) (Analysis 1.7), at four months the RR was 0.76 (95% CI 0.59 to 0.96; nine studies, 8770 participants) (Analysis 2.7), and at 12 months the RR was 0.61 (95% CI 0.33 to 1.12; four studies, 1130 participants) (Analysis 3.7). The heterogeneity among treatment effects was low at one month (I² = 0%), but we found moderate heterogeneity at four months (I² = 33%) and at 12 months (I² = 35%). This may be due to differences in the definition of the endpoint of unstable angina among trials (Table 5).
1.7. Analysis.

Comparison 1 Statins versus control at 1 month, Outcome 7 Unstable angina.
2.7. Analysis.

Comparison 2 Statins versus control at 4 months (3 to 6 months), Outcome 7 Unstable angina.
3.7. Analysis.
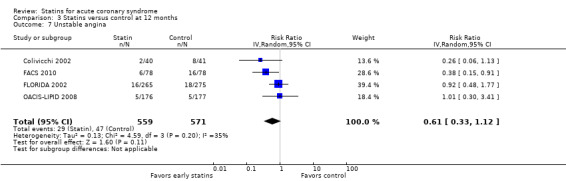
Comparison 3 Statins versus control at 12 months, Outcome 7 Unstable angina.
In sensitivity analyses, summary estimates for unstable angina at four months suggested smaller risk reductions for trials with higher methodological quality compared to trials that lacked a respective quality component. In trials with concealed allocation the summary RR for statins compared to control was 0.79 (95% CI 0.64 to 0.97; two studies, 7583 participants) (Analysis 5.7). For trials without concealed allocation the RR was 0.68 (95% CI 0.44 to 1.04; seven studies, 1187 participants) (Analysis 5.7). For trials with blinded outcome assessment the summary RR was 0.81 (95% CI 0.66 to 0.99; four studies, 8204 participants) (Analysis 5.9). For trials without blinded outcome assessment the RR was 0.59 (95% CI 0.35 to 1.00; five studies, 566 participants) (Analysis 5.9). For trials with blinding of patients and caregivers the summary RR was 0.85 (95% CI 0.64 to 1.14; five studies, 8378 participants) (Analysis 5.8). For trials without blinding of patients and caregivers the RR was 0.51 (95% CI 0.34 to 0.79; four studies, 392 participants) (Analysis 5.8).
5.7. Analysis.
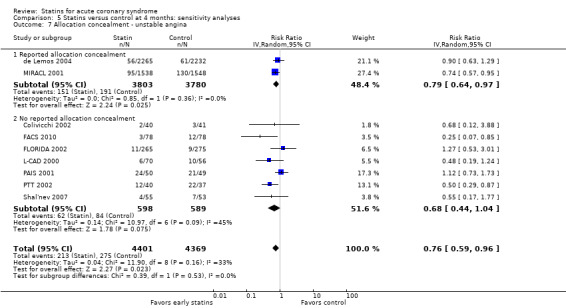
Comparison 5 Statins versus control at 4 months: sensitivity analyses, Outcome 7 Allocation concealment ‐ unstable angina.
5.9. Analysis.

Comparison 5 Statins versus control at 4 months: sensitivity analyses, Outcome 9 Blinded assessment ‐ unstable angina.
5.8. Analysis.

Comparison 5 Statins versus control at 4 months: sensitivity analyses, Outcome 8 Blinded patients and caregivers ‐ unstable angina.
When we additionally included 4.5‐month data from 3605 patients with ACS from the PRINCESS study in a sensitivity analysis (PRINCESS 2004), the summary RR for unstable angina was 0.78 (95% CI 0.65 to 0.95) (Analysis 5.13).
5.13. Analysis.

Comparison 5 Statins versus control at 4 months: sensitivity analyses, Outcome 13 Unstable angina including PRINCESS.
Overall, we found moderate quality evidence that early statin therapy leads to a relevant risk reduction of unstable angina within the first four months following ACS (see Table 1).
Acute heart failure
There was no statistically significant difference for acute (new or worsening) heart failure with early statin treatment compared to placebo or no treatment at one month (RR 0.85, 95% CI 0.63 to 1.14; five studies, 11,141 participants) (Analysis 1.8), four months (RR 0.86, 95% CI 0.65 to 1.15; two studies, 7583 participants) (Analysis 2.8), or at 12 months (RR 0.09, 95% CI 0.01 to 1.64; one study, 353 participants) (Analysis 3.8). The heterogeneity among treatment effects was low at one month (I² = 0%) and four months (I² = 0%); at 12 months there was only one study.
1.8. Analysis.

Comparison 1 Statins versus control at 1 month, Outcome 8 Acute heart failure.
2.8. Analysis.

Comparison 2 Statins versus control at 4 months (3 to 6 months), Outcome 8 Acute heart failure.
3.8. Analysis.

Comparison 3 Statins versus control at 12 months, Outcome 8 Acute heart failure.
Adverse events
Among all included trials, only three incidents of rhabdomyolysis were reported in patients treated with statins (0.04%); all occurred in the A‐to‐Z trial (RR 6.90, 95% CI 0.36 to 133.47; 4497 participants) (Analysis 4.1). There were nine individuals with myopathy (elevated creatinine kinase levels more than 10 times the upper limit of normal) in the statin groups versus one in the control groups (0.13% versus 0.015%); the summary RR for myopathy was significantly higher with statins than with control (RR 4.69, 95% CI 1.01 to 21.67; three studies, 4677 participants) (Analysis 4.2). All nine cases occurred beyond the first month of statin treatment and seven of the nine patients were treated with high‐dose simvastatin (80 mg/day). None of the nine patients died. The risk of elevated liver aminotransferase levels (ALT more than three times the upper limit of normal) was significantly higher in the early statin groups than the control groups (RR 2.49, 95% CI 1.16 to 5.32; five studies, 11,914 participants) (Analysis 4.3). The heterogeneity in the effects on elevated liver aminotransferase levels was moderate (I² = 48%). This may be due to differences in trial settings and included patients (Table 5). Due to serious concerns about the imprecision of the rhabdomyolysis results, we rated the available evidence as low quality (see Table 1).
4.1. Analysis.

Comparison 4 Statins versus control: adverse events, Outcome 1 Rhabdomyolysis.
4.2. Analysis.

Comparison 4 Statins versus control: adverse events, Outcome 2 Elevated CK > 10x upper‐limit of normal.
4.3. Analysis.

Comparison 4 Statins versus control: adverse events, Outcome 3 Elevated ALT > 3x upper‐limit of normal.
Patient‐perceived quality of life
None of the included trials reported patient‐perceived quality of life.
Discussion
Key findings
This systematic review of randomized controlled trials in over 14,000 patients with acute coronary syndrome (ACS) investigated whether early statin therapy compared to placebo or no treatment improves patient‐relevant outcomes shortly after ACS. The results of our meta‐analysis did not show a statistically significant reduction of the composite primary endpoint (death, myocardial infarction, or stroke) for patients treated early with statins at one month, four months, and 12 months following ACS. There was, however, a non‐significant trend towards risk reduction and this trend increased with time. There were non‐significant trends towards risk reductions for the secondary outcomes of total death, total myocardial infarction, total stroke, cardiovascular death, and acute heart failure at one month, four months, and 12 months following ACS. The only significant relative risk reductions were in unstable angina at four months (estimated relative risk reduction of 24%) and revascularization procedures at 12 months (estimated relative risk reduction of 30%) following ACS. There were few data available from included trials at 12 months. However, the vulnerable coronary situation following ACS usually stabilizes within three to four months and other studies have already shown significant risk reductions for hard clinical outcomes such as myocardial infarction, stroke, or death in patients with stable coronary heart disease (4S 1994; Briel 2004; LIPID Study Group 1998; Studer 2005). Our results at 12 months are compatible with these findings.
In terms of adverse events we found that the risk of elevated liver aminotransferase levels (ALT more than three times the upper limit of normal) was significantly higher in the early statin groups than the control groups, but serious events such as myopathy or rhabdomyolysis were rare (three reported incidents of rhabdomyolysis in patients treated early with simvastatin 80 mg (0.04%) and nine individuals with myopathy (elevated creatinine kinase levels more than 10 times the upper limit of normal) in statin‐treated patients (0.13%); seven of these nine individuals took simvastatin 80 mg).
Overall we rated the quality of evidence for all outcomes as moderate due to some concerns about risk of bias and imprecision of results (see Table 1), except for rhabdomyolysis for which we only found low quality evidence due to serious concerns about imprecision of results.
Why should effects on unstable angina be stronger than effects on myocardial infarction?
One could argue that statins might ameliorate coronary vascular endothelial function, but that doing so does not directly influence atherothrombosis. One might also posit that there actually are concordant effects on all of these endpoints (trends are all in a favorable direction with statins), but that the composite sample size and duration of observation in this group of trials are inadequate to ascertain an effect with sufficiently low type I error rate. Another important point is the biomarker methods used for ascertainment of myocardial infarction in many of these studies. In the late 1990s or even early 2000s, many sites continued to use CK‐MB or even total CK as the biomarker to detect myocardial injury. Multicenter trials generally do not specify one biomarker to define myocardial injury in endpoint events. Thus many endpoint events that would be associated with a small rise in troponin and today be categorized as acute myocardial infarction according to the current International Definition of Myocardial Infarction (Thygesen 2007), were likely to have been considered biomarker‐negative and categorized as unstable angina using the older and less sensitive biomarker methods prevalent during the conduct of the trials in question. Therefore, one might reasonably expect that had contemporary diagnostic criteria and methods been applied to the events in these trials, there might have been more events categorized as acute myocardial infarction (and fewer as unstable angina), affording greater power to detect an effect of early statin therapy on acute myocardial infarction.
Strengths and weaknesses
We have conducted an extensive literature search to retrieve all relevant eligible trials and collaborated with the investigators of the primary trials. This collaboration with experts in the field should minimize potential publication bias. In addition, formal testing indicated little evidence for such bias. In searching trial registries we found no evidence of ongoing eligible trials, which means that the trials gathered in this review may constitute the totality of the available evidence on the topic.
We were unable to include one small trial with 151 randomized individuals because the original investigators failed to clarify outcome events (Pedersen 2000). Two other trials including 3468 patients had a follow‐up of only one month (PACT 2004) and 1.5 months (RECIFE 1999). As a consequence, the power of our analysis at four months was compromised.
It may well be that early use of statins in ACS is associated with a beneficial effect on hard clinical outcomes such as total mortality, myocardial infarction, and stroke in the short term; summary estimates for all efficacy outcomes show a trend towards risk reduction with early statin therapy, but this meta‐analysis may lack the power to detect a significant risk reduction for hard outcomes. However, our sensitivity analyses indicated even smaller treatment effects when restricting the analysis to trials of adequate methodological quality, or when we additionally included secondary endpoint data from a large, prematurely terminated trial using cerivastatin in 3605 patients (PRINCESS 2004). To rule out effects of 10% risk reduction or less on our combined primary endpoint (death, myocardial infarction, and stroke), more than 34,000 patients with ACS would need to be randomized (Lachin 2000).
As expected, statins lowered low‐density lipoprotein (LDL) cholesterol levels more efficiently than placebo or usual care, and there were larger reductions in LDL cholesterol in trials using higher doses of statins. However, available data precluded adequate exploration of an association between clinical outcomes and the lipid‐lowering potency of different statin types and doses. It remains an open question whether potent statins at top doses provide clinical benefit that is not achieved with lower‐intensity statin therapy, since the only individual trials that have shown significant clinical benefit of statins in the early period following ACS are those that compared atorvastatin 80 mg with placebo (MIRACL 2001) or pravastatin 40 mg (Cannon 2004b). The possibility that high‐intensity, but not moderate‐intensity statin therapy is beneficial in the early period after ACS is supported by data from a meta‐analysis of five randomized controlled trials that compared high‐intensity with moderate‐intensity statin treatment in a total of 39,612 patients with coronary heart disease (Baigent 2010). Two of the five trials (A‐to‐Z (de Lemos 2004) and PROVE‐IT (Cannon 2004b)) included only ACS patients; two other included trials (SEARCH (Armitage 2010) and IDEAL (Pedersen 2005)) included some patients with recent ACS. The risk ratio (RR) for major vascular events among those treated with a high‐intensity regimen was 0.85 (95% confidence interval (CI) 0.82 to 0.89). In part, this finding led to the recent recommendation in the 2013 AHA/ACC Guideline for the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults that most patients with established coronary heart disease should be treated with an intensive statin regimen (Stone 2013).
Finally, this systematic review cannot address the benefit of the use of early statins in patients with ACS undergoing early percutaneous coronary intervention (PCI) of culprit lesions, since only a minority of patients in the included trials underwent PCI. Finally, as only one trial specified that it was unfunded (PTT 2002), and 11 trials specified direct industry sponsorship (de Lemos 2004; FACS 2010; FLORIDA 2002; L‐CAD 2000; LAMIL 1997; LIPS 2002; MIRACL 2001; PACT 2004; PAIS 2001; PRINCESS 2004; RECIFE 1999), the reader should be cautioned of a potential bias of interpretation of trial results (Als‐Nielsen 2003).
Comparison with other studies
Our findings contrast with results from published observational studies on the topic that suggest a lower risk of mortality with early statin therapy within one month following ACS (odds ratios as low as 0.4) (Aronow 2001; Fonarow 2005; Spencer 2004; Stenestrand 2001). Results from these observational studies, however, may be prone to bias due to survivor treatment selection (Glesby 1996), competing medical issues (Redelmeier 1998), or differences in unknown confounders between comparison groups (Laupacis 2004). Another large observational study that found no benefit of early statin initiation in a propensity and covariate‐adjusted analysis might have better captured potentially important confounders (Newby 2002). Our meta‐analysis of randomized controlled trials demonstrates that observational studies with insufficient control of confounders greatly overestimate the magnitude of effect from early statin therapy in ACS.
On first sight, our findings might appear to contrast with results from the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE‐IT) trial that randomized patients with ACS to atorvastatin 80 mg/day or pravastatin 40 mg/day (Cannon 2004a). In fact, however, there is no obvious discordance. In PROVE‐IT, Kaplan‐Meier curves of the primary composite endpoint appear to diverge as early as 30 days after ACS in favor of patients treated with atorvastatin, but the difference did not reach statistical significance until six months. It is important to note that the primary composite endpoint of PROVE‐IT comprised not only death, myocardial infarction, and stroke, but also recurrent unstable angina requiring re‐hospitalization and revascularization. If the more limited composite of death, myocardial infarction, and stroke is considered, there was no significant difference between the two treatment arms of PROVE‐IT. Unstable angina and revascularization were the most frequent events in PROVE‐IT and appeared to have driven the primary composite endpoint. Similarly, our meta‐analysis indicates that statins reduce the risk of unstable angina following ACS at four months. Although endpoints such as unstable angina depend at least in part on clinicians' judgment or action, and therefore may be less reliable (Freemantle 2003), our finding of a risk reduction for unstable angina of 19% (95% CI 1% to 34%) at four months in trials with blinded outcome assessment supports the validity of this result.
Authors' conclusions
Implications for practice.
Statins impact lipid profiles within days (Correia 2002), and in vitro studies show immediate inhibition of smooth muscle cell proliferation and stimulation of re‐endothelialization by statins (Walter 2004). These effects seem to translate into a reduction of unstable angina pectoris at four months following ACS, but not to the same extent into a reduction of death, myocardial infarction, or stroke.
In our meta‐analysis we considered only endpoint events that occurred during the period of randomized treatment. It is likely that the beneficial effects of statins are cumulative. In most of the landmark trials of statins in patients with chronic coronary heart disease a benefit of treatment was not evident until one to two years after randomization (4S 1994; LIPID Study Group 1998). Similarly, there appeared to be a delayed benefit of more intensive statin treatment, compared to less intensive statin treatment, in the late phase of the A‐to‐Z trial (de Lemos 2004). Therefore, some of the benefit of statin treatment in the period up to four months after ACS may only become manifest after four months.
This systematic review confirms that early treatment with statins in ACS can, in general, be considered safe and that the highest approved doses of potent statins may be considered in this context (Stone 2013). However, physicians and patients should pay close attention to muscle‐related symptoms, especially when maximum available doses ‐ in particular of simvastatin 80 mg ‐ are administered (Ara 2009), or when clinical risk factors for statin myopathy are present (e.g. advanced age, low body mass, impaired renal function).
There are concerns that when administered in clinical practice, long‐term adherence to statins among patients with recent onset of ACS is poor (Jackevicius 2002). Evidence from a small randomized trial and from observational studies suggests better adherence to statins when therapy is started in hospital shortly after an acute event (Nordmann 2000; Smith 2005).
In summary, initiation of statin therapy within 14 days following ACS produces favorable trends but does not significantly reduce death, myocardial infarction, or stroke up to four months after the index event. Early initiation of statin therapy does significantly reduce the occurrence of unstable angina at four months following ACS. Serious muscle toxicity was more common with early statin therapy than with placebo, but was rare and mostly limited to treatment with simvastatin 80 mg.
Implications for research.
A pooled analysis using individual patient data from eligible trials would be useful since it allows for pooled time‐to‐event analyses, powerful subgroup analyses, and multivariable regression analyses to clarify further the timing and mechanism by which statins confer cardiovascular benefits.
What's new
| Date | Event | Description |
|---|---|---|
| 21 April 2015 | Review declared as stable | No further evidence is expected to change the conclusion of this review. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 6, 2011
| Date | Event | Description |
|---|---|---|
| 8 April 2014 | New citation required but conclusions have not changed | The updated search found no new studies, so the review conclusions remain the same. |
| 8 April 2014 | New search has been performed | Search updated on 12 April 2013: no further eligible studies identified. |
| 9 August 2011 | Amended | Addition of a 'Summary of findings' (SoF) table. Previous tables erroneously published as SoF tables were moved to the 'Additional tables' section. |
Notes
None.
Acknowledgements
We are grateful to Peter L Thompson, MD, Michael A Blazing, MD, Gerrit‐Anne van Es, PhD, Meral Kayikcioglu, MD, Hans‐Richard Arntz, MD, Nic JGM Veeger, MSc, Jocelyn Dupuis, MD PhD, Shinya Okazaki, MD, R Scott Wright, MD, and the Cochrane Heart Group.
Appendices
Appendix 1. Search strategies 2010
Cochrane Central Register of Controlled Trials
1 exp Hydroxymethylglutaryl‐CoA Reductase Inhibitors/ 2 exp Hydroxymethylglutaryl CoA Reductases/ 3 (hydroxymethylglutaryl$ adj5 inhibitor$).mp. 4 (hmg coa$ adj5 inhibit$).mp. 5 statin$.mp. 6 simvastatin.mp. 7 fluvastatin.mp. 8 cerivastatin.mp. 9 lovastatin.mp. 10 pravastatin.mp. 11 atorvastatin.mp. 12 rosuvastatin.mp. 13 lipostat.mp. 14 lipitor.mp. 15 crestor.mp. 16 zocor.mp. 17 pravachol.mp. 18 baycol.mp. 19 lescol.mp. 20 mevacor.mp. 21 mevinolin.mp. 22 exp Anticholesteremic Agents/ or hypocholesterolemic agent:.mp. 23 or/1‐22 24 exp Myocardial Infarction/ 25 exp Coronary Thrombosis/ or coronary thrombosis.mp. 26 acute coronary.mp. 27 exp Angina, Unstable/ 28 Myocardial infarct$.mp. 29 heart infarct:.mp. 30 acs.mp. 31 ami.mp. 32 (coronary adj3 syndrome$).mp. 33 acute angina.mp. 34 (unstable adj3 angina).mp. 35 unstable coronary.mp. 36 or/24‐35 37 36 and 23
Ovid MEDLINE
1 exp Hydroxymethylglutaryl‐CoA Reductase Inhibitors/ 2 exp Hydroxymethylglutaryl CoA Reductases/ 3 (hydroxymethylglutaryl$ adj5 inhibitor$).mp. 4 (hmg coa$ adj5 inhibit$).mp. 5 statin$.mp. 6 simvastatin.mp. 7 fluvastatin.mp. 8 cerivastatin.mp. 9 lovastatin.mp. 10 pravastatin.mp. 11 atorvastatin.mp. 12 rosuvastatin.mp. 13 lipostat.mp. 14 lipitor.mp. 15 crestor.mp. 16 zocor.mp. 17 pravachol.mp. 18 baycol.mp. 19 lescol.mp. 20 mevacor.mp. 21 mevinolin.mp. 22 exp Anticholesteremic Agents/ 23 or/1‐22 24 exp Myocardial Infarction/ 25 exp Coronary Thrombosis/ 26 acute coronary.mp. 27 exp Angina, Unstable/ 28 Myocardial infarct$.mp. 29 heart infarct:.mp. 30 acs.mp. 31 ami.mp. 32 (coronary adj3 syndrome$).mp. 33 acute angina.mp. 34 (unstable adj3 angina).mp. 35 unstable coronary.mp. 36 or/24‐35 37 36 and 23 38 randomized controlled trial$.mp. 39 randomized controlled trial.pt. 40 double‐blind method/ 41 single‐blind method/ 42 controlled clinical trial.pt. 43 ((singl$ or double$ or trebl$ or tripl$) adj (blind$ or mask$)).mp. 44 or/38‐43 45 clinical trials.pt. or comparative study/ or follow‐up studies/ or comparative study.pt. 46 prospective studies/ or 45 47 (random: adj5 (controlled or clinical)).mp. 48 random$.mp. 49 46 and (47 or 48) 50 49 or 44 51 50 and 37 52 animals/ not humans/ 53 51 not 52
EMBASE
1 exp Hydroxymethylglutaryl Coenzyme a Reductase Inhibitor/ 2 exp Hydroxymethylglutaryl Coenzyme a Reductase/ 3 (hydroxymethylglutaryl$ adj5 inhibitor$).mp. 4 (hmg coa$ adj5 inhibit$).mp. 5 statin$.mp. 6 simvastatin.mp. 7 fluvastatin.mp. 8 cerivastatin.mp. 9 lovastatin.mp. 10 pravastatin.mp. 11 atorvastatin.mp. 12 rosuvastatin.mp. 13 lipostat.mp. 14 lipitor.mp. 15 crestor.mp. 16 zocor.mp. 17 pravachol.mp. 18 baycol.mp. 19 lescol.mp. 20 mevacor.mp. 21 mevinolin.mp. 22 Anticholesteremic agents.mp. or Hypocholesterolemic Agent/ 23 or/1‐22 24 exp Heart Infarction/ 25 coronary thrombosis.mp. or exp Coronary Artery Thrombosis/ 26 acute coronary.mp. 27 exp Unstable Angina Pectoris/ 28 Myocardial infarct$.mp. 29 heart infarct:.mp. 30 acs.mp. 31 ami.mp. 32 (coronary adj3 syndrome$).mp. 33 acute angina.mp. 34 (unstable adj3 angina).mp. 35 unstable coronary.mp. 36 or/24‐35 37 Randomized Controlled Trial/ 38 double‐blind method/ 39 single‐blind method/ 40 randomized controlled trial:.mp. 41 ((singl$ or double$ or trebl$ or tripl$) adj (blind$ or mask$)).mp. 42 controlled clinical trial/ 43 or/37‐42 44 Clinical Trial/ 45 exp comparative study/ 46 follow up/ 47 prospective study/ 48 or/44‐47 49 random:.mp. 50 (random: adj5 (controlled or clinical)).mp. 51 50 or 49 52 51 and 48 53 52 or 43 54 53 and 36 and 23 55 nonhuman/ not human/ 56 55 and 54 57 54 not 56
EBSCO host CINAHL
S58 S57 and S36 S57 S55 or S56 S56 S49 or S48 or S47 or S46 or S45 or S44 or S43 or S42 or S41 or S40 or S39 or S38 or S37 S55 S54 and S52 S54 S53 or S52 or S50 S53 PT clinical trial S52 "random*" S51 (MH "Prospective Studies+") S50 (MH "Comparative Studies") S49 "controlled clinical trial*" S48 TX trebl* w1 mask* S47 TX tripl* w1 mask* S46 TX double* w1 mask* S45 TX singl* w1 mask* S44 "singl* w1 mask*" S43 "doubl* w1 mask*" S42 doubl* w1 blind* S41 TX tripl* w1 blind* S40 TX trebl* w1 blind* S39 TX singl* w1 blind* S38 TX randomized controlled trial* S37 (MH "Clinical Trials+") S36 S35 and S22 S35 S34 or S33 or S32 or S31 or S30 or S29 or S28 or S27 or S26 or S25 or S24 or S23 S34 TX unstable coronary S33 TX unstable n3 angina S32 TX acute angina S31 TX coronary n3 syndrome* S30 TX ami S29 TX acs S28 TX Myocardial infarct* S27 (MH "angina, unstable+") S26 (MH "angina unstable+") S25 TX acute coronary S24 (MH "coronary thrombosis+") S23 (MH "Myocardial Infarction+") S22 S21 or S20 or S19 or S18 or S17 or S16 or S15 or S14 or S13 or S12 or S11 or S10 or S9 or S8 or S7 or S6 or S5 or S4 or S3 or S2 or S1 S21 TX Anticholesteremic S20 TX mevinolin S19 TX mevacor S18 TX lescol S17 TX baycol S16 TX Pravachol S15 TX zocor S14 TX crestor S13 TX lipitor S12 TX lipostat S11 TX rosuvastatin S10 TX atorvastatin S9 TX pravastatin S8 TX lovastatin S7 TX cerivastatin S6 TX fluvastatin S5 TX simvastatin S4 TX statin* S3 TX hmg coa* N5 inhibit* S2 TX hydroxymethylglutaryl* N5 inhibitor* S1 (MH "Statins+")
Appendix 2. Search strategies 2013
The RCT filter for MEDLINE is the Cochrane sensitivity‐maximizing RCT filter and for EMBASE the terms as recommended in the Cochrane Handbook for Systematic Reviews of Interventions have been applied. For both the reference is Lefebvre 2011.
CENTRAL
#1 MeSH descriptor: [Hydroxymethylglutaryl‐CoA Reductase Inhibitors] explode all trees #2 hydroxymethylglutaryl* #3 HMG‐CoA* #4 statin or statins #5 atorvastatin #6 cerivastatin #7 fluvastatin #8 lovastatin #9 pravastatin #10 simvastatin #11 lipitor #12 baycol #13 lescol #14 mevacor #15 altocor #16 pravachol #17 lipostat #18 zocor #19 mevinolin #20 compactin #21 fluindostatin #22 rosuvastatin #23 dalvastatin #24 cranoc #25 canef #26 locol #27 lochol #28 leucol #29 lescol #30 monacolin #31 medostatin #32 mevinacor #33 livalo #34 pitava #35 pitavastatin #36 pravasin #37 mevalotin #38 gerosim #39 lipex #40 zenas #41 crestor #42 meglutol #43 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 #44 #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 #45 #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 #46 #43 or #44 or #45 #47 MeSH descriptor: [Myocardial Infarction] explode all trees #48 MeSH descriptor: [Acute Coronary Syndrome] this term only #49 MeSH descriptor: [Coronary Thrombosis] this term only #50 coronary next thrombosis #51 acute next coronary #52 MeSH descriptor: [Angina, Unstable] explode all trees #53 myocardial next infarct* #54 heart next infarct* #55 acs #56 ami #57 coronary near/3 syndrome* #58 acute next angina #59 unstable near/3 angina #60 unstable next coronary #61 #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 #62 #46 and #61 from 2010 to 2013
MEDLINE OVID
1. exp Anticholesteremic Agents/ 2. exp Hydroxymethylglutaryl CoA Reductases/ 3. (hydroxymethylglutaryl* adj5 inhibitor*).mp. 4. (hmg‐coa* adj5 statin*).mp. 5. (hmg‐coa* adj5 inhibit*).mp. 6. anticholesteremic agent*.mp. 7. hypocholesterolemic agent*.mp. 8. 3‐hydroxy‐3‐methylpentanedioic acid.mp. 9. beta‐hydroxy‐beta‐methylglutarate.mp. 10. 3‐hydroxy‐3‐methylglutaric acid.mp. 11. statin*.mp. 12. (altoc?r or altoprev or artein or atorvastatin).mp. 13. (baycol or bristacol or "bay w 6228" or "bay w6228").mp. 14. (canef or cerivastatin or certa or compactin or cranoc or crestor or ci‐981 or ci981 or cs‐500 or cs500 or cs‐514 or cs514).mp. 15. (dalvastatin or denan).mp. 16. (elisor or epatostantin or eptastatin* or epistatin or fluindostatin or fluvastatin or gerosim or itavastatin).mp. 17. (lescol or leucol or lipemol or lipitor or lipibec or liplat or lipex or lipobay or lipovas or lipostat or livalo or loc?ol or lodales or lovacol or lovastatin or l‐654969 or l‐644128 or l644128).mp. 18. (mevastatin or mevastin or mevinolin or mona?olin or methylcompactin or mk‐803 or mk803 or mk‐0803 or mk0803 or msd‐803 or mevacor or mk‐733 or mk733 or meglutol or mevalotin or mevinacor or medostatin or ml‐236b or ml236b or medipo).mp. 19. (nk‐104 or nk104 or nks‐104 or nks104 or nisvastatin or neolipid).mp. 20. (pravastatin or prareduct or pravachol or pravacol or pravasin* or pitavastatin or pitava or pravachol).mp. 21. (rms‐431 or rms431 or ribar or rivastatin or rosuvastatin or RG‐12561).mp. 22. (sanaprav or selektine or simvastatin or sinvacor or s?nvinolin or sortis or sq‐31000 or sq31000 sq‐31,000 or sq31,000 or sri‐62320 or sri62320 or s‐4522 or s4522).mp. 23. (tahor or torvast).mp. 24. (vast?n or xu‐62320 or xu62320 or ym‐548 or ym548 or zarator or zenas or zocor? or zd‐4522 or zd4522).mp. 25. or/1‐24 26. Acute Coronary Syndrome/ 27. exp Myocardial Infarction/ 28. exp Coronary Thrombosis/ 29. coronary thrombosis.tw. 30. acute coronary.tw. 31. exp Angina, Unstable/ 32. myocardial infarct*.tw. 33. heart infarct*.tw. 34. acs.tw. 35. ami.tw. 36. (coronary adj3 syndrome*).tw. 37. acute angina.tw. 38. (unstable adj3 angina).tw. 39. unstable coronary.tw. 40. or/26‐39 41. 25 and 40 42. randomized controlled trial.pt. 43. controlled clinical trial.pt. 44. randomized.ab. 45. placebo.ab. 46. clinical trials as topic.sh. 47. randomly.ab. 48. trial.ti. 49. 42 or 43 or 44 or 45 or 46 or 47 or 48 50. exp animals/ not humans.sh. 51. 49 not 50 52. 41 and 51 53. ((2010* or 2011* or 2012* or 2013*) not (201001* or "20100201")).ed. 54. 52 and 53
EMBASE OVID
1. (hydroxymethylglutaryl* adj5 inhibitor*).mp. 2. (hmg‐coa* adj5 statin*).mp. 3. (hmg‐coa* adj5 inhibit*).mp. 4. anticholesteremic agent*.mp. 5. hypocholesterolemic agent*.mp. 6. 3‐hydroxy‐3‐methylpentanedioic acid.mp. 7. beta‐hydroxy‐beta‐methylglutarate.mp. 8. 3‐hydroxy‐3‐methylglutaric acid.mp. 9. statin*.mp. 10. (altoc?r or altoprev or artein or atorvastatin).mp. 11. (baycol or bristacol or "bay w 6228" or "bay w6228").mp. 12. (canef or cerivastatin or certa or compactin or cranoc or crestor or ci‐981 or ci981 or cs‐500 or cs500 or cs‐514 or cs514).mp. 13. (dalvastatin or denan).mp. 14. (elisor or epatostantin or eptastatin* or epistatin or fluindostatin or fluvastatin or gerosim or itavastatin).mp. 15. (lescol or leucol or lipemol or lipitor or lipibec or liplat or lipex or lipobay or lipovas or lipostat or livalo or loc?ol or lodales or lovacol or lovastatin or l‐654969 or l‐644128 or l644128).mp. 16. (mevastatin or mevastin or mevinolin or mona?olin or methylcompactin or mk‐803 or mk803 or mk‐0803 or mk0803 or msd‐803 or mevacor or mk‐733 or mk733 or meglutol or mevalotin or mevinacor or medostatin or ml‐236b or ml236b or medipo).mp. 17. (nk‐104 or nk104 or nks‐104 or nks104 or nisvastatin or neolipid).mp. 18. (pravastatin or prareduct or pravachol or pravacol or pravasin* or pitavastatin or pitava or pravachol).mp. 19. (rms‐431 or rms431 or ribar or rivastatin or rosuvastatin or RG‐12561).mp. 20. (tahor or torvast).mp. 21. exp hypocholesterolemic agent/ 22. hydroxymethylglutaryl coenzyme A reductase/ 23. or/1‐22 24. exp heart infarction/ 25. exp acute coronary syndrome/ 26. exp coronary artery thrombosis/ 27. coronary thrombosis.tw. 28. acute coronary.tw. 29. exp unstable angina pectoris/ 30. Myocardial infarct*.tw. 31. heart infarct*.tw. 32. acs.tw. 33. ami.tw. 34. (coronary adj3 syndrome*).tw. 35. acute angina.tw. 36. (unstable adj3 angina).tw. 37. unstable coronary.tw. 38. or/24‐37 39. 23 and 38 40. random$.tw. 41. factorial$.tw. 42. crossover$.tw. 43. cross over$.tw. 44. cross‐over$.tw. 45. placebo$.tw. 46. (doubl$ adj blind$).tw. 47. (singl$ adj blind$).tw. 48. assign$.tw. 49. allocat$.tw. 50. volunteer$.tw. 51. crossover procedure/ 52. double blind procedure/ 53. randomized controlled trial/ 54. single blind procedure/ 55. 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 56. (animal/ or nonhuman/) not human/ 57. 55 not 56 58. 39 and 57 59. ((2010* or 2011* or 2012* or 2013*) not ("201001" or "201002" or "201003" or "201004")).em. 60. 58 and 59 61. limit 60 to embase
CINAHL
S26 S24 AND S25 S25 EM 2010‐2013 S24 S9 AND S23 S23 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 S22 "unstable coronary" S21 unstable N3 angina S20 "acute angina" S19 coronary N3 syndrome* S18 acs or ami S17 "myocardial infarct*" S16 "myocardial infarct*" S15 (MH "Angina, Unstable") S14 "acute coronary" S13 "coronary thrombosis" S12 (MH "Coronary Thrombosis") S11 (MH "Acute Coronary Syndrome") S10 (MH "Myocardial Infarction+") S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 S8 livalo or pitava or pitavastatin or pravasin or mevalotin or gerosim or lipex or zenas or crestor or meglutol S7 locol or lochol or leucol or lescol or monacolin or medostatin or mevinacor S6 fluindostatin or rosuvastatin or dalvastatin or cranoc or canef S5 pravachol or lipostat or zocor or mevinolin or compactin S4 simvastatin or lipitor or baycol or lescol or mevacor or altocor S3 atorvastatin or atorvastatin or fluvastatin or lovastatin or pravastatin S2 statin* or hydroxymethylglutaryl* or HMG‐CoA* S1 (MH "Statins+")
Data and analyses
Comparison 1. Statins versus control at 1 month.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths | 13 | 13484 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.80, 1.08] |
| 2 Death from all causes | 13 | 13155 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.59, 1.03] |
| 3 Death from cardiovascular causes | 10 | 12387 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.60, 1.07] |
| 4 Fatal and non‐fatal myocardial infarction or reinfarction | 12 | 13074 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.82, 1.16] |
| 5 Fatal and non‐fatal stroke | 7 | 12147 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.47, 1.29] |
| 6 Revascularization procedures (bypass grafts, angioplasty) | 10 | 9668 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.86, 1.16] |
| 7 Unstable angina | 10 | 12181 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.76, 1.05] |
| 8 Acute heart failure | 5 | 11141 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.63, 1.14] |
Comparison 2. Statins versus control at 4 months (3 to 6 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths | 11 | 9625 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.81, 1.06] |
| 2 Death from all causes | 12 | 9733 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.70, 1.14] |
| 3 Death from cardiovascular causes | 8 | 9273 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.64, 1.09] |
| 4 Fatal and non‐fatal myocardial infarction or reinfarction | 10 | 9537 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.77, 1.06] |
| 5 Fatal and non‐fatal stroke | 7 | 8536 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.45, 1.16] |
| 6 Revascularization procedures (bypass grafts, angioplasty) | 9 | 9474 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.78, 1.08] |
| 7 Unstable angina | 9 | 8770 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.59, 0.96] |
| 8 Acute heart failure | 2 | 7583 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.65, 1.15] |
Comparison 3. Statins versus control at 12 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths | 6 | 2080 | Risk Ratio (IV, Random, 95% CI) | 0.80 [0.58, 1.11] |
| 2 Death from all causes | 6 | 2080 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.39, 1.20] |
| 3 Death from cardiovascular causes | 5 | 1954 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.28, 1.09] |
| 4 Fatal and non‐fatal myocardial infarction or reinfarction | 5 | 1954 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.61, 1.45] |
| 5 Fatal and non‐fatal stroke | 4 | 1130 | Risk Ratio (IV, Random, 95% CI) | 0.38 [0.13, 1.10] |
| 6 Revascularization procedures (bypass grafts, angioplasty) | 5 | 1999 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.52, 0.93] |
| 7 Unstable angina | 4 | 1130 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.33, 1.12] |
| 8 Acute heart failure | 1 | 353 | Risk Ratio (IV, Random, 95% CI) | 0.09 [0.01, 1.64] |
Comparison 4. Statins versus control: adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rhabdomyolysis | 1 | 4497 | Risk Ratio (IV, Random, 95% CI) | 6.90 [0.36, 133.47] |
| 2 Elevated CK > 10x upper‐limit of normal | 3 | 4677 | Risk Ratio (IV, Random, 95% CI) | 4.69 [1.01, 21.67] |
| 3 Elevated ALT > 3x upper‐limit of normal | 5 | 11914 | Risk Ratio (IV, Random, 95% CI) | 2.49 [1.16, 5.32] |
Comparison 5. Statins versus control at 4 months: sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Allocation concealment ‐ combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths | 11 | 9625 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.81, 1.06] |
| 1.1 Reported allocation concealment | 3 | 8407 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.79, 1.16] |
| 1.2 No reported allocation concealment | 8 | 1218 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.44, 1.14] |
| 2 Blinded patients and caregivers ‐ combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths | 11 | 9625 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.81, 1.06] |
| 2.1 Blinded patients and caregivers | 7 | 9271 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.82, 1.09] |
| 2.2 No blinded patients and caregivers | 4 | 354 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.21, 1.00] |
| 3 Blinded assessment ‐ combined outcome of non‐fatal myocardial infarction, non‐fatal stroke, and total number of deaths | 11 | 9625 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.81, 1.06] |
| 3.1 Blinded outcome assessors | 5 | 9028 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.82, 1.08] |
| 3.2 No blinded outcome assessors | 6 | 597 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.30, 1.22] |
| 4 Allocation concealment ‐ death from all causes | 12 | 9733 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.70, 1.14] |
| 4.1 Reported allocation concealment | 3 | 8407 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.72, 1.21] |
| 4.2 No reported allocation concealment | 9 | 1326 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.31, 1.31] |
| 5 Blinded patients and caregivers ‐ death from all causes | 12 | 9733 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.70, 1.14] |
| 5.1 Blinded patients and caregivers | 7 | 9271 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.72, 1.19] |
| 5.2 No blinded patients and caregivers | 5 | 462 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.21, 1.44] |
| 6 Blinded assessment ‐ death from all causes | 12 | 9733 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.70, 1.14] |
| 6.1 Blinded outcome assessors | 5 | 9028 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.71, 1.17] |
| 6.2 No blinded outcome assessors | 7 | 705 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.31, 1.90] |
| 7 Allocation concealment ‐ unstable angina | 9 | 8770 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.59, 0.96] |
| 7.1 Reported allocation concealment | 2 | 7583 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.64, 0.97] |
| 7.2 No reported allocation concealment | 7 | 1187 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.44, 1.04] |
| 8 Blinded patients and caregivers ‐ unstable angina | 9 | 8770 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.59, 0.96] |
| 8.1 Blinded patients and caregivers | 5 | 8378 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.64, 1.14] |
| 8.2 No blinded patients and caregivers | 4 | 392 | Risk Ratio (IV, Random, 95% CI) | 0.51 [0.34, 0.79] |
| 9 Blinded assessment ‐ unstable angina | 9 | 8770 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.59, 0.96] |
| 9.1 Blinded outcome assessors | 4 | 8204 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.66, 0.99] |
| 9.2 No blinded outcome assessors | 5 | 566 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.35, 1.00] |
| 10 Death from all causes including PRINCESS | 13 | 13338 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.78, 1.17] |
| 11 Fatal and non‐fatal myocardial infarction or reinfarction including PRINCESS | 11 | 13142 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.78, 1.03] |
| 12 Fatal and non‐fatal stroke including PRINCESS | 8 | 12141 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.52, 1.18] |
| 13 Unstable angina including PRINCESS | 10 | 12375 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.65, 0.95] |
| 14 Initiation of statins | 11 | 9625 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.81, 1.06] |
| 14.1 Statin initiated within 3 days | 6 | 1295 | Risk Ratio (IV, Random, 95% CI) | 0.82 [0.41, 1.64] |
| 14.2 Initiation of statins up to 14 days | 5 | 8330 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.81, 1.07] |
| 15 Types of statins | 11 | 9625 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.81, 1.06] |
| 15.1 Pravastatin | 4 | 371 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.29, 1.45] |
| 15.2 Fluvastatin | 3 | 1520 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.54, 2.53] |
| 15.3 Atorvastatin | 3 | 3237 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.72, 1.06] |
| 15.4 Simvastatin | 1 | 4497 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.80, 1.22] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Colivicchi 2002.
| Methods |
Design: single‐center randomized controlled trial Setting: 1 center in Italy Patient recruitment: January 1999 to July 2001 Blinding: outcome adjudicators Intention‐to‐treat: yes Follow‐up period: 12 months Lost to follow‐up: 0 |
|
| Participants |
Number randomized: statin 40, control 41 Mean age (SD) in years: statin 69 (14), control 68 (14) Men, n (%): statin 23 (58%), control 24 (59%) Diabetes, n (%): statin 22 (55%), control 24 (58%) Hypertension, n (%): statin 35 (87%), control 37 (90%) Current smoker, n (%): unspecified Prior myocardial infarction, n (%): statin 34 (85%), control 35 (85%) Index event, n (%):
Inclusion criteria: (1) angiographic evidence of severe and diffuse coronary artery disease, that was not amenable to direct revascularization by coronary artery bypass grafting or percutaneous transluminal coronary angioplasty, as determined by a cardiac surgeon and an interventional cardiologist during the index admission; (2) objective evidence of symptomatic reversible myocardial ischemia (0.1 mV ST‐segment depression on the electrocardiogram) at a low exercise workload while receiving medical treatment (2 antianginal medications at maximal tolerated doses), as assessed by treadmill ergometry (Bruce's protocol) before discharge; and (3) left ventricular ejection fraction 35% Exclusion criteria: presence of congestive heart failure, the need for continuous use of intravenous antianginal medications, and the presence of any major concurrent illness |
|
| Interventions | Atorvastatin 80 mg initiated on average 12 days of onset of ACS | |
| Outcomes |
Primary: composite of cardiovascular death, non‐fatal MI, readmission for ACS (requiring new electrocardiographic changes or cardiac marker elevation), and stroke Secondary: individual components of the primary endpoint |
|
| Source | Not reported | |
| Daily intervention | Adherence to the National Cholesterol Education Program Guidelines | |
| Control | Usual care including lipid‐lowering therapy | |
| Notes | Outcome data available at 1, 3, 6, and 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list but no explicit specification on blinding |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of participants or caregivers but outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up of patients |
| Selective reporting (reporting bias) | Low risk | Author contact established; data on relevant outcomes provided |
| Other bias | High risk | Stopped early for benefit after 5th interim analysis |
de Lemos 2004.
| Methods |
Design: multi‐center randomized controlled trial Setting: 322 centers in 41 countries Patient recruitment: December 1999 to January 2003 Blinding: patients, caregivers, and outcome adjudicators Intention‐to‐treat: yes Follow‐up period: 4 months for comparison simvastatin 40/80 mg versus placebo Lost to follow‐up: 44 (1%) |
|
| Participants |
Number randomized: statin 2265, control 2232 Mean age (SD) in years: statin 60 (11), control 61 (11) Men, n (%): statin 1716 (76%), control 1680 (75%) Diabetes, n (%): statin 529 (23%), control 530 (24%) Hypertension, n (%): statin 1131 (50%), control 1105 (50%) Current smoker, n (%): statin 926 (41%), control 915 (41%) Prior myocardial infarction, n (%): statin 409 (18%), control 355 (16%) Index event, n (%):
Inclusion criteria: patients with ACS, between the ages of 21 and 80 years with either non‐ST‐elevation ACS; total cholesterol of 250 mg/dL (6.48 mmol/L) or lower; stabilized for at least 12 consecutive hours within 5 days after symptom onset, and meeting at least one of the following high‐risk characteristics: age older than 70 years, diabetes mellitus, prior history of coronary artery disease, peripheral arterial disease, or stroke; elevation of serum creatine kinase‐MB or troponin levels; recurrent angina with ST‐segment changes; electrocardiographic evidence of ischemia on a pre‐discharge stress test; or multivessel coronary artery disease determined by coronary angiography Exclusion criteria: on statins at time of randomization, coronary artery bypass graft surgery planned, or PCI planned within the following 2 weeks; ALT level higher than 20% above the upper limit of normal; an increased risk of myopathy due to renal impairment (serum creatinine level > 2.0 mg/dL) or concomitant therapy with agents known to enhance myopathy risk, such as fibrates, cyclosporine, macrolide antibiotics, azole antifungals, amiodarone, or verapamil; or a prior history of non exercise‐related elevations in creatine kinase level or nontraumatic rhabdomyolysis |
|
| Interventions | Simvastatin 40 mg initiated within 5 days of onset of ACS and then titrated to 80 mg at 1 month | |
| Outcomes |
Primary: composite of cardiovascular death, non‐fatal MI, readmission for ACS (requiring new electrocardiographic changes or cardiac marker elevation), and stroke Secondary: individual components of the primary endpoint, revascularization due to documented ischemia, death from any cause, new‐onset congestive heart failure (requiring admission or initiation of heart failure medications), and cardiovascular rehospitalization |
|
| Source | Funded by Merck | |
| Daily intervention | All patients were encouraged to adopt an American Heart Association Step I diet | |
| Control | Placebo | |
| Notes | Outcome data for the review at 1 and 4 months of follow‐up provided by original investigators. After 4 months the control group received simvastatin 20 mg | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization using blinded allocation tables |
| Allocation concealment (selection bias) | Low risk | Adequate. Central randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded patients, caregivers, and outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Author contact established; data on relevant outcomes provided; study protocol available |
| Other bias | Low risk | Adhered to the intention‐to‐treat principle; not stopped early for benefit |
ESTABLISH 2004.
| Methods |
Design: single‐center randomized controlled trial Setting: 1 center in Japan Patient recruitment: November 2001 to August 2003 Blinding: statistician Intention‐to‐treat: unspecified Follow‐up period: 6 months Lost to follow‐up: 1 (1%) |
|
| Participants |
Number randomized: statin 35, control 35 Mean age (SD) in years: statin 61 (10), control 63 (11) Men, n (%): statin 30 (86%), control 30 (86%) Diabetes, n (%): statin 12 (34%), control 11 (31%) Hypertension, n (%): statin 19 (54%), control 19 (54%) Current smoker, n (%): statin 24 (69%), control 19 (54%) Prior myocardial infarction, n (%): statin 5 (14%), control 5 (14%) Index event, n (%):
Inclusion criteria: ACS with significant stenosis on initial coronary angiography and received PCI. ACS was defined as high‐risk unstable angina, non–ST‐elevated myocardial infarction (MI) or ST‐elevated MI. MI was diagnosed by the rise (2 times) in serum creatine phosphokinase and positivity for troponin T Exclusion criteria: failed PCI, diseased bypass graft, recommended CABG, cardiogenic shock, and administration of lipid‐lowering drugs (statin, clofibrate, probucol or analog, nicotinic acid, or other prohibited drug) before enrollment |
|
| Interventions | Atorvastatin 20 mg initiated within 14 days of ACS | |
| Outcomes |
Primary: % change in plaque volume Secondary: composite of death, non‐fatal MI, and non‐fatal stroke, death from any cause, cardiovascular death, fatal MI, non‐fatal MI, total stroke, revascularization procedures (CABG/PCI), and unstable angina |
|
| Source | Not reported | |
| Daily intervention | Aspirin 100 mg daily, ticlopidine 100 mg twice daily | |
| Control | Usual care including lipid‐lowering diet | |
| Notes | Outcome data available at 1, 4 and 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimization method of randomization |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of participants, caregivers, or outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 patient was lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Author contact established; data on relevant outcomes provided |
| Other bias | Unclear risk | Adherence to the intention‐to‐treat principle not reported |
FACS 2010.
| Methods |
Design: multi‐center randomized controlled trial Setting: 5 centers in Czech Republic and Slovakia Patient recruitment: November 2003 to February 2006 Blinding: participants, caregivers Intention‐to‐treat: yes Follow‐up period: 12 months Lost to follow‐up: 0 |
|
| Participants |
Number randomized: statin 78, control 78 Mean age (SD) in years: statin 61 (12), control 63 (11) Men, n (%): statin 55 (71%), control 51 (65%) Diabetes, n (%): statin 14 (18%), control 16 (21%) Hypertension, n (%): statin 40 (51%), control 40 (51%) Current smoker, n (%): statin 33 (42%), control 39 (50%) Prior myocardial infarction, n (%): statin 4 (5%), control 8 (10%) Index event, n (%):
Inclusion criteria: ST elevation ACS must have resting chest pain less than 12 hours before admission and either ≥ 1 mm ST‐segment elevation in 2 or more continuous leads or new left bundle branch block on ECG. Those with non‐ST elevation ACS must have resting chest pain during the previous 48 hours and either ≥ 1 mm ST segment depression or negative T waves in 2 or more continuous leads Exclusion criteria: < 18 years of age or if they have concomitant active liver disease or persistent elevation of transaminases (> 3 times the upper limit of normal), a history of lipid‐lowering therapy less than 30 days before the index event or a known allergy to fluvastatin or to any additives present in the drug. Other exclusions include inability to ingest oral medication, unwillingness to be followed for the duration of the study, muscle disease (e.g. myositis), and creatine kinase ≥ 5 times the upper limit of normal due to conditions other than myocardial infarction. Women of childbearing potential who are pregnant, nursing or who are not using effective contraception |
|
| Interventions | Fluvastatin 80 mg within 1 hour of ACS | |
| Outcomes |
Primary: levels of C‐reactive protein and IL‐6 Secondary: composite of death, non‐fatal MI, and non‐fatal stroke, death from any cause, cardiovascular death, fatal MI, non‐fatal MI, total stroke, revascularization procedures (CABG/PCI) |
|
| Source | Medication and Clinical Monitoring by Novartis Pharma CR, Laboratory investigations by grant from Czech Ministry of Health | |
| Daily intervention | Usual care | |
| Control | Placebo | |
| Notes | Outcome data available at 1, 3, and 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomization ‐ method of sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Reported blinding of participants and caregivers, but not outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up of patients |
| Selective reporting (reporting bias) | Low risk | Author contact established; data on relevant outcomes provided; study protocol available |
| Other bias | Low risk | Adherence to the intention‐to‐treat principle; not stopped early for benefit |
FLORIDA 2002.
| Methods |
Design: multi‐center randomized controlled trial Setting: 28 centers in The Netherlands Patient recruitment: July 1997 to May 1999 Blinding: patients, caregivers, and outcome adjudicators Intention‐to‐treat: unspecified Follow‐up period: 12 months Lost to follow‐up: 0 |
|
| Participants |
Number randomized: statin 265, control 275 Mean age (SD) in years: statin 61 (12), control 60 (11) Men, n (%): statin 214 (81%), control 234 (85%) Diabetes, n (%): statin 29 (11%), control 31 (11%) Hypertension, n (%): statin 67 (25%), control 65 (24%) Current smoker, n (%): statin 140 (53%), control 139 (51%) Prior myocardial infarction, n (%): statin 31 (12%), control 31 (11%) Index event, n (%):
Inclusion criteria: new or markedly increased chest pain lasting longer than 30 minutes, or a new pathological Q wave of 0.04 s duration, or 25% of the corresponding R wave amplitude, both in at least 2 contiguous leads Exclusion criteria: age < 18 years, use of lipid‐lowering agents within the previous 3 months, a high triglyceride level of > 4.5 mmol/l, known familial dyslipidemia, severe renal failure, known hepatic disease, signs and symptoms of severe heart failure (New York Heart Association class IV), a scheduled percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) and co‐medication that influences the ST‐segment (digoxin, quinidine or tricyclic antidepressants), atrial fibrillation, Wolff‐Parkinson‐White syndrome, complete left bundle‐branch block, known pre‐existent ST‐segment deviations before the qualifying MI, left ventricular hypertrophy with a pattern of strain or presence of a permanent pacemaker |
|
| Interventions | Fluvastatin 80 mg within 14 days of ACS | |
| Outcomes |
Primary: composite of death, non‐fatal MI, and non‐fatal stroke, death from any cause, cardiovascular death, fatal MI, non‐fatal MI, total stroke, revascularization procedures (CABG/PCI), unstable angina requiring emergency hospitalization Secondary: ischemia on the ambulatory ECG (without taking clinical events into account), change in ischemic burden, the time to a major clinical event and the 12‐month change in lipid profile |
|
| Source | Unrestricted Grant from AstraZeneca, The Netherlands | |
| Daily intervention | At discretion of attending cardiologist for starting standard medication including aspirin, beta‐blocking agents, and/or ACE‐inhibitors | |
| Control | Placebo | |
| Notes | Outcome data available at 1, 4, 6, and 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization sequence generation unspecified |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Reported blinding of participants, caregivers, and outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete patient follow‐up |
| Selective reporting (reporting bias) | Low risk | Author contact established; data on relevant outcomes provided |
| Other bias | Unclear risk | Adherence to intention‐to‐treat principle not reported |
L‐CAD 2000.
| Methods |
Design: single‐center randomized controlled trial Setting: 1 center in Germany Patient recruitment: unspecified Blinding: none Intention‐to‐treat: yes Follow‐up period: 24 months Lost to follow‐up: 0 |
|
| Participants |
Number randomized: statin 70, control 56 Mean age (SD) in years: statin 55 (10), control 59 (11) Men, n (%): statin 57 (81%), control 44 (79%) Diabetes, n (%): none Hypertension, n (%): statin 22 (31%), control 18 (32%) Current smoker, n (%): statin 49 (70%), control 36 (64%) Prior myocardial infarction, n (%): statin 45 (64%), control 39 (70%) Index event, n (%):
Inclusion criteria: total cholesterol of > 200 to < 400 mg/dL and low‐density lipoprotein (LDL) cholesterol of > 130 to < 300 mg/dL (after exclusion of secondary forms of hyperlipidemia) with an acute myocardial infarction (defined by new Q waves and increase of enzymes of > 3‐fold the normal value) and/or who underwent emergency percutaneous transluminal coronary balloon angioplasty due to severe or unstable angina pectoris (defined by clinical symptoms and electrocardiographic (ECG) alterations Exclusion criteria: > 75 years old, diabetics (fasting blood glucose > 125 mg/dL), patients with post‐coronary artery bypass graft, known malignant disease, serious kidney or liver dysfunction (creatinine > 1.5 mg/dL, alanine aminotransferase and aspartate aminotransferase > 2 times normal value), or women of child‐bearing age not using a reliable contraception |
|
| Interventions | Pravastatin 20 to 40 mg (8 of 70 individuals received additionally cholestyramine or nicotinic acid) within 1 to 11 days of ACS | |
| Outcomes |
Primary: composite of death, non‐fatal MI, and non‐fatal stroke Secondary: non‐fatal myocardial infarction, coronary balloon angioplasty or bypass grafting, stroke, new onset of occlusive peripheral vascular disease, cardiovascular death, and all‐cause mortality |
|
| Source | Bristol‐Myers Squibb Company, Munich, Germany | |
| Daily intervention | All patients received dietary counseling per the standards of the European Atherosclerosis Society | |
| Control | Usual care (including lipid‐lowering therapy) | |
| Notes | Outcome data available at 1, 4, 6,12, and 24 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified randomization ‐ sequence generation unspecified |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of participants, caregivers, or outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete patient follow‐up |
| Selective reporting (reporting bias) | Low risk | Author contact established; data on relevant outcomes provided |
| Other bias | Low risk | Adherence to intention‐to‐treat principle; no stopping early for benefit |
LAMIL 1997.
| Methods |
Design: multi‐center randomized controlled trial Setting: 10 centers in Belgium Patient recruitment: unspecified Blinding: participants and caregivers, but not outcome assessors Intention‐to‐treat: not reported Follow‐up period: 3 months Lost to follow‐up: 14 (20%) |
|
| Participants |
Number randomized: statin 36, control 33 Mean age (SD) in years: unspecified Men, n (%): unspecified Diabetes, n (%): unspecified Hypertension, n (%): unspecified Current smoker, n (%): unspecified Prior myocardial infarction, n (%): unspecified Index event, n (%):
Inclusion criteria: definite cases of acute MI using ECG and enzymatic criteria Exclusion criteria: unspecified |
|
| Interventions | Pravastatin 10 to 20 mg 3 days after ACS | |
| Outcomes |
Primary: lipid profile Secondary: composite of death, non‐fatal MI, and non‐fatal stroke, death from any cause, cardiovascular death, fatal MI, non‐fatal MI, total stroke, and revascularization procedures (CABG/PCI) |
|
| Source | Bristol‐Myers‐Squibb Pharmaceutical Company who also played a role in co‐ordinating the study | |
| Daily intervention | None specified, no other lipid‐lowering drugs allowed | |
| Control | Placebo | |
| Notes | Outcome data available at 1 and 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation unspecified |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Reported blinding of participants and caregivers, but not outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It remains unclear how many patients were lost to follow‐up for clinical events; 14 patients (20%) were not available for a follow‐up lipid profile |
| Selective reporting (reporting bias) | Unclear risk | Author contact failed; no protocol available |
| Other bias | Unclear risk | Adherence to the intention‐to‐treat principle not reported |
LIPS 2002.
| Methods |
Design: multi‐center randomized controlled trial Setting: 57 centers in 10 countries Patient recruitment: April 1996 to October 1998 Blinding: patients, caregivers, and outcome adjudicators Intention‐to‐treat: yes Follow‐up period: median of 3.9 years Lost to follow‐up: 0 up to 12 months of follow‐up |
|
| Participants |
Number randomized: statin 417, control 407 (these individuals just represent the subgroup of patients with unstable angina; the LIPS trial originally included another 853 individuals with stable angina) Mean age (SD) in years: statin 61 (10), control 60 (10) Men, n (%): statin 344 (83%), control 336 (83%) Diabetes, n (%): statin 65 (16%), control 34 (8%) Hypertension, n (%): statin not available, control not available Current smoker, n (%): statin not available, control not available Prior myocardial infarction, n (%): statin 184 (44%), control 172 (42%) Index event, n (%):
Inclusion criteria: patients with unstable angina, who had successfully undergone their first PCI of 1 or more lesions in the native coronary arteries. Successful PCI was defined as a reduction of the stenosis diameter to less than 50% in the target lesion without evidence of myocardial necrosis, need for repeat PCI or CABG, or death before hospital discharge. Any type of PCI was allowed and included balloon angioplasty with or without stent placement, rotational or directional atherectomy, laser ablation, transluminal extraction catheter, or cutting balloon. Patients further needed to have a total cholesterol level between 135 and 270 mg/dL (3.5 to 7.0 mmol/L), with fasting triglyceride levels of less than 400 mg/dL (4.5 mmol/L) before the index procedure. The upper total cholesterol limit for eligibility was 212 mg/dL (5.5 mmol/L) for patients whose baseline lipids were measured from blood drawn 24 hours to 4 weeks following MI and 232 mg/dL (6.0 mmol/L) for patients with type 1 or 2 diabetes mellitus Exclusion criteria: sustained systolic blood pressure of more than 180 mmHg and diastolic blood pressure of more than 100 mmHg despite medical therapy, left ventricular ejection fraction of less than 30%, a history of previous PCI or CABG, severe valvular disease, idiopathic cardiomyopathy or congenital heart disease, severe renal dysfunction (defined as serum creatinine level > 1.8 mg/dL, obesity (defined as a body mass index > 35 kg/m2), and the presence of malignant or other disease with a life expectancy of less than 4 years |
|
| Interventions | Fluvastatin 80 mg initiated at a median of 2 days after the index PCI | |
| Outcomes |
Primary: development of a major adverse cardiac event (MACE), defined as cardiac death (any death unless an unequivocal noncardiac cause could be established); non‐fatal MI (appearance of pathological Q waves that were absent at baseline or a total creatine kinase level > 2 times the upper limit of normal (ULN) with presence of CK isoenzyme MB higher than the ULN); or a re‐intervention procedure (CABG, repeat PCI, or PCI for a new lesion). Angiographic assessments without interventions were not included Secondary: MACE, excluding re‐intervention procedures (surgical or PCI) occurring in the first 6 months of follow‐up for lesions treated at the index procedure, cardiac mortality, non‐cardiac mortality, all‐cause mortality, combined cardiac mortality and MI, and combined all‐cause mortality and MI |
|
| Source | Funded by Novartis Pharma AG who also provided the fluvastatin and matching placebo | |
| Daily intervention | Dietary and lifestyle counseling | |
| Control | Placebo | |
| Notes | Outcome data for the subgroup of patients with unstable angina at 1, 4, and 12 months of follow‐up were provided by original investigators | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomization ‐ sequence generation unclear |
| Allocation concealment (selection bias) | Low risk | Adequate. Coded medication containers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded patients, caregivers, and outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete patient follow‐up within first 12 months |
| Selective reporting (reporting bias) | Low risk | Author contact established; data on relevant outcomes provided |
| Other bias | Low risk | Adherence to intention‐to‐treat principle; not stopped early for benefit |
Macin 2005.
| Methods |
Design: single‐center randomized controlled trial Setting: 1 center in Argentina Patient recruitment: December 1999 to November 2000 Blinding: patients and caregivers, but not outcome adjudicators Intention‐to‐treat: unspecified Follow‐up period: 1 month Lost to follow‐up: 0 |
|
| Participants |
Number randomized: statin 44, control 46 Mean age (SD) in years: statin 59 (13), control 61 (12) Men, n (%): statin 34 (77%), control 33 (72%) Diabetes, n (%): statin 10 (23%), control 11 (24%) Hypertension, n (%): statin 29 (66%), control 31 (67%) Current smoker, n (%): statin 18 (41%), control 19 (41%) Prior myocardial infarction, n (%): statin 5 (11%), control 7(15%) Index event, n (%):
Inclusion criteria: > 21 years old who fulfilled both of the following criteria: ACS within 48 hours of onset and CRP levels ≥ 1.4 mg/dl within 24 hours. Acute coronary syndrome was diagnosed in the presence of ischemic chest pain at rest lasting ≥ 20 minutes and at least 1 of the following: new or presumably new ST‐segment deviations on electrocardiogram (electrocardiographic evidence of ST‐segment elevation or depression), enzyme abnormalities (creatine kinase–MB above upper limit of reference in ≥ 2 samples obtained with an interval of > 6 hours), and/or troponin T ≥ 0.02 ng/mL Exclusion criteria: (1) use of 3‐hydroxy‐3‐methyl‐glutaryl coenzyme A reductase inhibitor or another lipid‐lowering agent at any time during the preceding 1 month; (2) history of active liver disease; (3) untreated endocrine disorder; (4) history of systemic inflammatory disease or cancer; (5) known hypersensitivity to statins; (6) patients judged to be unlikely to comply with the study drug regimen or study process; (7) known infectious disease in the last 30 days; (8) unwilling to provide written informed consent; and (9) cardiogenic shock or acute pulmonary edema |
|
| Interventions | Atorvastatin 40 mg within 96 hours of ACS | |
| Outcomes |
Primary: C‐reactive protein levels Secondary: lipid profile, acute phase reactants, composite of death, non‐fatal MI, and non‐fatal stroke, death from any cause, fatal MI, non‐fatal MI and unstable angina |
|
| Source | Unspecified. Laboratorio Elea Sacifya provided active drug and placebo | |
| Daily intervention | All patients received dietary counseling to promote compliance with National Cholesterol Education Program (NCEP) diet | |
| Control | Placebo | |
| Notes | Outcome data available at 1 month | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation unspecified |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded participants and caregivers, but not outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete patient follow‐up for clinical events |
| Selective reporting (reporting bias) | Low risk | Author contact established; data on relevant outcomes provided |
| Other bias | Unclear risk | Adherence to the intention‐to‐treat principle not reported |
MIRACL 2001.
| Methods |
Design: multi‐center randomized controlled trial Setting: 122 centers in Europe, North America, South Africa, Australasia Patient recruitment: May 1997 to September 1999 Blinding: patients, caregivers, and outcome adjudicators Intention‐to‐treat: yes Follow‐up period: 4 month Lost to follow‐up: 9 (0.4%) |
|
| Participants |
Number randomized: statin 1538, control 1548 Mean age (SD) in years: statin 65 (12), control 65 (12) Men, n (%): statin 992 (64%), control 1020 (66%) Diabetes, n (%): statin 342 (22%), control 373 (24%) Hypertension, n (%): statin 843 (55%), control 846 (55%) Current smoker, n (%): statin 429 (28%), control 430 (28%) Prior myocardial infarction, n (%): statin 382 (25%), control 392 (25%) Index event, n (%):
Inclusion criteria: 18 years or older with chest pain or discomfort of at least 15 minutes' duration that occurred at rest or with minimal exertion within the 24‐hour period preceding hospitalization and represented a change from their usual anginal pattern Exclusion criteria: serum total cholesterol level at screening exceeded 270 mg/dL (7 mmol/L) (sites in Poland and South Africa used levels of 310 mg/dL (8 mmol/L)), coronary revascularization was planned or anticipated at the time of screening, evidence of Q‐wave acute MI within the preceding 4 weeks; coronary artery bypass surgery within the preceding 3 months; percutaneous coronary intervention within the preceding 6 months; left bundle‐branch block or paced ventricular rhythm; severe congestive heart failure (New York Heart Association class IIIb or IV); concurrent treatment with other lipid‐regulating agents (except niacin at doses of 500 mg/d), vitamin E (except at doses #400 IU/d), or drugs associated with rhabdomyolysis in combination with statins; severe anemia; renal failure requiring dialysis; hepatic dysfunction (alanine aminotransferase greater than 2 times ULN); insulin‐dependent diabetes; pregnancy or lactation |
|
| Interventions | Atorvastatin 80 mg daily, starting at a mean of 3 days after onset of ACS | |
| Outcomes |
Primary: composite of death, non‐fatal MI, and non‐fatal stroke Secondary: death from any cause, cardiovascular death, fatal MI, non‐fatal MI, total stroke, revascularization procedures (CABG/PCI), unstable angina requiring emergency hospitalization |
|
| Source | Grant from Pfizer Inc. | |
| Daily intervention | All participants received instruction and counseling to promote compliance with NCEP step 1 diet | |
| Control | Placebo | |
| Notes | Outcome data available at 1 and 4 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified randomization ‐ sequence generation unspecified |
| Allocation concealment (selection bias) | Low risk | Central randomization stratified by study center |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Reported blinding of participants, caregivers, and outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0.4% of patients lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Author contact established; data on relevant outcomes provided; study protocol available |
| Other bias | Low risk | Adherence to the intention‐to‐treat principle; not stopped early for benefit |
OACIS‐LIPID 2008.
| Methods |
Design: multi‐center randomized controlled trial Setting: 14 centers in Japan Patient recruitment: May 2000 to December 2003 Blinding: outcome adjudicators Intention‐to‐treat: yes Follow‐up period: 9 months Lost to follow‐up: 5 (1%) |
|
| Participants |
Number randomized: statin 176, control 177 Mean age (SD) in years: statin 64 (10), control 63 (11) Men, n (%): statin 129 (73%), control 142 (80%) Diabetes, n (%): statin 52 (30%), control 59 (34%) Hypertension, n (%): statin 81 (46%), control 87 (49%) Current smoker, n (%): statin 98 (56%), control 105 (59%) Prior myocardial infarction, n (%): statin 16 (9%), control 19 (10%) Index event, n (%):
Inclusion criteria: (1) clinical history of central chest pressure, pain, or tightness lasting for 30 minutes or more, (2) typical electrocardiographic changes (i.e. ST‐segment elevation > 0.1 mV in at least 1 standard or 2 precordial leads, ST‐segment depression > 0.1 mV in at least 2 leads, abnormal Q wave, or T wave inversion in at least 2 leads), and (3) an increase in the serum creatine kinase activity more than twice the normal laboratory value. All patients presenting within 1 week after the onset of acute MI were registered prospectively Exclusion criteria: concurrent therapy with any statins or had a history of side effects associated with any statin. Evidence of life‐threatening arrhythmia, severe chronic congestive heart failure (New York Heart Association class III–IV), hepatic dysfunction, renal failure, cerebrovascular disease, poorly controlled diabetes, pregnancy, lactation, age < 20 years, and unable to take medication or absence of written informed consent. Patients whom the doctors consider inappropriate for any other reason were also not included |
|
| Interventions | Pravastatin 10 mg initiated on average within 7 days after onset of ACS | |
| Outcomes | Primary: combination of death, non‐fatal myocardial infarction, unstable angina, revascularization and non‐fatal stroke, and re‐hospitalization because of other cardiovascular diseases | |
| Source | Grant‐in‐Aid for Scientific Research from Japan Society for the Promotion of Science, Tokyo, Japan and from Japan Arteriosclerosis Prevention Fund, Tokyo, Japan | |
| Daily intervention | All patients received instructions and counseling to promote compliance with the National Cholesterol Education Program (NCEP) Step I diet | |
| Control | Usual care | |
| Notes | Outcome data available at 1 and 9 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation unspecified |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of participants or caregivers, but blinded outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1% of participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Author contact partially established; data on relevant outcomes provided |
| Other bias | Low risk | Adherence to intention‐to‐treat principle; not stopped early for benefit |
PACT 2004.
| Methods |
Design: multi‐center randomized controlled trial Setting: 6 centers in Australia Patient recruitment: unspecified Blinding: patients and caregivers, but not outcome adjudicators Intention‐to‐treat: yes Follow‐up period: 1 month Lost to follow‐up: 85 (2%) |
|
| Participants |
Number randomized: statin 1710, control 1698 Mean age (SD) in years: statin 62 (12), control 61 (12) Men, n (%): statin 1308 (76%), control 1285 (76%) Diabetes, n (%): statin 244 (14%), control 234 (14%) Hypertension, n (%): statin 700 (41%), control 714 (42%) Current smoker, n (%): statin 608 (36%), control 575 (34%) Prior myocardial infarction, n (%): statin 236 (14%), control 197 (12%) Index event, n (%):
Inclusion criteria: 24 hours of the onset of symptoms if they had electrocardiographic changes suggestive of an acute MI or unstable angina Exclusion criteria: taking statin therapy before their event (other lipid‐lowering therapies were permitted), participation in any other clinical trial or the taking of an investigational drug within the previous 30 days, planned coronary revascularization or cardiac transplantation, severe renal or hepatic disease or other severe disease, drug‐ or alcohol‐related problems, gastrointestinal disease or a history of gastrointestinal surgery that might affect drug absorption, and known hypersensitivity or previous serious adverse reactions to statin therapy Women of child‐bearing potential |
|
| Interventions | Pravastatin 20 to 40 mg | |
| Outcomes | Primary: composite of death, non‐fatal MI, and non‐fatal stroke, death from any cause, cardiovascular death, fatal MI, non‐fatal MI, total stroke, unstable angina requiring emergency hospitalization | |
| Source | Bristol‐Myers Squibb, Australia | |
| Daily intervention | Usual care | |
| Control | Placebo | |
| Notes | Outcome data available at 1 month; trial stopped early due to recruitment difficulties | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation unspecified |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Reported blinding of participants and caregivers, but not outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2% of participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Author contact established; data on relevant outcomes provided |
| Other bias | Low risk | Adherence to intention‐to‐treat principle; not stopped early for benefit |
PAIS 2001.
| Methods |
Design: single‐center randomized controlled trial Setting: 1 center in The Netherlands Patient recruitment: April 1997 to‐ January 1998 Blinding: patients and caregivers, but not outcome adjudicators Intention‐to‐treat: yes Follow‐up period: 3 months Lost to follow‐up: 2 (2%) |
|
| Participants |
Number randomized: statin 50, control 49 Mean age (SD) in years: statin 64 (1), control 63 (2) Men, n (%): statin 35 (70%), control 37 (76%) Diabetes, n (%): statin 8 (16%), control 5 (10%) Hypertension, n (%): statin 12 (24%), control 16 (33%) Current smoker, n (%): statin 17 (34%), control 17 (35%) Prior myocardial infarction, n (%): statin 14 (28%), control 12 (25%) Index event, n (%):
Inclusion criteria: men aged 18 to 80 or postmenopausal women with serum total cholesterol levels > 5.2 mmol/l and LDL cholesterol levels > 3.5 mmol/l; signs of either UA or acute MI (defined as ST segment elevations ≥ 1 mm in 2 leads or T‐wave inversion) Exclusion criteria: history of hypersensitivity to statins, severe congestive heart failure, cardiomyopathy, significant liver disease, significant gastrointestinal disease or abdominal surgery that might adversely influence drug absorption, substance or alcohol abuse, history or present use of any other lipid‐lowering or investigational agent, uncontrolled diabetes, thyroid disease, severe renal impairment, dysproteinemia, primary muscle disease |
|
| Interventions | Pravastatin 40 mg/d | |
| Outcomes | Primary: composite of death, non‐fatal MI, and non‐fatal stroke, death from any cause, cardiovascular death, fatal MI, non‐fatal MI, total stroke, revascularization procedures (CABG/PCI), "recurrent angina pectoris" | |
| Source | Funded by Bristol‐Myers Squibb, Woerden, The Netherlands | |
| Daily intervention | "Dispensed educational materials on appropriate dietary modification" | |
| Control | Placebo | |
| Notes | Outcome data available at 1 and 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation unspecified |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Reported blinding of participants and caregivers, but not outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2% of patients lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Author contact established; data on relevant outcomes provided |
| Other bias | Low risk | Adherence to intention‐to‐treat principle; not stopped early for benefit |
PRINCESS 2004.
| Methods |
Design: multi‐center randomized controlled trial Setting: 280 centers in 8 countries Patient recruitment: unspecified Blinding: patients, caregivers, and outcome adjudicators Intention‐to‐treat: yes Follow‐up period: 4.5 months (44% of the participants completed the 4.5 months of follow‐up at trial termination) Lost to follow‐up: unclear |
|
| Participants |
Number randomized: statin 1795, control 1810 Mean age (SD) in years: not available Men, n (%): not available Diabetes, n (%): not available Hypertension, n (%): not available Current smoker, n (%): not available Prior myocardial infarction, n (%): not available Index event, n (%):
Inclusion criteria: patients with ACS (myocardial infarction or unstable angina), no lower LDL cholesterol limit |
|
| Interventions | Cerivastatin 0.4 mg initiated within 2 days of onset of ACS | |
| Outcomes |
Primary: composite of cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke, hospitalization for unstable angina or heart failure Secondary: recurrent coronary ischemia including ischemic coronary revascularization, fatal and non‐fatal reinfarction and unstable angina, all‐cause mortality, myocardial infarction, and stroke |
|
| Source | Funded by Bayer | |
| Daily intervention | Unclear | |
| Control | Placebo (after 3 months the control group received cerivastatin 0.4 mg) | |
| Notes | The trial was prematurely interrupted on 8 August 2001 when Bayer withdrew cerivastatin from the worldwide market. Outcome data on mortality from any cause, fatal and non‐fatal myocardial infarction, and fatal and non‐fatal stroke at 4.5 months of follow‐up were provided by the original investigators | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation unspecified |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded patients, caregivers, and outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No full publication available |
| Selective reporting (reporting bias) | Low risk | Author contact established; data on relevant outcomes provided |
| Other bias | Low risk | Adherence to intention‐to‐treat principle; not stopped early for benefit |
PTT 2002.
| Methods |
Design: single‐center randomized controlled trial Setting: 1 center in Turkey Patient recruitment: June 1997 to September 1998 Blinding: none Intention‐to‐treat: unclear Follow‐up period: 6 months Lost to follow‐up: 0 |
|
| Participants |
Number randomized: statin 79, control 85 Mean age (SD) in years: statin 53 (11), control 52 (10) Men, n (%): statin 65 (82%), control 69 (81%) Diabetes, n (%): statin 14 (18%), control 13 (15%) Hypertension, n (%): statin 16 (20%), control 21 (25%) Current smoker, n (%): statin 63 (80%), control 66 (78%) Prior myocardial infarction, n (%): statin none, control none Index event, n (%):
Inclusion criteria: patients receiving fibrinolysis therapy within 6 hours of ST‐segment elevated acute MI Exclusion criteria: contraindications for thrombolytic therapy, age > 75 years, history of myocardial infarction, previous percutaneous transluminal coronary angioplasty, coronary artery bypass graft surgery, congestive heart failure, secondary hyperlipidemia, uncontrolled hypertension or diabetes mellitus, liver disease, thyroid dysfunction, use of anticoagulant drugs other than aspirin, use of steroids or hormone replacement therapy, women of childbearing potential and patients with physical or psychosocial disorders that could interfere with protocol adherence |
|
| Interventions | Pravastatin 40 mg/d | |
| Outcomes | Primary: composite of death, non‐fatal MI, and non‐fatal stroke, death from any cause, cardiovascular death, fatal MI, non‐fatal MI, total stroke, revascularization procedures (CABG/PCI), "recurrent angina pectoris" | |
| Source | Not funded | |
| Daily intervention | All patients received fibrinolysis therapy, intravenous nitrates, heparin infusions, a AHA step II diet and a daily 100 mg aspirin | |
| Control | Usual care | |
| Notes | Outcome data available at 1 and 6 months. Note: only a subgroup of 77 patients (40 intervention, 37 control, those who received additional coronary angioplasty) were followed up for 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation unspecified |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of participants, caregivers, or clinical outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for clinical outcomes |
| Selective reporting (reporting bias) | Low risk | Author contact established; data on relevant outcomes provided |
| Other bias | Unclear risk | Adherence to the intention‐to‐treat principle not reported |
RECIFE 1999.
| Methods |
Design: single‐center randomized controlled trial Setting: 1 center in Canada Patient recruitment: unspecified Blinding: participants and caregivers, but not outcome assessors Intention‐to‐treat: unclear Follow‐up period: 6 weeks Lost to follow‐up: 0 for clinical events |
|
| Participants |
Number randomized: statin 30, control 30 Mean age (SD) in years: statin 55 (2), control 56 (2) Men, n (%): statin 26 (93%), control 22 (81%) Diabetes, n (%): statin 1 (4%), control 0 (0%) Hypertension, n (%): statin 5 (18%), control 8 (29%) Current smoker, n (%): statin 14 (50%), control 17 (63%) Prior myocardial infarction, n (%): statin 1 (4%), control 2 (7%) Index event, n (%):
Inclusion criteria: diagnosis of acute myocardial infarction or unstable angina and admission total serum cholesterol ?5.2 mmol/L or LDL cholesterol ?3.4 mmol/L and serum triglycerides ?4.5 mmol/L Exclusion criteria: presence of heart failure with an ejection fraction of < 40%, administration of lipid‐lowering agents in the preceding 8 weeks, renal failure with serum creatinine level > 200 mmol/L, and patients requiring coronary artery bypass surgery, premenopausal women, postmenopausal women on hormone replacement therapy |
|
| Interventions | Pravastatin 40 mg/d | |
| Outcomes |
Primary: brachial artery flow Secondary: composite of death, non‐fatal MI, and non‐fatal stroke, death from any cause, cardiovascular death, fatal MI, non‐fatal MI, total stroke, revascularization procedures (CABG/PCI), unstable angina requiring emergency hospitalization |
|
| Source | Funded by Bristol‐Myers Squibb, Canada | |
| Daily intervention | American Heart Association step 2 diet | |
| Control | Placebo | |
| Notes | Outcome data available at 1.5 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation unspecified |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Reported blinding of participants and caregivers, but not outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for clinical events |
| Selective reporting (reporting bias) | Low risk | Author contact established; data on relevant outcomes provided |
| Other bias | Unclear risk | Adherence to the intention‐to‐treat principle not reported |
Ren 2009.
| Methods |
Design: single‐center randomized controlled trial Setting: 1 center in China Patient recruitment: March 2004 to July 2006 Blinding: probable blinding of participants and caregivers; blinding of outcome assessors not reported Intention‐to‐treat: unclear Follow‐up period: 4 weeks Lost to follow‐up: 0 |
|
| Participants |
Number randomized: statin 43, control 43 Mean age (SD) in years: statin 58 (11), control 59 (10) Men, n (%): statin 27 (63%), control 30 (70%) Diabetes, n (%): statin 12 (28%), control 10 (23%) Hypertension, n (%): statin 21 (49%), control 18 (42%) Current smoker, n (%): not available Prior myocardial infarction, n (%): statin 1 (4%), control 2 (7%) Index event, n (%):
Inclusion criteria: newly diagnosed unstable angina, age > 18 years, ischemic symptoms < 72 h, absence of cardiogenic shock, and not previously treated with statin Exclusion criteria: severe renal dysfunction, primary cardiomyopathy or COPD, taking inflammatory drugs other than aspirin, elevated cardiac markers |
|
| Interventions | Simvastatin 40 mg/d | |
| Outcomes |
Primary: plasma IL‐6 Secondary: death from any cause, cardiovascular death, fatal MI, non‐fatal MI, heart failure, revascularization procedures (CABG/PCI) |
|
| Source | Not reported | |
| Daily intervention | Not reported | |
| Control | Placebo | |
| Notes | Outcome data available at 1 month | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation unspecified |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participants and caregivers probable; blinding of clinical outcome assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for clinical events |
| Selective reporting (reporting bias) | Low risk | Author contact established; data on relevant outcomes provided |
| Other bias | Unclear risk | Adherence to the intention‐to‐treat principle not reported |
Sakamoto 2005.
| Methods |
Design: multi‐center randomized controlled trial Setting: 28 centers in Japan Patient recruitment: February 2002 to September 2004 Blinding: no blinding of participants or caregivers, but blinding of outcome assessors Intention‐to‐treat: unclear Follow‐up period: 24 months Lost to follow‐up: 35 (7%) |
|
| Participants |
Number randomized: statin 241, control 245 Mean age (SD) in years: statin 63 (11), control 65 (12) Men, n (%): statin 190 (80%), control 193 (79%) Diabetes, n (%): statin 83 (35%), control 61 (25%) Hypertension, n (%): statin 149 (63%), control 142 (58%) Current smoker, n (%): statin 131 (55%), control 130 (53%) Prior myocardial infarction, n (%): statin 10 (4%), control 15 (6%) Index event, n (%):
Inclusion criteria: qualifying AMI (increased creatinine phosphokinase‐MB and/or total creatinine phosphokinase level ≥ 2 times the upper limit of normal) and prolonged chest pain (> 30 minutes), objective evidence of myocardial ischemia based on dynamic or interval ST‐ or T‐wave changes in ≥ 2 contiguous electrocardiographic leads (≥ 0.1 mV ST elevation, ≥ 0.05 mV flat or downsloping ST depression at the J point and 80 ms after the J point, or ≥ 0.3 mV T‐wave inversion), or new left bundle branch block; serum total cholesterol levels were required to be 180 to 240 mg/dL on admission Exclusion criteria: < 18 years of age, use of lipid‐lowering agents within the previous 3 months,known familial dyslipidemia, severe renal failure, known hepatic disease, signs and symptoms of severe heart failure (Killip's class III or IV), a scheduled PCI or coronary artery bypass grafting (CABG), previous PCI (within 6 months) or CABG (within 3 months), and the presence of malignant disease or allergy to statins |
|
| Interventions | Any statin (pravastatin, atorvastatin, fluvastatin, simvastatin, or pitavastatin) Note: statin and dose at discretion of treating physician and could be switched or adjusted at any time but prohibited from using any other lipid‐lowering agent |
|
| Outcomes |
Primary: combination of cardiovascular death, non‐fatal MI, recurrent symptomatic myocardial ischemia with objective evidence that required emergency rehospitalization, congestive heart failure that required emergency rehospitalization, and non‐fatal stroke Secondary: CABG, PCI for a new lesion, and repeat PCI procedures for restenosis of infarct‐related or non‐infarct‐related lesions |
|
| Source | Funded by the Japan Heart Foundation and the Ministry of Health, Labour and Welfare, Tokyo, Japan | |
| Daily intervention | All patients received instruction and counseling to promote compliance with the Japan Atherosclerosis Society Guidelines for Diagnosis and Treatment of Atherosclerotic Cardiovascular Diseases Step I diet | |
| Control | Usual care excluding statins | |
| Notes | Outcome data available at 1 and 24 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation unspecified |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of participants or caregivers, but blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Follow‐up information was sought for all patients who were withdrawn early from the study." 35 patients (7%) dropped out (unclear if followed up or not) |
| Selective reporting (reporting bias) | Unclear risk | Author contact failed; no study protocol available |
| Other bias | Unclear risk | Adherence to intention‐to‐treat not reported |
Shal'nev 2007.
| Methods |
Design: single‐center randomized controlled trial Setting: 1 center in Russia Patient recruitment: unspecified Blinding: none Intention‐to‐treat: unclear Follow‐up period: 6 months Lost to follow‐up: 2 (2%) |
|
| Participants |
Number randomized: statin 55, control 53 Mean age (SD) in years: not available Men, n (%): not available Diabetes, n (%): not available Hypertension, n (%): not available Current smoker, n (%): not available Prior myocardial infarction, n (%): not available Index event, n (%):
Inclusion criteria: patients with acute coronary syndrome (myocardial infarction or unstable angina) |
|
| Interventions | Simvastatin 40 mg/d | |
| Outcomes | Primary: total death, unstable angina (hospitalized and non‐hospitalized), MI (acute and in later follow‐up) | |
| Source | Unspecified | |
| Daily intervention | Unspecified | |
| Control | Usual care | |
| Notes | Outcome data available at 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation unspecified |
| Allocation concealment (selection bias) | Unclear risk | Unreported |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of participants, caregivers, or outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2% of participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Author contact failed; no study protocol available |
| Other bias | Unclear risk | Adherence to intention‐to‐treat not reported |
ACE: angiotensin‐converting enzyme ACS: acute coronary syndrome AHA: American Heart Association ALT: aminotransferase CABG: coronary artery bypass grafting COPD: chronic obstructive pulmonary disease
CRP: C‐reactive protein d: day ECG: electrocardiogram IL‐6: interleukin‐6 LDL: low‐density lipoprotein
MACE: major adverse cardiac event MI: myocardial infarction
NCEP: National Cholesterol Education Program PCI: percutaneous coronary intervention
UA: unstable angina
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akasaka 2012 | Head to head comparison of statins (atorvastatin 20 mg versus atorvastatin 5 mg); no clinical outcomes reported |
| Barderas 2009 | No clinical outcomes reported |
| Bermejo 2006 | Not a randomized trial |
| Cannon 2004a | Head to head comparison of statins (atorvastatin 80 mg versus pravastatin 40 mg) |
| Chi 2007 | Treatment not initiated within 14 days |
| Chiodini 2007 | Treatment not initiated within 14 days |
| Chyrchel 2011 | Only 7 days of statin treatment and follow‐up; no clinical outcomes |
| Colivicchi 2010 | Comparison of high‐dose (80 mg/d) versus low‐dose (20 mg/d) atorvastatin |
| Colivicchi 2010a | Head to head comparison of statins (atorvastatin 80 mg versus atorvastatin 20 mg) |
| Colivicchi 2011 | Head to head comparison of statins (atorvastatin 80 mg versus atorvastatin 20 mg) |
| Correia 2002 | Follow‐up less than 30 days (only 5 days) |
| de Winter 2005 | Not a randomized trial |
| Dohi 2010 | Extended follow‐up of the already included ESTABLISH trial |
| FLAME 2006 | Head to head comparison of statins (simvastatin 20 mg versus simvastatin 40 mg) |
| Ge 2010 | Statin versus placebo only for pretreatment of PCI; after PCI both groups received statins |
| Guazzi 2007 | No clinical outcomes reported |
| Gómez‐Doblas 2006 | Follow‐up less than 30 days (only 8 days) |
| Gómez‐Hernández 2008 | No clinical outcomes reported |
| Hall 2009 | Head to head comparison of statins (simvastatin versus rosuvastatin) |
| He 2011 | Head to head comparison of statins (atorvastatin 40 mg versus atorvastatin 10 mg) |
| Hiro 2009 | Head to head comparison of statins (pitavastatin versus atorvastatin) |
| Hiro 2009a | Head to head comparison of statins (pitavastatin versus atorvastatin) |
| Hiro 2010 | Head to head comparison of statins (pitavastatin versus atorvastatin) |
| Kanadasi 2006 | Not a randomized trial |
| Kashima 2009 | Protocol for a head to head comparison of statins |
| Kuznetsova 2010 | Head to head comparison of statins (atorvastatin versus rosuvastatin) |
| Lablanche 2008 | Head to head comparison of statins |
| LAVA 2005 | No clinical outcomes reported; fluvastatin 80 mg within 12 hours versus 4 to 5 days following ACS |
| Lemos 2005 | Treatment not initiated within 14 days |
| Leone 2008 | Head to head comparison of statins (atorvastatin 80 mg versus atorvastatin 20 mg) |
| Li 2005 | No clinical outcomes reported |
| Li 2006 | Follow‐up less than 30 days (only 14 days) |
| Lim 2012 | Not a randomized trial |
| Link 2006 | No clinical outcomes reported |
| Link 2006a | No clinical outcomes reported |
| Link 2011 | No clinical outcomes reported |
| Liu 2012 | Head to head comparison of statins (atorvastatin 80 mg versus "usual care statin dose") |
| Miyauchi 2006 | Head to head comparison of statins (pitavastatin versus atorvastatin); design and rationale paper for Hiro T et al |
| Monteiro 2008 | No clinical outcomes reported |
| Nakamura 2008 | No clinical outcomes reported |
| Nakaya 2005 | Not a randomized trial |
| Ordulu 2008 | Head to head comparison of statins |
| Ostadal 2003 | Follow‐up less than 30 days (only 1 day) |
| Patti 2007 | Treatment not given for 30 days (only twice) |
| PEACE 2005 | Head to head comparison of statins (pravastatin 20 mg versus pravastatin 40 mg) |
| Pedersen 2000 | Outcome data not available per treatment group for the comparison simvastatin 40 mg versus usual care |
| Pedersen 2005 | Treatment not initiated within 14 days |
| Pitt 2008 | Head to head comparison of statins |
| Pitt 2012 | Head to head comparison of statins |
| Post 2012 | Only statin versus placebo for pretreatment of PCI; no clinical outcomes |
| Sakata 2005 | Not a randomized trial |
| Shah 2007 | Head to head comparison of statin combinations |
| Stefanadi 2009 | No clinical outcomes reported |
| Suh 2011 | Not a randomized trial |
| Teshima 2009 | Not a randomized trial |
| Tousoulis 2006 | No clinical outcomes reported |
| Tousoulis 2006a | No clinical outcomes assessed |
| Tousoulis 2006b | Treatment not initiated within 14 days |
| Xin‐wei 2009 | High versus low‐dose statin therapy in pre‐PCI in ACS patients |
| Yun 2009 | Only pre‐procedural comparison (rosuvastatin versus no statin); after PCI all patients received rosuvastatin |
| Zhang 2013 | Head to head comparison of statins (atorvastatin 80 mg versus atorvastatin 20 mg) |
| Zhao 2009 | Head to head comparison of statins |
| Zheng 2009 | Atorvastatin versus atorvastatin plus probucol |
| Zheng 2009a | Atorvastatin versus atorvastatin plus probucol |
ACS: acute coronary syndrome PCI: percutaneous coronary intervention
Differences between protocol and review
None.
Contributions of authors
NV: carried out study selection, quality assessment, and data extraction; drafted the review text. AJN: carried out study selection, quality assessment and data extraction; drafted the review text. GGS, JAdL, FC, PO, SMM, AL, EM, FdH, HCB: participated in collection of additional data and critically reviewed the manuscript for important intellectual content. MB: conceived and designed the review; carried out part of the study selection, quality assessment, and data extraction; drafted the review text.
Sources of support
Internal sources
Santésuisse, Switzerland.
Gottfried and Julia Bangerter‐Rhyner‐Foundation, Switzerland.
External sources
No sources of support supplied
Declarations of interest
Dr Vale declared no interests.
Dr Nordmann declared no interests.
Dr Schwartz has received honoraria and grant support from Pfizer.
Dr de Lemos has received honoraria from Merck, Pfizer, Merck/Schering, and Bristol‐Myers Squibb and grant support from Merck and Pfizer. He has also received consulting fees from Astra Zeneca for participation in an endpoint review committee for a product unrelated to the current manuscript.
Dr Colivicchi declared no interests.
Dr den Hartog declared no interests.
Dr Ostadal has received honoraria from Pfizer, Merck, and AstraZeneca and grant support from Novartis.
Dr Macin declared no interests.
Dr Liem has received honoraria from Merck/Schering and Astra Zeneca.
Dr. Mills has received grant support or consulting honoraria from Pfizer, Merck, Janssen, Astra Zeneca, Novartis, and Boehringer Ingelheim.
Mrs Bhatnagar declared no interests.
Dr Bucher has received grant support from Merck and Bristol‐Myers Squibb.
Dr Briel declared no interests.
None of the support or honoraria above were related to research or drugs that are subject of the current meta‐analysis. There was no pharmaceutical industry support for this review.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Colivicchi 2002 {published and unpublished data}
- Colivicchi F, Guido V, Tubaro M, Ammirati F, Montefoschi N, Varveri A, et al. Effects of atorvastatin 80 mg daily early after onset of unstable angina pectoris or non‐Q‐wave myocardial infarction. American Journal of Cardiology 2002;90(8):872–4. [DOI] [PubMed] [Google Scholar]
de Lemos 2004 {published and unpublished data}
- Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KAA, White HD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004;292(11):1307‐16. [DOI] [PubMed] [Google Scholar]
ESTABLISH 2004 {published and unpublished data}
- Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H, et al. Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation 2004;110(9):1061‐8. [DOI] [PubMed] [Google Scholar]
FACS 2010 {published and unpublished data}
- Ostadal P, Alan D, Hajek P, Vejvoda J, Mates M, Blasko P, et al. Fluvastatin in the therapy of acute coronary syndrome: rationale and design of a multicenter, randomized, double‐blind, placebo‐controlled trial (The FACS Trial). Current Controlled Trials in Cardiovascular Medicine 2005;6(1):4. [ISRCTN 81331696] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostadal P, Alan D, Vejvoda J, Kukacka J, Macek M, Hajek P, et al. Fluvastatin in the first‐line therapy of acute coronary syndrome: results of the multicenter, randomized, double‐blind, placebo‐controlled trial (the FACS‐trial). Trials 2010;11(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
FLORIDA 2002 {published and unpublished data}
- Liem AH, Boven AJ, Veeger NJGM, Withagen AJ, Robles de Medina RM, Tijssen JGP, et al. Effect of fluvastatin on ischaemia following acute myocardial infarction: a randomized trial. European Heart Journal 2002;23(24):1931‐7. [DOI] [PubMed] [Google Scholar]
LAMIL 1997 {published data only (unpublished sought but not used)}
- Kesteloot H, Claeys G, Blanckaert N, Lesaffre E. Time course of serum lipids and apolipoproteins after acute myocardial infarction: modification by pravastatin. Acta Cardiologica 1997;52(2):107–16. [PubMed] [Google Scholar]
L‐CAD 2000 {published and unpublished data}
- Arntz HR, Agrawal R, Wunderlich W, Schnitzer L, Stern R, Fischer F, et al. Beneficial effects of pravastatin (+/‐ colestyramine/niacin) initiated immediately after a coronary event (the randomized lipid‐coronary artery disease [L‐CAD] study). American Journal of Cardiology 2000;86(12):1293–8. [DOI] [PubMed] [Google Scholar]
LIPS 2002 {published and unpublished data}
- Serruys PW, Feyter P, Macaya C, Kokott N, Puel J, Vrolix M, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;287(24):3215. [DOI] [PubMed] [Google Scholar]
Macin 2005 {published and unpublished data}
- Macin SM, Perna ER, Farias EF, Franciosi V, Cialzeta JR, Brizuela M, et al. Atorvastatin has an important acute anti‐inflammatory effect in patients with acute coronary syndrome: results of a randomized, double‐blind, placebo‐controlled study. American Heart Journal 2005;149(3):451–7. [DOI] [PubMed] [Google Scholar]
MIRACL 2001 {published and unpublished data}
- Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001;285(13):1711. [DOI] [PubMed] [Google Scholar]
OACIS‐LIPID 2008 {published data only (unpublished sought but not used)}
- Sato H, Kinjo K, Ito H, Hirayama A, Nanto S, Fukunami M, et al. Effect of early use of low‐dose pravastatin on major adverse cardiac events in patients with acute myocardial infarction: the OACIS‐LIPID study. Circulation 2008;72(1):17. [DOI] [PubMed] [Google Scholar]
PACT 2004 {published and unpublished data}
- Thompson PL, Meredith I, Amerena J, Campbell TJ, Sloman JG, Harris PJ. Effect of pravastatin compared with placebo initiated within 24 hours of onset of acute myocardial infarction or unstable angina: the Pravastatin in Acute Coronary Treatment (PACT) trial. American Heart Journal 2004;148(1):91. [DOI] [PubMed] [Google Scholar]
PAIS 2001 {published and unpublished data}
- Hartog FR, Kalmthout PM, Loenhout TT, Schaafsma HJ, Rila H, Verheugt FW. Pravastatin in acute ischaemic syndromes: results of a randomised placebo‐controlled trial. International Journal of Clinical Practice 2001;55(5):300. [PubMed] [Google Scholar]
PRINCESS 2004 {unpublished data only}
- LaBlanche JM, Wright RS, Jukema JW, Charbonneau F, Creplet J, Eldar M, et al. Reductions in early recurrent coronary ischemia from early administration of statin therapy upon admission for myocardial infarction: results of the PRINCESS trial. Circulation 2004;110(17):2339. [Google Scholar]
- Wright RS. PRINCESS: Prevention of ischaemic events by early treatment of cerivastatin after acute myocardial infarction. Hotline session III, European Society of Cardiology. 2004.
PTT 2002 {published and unpublished data}
- Kayikcioglu M, Can L, Evrengul H, Payzin S, Kultursay H. The effect of statin therapy on ventricular late potentials in acute myocardial infarction. International Journal of Cardiology 2003;90(1):63‐72. [DOI] [PubMed] [Google Scholar]
- Kayikcioglu M, Turkoglu C, Kltrsay H, Evrengul H, Can L. The short term results of combined use of pravastatin with thrombolytic therapy in acute myocardial infarction [abstract]. Circulation 1999;100(Suppl 1):I‐303. Abstract 1586. [Google Scholar]
- Kayikcioglu M, Turkoglu C, Kultursay H, Harum H, Can L. Combined use of pravastatin and thrombolytic agents in acute myocardial infarction. Pravastatin turkish trial (PTT). Circulation 1999;100(Suppl 1):1‐303. [Google Scholar]
- Kayikçioğlu M, Can L, Kültürsay H, Payzin S, Turkoğlu C. Early use of pravastatin in patients with acute myocardial infarction undergoing coronary angioplasty. Acta Cardiologica 2002;57(4):295. [DOI] [PubMed] [Google Scholar]
RECIFE 1999 {published and unpublished data}
- Dupuis J, Tardif JC, Cernacek P, Theroux P. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes: the RECIFE (reduction of cholesterol in ischemia and function of the endothelium) trial. Circulation 1999;99(25):3227. [DOI] [PubMed] [Google Scholar]
Ren 2009 {published data only}
- Ren HZ, Ma LL, Wang LX. Effect of simvastatin on plasma interleukin‐6 in patients with unstable angina. Clinical and Investigative Medicine. Médecine Clinique et Experimentale 2009;32(4):E280. [DOI] [PubMed] [Google Scholar]
Sakamoto 2005 {published data only (unpublished sought but not used)}
- Sakamoto T, Kojima S, Ogawa H, Shimomura H, Kimura K, Ogata Y, et al. Effects of early statin treatment on symptomatic heart failure and ischemic events after acute myocardial infarction in Japanese. American Journal of Cardiology 2006;97(8):1165–71. [DOI] [PubMed] [Google Scholar]
Shal'nev 2007 {published data only}
- Shal'nev VI. The effects of early application of simvastatin on C‐reactive protein level, blood lipids, and the clinical course of acute coronary syndrome. Klinicheskaia Meditsina 2007;85(11):46‐50. [PubMed] [Google Scholar]
References to studies excluded from this review
Akasaka 2012 {published data only}
- Akasaka T, Komukai K, Ishibashi K, Tanimoto T, Matsuo Y, Ino Y, et al. Effect of intensive vs. moderate lipid‐lowering therapy with atorvastatin on the stabilization of atherosclerosis in acute coronary syndromes: serial optical coherence tomography analysis. European Heart Journal 2012;33:355. [Google Scholar]
Barderas 2009 {published data only}
- Barderas MG, Tunón J, Dardé VM, Cuesta F, Jiménez‐Nácher JJ, Tarin N, et al. Atorvastatin modifies the protein profile of circulating human monocytes after an acute coronary syndrome. Proteomics 2009;9(7):1982‐93. [DOI] [PubMed] [Google Scholar]
Bermejo 2006 {published data only}
- Bermejo J, Segovia J, Alfonso F. Summary of the Clinical Studies Reported in the Scientific Sessions in the American Heart Association 2005 (Dallas, Texas, USA, 13–16 November 2005). Revista Espanola de Cardiologia (Internet) 2006;59(2):143–53. [PubMed] [Google Scholar]
Cannon 2004a {published data only}
- Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. New England Journal of Medicine 2004;350(15):1495‐504. [DOI] [PubMed] [Google Scholar]
Chi 2007 {published data only}
- Chi H, Wang S, Chen J, Zhang J. Long‐term effects of simvastatin on protection against atrial fibrillation in patients with acute myocardial infarction. Journal of Geriatric Cardiology 2007;4(3):144. [Google Scholar]
Chiodini 2007 {published data only}
- Chiodini BD, Franzosi MG, Barlera S, Signorini S, Lewis CM, D'Orazio A, et al. Apolipoprotein E polymorphisms influence effect of pravastatin on survival after myocardial infarction in a Mediterranean population: the GISSI‐Prevenzione study. European Heart Journal 2007;28(16):1977‐83. [DOI] [PubMed] [Google Scholar]
Chyrchel 2011 {published data only}
- Chyrchel M, Dudek D, Rzeszutko L, Dziewierz A, Chyrchel B, Rakowski T, et al. Effects of short‐term anti‐inflammatory therapy on endothelial function in patients with non‐ST‐segment elevation acute coronary syndrome. Cardiovascular Revascularization Medicine 2011;12:2‐9. [DOI] [PubMed] [Google Scholar]
Colivicchi 2010 {published data only}
- Colivicchi F, Tubaro M, Mocini D, Genovesi EA, Strano S, Melina G, et al. Full‐dose atorvastatin versus conventional medical therapy after non‐ST‐elevation acute myocardial infarction in patients with advanced non‐revascularisable coronary artery disease. Current Medical Research and Opinion 2010;26(6):1277‐84. [DOI] [PubMed] [Google Scholar]
Colivicchi 2010a {published data only}
- Colivicchi F, Tubaro M, Mocini D, Genovesi Ebert A, Strano S, Melina G, et al. Full‐dose atorvastatin versus conventional medical therapy after non‐ST‐elevation acute myocardial infarction in patients with advanced non‐revascularisable coronary artery disease. Current Medical Research & Opinion 2010;26:1277‐84. [DOI] [PubMed] [Google Scholar]
Colivicchi 2011 {published data only}
- Colivicchi F, Colaiaco C, Golia E, Tubaro M, Aiello A, Aspromonte N, et al. Full‐dose atorvastatin reduces hospitalizations for heart failure after non‐ST‐elevation acute myocardial infarction in patients with advanced non‐revascularisable coronary artery disease. Giornale Italiano di Cardiologia 2011;1:15S. [Google Scholar]
Correia 2002 {published data only}
- Correia LC, Sposito AC, Passos LC, Lima JC, Braga JC, Rocha MS, et al. Short‐term effect of atorvastatin (80 mg) on plasma lipids of patients with unstable angina pectoris or non‐Q‐wave acute myocardial infarction. American Journal of Cardiology 2002;90(2):162‐4. [DOI] [PubMed] [Google Scholar]
de Winter 2005 {published data only}
- Winter RJ, Windhausen F, Cornel JH, Dunselman PHJM, Janus CL, Bendermacher PEF, et al. Early invasive versus selectively invasive management for acute coronary syndromes. New England Journal of Medicine 2005;353(11):1095. [DOI] [PubMed] [Google Scholar]
Dohi 2010 {published data only}
- Dohi T, Miyauchi K, Okazaki S, Yokoyama T, Yanagisawa N, Tamura H, et al. Early intensive statin treatment for six months improves long‐term clinical outcomes in patients with acute coronary syndrome (Extended‐ESTABLISH trial): a follow‐up study. Atherosclerosis 2010;210:497‐502. [DOI] [PubMed] [Google Scholar]
FLAME 2006 {published data only}
- Wojakowski W, Maslankiewicz K, Ochala A, Pyrlik A, Ciosek J, Wieja P, et al. Early treatment with simvastatin in myocardial infarction to reduce the levels of multiple inflammatory markers in 6‐month follow‐up. FLAME randomized clinical trial. Circulation 2006;114:II_650‐1. [Google Scholar]
Ge 2010 {published data only}
- Ge J, Kim YJ, Jang YS, Zhu J, Marschner IC, Lam W. Design and rationale of a study in Asia of atorvastatin pretreatment in patients undergoing percutaneous coronary intervention for non‐ST elevation acute coronary syndromes. Journal of Cardiology 2010;55:303‐8. [DOI] [PubMed] [Google Scholar]
Gómez‐Doblas 2006 {published data only}
- Gómez‐Doblas JJ, Jimenez‐Navarro MF, Garcia‐Pinilla JM, Rodriguez‐Bailon I, Robledo J, Cabrera F, et al. Effect of statin treatment begun early after acute myocardial infarction on endothelial function in patients with normal levels of cholesterol. VAATOPE Study (VAlue of ATOrvastatin in Postinfarction Endothelium) [Influencia del tratamiento temprano con estatinas en la función endotelial tras el infarto de miocardio en pacientes con valores normales de colesterol. Estudio VAATOPE]. Medicina Clinica 2006;126(9):325–8. [DOI] [PubMed] [Google Scholar]
Gómez‐Hernández 2008 {published data only}
- Gómez‐Hernández A, Sánchez‐Galán E, Ortego M, Martin‐Ventura JL, Blanco‐Colio LM, Tarin‐Vicente N, et al. Effect of intensive atorvastatin therapy on prostaglandin E2 levels and metalloproteinase‐9 activity in the plasma of patients with non‐ST‐elevation acute coronary syndrome. American Journal of Cardiology 2008;102:12‐8. [DOI] [PubMed] [Google Scholar]
Guazzi 2007 {published data only}
- Guazzi M, Tumminello G, Reina G, Vicenzi M, Guazzi MD. Atorvastatin therapy improves exercise oxygen uptake kinetics in post‐myocardial infarction patients. European Journal of Clinical Investigation 2007;37(6):454–62. [DOI] [PubMed] [Google Scholar]
Hall 2009 {published data only}
- Hall AS, Jackson BM, Farrin AJ, Efthymiou M, Barth JH, Copeland J, et al. A randomized, controlled trial of simvastatin versus rosuvastatin in patients with acute myocardial infarction: the Secondary Prevention of Acute Coronary Events‐‐Reduction of Cholesterol to Key European Targets Trial. European Journal of Cardiovascular Prevention & Rehabilitation 2009;16:712‐21. [DOI] [PubMed] [Google Scholar]
He 2011 {published data only}
- He XZ, Zhou SH, Wan XH, Wang HY, Zhong QH, Xue JF. The effect of early and intensive statin therapy on ventricular premature beat or nonsustained ventricular tachycardia in patients with acute coronary syndrome. Clinical Cardiology 2011;34:59‐63. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Hiro 2009 {published data only}
- Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN‐ACS [Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome] Study). Journal of the American College of Cardiology 2009;54(4):293–302. [DOI] [PubMed] [Google Scholar]
Hiro 2009a {published data only}
- Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN‐ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). Journal of the American College of Cardiology 2009;54(4):293‐302. [DOI] [PubMed] [Google Scholar]
Hiro 2010 {published data only}
- Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, et al. Diabetes mellitus is a major negative determinant of coronary plaque regression during statin therapy in patients with acute coronary syndrome‐‐serial intravascular ultrasound observations from the Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome Trial (the JAPAN‐ACS Trial). Circulation Journal 2010;74:1165‐74. [DOI] [PubMed] [Google Scholar]
Kanadasi 2006 {published data only (unpublished sought but not used)}
- Kanadaşı M, Çaylı M, Demirtaş M, Inal T, Demir M, Koç M, et al. The effect of early statin treatment on inflammation and cardiac events in acute coronary syndrome patients with low‐density lipoprotein cholesterol. Heart and Vessels 2006;21(5):291–7. [DOI] [PubMed] [Google Scholar]
Kashima 2009 {published data only}
- Kashima Y, Izawa A, Aizawa K, Koshikawa M, Kasai H, Tomita T, et al. Rationale and design of assessment of lipophilic vs. hydrophilic statin therapy in acute myocardial infarction (the ALPS‐AMI) study. Journal of Cardiology 2009;54(1):76–9. [DOI] [PubMed] [Google Scholar]
Kuznetsova 2010 {published data only}
- Kuznetsova MA, Vaulin NA, Masenko VP, Gratsianskii NA. Non‐ST‐elevation acute coronary syndrome. Comparison of effects of atorvastatin and rosuvastatin on blood levels of lipids and markers of inflammation. Kardiologiia 2010;50:21‐5. [PubMed] [Google Scholar]
Lablanche 2008 {published data only}
- Lablanche JM, Danchin N, Farnier M, Tedgui A, Vicaut E, Alonso J, et al. Effects of rosuvastatin and atorvastatin on the apolipoprotein B/apolipoprotein A‐1 ratio in patients with an acute coronary syndrome: the CENTAURUS trial design. Archives of Cardiovascular Diseases 2008;101(6):399–406. [DOI] [PubMed] [Google Scholar]
LAVA 2005 {published data only}
- Wojakowski W, Michalowska A, Majka M, Maslankiewicz K, Wyderka R, Krol M, et al. Early treatment with fluvastatin enhances the mobilization of CD34+, CD117+, CXCR4+, C‐met+ stem cells into peripheral blood in patients with acute myocardial infarction: LAVA trial. Journal of the American College of Cardiology 2005;45(Suppl A, February):A197‐8. [Google Scholar]
Lemos 2005 {published data only}
- Lemos PA, Feyter PJ, Serruys PW, Saia F, Arampatzis CA, Disco C, et al. Fluvastatin reduces the 4‐year cardiac risk in patients with multivessel disease. International Journal of Cardiology 2005;98(3):479–86. [DOI] [PubMed] [Google Scholar]
Leone 2008 {published data only}
- Leone AM, Rutella S, Giannico MB, Perfetti M, Zaccone V, Brugaletta S, et al. Effect of intensive vs standard statin therapy on endothelial progenitor cells and left ventricular function in patients with acute myocardial infarction: Statins for regeneration after acute myocardial infarction and PCI (STRAP) trial. International Journal of Cardiology 2008;130:457‐62. [DOI] [PubMed] [Google Scholar]
Li 2005 {published data only}
- Li JJ, Fang CH. Effects of 4 weeks of atorvastatin administration on the antiinflammatory cytokine interleukin‐10 in patients with unstable angina. Clinical Chemistry 2005;51(9):1735. [DOI] [PubMed] [Google Scholar]
Li 2006 {published data only}
- Li JJ, Li YS, Fang CH, Hui RT, Yang YJ, Cheng JL, et al. Effects of simvastatin within two weeks on anti‐inflammatory cytokine interleukin 10 in patients with unstable angina. BMJ 2006;92(4):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2012 {published data only}
- Lim SY, Bae EH, Choi JS, Kim CS, Park JW, Ma SK, et al. Effect on short‐ and long‐term major adverse cardiac events of statin treatment in patients with acute myocardial infarction and renal dysfunction. American Journal of Cardiology 2012;109:1425‐30. [DOI] [PubMed] [Google Scholar]
Link 2006 {published data only}
- Link A, Ayadhi T, Bohm M, Nickenig G. Rapid immunomodulation by rosuvastatin in patients with acute coronary syndrome. European Heart Journal 2006;27(24):2945. [DOI] [PubMed] [Google Scholar]
Link 2006a {published data only}
- Link A, Ayadhi T, Bohm M, Nickenig G. Rapid immunomodulation by rosuvastatin in patients with acute coronary syndrome. European Heart Journal 2006;27:2945‐55. [DOI] [PubMed] [Google Scholar]
Link 2011 {published data only}
- Link A, Selejan S, Hewera L, Walter F, Nickenig G, Bohm M. Rosuvastatin induces apoptosis in CD4(+)CD28 (null) T cells in patients with acute coronary syndromes. Clinical Research in Cardiology 2011;100:147‐58. [DOI] [PubMed] [Google Scholar]
Liu 2012 {published data only}
- Liu P, Jiang J, Li J, Hong T, Zhang Y, Yu R, et al. Intensive statin therapy for Chinese patients with coronary artery disease undergoing percutaneous coronary intervention (ISCAP study): rationale and design. Catheterization & Cardiovascular Interventions 2012;79:967‐71. [DOI] [PubMed] [Google Scholar]
Miyauchi 2006 {published data only}
- Miyauchi K, Kimura T, Morimoto T, Nakagawa Y, Yamagishi M, Ozaki Y, et al. Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome (JAPAN‐ACS): rationale and design. Circulation Journal 2006;70:1624‐8. [DOI] [PubMed] [Google Scholar]
Monteiro 2008 {published data only}
- Monteiro CMC, Oliveira L, Izar MCO, Santos AO, Povoa RMS, Fischer SM, et al. Early effects of lipid lowering treatment in subjects with metabolic syndrome and acute coronary syndromes. International Journal of Atherosclerosis 2008;3:93‐9. [Google Scholar]
Nakamura 2008 {published data only}
- Nakamura T, Obata J, Kitta Y, Takano H, Kobayashi T, Fujioka D, et al. Rapid stabilization of vulnerable carotid plaque within 1 month of pitavastatin treatment in patients with acute coronary syndrome. Journal of Cardiovascular Pharmacology 2008;51(4):365. [DOI] [PubMed] [Google Scholar]
Nakaya 2005 {published data only}
- Nakaya R, Uzui H, Shimizu H, Nakano A, Mitsuke Y, Yamazaki T, et al. Pravastatin suppresses the increase in matrix metalloproteinase‐2 levels after acute myocardial infarction. International Journal of Cardiology 2005;105(1):67–73. [DOI] [PubMed] [Google Scholar]
Ordulu 2008 {published data only}
- Ordulu E, Erdogan O. Early effects of low versus high dose atorvastatin treatment on coagulation and inflammation parameters in patients with acute coronary syndromes. International Journal of Cardiology 2008;128(2):282–4. [DOI] [PubMed] [Google Scholar]
Ostadal 2003 {published data only}
- Ostadal P, Alan D, Hajek P, Horak D, Vejvoda J, Trefanec J, et al. The effect of early treatment by cerivastatin on the serum level of C‐reactive protein, interleukin‐6, and interleukin‐8 in the patients with unstable angina and non‐Q‐wave myocardial infarction. Molecular and Cellular Biochemistry 2003;246(1‐2):45‐50. [PubMed] [Google Scholar]
Patti 2007 {published data only}
- Patti G, Pasceri V, Colonna G, Miglionico M, Fischetti D, Sardella G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA‐ACS randomized trial. Journal of the American College of Cardiology 2007;49(12):1272–8. [DOI] [PubMed] [Google Scholar]
PEACE 2005 {published data only}
- Min Z, Zhao M, Jia SQ. Pravastatin early use in acute coronary evaluation (PEACE) trial. American Journal of Cardiology 2005;95(Suppl 8, April):74A. [Google Scholar]
Pedersen 2000 {published data only}
- Pedersen TR, Jahnsen KE, Vatn S, Semb AG, Kontny F, Zalmai A, et al. Benefits of early lipid‐lowering intervention in high‐risk patients: the lipid intervention strategies for coronary patients study. Clinical Therapeutics 2000;22(8):949‐60. [DOI] [PubMed] [Google Scholar]
Pedersen 2005 {published data only}
- Pedersen TR, Faergeman O, Kastelein JJP, Olsson AG, Tikkanen MJ, Holme I, et al. High‐dose atorvastatin vs usual‐dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005;294(19):2437. [DOI] [PubMed] [Google Scholar]
Pitt 2008 {published data only}
- Pitt B, Loscalzo J, Ycas J, Raichlen JS. Lipid levels after acute coronary syndromes. Journal of the American College of Cardiology 2008;51(15):1440‐5. [DOI] [PubMed] [Google Scholar]
Pitt 2012 {published data only}
- Pitt B, Loscalzo J, Monyak J, Miller E, Raichlen J. Comparison of lipid‐modifying efficacy of rosuvastatin versus atorvastatin in patients with acute coronary syndrome (from the LUNAR study). American Journal of Cardiology 2012;109:1239‐46. [DOI] [PubMed] [Google Scholar]
Post 2012 {published data only}
- Post S, Post MC, Branden BJ, Eefting FD, Goumans MJ, Stella PR, et al. Early statin treatment prior to primary PCI for acute myocardial infarction: REPERATOR, a randomized placebo‐controlled pilot trial. Catheterization and Cardiovascular Interventions 2012;80:756‐65. [DOI] [PubMed] [Google Scholar]
Sakata 2005 {published data only}
- Sakata Y, Sato H, Kinjo K, Fujii K, Kodama K, Tanouchi J, et al. Rationale and design of the OACIS‐LIPID study that evaluates early use of pravastatin in acute myocardial infarction. Heart Drug 2005;5(4):193–6. [Google Scholar]
Shah 2007 {published data only}
- Shah HD, Parikh KH, Chag MC, Shah UG, Baxi HA, Chandarana AH, et al. Beneficial effects of the addition of fenofibrate to statin therapy in patients with acute coronary syndrome after percutaneous coronary interventions. Experimental and Clinical Cardiology 2007;12(2):91‐6. [PMC free article] [PubMed] [Google Scholar]
Stefanadi 2009 {published data only}
- Stefanadi E, Tousoulis D, Antoniades C, Katsi V, Bosinakou E, Vavuranakis E, et al. Early initiation of low‐dose atorvastatin treatment after an acute ST‐elevated myocardial infarction, decreases inflammatory process and prevents endothelial injury and activation. International Journal of Cardiology 2009;133(2):266‐8. [DOI] [PubMed] [Google Scholar]
Suh 2011 {published data only}
- Suh SY, Rha SW, Ahn TH, Shin EK, Choi CU, Oh DJ, et al. Long‐term safety and efficacy of pitavastatin in patients with acute myocardial infarction (from the Livalo Acute Myocardial Infarction Study [LAMIS]). American Journal of Cardiology 2011;108:1530‐5. [DOI] [PubMed] [Google Scholar]
Teshima 2009 {published data only}
- Teshima Y, Yufu K, Akioka H, Iwao T, Anan F, Nakagawa M, et al. Early atorvastatin therapy improves cardiac function in patients with acute myocardial infarction. Journal of Cardiology 2009;53(1):58‐64. [DOI] [PubMed] [Google Scholar]
Tousoulis 2006 {published data only}
- Tousoulis D, Antoniades C, Katsi V, Bosinakou E, Kotsopoulou M, Tsioufis C, et al. The impact of early administration of low‐dose atorvastatin treatment on inflammatory process, in patients with unstable angina and low cholesterol level. International Journal of Cardiology 2006;109:48‐52. [DOI] [PubMed] [Google Scholar]
Tousoulis 2006a {published data only}
- Tousoulis D, Antoniades C, Katsi V, Bosinakou E, Kotsopoulou M, Tsioufis C, et al. The impact of early administration of low‐dose atorvastatin treatment on inflammatory process, in patients with unstable angina and low cholesterol level. International Journal of Cardiology 2006;109(1):48–52. [DOI] [PubMed] [Google Scholar]
Tousoulis 2006b {published data only}
- Tousoulis D, Bosinakou E, Kotsopoulou M, Antoniades C, Katsi V, Stefanadis C. Effects of early administration of atorvastatin treatment on thrombotic process in normocholesterolemic patients with unstable angina. International Journal of Cardiology 2006;106(3):333–7. [DOI] [PubMed] [Google Scholar]
Xin‐wei 2009 {published data only}
- Xin‐wei JIA, Xiang‐hua FU, Jing Z, Xin‐shun GU, Wei‐ze FAN, Wei‐li WU, et al. Intensive cholesterol lowering with statin improves the outcomes of percutaneous coronary intervention in patients with acute coronary syndrome. Chinese Medical Journal 2009;122(6):659–64. [PubMed] [Google Scholar]
Yun 2009 {published data only}
- Yun KH, Jeong MH, Oh SK, Rhee SJ, Park EM, Lee EM, et al. The beneficial effect of high loading dose of rosuvastatin before percutaneous coronary intervention in patients with acute coronary syndrome. International Journal of Cardiology 2009;137(3):246‐51. [DOI] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang X, Wang H, Liu S, Gong P, Lin J, Lu J, et al. Intensive‐dose atorvastatin regimen halts progression of atherosclerotic plaques in new‐onset unstable angina with borderline vulnerable plaque lesions. Journal of Cardiovascular Pharmacology and Therapeutics 2013;18:119‐25. [DOI] [PubMed] [Google Scholar]
Zhao 2009 {published data only}
- Zhao Z, Geng J, Ge ZM, Wang W, Zhang Y, Kang WQ. Efficacy and safety of atorvastatin during early hospitalization in elderly patients with unstable angina. Clinical and Experimental Pharmacology & Physiology 2009;36(5‐6):554‐8. [DOI] [PubMed] [Google Scholar]
Zheng 2009 {published data only}
- Zheng XY, Liu L, Yang DG. Early effect of atorvastatin, alone and in combination with probucol, on endothelial dysfunction in patients with acute coronary syndrome. Atherosclerosis Supplements 2009;Conference. [PubMed] [Google Scholar]
Zheng 2009a {published data only}
- Zheng XY, Liu L, Zhao SP. Effects of atorvastatin, alone and in combination with probucol on endothelial function in patients with acute coronary syndrome. Chung‐Hua Hsin Hsueh Kuan Ping Tsa Chih [Chinese Journal of Cardiology] 2009;37:900‐3. [PubMed] [Google Scholar]
Additional references
4S 1994
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344(8934):1383‐9. [PubMed] [Google Scholar]
AHA 2007
- American Heart Association. Heart disease and stroke statistics ‐ 2007 update. Available at www.americanheart.org/statistics 2006 (accessed August 2007).
Als‐Nielsen 2003
- Als‐Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events?. JAMA 2003;290(7):921‐8. [DOI] [PubMed] [Google Scholar]
Ara 2009
- Ara R, Pandor A, Stevens J, Rees A, Rafia R. Early high‐dose lipid‐lowering therapy to avoid cardiac events: a systematic review and economic evaluation. Health Technology Assessment 2009;13(34):1‐118. [DOI] [PubMed] [Google Scholar]
Armitage 2010
- Armitage J, Bowman L, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double‐blind randomised trial. Lancet 2010;376(9753):1658‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aronow 2001
- Aronow HD, Topol EJ, Roe MT, Houghtaling PL, Wolski KE, Lincoff AM, et al. Effect of lipid‐lowering therapy on early mortality after acute coronary syndromes: an observational study. Lancet 2001;357(9262):1063‐8. [DOI] [PubMed] [Google Scholar]
Baigent 2010
- Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376(9753):1670‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Briel 2004
- Briel M, Studer M, Glass TR, Bucher HC. Effects of statins on stroke prevention in patients with and without coronary heart disease: a meta‐analysis of randomized controlled trials. American Journal of Medicine 2004;117(8):596‐606. [DOI] [PubMed] [Google Scholar]
Cannon 2004b
- Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. New England Journal of Medicine 2004;350(15):1495‐504. [DOI] [PubMed] [Google Scholar]
Deeks 1998
- Deeks J, Bradburn M, Localio R, Berlin J. Much ado about nothing: statistical methods for meta‐analysis with rare events. 6th Cochrane Colloquium, Baltimore, MD, USA. 1998.
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fonarow 2005
- Fonarow GC, Wright RS, Spencer FA, Fredrick PD, Dong W, Every N, et al. Effect of statin use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. American Journal of Cardiology 2005;96(5):611‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Freemantle 2003
- Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials: greater precision but with greater uncertainty?. JAMA 2003;289(19):2554‐9. [DOI] [PubMed] [Google Scholar]
Glesby 1996
- Glesby MJ, Hoover DR. Survivor treatment selection bias in observational studies: examples from the AIDS literature. Annals of Internal Medicine 1996;124(11):999‐1005. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
HPS 2002
- The Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet 2002;360(9326):7‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hulten 2006
- Hulten E, Jackson JL, Douglas K, George S, Villines TC. The effect of early, intensive statin therapy on acute coronary syndrome. A meta‐analysis of randomized controlled trials. Archives of Internal Medicine 2006;166:1814‐21. [DOI] [PubMed] [Google Scholar]
Jackevicius 2002
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002;288(4):462‐7. [DOI] [PubMed] [Google Scholar]
Kumar 2009
- Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clinic Proceedings 2009;84(10):917‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lachin 2000
- Lachin JM. Sample size, power, and efficiency. In: Lachin JM editor(s). Biostatistical Methods. 1st Edition. New York: John Wiley & Sons, 2000:61‐86. [Google Scholar]
Laupacis 2004
- Laupacis A, Mamdani M. Observational studies of treatment effectiveness: some cautions. Annals of Internal Medicine 2004;140(11):923‐4. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
LIPID Study Group 1998
- LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long‐Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. New England Journal of Medicine 1998;339(19):1349‐57. [DOI] [PubMed] [Google Scholar]
Montori 2005
- Montori VM, Permanyer‐Miralda G, Ferreira‐Gonzalez I, Busse JW, Pacheco‐Huergo V, Bryant D, et al. Validity of composite end points in clinical trials. BMJ 2005;330(7491):594‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrissey 2009
- Morrissey RP, Diamond GA, Kaul S. Statins in acute coronary syndromes: do the guideline recommendations match the evidence?. Journal of the American College of Cardiology 2009;54(15):1425‐33. [DOI] [PubMed] [Google Scholar]
Newby 2002
- Newby LK, Kristinsson A, Bhapkar MV, Aylward PE, Dimas AP, Klein WW, et al. Early statin initiation and outcomes in patients with acute coronary syndromes. JAMA 2002;287(23):3087‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nordmann 2000
- Nordmann A, Blattmann L, Gallino A, Khetari R, Martina B, Muller P, et al. Systematic, immediate in‐hospital initiation of lipid‐lowering drugs during acute coronary events improves lipid control. European Journal of Internal Medicine 2000;11(6):309‐16. [DOI] [PubMed] [Google Scholar]
Redelmeier 1998
- Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. New England Journal of Medicine 1998;338(21):1516‐20. [DOI] [PubMed] [Google Scholar]
Ridker 1998
- Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1998;98(9):839‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rosenson 1998
- Rosenson RS, Tangney CC. Antiatherothrombotic properties of statins: implications for cardiovascular event reduction. JAMA 1998;279(20):1643‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Serruys 2002
- Serruys PW, Feyter P, Macaya C, Kokott N, Puel J, Vrolix M, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;287(24):3215‐22. [DOI] [PubMed] [Google Scholar]
Shepherd 1995
- Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, Macfarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. New England Journal of Medicine 1995;333(20):1301‐7. [DOI] [PubMed] [Google Scholar]
Smith 2005
- Smith CS, Cannon CP, McCabe CH, Murphy SA, Bentley J, Braunwald E. Early initiation of lipid‐lowering therapy for acute coronary syndromes improves compliance with guideline recommendations: observations from the Orbofiban in Patients with Unstable Coronary Syndromes (OPUS‐TIMI 16) trial. American Heart Journal 2005;149(3):444‐50. [DOI] [PubMed] [Google Scholar]
Spencer 2004
- Spencer FA, Allegrone J, Goldberg RJ, Gore JM, Fox KA, Granger CB, et al. Association of statin therapy with outcomes of acute coronary syndromes: the GRACE study. Annals of Internal Medicine 2004;140(11):857‐66. [DOI] [PubMed] [Google Scholar]
Sposito 2002
- Sposito AC, Chapman MJ. Statin therapy in acute coronary syndromes: mechanistic insight into clinical benefit. Arteriosclerosis, Thrombosis & Vascular Biology 2002;22(10):1524‐34. [DOI] [PubMed] [Google Scholar]
Staffa 2002
- Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. New England Journal of Medicine 2002;346(7):539‐40. [DOI] [PubMed] [Google Scholar]
Stenestrand 2001
- Stenestrand U, Wallentin L. Early statin treatment following acute myocardial infarction and 1‐year survival. JAMA 2001;285(4):430‐6. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta‐analysis. BMJ 2001;323(7304):101‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 2013
- Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013 Nov 7 [Epub ahead of print]. [DOI: 10.1016/j.jacc.2013.11.002] [DOI]
Studer 2005
- Studer M, Briel M, Leimenstoll B, Glass TR, Bucher HC. Effect of different antilipidemic agents and diets on mortality: a systematic review. Archives of Internal Medicine 2005;165(7):725‐30. [DOI] [PubMed] [Google Scholar]
Thygesen 2007
- Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. European Heart Journal 2007;28(20):2525‐38. [DOI] [PubMed] [Google Scholar]
Walter 2004
- Walter DH. Insights into early and rapid effects of statin therapy after coronary interventions. Current Pharmaceutical Design 2004;10(4):369‐73. [DOI] [PubMed] [Google Scholar]
Wood 1998
- Wood D, Backer G, Faergeman O, Graham I, Mancia G, Pyorala K, et al. Prevention of coronary heart disease in clinical practice. Recommendations of the Second Joint Task Force of European and other Societies on coronary prevention. European Heart Journal 1998;19(10):1434‐503. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Briel 2006a
- Briel M, Schwartz GG, Thompson PL, DeLemos JA, Blazing MA, Es GA, et al. Effects of early treatment with statins on short‐term clinical outcomes in acute coronary syndromes. A meta‐analysis of randomized controlled trials. JAMA 2006;295:2046‐56. [DOI] [PubMed] [Google Scholar]
Briel 2006b
- Briel M, Schwartz GG, Bucher HC, Nordmann AJ. 5012 Effects of early treatment with statins on short‐term clinical outcomes in acute coronary syndromes: a systematic review and collaborative meta‐analysis of randomized trials. European Heart Journal 2006;27(Suppl 1):856. [Google Scholar]
Briel 2011
Vale 2011
- Vale N, Nordmann AJ, Schwartz GG, Lemos J, Colivicchi F, Hartog F, et al. Statins for acute coronary syndrome. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD006870.pub2] [DOI] [Google Scholar]


